1. Introduction
Liposomal doxorubicin (Doxil®) is a liposome formulation encapsulating the chemotherapeutic drug, doxorubicin, thereby decreasing its toxicity [1]. An ammonium sulfate (pH) gradient is used to load and stably retain doxorubicin in the liposome interior. Doxil also contains polyethylene glycol (PEG)-lipid resulting in pegylation of the liposome surface, which enables it to have a prolonged circulation and a reduced volume of distribution, thereby improving tumor uptake through the enhanced permeability and retention effect (EPR) and extending effective tumor therapy [1, 2]. Animal and human studies have shown that Doxil preferentially accumulated in tumor xenografts and human tumors. Doxil had a 20-30-hour blood half clearance time in tumor xenograft-bearing mice and rats and 50-60-hour half clearance time in humans [1-3]. Doxil is approved for the treatment of AIDS-related Kaposi's sarcoma, recurrent ovarian cancer, and metastatic breast cancer [2, 4].
Squamous cell carcinoma of the head and neck (HNSCC) accounts for nearly 5 % of the cancer cases in the United States [5]. Most HNSCC patients present with advanced stage disease (III/IV) [6]. Surgery and radiation therapy are the primary treatment modalities. Although good prognosis have been achieved for early stage disease (I/II), the 5-year survival rates are only 30-40 % for late stage disease due to local recurrence and distant metastases [7]. Combination therapy with various treatment modalities has been developed to improve survival and maintain critical functions [8]. Concomitant administration of chemo- and radiation therapy may enhance the tumor cytotoxicity from radiation, resulting in improved loco-regional tumor therapy but at the cost of increased normal tissue toxicity [7, 9]. High local drug concentration is required to prevent local recurrence and this can be achieved using liposomes as drug delivery systems as they have improved pharmacokinetic profile in comparison to the free drugs. Preclinical and clinical studies with Doxil have shown improved efficacy in head and neck tumors [10, 11] and enhancement of the effect of radiotherapy [12].
Liposomes have been labeled with diagnostic and therapeutic radionuclides [13]. Preclinical studies of liposomes labeled with 186Re [14-16] and 188Re [17-19] have been reported. Theoretical dosimetry studies have suggested that liposomes with therapeutic radionuclides when administered intravenously (iv) would deliver a high radiation absorbed dose to the tumor while sparing the bone marrow and controlling liver and spleen dose to acceptable levels [20, 21]. 186Re is an attractive radionuclide for imaging and therapy because of its 3.78-day half-life with 137 keV gamma emission for scintigraphic imaging and β emission of maximum 1.07 MeV energy with a tissue penetration depth range of 2-4 mm for tumor therapy [22].
Doxil uses an ammonium sulfate gradient to load and retain doxorubicin into the liposomes and the same gradient can be used to load and trap diagnostic radionuclide, technetium-99m (99mTc), and therapeutic radionuclides, 186Re and 188Re with complex of N,N-bis(2-mercaptoethyl)-N′,N′-diethylethylenediamine (BMEDA) [19, 23]. By trapping 186Re in Doxil, both doxorubicin and 186Re will be carried in the same liposome for combination chemo-radionuclide therapy. Although biodistribution of iv administered 99mTc-Doxil has been studied in normal rats [23], therapeutic applications of 186Re-Doxil in head and neck cancer xenografts have not been reported yet. PEG liposomes with a similar lipid composition and particle size, and with ammonium sulfate gradient were prepared as control. Prior to initiating chemo-radionuclide therapy studies, the in vitro stability, pharmacokinetics and biodistribution of iv administered 186Re-Doxil and 186Re-PEG liposomes were investigated. The distribution of both formulations in nude rats with head and neck xenografts was followed for 5 days post administration using micro-single photon emission tomography (SPECT)/computed tomography (CT). In this study, the feasibility of preparation of 186Re-Doxil with high efficiency and its in vitro serum stability, pharmacokinetics, imaging, and biodistribution in a HNSCC model are demonstrated.
2. Materials and methods
2.1. Animal Model
All animal experiments were conducted according to the National Institutes of Health Animal Use Guidelines and with prior approval of our Institutional Animal Care Committee. All experimental procedures were conducted while the animals were anesthetized with 1-3% isoflurane (Vedco, St Joseph, MO) in 100% oxygen using an anesthesia inhalation unit (Bickford, Wales Center, NY).
A previously characterized human head and neck cancer xenograft model in nude rats was used [24]. SCC-4 cell line (ATCC, Manassas, VA) was cultured and maintained at 37°C in an incubator with 5% CO2. When the cells were 80-90% confluent, they were collected and made into a single cell suspension in saline. Male rnu/rnu athymic nude rats (Harlan, Indianapolis, IN) at 4 – 5 weeks age (75 – 100 g) were inoculated subcutaneously with 5 × 106 of SCC-4 tumor cells in 0.20 ml of saline on the dorsum at the level of the scapulae. Tumor dimensions were determined by measuring length (l), width (w), and depth (d) of each tumor using digital calipers. The tumor volume was calculated using the ellipsoid volume formula, V = (π/6)lwd [25]. Animals were used for the study when tumor volume was ∼ 1.5 cm3, which typically occurred between 15 and 16 d after tumor cell inoculation.
2.2. Preparation of liposomes
Doxil®, a commercially available liposomal doxorubicin formulation manufactured by Johnson & Johnson (New Brunswick, NJ) was purchased from Oak Hills Pharmacy (San Antonio, TX). Doxil contains 2 mg/ml of doxorubicin, 3.19 mg/ml of N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (DSPE-PEG 2000), 9.58 mg/ml of fully hydrogenated soy phosphatidylcholine, and 3.19 mg/ml of cholesterol. Control PEG-Liposomes containing ammonium sulfate pH gradient were manufactured in house with the lipid composition and particle diameter in reference of Doxil's lipid composition and particle size (Table 1). PEG-liposomes containing 1,2-distearoyl-sn-glycero-phosphatidylcholine (DSPC) (Avanti Polar Lipids, Pelham, AL), DSPE-PEG 2000 (Avanti Polar Lipids), and cholesterol (Calbiochem, San Diego, CA) (weight ratio 3:1:1) was manufactured following previously reported method with minor modification [14, 26]. Lipid mixture dissolved in a chloroform/methanol mixture (2:1 v/v) [27] was dried to form a lipid thin film by rotary evaporation and desiccated overnight. The lipid film was rehydrated with 300 mM sucrose (Ferro Pfanstiehl Laboratories, Cleveland, OH) in sterile water for injection and warmed to 60°C for complete suspension of lipids and lyophilized overnight. The dried lipid-sucrose mixture was rehydrated with 240 mM ammonium sulfate (Sigma) in sterile water, then subjected to 5 freeze-thaw cycles at 60°C followed by extrusion through a series of polycarbonate filters (2 μm, 400nm, 200 nm, 2 passes each; 100 nm, 5 passes; 50nm, 10 passes) (Lipex Extruder and Whatman Nucleopore filters, Northern Lipids, Vancouver, Canada). The extruded liposome solution was stored at 4°C until needed.
Table 1.
Characteristics of Doxil and control PEG liposomes
| Doxil | PEG liposomes | |
|---|---|---|
| Diameter | 87.3 ± 8.5 nm | 91.3 ± 11.8 nm |
| Phospholipid | 9.58 mg.ml | 17.47 mg/ml |
| PEG 2000-DSPE | 3.19 mg/ml | 4.71 mg/ml |
| Cholesterol | 3.19 mg/ml | 4.71 mg/ml |
| Ammonium Sulfate gradient | 250 mM | 240 mM |
| Total lipid | 15.96 mg/ml | 26.89 mg/ml |
The diameter of Doxil and control PEG-liposomes were measured with 488-nm laser light scattering instrument (Brookhaven Instruments, Holtsville, NY). Phospholipid content was measured for the control PEG-liposomes using Stewart assay [28]. Control PEG-liposomes were checked for bacterial growth and pyrogenicity (University Hospital Pathology Laboratory, San Antonio, TX). No bacterial growth was detected within 14-day culture and endotoxin level was < 5 EU/ml.
