Abstract
The identification of neuroprotectin D1 (NPD1), a biosynthetic product of docosahexaenoic acid (DHA), in brain and retina as well as the characterization of its bioactivity, is generating a renewed interest in the functional role and pathophysiological significance of omega-3 fatty acids in the central nervous system.
Neurotrophins, particularly pigment epithelium-derived factor (PEDF), induce NPD1 synthesis and its polarized apical secretion, implying paracrine and autocrine bioactivity of this lipid mediator. Also, DHA and PEDF synergistically activate NPD1 synthesis and antiapoptotic protein expression and decreased proapoptotic Bcl-2 protein expression and caspase 3 activation during oxidative stress.
In experimental stroke, endogenous NPD1 synthesis was found to be upregulated, and the infusion of the lipid mediator into the brain under these conditions revealed neuroprotective bioactivity of NPD1.
The hippocampal CA1 region from Alzheimer’s disease (AD) patients (rapidly sampled) shows a major reduction in NPD1.
The interplay of DHA-derived neuroprotective signaling aims to counteract proinflammatory, cell-damaging events triggered by multiple, converging cytokine and amyloid peptide factors, as in the case of AD. Generation of NPD1 from DHA thereby appears to redirect cellular fate toward successful preservation of retinal pigment epithelial (RPE)-photoreceptor cell integrity and brain cell aging. The Bcl-2 pro- and antiapoptotic proteins, neurotrophins, and NPD1, lie along a cell fate-regulatory pathway whose component members are highly interactive, and have potential to function cooperatively in cell survival. Agents that stimulate NPD1 biosynthesis, NPD1 analogs, or dietary regimens may be useful as new preventive/therapeutic strategies for neurodegenerative diseases.
1. Introduction
One of the brain and retina early responses to injury and to the onset of neurodegeneration is the synthesis of stereospecific docosahexaenoic acid (DHA) derivatives, the bioactive neuroprotective docosanoids. Although it has been reported that DHA participates in retina and brain damage [1–5], our laboratory, in contrast, showed that the retina forms mono-, di- and trihydroxyl derivatives of DHA that may be neuroprotective [6]. Since lipoxygenase inhibitors were found to block this synthesis, an enzymatic process catalyzed by a lipoxygenase was implied [6] and the name “docosanoids” was suggested because they are derived from the 22C DHA. Upon the development of lipidomic analysis based on liquid chromatography, photodiode array, electrospray ionization, and tandem mass spectrometry (LC-PDA-ESI-MS-MS-based lipidomic analysis), a collaboration between the group of Charles Serhan (Harvard Medical School, http://hms.harvard.edu/hms/home.asp) and our group identified oxygenation pathways for the synthesis of the docosanoid neuroprotectin D1 (NPD1) and its bioactivity during brain ischemia-reperfusion [7] and in retinal pigment epithelial (RPE) cells challenged by oxidative stress [8]. We suggested the name ‘neuroprotectin D1’ [8] based upon its neuroprotective bioactivity in oxidatively stressed RPE cells and brain and its potent ability to inactivate pro-apoptotic and pro-inflammatory signaling. ‘D1’ refers to its being the first identified neuroprotective mediator derived from DHA. DHA belongs to the essential omega-3 fatty acid family. Activators of docosanoid synthesis include oxidative stress, cytokines and neurotrophins [5,9,10]. 10R,17S-DHA (NPD1) is the first characterized docosanoid. Here we provide a brief overview of the neuroprotective bioactivity of NPD1 in RPE cells, in experimental stroke, and in human neural progenitor brain cells in culture.
