Abstract
Diabetes is a chronic disease associated with hyperglycemia and altered bone metabolism that may lead to complications including osteopenia, increased risk of fracture and osteoporosis. Hyperglycemia has been implicated in the pathogenesis of diabetic bone disease; however, the biologic effect of glucose on osteoclastogenesis is unclear. In the present study, we examined the effect of high D(+)glucose (D-Glc) and L(−)glucose (L-Glc; osmotic control) on RANKL-induced osteoclastogenesis using RAW264.7 cells and Bone Marrow Macrophages (BMM) as models. Cells were exposed to sustained high glucose levels to mimic diabetic conditions. Osteoclast formation was analyzed using tartrate resistant acid phosphatase (TRACP) assay, expression of calcitonin receptor (CTR) and cathepsin K mRNAs, and cultures were examined for reactive oxygen species (ROS) using dichlorodihydrofluorescein diacetate (DCF-DA) fluorescence, caspase-3 and Nuclear Factor kappaB (NF-κB) activity. Cellular function was assessed using a migration assay. Results show, for the first time, that high D-Glc inhibits osteoclast formation, ROS production, caspase-3 activity and migration in response to RANKL through a metabolic pathway. Our findings also suggest that high D-Glc may alter RANKL-induced osteoclast formation by inhibiting redox-sensitive NF-κB activity through an anti-oxidative mechanism. This study increases our understanding of the role of glucose in diabetes-associated bone disease. Our data suggest that high glucose levels may alter bone turnover by decreasing osteoclast differentiation and function in diabetes and provide new insight into the biologic effects of glucose on osteoclastogenesis.
Keywords: osteoclast, glucose, RANKL, reactive oxygen species, differentiation
INTRODUCTION
Diabetes is becoming one of the leading disorders worldwide, reaching an estimated 300 million patients by 2010 [1]. Diabetes is associated with complications [2] such as altered bone metabolism that may lead to osteopenia, increased risk of fracture and osteoporosis [3–5]. However, the causal relationship between diabetes and bone loss has been controversial and the bone disease that develops in type 1 and type 2 diabetes may differ. In type 1 diabetic patients, bone mineral density is reduced by greater than 10% compared to nondiabetics and correlates with the duration of diabetes, whereas in type 2 diabetes bone density is more often increased [6,7]. In diabetics with low bone mass, histomorphometry studies suggested that osteopenia was due to decreased bone formation rate [8]. Hyperglycemia has been implicated in the pathogenesis of diabetic bone disease and, decreased activity of osteoblasts under diabetic conditions has been reported in both animal models and humans [9,10]. In vitro, incubation of cultured osteoblast-like cells with high glucose inhibits mineralization, Insulin-like Growth Factor-I (IGF-I)-stimulated growth and modulates osteoblast gene expression [11–14]. Studies are conflicting as to whether osteoclastogenesis is altered in diabetes [6]. Although glucose is the principal source of energy for osteoclast resorption [15], the effect of sustained high glucose levels on osteoclast differentiation and function has not been explored.
Osteoclasts are essential for bone remodeling. Receptor Activator of Nuclear Factor kappaB Ligand (NF-κB) is a key factor for differentiation of monocyte/macrophage osteoclast precursors into multinucleated osteoclasts and for activation of mature osteoclasts [16–18]. Binding of RANKL to its cognant receptor on osteoclast precursors leads to activation of NF-κB that is required for osteoclast differentiation [19,20]. Reactive oxygen species (ROS) are also crucial for RANKL-induced osteoclastogenesis [21–24] and NF-κB is among the redox-sensitive cell signaling pathways [25–27]. Recent studies indicate that ROS such as hydrogen peroxide produced in BMM cells stimulate osteoclast differentiation, whereas decreasing the level of ROS reverses RANKL responses [28]. RANKL signaling induces caspase-3, an enzyme involved in apoptotic and non-apoptotic events including cell cycle progression, activation and differentiation. Studies in procaspase-3 knockout mice and RAW264.7 cells indicate that osteoclasts fail to differentiate in response to RANKL in the absence of procaspase-3 or when caspase-3 activity is inhibited [29]. Terminal differentiation of osteoclasts is characterized by acquisition of mature phenotypic markers such as the calcitonin receptor (CTR), tartrate resistant acid phosphatase (TRACP) and ability to resorb bone. Resorption involves synthesis of cysteine proteinases, such as Cathepsin K, and matrix metalloproteinases (MMPs) [30,31]. In addition, MMP-9 and MMP-14 provide a stimulus for migration of osteoclasts that is relevant for access of osteoclasts to bone surfaces [32].
