Abstract
Purpose
Current therapy for lung cancer involves multimodality therapies. However, many patients are either refractory to therapy or develop drug resistance. KRAS and epidermal growth factor receptor (EGFR) mutations represent some of the most common mutations in lung cancer, and many studies have shown the importance of these mutations in both carcinogenesis and chemoresistance. Genetically engineered murine models of mutant EGFR and KRAS have been developed that more accurately recapitulate human lung cancer. Recently, using cell-based experiments, we showed that platinum-based drugs and the antidiabetic drug rosiglitazone (PPARg ligand) interact synergistically to reduce cancer cell and tumor growth. Here, we directly determined the efficacy of the PPARγ/carboplatin combination in these more relevant models of drug resistant non – small cell lung cancer.
Experimental Design
Tumorigenesis was induced by activation of either mutant KRAS or EGFR. Mice then received either rosiglitazone or carboplatin monotherapy, or a combination of both drugs. Change in tumor burden, pathology, and evidence of apoptosis and cell growth were assessed.
Results
Tumor burden remained unchanged or increased in the mice after monotherapy with either rosiglitazone or carboplatin. In striking contrast, we observed significant tumor shrinkage in mice treated with these drugs in combination. Immunohistochemical analyses showed that this synergy was mediated via both increased apoptosis and decreased proliferation. Importantly, this synergy between carboplatin and rosiglitazone did not increase systemic toxicity.
Conclusions
These data show that the PPARγ ligand/carboplatin combination is a new therapy worthy of clinical investigation in lung cancers, including those cancers that show primary resistance to platinum therapy or acquired resistance to targeted therapy.
Lung cancer is the leading cause of cancer-related deaths. There are over 210,000 cases of lung cancer diagnosed and over 160,000 deaths in the United States alone (1, 2). The most common type of lung cancer is non – small cell lung cancer (NSCLC), which comprises over 75% of the cases (3). Despite advances in multimodality therapies, <15% of patients with NSCLC survive beyond 5 years of initial diagnosis. Activating mutations of the KRAS proto-oncogene are among the most common genetic alterations in NSCLC (4-8). These mutations lead to the constitutive activation of downstream signaling transduction pathways including RAF and phosphatidylinositol-3-OH kinase. These pathways, in turn, regulate proliferation and survival. In addition to playing a role in the development of lung cancer, mutations in KRAS predict a poor outcome and a poor response to conventional therapy such as platinum-based drugs, as well as targeted therapy (4, 9-12). The epidermal growth factor receptor (EGFR) is another key signal transduction component that is commonly altered in >60% of NSCLC (13). Genomic amplification, point mutations, and autocrine loop activation are responsible for the increased activity of EGFR in many of these cancers. The EGFR has received a significant amount of attention in recent years because of the development of small molecule tyrosine kinase inhibitors (TKI). Although stable disease is observed in many patients after treatment with these TKIs, clinically objective responses are mainly observed in a subpopulation of patients (female, nonsmoker, Asian, and adenocarcinoma). One of the causes of tumor sensitivity to TKIs in these patients is an activating mutations in the kinase domain of the EGFR (14, 15). Despite the dramatic response of cancers with sensitizing EGFR mutations to TKIs, these tumors invariably develop drug resistance within 9 to 12 months (16 -18). In approximately half of cases with acquired resistance, there is a secondary mutation to the EGFR, T790M (19). This mutation has been shown in vitro to increase the EGFR kinase activity and to confer TKI resistance. There are few viable treatment options for these relapsed patients.
Translational Relevance.
This manuscript describes the use of genetically engineered mouse models to show the striking efficacy and lack of systemic toxicity of the PPARγligand/carboplatin combination therapy in the treatment of autochthonous murine lung adenocarcinomas. PPARγ ligands are clinically approved for the treatment of type II diabetes and have a favorable toxicity profile. Carboplatin is a conventional DNA adduct forming chemotherapeutic agent commonly used in the treatment of lung cancer and a variety of other solid tumors. Combination of carboplatin with other conventional chemotherapeutics in the clinical leads to only slight improved efficacy while increasing the overall toxicity profile of the treatment regimens. Here, we showed that the PPARγ ligand/carboplatin combination treatment leads to dramatic shrinkage of mutant K-Ras and epidermal growth factor receptor – induced murine lung adenocarcinomas. These mutations are associated with resistant to conventional as well as targeted therapeutics. Equally important, there is no increased systemic toxicity in these treated mice. This series of experiments are one of the first demonstrations for the use of genetically engineered mouse models to test the optimal combinations of conventional chemotherapeutics and provide a strong preclinical rationale for the testing of this combination regimen in human clinical trials.
PPARγ is a member of the nuclear hormone receptor superfamily of ligand-activated transcription factors that plays a critical role in the regulation of multiple cellular processes including energy metabolism and differentiation (20, 21). Agonist ligands for PPARγ including pioglitazone and rosiglitazone are widely and clinically used for the treatment of type 2 diabetes. Studies have shown that PPARγ can function as a tumor suppressor, and its ligands have antitumor activity in preclinical models (21 - 27). This is particularly attractive because the PPARγ ligands are extremely well-tolerated compared with conventional chemotherapeutics, and as such, they have considerable appeal as novel cancer therapeutics. Indeed, a recent report described a decreased risk of lung cancer in patients taking PPARγ ligands for control of diabetes (28). However, with the exception of an early trial in liposarcoma, exploratory clinical trials testing PPARγ ligands as monotherapy in advanced cancer failed to show a therapeutic benefit (29 - 33).
