Abstract
Krüppel-related zinc finger proteins (KRAB-zfps) comprise the largest mammalian transcription factor family, but their specific functions are largely unknown. Two KRAB-zfps, regulator of sex-limitation (Rsl) 1 and Rsl2, repress expression of the mouse sex-limited protein (Slp) gene, the hallmark of Rsl activity, as well as some other male-predominant liver genes. This phenotype suggests Rsl modifies sex-specific transcription. The scope of Rsl control was determined by expression profiling of liver RNA from wild-type (wt), rsl, and transgenic mice with hepatic overexpression of Rsl1 or Rsl2. About 7.5% of the liver transcriptome was Rsl-responsive. More genes in males than females were affected by the loss of Rsl (e.g., in rsl mice), whereas Rsl overexpression altered more transcripts in females than males. Rsl dramatically repressed some female-predominant genes, but most were modestly (1.25- to 2-fold) influenced. In males, most Rsl-responsive genes unexpectedly expressed at lower levels in rsl than wt, suggesting not all are direct targets of Rsl repression. Gene Ontology analysis showed Rsl targets enriched in pathways of cholesterol, steroid, and lipid metabolism, linking Rsl to energy balance. In accord with this, blood glucose levels were less in male rsl than wt mice, and less responsive to fasting and refeeding. rsl mice were also leaner than wt, consistent with their hepatic regulation of phosphoenolpyruvate carboxykinase 1 and stearoyl-Coenzyme A desaturase 1. Altogether, Rsl's effect on sexually dimorphic and metabolically sensitive liver gene expression suggests a role for KRAB-zfps as broad genetic modulators of individual adaptation.
Keywords: Rsl, Slp, KRAB-zfp, sexual dimorphism, metabolic homeostasis
krüppel-related AB-box (KRAB) zinc finger proteins (zfp) are the largest family of transcriptional regulators in humans and mice with >400 members in each species (11). Nevertheless, the physiological impact of individual genes is largely unknown. The KRAB-zfp structure is highly conserved and biochemically well-defined with potent KRAB transcriptional repression domains tethered to DNA by zinc fingers (18, 36). We cloned two paralogous KRAB-zfp genes based on their function as modifier loci of mouse sex-limited protein (Slp) (16). Slp is a diverged complement protein expressed in liver as well as other sites under complex hormonal regulation (27). In mice mutant at the regulator of sex-limitation (rsl) locus, the normally male-specific Slp is also expressed at high levels in females and about twofold higher in rsl males compared with wild type (wt). Other male-predominant liver genes, such as certain cytochrome P450 (Cyp) family members and major urinary proteins (MUPs), are also higher in rsl than wt mice although to a lesser extent than Slp (16, 34), suggesting Rsl is involved in sexually dimorphic liver gene expression. Congenic mouse strains, with the rsl locus in a wt (Rsl, B10.D2) genetic background, resolved a region of 25 clustered KRAB-zfps, among which only Rsl1 and Rsl2 had polymorphisms that segregated with the phenotype of aberrant female Slp expression. Bacterial artificial chromosome (BAC) transgenes encompassing Rsl1 or Rsl2 from wt strains rescued this phenotype by repressing Slp or MUP genes in females (16), thus confirming the function of these KRAB-zfps.
Sexually dimorphic gene expression in the liver is largely controlled by signal transducer and transactivator 5b (STAT5b), a transcriptional regulator that responds to sex-specific pulses of growth hormone (GH) (7, 35). In males, testosterone acts on the hypothalamic-pituitary axis to drive pronounced pulsatile GH secretion. Activated GH receptors on liver cell membranes phosphorylate STAT5b, which then enters the nucleus where it activates male-predominant and represses female-predominant genes (4). Other transcription factors modify STAT5b regulation. For example, STAT5a/b heterodimers are required for female-predominant expression of some Cyp2b isoforms (24) and HNF-4α acts in concert with STAT5b to activate several male-predominant Cyp genes (38). At some level, Rsl interacts with STAT5b to accentuate male-specific expression of Slp. Peroxisome proliferator-activated receptors (PPARs) also intersect STAT5b signaling by inhibiting its activation of targets involved in fatty acid metabolism, adipocyte differentiation, and cholesterol homeostasis (30). All of these factors also affect liver physiology in a sex-dependent manner (1, 24, 35, 38). As part of a large network of transcriptional regulators these factors maintain energy homeostasis, but how they contribute to sex differences in metabolic phenotypes (19, 20) and whether this impacts sex differences in metabolic dysfunction is not known.
To define the broader role of Rsl1/2 in the liver, we identified Rsl-dependent transcripts by gene expression profiling of RNA from the livers of wt and rsl mice, and transgenic (tg) mice with liver-targeted overexpression of Rsl1 or Rsl2. This analysis revealed that global liver gene expression was less sexually dimorphic in rsl mice than wt and a remarkable number of genes were affected in each sex. Unlike Slp and a small subset of other sexually dimorphic genes, most of the Rsl-responsive transcripts were more modestly affected by either the absence or overexpression of Rsl. Comparison of Rsl-responsive transcripts to those responsive to STAT5 revealed unexpectedly little overlap in these data sets. In contrast, genes associated with energy balance were enriched among Rsl-responsive transcripts, and subtle sex-specific differences in metabolic phenotypes were observed between rsl and wt mice. These findings suggest that Rsl1/2 act as subtle modulators of a broad set of genes involved in metabolic and reproductive physiology.
MATERIALS AND METHODS
Mice.
B10.D2 (D2) mice were purchased from Jackson Laboratories (stock #000463). B10.D2.PL (PL) mice were initially provided by Dr. Ray Miller (13) and are maintained in our mouse facility. PL mice possess ∼2.2 cM of chromosome 13 from the PL/J strain (stock #000680, Jackson Labs) on the D2 genetic background (13). Transgenic mice were created by the University of Michigan Transgenic Animal Model Core (TAMC) facility. Fertilized oocytes from D2 mice were injected with pTTR1ExV5/Rsl1 or pTTR1ExV5/Rsl2, plasmids encoding Rsl1 or Rsl2 cDNAs, respectively, under the control of the liver-specific transthyretin promoter (15). Transgenic founders were identified at 2 wk of age by tail biopsy and genotyping PCR (see below) according to protocols from the TAMC (http://www.med.umich.edu/tamc/). Tg founders were bred through two generations to the PL strain to place the transgenes onto a homozygous rsl background.
For metabolic studies, mice aged 10–12 wk were isolated in groups of three. Body weights and blood glucose levels from tail vein (Freestyle Blood Glucose Monitoring Strips; Abbott Labs, Alameda, CA), were measured before and after fasting and following refeeding. Food was removed at 7:30 PM (late in the animals' “lights-on” cycle). At 7:30 AM, groups were either euthanized by cervical dislocation or provided food ad libitum for 4 h and then euthanized. Water was provided continuously throughout. Body composition was measured by the University of Michigan Animal Metabolic Phenotyping Core using an NMR analyzer (Minispec LF90II; Bruker Optics, Billerica, MA). All animal experimentation protocols were approved by the University of Michigan Committee on Use and Care of Animals.
Genotyping.
Genotyping was performed by PCR on mouse genomic DNA as follows: 35 cycles of 94°C for 15 s, 62°C for 30 s, 72°C for 90 s. Marker D13Dmr14 (forward primer: 5′-GGAAACTGGAATGGGGCTAT-3′, reverse primer: 5′-GGGGGTGCACCTAGAAGAA-3′) was used to identify alleles. The D2 allele yields a 390 bp product; the PL allele yields a 327 bp product. Transgenes were detected with vector-specific forward primer TTR1Intron1Seq2-F: 5′-CAAGCCGGTTTACTCTGACC-3′ and Rsl1-specific reverse primer Rslcan9spp-R: 5′-TCTGTTGACACATTGTTCATGCT-3′, or Rsl2-specific reverse primer Rslcan4spp-R: 5′-GAAATCTTTTGACACATTCTTCATACA-3′. The primer TTR1Intron2-R: 5′-GATGCCTGCATGTGAATTTG-3′ was included to amplify the endogenous transthyretin gene as an internal positive control.
RNA analysis.
Total liver RNA was isolated by the guanidinium isothiocyanate method (6). Transgene expression was detected in 2 μg total liver RNA by RT-PCR with Superscript II Reverse Transcriptase according to manufacturer's instructions (Invitrogen, Carlsbad, CA). Semiquantitative PCR was performed with reverse primer Rslcan9spp-R or Rslcan4spp-R and radiolabeled forward primers (Rsl1-specific Can9E1-F: 5′-GGTCTGTACTCGTGCGTCTTT-3′ or Rsl2-specific Can4E1-F: 5′-TGCCTCTTCCTCAAAGTTGG-3′), end-labeled with [γ-32P]ATP (MP Biomedicals, Solon, OH) and T4 polynucleotide kinase [New England Biolabs (NEB), Ipswich, MA] prior to PCR, according to instructions from NEB. PCR conditions were as described above for genotyping with cycles reduced to 25 to allow semiquantitative comparisons. β-Actin, GenBank accession #NM_007393, was used as an internal control (PCR for 20 cycles with MusβAct-F: 5′-CGGGATCCCAGCTTCTTTGCAGCTCCTT-3′ and MusβAct-R: 5′-GGAATTCAGTCCGCCTAGAAGCACTTG-3′). Products were separated on 5% polyacrylamide/7 M urea sequencing gels followed by autoradiography and PhosphorImager analysis.
