Summary
The presence of phenylalanine (F) at the 6′ position of transmembrane domain 2 (TM2) in the α4 subunit of α4β2 nicotinic receptors enhances desensitization. As the GABAA receptor affords the ability to study the influence of as few as one and as many as five Fs at this position, we have used it to investigate potential subunit- and stoichiometry-dependent effects of the TM2 6′F mutation on desensitization. Whereas the presence of one F at this position decreased extent of desensitization, desensitization was increased in all configurations that included two or more Fs at the TM2 6′ position; desensitization was particularly rapid with 3 or 4 F residues present. Our results demonstrate the ability of phenylalanine residues at the TM2 6′ position to modulate desensitization is likely conserved in the cys-loop family of ligand-gated ion channels. Moreover, our findings demonstrate both stoichiometric-and subunit-dependent effects of the ability of this mutation to regulate desensitization in GABAA receptors.
Keywords: GABAA receptor, ligand-gated ion channel, desensitization, Cl− channel
Introduction
The GABAA receptor is a member of the cys-loop ligand-gated ion channel superfamily, and is the major inhibitory ion channel in the central nervous system. Each subunit of the pentameric receptor has four transmembrane domains, and the second transmembrane domain (TM2) lines the channel pore [4]. The receptor is encoded by numerous isoforms of multiple subunits; this allows for formation of many receptor subtypes, the α1β2γ2 subtype being the most prevalent [9].
Residues in the TM2 domain are known to influence channel desensitization kinetics. Of particular relevance to this investigation is the finding that autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE) is caused by a genetic mutation of the TM2 6′ residue (S → F) in the nicotinic α4 subunit [15]. This mutation results in enhanced desensitization kinetics [3, 16].
The degree to which the presence of F at the TM2 6′ position affects other members of the cys-loop superfamily has not been assessed. In the present study, we tested the hypothesis that the introduction of F at the TM2 6′ position affects desensitization of GABAA receptors. Moreover, as the GABAA receptor can be manipulated to express anywhere from zero to five phenylalanines at this position, we tested whether effects of the TM2 6′ F mutation are subunit- and/or stoichiometry-dependent.
Materials and methods
Site-directed mutagenesis and transient transfection
Rat isoforms of the α1, β2, and γ2 (short form) GABAA receptor subunits were generously provided by Cynthia Czajkowski (Madison, WI) and subcloned into the mammalian transfection vector pCDNA3.1 (Invitrogen). The threonine at the TM2 6′ position in each subunit was mutated to phenylalanine (Quikchange, Stratagene). Mutated cDNA was confirmed by sequencing (Core Facility, Texas Tech University). Transient transfection was performed in human embryonic kidney cells (HEK 293T) plated on poly-L-lysine treated 25 mm coverslips. GABAA α1β2 and α1β2γ2 receptors were transfected at a cDNA ratio of 1:1 or 1:1:5 μg per subunit, respectively, using the modified calcium phosphate precipitation method [6]. Electrophysiological experiments were performed 24–48 hours after transfection.
Electrophysiology
Glass pipettes were pulled from thick walled borosilicate glass using a horizontal pipette puller (Sutter Instruments P-87, Novato, CA). Pipettes were filled with intracellular recording solution consisting of (in mM): CsCl (140), Mg2+-ATP (4), EGTA (10), HEPES-Na+ (10), pH 7.2. Coverslips were transferred to a recording chamber for electrophysiological recording and were perfused with extracellular recording solution containing (in mM): NaCl (125), HEPES-Na+ (20), KCl (5.5), MgCl2 (0.8), CaCl2 (3), glucose (10), pH 7.3. Whole-cell patch clamp currents were recorded using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA, USA) equipped with a CV-401AU headstage on an air-table supported inverted microscope (Olympus IMT-2; Olympus, Tokyo, Japan). All currents were low-passed filtered at 5 kHz, recorded on a digital oscilloscope and thermal-head pen recorder (Gould TA240; Gould, Cleveland, OH, USA), and stored on a computer using an online data acquisition system (pClamp 6.0, Axon Instruments). When a change in access resistance was noted, the recording was aborted. Each experiment was conducted at room temperature and at a holding potential of −60 mV.