In the present study, we examined the effect of high D(+)glucose (D-Glc) and high L(−)glucose (L-Glc) (osmotic control) on RANKL-induced osteoclastogenesis using RAW264.7 and BMMs as models. Cells were exposed to sustained high glucose levels often observed in diabetic subjects. Osteoclast formation was analyzed using TRACP assay, expression of CTR and cathepsin K mRNAs by RT-PCR, ROS generation by dichlorodihydrofluorescein diacetate (DCF-DA) as well as dihydroethidium (DHE) fluorescence and caspase-3 activity. Cell migration was assessed in wounded cultures. Results show, for the first time, that high D-Glc inhibits osteoclast formation, ROS production, caspase-3 activity and migration in response to RANKL through a metabolic pathway. Our findings also suggest high D-Glc may alter RANKL-induced osteoclast formation by inhibiting NF-κB activity through a ROS-dependent mechanism.
MATERIALS AND METHODS
RAW264.7 cell culture and osteoclast differentiation
The murine monocytic cell line RAW264.7 (ATCC #TIB-71, Manassas, VA, USA) was maintained in α-MEM (containing 5.5mM D(+)glucose) supplemented with 10% FCS and incubated at 37°C in 5% CO2. Osteoclastogenesis experiments were performed as previously described [33]. Briefly, cells were seeded at a density of 1×104/cm2 and cultured for 6 days in differentiation medium: phenol red-free α-MEM supplemented with 2% fetal calf serum (FCS) and 50 ng/ml murine recombinant RANKL (mrRANKL) (RD systems).
Murine bone marrow macrophage (BMM)-derived osteoclasts
Bone marrow cells were isolated from 4-wk-old C57/Bl mice. Briefly, femurs were excised and the marrow cavity was flushed with phenol red-free α-MEM supplemented with 10% FCS. Cells were washed, plated in the same medium at a density of 2×105 cells/cm2 and incubated in the presence of mrM-CSF (20ng/ml) (RD systems). After 18 hrs, non-adherent cells were collected and washed. To obtain osteoclasts, cells were seeded at 5×104 cells/cm2 and incubated for 6 days in differentiation medium containing RANKL (50 ng/ml) and mrM-CSF (5 ng/ml).
High glucose incubation conditions
To determine the effect of glucose on osteoclastogenesis in RAW264.7 and BMM cultures, cells were incubated in the presence or absence of increasing doses of glucose for 6 days throughout differentiation. Cultures were divided into four treatment groups: Untreated (phenol red-free α-MEM supplemented with 2% fetal calf serum), differentiation medium (Control), differentiation medium with 10–25 mM D(+)glucose (high D-Glc) or differentiation medium with 10–25 mM L(−)glucose (high L-Glc) used as an osmotic control. Addition of glucose (10–25 mM) to the alpha-MEM culture medium led to a final concentration of 15.5 to 30.5 mM. Medium was changed every 2 days.
Tartrate-resistant acid phosphatase (TRACP) assay and cytochemistry
TRACP activity was assayed according to standard methods using p-nitrophenyl phosphate as a substrate [34]. Cell lysates were prepared and samples were incubated in buffer solution containing 125mM sodium acetate buffer, pH 5.2, 100mM p-nitrophenyl phosphate (Sigma-Aldrich) and 1mM L(+) sodium tartrate. The p-nitrophenol liberated was determined in 96-well plates by measuring the absorbance at 405 nm at 37°C. Protein amounts were quantified using the Bicinchoninic Acid Kit (Sigma-Aldrich) to determine the specific activity. Data is expressed as the mean optical density (O.D.)/min/mg protein. In parallel experiments, cellular TRACP activity was analyzed using the Leukocyte Acid Phosphatase kit (Sigma-Aldrich). Briefly, the cells were washed twice with PBS, fixed for 5 minutes with citrate/paraformaldehyde/acetone solution and stained according to the manufacturer’s instructions.