Real-time quantitative RT-PCR (Q-RT-PCR) was performed by first synthesizing cDNA using 2.5 μg total liver RNA with the High Capacity cDNA Archive Kit (Applied Biosystems, Warrington, UK) according to the manufacturer's instructions. PCR was performed in the Applied Biosystems 7500 thermocycler with SYBR Green (Applied Biosystems) reagent according to the manufacturer's protocol. Primers specific to each gene were designed using the Primer3 program (http://frodo.wi.mit.edu) and the National Center for Biotechnology Information (NCBI) mouse genome sequence (Build 36.1). Primer sequences are available upon request. Reactions were performed in duplicate, and cycle threshold (Ct) values were converted to relative expression levels using the 2−ΔΔCt method (26) with 18S rRNA as the normalization standard and an arbitrarily chosen D2 male as the “calibrator.” Relative expression values were validated individually in all mice used in the microarray as well as in three or more additional mice per sex and genotype.
Microarray gene expression profiling.
RNA quality assessment and all other procedures specific to the handling of microarray chips and reagents were performed by the University of Michigan Comprehensive Cancer Center Microarray Core facility (http://www.umich.edu/∼caparray). Complementary DNA probes were synthesized from pooled RNA, which were grouped as shown in Supplemental Table S1,1 and hybridized in duplicate to Affymetrix Mouse Genome 430 2.0 Arrays. The data were first analyzed for quality and converted to relative expression values using the robust multiarray average (RMA) method of Irizarry et al. (12). Results were deposited into the NCBI Gene Expression Omnibus (GEO) database (GEO accession #GSE12712). Out of 45,101 probe sets, 20,393 met a relative minimum expression threshold value of 26 in at least two of the samples. The next analysis involved four pairwise comparisons (1, wt male vs. wt female; 2, rsl male vs. rsl female; 3, wt male vs. rsl male; 4: wt female vs. rsl female). To examine these comparisons side-by-side, mean expression ratios were visually represented as heat maps.
The vertical position of each probe set within the heat map was based on the relative z-score across all four comparisons. The z-score is an estimate of how well the data fit a normal distribution, with better fit leading to smaller z-scores and segregation toward the ends of the heat map. This effectively sorted the data according to the magnitude of the differential expression between wt males and wt females. For instance, probe sets at the top and bottom of the heat map represent transcripts for which the magnitude of sex-biased expression in wt mice is the greatest and the degree of statistical confidence in the relative position within the map is the highest (e.g., Ddx3y, Xist). This analysis produced a profile of sex-biased gene expression in wt mice that is supported statistically by incorporating variability within both wt and rsl datasets.
Next, the data, including that from the tg mice, were further selected for duplicate consistency by fitting a linear model and selecting differentially expressed probe sets using a nested F-test (P < 0.05) (31), resulting in 1,528 probe sets in which one or more groups had a significantly different expression value. Of these 1,528 probe sets, 907 differed between wt and rsl males, regardless of the female pattern and 811 differed between wt and rsl females, regardless of the male pattern. The entire 1,528 set was analyzed for Gene Ontology (GO) term enrichment using the GOstat program (http://gostat.wehi.edu.au). A significance cutoff was set at P <0.05 with the multiple testing error controlled by the method of Benjamini and Hochberg (2).
Protein analysis.
We prepared liver protein extracts by homogenizing ∼0.2 g liver in 3 ml ice-cold homogenization buffer [10 mM HEPES (pH 7.9), 300 mM NaCl, 25 mM KCl, 1.5 mM MgCl2, 1.0 mM EDTA, 0.5 mM DTT, 0.5 mM spermidine, 0.15 mM spermine, 10% glycerol, 200 mM PMSF, and 2.0 mg/ml leupeptin] in a 15 ml Dounce homogenizer. Cellular debris was cleared by microfuge (13,000 rpm) at 4°C for 10 min. Protein concentration of the supernatant was determined by Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA) and a BSA standard curve. For Western blotting, 50 μg liver proteins were separated on 12% SDS-PAGE gels and transferred to PVDF membranes. Blots were probed with Scd1 (S-15) (Santa Cruz Biotechnology, Santa Cruz, CA) and GAPDH (6C5) antibodies (Millipore, Billerica, MA). Proteins were detected by horseradish peroxidase-conjugated secondary antibody (Santa Cruz, sc-2020) and enhanced chemiluminescent (Pierce Biotechnology, Rockford, IL) images on autoradiography film (Denville Scientific, Metuchen, NJ). Band intensities were quantified with the ImageJ program.
Statistics.
All bar graphs reflect the mean values (± SE). Student's t-tests were used to determine significant differences presented in scatter plots and bar graphs. Data from multiple genotypes (i.e., Q-RT-PCR, weight, percent body fat, blood glucose levels) were analyzed by single-factor ANOVA followed by post hoc pairwise comparisons with Bonferroni correction.
RESULTS
Slp expression is extinguished by Rsl overexpression.
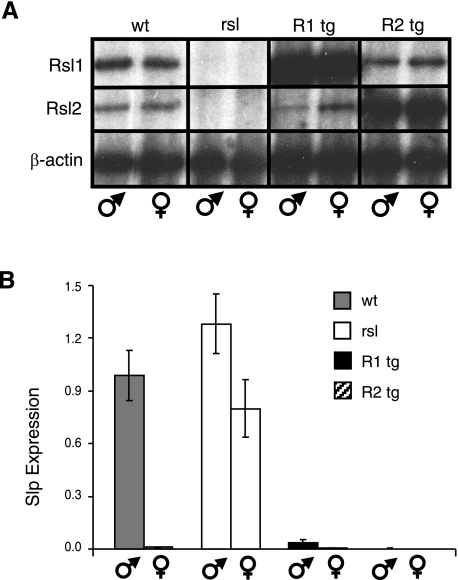
Previously, we showed that Rsl1/2 regulate a small set of male-predominant genes in the liver by comparing B10.D2 (D2, wt) mice to the congenic B10.D2.PL (PL, rsl) strain that has inactivating mutations in both Rsl1 and Rsl2 (16). To magnify the effects of Rsl1/2, tg mice were created in the D2 background that overexpress either Rsl1 or Rsl2, using the transthyretin liver-specific promoter to drive cDNAs (15). These mice were maintained on a pure genetic background by breeding for two generations to the PL strain and animals that were rsl-null at the endogenous Rsl locus were chosen for further studies. Semiquantitative (Fig. 1A) and real-time Q-RT-PCR results (data not shown) confirm as expected that male and female expression of Rsl1/2 were similar and that these transgenes were expressed in the liver at levels ∼100 times that of their endogenous counterparts.
Fig. 1.
Supraphysiological levels of liver-specific regulator of sex limitation (Rsl) extinguish sex-limited protein (Slp) expression in both sexes. A: semiquantitative RT-PCR indicates tg expression is ∼100-fold higher than the endogenous genes. Unlike the homozygous wild-type (wt) and rsl mice, the transgenic (tg) mice analyzed here (R1 and R2) are heterozygous at the Rsl locus. Gross overexpression from the transgenes in these mice had little influence on the endogenous levels of Rsl1 or Rsl2. B: Slp expression is dramatically repressed in mice with excess Rsl. Slp mRNA was assayed by real-time quantitative (Q)-RT-PCR and values were normalized to wt males. Slp was virtually undetectable in RNA from Rsl females, Rsl1 tg females, and both sexes of Rsl2 tg mice. Bars represent means ± SE. Rsl males, n = 5; Rsl females, n = 6; rsl males, n = 5; rsl females, n = 7; each sex of tg mice, n = 6.
To begin assessing liver gene regulation, we measured Slp mRNA in the liver of male and female mice by real-time Q-RT-PCR. Slp expression is normally restricted to male liver, and the presence of Slp in females is indicative of the rsl phenotype. Previous results showed that low levels of Rsl1, but not Rsl2, conferred by BAC transgenes were able to repress Slp in females (16). The current results showed that overexpression of Rsl2 could also extinguish Slp. Moreover, whereas modest Rsl1 expression from the BAC transgenes had little effect on Slp in male mice (16), excess Rsl1 or 2 resulted in dramatic Slp repression in males as well as females (Fig. 1B). Thus, Slp is a highly sensitive indicator of Rsl action. Despite the profound effect on Slp, the tg mice appeared normal with no obvious deficits in health or reproduction.
