Abstract
Objective
Hypertriglyceridemia is a risk factor for coronary heart disease. The aim of this study was to determine the effect of AA supplementation on plasma, liver and muscle lipid concentrations and insulin sensitivity in elderly.
Methods
Twelve impaired glucose tolerant elderly (67.0 ± 5.6 (SD) years, 7 females, 5 males) ingested 11 g of essential AA + arginine twice a day for 16 weeks, after a 7 week control run in. Diet and activity were not otherwise modified. Plasma lipid concentrations and oral glucose tolerance were measured every 4th week, and tissue lipid concentrations (magnetic resonance spectroscopy) every 8th week.
Results
No changes in plasma lipids were observed during the control run-in. AA supplementation lowered plasma triglyceride (TG) (P < 0.001), total cholesterol (P = 0.048) and very low density lipoprotein (VLDL)-cholesterol (P < 0.001) concentrations. Plasma TG dropped ~20% from the initial value of 1.45 ± 0.18 (SE) mmol/l (128 ± 16 mg/dl), with greatest decrease in the subjects starting out with highest concentrations (r = −0.83). Similarly, liver fat content (liver TG/intralipid standard) decreased ~50% from the initial value of 0.34 ± 0.06 (P = 0.021; n = 9), with greatest decrease in the subjects that initially had highest values (r = −0.86). Intramuscular fat content and insulin sensitivity did not change.
Conclusion
Diet supplementation with AA lowers plasma TG, total cholesterol and VLDL-cholesterol concentrations, and liver lipid content in impaired glucose tolerant elderly. AA supplementation may have a potential role in treatment of hypertriglyceridemia or hepatic steatosis.
Keywords: elderly, amino acids, plasma lipids, liver lipids, insulin sensitivity
INTRODUCTION
Hypertriglyceridemia has been shown to be a significant independent risk factor for coronary artery disease [1–3], and treatment for hypertriglyceridemia has been included in The Adult Treatment Panel III (ATP III) of the National Cholesterol Education Program [4].
Low carbohydrate diets have been shown to positively affect plasma lipid concentrations, resulting in decreased triglyceride (TG) and increased high density lipoprotein (HDL) concentrations both in the presence of weight loss and compared to low fat diets [5,6]. However, since dietary fat, carbohydrate and protein are the primary energy-containing macronutrients, it is difficult to study the independent effects of changing one of the components of the diet [7]. If the intake of one of them is decreased, compensatory adjustments in one or both of the others must occur to maintain energy balance. Thus, most of the low carbohydrate diets have elevated protein intake, and it is difficult to separate the independent effects of absence of carbohydrates in the diet vs. elevated protein intake. In accordance with this, studies of high-protein diets with or without weight-loss also suggest a lowering effect on plasma TG concentration [8–13]. Again it has been difficult to separate the independent effects of protein to those of absence of carbohydrate or change in body weight. Finally, a further complication is the type of individual macronutrient in the diet, e.g., protein of plant vs. animal origin, carbohydrate with low vs. high glycemic index, and saturated vs. unsaturated fat. For examples, the vegetable plant protein soy protein seems to have unique effects on plasma lipoproteins [14].
Thus, in an effort to clarify the potential independent effect of protein on plasma and tissue lipids, we supplemented a normal weight-maintaining diet with a relatively small amount of amino acids (~90 kcal/day) between meals in the present study. We measured tissue lipids in addition to plasma lipids since the increase in insulin resistance with aging has been linked to increased fat accumulation in muscle and liver tissue [15,16]. Increased protein intake in the context of weight loss improves glucose control [17], but the effect of amino acid supplementation during a weight-maintaining diet is not known. Further, it has repeatedly been shown that amino acid intake stimulates muscle protein synthesis and improves muscle protein net balance [18]. Since muscle is the main tissue for insulin-mediated glucose uptake, it may be hypothesized that an increase in muscle mass will improve insulin action at the whole body level.
The overriding hypothesis of this study was that supplementation of the normal diet with a mixture of amino acids will reduce circulating and tissue TG concentrations and improve insulin sensitivity in elderly subjects with impaired glucose tolerance. The aim of the study was to investigate the effect of supplementation of the diet with essential amino acids + arginine on plasma, liver and muscle lipids and insulin sensitivity in impaired glucose tolerant elderly.
SUBJECTS AND METHODS
Study Design
Twelve elderly volunteers participated in an approximately 24 week study period. After a run-in period of observation of ~7 weeks, they ingested 11 g of essential amino acids (EAA) + arginine two times a day, between meals for 16 weeks. Diet and activity were not otherwise modified. Every 4th week after the start of amino acid ingestion, the subjects were admitted to the General Clinical Research Center (GCRC) at UTMB, Galveston. Body composition was measured by a full-body dual-energy x-ray absorptiometry (DEXA) scan, and insulin sensitivity was measured by an oral glucose tolerance test. In addition, plasma lipid panel was determined. Muscle and liver lipids were measured by magnetic resonance spectroscopy (MRS) every 8th week. At weeks 0 and 16, a muscle biopsy was also collected from m. vastus lateralis for measurement of activities of oxidative enzymes. The protocol was approved by the Institutional Review Board at UTMB and the General Advisory Committee of the GCRC at UTMB.