RNA extraction and RT-PCR
Alterations in expression of CTR, cathepsin K, MMP-9 and MMP-14 were examined by RT-PCR. Total RNA was isolated from RAW264.7 cells using TRIzol reagent (GIBCO BRL) and treated with DNase I (1 U/μg) to remove any contaminating genomic DNA. First-strand cDNA was reverse-transcribed from 2 μg of total RNA using Murine Moloney Leukemia Virus-Reverse Transcriptase, RNAsine, olig-dT and dNTP mix (Invitrogen) according to manufacturer’s protocol. Two microliters of RT reaction mixture were subjected to PCR using specific primers (30 pmol each, Table 1) and TaqPlatinum Supermix (Invitrogen) in a final volume of 50 μl. Primers for 18S were used to ascertain that an equivalent amount of cDNA was synthesized. PCR amplification was performed over a range of cycle numbers to ensure that amplification was in the linear range of the curve. PCR products were analyzed in 1% agarose gels, stained with ethidium bromide and photographed. The density of each band was measured using ImageJ software (NIH, Bethesda, MD, USA). The densities of osteoclast marker mRNA bands were normalized relative to those of 18S mRNA bands. Data are expressed as relative mRNA expression where the ratio of the CTR, cathepsin K, MMP9 or MMP-14 band density in control cultures to the corresponding 18S signal was arbitrarily set at 1.
Table 1.
Primer sequences and PCR conditions.
| Genes | Strand | Sequences | Size | Cycles | T°C |
|---|---|---|---|---|---|
| CTR | + | 5′ tgctatgaccggattcatca 3′ | 383 | 35 | 55 |
| − | 5′ gtcaccctctggcagctaag 3′ | ||||
| Cathepsin K | + | 5′ cttccaatacgtgcagcaga 3′ | 382 | 20 | 60 |
| − | 5′ ccgagccaagagagcatatc 3′ | ||||
| MMP-9 | + | 5′ ccaccacaactgaaccacag 3′ | 356 | 30 | 60 |
| − | 5′ agtaaggaaggggccctgta 3′ | ||||
| MMP-14 | + | 5′ ggcctggaacattctaacga 3′ | 331 | 30 | 60 |
| − | 5′ gcattgggtatccatccatc 3′ | ||||
| 18S | + | 5′ gaccataaacgatgccgact 3′ | 389 | 20 | 60 |
| − | 5′ gaacgccacttgtccctcta 3′ | ||||
Measurement of intracellular reactive oxygen species
Dichlorofluorescein diacetate fluorescence
ROS generation was determined using the peroxide-sensitive probe 2′7′-dichlorofluorescein diacetate (DCF-DA) dye as we have previously described [35]. DCF-DA is a membrane-permeable dye that diffuses across lipid membranes and is subsequently converted into a membrane-impermeable form, DCF, by deacetylation. The non-fluorescent DCF is oxidized by intracellular ROS and forms the highly fluorescent DCF. The effect of glucose on ROS production during differentiation was analyzed in cultures incubated in differentiation medium for 6 days in the presence or absence of glucose. To determine whether glucose alters ROS production in mature osteoclast cells, RAW264.7 cells, incubated in differentiation medium alone for 6 days, were either treated on not treated with glucose for 24 hr. After washing in pre-warmed serum-free medium without phenol red, cells were incubated with 1 μM of DCF-DA for 30 min at 37°C, 5% CO2, and then washed in PBS to remove excess dye. ROS levels were determined by analyzing the fluorescence intensity in random microscopic fields (10–20X magnification) using an inverted fluorescent microscope. The mean values were calculated using ImageJ software and data are expressed as relative fluorescence/pixel.
Dihdroethidium fluorescence
Superoxide anion concentrations within cells were monitored by measuring the changes in fluorescence resulting from the oxidation of dihydroethidium (DHE) (Invitrogen/Molecular Probes). DHE can enter the cell and be oxidized by superoxide to yield ethidium (Eth), which binds to DNA to produce bright red fluorescence. The increase in Eth-DNA fluorescence is suggestive of superoxide production within cells. Cells were loaded with 50 uM DHE for 10–30 min at 37°C as described [36]. Fluorescence was monitored by laser confocal fluorescence microscopy.