Impact of Rsl deficiency or excess.
To expand the view of Rsl1 and 2 in gene regulation, liver gene expression profiling was performed with total RNA isolated from livers of adult (>10 wk) wt, rsl, and Rsl1 and Rsl2 tg mice. For wt or rsl mice, 10 individuals of each sex were arbitrarily assigned to duplicate groups, A and B, with five mice per group. The tg mice were similarly segregated by sex; however, each group, A or B, was assembled from a separate tg line (Supplemental Table S1). Total liver RNA was analyzed individually for integrity, and equivalent amounts per mouse were pooled. Complementary DNA probes were synthesized and hybridized in duplicate (groups A and B) to the whole mouse genome microarray from Affymetrix. The array does not contain a probe set for Slp, precluding its use as a positive control, but the raw data were highly congruent between duplicate pools and of high quality overall (12) (Supplementary Fig. S1). Out of 45,101 probe sets, 20,393 provided gene expression data.
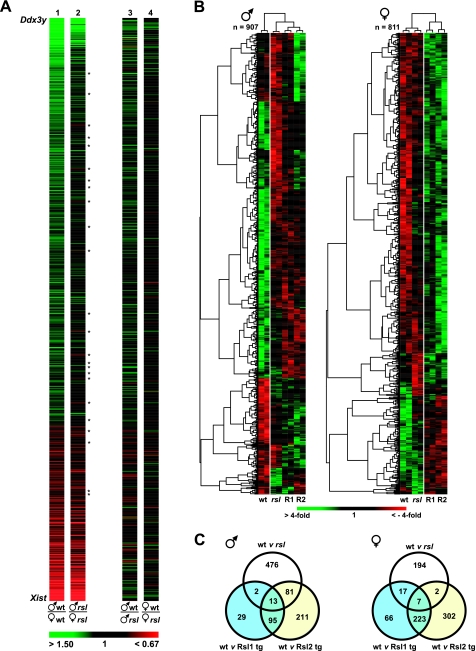
The 20,393 probe sets that identified transcripts found in mouse liver were first organized according to sex-predominant expression (those highest in males to those highest in females) in wt mice and are displayed in the false color heat map shown in Fig. 2A. Transcripts with the greatest differential in sex-specific expression and most statistical significance segregate to the extremes. Y-chromosome linked Ddx3y is at the top, and X-chromosome linked Xist is at the bottom. Bright green indicates transcripts in which the male-to-female ratio is ≥1.5-fold, whereas bright red indicates transcripts in which the female-to-male ratio is ≥1.5-fold. The same arrangement of transcripts was analyzed similarly in male vs. female rsl mice and in wt vs. rsl males and in wt vs. rsl females (Fig. 2A). The overall pattern of sex-specific genes is largely the same between wt and rsl mice. However, the reduction in color in the rsl column suggests the breadth and magnitude of sexually dimorphic gene expression are reduced as a consequence of mutations in the Rsl genes. For several sex-dependent transcripts, absence of Rsl (in rsl mice) appeared to invert the expression bias; i.e., expression of some transcripts that appeared male-predominant in wt mice was female-predominant in rsl mice and vice versa (Fig. 2A, Supplemental Table S2). This resulted from changes of greater magnitude in one sex than the other, or in some cases, changes in both sexes, but in opposite directions.
Fig. 2.
Expression profiling of RNA from wt, rsl, and transgenic mouse liver. A: false-color heat map highlighting sex-specific gene expression in wt and rsl liver. We found 20,393 probe sets surpassed the minimum expression threshold and were arranged according to their magnitude of sex-biased expression determined by the male-to-female ratio of their average expression values. Male-predominant genes are in green, female-predominate genes are in red, and the magnitude of the expression ratio is according to the color bar shown at bottom. At the extremes are the Y-chromosome Ddx3y and X-chromosome Xist genes. Twenty-four probe sets appear inverted in their sex specificity in rsl relative to wt mice (*). The greater percentage of black in the rsl column (column 2) indicates that liver gene expression is less sex-biased in rsl than in wt mice. The same arrangements of probe sets were similarly color coded for wt/rsl male and wt/rsl female comparisons. B: false color heat maps of probe sets that differ from wt mice in at least 1 mouse genotype (P < 0.05). To visualize sex-dependent expression patterns, the probe sets were organized independently in males and females. The color intensity reflects the abundance of transcript relative to the spot average. Red is below the mean, and green is above the mean, with fold deviation in accord with the color bar at bottom. Labels below the columns identify results from duplicate pools [A (left) and B (right)]. The dendrograms at top, also highlighted by the vertical white lines, indicate that tg mice (R1 and R2) retained an overall pattern of expression similar to rsl rather than wt males, whereas in females, tg mice were similar to each other but differed considerably from either nontransgenic parental strain. C: Venn diagrams showing the distribution of expression patterns in B across genotypes. Males are on the left; females are on the right.
Extension of the comparisons to mice of the same sex but different genotypes (wt vs. rsl) highlighted the sex-specific effects of Rsl. For instance, in males (Fig. 2A), male-predominant transcripts were largely expressed at higher levels in wt than rsl, while at the same time, the majority of female-predominant transcripts appeared the same between wt and rsl mice, except for those with the greatest magnitude of female-specific expression, which were also expressed at higher levels in wt than rsl mice. In females, however, the effect of Rsl was noticeably less overall (Fig. 2A) and largely equivalent in terms of the numbers of male-predominant, female-predominant, and nondimorphic transcripts affected.
To explore whether overexpression exaggerated the Rsl influence, expression profiling data analysis included results from the tg mice. Data filtering (31), to ensure at least one of the groups differed significantly from wt, reduced the unique informative transcripts to 1,528. To further examine sex-specific influences, transcripts that differed in males (n = 907) were arranged independently from those that differed in females (n = 811) (Fig. 2B); 190 transcripts were present in both categories. Unsupervised hierarchical clustering sorted the samples by genotype and, independently, into groups of genes related by similar expression patterns. Globally, male gene expression (Fig. 2B, left) was more affected by the loss of Rsl (cf. wt to rsl) than female gene expression. Overexpression of Rsl only partially restored wt expression patterns. Clustering indicated rsl males share more patterns in common with both Rsl1-tg and Rsl2-tg males than with wt males. Female gene expression, on the other hand, was less affected by the loss of Rsl (cf. wt to rsl) yet dramatically influenced by Rsl excess, as seen in the dendrogram segregating non-tg from tg females. In contrast, examining the gene names that were clustered by similar expression patterns (Fig. 2B, left) did not reveal obvious biological connections.
Venn diagrams (Fig. 2C) were used next to examine how the differential gene expression was distributed among wt, rsl and tg mice. In males, 63% (572/907) of the genes differed significantly in expression between wt and rsl mice. In females this differential was only 27% (220/811), again reflecting greater sensitivity in males to the loss of Rsl. Nearly one-third (292/907) of the transcripts differed in expression between wt males and males with excess Rsl2, exclusively, in contrast to only 3.4% (31/907) for males with excess Rsl1. A similar trend was evident in females with 37% (304/811) of the transcripts differing in expression between wt and Rsl2-tg, exclusively, and only 10% (83/811) for females with excess Rsl1. These results also suggest Rsl1 functions are largely a subset of Rsl2 (i.e., ∼75% of Rsl1-responsive genes are also responsive to Rsl2).
Liver gene expression in mice with Rsl deficiency or excess.
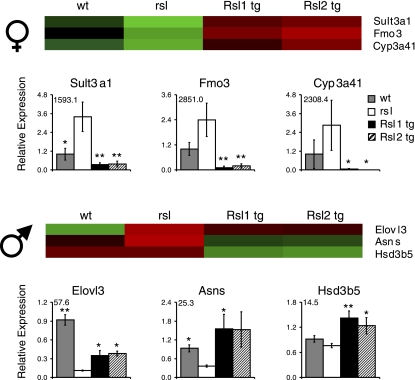
Previously, we demonstrated that Rsl1 and Rsl2 regulate male-predominant MUPs and some cyp genes, in addition to Slp (16, 34). Genes with the most sex-biased expression in wt mice may be additional targets of Rsl. The top 20 female- and male-predominant genes are listed in Tables 1 and 2, respectively. Rsl-responsive genes are in bold and most were validated by Q-RT-PCR (Fig. 3, Supplemental Fig. S2). Many of these genes are associated with metabolic homeostasis and reside in pathways responsible for steroid hormone or lipid metabolism. For instance, within the top 20 female-biased genes that are Rsl-regulated are sulfotransferase (Sult) 2a2, Sult3a1, flavin monoxygenase 3 (Fmo3), acyl-CoA thioesterase 3 (Acot3), Cyp2b13, Cyp3a41, Cyp17a1, and solute carrier 22a20 (Slc22a20). Expression patterns for all these genes in females (Fig. 3A, Supplemental Fig. S2) was reminiscent of Slp regulation in that expression was higher in rsl than wt females. The top male-predominant genes regulated by Rsl are also associated with metabolic pathways, but their expression patterns were distinctly different from the female-predominant genes. For example, elongation of long-chain fatty acids like 3 (Elovl3) and asparagine synthetase (Asns) were reduced in expression in rsl males and elevated in males with excess Rsl. Hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 5 (Hsd3b5) did not differ significantly between wt and rsl mice, however, Rsl overexpression resulted in an elevated expression level (Fig. 3B).