2. Docosahexaenoic acid (DHA) is concentrated in photoreceptors
Rods and cones (photoreceptors) require outer segment phagocytosis to function properly. Photoreceptor cells are specialized and differentiated neurons with stacks of photosensitive membrane disks (outer segments) which contain rhodopsin as well as other proteins. This part of the photoreceptor, in which rhodopsin and other proteins perform their functions, contains the highest amounts of DHA-rich phospholipids in the human body and tenaciously retain DHA even during very prolonged periods of omega-3 fatty acid deprivation [5,11,12]. Interestingly, there are also omega-3 fatty acid derivatives longer than C22. Essentially, all are esterified in phospholipids. In some phospholipids, there are two omega-3 fatty acids esterified at both sn-1 and sn-2 of the same glycerol backbone (the supraenoic or supraenes molecular species of phospholipids). The supraenoic phosphatidylcholines that contain DHA (at position sn-2) and the 24:6–36:6 elongation products of the omega-3 fatty acid family series (at position sn-1) are tightly bound to rhodopsin, and may restrict rhodopsin motion [13]. Phospholipids containing DHA conform a favorable environment within which G-protein-coupled events can occur. Another aspect is the physiologically selective enrichment of DHA in phosphatidylserine in neural cells, which positively modulates Akt survival signaling [14,15]. These actions of DHA are not mutually exclusive, and contribute specific functions for membrane DHA.
Under normal conditions, DHA is retained and protected from peroxidation. However, when lipid peroxidation takes place in experimental models of retinal degeneration, perturbation of photoreceptor function, damage, and cell death occur. In several forms of retinitis pigmentosa (RP) and in Usher’s syndrome, a decrease of DHA content in blood has been reported [16]. A possible implication of these studies is that decreased DHA supply to the retina may impair photoreceptor function by decreasing the availability of DHA to photoreceptors. However, the relationship between decreased DHA blood supply and disease initiation and progression remains unclear. Rats overexpressing rhodopsin mutations homologous to human RP display decreased amounts of DHA in photoreceptors [12]. This could represent a retinal response to metabolic stress, whereby decreasing the amount of the major target of lipid peroxidation (DHA) contributes to the protection of photoreceptors. In addition, in constant-light-mediated retinal degeneration, there is loss of DHA from photoreceptors. Rats reared in bright cyclic light are protected from such loss and degeneration, suggesting that there is adaptation and/or a plasticity mechanism [17].
Is the shortage of DHA in the blood of Usher’s syndrome and RP patients reflected in the close relationship of very-long-chain DHA-derived acyl groups with rhodopsin? Or, as shown in experimental retinal degeneration, is the peroxidation of DHA closely associated with rhodopsin, perhaps impairing the function of this protein? Or alternatively (or in addition), does DHA serve as a precursor for a survival mediator, and is the regulation of this pathway perturbed? These questions have not yet been answered. However, DHA has been shown to promote survival and inhibit apoptosis of photoreceptors [18,19]. In an Alzheimer’s disease mouse model, DHA exerts neural protection, and several studies have shown neuroprotective properties of DHA. Here, we review recent results that demonstrate that DHA is the precursor of neuroprotectin D1, a potent promoter of photoreceptor cell survival, which also promotes protection of neural cells.
3. Photoreceptor outer segment phagocytosis selectively attenuates oxidative stress-induced apoptosis in retinal pigment epithelial cells
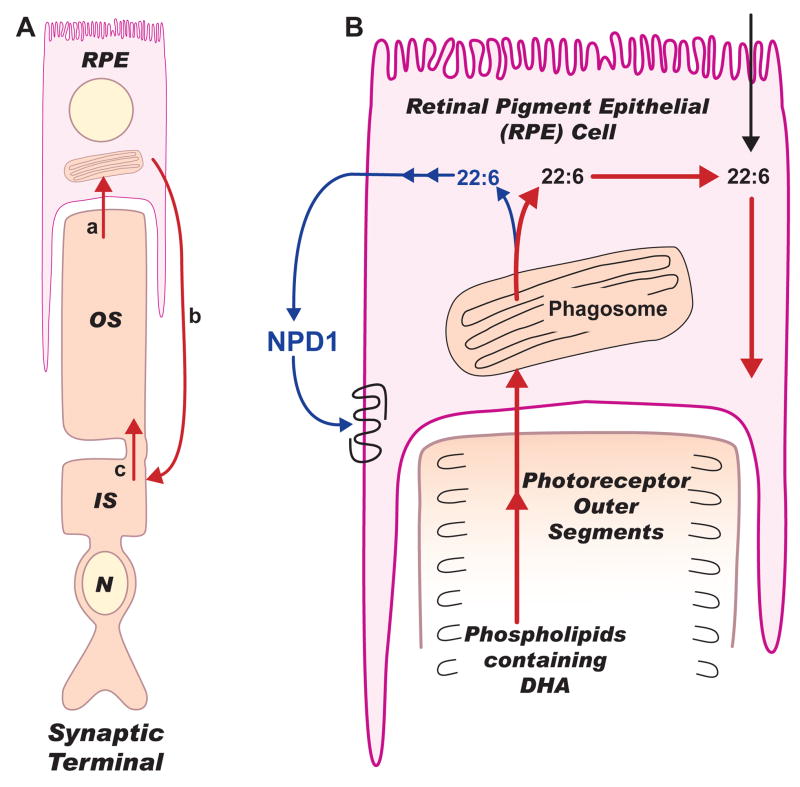
Photoreceptor cells undergo a constant renewal of their outer segments mediated by RPE cells in a daily and circadian manner (Figure 1A). Proteins are turned over and replaced rapidly; however, this is not the case with phospholipids since they are retained during renewal. DHA is recycled back from the RPE cells to the photoreceptors (Figure 1B). Photoreceptor phospholipids are enriched with DHA, which is essential and necessary for photoreceptor function and survival.