Experimental protocol
GABA was dissolved in external recording solution and applied to patched cells using a modified Y-tube placed within 100μm of the target cell. Using this system, the 10–90% rise time of the junction potential at the open tip averages 30 ms [10]. To study desensitization, we utilized a saturating concentration of GABA, which was applied for 5 sec to the cell under study.
Chemicals
GABA was obtained from Sigma (St. Louis, MO, USA). GABA was dissolved into ultrapure H20.
Data analysis
Concentration-response profiles for GABA were analyzed and an EC50 concentration was determined for each receptor using the following logistic equation:
| equation 1 |
where I is the peak Cl- current amplitude normalized to the control. EC50 is the half-maximal concentration, and n is the Hill coefficient.
Effect of the TM2 T6′F mutant on desensitization was quantified by measuring both rate and extent of desensitization. Desensitization rate was determined by fitting decaying currents with bi-exponential function, using the following equation:
| equation 2 |
where It = current amplitude t seconds after the peak response, A1 is amplitude at start of first phase, A2 is amplitude at start of 2nd phase, t is time, t is time constant, and y0 is plateau current level
Extent of desensitization was determined using the formula %D = 100 × (Ipeak−It)/It, where Ipeak is the current at the peak response, and It is the residual current remaining either 0.5 or 5 sec following continuous ligand application.
Structural modeling
The second transmembrane domains of the GABAAR channel were aligned with the nicotinic acetylcholine receptor TM2 domain backbone (PDB accession code 1EQ8) determined by NMR spectroscopy [13]. The model was constructed using Pymol [8]. The 6′ residues were rendered in space-fill mode, while ribbons represent the remaining TM2 domain residues, viewed from the extracellular side of the membrane. A threonine replaced the 6′ serine residue in each of the TM2 domains. Phenylalanines replaced threonines in the subsequent models in three and five subunits.
Results
Influence of the T6′F mutant on GABA sensitivity
In α1β2 receptors, the presence of phenylalanine in the β2 subunit (α1β2(T6′F) receptors) resulted in a two- fold shift in GABA EC50. In contrast, its presence in the α1 subunit (α1(T6′F)β2 receptors) had no effect on GABA sensitivity (Table 1). We attempted to also assess α1(T6′F)β2(T6′F) receptors, but could not reliably detect GABA-gated current up to a GABA concentration of 1 mM.
Table 1.
GABA sensitivity in α1β2 and α1β2γ2 GABAA receptors carrying the T6′F mutant in one or more subunits.
| Receptor | GABA EC50 (μM) | GABA Hill coefficient |
|---|---|---|
| α1β2 | 1.6 ± 0.2 | 1.3 ± 0.1 |
| α1(T6′F) β2 | 1.7 ± 0.3 | 0.9 ± 0.1 |
| α1β2(T6′F) | 3.6 ± 0.5* | 1.1 ± 0.1 |
| α1(T6′F) β2(T6′F) | ND | ND |
| α1β2γ2 | 19.5 ± 1.8 | 1.3 ± 0.1 |
| α1(T6′F)β2γ2 | 39.5 ± 1.1* | 1.2 ± 0.03 |
| α1β2(T6′F)γ2 | 9.4 ± 0.4 | 1.4 ± 0.1 |
| α1β2γ2(T6′F) | 9.4 ± 1.4 | 0.8 ± 0.1 |
| α1(T6′F)β2γ2(T6′F) | 16.7 ± 2.0 | 0.9 ± 0.1 |
| α1(T6′F)β2(T6′F)γ2 | 12.4 ± 0.8 | 0.58 ± 0.03* |
| α1(T6′F)β2(T6′F)γ2(T6′F) | ND | ND |
denotes significantly different (p < 0.05, unpaired t-test) from wild type receptor. N = a minimum of four cells for all receptor configurations. ND, not determined due to inability to detect GABA-gated currents.