Table 1.
Female-predominant liver genes in wt mice
| Gene | Accession Number |
Expression Ratio (average) |
|||
|---|---|---|---|---|---|
| F-wt/M-wt | F-rsl/F-wt | F-R1/F-rsl | F-R2/F-rsl | ||
| Xist | L04961 | 302.05 | 1.25 | 0.84 | 1.03 |
| >Sult2a2 | NM_009286 | 249.96 | 0.61 | 0.06 | 0.04 |
| >Sult3a1* | NM_020565 | 140.04 | 2.33 | 0.23 | 0.21 |
| >Fmo3* | NM_008030 | 113.21 | 1.79 | 0.07 | 0.04 |
| Cyp2b9 | NM_010000 | 73.01 | 0.85 | 0.75 | 0.78 |
| >Acot3* | BB505010 | 69.14 | 1.89 | 0.38 | 0.37 |
| >Cyp2b13 | NM_007813 | 68.21 | 0.94 | 0.19 | 0.46 |
| BC014805 | AJ132857 | 63.09 | 1.02 | 0.65 | 0.70 |
| AI132709 | AI266897 | 49.22 | 0.85 | 0.61 | 0.63 |
| >Cyp3a41* | NM_017396 | 31.80 | 0.50 | 0.14 | 0.07 |
| Mt2 | AA796766 | 27.04 | 0.88 | 1.02 | 1.06 |
| Mt1 | NM_013602 | 17.39 | 0.94 | 0.73 | 0.87 |
| Cutl2 | BB129488 | 12.41 | 1.27 | 0.69 | 0.55 |
| >Cyp17a1* | NM_007809 | 11.80 | 1.52 | 0.35 | 0.43 |
| Abcd2 | BB197269 | 10.33 | 1.01 | 0.84 | 0.81 |
| >Npal1 | AK014427 | 9.91 | 0.54 | 1.82 | 1.28 |
| >Slc22a20 (mOAT6)* | NM_134256 | 8.58 | 1.14 | 0.30 | 0.31 |
| Cyp4a14 | AI327006 | 7.88 | 1.00 | 0.89 | 1.08 |
| Ly6i | AI789751 | 7.37 | 1.47 | 0.58 | 0.62 |
| >Hao3 | NM_019545 | 6.23 | 1.45 | 0.24 | 0.33 |
Shown are the top 20 female-predominant genes in wild-type (wt) mice. In bold are genes that also appear to be regulator of sex limitation (Rsl)-regulated due to their differential expression in rsl and Rsl1 (R1) and Rsl2 (R2) transgenic mice. >, Female-predominant genes regulated by Rsl.
Expression patterns have been validated by quantitative (Q)-RT-PCR.
Table 2.
Male-predominant liver genes in wt mice
| Gene | Accession Number |
Expression Ratio (average) |
|||
|---|---|---|---|---|---|
| M-wt/F-wt | M-rsl/M-wt | M-R1/M-rsl | M-R2/M-rsl | ||
| Ddx3y | AA210261 | 130.62 | 1.05 | 0.85 | 0.93 |
| Moxd1 | NM_021509 | 52.73 | 1.12 | 2.30 | 2.60 |
| Eif2 s3y | NM_012011 | 41.25 | 1.03 | 1.06 | 1.03 |
| >Elovl3* | BC016468 | 33.51 | 0.30 | 1.83 | 2.37 |
| Cyp4a12 | BC025936 | 22.82 | 1.19 | 0.82 | 0.80 |
| >Asns* | AV212753 | 14.41 | 0.40 | 3.01 | 2.88 |
| >Ugt2b38 | AI118428 | 12.66 | 2.15 | 0.69 | 0.81 |
| Jarid1d | AF127244 | 12.25 | 0.67 | 1.24 | 1.34 |
| >Hsd3b5* | NM_008295 | 10.33 | 1.00 | 0.99 | 1.02 |
| E130013N09Rik | BI731047 | 9.77 | 0.40 | 1.24 | 1.02 |
| >Cml4 | NM_023455 | 9.29 | 0.61 | 1.14 | 1.04 |
| Olig1 | AB038696 | 7.99 | 0.45 | 0.67 | 0.71 |
| >Serpine2 | NM_009255 | 7.51 | 0.55 | 0.94 | 0.69 |
| Serpina12 | AK014346 | 7.25 | 0.97 | 1.19 | 1.15 |
| Uty | BB207470 | 7.12 | 0.94 | 0.92 | 1.02 |
| Gnai1 | BQ174580 | 7.00 | 0.50 | 0.82 | 0.69 |
| 9330164H19Rik | BB768758 | 5.85 | 0.77 | 0.80 | 1.59 |
| C6 | NM_016704 | 5.63 | 1.26 | 0.99 | 1.01 |
| Keg1 | AI746421 | 5.50 | 0.47 | 1.36 | 0.94 |
| Cml5 | BC024605 | 5.26 | 0.62 | 1.33 | 1.15 |
Shown are the top 20 male-predominant genes in wt mice. In bold are genes that also appear to be Rsl-regulated due to their differential expression in rsl and Rsl1 (R1) and Rsl2 (R2) transgenic mice. >, Male-predominant genes regulated by Rsl.
Expression patterns have been validated by Q-RT-PCR. Hsd3b5 is male-predominant, however, by microarray, appeared Rsl-regulated only in females.
Fig. 3.
Real-time Q-RT-PCR validation of sexually dimorphic Rsl-responsive genes identified by microarray. Female-predominant genes are on top and male-predominant genes are below. Above the graphs are close-up images taken from the heat maps in Fig. 2B. To simplify the comparison, the color intensity of a single bar per genotype was adjusted to approximate the mean of pool A and pool B. Below the heat maps are real-time Q-RT-PCR results normalized to the mean expression in wt mice. Sex-specific expression ratios in wt mice (top, female/male; bottom, male/female) are in the top left corner. Bars represent means ± SE. *Mice that differ from rsl, *P < 0.05 and **P < 0.01. wt mice, n = 10; rsl males, n = 10; rsl females, n = 9; tg males, n = 10, Rsl1-tg females, n = 17, Rsl2-tg females, n = 13.
To take a broader view of Rsl liver gene regulation, we performed pairwise comparisons by genotype (wt, rsl, or tg) and filtered the data to identify transcripts that differ by 1.5-fold or more (Table 3). For each pairwise comparison, the genes affected by Rsl in both sexes were subtracted to create three mutually exclusive categories: genes affected in both sexes, genes affected in males only and genes affected in females only. This allowed us to examine gene categories in which magnitude and direction of expression differences depended on sex, Rsl, or both.
Table 3.
Impact of Rsl Deficiency or Excess on Mouse Liver Gene Expression
|
wt : rsl |
wt : R1-tg
|
wt : R2-tg
|
rsl: R1-tg
|
rsl: R2-tg
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Gene Count | Gene Count | Gene Count | Gene Count | Gene Count | |||||
| Both sexes | Both sexes | Both sexes | Both sexes | Both sexes | |||||
| wt < rsl | 26* | wt > R1tg | 45* | wt > R2tg | 38* | rsl > R1tg | 22* | rsl > R2tg | 25* |
| wt > rsl | 29 | wt < R1tg | 52 | wt < R2tg | 72 | rsl < R1tg | 35 | rsl < R2tg | 56 |
| <1.5-fold Δ | 135 | <1.5-fold Δ | 103 | <1.5-fold Δ | 80 | <1.5-fold Δ | 133 | <1.5-fold Δ | 109 |
| Total | 190 | Total | 190 | Total | 190 | Total | 190 | Total | 190 |
| Male only | Male only | Male only | Male only | Male only | |||||
| wt < rsl | 59 | wt > R1tg | 230 | wt > R2tg | 155 | rsl > R1tg | 22 | rsl > R2tg | 37 |
| wt > rsl | 281† | wt < R1tg | 73 | wt < R2tg | 113 | rsl < R1tg | 46 | rsl < R2tg | 182 |
| <1.5-fold Δ | 377 | <1.5-fold Δ | 514 | <1.5-fold Δ | 449 | <1.5-fold Δ | 648 | <1.5-fold Δ | 498 |
| Total | 717 | Total | 717 | Total | 717 | Total | 717 | Total | 717 |
| Female only | Female only | Female only | Female only | Female only | |||||
| wt < rsl | 39* | wt > R1tg | 25 | wt > R2tg | 49 | rsl > R1tg | 20 | rsl > R2tg | 26 |
| wt > rsl | 8 | wt < R1tg | 117 | wt < R2tg | 253 | rsl < R1tg | 63 | rsl < R2tg | 141 |
| <1.5-fold Δ | 524 | <1.5-fold Δ | 479 | <1.5-fold Δ | 319 | <1.5-fold Δ | 538 | <1.5-fold Δ | 454 |
| Total | 621 | Total | 621 | Total | 621 | Total | 621 | Total | 621 |
Genes displayed in the heat map in Fig. 2B were compared pairwise by genotype and sorted based on directionality and magnitude of differential expression (i.e., greater than, >, or less than, <, a 1.5-fold differential).