2.3. Preparation of 186Re-Doxil / 186Re-PEG-liposomes
To a vial containing 50 mg glucoheptonate (GH) (Sigma-Aldrich, St Louis, MO), and 3.0 μl BMEDA (ABX, Radeberg, Germany), 2.0 ml of nitrogen-degassed saline was added. The mixture was mixed by magnetic stirring for 20 min followed by the addition of 240 μl of freshly prepared stannous chloride solution (15 mg/ml). An aliquot of 1.0 ml of the GH-BMEDA-stannous chloride mixture was placed in a new vial after adjusting the pH of the mixture to 5.0. The vial was flushed with nitrogen and sealed. 186Re-perrhenate solution (∼2.96 GBq (80 mCi); University of Missouri Research Reactor, Columbia, MO) was added to the vial and incubated at 80°C for 1 h. After incubation, the 186Re-BMEDA solution was cooled to 25°C before adjusting the pH of the solution to 7.0. Immediately before using for radiolabelling, Doxil or PEG-liposomes were eluted with PBS (pH 7.4) through a PD-10 column (GE Healthcare, Piscataway, NJ) to create the ammonium sulfate pH gradient by removing free ammonium sulfate from liposome exterior. Eluted Doxil or PEG-liposomes were added to 186Re-BMEDA solution and incubated at 37°C for 1 h. Finally, labeled 186Re-Doxil or 186Re-PEG-liposomes were separated from free 186Re-BMEDA by eluting through PD-10 columns with PBS (pH 7.4). The labeling efficiency was calculated by dividing the 186Re-activity in Doxil / PEG-liposomes after separation by the total 186Re-activity before separation.
2.4. In vitro labeling stability studies
An aliquot of final 186Re-Doxil / 186Re-PEG-liposome sample was added to an aliquot of fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA) in a 1:1 volume ratio and the solution was incubated at 37°C. The amount of 186Re-activity associated with the liposomes at different time points was measured using ACL 4% crosslinked agarose gel (Sooner Scientific, Garvin, OK) spin column [23, 29]. Agarose gel (2.0 ml) was packed in a microcolumn (Bio-Rad, Hercules, CA) by centrifugation at 1500 rpm for 2 min. Then, 10 column volumes of PBS (pH 7.4) were used to equilibrate the column. At the desired time points (1, 4, 24, 48, 72, and 96 h), 100 μl of 186Re-Doxil / 186Re-PEG-liposome serum solution was added to the equilibrated spin column, centrifuged at 1500 rpm for 1 min and the first fraction collected in a tube. Then, 100 μl of PBS (pH 7.4) was added to the column, centrifuged at 1500 rpm for 1 min, and the second fraction collected in a new tube. This elution process was repeated 19 times and each fraction collected in a new tube after centrifugation. The 186Re-activity in each fraction was counted using a Minaxi γ A5550 gamma counter (PerkinElmer Life and Analytical Sciences, Boston, MA). The percentage of 186Re-activity associated with Doxil / PEG-liposomes was calculated by summing the activity in the first 8 fractions divided by the total activity in all 20 fractions. The above procedure was repeated with 186Re-Doxil and 186Re-PEG-liposomes stored in PBS (pH 7.4) at 25°C at the same time points (1, 4, 24, 48, 72, and 96 h).
2.5. Biodistribution and pharmacokinetic studies
Fourteen male nude rats with HNSCC xenografts were used for this study. 186Re-Doxil (n = 7) or 186Re-PEG-liposomes (n = 7) was intravenously (iv) injected through the tail vein at the dose level of 555 MBq/kg (15 mCi/kg) under anesthesia. The total lipid dose for both groups was maintained at 52 mg/kg. The doxorubicin dose was maintained at 6.5 mg/kg for the 186Re-Doxil group. Rats were sacrificed at 120 h post injection by cervical dislocation. All major organs and tissues were collected in 10% buffered formalin (Fisher Scientific, Pittsburgh, PA). The organs were weighed and counted for 186Re-activity using Wallac Wizard automatic gamma counter (PerkinElmer Life and Analytical Sciences, Boston, MA). A 50 μl standard of 186Re-Doxil or 186Re-PEG-liposomes was also counted and used for decay correction. Data are expressed as percentage of injected dose per gram (%ID/g) and percentage of injected dose per organ (%ID/ organ).
The pharmacokinetics of 186Re-Doxil and 186Re-PEG-liposomes were determined by collecting blood samples in a micro-centrifuge tube through the tail vein at 0.08 (5 min), 0.5, 1, 2, 4, 8.5, 24, 48, 72, 96, and 120 h after iv injection. The weight of each blood sample was determined by weighing the micro-centrifuge tube before and after blood collection. The concentrations of radioactivity in blood were calculated as %ID/g and the %ID/g at 5 min was normalized to 100%. The blood clearance patterns of 186Re-Doxil and 186Re-PEG-liposomes were simulated using Origin software, version 7.5 (Origin Lab, Northampton, MA). A dual exponential equation Y = b1 × e-c1 ×t + b2× e-c2 ×t was used. Here Y is the %ID in blood; t is the time after injection; b1, b2, c1, and c2 are constants. The two-phase blood clearance half-times [(t1/2)1 and (t1/2)2] were calculated from the simulated dual-exponential curves as follows: (t1/2)1 = 0.693/c1; (t1/2)2 = 0.693/c2 [23].
2.6. Micro-SPECT/CT imaging and image analysis
186Re has a penetrative 137 keV gamma emission that allows for monitoring of the biodistribution of labeled liposomes using gamma scintigraphy. High resolution parallel hole collimator (HRES) planar gamma camera images and SPECT images were acquired in 137 (±10%) keV energy window using a microSPECT scanner equipped with dual cadmium zinc telluride (CZT) detectors (FLEX SPECT/CT/PET, Gamma Medica, Northridge, CA). Static planar images were acquired in two views, anterior-posterior (AP) and lateral at baseline, 4, 20, 46, 70, 96, and 118 h after 186Re-Doxil/PEG-liposome injection. A standard source of 186Re-Doxil or 186RePEG-liposomes of ∼ 0.26 MBq (∼70 μCi) was placed in the field of view but outside the position of the rat during static planar image acquisition for image quantification. Tomographic images with parallel hole collimators (32 projections, 7000 counts/projection) were acquired at the same time points as the planar static images and also acquired with multi-pinhole (MPH) collimators (32 projections, 7000 counts/projection, Radius of rotation (ROR) = 5 cm, Field of View (FOV) = 9.78 cm) at 20 h. SPECT images reconstructed using the Lumagen® processing software available with the system had a matrix size of 80 × 80 × 80 and voxel dimension of 1.6 mm. CT images were acquired at 20 h and reconstructed at matrix size of 512 × 512 × 512 with 0.17 mm voxel dimension using the software available with the scanner.
The planar images acquired at each time point were analyzed to determine the %ID/g in blood, liver, spleen, kidneys, intestines, and tumor. Region of interest (ROI) was drawn around the standard source to obtain counts to activity (mCi) conversion factor. To determine the %ID/g in tumor at 4 h, ROI was drawn over the tumor in the lateral images and the counts obtained were converted to activity. Using the weight of the tumor obtained at 120 h after biodistribution and injected activity, %ID/g in tumor at 4 h was determined. No blood pool correction was applied. The %ID/g for the listed organs at each time point for both 186Re-Doxil and 186Re-PEG-liposomes were determined as above.