GABA EC50 values for all α1β2γ2 receptor combinations studied are shown in Table 1. In contrast to what was observed in α1β2 receptors, the presence of F at the 6′ position in the β2 subunit of α1β2γ2 receptors did not shift the EC50. Instead, a two-fold decrease in agonist sensitivity was observed with the 6′F present in the α1 subunit. As in α1β2 receptors, no GABA current was detected when five phenylalanines (α1(T6′F) β2(T6′F)γ2(T6′F) receptors) were present at the 6′ position.
Effect of the TM2 T6′F mutation on desensitization in α1(T6′F)β2 and α1β2(T6′F) GABAA receptors
Extent of desensitization at 0.5 sec and 5 sec, rate(s) of desensitization (τ), and the relative weight of observed time constants were determined. In wild type α1β2 receptors, current amplitude decayed to 8 ± 1.4 and 43 ± 3.8%, at 0.5 and 5 sec of GABA application, respectively. Decaying currents could be best fitted with a bi-exponential function, with the slower time constant contributing only minimally to the overall decrease in current decay. In α1β2 receptors, the introduction of the TM2 6′ F mutant in both theα1(T6′F)β2 and α1β2(T6′F) receptor promoted enhanced desensitization in response to application of an EC50 concentration of GABA (Fig. 1). In α1β2(T6′F) receptors, the fast decay time constant was significantly more rapid, and its relative contribution to the rate of current decay was dramatically increased in these receptors (Fig. 1A, Table 2). Correspondingly, desensitization extent was significantly increased at both 0.5 and 5 sec, respectively. Similar results were found in receptors expressing the mutant F residue in the α1 subunit (α1(T6′F)β2 receptors).
Figure 1. Stoichiometric effects of the TM2 T6′F mutation on desensitization.
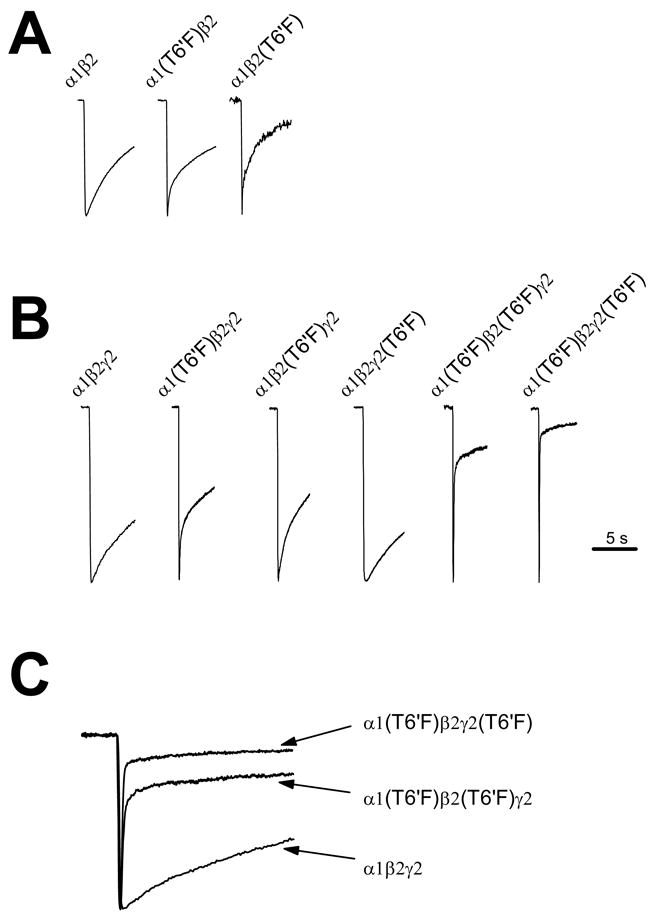
Desensitization in response to saturating GABA in α1β2 (A) or α1β2γ2 receptors (B, C) with the T6′F mutation present in one or more subunits. In α1β2 receptors, presence of F in either subunit significantly enhanced the rapid time constant, and its relative contribution to current decay (A). Typical traces from wild type and mutant receptors are shown. (B) In α1β2γ2 receptors, the presence of F in the γ2 subunit slowed desensitization, while its presence in either the α1 or β2 subunit markedly enhanced the early phase and/or extent of desensitization. Desensitization was particularly rapid when 3 (i.e., α1(T6′F) β2γ2(T6′F) receptors) or 4 (i.e., α1(T6′F)β2(T6′F)γ2 receptors) Fs were present. In (C), same traces as those shown in (B) are superimposed for comparison. In all cases, traces are illustrated only through the ligand application period and trace amplitudes have been normalized to better permit comparison of decay kinetics. See table 2 for mean values.
