Abstract
Purpose
We compared the prevalence, level of bother and effect on daily activities of urinary incontinence among women with type 1 diabetes enrolled in the Epidemiology of Diabetes Interventions and Complications study to a population based sample of women with normal glucose.
Materials and Methods
We performed a cross-sectional analysis of women with type 1 diabetes and normal glucose tolerance using 2 study populations. The Diabetes Control and Complications Trial cohort followup, Epidemiology of Diabetes Interventions and Complications, began in 1994. In 2004 women participants (550) completed a self-administered questionnaire on urinary incontinence. Our primary outcome was weekly or greater incontinence, overall and by type. Prevalence of urinary incontinence was compared to a subgroup of women with normal glucose in the 2001 to 2002 National Health and Nutrition Examination Survey (NHANES).
Results
Overall 65% of women with type 1 diabetes reported any urinary incontinence (17% reported weekly incontinence). Nearly 40% of these women were greatly bothered by their incontinence and 9% believed it affected their day-today activities. Women with type 1 diabetes had a nearly 2-fold greater prevalence of weekly urge incontinence compared to those without diabetes in the NHANES cohort (8.8% vs 4.5%, p = 0.01).
Conclusions
Urinary incontinence is common in women with type 1 diabetes and the prevalence of weekly urge incontinence is far greater compared to that in women with normal glucose levels. Moreover, the prevalence of urinary incontinence in women with type 1 diabetes was greater than that of neuropathy, retinopathy and nephropathy. These findings highlight the importance of screening for urinary incontinence among women with type 1 diabetes. Studies examining factors associated with urinary incontinence in women with type 1 diabetes are warranted.
Keywords: women, urinary incontinence, diabetes mellitus, type 1, prevalence
Urinary incontinence, defined as “the complaint of any involuntary leakage of urine” is one of the most prevalent chronic conditions in the elderly. Although the estimated prevalence of urinary incontinence varies depending on the definition applied and the age range of the population under study, on average 30% to 40% of middle-aged women report having UI leading to significant distress and poorer quality of life.1,2 While several risk factors for UI have been examined, recent evidence suggests that UI is more common in women with type 2 diabetes and that diabetes is an independent risk factor for incontinence.3–5 However, there has been limited research among middle-aged women with type 1 diabetes to estimate the prevalence of incontinence and how it may differ in comparison to women with normal glucose.
While there are several studies of incontinence in women with diabetes, they are limited by their inclusion of primarily small samples from selected populations, inclusion of mostly women with type 2 diabetes, and observational study designs lacking adjustment for potential confounding variables such as age, body weight and history of childbirth.6–8 Distinguishing between type 1 and type 2 diabetes would allow for further understanding of the contribution of mechanisms dependent on hyperglycemia to urinary dysfunction.
To determine the prevalence of UI among 550 women with T1D we analyzed data from the Uro-EDIC study. We compared the prevalence and impact of incontinence among women in Uro-EDIC to a subsample of women without diabetes from the 2001 to 2002 NHANES who were selected to be of similar age and race.
MATERIALS AND METHODS
The Diabetes Control and Complications Trial
Details regarding the inclusion and exclusion criteria for the DCCT and the treatment protocol are described elsewhere.9 A total of 1,441 subjects with T1DM 13 to 39 years old were recruited into the DCCT between 1983 and 1989 in 2 cohorts. The primary prevention cohort had T1DM for 1 to 5 years, no retinopathy and urinary albumin excretion less than 40 mg/24 hours at baseline. The secondary intervention cohort had T1DM for 1 to 15 years, mild to moderate nonproliferative retinopathy and urinary albumin excretion of 200 mg/24 hours or less at baseline. A total of 711 participants were randomly assigned to receive intensive treatment which consisted of insulin administered 3 or more times per day by injection or by continuous subcutaneous infusion with an external pump. The conventional therapy group (730 participants) received 1 or 2 daily insulin injections with a goal of freedom from symptoms of hypoglycemia and frequent or severe hypoglycemia. The intensive and conventional treatment groups maintained a separation of median HbA1c level of 2% throughout followup. The DCCT was terminated in 1993 after 6.5 years of mean followup when it was proven that intensive treatment reduced the risks of retinopathy, nephropathy and neuropathy by 35% to 90% compared with conventional treatment. Intensive therapy was subsequently recommended for all subjects who returned to their health care providers for diabetes care.