2.7. Autoradiography and Histopathology
From the tumor excised at 120 h, a thin slice (∼ 1 mm) was sectioned along the longest dimension of tumor for autoradiography. The thin section was placed on a reusable phosphor imaging plate (DenOptix®, Gendex Dental Systems, Lake Zurich, IL) at -20°C to obtain the autoradiography image. The plate was exposed to the tumor section for 2 h for 186Re-Doxil and for 20 h for 186Re-PEG-liposomes. The latent image was converted to digital image by laser photostimulation scanning (Gendex Dental Systems, Lake Zurich, IL). The same tumor section was then fixed in 10% buffered formalin for 48 h and embedded in paraffin. Four μm-thick sections of each tumor specimen was prepared and stained with hematoxylin and eosin (H&E) for histopathological examination and comparison with the autoradiography images.
2.8. Statistical analysis
The data are shown as average ± standard deviation (SD). Group comparisons were performed using ANOVA with Origin software (Origin Lab, Northampton, MA). P < 0.05 was considered significant.
3. Results
3.1. Labeling efficiencies
The labeling efficiencies of 186Re-Doxil and 186Re-PEG-liposomes were 76.1± 8.3 % (n=4) and 77.1 ± 8.4 % (n=4) respectively. There was no significant difference in labeling efficiencies for 186Re-Doxil and 186Re-PEG-liposomes when either 4.44 GBq or 2.22 GBq of 186Re-activity was used for liposome labeling.
3.2. In vitro stability studies
The in vitro stabilities of 186Re-Doxil and 186Re-PEG-liposomes following incubation in FBS at 37°C are shown in Fig. 1A. The 186Re-activity associated with Doxil was 80.42 ± 4.27 % at 4 h and 12.25 ± 1.67 % at 48 h. The 186Re-activity associated with PEG-liposomes was 81.28 ± 4.44 % and 6.06 ± 1.78 % at 4 and 48 h respectively. The 186Re activity associated with Doxil and PEG-liposomes after storing in PBS, pH 7.4 at 25°C is shown in Fig. 1B. After storing 186Re-Doxil and 186Re-PEG-liposomes at 25°C in PBS, pH 7.4, for 24 h, there was 26.39 ± 4.00% and 40.25 ± 5.44% of 186Re-activity associated with Doxil and PEG-liposomes respectively.
Figure 1.
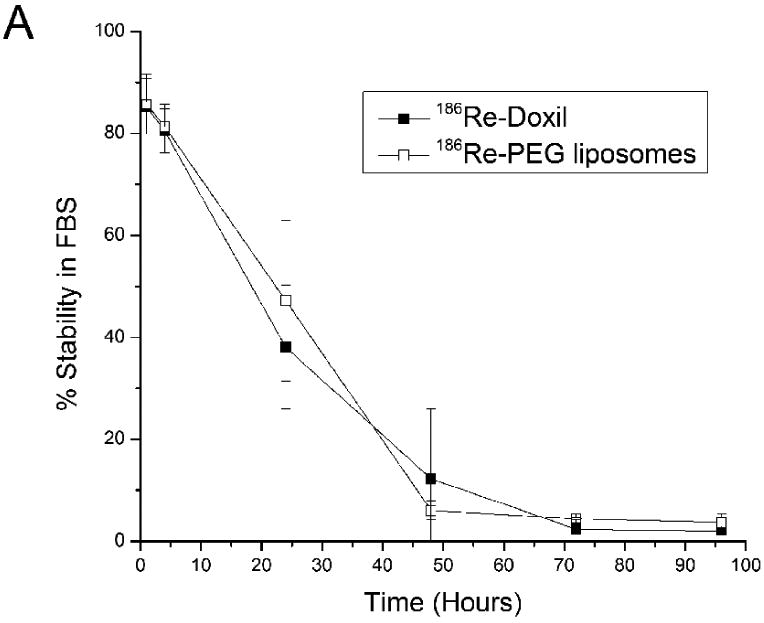
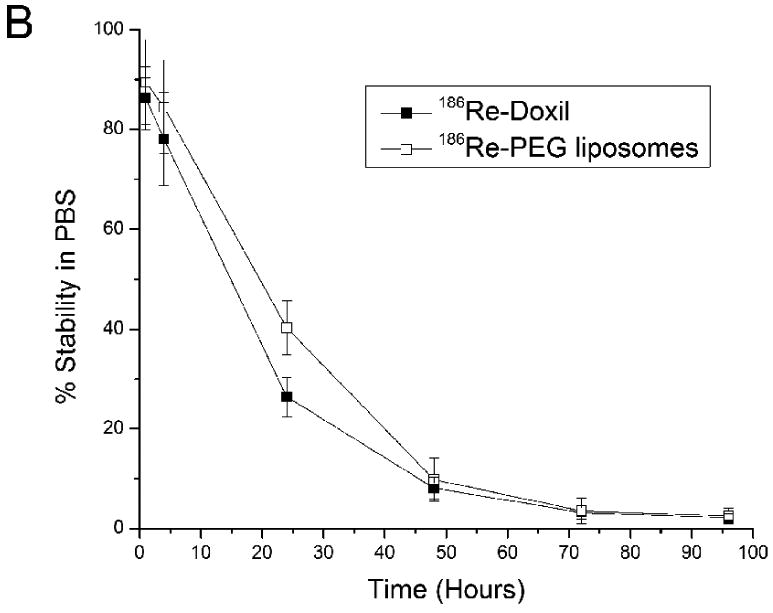
In vitro stability of 186Re-Doxil and 186Re-PEG liposomes at different times after incubation in FBS at 37°C (A) or in PBS, pH 7.4 at 25°C (B) (mean ± SD, n=3).
3.3. Pharmacokinetic studies
The blood clearance curves of 186Re-Doxil and 186Re-PEG-liposomes from baseline to 120 h are shown in Fig. 2. The maximum radioactivities in blood were determined as 2.64 ± 0.09 %ID/g and 3.22 ± 0.39 %ID/g at 0.08 h for 186Re-Doxil and 186Re-PEG-liposomes respectively. 186Re-Doxil showed a slow blood clearance with 1.31 ± 0.07 %ID/g remaining in blood at 24 h. In contrast, 186Re-PEG-liposomes showed a more rapid clearance with 1.66 ± 0.12 % ID/g and 0.11 ± 0.01 % ID/g remaining in blood at 0.5 h and 24 h respectively. Exponential curve-fitting analysis of the clearance curves showed a two-phase blood clearance for both 186Re-Doxil and 186Re-PEG-liposomes. For 186Re-Doxil, 17.04 % of the injected activity had a half-clearance time of 0.8 h and 83.2% of the injected activity had a half-clearance time of 28.2 h. For 186Re-PEG-liposomes, 84.01 % of the injected activity was cleared with a half-clearance of 0.42 h and 14.9% of the injected activity had a half-clearance time of 18.6 h. The half-clearance time for 186Re-Doxil is similar to the reported half-clearance time of Doxil [2], which suggests that the in vivo stability of 186Re-Doxil might be better than that of the in vitro incubation. The half-clearance time of 186Re-Doxil was significantly longer than 186Re-PEG-liposomes. This long circulation time could deliver a higher concentration of 186Re-Doxil into the tumor; thereby improve tumor therapy by chemotherapy from doxorubicin and radionuclide therapy from 186Re.
Figure 2.