Table 2.
Stoichiometric determinants of the T6′F mutation on desensitization.
Currents were activated by using a saturating concentration of GABA; decaying currents were fit with a bi-exponential function. A1/A2 is ratio of amplitude of decaying current fit to the data to determine τ1 compared to the amplitude of decaying current fit to the data to determine τ2. %D is extent of desensitization (measured at 0.5 and 5 s).
| Receptor | τ1 (ms) | τ2 (ms) | A1/A2 | %D0.5 s | %D5 s | n | Proposed number of T6′F |
|---|---|---|---|---|---|---|---|
| α1β | 342 ± 33 | 4012 ± 366 | 0.042 ± 0.012 | 8 ± 1.4 | 43 ± 3.8 | 6 | 0 |
| α1β2(T6′F) | 167 ± 44 * | 2870 ± 174 | 0.30 ± 0.05** | 29 ± 3.9** | 73 ± 5.5** | 4 | 3 or 2 |
| α1(T6′F)β2 | 190 ± 30** | 4677 ± 1039 | 0.20 ± 0.07* | 23 ± 2.8** | 54 ± 5.5 | 5 | 2 or 3 |
| α1β2γ2 | 182 ± 41 | 3479 ± 1429 | 0.068 ± 0.034 | 11 ± 2.9 | 43 ± 8.7 | 5 | 0 |
| α1(T6′F)β2γ2 | 148 ± 22 | 3497 ± 507 | 0.63 ± 0.13**,# | 30 ± 3.9**,# | 57 ± 5.6 | 8 | 2 |
| α1β2(T6′F)γ2 | 170 ± 55 | 4126 ± 1323 | 0.12 ± 0.037 | 16 ± 1.4* | 49 ± 3.0 | 7 | 2 |
| α1β2γ2(T6′F) | 403 ± 174 | 5867 ± 1391 | 0.044 ± 0.018 | 4.2 ± 0.9* | 24 ± 3.5 | 6 | 1 |
| α1(T6′F)β2(T6′F)γ2 | 83 ± 9.0* | 2174 ± 183 | 5.97 ± 1.02** | 70 ± 4.2** | 81 ± 3.0** | 6 | 4 |
| α1(T6′F)β2γ2(T6′F) | 50 ± 5.8* | 2313 ± 477 | 9.14 ± 2.13** | 78 ± 6.0** | 87 ± 4.4** | 7 | 3 |
p<0.05;
p<0.01, unpaired t-test, compared to wild type;
significantly different (p < 0.01) compared to α1(T6′F)β2
Stoichiometric assessment of the effect of the TM2 T6′F mutation on desensitization in α1β2γ2 GABAA receptors
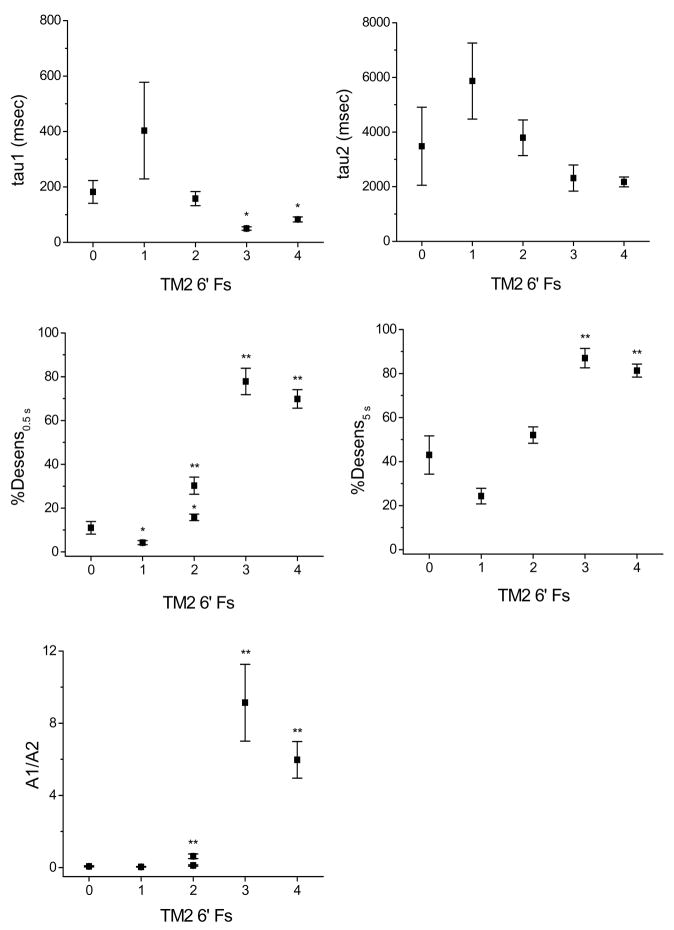
To gain a better understanding of stoichiometry-dependent effects of the TM2 6′F mutation on desensitization, we assessed this trait in αβγ-containing receptors with the mutant F expressed in one or more subunits. The following receptors were assessed: α1β2γ2, α1(T6′F)β2γ2, α1β2(T6′F)γ2, α1β2γ2(T6′F), α1(T6′F)β2(T6′F)γ2, and α1(T6′F)β2γ2(T6′F). In wild type receptors, current amplitude decreased to 11 ± 2.9 and 43 ± 8.7%, at 0.5 