Figure 1. Omega-3 fatty acids in photoreceptors and retinal pigment epithelial cells.
In A, a photoreceptor cell and a retinal pigment epithelial (RPE) cell are illustrated. In B, the recycling of docosahexaenoic acid (DHA) after phagosomal digestion and neuroprotection D1 (NPD1) synthesis are depicted. Dietary 18:3,n-3 or 22:6,n-3 (DHA) are actively taken up by hepatocytes where elongation and desaturation of 18:3 to DHA takes place, catalyzed by enzymes located in the endoplasmic reticulum and peroxisomes. 22:6 then is activated (22:6-CoA) and acylated mainly into phospholipids that are secreted into the blood stream as lipoproteins. DHA from lipoproteins is taken up by RPE cells (pink) through the choriocapillaris and then channeled through the interphotoreceptor matrix (IPM) to the inner segments (IS) of photoreceptors (brown). In the IS, phospholipid biosynthesis utilizes DHA and in turn provides phospholipids containing DHA to the biogenesis of outer segment membranes (A: c). The photoreceptor cell is connected to the RPE cell by red arrows that indicate the omega-3 fatty acids conservation route (short loop) during photoreceptor outer segment renewal (A: a,b,c). Thus, a phagosome is shown in the RPE from where red arrows (A and B) follow this conservation route, while a blue arrow indicates DHA being used for NPD1 synthesis (A). This is consistent with the recent demonstration that outer segment phagocytosis selectively increases NPD1 synthesis in the RPE cell [20]. NPD1 is illustrated as acting on a putative receptor on the RPE cell. Signaling evolving from this receptor is shown in Figure 2.
Experimental phagocytosis of photoreceptor outer segments and polystyrene microspheres showed that RPE cells fed only with outer segments were more resistant to oxidative stress-induced apoptosis [20]. On the other hand, RPE cells that effectively phagocytized microspheres when exposed to oxidative stress showed apoptosis. The synthesis of NPD1 was selectively enhanced during outer segment phagocytosis. The highest amount of NPD1 was seen in RPE cells that phagocytized outer segments and were exposed to oxidative stress. A three-fold accumulation of NPD1 was observed as compared to cells that did not phagocytize outer segments. The increase in NPD1 was almost six times higher than that of RPE cells exposed to microspheres. Outer segment phagocytosis also triggered early response gene induction [21], as well as COX-2 [22] and PPARγ [23] expression.
Thus, outer segment phagocytosis enhances RPE cell survival during oxidative stress preceded by increases of free DHA and NPD1. The RPE cells utilize free DHA for NPD1 synthesis when exposed to oxidative stress that, in turn, exerts antiinflammatory and antiapoptotic signaling. We can finally distinguish the homeostasis of DHA-NPD1 regulation that, if altered, could lead to the initiation or prolongation of retinal degenerative diseases due to loss of the RPE cells.