We recently discovered that the combination of PPARγ ligands and platinum-based drugs caused a significant and synergistic reduction in the growth of several human cancer cells, including NSCLC cell line xenografts in nude mice (34). However, these cell lines might not be representative of primary lung cancer cells, as they have been kept in cell culture for extended periods of time and may have evolved many additional genetic alterations. Additionally, xenograft experiments often do not fully recapitulate the immune and stromal-tumor interactions that might impact on the differential responses to therapeutic treatments (35).
Several laboratories have recently developed mouse models of NSCLC cancer based on specifically defined oncogenic alterations that are associated with lung cancer (35, 36). These tumors are certainly more similar to human lung cancer than xenograft models and provide a more rigorously defined pre clinical model for the testing of novel therapeutics. These models also represent drug-resistant lung cancer seen in patients for whom there are presently no good therapeutic options (37, 38). In this study, we have applied the carboplatin/PPARγ combination therapy to two different autochthonous models of lung cancer driven by mutant KRAS or EGFR. The combination of PPARγ agonist and a platinum chemotherapy agent led to significant tumor shrinkage without an increase in systemic toxicity in both of these models. These data show the feasibility of this combination regimen of PPARγ agonist and platinum-based chemotherapy drugs in the treatment of NSCLC patients, especially patients with tumors refractory to conventional and other molecularly targeted therapies.
Materials and Methods
Induction of lung tumors
Tet-op EGFR T790M-L858R (EGFR-TL) mice were generated as previously described (39). The CCSP-rtTA mice were generously provided by Dr. Jeffery Whitsett at University of Cincinnati, Cincinnati, OH (40). Bitransgenic mice (EGFR-TL and CCSP-rtTA) were administered doxycycline beginning at age 4 wk as previously described (37). After 6 wk on doxycycline, bitransgenic mice (EGFR-TL and CCSP-rtTA) were subjected to magnetic resonance imaging (MRI) to document the lung tumor burden (41). The Lox-StopLox K-ras G12D (LSL-KrasG12D) mice were generously provided by Dr. Tyler E. Jacks (Massachusetts Institute of Technology, Cambridge, MA). LSL-kras mice were infected with adenovirus Cre recombinase at ages 6 to 8 wk as previously described, and tumor burden was confirmed by MRI (39). All mice were housed in the pathogen-free environment at the Harvard School of Public Health. The mice were handled in strict accord with good animal practice as defined by The Center for Animal Resources and Comparative Medicine at Harvard Medical School, and all animal work was done with Animal Resources and Comparative Medicine approval.
Cancer therapy using carboplatin and the PPARγ agonist drug rosiglitazone in vivo
Carboplatin (Sigma) was reconstituted in double distilled water. Mice were dosed at 50 mg/kg thrice a week via i.p. injection. Rosiglitazone pellets were synthesized and obtained from Bio-Serv. Control laboratory chow pellets and rosiglitazone pellets at a dose of 25 mg/kg/d. After treatment, mice were analyzed by MRI at different time points to determine the change in tumor burden.
Histology and immunohistochemistry
Mice were euthanized after confirming tumor burden with MRI. Left lungs were dissected and snap frozen for biochemical analysis as described previously. The remaining lung was inflated with neutral buffered 10% formalin for 10 min and then fixed in 10% formalin overnight at room temperature. After fixation, tissues were washed in PBS, placed in 75% ethanol, embedded in paraffin, and 5-μm sections were cut and stained with H&E. Sectioning staining and immunohistochemistry were done by the Department of Pathology at Brigham and Women's Hospital using antibodies against cleaved poly (ADP-ribose) polymerase, terminal deoxynucleotidyl-transferase – mediated dUTP nick-end labeling (TUNEL), Ki67, and proliferating cell nuclear antigen as previously described (39).
Analysis of carboplatin induced toxicity
Mice were given control chow, chow containing rosiglitazone (25 mg/kg/d), or carboplatin (50 mg/kg 3×/wk i.p.) alone or in combination for 2 wk. Mice were euthanized, blood was collected, and CBC and Chem7 were done by the Clinical Chemistry Lab at Children's Hospital, Boston.