and 5 sec of GABA application, respectively, and decaying currents could be fit well with a bi-exponential function (Fig. 1B, Table 2). As in α1β2 receptors, the contribution of the rapid component of desensitization was minimal in wild type α1β2γ2 receptors. Compared to the wild type configuration, more extensive desensitization was observed at 0.5 sec in both mutant configurations expressing 2 Fs at the 6′ position (α1(T6′F)β2γ2, α1β2(T6′F)γ2). The rapid time constant and/or the relative contribution of the rapid time constant was also enhanced in these receptors. There was a larger effect on both the ratio of the rapid to slow time constant and desensitization extent at 0.5 s in α1(T6′F)β2γ2 compared to α1β2(T6′F)γ2(p<0.01 in both cases, Table 2.). In α1β2γ2 receptors expressing 3 or 4 F residues, both the rate and extent of desensitization were particularly enhanced. Interestingly, the introduction of a single F (α1β2γ2 (T6′F) receptors) slowed desensitization. Figure 2 demonstrates the relationship between the number of TM2 6′ Fs and desensitization parameters. A linear correlation could not be observed; instead, there appears to be a threshold for rapid desensitization (2 Fs), and it is further enhanced equally with 3 or 4 Fs present at this position.
Figure 2. Effect on desensitization of number of Fs at the TM2 6′ position.
The extent to which the TM2 6′ F mutations impacted desensitization depended on the number of Fs introduced. For several parameters, a significant facilitative effect was observed with 2 Fs present. The shift to very rapid desensitization was seen with 3 or 4 Fs present. The presence of a single F had the opposite effect, and slowed desensitization. For %Desensitization at 0.5 s and A1/A2 ratio, two values are plotted for TM2 = 2 because there was a significant difference with two Fs present in the α1 versus the β2 subunit (*, p < 0.05; **, p < 0.01; see also Table 2). Because the stoichiometry of α1β2 receptors remains in question, mutants from that configuration are not included in these data.