Subjects
Twelve impaired glucose tolerant elderly (7 females, 5 males, 67.0 ± 5.6 (SD) years, 164.5 ± 16.4 cm; 74.3 ± 19.7 kg at baseline) participated in the study. They were fully informed about the purpose and procedures of the study before written consent was obtained. Each subject had a complete medical screening at the GCRC prior to participation in the experiments (51 ± 9 days before start of supplementation), including vital signs, blood tests, urine tests, and a 12-lead electrocardiogram, and these results were used as a baseline for the placebo run-in period for those subjects that qualified to participate in the study. Exclusion criteria included evidence for heart disease, hyperlipidemia, kidney or liver disease, or any other disease that might influence the results of the study. Hyperlipidemia was excluded, as we wanted to examine the effect of protein in elderly, without the confounding variable of pre-existing hyperlipidemia. The subjects also underwent a standard oral glucose tolerance test (OGTT) using 75 g of glucose. Only subjects with impaired glucose tolerance defined as a plasma glucose concentration >10 mmol/l (180 mg/dl) at 1 hr or >7.8 mmol/l (140 mg/dl) at 2 hr after oral intake of 75 g glucose, were included. Diabetic subjects (plasma glucose concentration >11.1 mmol/l (200 mg/dl) at 1 hr or 2 hr after glucose intake) with a reduced insulin production, and subjects taking any medication to treat abnormal blood lipid levels were not included in the study.
Nutritional Supplement
Each dose of the nutritional supplement consisted of 11 g of amino acids with the following composition: 0.36 g histidine, 0.94 g isoleucine, 3.95 g leucine, 1.88 g lysine, 0.39 g methionine, 0.51 g phenylalanine, 1.05 g threonine, 0.82 g valine, 1.10 g arginine. The choice of amino acids was based on our numerous previous findings in muscle that only EAA are needed for stimulation of protein synthesis [e.g., 19,20]. Thus, we expected that only the EAA were needed to achieve effect, and the amount given (g or kcal) could be restricted. We included arginine in addition to the EAA since arginine may have unique anabolic effects [21,22]. The supplement was taken in two daily doses in the form of capsules, and recorded in a diary. The first dose was taken between breakfast and lunch, and the second dose was ingested between lunch and dinner. The subjects visited the hospital every two weeks to pick up a new supply of supplements. In the weeks with no hospital visits, the subjects were given follow-up calls to check on the intake of the supplements, as well as on diet, activity and anything else (sickness, etc.) that might influence the results of the study.
Diet and Physical Activity
Before the start of the study the subjects were counseled to maintain their typical dietary intake and physical activity pattern. During their visits to the hospital and in telephone calls between visits, they were asked about this and reminded to not make any changes. The Physical Activity Scale for Elderly (PASE) was used to measure their physical activity during the study period. Further, at the start of the study the subjects were instructed by the dietician at the GCRC on how to complete a diet diary. In the week before the first overnight stay and every 4th week thereafter (week 3, 7, 11, and 15), the participants recorded their diet for 3 days (two week-days and one weekend-day).
Magnetic Resonance Spectroscopy (MRS)
Every 8th week, the intramuscular lipid concentration of m. soleus was measured with a 1H knee coil on a GE Advantage 1.5 Tesla whole-body imager (General Electric, Milwaukee, WI), as previously described [16]. The widest part of the calf was located during the first study, and measured from the floor and marked. A marker was placed at the location during the scan, and this slice of leg was always used for scans. Four areas were selected from the coronal slice localizer and were traced onto a transparency along with multiple anatomic landmarks. These four areas were then rescanned during each subsequent MRS analysis. A tube of 20% Intralipid (i.v. high-fat total parenteral feeding solution; Baxter Healthcare, Deerfield Park, IL) was placed inside the knee coil to obtain a standard external reference [23]. After a preliminary localization image, three to seven voxels (~7 mm × 7 mm × 10 mm each) were chosen in m. soleus free from fascia, gross fat marbling, and vessels. The exact voxel volumes were recorded. A voxel was also chosen from the Intralipid external reference. An optimized PRESS (Point RESolved Spectroscopy) sequence with a repetition time of 2000 ms and an echo time of 35 ms was run. Peak positions and areas of interest [extramuscular (CH2)n, intramuscular (CH2)n, extramuscular CH3, intramuscular CH3, total creatine, and trimethylamines] were determined by time domain fitting using jMRUi [24,25]. In brief, all water-suppressed free induction decay (FID) (metabolite FID) were deconvoluted with the water-unsuppressed FID (water FID) acquired from the same voxel to correct for zero-order phasing and removal of eddy current-induced artifacts [26]. The resulting metabolite FIDs were analyzed with AMARES (Method of Accurate, Robust and Efficient Spectral fitting), a nonlinear least-square-fitting algorithm operating in the time domain [16,27,28]. Spectra from voxels, which did not have optimal shimming or clear intracellular and extracellular lipid peak resolution, were not used in the AMARES fitting analysis. This process was repeated for the intralipid phantom. The TG levels were computed as a ratio relative to the Intralipid standard using the following formula: TG = [(PM/VM)/(PI/VI)], where PM is the methylene peak area, VM is the total measured tissue voxel volume, PI is the Intralipid peak area, and VI is the Intralipid voxel volume. This measurement is a TG concentration normalized to Intralipid concentration, and thus it is unitless.