Cell transfection and NF-κB-dependent luciferase activity
RAW264.7 cells were transfected after four days of culture with 80ng/cm2 of NF-κBRE/pGL3 Basic-luciferase vector using Fugene HD transfection reagent (Roche) according to published methods [37]. Cell lysates were prepared on day 6 and luciferase activity was quantified using the Luciferase assay kit (Promega). To analyze the effect of hydrogen peroxide on NF-kB-dependent luciferase activity, cells were incubated with or without H2O2 (100μM) on day 5 for 24 hours prior to harvesting for luciferase activity. In separate experiments, cells were incubated for 6 days in the presence or absence of RANKL (50ng/ml), with or without 25 mM L-Glc or D-Glc. For each condition, data are expressed as relative light units/mg protein.
Caspase-3 activity assay
The CaspACE™ assay system (Promega) was used to quantify caspase-3 activity in cell lysates following the manufacturer’s protocol. Briefly, RAW264.7 or BMM cells were harvested after six days in culture, washed with ice-cold PBS and lysed in ice-cold lysis buffer (NaCl 150 mM, Tris 50 mM, Nonidet P-40 1%, sodium deoxycholate 0.25%, NaF 1 mM, NaVO4 1 mM, leupeptine 10 μg/ml, aprotinin 10 μg/ml, PMSF 0.5 mM). For each treatment group, an equal amount of soluble protein was incubated with 50 μM acetyl-Asp-Glu-Val-Asp 7-amino-4-methyl coumarin (Promega), a fluorogenic substrate for caspase-3, with or without 50 μM acetyl-Asp-Glu-Val-Asp aldehyde, a specific caspase-3 inhibitor (Promega). Cell lysates were pre-incubated with the inhibitor for 30 min prior to adding the substrate and cleavage of the substrate was analyzed using a fluorometer excitation wavelength 360 nm, detection wavelength 460 nm. Caspase-3 activity in high L-Glc and high D-Glc-treated cultures are expressed as percent of the control that was arbitrarily set at 100%.
Cell migration assay
RAW264.7 cells were seeded into 6-well plates at 1×105 cells/well and incubated in differentiation medium with or without high L-Glc and D-Glc. On day 6, monolayers were disrupted (wounded) by scraping with a 1000-μL pipette tip that resulted in an approximately 500-μm cell-free lane. Cells were then washed with serum-free αMEM and incubated for an additional 6 days under the same conditions used during the first six days of culture. The medium was changed every 2 days and photographic images were taken on day 6, immediately after wounding, and on day 12. Cell migration was quantified by counting the number of cells present in the wounded area per field on day 12.
Statistical analysis
Results were analyzed by one-way ANOVA Newman-Keuls’ test using Prism4 software (Graphpad). Data represent the mean +/− S.E. of three separate experiments. Significance was determined as probability (p)<0.05.