The Epidemiology of Diabetes Interventions and Complications Study
The EDIC study began in January 1994 after the closeout of the DCCT and is a prospective multicenter 20-year observational study of the DCCT cohort. EDIC focuses on the interactions between established putative risk factors for long-term microvascular, neurological and cardiovascular outcomes of T1D including prior diabetes treatment and the level of glycemic control during the DCCT. In 1994, 1,375 (96%) of the surviving members of DCCT volunteered to participate in the EDIC observational followup study.10
Uro-EDIC Study
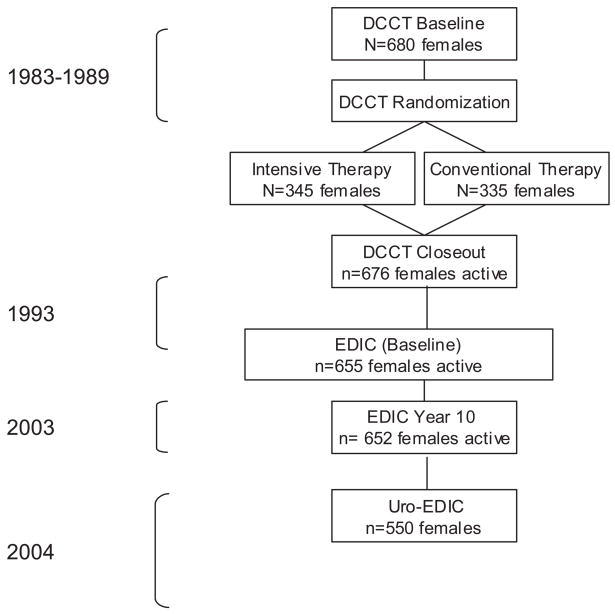
In 2001 the Urological Complications of Diabetes Group was formed by the Division of Urology at the NIDDK. In response to the group’s findings that complications in T1D were largely under studied the NIDDK supported efforts to examine urological complications in the EDIC cohort. During study visit 10 of EDIC (September 1, 2002 to April 30, 2004) 550 of the 652 (84%) women in the EDIC agreed to participate in the Uro-EDIC study and responded to questions about UI. The figure presents the flow of the women participants from entry into DCCT to EDIC year 10 and the completion of the Uro-EDIC questionnaire.
Peripheral neuropathy for this study was defined by responses to the Michigan Neuropathy Screening Instrument (more than 6 positive responses on the questionnaire or a score greater than 2 on the examination), thresholds defined by prior validation studies.11,12 Retinopathy was assessed using fundus photographs that were centrally graded using the Early Treatment Diabetic Retinopathy Study scale of 0 to 23 (less than 12—nonproliferative, 12 or more—proliferative).13 Nephropathy was defined as microalbuminuria (AER 40 to 300 mg/24 hours) or albuminuria (AER greater than 300 mg/24 hours).
National Health and Nutrition Examination Survey 2001 to 2002
To obtain a population based comparison group of women without diabetes, we selected a group of women with normal glucose included in the NHANES 2001 to 2002 sample (http://www.cdc.gov/nchs/about/major/nhanes/nhanes01-02.htm). NHANES 2001 to 2002 is a nationally representative probability sample of noninstitutionalized civilians, obtained using a stratified, multistage, cluster sampling design. Because women in Uro-EDIC are white and 20 to 59 years old, we restricted our NHANES sample to women who were white by self-report, 20 to 59 years old, not pregnant, without a diagnosis of diabetes and had a fasting glucose less than 100 mg/dl.