Results
The combination of PPARγ agonist rosiglitazone with carboplatin causes tumor shrinkage in Kras-driven tumors
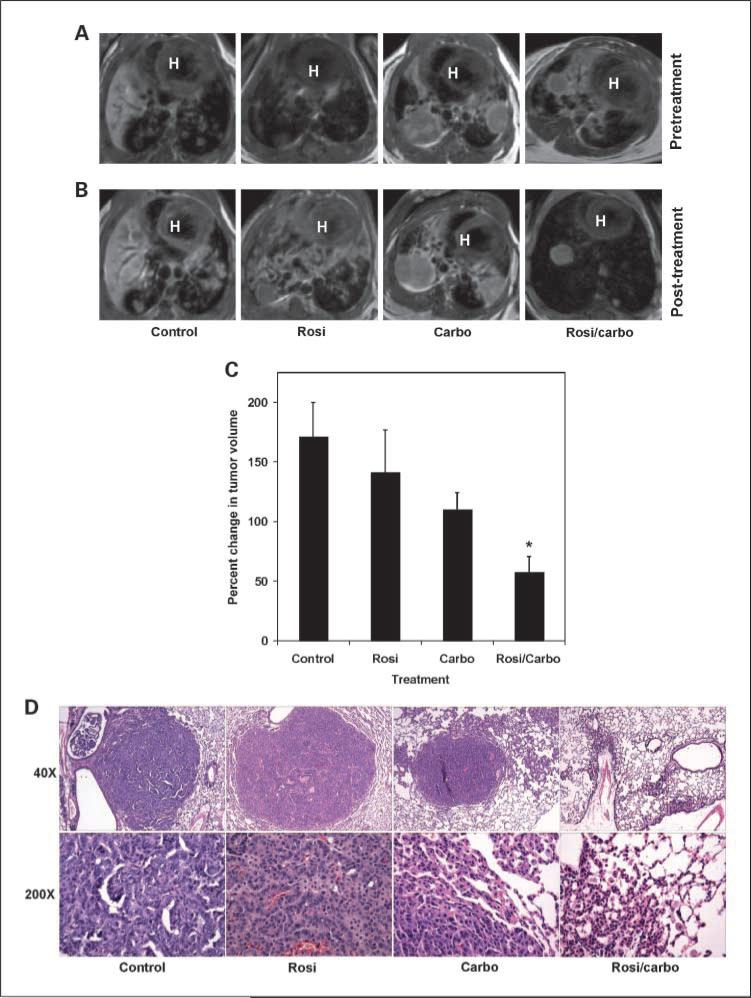
We used the LSL-KrasG12D conditional mutant mice to model KRAS-driven human lung cancer (42). These mice proceed to develop lung tumors in a time- and dose-dependent fashion that recapitulates the human condition. Tumors were induced and mice were imaged in a cohort of LSL-KrasG12D mice as described in Materials and Methods (Fig. 1A). Mice then received control, rosiglitazone monotherapy, carboplatin monotherapy, or rosiglitazone and carboplatin combination therapy for 11 days. Mice were imaged again to document change in tumor burden. Tumor burden increased in the control and mono-therapy rosiglitazone or carboplatin-treated mice ∼80% (Fig. 1B and C). However, tumors from rosiglitazone and carboplatin-treated mice did not seem to increase as much as tumors from control mice, although this difference was not statistically significant (P = 0.31 and 0.07, respectively). In striking contrast, the combination of rosiglitazone and carboplatin led to a significant decrease in tumor burden (Fig. 1B). There was a >40% reduction in average tumor volume in these mice after the combination treatment (Fig. 1C; P < 0.005).
Fig. 1.
Treatment of mice with the combination of rosiglitazone and carboplatin dramatically reduce mutant KRAS – induced lung tumors. Eighteen weeks after administration of adenovirus Cre by nasal installation, LSL-KrasG12D mice were imagined by MRI. Mice were treated with control chow, chow containing rosiglitazone (25 mg/kg/d), or carboplatin (50 mg/kg 3×/wk i.p.) alone or in combination for 11d, and then mice were reimaged. A, MRI of tumor burden before indicated treatment. B, MRI of tumor burden after indicated treatment. Red H, heart for anatomic orientation. C, average change in tumor volume compared with pretreatment volume was determined as previously described (*, P < 0.005; ref. 39). D, histopathology of Kras induced lung tumors after treatment. Top, ×40 magnification of representative tumor. Bottom, ×200 magnification.
We next examined the pathology of the tumors after the treatment described above (Fig. 1D). The tumors in the placebo group were composed of parenchymal and bronchial adenocarcinomas. The parenchymal adenocarcinomas displayed a mixture of bronchioalveolar, acinar, and solid patterns with occasional signet ring cells and numerous mitotic figures. The airway tumors were predominantly papillary in nature. The tumors from the rosiglitazone-treated mice were similar in number, size, and histopathologic features to the placebo group, with minimal, if any, treatment effects. The parenchymal tumors in the carboplatin group did show a mild treatment effect, with only occasional tumors showing signs of regression. In contrast to the above three groups, tumors from the combination rosiglitazone/carboplatin-treated group showed a dramatic reduction in parenchymal tumor burden with fewer and smaller tumor nodules. There were numerous areas that showed thickened alveolar walls with reactive type II pneumocytes, indicative of healing and resolution of an area previously occupied by tumor. Furthermore, both mitotic activity and the amount of airway papillary tumor were also decreased (Fig. 1D). There did not seem to be any effect on normal alveolar cells by the combination. Some areas of lung contained extensive eosinophilic intraalveolar macrophages, likely as a reactive process. These areas may have given the impression of being tumor by MRI because they would appear as areas of increased density. This suggests that the MRI analysis of mice treated with the combination may be actually overestimating the amount of tumor burden. Hence, these data show that combining the PPARγ ligand rosiglitazone with carboplatin leads to a significant reduction in gross and microscopic tumor burden induced by a mutation commonly associated with platinum drug resistance.