Categories containing genes with expression patterns similar to Slp. †Large percentage of Rsl-responsive genes affected in males only are expressed at higher levels in wt than rsl.
Given that Rsl1/2 encode transcriptional repressor proteins and that the defining Rsl-target, Slp, is male-predominant, we first looked at the category with expression patterns most similar to Slp i.e., higher expression in both sexes of rsl mice and lower expression in both sexes of tg mice, relative to wt (Table 3). Transcripts in this category are suspected to be direct targets of Rsl repression. Only 26 out of 190 (13.7%) showed expression trends, in both sexes, similar to Slp (rsl > wt) (Table 3). However, because the difference in Slp expression between wt and rsl males is relatively small (Fig. 1B), we also considered the “female only” category as likely to contain direct targets of Rsl repression. For females, 39 transcripts were higher in rsl than wt, while 8 were higher in wt than rsl mice. In the “male only” category, 59 were higher in rsl than wt, and a remarkable 281 were higher in wt than rsl (Table 3). These findings suggest that the majority of Rsl effects in males (83%) may be via Rsl's repression of downstream regulators; in contrast in females, the majority of Rsl effects (81%) are consistent with direct transcriptional repression.
Next, we focused on transcripts with higher expression in wt or rsl mice than in tg mice. Because the Rsl transgenes are greatly overexpressed, we anticipated a considerable increase in the number of genes that would be lower in expression, similar to Slp (Fig. 1B). Remarkably, among all four tg to non-tg comparisons, the number of transcripts expressed at lower levels in the tg mice only ranged from 22 to 45 (Table 3). Even more surprising was the number that showed a higher level of expression in the tg mice relative to wt or rsl mice (52 for wt < Rsl1-tg, 72 for wt < Rsl2-tg, 35 for rsl < Rsl1-tg and 56 for rsl < Rsl2-tg). In addition, that the majority of gene expression differences, even in the overexpressing tg mice, are <1.5-fold, supports a modulatory role for Rsl, globally, rather than one of dramatic repression seen for Slp. Because gross overexpression of the transgenes is restricted to the liver and thus may complicate further physiological analyses, subsequent comparisons focused only on wt and rsl mice.
Sexually dimorphic expression in mice with Rsl deficiency.
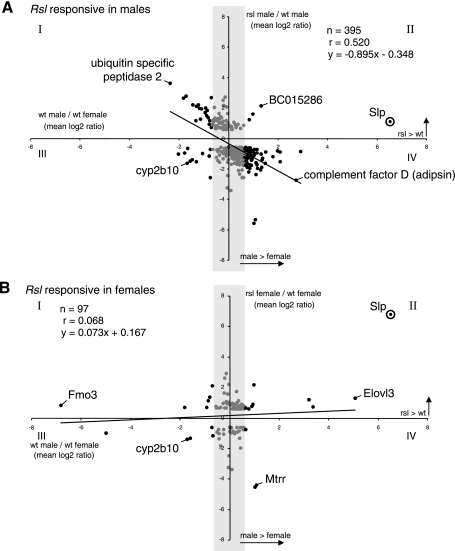
STAT5b has a dominant effect on male-specific liver gene expression (7, 35) including Slp, suggesting the absence of Rsl might correlate inversely with effects of STAT5b loss, as seen in knockout mice (35). To test this, average log2 ratios of the 1,528 Rsl-responsive genes in wt males and females (Fig. 4, x-axis) were plotted against their average log2 ratios between wt and rsl mice (Fig. 4; y-axis, −0.58 rsl-biased, >0.58 Rsl-biased). In males, 395 transcripts were >1.5-fold different in expression between wt and rsl, and 137 of these were also sex-biased in wt liver (Fig. 4A). In females, 97 transcripts were >1.5-fold different in expression between wt and rsl and 24 of these were also sex-biased in wt liver (Fig. 4B). If the magnitude of sex-specific expression were highly dependent on Rsl, sexually dimorphic transcripts would align along diagonals radiating from the origin into quadrants I and II of the scatter plots. For instance, Slp expression, assessed by Q-RT-PCR, mapped to quadrant II. Surprisingly, a large percentage (98 out of 102) of male-predominant genes were, unlike Slp, expressing at higher levels in wt than rsl liver (Fig. 4A). By contrast, in females, a nearly equal number of female-predominant genes were split between genotypes (Fig. 4B). The weak correlation (males, r = 0.52; females, r = 0.07) between the effect of sex and genotype suggests that Rsl1/2 are not key determinants of sex-biased gene expression. rsl males are somewhat “feminized” by the loss of Rsl, while females merely exhibit a modest loss of repression (except for Slp, in which loss of repression is severe) that is not sex-dependent. Overall, the liver gene expression of rsl mice is less sexually dimorphic than wt, due in large part to reduced expression of numerous genes in males.
Fig. 4.
Scatter plots to determine correlation of sex-biased liver gene expression with the presence or absence of Rsl. A: the log2 ratios for the 907 Rsl-responsive probe sets in males were plotted according to their sex-biased expression in wt mice (x-axis) relative to their differential expression in wt vs. rsl mice (y-axis). Only genes for which the wt to rsl ratio is >1.5-fold are shown. Female-predominant genes are on the left (quadrants I and III). Male-predominant genes are on the right (quadrants II and IV). The shaded region highlights genes that are sex-neutral (< 1.5-fold Δ males v. females). Slp was not on the microarray but is plotted here for reference with relative expression values determined by real-time Q-RT-PCR. The trend line indicates the best-fit linear representation of the 395 probe sets. The low correlation coefficient (r = 0.52) suggests male-specific gene expression is not tightly linked to the presence of Rsl. The majority of points (67%) in quadrant IV suggest that most male-predominant liver genes in males, unlike Slp, are diminished in expression due to the loss of Rsl. B: the log2 ratios for the 811 Rsl-responsive probe sets in females were plotted according to their sex-biased expression in wt and rsl mice. Axes, shading, and labeling are as in A. The majority of points (44%) in quadrant II suggest that nearly half of all male-predominant liver genes in females, similar to Slp, are increased in expression due to the loss of Rsl.
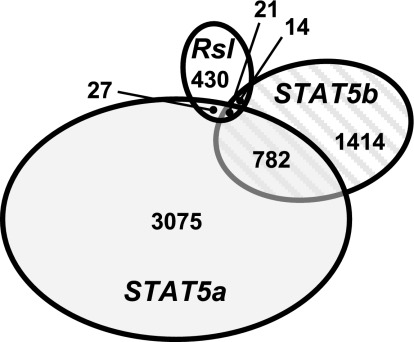
To specifically address Rsl and STAT5 interaction we compared the 492 Rsl-responsive transcripts with the STAT5-responsive transcripts reported by Clodfelter et al. (7, 8) (Fig. 5). While STAT5b plays a major role in enforcing male-specific liver gene expression, STAT5a only affects about 4% of sexually dimorphic liver genes and most are female-predominant (8). Rsl-responsive genes intersect with ∼1.2% of STAT5a-responsive and ∼1.5% of STAT5b-responsive genes. Given this low level of overlap, Rsl regulation does not seem to be restricted to STAT5-dependent target genes.
Fig. 5.
Comparison of genes responsive to Rsl and/or STAT5. The 492 Rsl-responsive transcripts were compared by GenBank accession number to the STAT5-responsive transcripts reported by Clodfelter et al. 2006 (7) and Clodfelter et al. 2007 (8). The overlapping gene numbers may be slightly underestimated due to differences in accession numbers for identical genes on the Affymetrix array, used here, and the Agilent Rosetta/Merck Mouse TOE 75k Array I used elsewhere (7, 8). For all data sets, significant difference thresholds were 1.5-fold (P < 0.05). While each study was comprehensive, the total number of transcripts analyzed differed slightly. Here we observed 20,393 unique liver transcripts. The STAT5a study reported 18,204, and the STAT5b study reported 23,455.
Impact of Rsl on genes that are not sexually dimorphic.