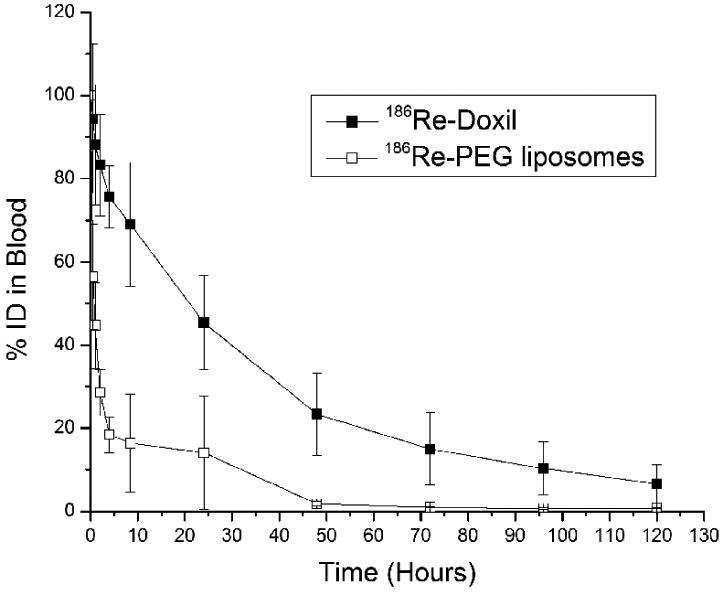
Blood clearance curves of 186Re-Doxil and 186Re-PEG-liposomes after iv administration in HNSCC tumor bearing rats. Data represented as mean %ID ± SD. (n=7 at each time point for each group).
3.4. MicroSPECT/CT imaging and planar image analysis
MicroSPECT/CT images of 186Re-Doxil and 186Re-PEG-liposomes acquired using MPH collimator at 20 h after administration are shown in Fig. 3. Prolonged blood retention, decreased liver uptake and increased tumor uptake was observed in images of rats receiving 186Re-Doxil. Lateral planar scintigraphic images of 186Re-Doxil and 186Re-PEG-liposomes acquired at different time points after intravenous injection are shown in Fig. 4. 186Re-Doxil showed slow blood clearance indicated by high level of radioactivity in the heart, low liver uptake, and high spleen accumulation. 186Re-Doxil also had a consistent high level of accumulation in the abdominal region. In comparison, 186Re-PEG-liposomes showed a more rapid blood clearance and high liver and spleen accumulation. 186Re-Doxil and 186Re-PEG-liposomes had a similar excretion pattern through the kidneys. The %ID/g in tumor and other organs at the different time points determined from image analysis for 186Re-Doxil and 186Re-PEG-liposomes are shown in Fig. 5. The results showed that 186Re-Doxil had significantly higher %ID/ g in blood than 186Re-PEG-liposomes at all time points (p<0.01). The maximum radioactivities in blood were 2.57 ± 0.16 %ID/g and 0.52 ± 0.05 %ID/g at 4 h for 186Re-Doxil and 186Re-PEG-liposomes respectively. For 186Re-PEG-liposomes, the radioactivity in liver, spleen, and tumor reached maximum levels of 3.65 ± 0.42 %ID/g, 1.85 ± 0.85 %ID/g and 0.86 ± 0.43 %ID/g respectively, at 4 h. For 186Re-Doxil, the radioactivity in liver, spleen and tumor reached maximum levels of 1.72 ± 0.11 %ID/g at 20 h, 5.24 ± 1.86 %ID/g at 46 h and 2.06 ± 0.52 %ID/g at 4 h, respectively. The maximum radioactivity in kidney was 7.49 ± 1.32 %ID/g at 46 h for 186Re-Doxil and 8.81 ± 2.03 %ID/g for 186Re-PEG-liposomes at 20 h.
Figure 3.
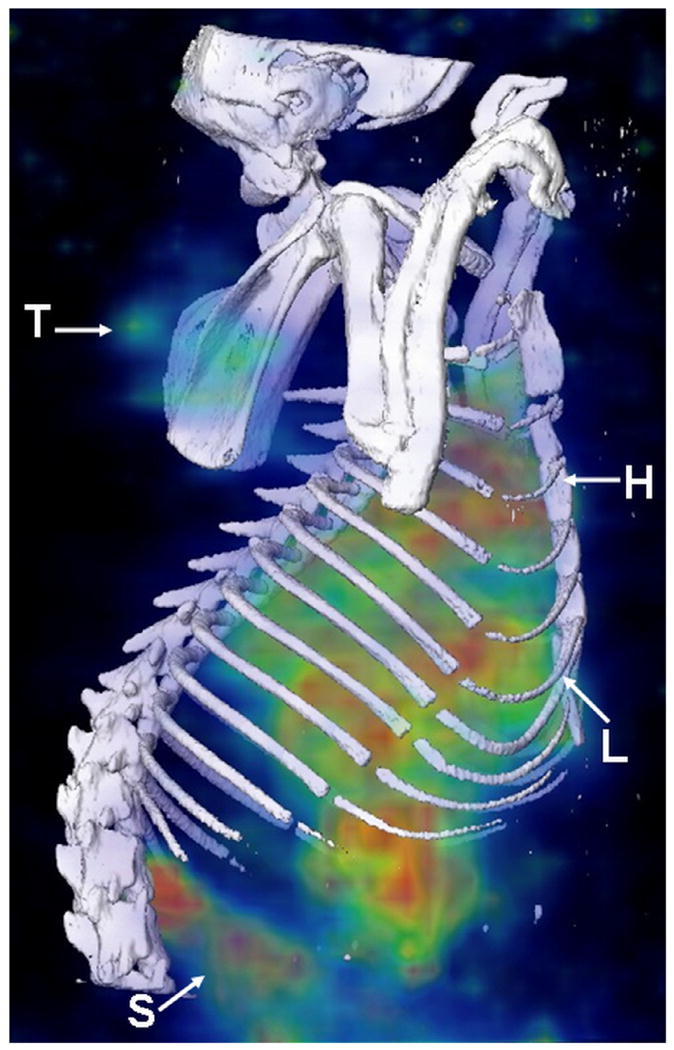
MicroSPECT/CT images acquired at 20 h post administration of 186Re-Doxil using MPH collimator. Three dimensional (3D) volume rendered SPECT image of 186Re-Doxil overlaid with CT isosurface displayed in bone window shows the accumulation in Tumor (T), liver (L), spleen (S) and circulation through heart (H).
Figure 4.
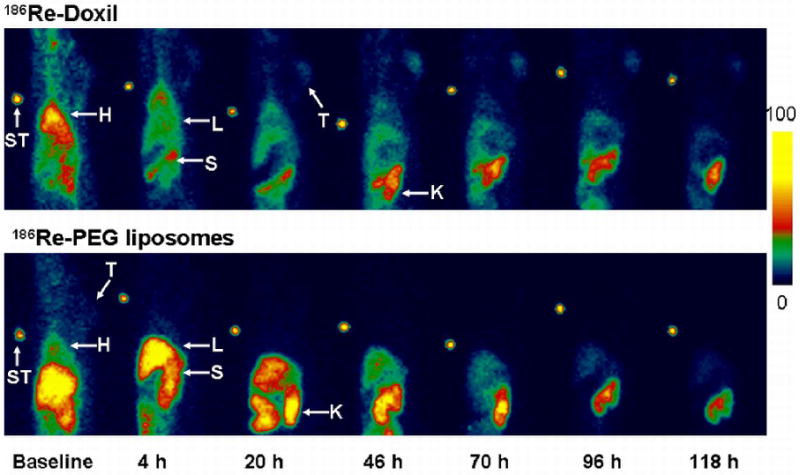
Lateral planar scintigraphic images depicting the distribution of 186Re-Doxil (upper panel) and 186Re-PEG-liposomes (lower panel) at various time points after injection. The slow clearance of 186Re-Doxil, low accumulation in liver and high accumulation in tumor is seen. (H-Heart, L-Liver, S-Spleen, K-Kidney, T-Tumor, STD-Standard).
Figure 5.