The rosiglitazone/carboplatin combination alters tumor cell survival and proliferation
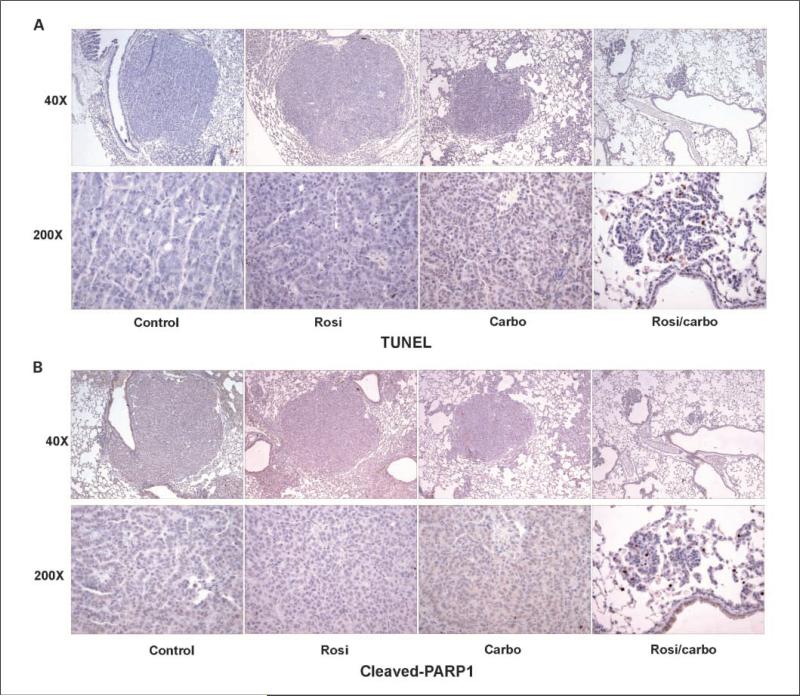
Our previous work indicated that the rosiglitazone/carboplatin combination was inhibiting cancer cell growth in culture via alterations in both apoptosis and proliferation (34). As shown in Fig. 2A, tumors from control mice and mice treated with rosiglitazone or carboplatin monotherapy showed very little evidence of apoptosis as determined by TUNEL staining. However, tumors from mice treated with the rosiglitazone/carboplatin combination showed extensive TUNEL-positive cells indicating that this combination dramatically increased apoptosis. Cleavage of Poly (ADP-ribose) polymerase by the effector caspase, caspase-3, is a useful molecular marker of apoptosis. In agreement with the TUNEL staining, we saw very little cleaved poly (ADP-ribose) polymerase staining in control or after rosiglitazone and carboplatin monotherapy (Fig. 2B). In contrast, tumors from combination-treated mice showed extensive staining of cleaved poly (ADP-ribose) polymerase.
Fig. 2.
Rosiglitazone and carboplatin in combination dramatically increase apoptosis. Tumors from control, rosiglitazone, carboplatin-treated mice were fixed, paraffin embedded, and 5-μm sections cut. Sections were stained for (A) TUNEL-positive cells or (B) cleaved poly (ADP-ribose) polymerase as described in Materials and Methods. Top, ×40 magnification of representative tumor. Bottom, ×200 magnification.
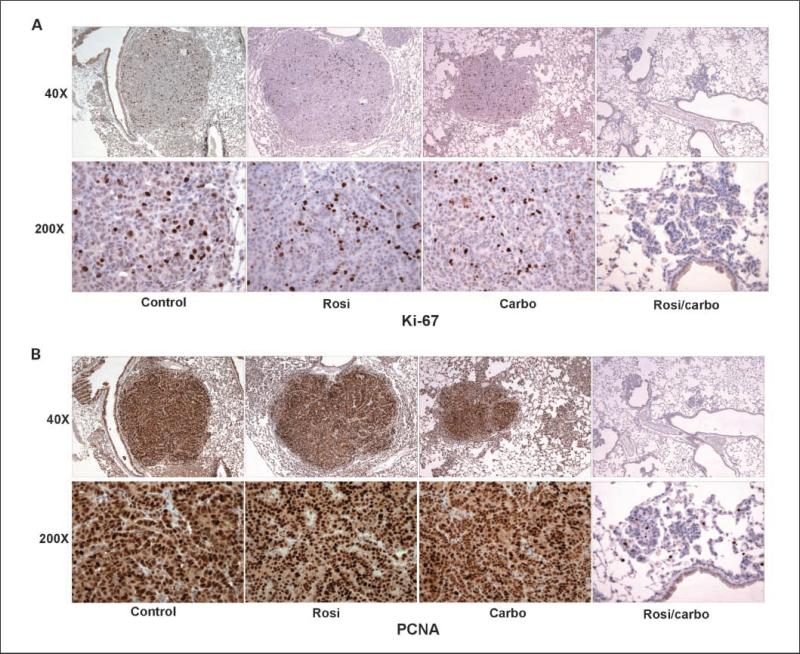
Carboplatin is known to alter cell cycle kinetics (43). Our previous data showed that rosiglitazone augments the ability of carboplatin to reduce cell proliferation. In the tumors studied here, there was a small decrease in Ki67 staining from tumors of mice treated with rosiglitazone or carboplatin monotherapy (Fig. 3A). Interestingly, compared with control mice, we did not observe a difference in PNCA staining after these treatments alone (Fig. 3B). In contrast, tumors from mice treated with the rosiglitazone and carboplatin combination showed a dramatic reduction in both Ki67 and proliferating cell nuclear antigen staining. This reinforces the dramatic reduction in mitotic figures observed by histopathology. These data strongly suggest that the reduction in tumor burden we observed by MRI and pathology after treatment with a combination of carboplatin and rosiglitazone is the result of both increased apoptosis and decreased proliferation.
Fig. 3.
Rosiglitazone and carboplatin in combination dramatically reduce tumor proliferation. Tumors from control, rosiglitazone, carboplatin-treated mice were fixed, paraffin embedded, and 5-μm sections cut. Sections were stained for (A) Ki67 or (B) PNCA as described in Materials and Methods. Top, ×40 magnification of representative tumor. Bottom, ×200 magnification.
Tumor shrinkage by the combination of rosiglitazone and carboplatin in TKI resistant lung cancer
Acquisition of drug resistance in lung cancer remains a difficult clinical problem. Recently, we developed a mouse model of NSCLC that is resistant to EGFR inhibition (37). Mice are engineered with a construct that allows for doxycycline-inducible expression of the EGFR with the TKI-sensitizing mutation, L858R, as well as the T790M mutation, one of the alterations responsible for TKI resistance (37). Mice with a single mutation (L858R) respond to EGFR inhibition by small TKI, whereas mice harboring the double mutant allele (T790M-L858R mutant EGFR) do not.