A considerable number of genes that differed between wt and rsl mice (264 in males and 73 in females) were not sexually dimorphic (Fig. 4, Supplemental Tables S3–S6). Two genes expressed at higher levels in both sexes of wt mice are members of the tripartite motif-containing (TRIM) family, Trim 30 and Trim 34. Two additional genes that were not sexually dimorphic but similarly higher in both sexes of wt mice are GTPase, very large interferon inducible 1 (Gvin1), and virus inducible noncoding RNA (VINC, Riken cDNA 2310043N10). Together Trim30, Trim34, Gvin1, and VINC are associated with viral resistance (14, 23, 25, 29). Several other nondimorphic genes that appear Rsl-regulated are involved in fatty acid metabolism (Supplemental Tables S3–S6), such as the fatty acid transporter CD36, mevalonate (diphospho) decarboxylase (Mvd), phosphomevalonate kinase (Pmvk), squalene epoxidase (Sqle), stearoyl-CoA desaturase 1 (Scd1), and angiopoietin-like 4 (Angptl4). Interestingly, some of these genes have been reported to be female-predominant in other rodent models (7, 28, 32). These differences likely reflect strain or species variations.
Enrichment of GO terms among Rsl-regulated genes.
The GOstat program (2) was used next to identify GO terms enriched among the Rsl-regulated genes (Table 4). Out of 1,528 informative transcripts, 1,491 come from unique genes, and 732 were annotated within the GOstat database. Eleven GO terms were identified as enriched based on P values <0.05. The majority of enriched terms identify pathways of lipid or steroid metabolism, such as “steroid biosynthetic process,” “cholesterol metabolic process,” and “lipid biosynthetic process.” Because many of these same terms are found in the annotations of sexually dimorphic genes in general (7, 8, 39), the 1,528 transcripts were reanalyzed separately by sex (i.e., 907 Rsl-regulated in males and 811 Rsl-regulated in females) to enhance the visibility of Rsl effects that may be sex-specific. Surprisingly, no GO terms were enriched at a P value <0.05 for the female set. In addition to the list of GO terms obtained with the total gene set, three new terms “cellular lipid metabolic process,” “lipid metabolic process,” and “pyrimidine base metabolic process” were identified when the male genes were analyzed, separately. Two of these terms are associated with fatty acid metabolism whereas the broader terms “microsome” and “regulation of catalytic activity” were no longer significant (P >0.05). These results again highlight the broader impact of the absence of Rsl in males compared with females and suggest the presence or absence of Rsl may alter liver metabolism of xenobiotics, steroids, cholesterol, and lipids.
Table 4.
GO terms enriched within Rsl-regulated genes
| GO Term | Overall P Value | Overlap Gene Counta | Male P Value | Overlap Gene Countb | Set Gene Countc |
|---|---|---|---|---|---|
| Steroid biosynthetic process | 2.75E-06 | 18 | 2.86E-07 | 16 | 62 |
| Sterol biosynthetic process | 3.36E-06 | 12 | 4.67E-07 | 11 | 27 |
| Steroid metabolic process | 2.86E-05 | 20 | 1.55E-04 | 17 | 121 |
| Cholesterol biosynthetic process | 2.86E-05 | 10 | 9.06E-06 | 9 | 22 |
| Sterol metabolic process | 1.63E-04 | 15 | 3.85E-05 | 13 | 62 |
| Alcohol metabolic process | 2.16E-04 | 29 | 5.50E-05 | 24 | 232 |
| Vesicular fraction | 0.00103 | 19 | 0.023 | 14 | 132 |
| Cholesterol metabolic process | 0.00133 | 13 | 5.90E-04 | 11 | 57 |
| Lipid biosynthetic process | 0.00141 | 26 | 1.87E-05 | 22 | 214 |
| Microsome | 0.0146 | 17 | 0.099 | 12 | 128 |
| Regulation of catalytic activity | 0.0489 | 28 | >0.1 | 276 | |
| Cellular lipid metabolic process | 0.00126 | 33 | 457 | ||
| Lipid metabolic process | 0.00364 | 36 | 536 | ||
| Pyrimidine base metabolic process | 0.023 | 4 | 9 |
Out of 1,528 microarray spots, 1,491 correspond to unique genes and 732 had annotated Gene Ontology (GO) terms. The 732 genes were analyzed for GO term enrichment against the GO database consisting of 14,331 genes. P values were calculated to identify enrichment (P < 0.05) and the false discovery rate was controlled by the method of Benjamini. P values in italics are greater than the cutoff for significance of 0.05.
The number of genes within the 732 annotated, Rsl-regulated genes that are annotated with the given category.
The number of genes within the 481 annotated, Rsl-regulated genes in males that are annotated with the given category.
The number of genes within all 14,331 annotated genes in the GOstat database that are annotated with the given category.
Rsl mice differ from wt mice in glucose and lipid metabolism.
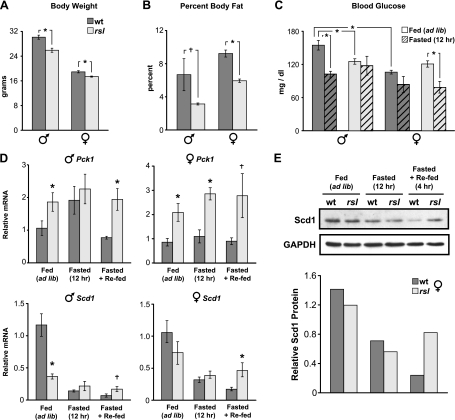
Based on the enrichment of metabolic GO terms among Rsl-responsive genes, we asked whether Rsl affects intermediary metabolism by comparing indicators of metabolic homeostasis in wt and rsl mice. In age-matched adults, both sexes of rsl mice weighed somewhat less than wt (Fig. 6A) and body composition scans (Fig. 6B) confirmed that rsl mice had a smaller percentage of body fat. In mice fed ad libitum, blood glucose levels were ∼1.5-fold higher in wt males than females, but did not differ by sex in rsl mice (Fig. 6C). Following a 12 h fast, wt males responded as expected with a 25% drop in glucose levels, while rsl male levels were not significantly different. Females demonstrated a fasting-induced drop in blood glucose, but the magnitude of change was more pronounced in rsl than wt (Fig. 6C). Together, these results suggest blood glucose levels are both sex- and Rsl-dependent and overall less sexually dimorphic in rsl than wt mice.
Fig. 6.
Metabolic homeostasis differs between wt and rsl mice. Body weight (n = 9 for each sex and genotype, A) and percent body fat (n = 4 for each sex and genotype, B) were measured in age-matched adults of both sexes of wt and rsl mice. C: blood glucose levels were measured at 7:30 PM (late in the “lights-on” phase) in wt and rsl mice, fed ad libitum (n = 9). Cohorts of wt and rsl mice (n = 3) were fasted for 12 h or given chow for 4 h after fasting. D: expression of Pck1 and Scd1, in mice of each sex and genotype, fed ad libitum (n = 7), fasted (n = 3), or fasted and refed (n = 3). Real-time Q-RT-PCR measured mRNA levels and values were normalized to wt males fed ad libitum. All bars represent means ± SE. †P < 0.10, *P < 0.05, wt vs. rsl. E: validation of changes in Scd1 protein. Representative females with relative Scd1 mRNA values near the mean were assayed by Western blot for Scd1 protein in liver extracts. For relative quantification, Scd1 band intensities were normalized to GAPDH.
Metabolic stability is tied to the appropriate expression of key enzymes in gluconeogenesis and lipogenesis [e.g., phosphoenolpyruvate carboxykinase 1 (Pck1) and stearoyl-CoA desaturase-1 (Scd1), respectively] (9). To further investigate the role of Rsl in metabolic control, we compared the expression of Pck1 and Scd1 in wt and rsl mice (Fig. 6D). Scd1 was identified as Rsl-responsive by microarray (Supplemental Table S4); however, Pck1 was not, perhaps due to greater individual variability. Expression levels for both genes by Q-RT-PCR were both Rsl- and sex-dependent (Fig. 6D). Pck1 mRNA in rsl mice was nearly twice the wt level, except in males following a 12 h fast. This male-specific effect was due to a diminished fasting/refeeding response in rsl males. In females of either genotype, the fasting/refeeding response was noticeably less than that observed in wt males. Sex- and genotype-dependent expression patterns were also seen with the Scd1 gene. Scd1 mRNA in rsl males was only ∼25% that of the wt level during fed conditions, consistent with the microarray results (Supplemental Table S4). In response to a 12 h fast, Scd1 levels dropped in all mice, as expected (17), but genotypic differences (rsl > wt) were again apparent after refeeding for 4 h.
The Scd1 protein has a short half-life and is regulated at the level of degradation (33). To test whether Rsl affected protein stability, Scd1 protein levels were assayed in livers of mice fasted and refed as above. Results indicated that protein abundance closely paralleled the mRNA pattern (Fig. 6E), suggesting that Rsl's effect on Scd1 expression is predominantly at the level of transcription. Taken together, these subtle differences in gross physiology and gene expression profiles suggest metabolism in rsl mice may differ from wt in response to environmental stress.