4. Neurotrophins are neuroprotectin D1 synthesis activators
The retinal pigment epithelium (RPE) serves a crucial role in the maintenance of healthy photoreceptors and, consequently, vision. The basolateral membrane of the RPE is in contact with the choriocapillaris, thereby allowing nutrients to pass from the blood stream into the eye. This semi-permeable epithelium allows all-trans retinol (Vitamin A), DHA, and other molecules to be captured and delivered to photoreceptors. The apical aspect of the RPE is in contact with the tip of the outer segments of photoreceptor cells. During photoreceptor outer segment renewal, the RPE phagocytizes the old disks discarded by the distal outer segment. These old disks once housed the phototransduction machinery for the eye, as well as DHA, and get recycled back to the RPE (Figure 1, A and B).
NPD1 also cytoprotects against the apoptotic effects of the accumulated bispyridinium bisretinoid A2E. A2E is a byproduct of phototransduction that becomes toxic when it accumulates in RPE cells during aging, in age-related macular degeneration, and in Stargardt macular dystrophy. It is interesting to note that there is a six hour window in which these neuroprotective effects can be seen. NPD1 also neuroprotects against oxidative stress generated by serum starvation/H2O2 (600μM)/TNFα (10 ng/ml), and does so for an eight-hour time frame. In looking at the pathway for cytoprotection, NPD1 (50 nM) did not affect the conversion of A2E to A2E oxiranes. The action of NPD1 was found to occur at the premitochondrial stage of the apoptosis pathway via decreasing caspase-3 cleavage, decreasing Bax expression, and increasing Bcl-2 expression [10].
Neurotrophins stimulated production and release of NPD1 from the RPE. Human RPE cells were used in monolayers to define whether the NPD1 was secreted on the apical or the basal side of the RPE. Glial cell line-derived neurotrophic factor (GDNF), leukemia inhibitory factor (LIF), neurotrophin-3 (NT3), ciliary neurotrophic factor (CNTF), nerve growth factor (NGF), fibroblast growth factor (FGF), brain-derived neurotropic factor (BDNF), Persephin, and pigment epithelium-derived factor (PEDF) all produced an increase of NPD1 through the apical surface. PEDF elicited the largest synthesis and release of NPD1. Increasing concentrations of PEDF or ciliary neurotrophic factor (CNTF), when added to the basal medium, induced less apical incubation of NPD1, whereas adding the neurotrophins to the apical medium yielded a concentration-dependent increase in apical NPD1 levels [10].
DHA potentiates PEDF-induced release of NPD1 through the apical surface of RPE cells. NPD1 concentration is dependent on whether the PEDF was placed in the media on the apical or basolateral side, with the apical side resulting in more NPD1 production. The addition of DHA to either side of the monolayer produced a PEDF-potentiated increase of NPD1 only on the apical side. Increasing concentrations of DHA on both sides of the monolayer yielded a stepwise increase in apical NPD1 concentration up to a point (50 nM), whereas the basolateral NPD1 concentration had the opposite effect. The addition of PEDF (50 ng/ml) to the increasing DHA concentration synergistically augmented apical NPD1 concentration.
When presented with oxidative stress (serum starvation/H2O2 (600 μM)/TNFα (10 ng/ml)), DHA and PEDF synergistically act to protect human RPE cells in culture. The concentration of NPD1 is greater when DHA and PEDF are added together than when either DHA or PEDF are added alone. Moreover, DHA and PEDF synergistically increased the expression of antiapoptotic proteins Bcl-2 and Bfl-1 while concomitantly decreasing the proapoptotic proteins Bid, Bax, and Bad during oxidative stress conditions. DHA alone, when increased from 10 to 50 nM, amplified Bcl-2 and Bfl-1 production. Though PEDF by itself does not modify expression of the Bcl-2 family of proteins, PEDF synergistically acts with DHA to increase antiapoptotic protein expression and decrease proapoptotic protein expression. Caspase-3 cleavage increases with increased apoptosis (as seen by Hoechst-positive RPE cells) under oxidative stress. Caspase-3 decreases with the addition of 10 to 50 nM DHA, and this effect is potentiated by the addition of PEDF.
5. Experimental stroke
In experimental stroke, endogenous NPD1 synthesis was found to be upregulated during the initial eight hours of reperfusion [7]. When this lipid mediator was infused into the brain during ischemia-reperfusion, neuroprotective bioactivity was revealed [7]. Furthermore, when DHA was administered i.v. after middle-cerebral artery occlusion, protection was concomitant with NPD1 synthesis on the ipsilateral brain side [24].