RESULTS
High D-Glc inhibits RANKL-induced TRACP activity
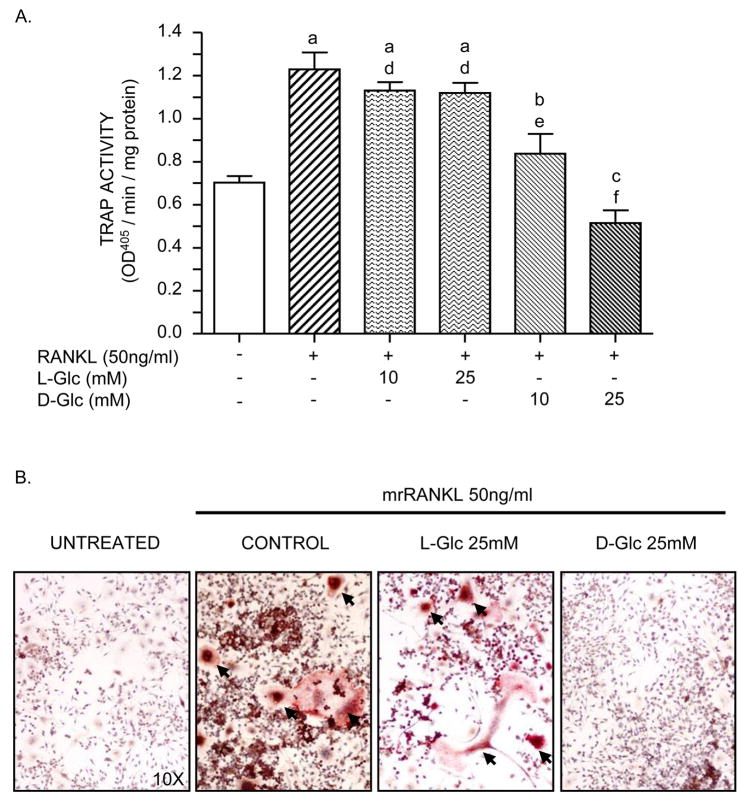
To determine the effect of sustained high glucose levels on osteoclastogenesis, RAW264.7 cells, incubated in differentiation medium, were exposed to 10–25mM of D(+)glucose or L(−)glucose (used as an osmotic control) for 6 days. Figure 1A shows that TRACP specific activity increased 2-fold when cells were differentiated in the presence of RANKL alone. This increase in TRACP activity was reduced by the addition of 10mM of D-Glc to the culture medium and was abolished in presence of the highest concentration of D-Glc (25mM; high D-Glc). Unlike D-glucose, TRACP activity was similar to control when cells were treated with either 10mM or 25mM of L-Glc. To confirm the inhibitory effect of D-Glc on RANKL-induced TRACP activity at the cellular level, cells were incubated in differentiation medium with an optimal concentration (25 mM) of L-Glc or D-Glc and stained for TRACP. Figure 1B shows that TRACP-positive multinucleated osteoclast-like cells formed in control cultures. In contrast, cultures exposed to high D-Glc showed a profound decrease in TRACP-positive multinucleated cells and a predominance of mononuclear cells. High L-Glc had no significant effect on TRACP-positive multinucleated cell formation compared to control cultures. Significant osteoclast formation was not identified in cultures incubated in medium without RANKL (Untreated).
Figure 1. Effect of glucose on TRACP activity.
RAW264.7 cells were seeded at a density of 1×104 cells/cm2 and incubated 6 days in: alpha-MEM, 2% FCS (untreated), differentiation medium (RANKL 50ng/ml; control), differentiation medium with L-Glc or differentiation medium with D-Glc (10mM and 25 mM in A; 25 mM in B). The medium was changed every 2 days. A) TRACP assay. TRACP activity was assayed using p-nitrophenyl phosphate as a substrate and the optical density at 405 nm was determined by spectrophometry (100μl final). B) TRACP histochemical stain. Cells were fixed and stained to identify TRACP-positive multinucleated cells (arrows). Photomicrographs of TRAP stained cultures are representative of three independent experiments. Values are means +/− S.E. of three independent experiments. a (p)<0.001, b (p)<0.05, c (p)>0.05 vs untreated cells; d (p)>0.05, e (p)<0.01, f (p)< 0.001 vs control cells.
High D-Glc inhibits RANKL-induced osteoclast differentiation
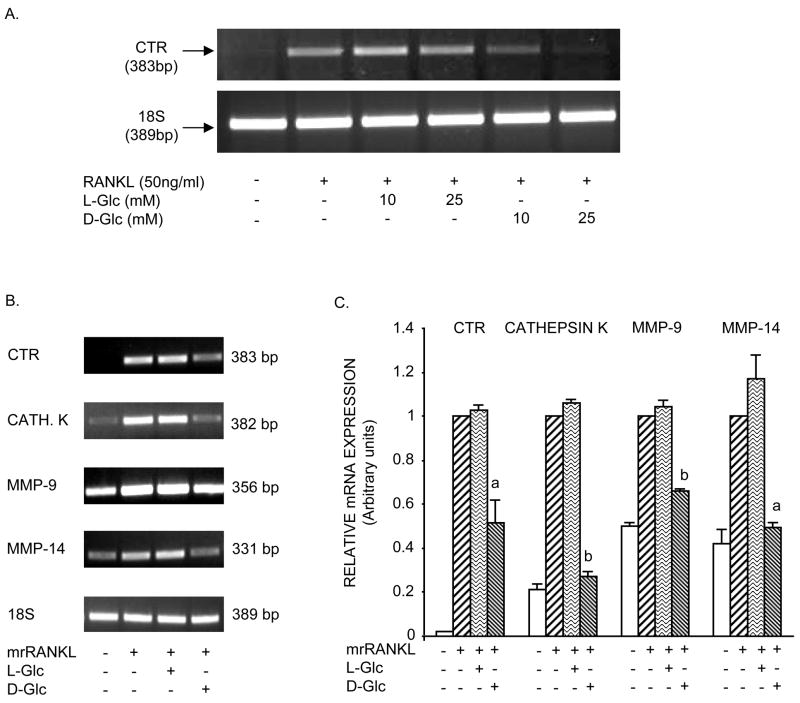