Liver lipid concentration was measured with a 1H whole-body coil on the same system. Hepatic measurements were performed in the middle right lobe [29]. The scans were localized to the same area of the liver via anatomic landmarking of the hepatic blood flow and the ribs, so that approximately the same area of liver was scanned with each study. A tube of Intralipid was again used for reference. After a preliminary localization scan, a voxel (~30 mm × 30 mm × 20 mm) was chosen at a location free from large vessels. An optimized PRESS sequence was run 256 times without respiratory gating. These spectra represent an average lipid concentration measurement over the mid-right lobe. Spectra were manually phased, and final analysis was then performed with jMRUI.
Dual-Energy X-ray Absorptiometry (DEXA)
The subjects underwent a full-body DEXA scan every 4th week to determine body composition. All DEXA scans were performed on a Hologic QDR 4500 A system (Hologic, Inc., Bedford, MA).
Oral Glucose Tolerance Test (OGTT)
An OGTT was performed every 4th week to determine insulin sensitivity. The subjects were fasted for about 12 hours prior to the OGTT. A catheter was placed in a vein in the arm, and two background blood samples were drawn. Thereafter, the subjects received 75 g of glucose (Glucola, Fisherbrand LIMEONDEX, Fisher HealthCare, Houston, TX). Blood samples were collected at 30, 60, 90, and 120 min following the glucose ingestion for the determination of plasma glucose, insulin and glycerol concentrations. The first blood sample was also analyzed for plasma lipid panel, and in week 0 and 16, amino acid concentrations.
Muscle Biopsy
In week 0 and 16, a muscle biopsy was taken after the OGTT under local anesthesia, from the lateral portion of the vastus lateralis, approximately 10–15 cm above the knee. A 5 mm Bergstrom biopsy needle (Depuy, Warsaw, IN) was used to sample approximately 30–50 mg of mixed muscle tissue. The sample was quickly rinsed, blotted, and immediately frozen in liquid nitrogen and stored at −80°C for later analysis of citrate synthase, cytochrome c oxidase, and beta-hydroxyacyl-CoA dehydrogenase (beta-HAD) activities.
Sample Analyses
Plasma glucose concentration was determined enzymatically (YSI 1500, Yellowspring Instruments, Yellowspring, OH, USA). Plasma insulin concentration was determined by a radioimmunoassay method (Diagnostic Products Corporation, Los Angeles, CA, USA). Plasma amino acid concentrations were analyzed by high-performance liquid chromatography (Waters Alliance® HPLC System 2690, Milford, MA). Enzymatic methods were used to determine plasma FFA (NEFA-C, Wako Chemicals GmbH, Neuss, Germany) and glycerol (Sigma-Aldrich, St. Louis, MO) concentrations. The lipid panel was comprised of triglycerides (TG), total cholesterol, and HDL-cholesterol concentrations. They were all measured on a Vitros 950 system (Ortho-Clinical Diagnostics, Raritan, NJ). The HDL-cholesterol was measured by precipitation of the LDL and VLDL, and the cholesterol left in the supernatant (HDL) was then determined. The LDL-cholesterol (mg/dl) was calculated using the Friedewald equation ([LDL-cholesterol] = [Total cholesterol] – [HDL-cholesterol] – ([TG]/5) [30]. Thus, the quotient [TG]/5 is used as an estimate of VLDL-cholesterol concentration. The cholesterol concentrations (mg/dl) were then multiplied by 0.02586 for conversion to SI units (mmol/l). Similarly, TG concentrations were multiplied by 0.01129 for conversion.
Mitochondrial enzyme activities of citrate synthase, cytochrome c oxidase, and beta-HAD were measured from homogenates of m. vastus lateralis biopsies in a sucrose/EDTA/Tris buffer, as previously described [31].
Calculations
Whole-body insulin sensitivity following a 75 g glucose load was estimated by the composite model for insulin sensitivity designed by Matsuda and DeFronzo [32] as the Insulin Sensitivity Index (ISI) = 10000/square root of ([fasting glucose x fasting insulin] × [mean glucose × mean insulin during OGTT]). The value derived from this equation is an M value of glucose uptake in mg · (m2 · min)−1, which is approximated to the results that would likely have been obtained if a more invasive hyperinsulinemic-euglycemic clamp test had been performed. The range of values is 0–14, with insulin sensitivity being better the higher the ISI index. The area under the curve for plasma glucose and insulin concentrations was also determined, with the average pre-OGTT concentration used as the zero point.