6. Aging
The aging of human neural progenitor cells (HN cells, neurons and glia) in primary culture during eight weeks is accompanied by an eight-fold enhanced synthesis and release of Aβ40 and Aβ42 peptides that resembles processes that involve Aβ amyloid peptides in Alzheimer’s disease (AD). No changes in neuronal and glial morphology after 18 hours of exposure to Aβ42 took place. This suggests that although Aβ42 is activating cell-damaging signals, and yet it is unable to overwhelm endogenous survival signaling. Also, there was an early onset of apoptosis and changes in gene-expression patterns that resemble neurodegenerative events characteristic of AD [25]. Accumulation of secreted Aβ40 and Aβ42 peptides during HN cell aging has implications in the development of Aβ-related neuropathology. The proinflammatory cytokine IL-1β enhances Aβ40 and Aβ42 secretion as a function of HN cell aging. IL-1β stimulates gamma-secretase-mediated cleavage of βAPP into Aβ peptides. Conversely, DHA suppresses both Aβ40 and Aβ42 peptide release with concomitant NPD1 synthesis [25]. Moreover, NPD1 inhibits Aβ42-induced apoptosis in HN cells. Therefore, DHA neuroprotection in a model of human brain cell aging in vitro involves NPD1 synthesis. To further explore cell survival signaling in the same cell culture model, pro- and antiapoptotic Bcl-2 proteins were studied. These proteins are modulators of pre-mitochondrial check points of apoptosis. Proapoptotic Bik and Bax are enhanced by Aβ42, but not by DHA or NPD1, whereas Bcl-2, Bcl-xL, and Bfl-1(A1) increase in the presence of DHA. NPD1, on the other hand, promotes a much larger increase in antiapoptotic Bcl-2 proteins. Bfl-1(A1) increased almost 6-fold. These modulatory actions of NPD1 may play critical roles in the survival of aged and terminally differentiated cells and break the mechanistic link between inflammatory signaling and apoptosis. In fact, NPD1 also induces the antiapoptotic Bcl-2 family proteins Bcl-2 and Bcl-xL in oxidatively challenged human RPE cells and promotes cytoprotection [8,10]. A further suggestion of the significance of NPD1 in AD is the finding that hippocampal CA1 from AD patients shows a major reduction in NPD1 [25]. Thus, the interplay of DHA-derived neuroprotective signaling aims to counteract proinflammatory, cell-damaging events triggered by multiple, converging cytokine and amyloid peptide factors in AD. Amyloid peptides mediate oxidative stress and microglial-derived cytokines, such as IL-1β and TNFα, support progressive inflammatory episodes in AD. These noxious stimuli further orchestrate pathogenic gene-expression programs in stressed brain cells, thereby linking a cascade of cell death pathways with apoptosis and neuronal demise. Neural mechanisms leading toward NPD1 generation from DHA thereby appear to redirect cellular fate toward successful preservation of RPE-photoreceptor cell integrity [10,20,26] and brain cell aging [25]. The Bcl-2 pro- and antiapoptotic gene families, sAPP alpha (and/or other neurotrophins) and NPD1, lie along a cell fate-regulatory pathway whose component members are highly interactive, and have potential to function cooperatively in brain and retina cell survival. Activators of NPD1 biosynthesis, NPD1 analogs or dietary regimens may be useful for exploring new preventive/therapeutic strategies for neurodegenerative diseases.