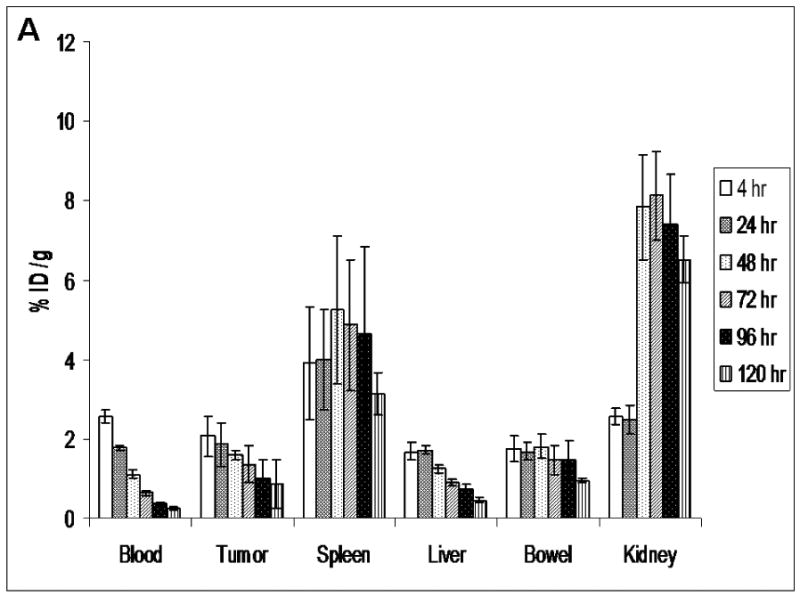
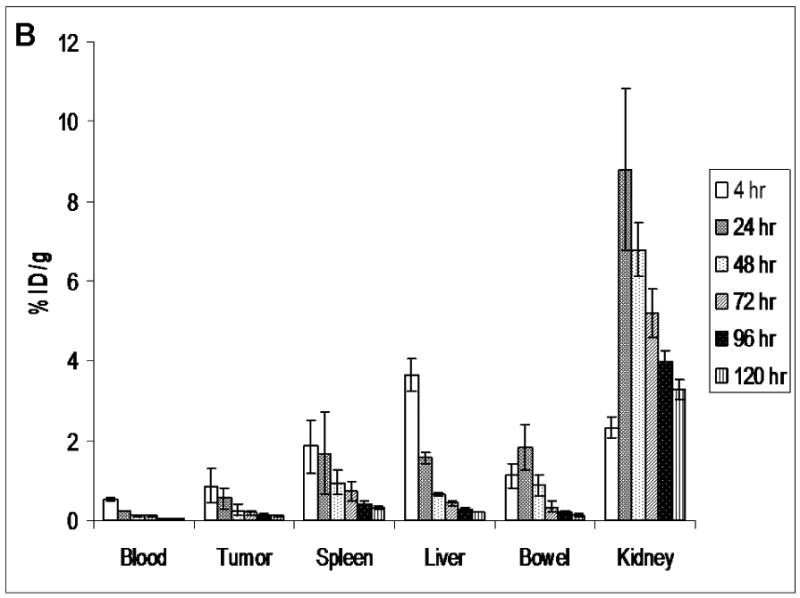
The distribution of 186Re-Doxil (A) and 186Re-PEG-liposomes (B) at various time points determined from planar image analysis. The %ID/g in blood, tumor and spleen are significantly higher for 186Re-Doxil. Data represented as mean ± SD. (n=4 at each time point and for each group).
3.5. Biodistribution studies
The biodistribution values at 120 h after administration of 186Re-Doxil and 186Re-PEG-liposomes are shown in Table 2. Comparison of the %ID per organ for 186Re-Doxil and 186Re-PEG-liposomes showed that the radioactivity in blood was 1.70 ± 1.20 %ID and 0.09 ± 0.07 %ID for 186Re-Doxil and 186Re-PEG-liposomes respectively. The %ID in blood was 20-fold higher for 186Re-Doxil in comparison to 186Re-PEG-liposomes (P < 0.01). Similarly, the %ID in tumor showed a 20-fold increased accumulation for 186Re-Doxil in comparison to 186Re-PEG-liposomes (P < 0.05). The %ID in spleen, muscle, skin, testis, intestines, bone with bone marrow, and feces was significantly higher for 186Re-Doxil in comparison to 186Re-PEG-liposomes (P < 0.001 for spleen, intestines and feces, P < 0.01 for testis and bone with bone marrow, P < 0.05 for muscle and skin). There was no significant difference in the %ID in liver, kidney, and urine at 120 h between 186Re-Doxil and 186Re-PEG-liposomes.
Table 2.
Biodistribution of 186Re-PEG-liposomes and 186Re-Doxil at 120 h after injection (n=7 in each group).
| %ID per organ (mean ± SD) | %ID per gram (mean ± SD) | |||
|---|---|---|---|---|
| Organ | 186Re-Doxil | 186Re-PEG liposomes | 186Re-Doxil | 186Re-PEG liposomes |
| Blood | 1.70±1.20 ** | 0.09±0.07 | 0.14±0.09 | 0.01±0.01 |
| Tumor | 0.36±0.33 * | 0.02±0.01 | 0.37±0.17 | 0.02±0.01 |
| Skin | 2.70±1.85 * | 0.34±0.22 | 0.1±0.06 | 0.01±0.01 |
| Bladder | 0.06±0.04 | 0.03±0.03 | 0.21±0.13 | 0.03±0.04 |
| Stomach | 1.30±0.68 *** | 0.11±0.09 | 0.16±0.09 | 0.02±0.01 |
| Intestines | 0.99±0.27 *** | 0.13±0.07 | 0.20±0.08 | 0.02±0.01 |
| Kidney | 4.24±1.73 | 4.90±2.33 | 5.29±2.06 | 5.51±2.71 |
| Spleen | 0.83±0.33 *** | 0.16±0.1 | 5.27±3.39 | 0.55±0.27 |
| Liver | 4.74±0.52 | 3.77±1.61 | 0.56±0.10 | 0.37±0.14 |
| Lung | 0.14±0.05 *** | 0.03±0.02 | 0.13±0.03 | 0.03±0.02 |
| Heart | 0.03±0.02 ** | 0.01±0.01 | 0.05±0.03 | 0.01±0.01 |
| Testis | 0.03±0.02 ** | 0.01±0.01 | 0.02±0.01 | 0.01±0.004 |
| Bone | 1.84±1.08 ** | 0.20±0.11 | 0.09±0.05 | 0.01±0.005 |
| Muscle | 1.11±0.95 * | 0.26±0.19 | 0.01±0.01 | 0.003±0.002 |
| Brain | 0.01±0.01 | 0.003±0.002 | 0.006±0.004 | 0.002±0.001 |
| Urine | 11.51±2.19 | 11.93±1.96 | 3.14±0.75 | 1.60±0.18 |
| Feces | 14.80±0.43 *** | 20.75±0.35 | 11.27±3.71 | 6.52±0.26 |
Compared with 186Re-PEG liposomes uptake in the same organ;
p<0.05,
p<0.01,
p<0.001.
3.6. Comparison of histopathology and autoradiography images
To determine the distribution of 186Re-Doxil and 186Re-PEG-liposomes in tumor at 120 h after iv administration, the H&E stained specimens were compared with the corresponding autoradiographic images (Fig. 6). Autoradiographically, a higher accumulation of 186Re occurred at the periphery of the tumor than in the center. Overlay of the H&E image and the autoradiographic images showed that the distribution of the radioactivity was in the region of expansion likely a reflection of blood supply. There was decreased accumulation in the tumor center compared to the periphery. This distribution was similar for both 186Re-Doxil and 186Re-PEG-liposomes. It has been reported that the peripheral tumor capsule of this xenograft had increased vascularity as compared to the central portions of the tumor as demonstrated with immunohistochemical stains for endothelial cell markers CD31 and CD34 [24]. The increased extravasation and accumulation of 186Re-Doxil and 186Re-PEG-liposomes in the tumor periphery is expected due to the increased vascularity and integrity of the blood vessels in this region.