To examine the effect of high glucose on osteoclast differentiation, cells were analyzed by RT-PCR for expression of osteoclast markers. Figure 2A shows that RANKL increased CTR transcripts, a reliable marker of mature osteoclasts, in RAW264.7cells at day 6. This effect was partially to completely inhibited by the addition of 10mM and 25mM of D-glucose, respectively. In contrast, cultures incubated with either 10mM or 25mM of L-Glc (osmotic control) showed no inhibition of RANKL-induced CTR expression. Using an optimal dose of D-glucose (25mM), RT-PCR analysis was extended to include cathepsin K, MMP-9 and MMP-14. Figure 2B shows, that in addition to CTR, cathepsin K, MMP-9 and MMP-14 expressed by mature osteoclasts, were also up-regulated in response to RANKL compared to untreated cells. Exposure of cells to high D-Glc, markedly inhibited RANKL-induced osteoclast differentiation as demonstrated by reduced expression of CTR and cathepsin K. MMP-14 and, to a lesser extent, MMP-9 also declined in the presence of high D-Glc. Semi-quantitative RT-PCR analysis shown in Figure 2C confirmed that high D-Glc significantly decreased RANKL-induced CTR, cathepsin K, MMP-9 and MMP-14 mRNAs approximately 50%, 70%, 35% and 55%, respectively, whereas exposure of cells to high L-Glc did not have a significant effect on transcript levels. These data correlate with the effect of D-Glc and L-Glc on TRACP-positive multinucleated cell formation (Figure 1). The decline in osteoclast formation in the presence of high D-Glc, but not high L-Glc (osmotic control), indicates a metabolic effect of D-Glc on osteoclastogenesis.
Figure 2. Effect of glucose on expression of osteoclast markers.
A) Cells were incubated under the same conditions described in Figure 1A. Total RNA was extracted and subjected to RT-PCR for CTR. B) Cells were incubated under the same conditions described in Figure 1B. RT-PCR for CTR, cathepsin K, MMP-9 and MMP-14. Representative results of RT-PCR analysis were obtained from three independent experiments. C) Semi-quantitative analysis of osteoclast marker expression shown in B. Values are means +/− S.E. of three independent experiments. a (p)<0.05; b (p)<0.001 vs control cells or L-Glc-treated cells.
High D-Glc inhibits ROS production in RAW264.7 cultures
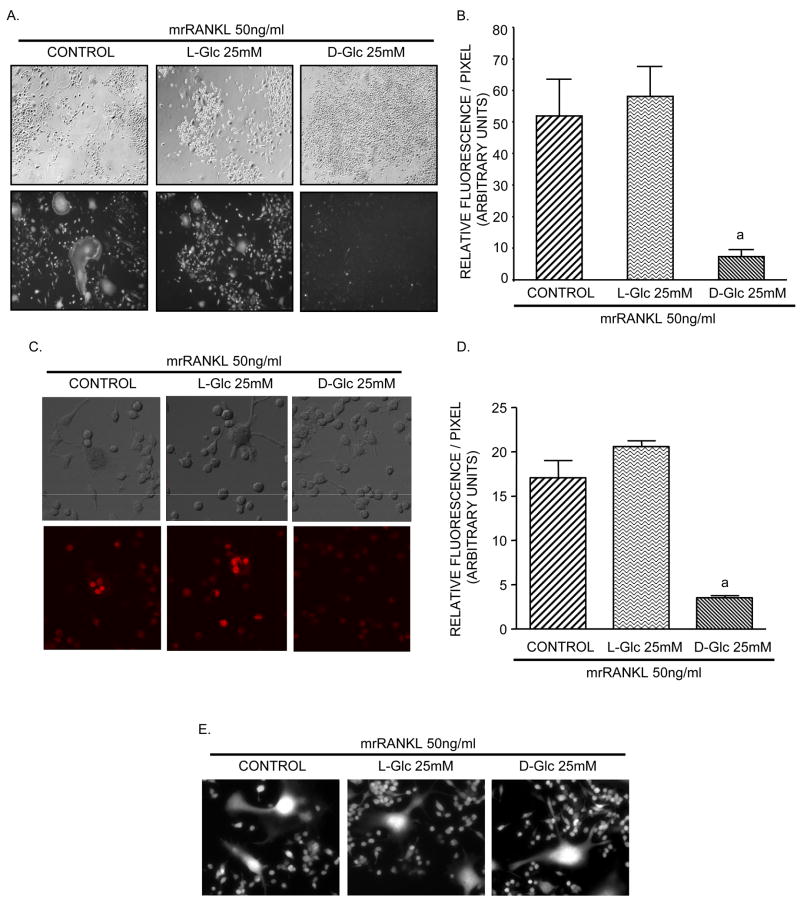
ROS are important for stimulating osteoclast differentiation and function [21–24]. The effect of high glucose on intracellular ROS production in RAW264.7 cultures was analyzed using the peroxide-sensitive DCF-DA probe. To determine the effect of glucose on ROS production during osteoclast differentiation, cells were incubated in differentiation medium in the presence or absence of 25 mM D-Glc or L-Glc for 6 days. The phase contrast and corresponding fluorescent images of these cultures is shown in Figure 3A. In cultures incubated with RANKL alone, abundant DCF-DA fluorescence was detected in multinucleated osteoclast-like cells and mononuclear