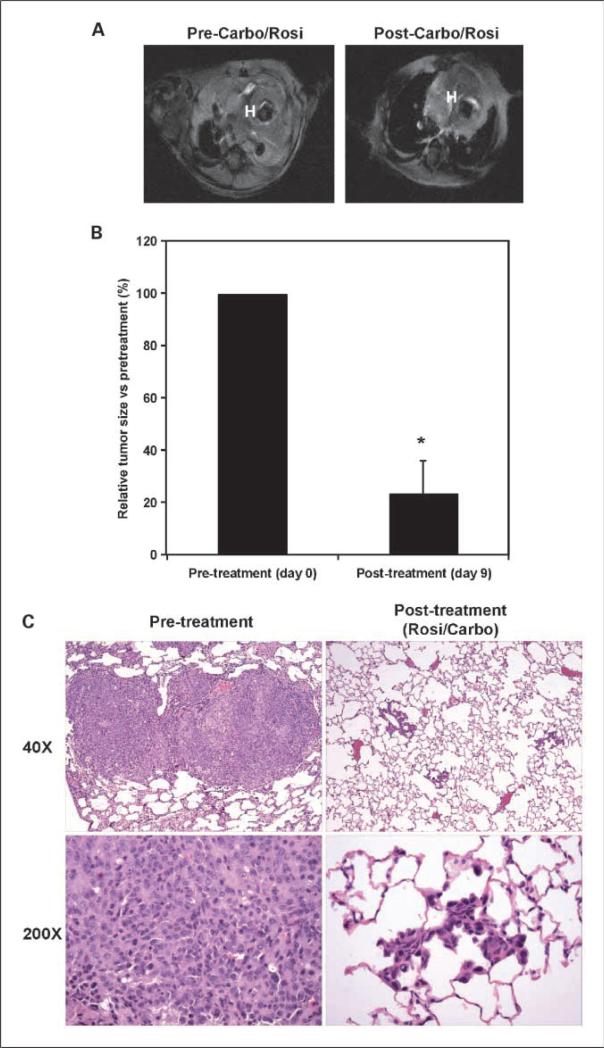
EGFR-TL mice with confirmed tumor burden were treated for 9 days with either rosiglitazone or carboplatin alone or in combination to examine effects on tumors driven by EGFR mutations with secondary TKI resistance. Tumors from control and mice treated with single agents increased during the course of the experiment (data not shown). In contrast, treatment of mice with the combination of rosiglitazone and carboplatin led to a significant 80% reduction in tumor burden (Fig. 4A and B; P < 0.0001). Pathologic analysis revealed that the tumors from the untreated mice were parenchymal adenocarcinomas with solid and bronchioloalveolar features without prominent airway tumors (Fig. 4C). Consistent with the imaging data, the tumor number and size decreased significantly with the combination treatment. Although some solid tumor nodules remained, the bronchioloalveolar tumor burden was markedly decreased with focal alveolar wall thickening, type II pneumocyte hyperplasia, and absence of tumor cells.
Fig. 4.
Treatment of mice with the combination of rosiglitazone and carboplatin dramatically shrinks mutant EGFR – induced TKI-resistant lung tumors. Bitransgenic CCSP-rTA/EGFR-TL mutant mice were treated with doxycline for 9 wk and then imaged by MRI. Mice were treated with control chow, chow containing rosiglitazone (25 mg/kg/d), or carboplatin (50 mg/kg 3×/wk i.p.) alone or in combination for 9 d, and then mice were reimaged. A, a representative MRI from a CCSP-rTA/EGFR-TL mutant mouse prior (left) and after (right) treatment with a combination of rosiglitazone and carboplatin. Red H, heart for anatomic orientation. B, average change in tumor volume compared with pretreatment volume was determined as previously described (*, P < 0.0001;ref.39). C, histopathology of TKI-resistant EGFR-TL mutant induced lung tumor taken from mice euthanized prior (left) to treatment and after (right) the carboplatin/rosiglitazone combination treatment. Top, 40 magnification of representative tumor. Bottom, ×200 magnification.
PPARγ agonist rosiglitazone does not increase myelosuppressive side effects when coadministered with carboplatin
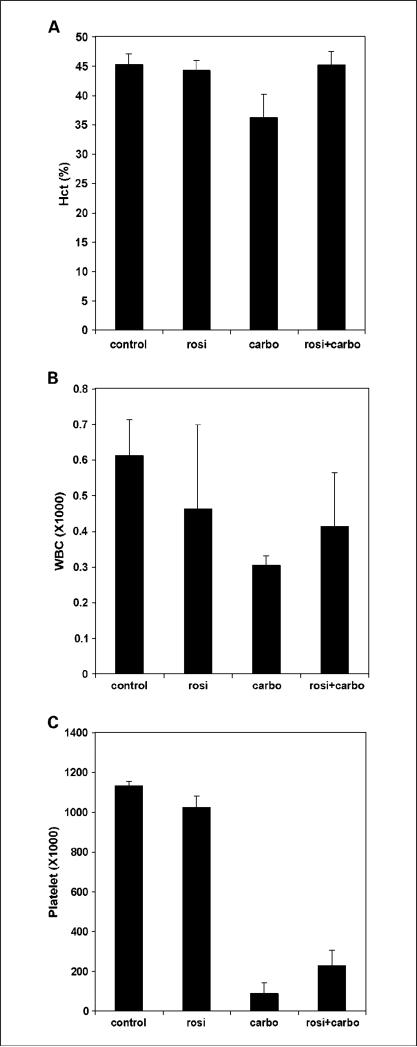
Extensive toxicities place an upper limit on the amount of platinum-based drugs that can safely be used in patients (44). Myelosuppression is a particularly common side effect associated with carboplatin therapy. One serious concern arising from our studies is that the PPARγ ligands might increase both the efficacy and toxicity of the platinum drugs. To critically investigate the toxicity of these drugs, we did a complete blood count on mice after treatment with rosiglitazone or carboplatin monotherapy, or in combination. Rosiglitazone monotherapy had little to no effect on hematocrit, WBC, or platelet count (Fig. 5). In contrast, carboplatin alone had a significant myelosuppressive effect with a slight decrease in hematocrit levels and a significant decrease in WBC and platelet count. Importantly, we did not observe a further decrease in these variables when mice were treated with a combination of carboplatin and rosiglitazone at exactly the doses that yielded improved therapeutic effects on tumors. Although nephrotoxicity is more commonly associated with cisplatin rather than carboplatin, we also examined kidney function by measuring BUN and creatine levels in the blood of mice after these treatments. Carboplatin and rosiglitazone monotherapy did not have a significant effect on BUN or creatine levels (data not shown). These variables were also not altered when carboplatin and rosiglitazone were administered in combination. Therefore, these data indicate that the synergy between the PPARγ agonist rosiglitazone with carboplatin in therapeutic effects does not cause significant increases in systemic toxicities associated with platinum-based drug use.