Figure 6.
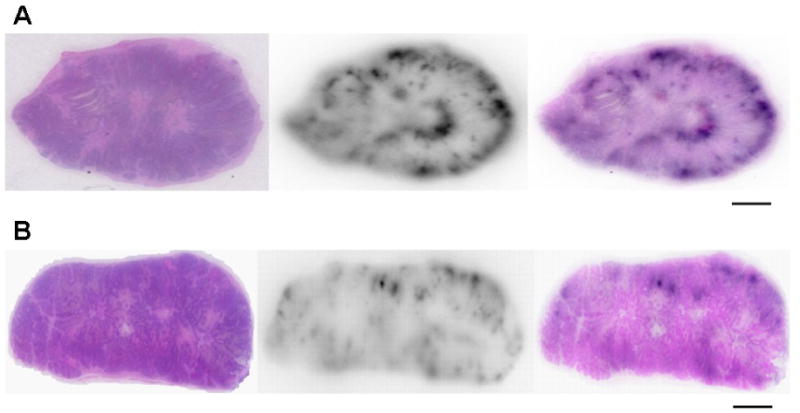
Comparison of H&E stained paraffin sections and autoradiographic images to determine the microdistribution of 186Re in the tumor at 120 h post administration of 186Re-Doxil (A) and 186Re-PEG-liposomes (B). 186Re is in the periphery of both tumor specimens. 186Re-Doxil accumulation was increased in tumor compared to 186Re-PEG-liposomes. (Scale bar: 3 mm).
4. Discussion
Liposomes can be labeled with radioisotopes by trapping them within the inner space, intercalated within the double membrane, or connected to the surface of the liposomes [30]. Liposomes have been labeled with β-emitting therapeutic radionuclides, such as 131I, 90Y, 186Re, and 177Lu [21, 31, 32]. As the method reported by Hafeli et al. [31] required that 186Re/188Re complex be labeled by incorporating it into liposomes during liposome manufacturing, this labeling method was clinically impractical. A method of encapsulating 186Re/188Re in liposomes with high efficiency, good stability and convenience was used in the present studies [14]. Biodistribution and therapy studies have been reported with 186Re-neutral liposomes in normal rats [14] and in tumor bearing rats [15, 16], respectively. Although labeling of Doxil with 99mTc and 111In-oxine has been reported [23, 33], the labeling method used in this paper could directly load therapeutic 186Re/188Re into Doxil without pre-labeling modification of the liposomes.
In this study, the feasibility and characterization of Doxil labeled with 186Re was determined for future tumor chemo-radionuclide therapy studies. According to the method reported by Bao et al. [14, 23], Doxil was labeled using 186Re-BMEDA complex. The 186Re-BMEDA complex was entrapped in Doxil by the ammonium sulfate gradient. Control PEG-liposomes with a similar lipid composition and concentration, ammonium sulfate (pH) gradient and particle size as Doxil was prepared (Table 1) and labeled using 186Re-BMEDA complex. High labeling efficiencies were achieved for 186Re-Doxil (76.1± 8.3%) and 186Re-PEG-liposomes (77.1 ± 8.4%). The in vitro serum stability of 186Re-Doxil was also investigated (Fig. 1). There was almost 40% of 186Re activity associated with Doxil at 24 h. The high stability maintained up to 24 h allows for sufficient accumulation of 186Re-Doxil in the tumor. Leakage of the contents of 186Re-Doxil after 24 h helps release doxorubicin from the liposomes accumulated in the tumor, thus potentially leading to therapeutic effects from both doxorubicin and 186Re in the tumor.
The in vitro stability achieved with 186Re-Doxil and 186Re-PEG-liposomes is different from those reported for 186Re-neutral liposomes [14]. This could be potentially due to difference in liposome formulation between Doxil and neutral liposomes. Since Doxil has a lower amount of cholesterol 186Re could be released earlier from Doxil/PEG-liposomes. Also, 186Re is loaded into an ammonium sulfate gradient occupied by doxorubicin in Doxil and hence has less gradient available for radiolabeling. Another reason could be that higher initial amounts of 186Re-activity per mg of phospholipid were used for this study compared with previous neutral liposome study. Finally, 186Re radioactivity also includes stable 185Re nuclide, which can lead to technical challenges in labeling efficiency and stability compared with the labeling of Doxil using carrier-free 99mTc or 188Re based on the same BMEDA chemistry. The current studies have shown that the high labeling efficiency was not influenced by 185Re carrier; however, the in vitro stability decreased compared with previous reports using carrier-free 188Re activity [17-19].
Although similar in vitro stabilities were observed for 186Re-Doxil and 186Re-PEG-liposomes, the in vivo behavior of 186Re-Doxil and 186Re-PEG-liposomes had a profound difference in blood clearance. Pharmacokinetic studies of 186Re-Doxil showed prolonged blood retention of 186Re-activity with 83.17% of the injected 186Re-Doxil having a clearance time of 28.12 h. The half-clearance time of 186Re-Doxil is similar to that of 20-30 h for Doxil itself as reported previously, which suggests an excellent in vivo stability of 186Re-Doxil [2]. In contrast, 186Re-PEG-liposomes had a rapid clearance from blood and accumulation into the liver. As the tumors in this study were large (1.5 cm3), they were characterized by hypovascular areas and increased interstitial pressure which interfere with the passive targeting and hence decreased liposome uptake [34]. The slow clearance rate allowed for the higher bioavailability of 186Re-Doxil and thus increased passive targeting of 186Re-Doxil to tumors. Control PEG-liposomes were prepared with DSPC which has been shown to have a longer circulation time in mice [35]. In spite of similar lipid composition, ammonium sulfate gradient, and diameter as Doxil, 186Re-PEG-liposomes had a very rapid clearance from the blood (Fig. 3). It has been reported that for liposomes of about 100 nm, 9.6 mol% of DPSE-PEG 2000 is required to achieve optimal blood circulation and reduced uptake in liver and spleen [36]. Our control PEG liposomes had only 5 mol% of DSPE-PEG 2000 and this could be one reason for its faster clearance. The presence of doxorubicin in a crystallized form in Doxil probably renders the liposomes more rigid and stable and hence the 5 mol% of DSPE-PEG 2000 is likely enough to make it invisible to the reticuloendothelial system (RES) of liver and spleen. Further studies are required with PEG-liposomes prepared with increased mol% of DSPE-PEG 2000 or substitution with DSPE-PEG 5000 [37] to match the circulation time of Doxil to determine the pharmacokinetics and accumulation in tumor for potential therapy.
Molecular imaging has been increasingly applied for drug development in preclinical and clinical studies [38] by allowing for the non-invasive assessment of drug efficacy, pharmacokinetics and distribution in the body. 186Re is a therapeutic radionuclide with a 10% γ-emission at 137 keV which allows for the diagnostic imaging and therapy with 186Re-Doxil. In this study, imaging of 186Re-Doxil using planar scintigraphy at various time points and microSPECT/CT at 20 h after iv administration depicted non-invasively the slow blood clearance and low accumulation in the liver. In addition, 186Re-Doxil also accumulated in the intestine and surrounding tissues, which is visible from 4 h after administration and the accumulation is stable up to 120 h. Activity in kidney was seen by 46 h for 186Re-Doxil and 186Re-PEG liposomes which suggests that some of the 186Re-BMEDA released from the metabolized liposomes excreted through the kidney. Higher accumulation was seen in the tumor for 186Re-Doxil in comparison to 186Re-PEG-liposomes. The %ID/g in blood, tumor, liver, spleen, kidney and bowel were determined from planar scintigraphic images for 186Re-Doxil and 186Re-PEG-liposomes. Comparison of %ID/g obtained for the above organs from imaging and biodistribution at 120 h for 186Re-Doxil and 186Re-PEG-liposomes showed that the values were similar to each other. Thus %ID/g obtained at the other time points (4, 20, 46, 70, and 96 h) from imaging would reflect the trend in the accumulation of radioactivity in the organs after administration and hence imaging can be used as a tool for real-time assessment of tumor targeting, distribution, and pharmacokinetics of radiolabeled therapeutic liposomes.