cells. In high D-Glc-treated cultures, the fluorescent signal was markedly reduced, correlating with decreased multinucleated osteoclast-like cells, and little or no fluorescence was identified in the mononuclear cells. High L-Glc did not significantly alter DCF-DA fluorescence compared to control. Quantification of DCF-DA fluorescence (Figure 3B) correlated with the pattern of fluorescent signals visualized in Panel A. Compared to control, high D-Glc decreased ROS levels by 90%, whereas high L-Glc had no effect on ROS production. Alternatively, in cultures incubated under the same conditions shown in Panel A, we evaluated ROS generation using the superoxide-sensitive fluorophore dihydroethidium (DHE). Similar to DCF-DA, exposure of the cells to 25 mM D-Glc, but not L-Glc, caused a decrease in DHE fluorescence (Figures 3C and 3D). In separate experiments, the effect of glucose on ROS production in differentiated osteoclast cultures was also assessed. Figure 3E shows that mature osteoclasts incubated with RANKL alone for 6 days have abundant ROS production and that treatment of these cells with high L-Glc or D-Glc for 24 hrs did not significantly alter the production of ROS.
Figure 3. Effect of glucose on ROS production and NF-κB-dependent luciferase activity.
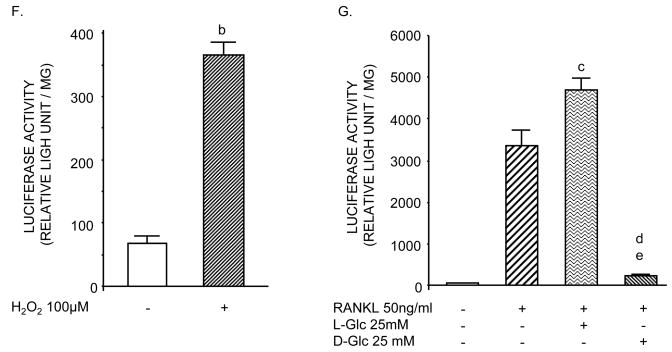
Analysis of ROS production. A–D) RAW264.7 cells were seeded into chamber slides and incubated in differentiation medium with or without 25 mM L-Glc or D-Glc for 6 days or E) incubated in differentiation medium alone for 6 days and then treated or not treated with 25 mM L-Glc or D-Glc for 24 hr. A,C) Phase contrast and corresponding DCF-DA fluorescence (A) and DHE fluorescence (C) of cultures. ROS production was visualized at 10–20X magnification using inverted fluorescent optic microscope (A) or with a confocal laser scanning fluorescence microscope (C). Photomicrographs are representative of three independent experiments. B,D) Quantification of DCF-DA or DHE fluorescence intensity shown in A and C, respectively. Values are means +/− S.E. of three independent experiments. a (p)<0.001 vs control cells or L-Glc-treated cells. NF-κB-dependent luciferase activity in RAW264.7 cells. Cells were transfected after 4 days of culture with 80 ng/cm2 of NF-κB-RE/pGL3 Basic-luciferase vector and luciferase activity was assayed on day 6. F) Analysis of luciferase activity after H2O2 treatment for 24 hr. G) Analysis of luciferase activity in cultures treated for 6 days with or without 25 mM L-Glu or D-Glc. Values are means +/− S.E. of three independent experiments. b (p)<0.001 vs untreated cells, c (p)<0.01 vs control cells; d (p)<0.001 vs control cells or L-Glc-treated cells; e (p)>0.05 vs untreated cells.