Fig. 5.
Rosiglitazone does not increase the myelosuppressive effects of carboplatin. Mice were treated with control chow, chow containing rosiglitazone (25 mg/kg/d), or carboplatin (50 mg/kg 3×/wk i.p.) alone or in combination for 2 wk. A, hematocrit, (B) WBC, and (C) platelet counts were determined as described in Materials and Methods. Columns, mean (n = 4); bars, SD.
Discussion
We have previously shown that the combination of PPARγ ligand and carboplatin synergize to reduce the growth of human lung tumors transplanted into nude mice (34). Although those studies suggested a new therapeutic approach to the treatment of lung cancer, the use of established human lung cell lines in a xenograft setting does not recapitulate the human condition with adequate fidelity to predict therapeutic utility in patients (35). The use of genetically engineered mice with specific lesions has been crucial to defining oncogenic pathways and their mechanism of action in cancer (35, 36, 45). The improved ability of these models to recapitulate the human condition and response to therapy underscores the utility of these mice. In addition, these models have recently proven useful in preclinical testing before advancing to human clinical trials for new cancer treatments (37). Although the models used here represent a subset of NSCLC, those with either KRAS or EGFR mutations, they also represent the tumor subset with the greatest clinical challenge. In this article, we describe the ability of a PPARγ agonist, rosiglitazone, to reduce autochthonous lung tumor burden in these genetically engineered murine models of human lung cancer when administered in combination with carboplatin. Based on these data, the combination of PPARγ activation by rosiglitazone and carboplatin represents a potentially new mode of therapy to increase chemosensitivity in lung cancer and other malignancies for which platinum-based regimens are used in clinical oncology.
Naïve and acquired resistance to cancer chemotherapy represent a significant obstacle, which prevents long term tumor control in patients with lung cancer (46). Mutations to a number of oncogenes underlie the resistance of many tumors to current chemotherapy. The ability to genotype tumors has enabled clinicians to identify mutations in human cancer and predict the role of these mutations in response to chemotherapy. RAS mutations are found in roughly 30% of all lung cancers (4 - 8). Mutations to the EGFR have been described in ∼8% to 15% of tumors from lung cancer patients (38, 47). However, the incidence increases for certain populations such as women, Asians, nonsmokers, and adenocarcinoma. Interestingly, these oncogenic EGFR mutations also sensitize the tumors to small molecule TKI that target the EGFR (14, 15). Unfortunately, these responses are short lived. Many of these tumors develop resistance to EGFR inhibition due to the development of a secondary mutation (16, 18, 37). The development of KRAS and mutant EGFR – driven lung tumor models has allowed us to study the role of the PPARγ ligand/carboplatin combination in these better-defined models (37, 42). Tumors increased in size in control and single agent – treated mice. Pathologic analysis confirmed that carboplatin, but not rosiglitazone treatment, led to a small degree of tumor regression. This partial response highlights the utility of this model to recapitulate chemoresistant human lung cancer because response to platinum-based therapy in humans is <30% (48). Although carboplatin alone produced only a partial response, we observed a significant regression of tumors after treatment with rosiglitazone and carboplatin, both in terms of gross tumor volume and microscopically. This effect was a result of both increased apoptosis and decreased proliferation.
It should be noted that PPARγ ligands are already in clinical use for the management of type 2 diabetes mellitus and therefore are readily available for human clinical research studies in cancer. Indeed, almost 10 million people in the United States are treated with rosiglitazone or pioglitazone for control of their diabetes. Importantly, these drugs have a fairly favorable toxicity profile, especially when compared with most cancer chemotherapy agents. However, a recent report also suggested increased cardiotoxicity in patients taking rosiglita-zone (49). Interestingly, pioglitazone, another Food and Drug Administration – approved PPARγ ligand, has not been reported to cause cardiotoxicity. Therefore, there remains a serious concern that PPARγ agonist ligands might increase the overall toxicities of carboplatin chemotherapy, or that combination dosing in humans might be associated with novel toxicities not seen with either drug individually. Myelosuppression, especially in the form of thrombocytopenia, is the most common side of effect of carboplatin (50, 51). Our data indicate that combination dosing of the PPARγ agonist ligand, rosiglitazone, and carboplatin does not increase the myelosuppression or other toxic effects of carboplatin. Although beyond the scope of the work presented, addition of a PPARγ ligand may actually reduce the myelosuppression caused by carboplatin when dosed in combination with rosiglitazone. Indeed, other groups have shown that PPARγ ligands actually protect against the nephrotoxic and myelosuppressive effects of cisplatin and 5-fluorouracil, respectively (41, 51). We have previously suggested that PPARγ-mediated attenuation of inflammatory pathways may mediate the effect between PPARγ ligands and carboplatin (34, 52). This same mechanism may be functioning to protect normal tissues as well. Additional studies will be needed to evaluate these observations of potential normal tissue protection and are currently exploring whether the synergy and potential protection are mediated by similar pathways.