Discussion
Our interest in studying the influence of TM2 6′ phenylalanine on desensitization of GABAA receptors was driven by the observation that in individuals afflicted with autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE), the nicotinic α4 subunit TM2 6′ serine is mutated to phenylalanine [15]. When coexpressed with wild type β2 subunits, these mutant receptors display enhanced desensitization [3, 16]. This mutation presumably causes epileptic seizures since overall activity of the α4β2 nicotinic receptors would be diminished.
As in the neuronal nAchR, presence of F at the TM2 6′ position in GABAA receptors also facilitates desensitization. As the neuronal α4β2 nicotinic receptor likely expresses two α subunits [12], the presence of 2 Fs is sufficient to confer accelerated desensitization in that receptor as well. The fact that introduction of F at the TM2 6′ position has comparable effects in nicotinic and GABAA receptors suggests its ability to promote desensitization may be common to the cys-loop receptor superfamily. Interestingly, introducing an F residue at the TM2 6′ position in α7 nAChRs expressed in Xenopus oocytes resulted in a receptor with slow desensitization kinetics [14]. We could not detect GABA-gated current in cells transfected with mutant receptors expressing 5 Fs at this position. Thus, we cannot directly compare our findings to those of Placzek et al. [14]. It is worth noting that in the same report, Placzek et al. could also not record detectable currents when the α7 TM2 6′F mutant was transfected into mammalian cells.
For the GABAA receptor, our results also indicate the effect of the TM2 6′ F mutation is influenced by the subunit in which it is expressed. Whereas we observed at least some enhancement of desensitization (extent and/or rate) when F was present in either the α or β subunit of αβγ-expressing receptors (known stoichiometry of 2:2:1), its effect was greatest when present in the α subunit. In contrast, in αβ-expressing receptors, desensitization was more significantly enhanced when F was present in the β subunit. There continues to be some debate regarding the stoichiometry of αβ-expressing receptors [1, 2, 5, 7, 11]. Although not definitive, our studies support the presence of 3 β and 2 α subunits in these receptors, since desensitization was more significantly enhanced in α1β2(T6′F) receptors compared to α1(T6′F)β2 receptors. This could be most readily explained by the presence of a third F (thus a 3rd β2 subunit) in α1β2(T6′F) receptors.
The effect of the 6′ F was particularly striking in receptors expressing 3 or 4 Fs. In both α1(T6′F)β2γ2(T6′F) and α1(T6′F)β2(T6′F)γ2 receptors, (3 and 4 Fs, respectively), the rapid time constant was very short-lived and clearly predominant relative to the longer time constant. Thus, two Fs appears to be the threshold for enhanced desensitization, the effect is considerably augmented with 3 Fs, but further enhancement is not gained with additional Fs beyond three.
Channel lining mutations within the Cys-loop family of ion channels can influence agonist sensitivity, and these effects may be subunit-specific. In α1β1 GABAA receptors, mutating the conserved TM2 9′ leucine in the β1 subunit to F has no effect on GABA EC50 [7]. The same mutation, when instead introduced into the α1 subunit, causes a five-fold decrease in agonist sensitivity. Of the seven different mutant configurations we tested in the present investigation, only two (α1β2(T6′F) and α1(T6′F)β2γ2 receptors) had a significantly different GABA EC50 when compared to the respective wild type receptor (Table 1). In both cases, the shift in agonist sensitivity was modest (two-fold decrease). It is of some interest that the shift in EC50 was associated with a different subunit in αβ compared to αβγ receptors. Interestingly, the shift in EC50 seen in α1(T6′F)β2γ2 receptors was “restored” to the wild type value when the T6′F mutation was also present in either the β2 or γ2 subunit (see Table 2). This is equivalent to the results of Dalziel et al. [7], who found that when the TM2 9′ L→F mutation was present in both α1 and β1 subunits, the EC50 was unchanged from control. As noted, however, the shifts in EC50 in the present investigation were modest and present in only a minority of receptor populations, indicating considerable tolerance for mutations at the 6′ position with regard to agonist sensitivity.