Urinary Incontinence Measurements
Urinary incontinence was assessed in Uro-EDIC by a self-administered questionnaire which included validated instruments used in previous studies.14,15 The sequence of incontinence questions began with “During the past 12 months how often have you leaked even a small amount of urine….” Frequency of incontinence was ascertained as every day, 1 or more times per week, 1 or more times per month, or less than once per month. The primary outcome of interest was weekly or more frequent incontinence which we consider clinically significant. Among women with weekly or greater incontinence the type of incontinence in the last week was classified by the addition of questions “… during activities like coughing, sneezing, lifting, or exercise?” (stress incontinence) and “… with an urge to urinate and could not get to the bathroom fast enough?” (urge incontinence).
Severity of incontinence was determined based on incontinence frequency and amount of urine lost per episode (drops, small splashes, more), using the validated Sandvik Severity score.16 The Sandvik Scale is calculated from frequency and amount of urine loss on a scale of 1 to 12 (mild—1 to 2, moderate—3 to 6, severe—8 to 9, very severe—12). The women also reported the level of bother of incontinence and whether it interfered with their activities. Specifically participants were asked during the last 12 months “how much did your leakage of urine bother you?” and “how much did your leakage of urine affect your day-to-day activities?” Response options included not at all, only a little, somewhat, very much or greatly.
In NHANES urinary incontinence was assessed by the questions “During the past 12 months, how frequently have you leaked or lost control of even a small amount of urine with an activity like coughing, lifting or exercising?” (stress incontinence), “with an urge or pressure to urinate and could not get to the toilet fast enough?” (urge incontinence) and “without an activity like coughing, lifting or exercising or an urge to urinate?” (other incontinence). Frequency of incontinence for all 3 types was ascertained as every day, a few times a week, a few times a month or a few times a year. To be directly comparable to Uro-EDIC overall weekly or greater incontinence in NHANES was defined as weekly or more frequent incontinence for any of the types assessed. Bother and impact due to UI were assessed in the same manner as in Uro-EDIC. Severity of incontinence was not assessed in NHANES.
Statistical Analysis
Characteristics of the Uro-EDIC and NHANES samples were summarized using means, standard deviations and proportions as appropriate. In the analysis of the combined Uro-EDIC and NHANES samples we used the survey functions in SAS® to accommodate features of the complex NHANES survey design including stratification, clustering within PSUs and probability weights. The NHANES weights were rescaled to sum to the number of NHANES women included in the analysis, while weights for the Uro-EDIC subjects were set to 1. This ensured that each of the subsamples influenced the results in proportion to sample size while retaining the differences among the NHANES weights. The Uro-EDIC sample was treated as a single additional stratum and each of the 29 clinic sites treated as a PSU. We estimated the adjusted prevalence of incontinence in the Uro-EDIC cohort that would be expected if covariate patterns were the same as in the NHANES sample using the weighted NHANES prevalence and the adjusted odds ratio for the contrast between Uro-EDIC and NHANES from a survey logistic model adjusting for age, BMI, parity, hysterectomy and current smoking. SAS version 9.13 was used to perform the analyses.
RESULTS
There were no significant differences in participant characteristics presented in table 1 between the 550 Uro-EDIC women who completed the self-reported questionnaire and the 102 women who did not complete the questionnaire (data not shown). Uro-EDIC women had a mean age of 44 years (SD ±7) and approximately two-thirds were overweight or obese (table 1). Nearly a third of the women were nulligravid, a quarter were postmenopausal and 12% reported a prior hysterectomy. Median duration of diabetes in this cohort was approximately 22 years with the prevalence of diabetic complications 31% retinopathy (more than moderate NPDR), 25% nephropathy (microalbuminuria or albuminuria) and 32% peripheral neuropathy.
Table 1.