These series of experiments are one of the first demonstrations of the use of genetically engineered mouse models to test the optimal combinations of conventional chemotherapeutics. Importantly, we show that the rosiglitazone/carboplatin combination represents a more powerful anticancer treatment modality compared with either agent alone; this should have practical implications because we show that these agents can be administered without increasing overall toxicity. Finally, many pathways are involved in chemosensitivity and chemoresistance. The ability of the rosiglitazone/carboplatin combination regimen to synergistically inhibit tumor growth in different genetically engineered mouse models of lung tumorigenesis shows the potential for a broadly effective anticancer strategy. Clinical trials to test the safety and efficacy of this combination regimen in cancer patients are also planned based on this work.
Acknowledgments
We thank Dr. Jeffrey Whitsett for providing the CCSP-rtTA transgenic mice and Dr. Tyler E. Jacks for providing lox-stop-lox KRas G12D (Lsl-kras) mice.
Grant support: NIH grant K08 AG024004, R01 CA122794, R01 AG2400401, the Sidney Kimmel Foundation for Cancer Research, the Joan Scarangello Foundation to Conquer Lung Cancer, the Cecily and Robert Harris Foundation, and the Flight Attendant Medical Research Institute (K.K. Wong); NIH grant K01 DK064685, and the University of Maryland/Greenebaum Cancer Center CRF (G.D. Girnun); NIH MERIT award (B.M. Spiegelman); and R37DK31405 (J. Silvaggi).
Footnotes
Note: G.D. Girnun and L. Chen contributed equally to this work.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:29–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 3.Tyson LB. Non-small cell lung cancer: new hope for a chronic illness. Oncol Nurs Forum. 2007;34:963–70. doi: 10.1188/07.ONF.963-970. [DOI] [PubMed] [Google Scholar]
- 4.Aviel-Ronen S, Blackhall FH, Shepherd FA, Tsao MS. K-ras mutations in non-small-cell lung carcinoma: a review. Clin Lung Cancer. 2006;8:30–8. doi: 10.3816/CLC.2006.n.030. [DOI] [PubMed] [Google Scholar]
- 5.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–9. [PubMed] [Google Scholar]
- 6.Rodenhuis S, Slebos RJ. The ras oncogenes in human lung cancer. Am Rev Respir Dis. 1990;142:S27–30. doi: 10.1164/ajrccm/142.6_Pt_2.S27. [DOI] [PubMed] [Google Scholar]
- 7.Rodenhuis S, Slebos RJ, Boot AJ, et al. Incidence and possible clinical significance of K-ras oncogene activation in adenocarcinoma of the human lung. Cancer Res. 1988;48:5738–41. [PubMed] [Google Scholar]
- 8.Suzuki Y, Orita M, Shiraishi M, Hayashi K, Sekiya T. Detection of ras gene mutations in human lung cancers by single-strand conformation polymorphism analysis of polymerase chain reaction products. Oncogene. 1990;5:1037–43. [PubMed] [Google Scholar]
- 9.Pao W. Defining clinically relevant molecular subsets of lung cancer. Cancer Chemother Pharmacol. 2006;58(Suppl 1):s11–5. doi: 10.1007/s00280-006-0310-x. [DOI] [PubMed] [Google Scholar]
- 10.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–9. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 11.Huncharek M, Muscat J, Geschwind JF. K-ras oncogene mutation as a prognostic marker in non-small cell lung cancer: a combined analysis of 881 cases. Carcinogenesis. 1999;20:1507–10. doi: 10.1093/carcin/20.8.1507. [DOI] [PubMed] [Google Scholar]
- 12.Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2005;92:131–9. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukohara T, Kudoh S, Yamauchi S, et al. Expression of epidermal growth factor receptor (EGFR) and downstream-activated peptides in surgically excised non-small-cell lung cancer (NSCLC). Lung Cancer. 2003;41:123–30. doi: 10.1016/s0169-5002(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 14.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 15.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 16.Inukai M, Toyooka S, Ito S, et al. Presence of epidermal growth factor receptor geneT790M mutation as a minor clone in non-small cell lung cancer. Cancer Res. 2006;66:7854–8. doi: 10.1158/0008-5472.CAN-06-1951. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 18.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinibor erlotinibis associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson BE, Jackman D, Janne PA. Cancer Chemother Pharmacol. Vol. 58. Suppl 7: 2006. Impact of EGFR mutations on treatment of non-small cell lung cancer. pp. 5–9. [Google Scholar]
- 20.Lehrke M, Lazar MA. The many faces of PPARγ. Cell. 2005;123:993–9. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Michalik L, Desvergne B, Wahli W. Peroxisomeproliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 22.Koeffler HP. Peroxisome proliferator-activated receptor γ and cancers. Clin Cancer Res. 2003;9:1–9. [PubMed] [Google Scholar]