A fundamental question that arises from the current study is the mechanism by which phenylalanine affects desensitization. Weiland et al. [16] suggested the hydrophobic nature of phenylalanine may be involved in its ability to facilitate desensitization in α4β2 nicotinic receptors. The TM2 6′ residue is exposed to the channel pore in both the closed and open state, although likely more centrally directed in the open state [17]. With multiple Fs existing in this position, once the channel opens they may interact with one another hydrophobically and stabilize the channel in an inactive, non-conducting state. In the closed state, the orientation of the residues is likely such that hydrophobic interactions are minimal, if present at all. Figure 3 is a space-filled model of the GABAA receptor pore (open state) at the TM2 6′ level with zero, three and five F residues present. The potential hydrophobic interactions of the F side chain are evident. Our finding that a single F did not promote enhanced desensitization would be consistent with the hypothesis of Weiland et al. [16], as a single phenylalanine would not have a companion residue with which to interact hydrophobically. In addition, the fact that we observed the most dramatic effects on desensitization with three or more residues mutated to F is also consistent with this idea, as this would provide additional potential hydrophobic interactions. The nearly complete occlusion of the pathway with five phenylalanines present provides a plausible explanation for our inability to record detectable current in receptors expressing five TM2 6′ phenylalanine residues.
Figure 3. Structural model of the GABAA receptor second transmembrane domain.
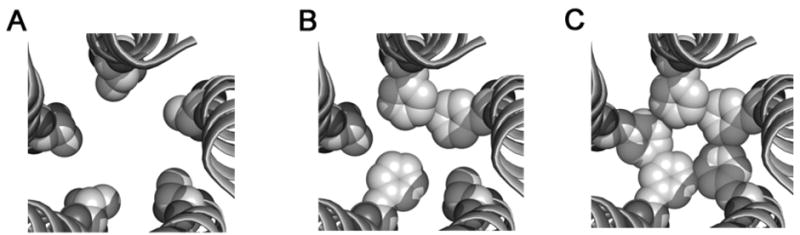
Proposed model of the TM2 domains in the open state containing five threonines (A), three (B) and five phenylalanines (C) at the 6′ position. The residue at this position (space-filled representation) is channel-lining [17]. The pore is larger with the five threonines (A) and constricts as more 6′ residues are converted to phenylalanines (C), as what may be observed for α1(T6′F)β2(T6′F) or α1(T6′F) β2 (T6′F)γ2(T6′F) mutant receptors. The inclusion of 2 or more phenylalanines could interact hydrophobically to move the receptor from the open state to a desensitized state.
In summary, we have characterized the subunit and stoichiometry-dependent actions of the TM2 6′ phenylalanine on desensitization of GABAA receptors. Multiple phenylalanines are required at this position to enhance desensitization of the channel, presumably due to hydrophobic interactions of the pore-projecting residues. These studies provide additional insight into the critical role of this residue in kinetics and pharmacology of the cys-loop family of ligand-gated ion channels.
Acknowledgments
We thank Dr. Cynthia Czajkowski for providing the GABAA receptor subunit cDNA. This work was supported by National Institutes of Health Grant ES07904 (to GHD).