DISCUSSION
Gene expression profiling with genomic microarrays has become a common comprehensive method for linking transcriptional regulatory proteins with physiological pathways (3, 7, 8, 39). Here we have applied this approach to mice that differ genetically in their ability to express the KRAB zinc finger transcriptional repressors Rsl1 and Rsl2. Because mice with mutations in both Rsl1 and 2 exhibit a liver phenotype (i.e., Slp expression in females) (34), we investigated the complete liver transcriptome to view Rsl's broad influence. Out of the nearly 20,000 transcripts that met the minimum threshold for expression, 7.5% differed significantly in accord with Rsl genotype. Within this list of transcripts were genes that were male-predominant, female-predominant, or not sexually dimorphic (Tables 1 and 2, Supplemental Tables S3–S6). Based on the expression pattern of Slp (Fig. 1B), we anticipated similar Rsl repression for other male-predominant genes. This was evident in females, where 10 out of 13 male-predominant transcripts were higher in rsl than wt (Fig. 4B), but much less evident in males where only 4 out of 102 transcripts were higher in rsl than wt (Fig. 4A). These findings highlight the sex-specific activity of Rsl and also demonstrate that the majority of Rsl-responsive transcripts, particularly in males, are influenced in a more complex manner than is Rsl's regulation of Slp.
Based on initial characterization (16), Rsl appeared to be largely involved in male-specific gene expression, and thus an interaction with STAT5b was expected. With the exception of Slp, the genes with the greatest magnitude of response to Rsl are female-predominant (e.g., Sult2a2, Sult3a1, Fmo3, Acot3, Cyp2b13, Cyp3a41, Cyp17a1, Slc22a20, Hao3; Table 1) and are located in the regions of overlap in Fig. 5. Furthermore, the direction of the Rsl-effect is consistent with a mechanism of direct repression. Therefore, Rsl, like STAT5a in the liver, modulates the expression of many genes, regardless of their sex specificity, and directly regulates a small group of mostly female-predominant genes. Counter to previous expectations, this suggests that a relatively small group of genes are both activated by STAT5 and repressed by Rsl. For many of these genes, sequences resembling STAT5 binding sites have been identified in the vicinity of their transcription start sites (unpublished observation). The precise mechanism of Rsl regulation of these targets awaits future studies.
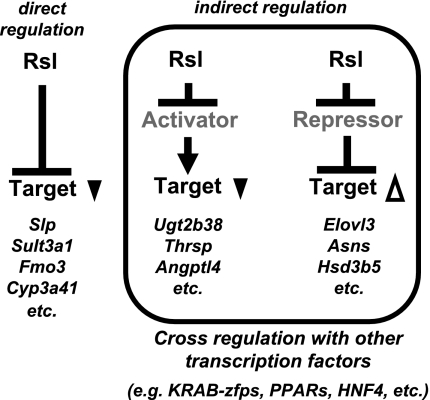
Rsl1 and Rsl2, like the other KRAB-zfps studied to date, are transcriptional repressors based on in vitro analysis and many genes here evidence direct repression (18). In addition to Slp, genes directly repressed by Rsl include Sult3a1, Fmo3, and Cyp3a41, all of which are higher in rsl females compared with wt and severely reduced by Rsl transgene overexpression (Fig. 3B). Genes such as Elovl3 and Asns are also responsive to Rsl, but their reduced expression in male rsl mice and elevated expression in the tg mice (Fig. 3A) suggests these differences are likely due to Rsl's influence on downstream transcriptional regulators (Fig. 7), through either protein-DNA or protein-protein interactions. Finding KAP1, the obligate corepressing partner of KRAB-zfps, enriched at 3′ ends of KRAB-zfp genes in chromatin suggests KRAB-zfps regulate each other (22). This mechanism, in which Rsl represses a repressor (Fig. 7), may account for the paradoxical results seen for male-specific genes such as Elovl3, Asns, and Hsd3b5, in which expression is lower in rsl relative to wt mice (Fig. 3, Supplemental Fig. S2). Given that the majority of Rsl-responsive genes in males have expression patterns similar to Elovl3 (Table 3; wt > rsl = 281 out of 340), this mode of regulation may be prevalent in males but not in females (Table 3, wt > rsl = 8 out of 47). Cross-regulation among KRAB-zfp family members may be responsible; however, no candidate is apparent from the microarray results or from previous examination of other KRAB-zfps within the Rsl locus (16). In addition, Rsl1 and Rsl2 do not cross-regulate, since overexpression of one, as a transgene, does not affect endogenous levels of the other (Fig. 1A).
Fig. 7.
Rsl-dependent liver gene regulation. Examples of targets that are likely regulated by direct repression include Slp, Sult3a1, Fmo3, and Cyp3a41. Genes such as Ugt2b38, Thrsp, and Angptl4 had expression patterns similar to Slp (i.e., higher in rsl than wt mice) but were only partially restored to wt levels by Rsl overexpression. Unexpected was the large proportion of genes that had the inverse expression pattern (i.e., lower in rsl than wt mice and elevated by Rsl overexpression) (e.g., Elovl3, Asns, Hsd3b5, etc.). Together these latter groups are likely controlled by indirect regulation via activators or repressors in pathways downstream from Rsl repression.
The male-biased mRNAs MUP1/2 and MUP3 are more abundant in rsl compared with wt liver by northern blotting and thus likely targets of Rsl repression (16, 34). The Affymetrix mouse genome array used here contains four probe sets that detect MUP1 but only one shows a male-biased expression pattern in wt mice. Furthermore, all the spot intensity values for probe sets specific to MUP1, 2, and 3 are near the array maximum and thus not reliably quantifiable. By Q-RT-PCR, however, we confirmed a two- to fourfold elevation of MUP1, MUP2, and MUP3 in rsl relative to wt mice (data not shown) but with considerable individual variability. Thus, while Rsl appears to modulate MUP gene regulation, the specific mechanistic details remain to be determined.
Another previously identified Rsl target is male-predominant Cyp2d9 (34). The microarray results confirm previous findings but indicate Cyp2d9 is only ∼1.3-fold higher in rsl than wt mice and not significantly less in the tg animals. Changes in gene expression above the 1.5-fold filtering cut-off and response to tg overexpression, however, were observed for other steroid metabolizing enzymes such as Sult3a1, Fmo3, and Cyp3a41.
Rsl regulates many genes that are not sexually dimorphic (see shaded area of Fig. 4), sometimes in both sexes and sometimes only in one (Supplemental Tables S3–S6). For TRIM30, TRIM34, Gvin1 and VINC expression is reduced in both sexes of rsl mice and elevated in both tg groups, indicating Rsl likely acts through other regulators. The TRIM family has been associated with a wide range of cellular processes including epigenetic control of transcription, apoptosis, and cell cycle regulation. TRIM30, TRIM34, Gvin1, and VINC all function in pathways associated with cellular resistance to viral infection (14, 23, 25, 29), suggesting Rsl proteins may impact cellular immunity. Interestingly, the KRAB-associated corepressor KAP1 is also a member of the TRIM family, but its transcript level does not vary with sex or Rsl genotype.
GO analysis indicates Rsl-responsive genes are significantly enriched in processes of steroid, cholesterol, and lipid metabolism (Table 4). Although the health of the mice in a laboratory setting is not obviously compromised due to either the loss or overexpression of Rsl1/2, subtle expression differences of genes involved in energy homeostasis could influence how the animals respond to environmental challenges. By comparing wt to rsl mice, we noticed a slight but statistically significant difference in body weight, body composition, and blood glucose levels (Fig. 6, A–C), as well as differential regulation of genes involved in metabolic homeostasis (e.g., Pck1 and Scd1) (Fig. 6, D and E). Elevated Pck1 and reduced Scd1 expression have been used as indicators of the fasted state (9, 10, 21). Together, these results indicate rsl mice are leaner than wt and may be diminished in their capacity to respond to dietary stress. The liver, most notably in male rsl mice, may be somewhat insulin resistant and compensates for the perceived glucose deficiency by mobilizing fatty acids for energy. This shift in physiology in rsl males toward that of wt females is consistent with the loss of sexually dimorphic gene expression (Fig. 2A) observed globally in rsl mice. Alternatively, a reduction in food consumption or an increase in locomotor activity could occur in rsl compared with wt mice. Additional influences due to Rsl expression outside the liver, such as in pancreas, muscle, or adipose tissue, are also possible.
Sex-specific GH secretion from the pituitary has recently been shown to produce sexually dimorphic expression patterns of metabolic genes including SREBP-1c, LXRα, and Scd1 (1). In the liver, many GH effects, particularly those that are sex-specific, are transmitted to the nucleus via STAT5b (4, 7, 35). For instance, Slp is male-specific in the liver due in large part to two STAT5b binding sites upstream from the transcription start site (27, 37). In the regulation of Slp, Rsl1/2 appears to antagonize STAT5b (directly or indirectly) by attenuating Slp expression in wt mice, especially females, and virtually extinguishing Slp expression in tg mice that overexpress Rsl in the liver (Fig. 1). Rsl1/2 may have a similar, yet less pronounced, role in intermediary metabolism. Previous studies suggest that feminization of the GH plasma pattern increases insulin resistance (1), and our microarray results indicate that the loss of Rsl reduces sexually dimorphic liver gene expression, particularly in rsl males compared with wt (Fig. 2). Future studies will examine the role of KRAB-zfp repressors within the network of transcriptional regulators that influence sexual dimorphism, homeostasis, and metabolic disease susceptibility (e.g., PPAR-α, PPAR-γ, SREBP-1c, LXR, PXR).