- 23.Girnun GD, Smith WM, Drori S, et al. APC-dependent suppression of colon carcinogenesis by PPARγ. Proc Natl Acad Sci U S A. 2002;99:13771–6. doi: 10.1073/pnas.162480299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller E, Sarraf P, Tontonoz P, et al. Terminal differentiation of human breast cancer through PPAR γ. Mol Cell. 1998;1:465–70. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- 25.Mueller E, Smith M, Sarraf P, et al. Effects of ligand activation of peroxisome proliferator-activated receptor γ in human prostate cancer. Proc Natl Acad Sci U S A. 2000;97:10990–5. doi: 10.1073/pnas.180329197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarraf P, Mueller E, Jones D, et al. Differentiation and reversal of malignant changes in colon cancer through PPARγ. Nat Med. 1998;4:1046–52. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 27.Elstner E, Muller C, Koshizuka K, et al. Ligands for peroxisome proliferator-activated receptorγ and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci U S A. 1998;95:8806–11. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Govindarajan R, Ratnasinghe L, Simmons DL, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007;25:1476–81. doi: 10.1200/JCO.2006.07.2777. [DOI] [PubMed] [Google Scholar]
- 29.Burstein HJ, Demetri GD, Mueller E, Sarraf P, Spiegelman BM, Winer EP. Use of the peroxisome proliferator-activated receptor (PPAR) γ ligand troglitazone as treatment for refractory breast cancer: a phase II study. Breast Cancer Res Treat. 2003;79:391–7. doi: 10.1023/a:1024038127156. [DOI] [PubMed] [Google Scholar]
- 30.Kulke MH, Demetri GD, Sharpless NE, et al. A phase II study of troglitazone, an activator of the PPARγ receptor, in patients with chemotherapy-resistant meta-static colorectal cancer. Cancer J. 2002;8:395–9. doi: 10.1097/00130404-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Smith MR, Manola J, Kaufman DS, et al. Rosiglitazone versus placebo for men with prostate carcinoma and a rising serum prostate-specific antigen level after radical prostatectomy and/or radiation therapy. Cancer. 2004;101:1569–74. doi: 10.1002/cncr.20493. [DOI] [PubMed] [Google Scholar]
- 32.Debrock G, Vanhentenrijk V, Sciot R, Debiec-Rychter M, Oyen R, Van Oosterom A. A phase II trial with rosiglitazone in liposarcoma patients. Br J Cancer. 2003;89:1409–12. doi: 10.1038/sj.bjc.6601306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demetri GD, Fletcher CD, Mueller E, et al. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-γ ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci U S A. 1999;96:3951–6. doi: 10.1073/pnas.96.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Girnun GD, Naseri E, Vafai SB, et al. Synergy between PPARγ ligands and platinum-based drugs in cancer. Cancer Cell. 2007;11:395–406. doi: 10.1016/j.ccr.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov. 2006;5:741–54. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 36.Dutt A, Wong KK. Mouse models of lung cancer. Clin Cancer Res. 2006;12:4396–402s. doi: 10.1158/1078-0432.CCR-06-0414. [DOI] [PubMed] [Google Scholar]
- 37.Li D, Shimamura T, Ji H, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J Clin Oncol. 2005;23:2556–68. doi: 10.1200/JCO.2005.07.799. [DOI] [PubMed] [Google Scholar]
- 39.Li D, Ji H, Zaghlul S, et al. Therapeuticanti-EGFR antibody 806 generates responses in murine de novo EGFR mutant-dependent lung carcinomas. J Clin Invest. 2007;117:346–52. doi: 10.1172/JCI30446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher GH, Wellen SL, Klimstra D, et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15:3249–62. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benko I, Djazayeri K, Abraham C, Zsuga J, Szilvassy Z. Rosiglitazone-induced protection against myelotoxicity produced by 5-fluorouracil. Eur J Pharmacol. 2003;477:179–82. doi: 10.1016/j.ejphar.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 42.Jackson EL, Willis N, Mercer K, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–8. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eastman A. The mechanism of action of cisplatin: From adducts to apoptosis. In: Lippert B, editor. Cisplatin Chemistry and Biochemistry of a Leading Anti-cancer Drug. Wiley-VCH; Basel (Switzerland): 1999. pp. 111–34. [Google Scholar]
- 44.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 45.Van Dyke T, Jacks T. Cancer modeling in the modern era: progress and challenges. Cell. 2002;108:135–44. doi: 10.1016/s0092-8674(02)00621-9. [DOI] [PubMed] [Google Scholar]
- 46.Wilson TR, Longley DB, Johnston PG. Chemoresistance in solid tumours. Ann Oncol. 2006;17(Suppl 10):x315–24. doi: 10.1093/annonc/mdl280. [DOI] [PubMed] [Google Scholar]
- 47.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 48.Cosaert J, Quoix E. Platinum drugs in the treatment of non-small-cell lung cancer. Br J Cancer. 2002;87:825–33. doi: 10.1038/sj.bjc.6600540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nathan DM. Rosiglitazone and cardiotoxicity-weighing the evidence. N Engl J Med. 2007;357:64–6. doi: 10.1056/NEJMe078117. [DOI] [PubMed] [Google Scholar]
- 50.Djazayeri K, Szilvassy Z, Benko K, et al. Effect of rosiglitazone, an insulin sensitizer, on myelotoxicity caused by repeated doses of 5-fluorouracil. Pharmacol Res. 2006;53:156–61. doi: 10.1016/j.phrs.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Lee S, Kim W, Moon SO, et al. Rosiglitazone ameliorates cisplatin-induced renal injury in mice. Nephrol Dial Transplant. 2006;21:2096–105. doi: 10.1093/ndt/gfl194. [DOI] [PubMed] [Google Scholar]
- 52.Arlt A, Schafer H. NFκB-dependent chemoresistance in solid tumors. Int J Clin Pharmacol Ther. 2002;40:336–47. doi: 10.5414/cpp40336. [DOI] [PubMed] [Google Scholar]