Abbreviations
- GABA
γ-aminobutyric acid
- GABAA
GABA type-A receptor
- DMSO
dimethylsulfoxide
- EGTA
ethylene glycol-bis(β-aminoethyl ether)N, N, N′, N′-tetraacetic acid
- HEK
human embryonic kidney
- HEPES
N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baumann SW, Baur R, Sigel E. Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–5. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- 2.Baumann SW, Baur R, Sigel E. Subunit arrangement of gamma-aminobutyric acid type A receptors. J Biol Chem. 2001;276:36275–80. doi: 10.1074/jbc.M105240200. [DOI] [PubMed] [Google Scholar]
- 3.Bertrand S, Weiland S, Berkovic SF, Steinlein OK, Bertrand D. Properties of neuronal nicotinic acetylcholine receptor mutants from humans suffering from autosomal dominant nocturnal frontal lobe epilepsy. Br J Pharmacol. 1998;125:751–60. doi: 10.1038/sj.bjp.0702154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betz H. Ligand-gated ion channels in the brain: the amino acid receptor superfamily. Neuron. 1990;5:383–92. doi: 10.1016/0896-6273(90)90077-s. [DOI] [PubMed] [Google Scholar]
- 5.Boileau AJ, Pearce RA, Czajkowski C. Tandem subunits effectively constrain GABAA receptor stoichiometry and recapitulate receptor kinetics but are insensitive to GABAA receptor-associated protein. The Journal of neuroscience. 2005;25:11219–30. doi: 10.1523/JNEUROSCI.3751-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Okayama H. High efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2750. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalziel JE, Cox GB, Gage PW, Birnir B. Mutating the highly conserved second membrane-spanning region 9′ leucine residue in the alpha(1) or beta(1) subunit produces subunit-specific changes in the function of human alpha(1)beta(1) gamma-aminobutyric Acid(A) receptors. Mol Pharmacol. 2000;57:875–82. [PubMed] [Google Scholar]
- 8.DeLano WL. The Pymol Molecular Graphics System. 2002. [Google Scholar]
- 9.Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- 10.Huang RQ, Dillon GH. Effect of extracellular pH on GABA-activated current in rat recombinant receptors and thin hypothalamic slices. J Neurophysiol. 1999;82:1233–43. doi: 10.1152/jn.1999.82.3.1233. [DOI] [PubMed] [Google Scholar]
- 11.Im WB, Pregenzer JF, Binder JA, Dillon GH, Alberts GL. Chloride channel expression with the tandem construct of alpha 6-beta 2 GABAA receptor subunit requires a monomeric subunit of alpha 6 or gamma 2. J Biol Chem. 1995;270:26063–6. doi: 10.1074/jbc.270.44.26063. [DOI] [PubMed] [Google Scholar]
- 12.Lukas RJ, Bencherif M. Recent developments in nicotinic acetylcholine receptor biology. In: Arias HR, editor. Biological and Biophysical Aspects of Ligand-Gated Ion Channel Receptor Superfamilies. Research Signpost; Kerala: 2006. [Google Scholar]
- 13.Opella SJ, Marassi FM, Gesell JJ, Valente AP, Kim Y, Oblatt-Montal M, Montal M. Structures of the M2 channel-lining segments from nicotinic acetylcholine and NMDA receptors by NMR spectroscopy. Nature structural biology. 1999;6:374–9. doi: 10.1038/7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Placzek AN, Grassi F, Papke T, Meyer EM, Papke RL. A single point mutation confers properties of the muscle-type nicotinic acetylcholine receptor to homomeric alpha7 receptors. Mol Pharmacol. 2004;66:169–77. doi: 10.1124/mol.66.1.169. [DOI] [PubMed] [Google Scholar]
- 15.Steinlein OK, Mulley JC, Propping P, Wallace RH, Phillips HA, Sutherland GR, Scheffer IE, Berkovic SF. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nature genetics. 1995;11:201–3. doi: 10.1038/ng1095-201. [DOI] [PubMed] [Google Scholar]
- 16.Weiland S, Witzemann V, Villarroel A, Propping P, Steinlein O. An amino acid exchange in the second transmembrane segment of a neuronal nicotinic receptor causes partial epilepsy by altering its desensitization kinetics. FEBS Lett. 1996;398:91–6. doi: 10.1016/s0014-5793(96)01215-x. [DOI] [PubMed] [Google Scholar]
- 17.Xu M, Akabas MH. Identification of channel-lining residues in the M2 membrane-spanning segment of the GABA(A) receptor alpha1 subunit. J Gen Physiol. 1996;107:195–205. doi: 10.1085/jgp.107.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]