Statistical Methods
Overall significance of differences in response of diet intake, ISI, tissue lipids, and fasting plasma lipids, insulin, and glucose concentrations with time was tested by one-way repeated measures analysis of variance (ANOVA) followed by Dunnett’s test with week 0 as control (SigmaStat 2.03, SPSS Inc, Chicago, IL). The correlation between plasma TG concentrations at week 0 and changes in plasma TG concentration during the supplementation period was measured by Spearman rank correlation coefficient, whereas the corresponding correlation for liver lipid content was determined by linear regression analysis. Significance of changes in the time course of glycerol, insulin, and glucose concentrations during the OGTT was tested by two-way repeated measures ANOVA, with week 0 and pre-OGTT values as control. Changes in amino acid concentration or muscle oxidative enzymes from week 0 to 16 were tested by paired t-tests. Comparisons of plasma lipid concentrations and ISI at screening (week -7) and week 0 were also done by paired t-tests. Results were considered significant if P < 0.05. The results are presented as means ± SE unless otherwise noted.
RESULTS
The amino acid supplementation was well tolerated by the subjects, and there were no overall changes in physical activity or diet during the study period. The dietary intake was 1733 ± 226 kcal/day when no supplement was taken vs. an average of 1735 ± 176 kcal/day during the supplementation period (n = 9). Corresponding values for protein intake were 1.03 ± 0.19 vs. 0.99 ± 0.21 g · kg−1 · day−1, fat intake was 0.86 ± 0.15 vs. 0.86 ± 0.09 g · kg−1 · day−1, and carbohydrate intake was 3.05 ± 0.70 vs. 3.11 ± 0.70 g · kg−1 · day−1.
The amino acid supplementation did not lead to changes in overall body mass (week 0 vs. 16: 74.31 ± 5.67 vs. 74.60 ± 5.62 kg), body mass index (27.9 ± 1.8 vs. 28.0 ± 1.9 kg/m2), total fat mass (24.19 ± 3.59 vs. 23.90 ± 3.70 kg), or trunk fat mass (11.89 ± 1.76 vs. 11.67 ± 1.78). Fasting plasma amino acid concentration did not change during the study.
Plasma Lipids
There were no changes in plasma TG, total cholesterol, LDL-, HDL-, or VLDL-cholesterol concentrations during the run-in period from the time of screening until the start of the supplementation period (51 ± 9 days without supplementation; Table 1).
Table 1.
Plasma lipid concentrations and Insulin Sensitivity Index in IGT elderly (n = 12) at baseline, and after 4, 8, 12, and 16 weeks of amino acid supplementation.
| Screening (~week -7) | Week 0 | Week 4 | Week 8 | Week 12 | Week 16 | |
|---|---|---|---|---|---|---|
| Triglycerides (mmol/l)† | 1.43 ± 0.16 | 1.45 ± 0.18 | 1.19 ± 0.12* | 1.26 ± 0.17* | 1.21 ± 0.15* | 1.15 ± 0.16* |
| Total cholesterol (mmol/l)‡ | 5.15 ± 0.28 | 5.17 ± 0.28 | 5.15 ± 0.28 | 4.86 ± 0.28 | 4.91 ± 0.23 | 4.81 ± 0.31 |
| HDL-cholesterol (mmol/l) | 1.47 ± 0.16 | 1.50 ± 0.16 | 1.60 ± 0.18 | 1.42 ± 0.13 | 1.45 ± 0.16 | 1.55 ± 0.16 |
| LDL-cholesterol (mmol/l) | 3.00 ± 0.18 | 3.00 ± 0.21 | 3.03 ± 0.21 | 2.84 ± 0.21 | 2.92 ± 0.18 | 2.74 ± 0.21 |
| VLDL-cholesterol (mmol/l)† | 0.65 ± 0.08 | 0.67 ± 0.10 | 0.57 ± 0.08* | 0.59 ± 0.08* | 0.54 ± 0.05* | 0.54 ± 0.08* |
| FFA (mEq/l) | --- | 0.76 ± 0.06 | 0.60 ± 0.06 | 0.64 ± 0.05 | 0.62 ± 0.05 | 0.66 ± 0.04 |
| Insulin Sensitivity Index | 4.30 ± 0.91 | 4.54 ± 0.74 | 4.37 ± 0.80 | 5.37 ± 0.85 | 5.35 ± 0.99 | 5.17 ± 0.80 |
Data are mean ± SE.
ANOVA: P<0.001;
ANOVA: P<0.05;
P<0.05 vs. week 0. To convert from mmol/l to mg/dl, divide triglycerides by 0.01129 and cholesterol by 0.02586.
Significant decreases were found in plasma TG (P <0.001), total cholesterol (P = 0.048) and VLDL-cholesterol (P <0.001) concentrations during the amino acid supplementation (Table 1). For TG and VLDL-cholesterol the changes from baseline were significant at all time points, whereas they did not reach significance at any specific time point for total cholesterol concentration.