Characteristics of women with type 1 diabetes in the Uro-EDIC cohort
| No. (%)* | |
|---|---|
| Primary prevention cohort of DCCT: | |
| Conventional | 132 (48) |
| Intensive | 146 (53) |
| Secondary intervention cohort of DCCT: | |
| Conventional | 138 (51) |
| Intensive | 134 (49) |
| Yrs diabetes: | |
| Median or greater (21.75) | 290 (53) |
| Less than median (21.75) | 260 (47) |
| Hba1c: | |
| First quartile (4.4–7.0) | 126 (23) |
| Second quartile (7.0–7.6) | 149 (28) |
| Third quartile (7.6–8.6) | 128 (24) |
| Fourth quartile (8.6–13.6) | 135 (25) |
| Attained age: | |
| 20–29 | 8 (2) |
| 30–39 | 169 (31) |
| 40–49 | 248 (45) |
| 50–59 | 125 (23) |
| Current smoking | 74 (14) |
| Body mass index (kg/m2): | |
| Normal (less than 25) | 183 (34) |
| Overweight (25–less than 30) | 222 (42) |
| Obese (30 or greater) | 130 (24) |
| Waist circumference (cm): | |
| Median (82.2) or greater | 260 (50) |
| Less than median (82.2) | 259 (50) |
| Postmenopausal† | 124 (23) |
| Prior hysterectomy‡ | 66 (12) |
| Parity (No. live births): | |
| 0 | 165 (30) |
| 1 | 112 (20) |
| 2 or More | 273 (50) |
| Peripheral neuropathy§ | 165 (32) |
| Retinopathy:|| | |
| None | 18 (3) |
| Microaneurysm only | 143 (26) |
| Very mild NPDR | 215 (39) |
| Moderate or greater NPDR | 174 (31) |
| Nephropathy EDIC yrs 9 + 10:¶ | |
| Normal (AER less than 40) | 415 (76) |
| Microalbuminuria (AER 40–less than 300) | 110 (20) |
| Albuminuria (AER 300 or greater) | 25 (5) |
All totals that do not add up to 550 imply that the information is missing for those subjects.
Defined as a subject’s menstrual periods ceasing and considered permanent.
Defined as a subject’s menstrual period ceasing and considered permanent due to surgery.
Defined in EDIC by Michigan Neuropathy Screening Instrument as more than 6 positive responses on the questionnaire or a score greater than 2 on the examination.
Determined by Early Treatment Diabetic Retinopathy Study score.
EDIC years 9 and 10 have been combined to represent the entire study sample.
The prevalence of UI reported by women in the Uro-EDIC cohort is presented in table 2. Nearly 40% of Uro-EDIC women reported monthly or more frequent incontinence with daily incontinence reported by 4% and weekly incontinence by 13%. Only approximately a third of the women reported being continent. Among those who reported weekly or greater incontinence 22% reported stress incontinence and 10% urge incontinence. More than a third of the women with incontinence had moderate or more severe incontinence. Approximately 38% of the women were very much or greatly bothered by their incontinence and 9% believed it very much or greatly affected their day-to-day activities.
Table 2.
Urinary incontinence in the last 12 months in women with type 1 diabetes in the Uro-EDIC cohort
| No. (%) | |
|---|---|
| Frequency of incontinence: | |
| None | 191 (35) |
| Less than monthly | 152 (28) |
| Monthly | 113 (21) |
| Weekly | 69 (13) |
| Daily | 24 (4) |
| Severity of incontinence:* | |
| Mild | 229 (65) |
| Moderate | 101 (29) |
| Severe/very severe | 21 (6) |
| Type of incontinence:† | |
| Stress‡ | 116 (22) |
| Urge‡ | 49 (10) |
| Level of bother:† | |
| None | 3 (3) |
| Only a little | 23 (24) |
| Somewhat | 32 (34) |
| Very much | 25 (27) |
| Greatly | 10 (11) |
| Interferes with activities:† | |
| None | 42 (45) |
| Only a little | 27 (29) |
| Somewhat | 16 (17) |
| Very much | 6 (7) |
| Greatly | 2 (2) |
Sandvik Severity Scale calculated from frequency and amount of urine loss on a scale of 1 to 12 (mild— 1 to 2, moderate— 3 to 6, severe/very severe— 8 to 12).
Among women with weekly or daily incontinence.
Stress UI defined as weekly or greater UI during activities like coughing, sneezing, lifting or exercise. Urge UI defined as weekly or greater urge to urinate with inability to reach toilet.