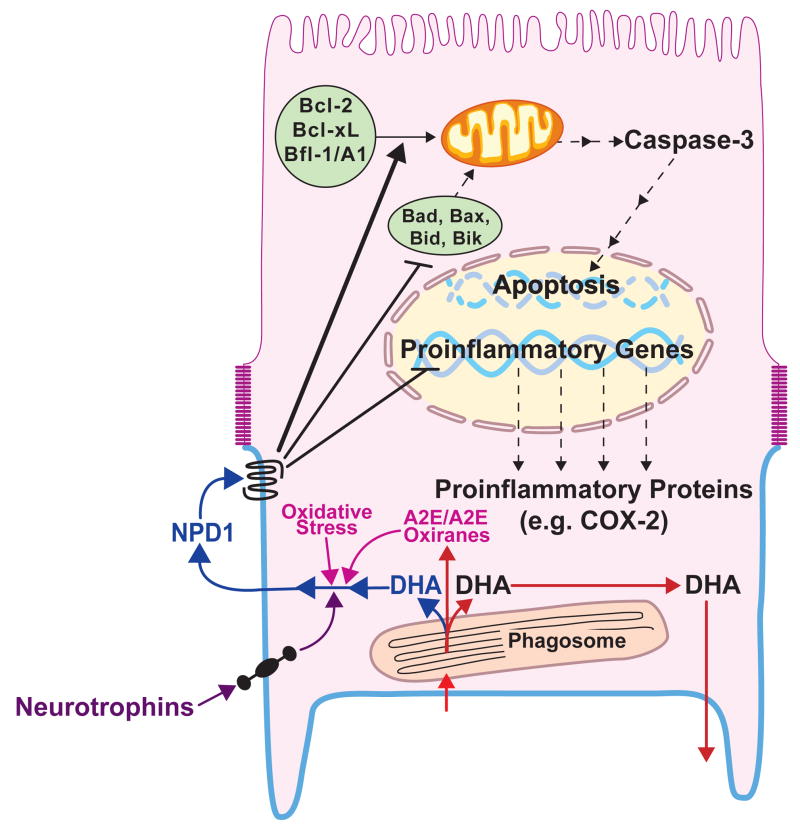
Figure 2. Neuroprotectin D1 bioactivity in the RPE cell.
Neurotrophins are depicted to induce NPD1 synthesis and its apical release [10]. Oxidative stress or A2E (N-retinyl-N-retinylidene ethanolamine)/A2E oxiranes (epoxides) are activators of neuroprotectin D1 (NPD1) synthesis as well (in purple). Docosahexaenoic acid (DHA) is shown to arrive to the RPE as part of the phagosome (DHA-phospholipids). After phagolysosomal digestion, most of the DHA is recycled back to the inner segments of photoreceptors through the interphotoreceptor matrix (red arrows). The precise phospholipid-DHA molecular species that is hydrolyzed to generate the free DHA pool precursor of NPD1 has not been identified (NPD1 synthesis pathway, blue color). NPD1 is released through the apical cellular side and recognizes a putative receptor. Intracellular signaling then inhibits pro-inflammatory gene expression. As a consequence, a decrease in proinflammatory proteins takes place that, when available, plays a role in choroidal neovascularization and cell injury. In addition, NPD1-triggered signaling upregulates antiapoptotic Bcl-2 family protein expression and downregulates pro-apoptotic Bcl-2 family protein expression. As a result, caspase 3 activity is decreased and apoptosis reduced.
Acknowledgments
Supported by National Institutes of Health, National Eye Institute grant EY005121, National Center for Research Resources grant P20 RR016816, and National Institute of Neurological Disorders and Stroke grant NS046741.
Footnotes
Conflicts of Interest: N.G. Bazan – Resolvyx Pharmaceuticals, Bedford, MA (consultant); Louisiana State University Health Sciences Center (patents assignee).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petursdottir AL, Farr SA, Morley JE, Banks WA, Skuladottir GV. Lipid peroxidation in brain during aging in the senescence-accelerated mouse (SAM) Neurobiol Aging. 2007;28:1170–1178. doi: 10.1016/j.neurobiolaging.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 2.Reich EE, Markesbery WR, Roberts LJ, 2nd, Swift LL, Morrow JD, Montine TJ. Brain regional quantification of F-ring and D-/E-ring isoprostanes and neuroprostanes in Alzheimer’s disease. Am J Pathol. 2001;158:293–297. doi: 10.1016/S0002-9440(10)63968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res. 2004;45:205–213. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Organisciak DT, Darrow RM, Jiang YL, Blanks JC. Retinal light damage in rats with altered levels of rod outer segment docosahexaenoate. Invest Ophthalmol Vis Sci. 1996;37:2243–2257. [PubMed] [Google Scholar]
- 5.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–271. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Bazan NG, Birkle DL, Reddy TS. Docosahexaenoic acid (22:6, n-3) is metabolized to lipoxygenase reaction products in the retina. Biochem Biophys Res Commun. 1984;125:741–747. doi: 10.1016/0006-291x(84)90601-6. [DOI] [PubMed] [Google Scholar]
- 7.Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. Erratum in: J Biol Chem 278 (2003) 51974. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci USA. 2007;104:13152–13157. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Anderson RE, Maude MB, McClellan M, Matthes MT, Yasumura D, LaVail MM. Low docosahexaenoic acid levels in rod outer segments of rats with P23H and S334ter rhodopsin mutations. Mol Vis. 2002;8:351–358. [PubMed] [Google Scholar]