Previous biodistribution studies of 186Re-neutral liposomes showed high radioactivity in spleen, liver and kidney at 72 h [14]. The high level of RES organ uptake with the liposomes is a limitation of liposomal radionuclide therapy as high radiation absorbed dose could be delivered to these organs. In this study, biodistribution at 120 h for 186Re-Doxil showed high %ID/g in spleen and kidney (Table 2). On the basis of results from biodistribution and imaging, kidney would receive a high radiation absorbed dose from 186Re-BMEDA released from the metabolized liposomes and excreted through the kidneys. Thus, kidney would be the dose limiting organ of 186Re-Doxil chemo-radionuclide therapy. The dose may be reduced by using peptides to help remove 186Re activity from the kidney [39, 40].
Active targeting and improved therapeutic efficacy of tumor could be achieved by conjugation of Doxil to a ligand, peptide or antibodies for immunoliposome drug delivery [41]. Active targeting could also improve the distribution and retention of 186Re-Doxil in the tumor. The comparison of 186Re-Doxil H&E and autoradiography images (Fig. 6) showed higher accumulation of radioactivity in the tumor periphery likely a reflection of increased blood supply. Use of antibodies to target 186Re-Doxil to tumor could help achieve homogeneous distribution and retention due to internalization of the targeted Doxil [42]. Preclinical and clinical studies in breast cancer over-expressing HER2 showed that HER2 scFv-targeted liposomal doxorubicin increased the bioavailability and concentration of doxorubicin in tumors which was associated with improved tumor control [41, 43]. By using antibodies to actively target 186Re-Doxil, the radiation absorbed dose to the tumor could result in increased DNA damage due to internalization and improved tumor accumulation.
Systemic radionuclide therapy has been effective for hematologic malignancies but less effective for solid tumors [44]. The low tumor-to-normal tissue radioactivity ratio could be improved by the use of liposomes as delivery vehicles. In this study, we showed that a commercial liposome formulation Doxil could be radiolabeled with 186Re for chemo-radionuclide therapy studies. The use of therapeutic radionuclide 186Re allowed for the simultaneous imaging of distribution of 186Re-Doxil and therapy of tumor. The experimental results from the pharmacokinetic study revealed a slow clearance rate of 186Re-Doxil from blood which allowed for higher passive targeting to tumor. The biodistribution study at 120 h showed a 20-fold increased %ID in blood and tumor for 186Re-Doxil in comparison to 186Re-PEG-liposomes. These findings suggest that the increased passive targeting and concentration of 186Re-Doxil in tumor could result in the achievement of a better therapeutic effect than doxorubicin and 186Re delivered individually in liposomes. Further studies are required to evaluate the therapeutic efficacy and toxicity of iv administered 186Re-Doxil in tumor bearing rats.
Conclusions
Our studies revealed that high labeling efficiency of 186Re-Doxil was achieved. 186Re-Doxil was reasonably stable in 50% FBS and had a long half-clearance time in the body similar to unlabeled Doxil. The results also demonstrated the importance of prolonged circulation time in order to achieve improved EPR-based accumulation in tumor. The biodistribution, pharmacokinetics and imaging studies of 186Re-Doxil in a HNSCC rat xenograft model demonstrated good bioavailability, tumor targeting and localization. Thus 186Re-Doxil may be used for effective chemo-radionuclide therapy with doxorubicin and 186Re being delivered simultaneously in the same liposome. The therapeutic efficacy of 186Re-Doxil will be evaluated in the HNSCC tumor xenograft model in our future investigations.
Acknowledgments
This project was funded by the NIH 5P30CA054174 Supplement Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gabizon AA. Pegylated liposomal doxorubicin: metamorphosis of an old drug into a new form of chemotherapy. Cancer Invest. 2001;19:424–36. doi: 10.1081/cnv-100103136. [DOI] [PubMed] [Google Scholar]
- 2.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42:419–36. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 3.Gabizon AA, Barenholz Y, Bialer M. Prolongation of the circulation time of doxorubicin encapsulated in liposomes containing a polyethylene glycol-derivatized phospholipid: pharmacokinetic studies in rodents and dogs. Pharm Res. 1993;10:703–8. doi: 10.1023/a:1018907715905. [DOI] [PubMed] [Google Scholar]
- 4.Gabizon AA, Shmeeda H, Zalipsky S. Pros and cons of the liposome platform in cancer drug targeting. J Liposome Res. 2006;16:175–83. doi: 10.1080/08982100600848769. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 6.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–94. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 7.Kies MS, Bennett CL, Vokes EE. Locally advanced head and neck cancer. Curr Treat Options Oncol. 2001;2:7–13. doi: 10.1007/s11864-001-0012-x. [DOI] [PubMed] [Google Scholar]
- 8.Salama JK, Seiwert TY, Vokes EE. Chemoradiotherapy for locally advanced head and neck cancer. J Clin Oncol. 2007;25:4118–26. doi: 10.1200/JCO.2007.12.2697. [DOI] [PubMed] [Google Scholar]
- 9.Vokes EE. Interactions of chemotherapy and radiation. Semin Oncol. 1993;20:70–9. [PubMed] [Google Scholar]
- 10.Harrington KJ, Lewanski C, Northcote AD, Whittaker J, Peters AM, Vile RG, et al. Phase II study of pegylated liposomal doxorubicin (Caelyx) as induction chemotherapy for patients with squamous cell cancer of the head and neck. Eur J Cancer. 2001;37:2015–22. doi: 10.1016/s0959-8049(01)00216-7. [DOI] [PubMed] [Google Scholar]
- 11.Harrington KJ, Rowlinson-Busza G, Uster PS, Stewart JS. Pegylated liposome-encapsulated doxorubicin and cisplatin in the treatment of head and neck xenograft tumours. Cancer Chemother Pharmacol. 2000;46:10–8. doi: 10.1007/s002800000128. [DOI] [PubMed] [Google Scholar]
- 12.Harrington KJ, Rowlinson-Busza G, Syrigos KN, Vile RG, Uster PS, Peters AM, et al. Pegylated liposome-encapsulated doxorubicin and cisplatin enhance the effect of radiotherapy in a tumor xenograft model. Clin Cancer Res. 2000;6:4939–49. [PubMed] [Google Scholar]
- 13.Mitra A, Nan A, Line BR, Ghandehari H. Nanocarriers for nuclear imaging and radiotherapy of cancer. Curr Pharm Des. 2006;12:4729–49. doi: 10.2174/138161206779026317. [DOI] [PubMed] [Google Scholar]
- 14.Bao A, Goins B, Klipper R, Negrete G, Phillips WT. 186Re-liposome labeling using 186Re-SNS/S complexes: in vitro stability, imaging, and biodistribution in rats. J Nucl Med. 2003;44:1992–9. [PubMed] [Google Scholar]