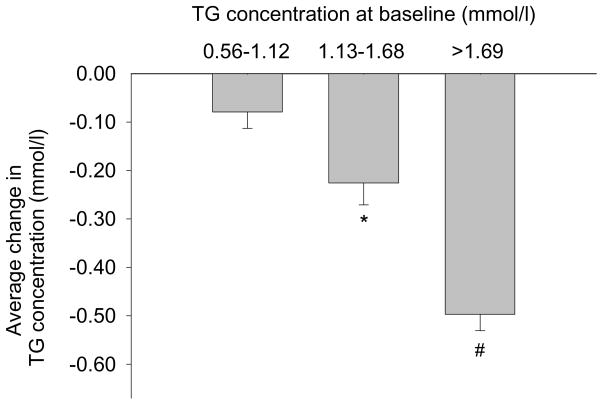
The changes in plasma TG concentrations during the study were related to starting level, with the greatest decrease in the subjects that initially had the highest plasma TG concentrations (Fig. 1). The correlation was not linear, therefore we calculated the Spearman rank correlation coefficient, which was r = −0.828 between the starting value and the average change from baseline at 4, 8, 12 and 16 weeks (P < 0.001). Most of the concentration changes occurred somewhere between 0–4 weeks (Table 1). Spearman rank correlation coefficient between the start value and the change from 0–4 weeks was −0.872 (P < 0.001).
Fig. 1.
The average plasma triglyceride concentration changes from baseline during 16 weeks of amino acid supplementation in elderly that had baseline values between 0.56–1.12 mmol/l (50–99 mg/dl; left; n = 4), between 1.13–1.68 mmol/l (100–149 mg/dl; middle; n = 5) and >1.69 mmol/l (>150 mg/dl; right; n = 3). Normal reference range: 0.34–1.92 mmol/l (30–170 mg/dl). Data are mean ± SE; *P = 0.01 vs. zero; #P = 0.004 vs. zero.
No changes were found in plasma FFA, and LDL- and HDL-cholesterol concentrations during the study (Table 1).
Liver and Muscle Lipids
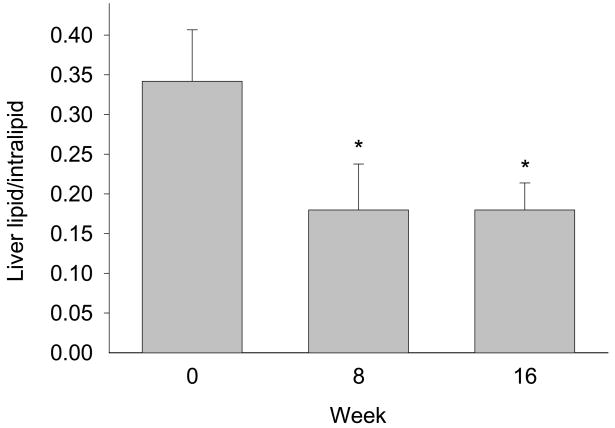
At the start of the study, there was a linear correlation between liver fat content and plasma TG concentration (r = 0.85; P = 0.007). Amino acid supplementation caused the liver fat content (liver TG/intralipid standard) to drop about 50% from the initial value of 0.34 ± 0.06 at week 0 (P = 0.021; n = 8 at week 0 and 16, n = 6 at week 8; Fig. 2). The change in liver fat content was most dramatic for the subjects starting out with the highest level (r = −0.86; P = 0.006). No significant changes were observed in intramuscular fat content.
Fig. 2.
Liver lipids (liver TG/intralipid standard) at baseline, and after 8 and 16 weeks of amino acid supplementation (mean ± SE; n = 8 at week 0 and 16, n = 6 at week 8); *P < 0.05 vs. baseline.
Muscle Oxidative Enzymes
No significant changes were found in the mitochondrial enzyme activities of citrate synthase (week 0 vs. 16: 0.22 ± 0.02 vs. 0.24 ± 0.03 μmol · min−1 · mg−1), cytochrome c oxidase (0.20 ± 0.02 vs. 0.19 ± 0.03 μmol · min−1 · mg−1), or beta-HAD (130.3 ± 9.8 vs. 123.8 ± 12.3 μmol · min−1 · mg−1).
Oral Glucose Tolerance Test (OGTT)
No significant effect of AA supplementation on the Insulin Sensitivity Index (Matsuda score) was found (Table 1). Further, no correlations were found between starting values and changes in ISI, or between changes in ISI and lean body mass, or leg lean mass, respectively.
In agreement with the ISI index, no overall changes in the area under the curve (AUC) of glucose or insulin were found during the study. Further, whereas the glycerol concentration during the OGTT decreased (P < 0.001), there was no interaction effect between week of supplementation and time course of glycerol during the OGTT.
DISCUSSION
The main finding in this study was that supplementation of a normal weight-maintaining diet with essential amino acids (EAA) + arginine decreased plasma and liver TG content, and also total cholesterol and VLDL-cholesterol concentrations, in elderly. Further, the decreases in plasma and liver TG concentrations were related to the starting level, suggesting a potential role for amino acids in the treatment of hypertriglyceridemia. Insulin sensitivity was not significantly improved as a result of amino acid supplementation.
The magnitude of change in liver lipid concentration was large (approximately 50%), and the response was rapid. The maximal decrease was achieved by 8 weeks. The rapid response of liver lipids is consistent with previous work, as in burn patients hepatic fat content may increase three- to fourfold within one week of the initial injury [33]. The values of liver TG in the current study were as expected based on our previous measurements in insulin resistant elderly, and higher than values in elderly with normal glucose tolerance [34]. However, in our previous work we found a correlation between the extent of insulin resistance and the amount of liver TG [16], whereas in the present study reduction of liver fat failed to impact insulin sensitivity. It appears that liver TG is not the sole determinant of insulin sensitivity in elderly individuals.