This study links Rsl to regulation of genes involved in immunity, steroid/xenobiotic metabolism and metabolic balance, pathways that interface closely with the environment. Whether it is a viral infection, exposure to toxins, or changes in the composition of the food source, the presence or absence of Rsl may determine how the liver responds to external insult. The Rsl genes serve as unique paradigms of the modulatory capacity of the much larger KRAB-zfp family. Polymorphisms or mutations in KRAB-zfp genes, such as those identified in Rsl (16), might underlie individual differences in response to environmental stress. These differences, along with expansion and diversification of KRAB-zfp genes over time, allow adaptation of physiology to fit the environment. In humans, variations in the KRAB-zfp family may also contribute, genetically, to differential susceptibility to common diseases such as metabolic syndrome, obesity, and type 2 diabetes.
GRANTS
This work was supported by a University of Michigan Gastrointestinal Peptide Research Center Pilot/Feasability Award (5P30DK034933) to C. J. Krebs and by NIDDK Grant DK-053998 to D. M. Robins.
Supplementary Material
Acknowledgments
The authors acknowledge the Michigan Diabetes Research and Training Center [National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant 5P60DK-20572] and the Michigan Metabolomics and Obesity Center for animal phenotyping. We also thank Drs. Jun Li and Charles Burant for helpful comments on the manuscript.
Address for reprint requests and other correspondence: D. M. Robins, Dept. of Human Genetics, Univ. of Michigan Medical School, 1241 E. Catherine St., Ann Arbor, MI 48109-0618 (e-mail: drobins@umich.edu).
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Ameen C, Linden D, Larsson BM, Mode A, Holmang A, Oscarsson J. Effects of gender and GH secretory pattern on sterol regulatory element-binding protein-1c and its target genes in rat liver. Am J Physiol Endocrinol Metab 287: E1039–E1048, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Beissbarth T, Speed TP. GOstat: find statistically overrepresented Gene Ontologies within a group of genes. Bioinformatics 20: 1464–1465, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38: 1289–1297, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Choi HK, Waxman DJ. Pulsatility of growth hormone (GH) signalling in liver cells: role of the JAK-STAT5b pathway in GH action. Growth Horm IGF Res 10, Suppl B: S1–S8, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analyt Biochem 162: 156–159, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol 20: 1333–1351, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Clodfelter KH, Miles GD, Wauthier V, Holloway MG, Zhang X, Hodor P, Ray WJ, Waxman DJ. Role of STAT5a in regulation of sex-specific gene expression in female but not male mouse liver revealed by microarray analysis. Physiol Genomics 31: 63–74, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev 86: 465–514, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Granner D, Pilkis S. The genes of hepatic glucose metabolism. J Biol Chem 265: 10173–10176, 1990. [PubMed] [Google Scholar]
- 11.Huntley S, Baggott DM, Hamilton AT, Tran-Gyamfi M, Yang S, Kim J, Gordon L, Branscomb E, Stubbs L. A comprehensive catalog of human KRAB-associated zinc finger genes: insights into the evolutionary history of a large family of transcriptional repressors. Genome Res 16: 669–677, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Jiang PP, Frederick K, Hansen TH, Miller RD. Localization of the mouse gene releasing sex-limited expression of Slp. Proc Natl Acad Sci USA 93: 913–917, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klamp T, Boehm U, Schenk D, Pfeffer K, Howard JC. A giant GTPase, very large inducible GTPase-1, is inducible by IFNs. J Immunol 171: 1255–1265, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Krebs CJ, Khan SM, Mollard B, Robins DM. Adapter annealing to engineer restriction enzyme sites at cloning junctions. Anal Biochem 350: 313–315, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Krebs CJ, Larkins LK, Price R, Tullis KM, Miller RD, Robins DM. Regulator of sex-limitation (Rsl) encodes a pair of KRAB zinc-finger genes that control sexually dimorphic liver gene expression. Genes Dev 17: 2664–2674, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefevre P, Diot C, Legrand P, Douaire M. Hormonal regulation of stearoyl coenzyme-A desaturase 1 activity and gene expression in primary cultures of chicken hepatocytes. Arch Biochem Biophys 368: 329–337, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Margolin J, Friedman J, Meyer W, Vissing H, Thiesen HJ, Rauscer FJ 3rd. Krüppel-associated boxes are potent transcriptional repression domains. Proc Natl Acad Sci USA 91: 4509–4513, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittendorfer B Insulin resistance: sex matters. Curr Opin Clin Nutr Metab Care 8: 367–372, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Mittendorfer B Sexual dimorphism in human lipid metabolism. J Nutr 135: 681–686, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Ntambi JM Dietary regulation of stearoyl-CoA desaturase 1 gene expression in mouse liver. J Biol Chem 267: 10925–10930, 1992. [PubMed] [Google Scholar]
- 22.O'Geen H, Squazzo SL, Iyengar S, Blahnik K, Rinn JL, Chang HY, Green R, Farnham PJ. Genome-wide analysis of KAP1 binding suggests autoregulation of KRAB-ZNFs. PLoS Genet 3: e89, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orimo A, Tominaga N, Yoshimura K, Yamauchi Y, Nomura M, Sato M, Nogi Y, Suzuki M, Suzuki H, Ikeda K, Inoue S, Muramatsu M. Molecular cloning of ring finger protein 21 (RNF21)/interferon-responsive finger protein (ifp1), which possesses two RING-B box-coiled coil domains in tandem. Genomics 69: 143–149, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Park SH, Liu X, Hennighausen L, Davey HW, Waxman DJ. Distinctive roles of STAT5a and STAT5b in sexual dimorphism of hepatic P450 gene expression. Impact of STAT5a gene disruption. J Biol Chem 274: 7421–7430, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Patarca R, Freeman GJ, Schwartz J, Singh RP, Kong QT, Murphy E, Anderson Y, Sheng FY, Singh P, Johnson KA, Guarnagia SM, Durfee T, Blattner F, Cantor H. rpt-1, an intracellular protein from helper/inducer T cells that regulates gene expression of interleukin 2 receptor and human immunodeficiency virus type 1. Proc Natl Acad Sci USA 85: 2733–2737, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaffl MW A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robins DM Multiple mechanisms of male-specific gene expression: lessons from the mouse sex-limited protein (Slp) gene. Prog Nucleic Acid Res Mol Biol 78: 1–36, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Rogers AB, Theve EJ, Feng Y, Fry RC, Taghizadeh K, Clapp KM, Boussahmain C, Cormier KS, Fox JG. Hepatocellular carcinoma associated with liver-gender disruption in male mice. Cancer Res 67: 11536–11546, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Saha S, Murthy S, Rangarajan PN. Identification and characterization of a virus-inducible non-coding RNA in mouse brain. J Gen Virol 87: 1991–1995, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Shipley JM, Waxman DJ. Simultaneous, bidirectional inhibitory crosstalk between PPAR and STAT5b. Toxicol Appl Pharmacol 199: 275–284, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Smyth GK Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article3, 2004. [DOI] [PubMed]
- 32.Stahlberg N, Rico-Bautista E, Fisher RM, Wu X, Cheung L, Flores-Morales A, Tybring G, Norstedt G, Tollet-Egnell P. Female-predominant expression of fatty acid translocase/CD36 in rat and human liver. Endocrinology 145: 1972–1979, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Toyama T, Kudo N, Mitsumoto A, Hibino Y, Tsuda T, Kawashima Y. Stearoyl-CoA desaturase activity is elevated by the suppression of its degradation by clofibric acid in the liver of rats. J Pharm Sci 103: 383–390, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Tullis KM, Krebs CJ, Leung JY, Robins DM. The regulator of sex-limitation gene, rsl, enforces male-specific liver gene expression by negative regulation. Endocrinology 144: 1854–1860, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA 94: 7239–7244, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urrutia R KRAB-containing zinc-finger repressor proteins. Genome Biol 4: 231, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varin-Blank N, Dondi E, Tosi M, Hernandez C, Boucontet L, Gotoh H, Shiroishi T, Moriwak K, Meo T. Male-specific transcription initiation of the C4-Slp gene in mouse liver follows activation by STAT5. Proc Natl Acad Sci USA 95: 8750–8755, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiwi CA, Gupte M, Waxman DJ. Sexually dimorphic P450 gene expression in liver-specific hepatocyte nuclear factor 4alpha-deficient mice. Mol Endocrinol 18: 1975–1987, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res 16: 995–1004, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.