A total of 383 women with complete incontinence information were included in our NHANES comparison sample (table 3). Compared to women in the NHANES cohort women in Uro-EDIC were slightly older (mean ± SD age 44 ± 7 years vs 39 ± 16, p <0.001), less likely to be postmenopausal, more likely to be nulligravid (30% vs 24%, p = 0.05) and tended to be overweight or obese (66% vs 50%, p <0.001).
Table 3.
Relevant characteristics of women with type 1 diabetes in the Uro-EDIC cohort and those with normal glucose in the NHANES 2001–2002 comparison sample
| No. Uro-EDIC (%) | No. NHANES 2001–2002 (%) | p Value | |
|---|---|---|---|
| Age: | |||
| 20–29 | 8 (2) | 88 (23) | <0.001 |
| 30–39 | 169 (31) | 105 (27) | <0.001* |
| 40–49 | 248 (45) | 101 (27) | |
| 50–59 | 125 (23) | 89 (23) | |
| Postmenopausal | 124 (23) | 115 (31) | 0.006 |
| Parity (No. live births): | |||
| 0 | 165 (30) | 93 (24) | 0.05 |
| 1 | 112 (20) | 68 (18) | 0.002* |
| More than 2 | 273 (50) | 222 (58) | |
| Prior hysterectomy | 66 (12) | 58 (15) | 0.2 |
| Body mass index: | |||
| Less than 25 | 183 (34) | 188 (50) | <0.001 |
| 25–29.9 | 222 (42) | 92 (24) | 0.31* |
| Greater than 30 | 130 (24) | 99 (26) | |
| Waist circumference (cm) | 83 (1) | 89 (1) | <0.001 |
| Hypertension | 172 (32) | 35 (9) | <0.001 |
| Current smoker | 74 (14) | 110 (29) | <0.001 |
Wald chi-square test on 1 df used to test for trend using the continuous form of the variable.
Table 4 demonstrates the prevalence and consequences of weekly or greater UI, overall and by type, among Uro-EDIC women and the NHANES comparison sample after adjustment for age, BMI, parity, hysterectomy and current smoking. Women with T1D had a 2-fold greater prevalence of weekly or greater urge incontinence (adjusted prevalence 8.8% vs 4.5%; adjusted OR 2.05; 95% CI 1.15, 3.65; p = 0.01). Women with T1D also had an increased prevalence of stress incontinence (adjusted prevalence 18.5% vs 13.4%; adjusted OR 1.47; 95% CI 0.97, 2.25; p = 0.07). Levels of overall weekly or greater incontinence, bother and impact of weekly or greater incontinence were similar among women with T1D and those with normal glucose.
Table 4.
Adjusted prevalence and consequences of weekly UI in women with type 1 diabetes in the Uro-EDIC cohort and women with normal glucose in the NHANES 2001–2002 comparison sample
| UI Outcome | Adjusted Prevalence (%) | Adjusted Odds Ratio | 95% CI | p Value |
|---|---|---|---|---|
| Weekly UI:* | ||||
| Uro-EDIC | 18.8 | 1.30 | 0.90–1.88 | 0.16 |
| NHANES | 15.1 | Ref | — | — |
| Weekly stress UI:† | ||||
| Uro-EDIC | 18.5 | 1.47 | 0.97–2.25 | 0.07 |
| NHANES | 13.4 | Ref | — | — |
| Weekly urge UI:‡ | ||||
| Uro-EDIC | 8.8 | 2.05 | 1.15–3.65 | 0.01 |
| NHANES | 4.5 | Ref | — | — |
| Bother (very much or greater): | ||||
| Uro-EDIC | 35.7 | 1.69 | 0.82–3.47 | 0.15 |
| NHANES | 24.7 | Ref | — | — |
| Impact (very much or greater): | ||||
| Uro-EDIC | 5.4 | 1.05 | 0.27–4.10 | 0.95 |
| NHANES | 5.2 | Ref | — | — |
Weekly UI defined as weekly or greater leakage of urine during the last 12 months.
Stress UI defined as weekly or greater UI during activities like coughing, sneezing, lifting or exercise.
Urge UI defined as weekly or greater urge to urinate with inability to reach toilet.