The mechanisms underlying the effect of amino acid supplementation on liver and plasma lipid concentrations are not known. The amount of liver lipids can be influenced by the rate of uptake (and hence delivery) of plasma fatty acids, the rate of hepatic fatty acid oxidation and the rate of secretion of TG in VLDL. Since there was no change in plasma FFA concentration and no detectable change in whole body or trunk fat mass, it seems unlikely that changes in rate of lipolysis and thus delivery of fatty acids to the liver can explain the 50% reduction in liver lipids. It is possible that a greater percentage of FFA taken up in the liver was oxidized, but since most energy in the liver is normally derived from fat oxidation [35] it is unclear if EAA could further stimulate fat oxidation sufficiently to reduce the intracellular stores of TG. Thus, the most likely explanation for the decrease in hepatic TG is an accelerated secretion in the form of VLDL-TG, possibly by EAA stimulating synthesis of VLDL-apoproteins.
An increased efficiency in TG secretion from the liver implies that peripheral clearance of TG was increased, since the plasma TG level fell significantly. It is further possible that if clearance was accelerated, the reduction in plasma TG concentration would in turn be a stimulus for a greater rate of secretion of TG from the liver (thereby explaining the reduction in hepatic TG). We found no effect of the EAA on muscle tissue citrate synthase, cytochrome c oxidase, and beta-hydroxyacyl-CoA dehydrogenase (beta-HAD), suggesting that mitochondrial number or oxidative capacity remained unchanged. It therefore seems unlikely that increased oxidation of fatty acids in the muscle could explain enhanced peripheral TG clearance. Further, there was no change in the size of the intramuscular TG pool. Rather than muscle, adipose tissue may be the principal site of increased TG clearance. Under normal conditions about 70% of fatty acids released from adipose tissue as FFA are incorporated into TG in the liver, and transported back to the adipose tissue in the form of TG [36]. At the adipose tissue the fatty acids from the circulating TG are reincorporated into TG for storage. This pathway has been termed the “extracellular” pathway of TG-fatty acid substrate cycling [37]. A stimulation of lipoprotein lipase activity at the adipose tissue level would explain a stimulation of TG clearance by EAA-intake via the “extracellular” route of substrate cycling. Further studies will be required to determine in such a stimulation occurs.
A decrease in plasma TG concentrations have been observed in relation to high protein/low carbohydrate diets [5], but the independent effects of low carbohydrate intake, increased protein intake and changes in body weight are unclear. In a cross-over feeding study, it was found that in the setting of a healthy diet reduced in saturated fat, a diet rich in protein (approximately half from plant sources) significantly lowered plasma TG levels compared with a carbohydrate rich diet [13]. Since the effect was also significantly greater than what was seen after a diet rich in unsaturated fat, the authors suggested that protein may have a direct TG lowering effect beyond that of replacing carbohydrates, which is known to increase TG levels. To our knowledge there are no previous studies of the effect of amino acids on plasma and tissue lipids, except that amino acid solutions have been given to dialysis patients. Thus, patients on peritoneal dialysis receiving 1.1% solution of amino acids instead of all glucose experienced a 13% decrease in plasma TG concentration within one month of the solution change [38]. In a longer study using amino acid dialysate for three years, patients experienced a significant decrease in plasma TG concentration [39].
The current study shows that the TG-lowering effect can be achieved by supplementing the normal diet with a small amount (expressed as g or kcal) of amino acids. Further, an intriguing finding is that the effect was greater at higher TG levels. The use of amino acid supplementation as treatment for patients with hypertriglyceridemia or hepatic steatosis merits further studies.
Insulin action declines with age [40]. This has been linked to increased fat accumulation in muscle and liver tissue [15,16]. However, the decrease of liver and plasma lipids in the current study did not translate to an improvement in insulin sensitivity. This implies that muscle fat accumulation (or muscle lipid metabolism) may be more important for insulin resistance than liver fat accumulation. This may be expected since muscle is the primary site of insulin-stimulated glucose disposal.
We assumed that no improvement in plasma or tissue lipids would occur over 16 weeks without intervention. This assumption is supported by the stability of the lipid values over the seven weeks run-in period preceding the study (Table 1). Further, the subjects had no conscious control over the measured endpoints, except that modifying activity or food intake could have potentially influenced the results. We did not find any changes in activity or food intake during the supplementation period, and the measurements of lipid parameters were made under the same standardized conditions each time. Consequently, we feel confident that the changes observed in this study were due to the ingestion of the amino acids.
The potential detrimental effects of high levels of protein intake have been dealt with extensively in the Dietary Reference Intakes report of the Food and Nutrition Board [41]. The report concluded that there is no documented adverse effects of high protein intake, and consequently set no upper limit on safe protein intake. There is no such data regarding ingestion of amino acid formulations, but one would expect a similar lack of detrimental effects.