- 13.Aveldano MI. Phospholipid species containing long and very long polyeonic fatty acids remain with rhodopsin after hexane extraction of photoreceptor membranes. Biochemistry. 1988;27:1229–1239. doi: 10.1021/bi00404a024. [DOI] [PubMed] [Google Scholar]
- 14.Kim HY. Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem. 2007;282:18661–18665. doi: 10.1074/jbc.R700015200. [DOI] [PubMed] [Google Scholar]
- 15.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazan NG, Scott BL, Reddy TS, Pelias MZ. Decreased content of docosahexaenoate and arachidonate in plasma phospholipids in Usher’s syndrome. Biochem Biophys Res Commun. 1986;141:600–604. doi: 10.1016/s0006-291x(86)80215-7. [DOI] [PubMed] [Google Scholar]
- 17.Li F, Cao W, Anderson RE. Protection of photoreceptor cells in adult rats from light-induced degeneration by adaptation to bright cyclic light. Exp Eye Res. 2001;73:569–577. doi: 10.1006/exer.2001.1068. [DOI] [PubMed] [Google Scholar]
- 18.Rotstein NP, Aveldano MI, Barrantes FJ, Politi LE. Docosahexaenoic acid is required for the survival of rat retinal photoreceptors in vitro. J Neurochem. 1996;66:1851–1859. doi: 10.1046/j.1471-4159.1996.66051851.x. [DOI] [PubMed] [Google Scholar]
- 19.Rotstein NP, Aveldano MI, Barrantes FJ, Roccamo AM, Politi LE. Apoptosis of retinal photoreceptors during development in vitro: protective effect of docosahexaenoic acid. J Neurochem. 1997;69:504–513. doi: 10.1046/j.1471-4159.1997.69020504.x. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee PK, Marcheselli VL, de Rivero Vaccari JC, Gordon WC, Jackson FE, Bazan NG. Photoreceptor outer segment phagocytosis selectively attenuates oxidative stress-induced apoptosis with concomitant neuroprotectin D1 synthesis. Proc Natl Acad Sci USA. 2007;104:13158–13163. doi: 10.1073/pnas.0705963104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ershov AV, Lukiw WJ, Bazan NG. Selective transcription factor induction in retinal pigment epithelial cells during photoreceptor phagocytosis. J Biol Chem. 1996;271:28458–28462. doi: 10.1074/jbc.271.45.28458. [DOI] [PubMed] [Google Scholar]
- 22.Ershov AV, Bazan NG. Induction of cyclooxygenase-2 gene expression in retinal pigment epithelium cells by photoreceptor rod outer segment phagocytosis and growth factors. J Neurosci Res. 1999;58:254–261. [PubMed] [Google Scholar]
- 23.Ershov AV, Bazan NG. Photoreceptor phagocytosis selectively activates PPARgamma expression in retinal pigment epithelial cells. J Neurosci Res. 2000;60:328–337. doi: 10.1002/(SICI)1097-4547(20000501)60:3<328::AID-JNR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Belayev L, Marcheselli VL, Khoutorova L, et al. Docosahexaenoic acid complexed to albumin elicits high-grade ischemic neuroprotection. Stroke. 2005;36:118–123. doi: 10.1161/01.STR.0000149620.74770.2e. [DOI] [PubMed] [Google Scholar]
- 25.Lukiw WJ, Cui JG, Marcheselli VL, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bazan NG. Homeostatic regulation of photoreceptor cell integrity: Significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid. The Proctor Lecture. Invest Ophthalmol Vis Sci. 2007;48:4866–4881. doi: 10.1167/iovs.07-0918. [DOI] [PubMed] [Google Scholar]