DISCUSSION
Among women with T1D in the Uro-EDIC study we found UI to be highly prevalent with nearly 40% reporting monthly or more frequent incontinence. Furthermore, nearly 40% of these women were greatly bothered by their incontinence and 9% believed it affected their day-to-day activities. Incontinence was more prevalent than other commonly recognized diabetes associated complications such as retinopathy, nephropathy and neuropathy. Importantly women with T1D had a nearly 2-fold greater prevalence of weekly urge incontinence compared to women without diabetes.
Our finding of a 2-fold increased risk of urge incontinence among women with T1D is similar to several studies that have identified urge incontinence was increased among women with type 2 diabetes.8 Because women in these prior studies had mostly type 2 diabetes, it is likely that Uro-EDIC women using insulin had more severe diabetes with more advanced neurovascular complications affecting the innervation of the bladder which might present in the early stages as urge incontinence.10
Among women without diabetes parity is a well-known and major risk factor for development of UI in middle-aged women, and that risk increases with the number of births.17 Interestingly women in Uro-EDIC were more commonly nulligravid and had fewer births than those with normal glucose in the NHANES, yet the Uro-EDIC women had a 2-fold greater odds of urge incontinence and 50% greater odds of stress incontinence than those with normal glucose after adjustment for parity. This finding suggests that other factors, potentially those associated with diabetes, may explain the increased prevalence of incontinence in this cohort.
The biology of diabetes associated bladder complications can be due to an alteration in the detrusor smooth muscle, neuronal dysfunction and urothelial dysfunction. Specifically it is hypothesized that microvascular complications might damage the innervation of the bladder or alter bladder muscle function.18 Early evidence demonstrated that morphological changes in efferent and afferent pathways to the bladder were closely related to bladder dysfunction, and these periperhal axonopathies were specifically induced in diabetic animal studies.19 In later studies of patients with diabetes others have found significant associations between sympathetic skin response and bladder dysfunction suggesting that diabetic cystopathy may in fact be a manifestation of peripheral neuropathy induced by diabetes.20
Although our study provides information on the prevalence, level of bother and effect on daily activities of UI among women with T1D, there are several limitations that should be considered. The Uro-EDIC women participated in the DCCT and subsequently the EDIC study and, thus, may not be representative of the general population of women with T1D. It is likely that among participants in the DCCT/EDIC diabetic management was good, perhaps better than in a population based sample of women with T1D. If diabetes associated factors such as poorly controlled glycemic levels affect development of incontinence among women with T1D as with other complications (nephropathy, neuropathy and retinopathy) then our reported incontinence prevalence may be underestimated. In addition, it is possible Uro-EDIC participants may have been followed more closely and more likely treated for incident incontinence which would result in our reported estimate of prevalence in this cohort to be further underestimated. While measures of overall incontinence and its bother and impact were measured identically in both cohorts, the frequency of stress and urge incontinence was assessed during the last year in NHANES and during the last 7 days in Uro-EDIC, which may introduce the potential for measurement error in the comparison of differences between cohorts. Finally, because the participants in the current study are mostly white, generalizing the findings to persons of other races may not be appropriate.
CONCLUSIONS
We found that young and middle-aged women with T1D have a markedly higher risk of weekly urge incontinence compared with women with normal glucose levels. Moreover, the prevalence of UI among women with T1D was higher than retinopathy, nephropathy and neuropathy. Physicians treating women with T1D should be alert for incontinence, which is often unrecognized and, therefore, under treated among women with diabetes.
Flow of female participants through DCCT and EDIC to Uro-EDIC
Acknowledgments
Supported by contracts with the Division of Diabetes, Endocrinology and Metabolic Disease of the National Institute of Diabetes and Digestive and Kidney Diseases, and the General Clinical Research Center Program, National Center for Research Resources.