Conclusion
Supplementation of a normal weight-maintaining diet with essential amino acids + arginine decreases liver and plasma TG concentrations in impaired glucose tolerant elderly. The effect is related to the starting level of plasma TG, thus, amino acid supplementation may have a potential role in the treatment of hypertriglyceridemia or hepatic steatosis. Since insulin sensitivity was not improved despite the reduction in liver fat content, the storage of fat in the liver is not the primary determinant of insulin sensitivity. Finally, plasma total cholesterol and VLDL-cholesterol is also reduced by the amino acid supplementation.
Acknowledgments
The authors thank the subjects who participated in the study for their time and dedication. We thank Sue Minello R.N., Roxana Hirst M.S., and Nancy Poore at the Pepper Center for help in recruiting the volunteers, and the nurses, dieticians and the staff at the General Clinical Research Center (GCRC) at the University of Texas Medical Branch (UTMB) at Galveston, TX. We thank Kendrick Armstrong, Melissa Bailey, Donovan Randolph B.S., Stephaine J. Blase, Tara Cocke, Daniel L. Creson, Christopher P. Danesi, Scott Schutzler, and Paulette Rousset for skillful technical assistance. We acknowledge Elizabeth Protas PhD for support of Sandrine Tissier.
The study was supported by P30 AG024832, Shriners Grant 8490, and Ajinomoto Co, Inc (provided the amino acids). Studies were performed at GCRC at UTMB Galveston supported by M01 RR 000073.
Footnotes
Role of authors: RRW, AAF, HK, and EB designed the study. Q-YTB, ST and EB organized the study and carried out the experiments. Q-YTB and OR provided medical coverage. MGC performed all the MRS scans and analyses. BRN directed the MRS scans and the analyses of these. BM directed the analyses and interpretations of oxidative enzymes. EB performed the data handling and statistical analyses, and also wrote the manuscript with the assistance of the others.
Financial disclosure: Hisamine Kobayashi is working for AminoScience Laboratories that is part of Ajinomoto Co which is the company that provided the free amino acids in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Assmann G, Schulte H, Funke H, von Eckardstein A. The emergence of triglycerides as a significant independent risk factor in coronary artery disease. Eur Heart J. 1998;(19 Suppl M):M8–14. [PubMed] [Google Scholar]
- 2.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81(4A):7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 3.Ebenbichler CF, Kirchmair R, Egger C, Patsch JR. Postprandial state and atherosclerosis. Curr Opin Lipidol. 1995;6(5):286–290. doi: 10.1097/00041433-199510000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 5.Volek JS, Sharman MJ, Forsythe CE. Modification of lipoproteins by very low-carbohydrate diets. J Nutr. 2005;135(6):1339–1342. doi: 10.1093/jn/135.6.1339. [DOI] [PubMed] [Google Scholar]
- 6.Volek JS, Feinman RD. Carbohydrate restriction improves the features of Metabolic Syndrome. Metabolic Syndrome may be defined by the response to carbohydrate restriction. Nutr Metab (Lond) 2005;2:31. doi: 10.1186/1743-7075-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lichtenstein AH. Thematic review series: patient-oriented research. Dietary fat, carbohydrate, and protein: effects on plasma lipoprotein patterns. J Lipid Res. 2006;47(8):1661–1667. doi: 10.1194/jlr.R600019-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe BM, Giovannetti PM. Short-term effects of substituting protein for carbohydrate in the diets of moderately hypercholesterolemic human subjects. Metabolism. 1991;40(4):338–343. doi: 10.1016/0026-0495(91)90142-j. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe BM, Giovannetti PM. High protein diet complements resin therapy of familial hypercholesterolemia. Clin Invest Med. 1992;15(4):349–359. [PubMed] [Google Scholar]
- 10.Wolfe BM. Potential role of raising dietary protein intake for reducing risk of atherosclerosis. Can J Cardiol. 1995;11(Suppl G):127G–131G. [PubMed] [Google Scholar]
- 11.Wolfe BM, Piche LA. Replacement of carbohydrate by protein in a conventional-fat diet reduces cholesterol and triglyceride concentrations in healthy normolipidemic subjects. Clin Invest Med. 1999;22(4):140–148. [PubMed] [Google Scholar]
- 12.Brinkworth GD, Noakes M, Keogh JB, Luscombe ND, Wittert GA, Clifton PM. Long-term effects of a high-protein, low-carbohydrate diet on weight control and cardiovascular risk markers in obese hyperinsulinemic subjects. Int J Obes Relat Metab Disord. 2004;28(5):661–670. doi: 10.1038/sj.ijo.0802617. [DOI] [PubMed] [Google Scholar]
- 13.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, III, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294(19):2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;333(5):276–282. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 15.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cree MG, Newcomer BR, Katsanos CS, Sheffield-Moore M, Chinkes D, Aarsland A, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89(8):3864–3871. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- 17.Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, et al. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133(2):411–417. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe RR. Regulation of muscle protein by amino acids. J Nutr. 2002;132(10):3219S–3224S. doi: 10.1093/jn/131.10.3219S. [DOI] [PubMed] [Google Scholar]