- 15.Wang SX, Bao A, Herrera SJ, Phillips WT, Goins B, Santoyo C, et al. Intraoperative 186Re-liposome radionuclide therapy in a head and neck squamous cell carcinoma xenograft positive surgical margin model. Clin Cancer Res. 2008;14:3975–83. doi: 10.1158/1078-0432.CCR-07-4149. [DOI] [PubMed] [Google Scholar]
- 16.Zavaleta CL, Goins BA, Bao A, McManus LM, McMahan CA, Phillips WT. Imaging of 186Re-liposome therapy in ovarian cancer xenograft model of peritoneal carcinomatosis. J Drug Target. 2008;16:626–37. doi: 10.1080/10611860802230372. [DOI] [PubMed] [Google Scholar]
- 17.Chang YJ, Chang CH, Chang TJ, Yu CY, Chen LC, Jan ML, et al. Biodistribution, pharmacokinetics and microSPECT/CT imaging of 188Re-bMEDA-liposome in a C26 murine colon carcinoma solid tumor animal model. Anticancer Res. 2007;27:2217–25. [PubMed] [Google Scholar]
- 18.Chen LC, Chang CH, Yu CY, Chang YJ, Hsu WC, Ho CL, et al. Biodistribution, pharmacokinetics and imaging of (188)Re-BMEDA-labeled pegylated liposomes after intraperitoneal injection in a C26 colon carcinoma ascites mouse model. Nucl Med Biol. 2007;34:415–23. doi: 10.1016/j.nucmedbio.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Chen LC, Chang CH, Yu CY, Chang YJ, Wu YH, Lee WC, et al. Pharmacokinetics, micro-SPECT/CT imaging and therapeutic efficacy of (188)Re-DXR-liposome in C26 colon carcinoma ascites mice model. Nucl Med Biol. 2008;35:883–93. doi: 10.1016/j.nucmedbio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Emfietzoglou D, Kostarelos K, Sgouros G. An analytic dosimetry study for the use of radionuclide-liposome conjugates in internal radiotherapy. J Nucl Med. 2001;42:499–504. [PubMed] [Google Scholar]
- 21.Kostarelos K, Emfietzoglou D. Tissue dosimetry of liposome-radionuclide complexes for internal radiotherapy: toward liposome-targeted therapeutic radiopharmaceuticals. Anticancer Res. 2000;20:3339–45. [PubMed] [Google Scholar]
- 22.Zweit J. Radionuclides and carrier molecules for therapy. Phys Med Biol. 1996;41:1905–14. doi: 10.1088/0031-9155/41/10/004. [DOI] [PubMed] [Google Scholar]
- 23.Bao A, Goins B, Klipper R, Negrete G, Phillips WT. Direct 99mTc labeling of pegylated liposomal doxorubicin (Doxil) for pharmacokinetic and non-invasive imaging studies. J Pharmacol Exp Ther. 2004;308:419–25. doi: 10.1124/jpet.103.059535. [DOI] [PubMed] [Google Scholar]
- 24.Bao A, Phillips WT, Goins B, McGuff HS, Zheng X, Woolley FR, et al. Setup and characterization of a human head and neck squamous cell carcinoma xenograft model in nude rats. Otolaryngol Head Neck Surg. 2006;135:853–7. doi: 10.1016/j.otohns.2006.06.1257. [DOI] [PubMed] [Google Scholar]
- 25.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–54. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed M, Lukyanov AN, Torchilin V, Tournier H, Schneider AN, Goldberg SN. Combined radiofrequency ablation and adjuvant liposomal chemotherapy: effect of chemotherapeutic agent, nanoparticle size, and circulation time. J Vasc Interv Radiol. 2005;16:1365–71. doi: 10.1097/01.RVI.0000175324.63304.25. [DOI] [PubMed] [Google Scholar]
- 27.Gabizon A, Shiota R, Papahadjopoulos D. Pharmacokinetics and tissue distribution of doxorubicin encapsulated in stable liposomes with long circulation times. J Natl Cancer Inst. 1989;81:1484–8. doi: 10.1093/jnci/81.19.1484. [DOI] [PubMed] [Google Scholar]
- 28.Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980;104:10–4. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- 29.Chonn A, Semple SC, Cullis PR. Separation of large unilamellar liposomes from blood components by a spin column procedure: towards identifying plasma proteins which mediate liposome clearance in vivo. Biochim Biophys Acta. 1991;1070:215–22. doi: 10.1016/0005-2736(91)90167-7. [DOI] [PubMed] [Google Scholar]
- 30.Phillips WT. Delivery of gamma-imaging agents by liposomes. Adv Drug Deliv Rev. 1999;37:13–32. doi: 10.1016/s0169-409x(98)00108-2. [DOI] [PubMed] [Google Scholar]
- 31.Hafeli U, Tiefenauer LX, Schbiger PA, Weder HG. A lipophilic complex with 186Re/188Re incorporated in liposomes suitable for radiotherapy. Int J Rad Appl Instrum B. 1991;18:449–54. doi: 10.1016/0883-2897(91)90104-s. [DOI] [PubMed] [Google Scholar]
- 32.McQuarrie S, Mercer J, Syme A, Suresh M, Miller G. Preliminary results of nanopharmaceuticals used in the radioimmunotherapy of ovarian cancer. J Pharm Pharm Sci. 2005;7:29–34. [PubMed] [Google Scholar]
- 33.Laverman P, Carstens MG, Boerman OC, Dams ET, Oyen WJ, van Rooijen N, et al. Factors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injection. J Pharmacol Exp Ther. 2001;298:607–12. [PubMed] [Google Scholar]
- 34.Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2001;46:149–68. doi: 10.1016/s0169-409x(00)00131-9. [DOI] [PubMed] [Google Scholar]
- 35.Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci U S A. 1988;85:6949–53. doi: 10.1073/pnas.85.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CM, Choi Y, Huh EJ, Lee KY, Song HC, Sun MJ, et al. Polyethylene glycol (PEG) modified 99mTc-HMPAO-liposome for improving blood circulation and biodistribution: the effect of the extent of PEGylation. Cancer Biother Radiopharm. 2005;20:620–8. doi: 10.1089/cbr.2005.20.620. [DOI] [PubMed] [Google Scholar]
- 37.Awasthi VD, Garcia D, Goins BA, Phillips WT. Circulation and biodistribution profiles of long-circulating PEG-liposomes of various sizes in rabbits. Int J Pharm. 2003;253:121–32. doi: 10.1016/s0378-5173(02)00703-2. [DOI] [PubMed] [Google Scholar]
- 38.Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 39.Behr TM, Goldenberg DM, Becker W. Reducing the renal uptake of radiolabeled antibody fragments and peptides for diagnosis and therapy: present status, future prospects and limitations. Eur J Nucl Med. 1998;25:201–12. doi: 10.1007/s002590050216. [DOI] [PubMed] [Google Scholar]
- 40.Vegt E, van Eerd JE, Eek A, Oyen WJ, Wetzels JF, de Jong M, et al. Reducing renal uptake of radiolabeled peptides using albumin fragments. J Nucl Med. 2008;49:1506–11. doi: 10.2967/jnumed.108.053249. [DOI] [PubMed] [Google Scholar]
- 41.Park JW, Benz CC, Martin FJ. Future directions of liposome- and immunoliposome-based cancer therapeutics. Semin Oncol. 2004;31:196–205. doi: 10.1053/j.seminoncol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–40. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 43.Laginha KM, Moase EH, Yu N, Huang A, Allen TM. Bioavailability and therapeutic efficacy of HER2 scFv-targeted liposomal doxorubicin in a murine model of HER2-overexpressing breast cancer. J Drug Target. 2008;16:605–10. doi: 10.1080/10611860802229978. [DOI] [PubMed] [Google Scholar]
- 44.DeNardo SJ, Denardo GL. Targeted radionuclide therapy for solid tumors: an overview. Int J Radiat Oncol Biol Phys. 2006;66:S89–95. doi: 10.1016/j.ijrobp.2006.03.066. [DOI] [PubMed] [Google Scholar]