Abbreviations and Acronyms
- AER
albumin excretion rate
- DCCT
Diabetes Control and Complications Trial
- EDIC
Epidemiology of Diabetes Interventions and Complications
- ETDRS
Early Treatment Diabetic Retinopathy Study
- NHANES
National Health and Nutrition Examination Survey
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- NPDR
nonproliferative diabetic retinopathy
- PSU
primary sampling unit
- T1D
type 1 diabetes
- T1DM
type 1 diabetes mellitus
- UI
urinary incontinence
- Uro-EDIC
Urologic Complications of Epidemiology of Diabetes Interventions and Complications
References
- 1.Hunskaar S, Arnold EP, Burgio K, Diokno AC, Herzog AR, Mallett VT. Epidemiology and natural history of urinary incontinence. Int Uro-gynecol J Pelvic Floor Dysfunct. 2000;11:301. doi: 10.1007/s001920070021. [DOI] [PubMed] [Google Scholar]
- 2.Samuelsson E, Victor A, Tibblin G. A population study of urinary incontinence and nocturia among women aged 20–59 years. Prevalence, well-being and wish for treatment Acta Obstet Gynecol Scand. 1997;76:74. doi: 10.3109/00016349709047789. [DOI] [PubMed] [Google Scholar]
- 3.Lifford KL, Curhan GC, Hu FB, Barbieri RL, Grodstein F. Type 2 diabetes mellitus and risk of developing urinary incontinence. J Am Geriatr Soc. 2005;53:1851. doi: 10.1111/j.1532-5415.2005.53565.x. [DOI] [PubMed] [Google Scholar]
- 4.Jackson SL, Scholes D, Boyko EJ, Abraham L, Fihn SD. Urinary incontinence and diabetes in post-menopausal women. Diabetes Care. 2005;28:1730. doi: 10.2337/diacare.28.7.1730. [DOI] [PubMed] [Google Scholar]
- 5.Brown JS, Vittinghoff E, Lin F, Nyberg LM, Kusek JW, Kanaya AM. Prevalence and risk factors for urinary incontinence in women with type 2 diabetes and impaired fasting glucose: findings from the National Health and Nutrition Examination Survey (NHANES) 2001–2002. Diabetes Care. 2006;29:1307. doi: 10.2337/dc05-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishigooka M, Suzuki Y, Hayami S, Ichiyanagi O, Hashimoto T, Nakada T. Role of symptom scoring and uroflowmetry in patients with diabetic cystopathy. Int Urol Nephrol. 1996;28:761. doi: 10.1007/BF02550724. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan SA, Te AE, Blaivas JG. Urodynamic findings in patients with diabetic cystopathy. J Urol. 1995;153:342. doi: 10.1097/00005392-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Goldman HB, Appell RA. Voiding dysfunction in women with diabetes mellitus. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:130. doi: 10.1007/s001920050032. [DOI] [PubMed] [Google Scholar]
- 9.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 10.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 12.The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT) Diabetologia. 1998;41:416. doi: 10.1007/s001250050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991;98:741. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 14.Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag J Clin Epidemiol. 2000;53:1150. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 15.Sandvik H, Hunskaar S, Vanvik A, Bratt H, Seim A, Hermstad R. Diagnostic classification of female urinary incontinence: an epidemiological survey corrected for validity. J Clin Epidemiol. 1995;48:339. doi: 10.1016/0895-4356(94)00147-i. [DOI] [PubMed] [Google Scholar]
- 16.Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47:497. doi: 10.1136/jech.47.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danforth KN, Townsend MK, Lifford K, Curhan GC, Resnick NM, Grodstein F. Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol. 2006;194:339. doi: 10.1016/j.ajog.2005.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshimura N, Chancellor MB, Andersson KE, Christ GJ. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 2005;95:733. doi: 10.1111/j.1464-410X.2005.05392.x. [DOI] [PubMed] [Google Scholar]
- 19.Paro M, Prosdocimi M, Zhang WX, Sutherland G, Sima AA. Autonomic neuropathy in BB rats and alterations in bladder function. Diabetes. 1989;38:1023. doi: 10.2337/diab.38.8.1023. [DOI] [PubMed] [Google Scholar]
- 20.Ueda T, Yoshimura N, Yoshida O. Diabetic cystopathy: relationship to autonomic neuropathy detected by sympathetic skin response. J Urol. 1997;157:580. doi: 10.1016/s0022-5347(01)65209-1. [DOI] [PubMed] [Google Scholar]