- 19.Tipton KD, Gurkin BE, Matin S, Wolfe RR. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem. 1999;10(2):89–95. doi: 10.1016/s0955-2863(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 20.Børsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283(4):E648–E657. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- 21.Paddon-Jones D, Børsheim E, Wolfe RR. Potential ergogenic effects of arginine and creatine supplementation. J Nutr. 2004;134(10 Suppl):2888S–2894S. doi: 10.1093/jn/134.10.2888s. [DOI] [PubMed] [Google Scholar]
- 22.Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Nakajo T, Takaoka M, et al. Arginine and Leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int J Mol Med. 2004;13(4):537–543. [PubMed] [Google Scholar]
- 23.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48(8):1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 24.van den Boogaart A, Van Hecke A, Van Huffel P, Graveron-Demilly S, vanOrmondt D, de Beer R. MRUI: a graphical user interface for accurate routine MRS data analysis. Proc 13th Annual Meeting of the European Society forMagnetic Resonance in Medicine and Biology; 1996. p. 318. Abstract. [Google Scholar]
- 25.van den Boogaart A. MRUI Manual V.96.3. A user’s guide to the Magnetic Resonance User Interface Software Package. Delft, the Netherlands: Technical University Press; 1997. [Google Scholar]
- 26.Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn Reson Med. 1990;14(1):26–30. doi: 10.1002/mrm.1910140104. [DOI] [PubMed] [Google Scholar]
- 27.Vanhamme L, van den BA, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129(1):35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 28.Rico-Sanz J, Thomas EL, Jenkinson G, Mierisova S, Iles R, Bell JD. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by (1)H-MRS. J Appl Physiol. 1999;87(6):2068–2072. doi: 10.1152/jappl.1999.87.6.2068. [DOI] [PubMed] [Google Scholar]
- 29.Tarasow E, Siergiejczyk L, Panasiuk A, Kubas B, Dzienis W, Prokopowicz D, et al. MR proton spectroscopy in liver examinations of healthy individuals in vivo. Med Sci Monit. 2002;8(2):MT36–MT40. [PubMed] [Google Scholar]
- 30.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 31.Morio B, Hocquette JF, Montaurier C, Boirie Y, Bouteloup-Demange C, McCormack C, et al. Muscle fatty acid oxidative capacity is a determinant of whole body fat oxidation in elderly people. Am J Physiol Endocrinol Metab. 2001;280(1):E143–E149. doi: 10.1152/ajpendo.2001.280.1.E143. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 33.Cree MG, Zwetsloot JJ, Fram RY, Herndon DN, Newcomer BR, Angel C, et al. Insulin sensitivity is related to fat oxidation and protein kinase C in children with acute burn injury. J Burn Care Res. 2008;29(4):585–594. doi: 10.1097/BCR.0b013e31817db88f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cree MG, Newcomer BR, Read LK, Sheffield-Moore M, Paddon-Jones D, Chinkes D, et al. Plasma triglycerides are not related to tissue lipids and insulin sensitivity in elderly following PPAR-alpha agonist treatment. Mech Ageing Dev. 2007;128(10):558–565. doi: 10.1016/j.mad.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidossis LS, Mittendorfer B, Walser E, Chinkes D, Wolfe RR. Hyperglycemia-induced inhibition of splanchnic fatty acid oxidation increases hepatic triacylglycerol secretion. Am J Physiol. 1998;275(5 Pt 1):E798–E805. doi: 10.1152/ajpendo.1998.275.5.E798. [DOI] [PubMed] [Google Scholar]
- 36.Wolfe RR, Klein S, Carraro F, Weber JM. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am J Physiol. 1990;258(2 Pt 1):E382–E389. doi: 10.1152/ajpendo.1990.258.2.E382. [DOI] [PubMed] [Google Scholar]
- 37.Wolfe RR, Peters EJ. Lipolytic response to glucose infusion in human subjects. Am J Physiol. 1987;252(2 Pt 1):E218–E223. doi: 10.1152/ajpendo.1987.252.2.E218. [DOI] [PubMed] [Google Scholar]
- 38.Brulez HF, van Guldener C, Donker AJ, ter Wee PM. The impact of an amino acid-based peritoneal dialysis fluid on plasma total homocysteine levels, lipid profile and body fat mass. Nephrol Dial Transplant. 1999;14(1):154–159. doi: 10.1093/ndt/14.1.154. [DOI] [PubMed] [Google Scholar]
- 39.Li FK, Chan LY, Woo JC, Ho SK, Lo WK, Lai KN, et al. A 3-year, prospective, randomized, controlled study on amino acid dialysate in patients on CAPD. Am J Kidney Dis. 2003;42(1):173–183. doi: 10.1016/s0272-6386(03)00421-9. [DOI] [PubMed] [Google Scholar]
- 40.Ferrannini E, Vichi S, Beck-Nielsen H, Laakso M, Paolisso G, Smith U. Insulin action and age. European Group for the Study of Insulin Resistance (EGIR) Diabetes. 1996;45(7):947–953. doi: 10.2337/diab.45.7.947. [DOI] [PubMed] [Google Scholar]
- 41.Otten JJ, Pitzi Hellwig J, Meyers LD, editors. Food and Nutrition Board; Institute of Medicine of the National Academies. National Academies Press; Washington DC: 2006. The Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. [Google Scholar]