High D-Glc alters RANKL-induced NF-κB activity
To examine the mechanism by which high D-Glc may alter osteoclast differentiation, the effect of H2O2, RANKL and glucose on NF-κB transcriptional activity was analyzed. RAW264.7 cells were transiently transfected with a vector containing NF-κB response elements linked to the luciferase reporter gene. To test NF-κB sensitivity to ROS, cells incubated in alpha-MEM, 2% FCS for 5 days were treated or not treated with 100μM Hydrogen peroxide (H2O2) for 24 hours prior to harvesting for luciferase activity. Figure 3F shows that H2O2 caused an 8-fold increase in luciferase activity compared to untreated cells, indicating that ROS directly affects NF-κB activity. When cells were incubated under differentiation conditions shown in Figure 3G, RANKL increased luciferase activity 30-fold compared to untreated cultures. In contrast, high D-Glc was a potent inhibitor of RANKL-induced NF-κB transcriptional activity as shown by decreased luciferase activity. The osmotic control high L-Glc only slightly enhanced luciferase activity. Taken together, these data suggest that D-Glc may exert its inhibitory action on NF-κB via blockade of intracellular oxidant release.
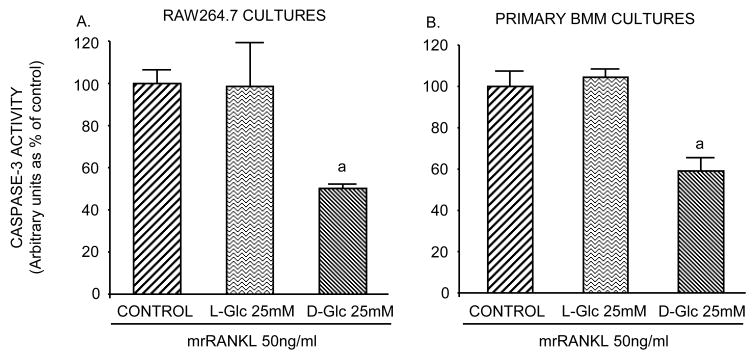
High D-Glc decreases caspase-3 activity
To determine whether glucose alters RANKL-induced caspase-3 activity during differentiation, RAW264.7 and BMM cells were analyzed for caspase-3 activity after incubation with RANKL for 6 days in the presence and absence of high L-Glc or D-Glc. Figure 4A and B show that caspase-3 activity declined approximately 40 to 50% in cultures containing high D-Glc, whereas activity in high L-Glc-treated cultures was similar to control. These data suggest that, in addition to blockade of intracellular oxidant release, high D-Glc may inhibit RANKL-induced osteoclast formation by decreasing caspase-3 activity through a metabolic pathway.
Figure 4. Effect of glucose on caspase-3 activity.
Cells were seeded in 6 well/plates (1×105 and 5×105 cells/well for RAW264.7 cells and BMM, respectively) and incubated in differentiation medium with or without 25 mM L-Glc or D-Glc. A) Measurement of caspase-3 activity. On day 6, cells were washed with ice-cold PBS, and lysed in ice-cold lysis buffer. For each condition, an equal amount of soluble protein was incubated with 50 μM of a caspase fluorogenic substrate, with or without 50 μM of a specific caspase-3 inhibitor. The cleavage of substrates was analyzed by a fluorometer at 460 nm. Values are means +/− S.E. of three independent experiments. a (p)<0.05 vs control or L-Glc-treated cells.
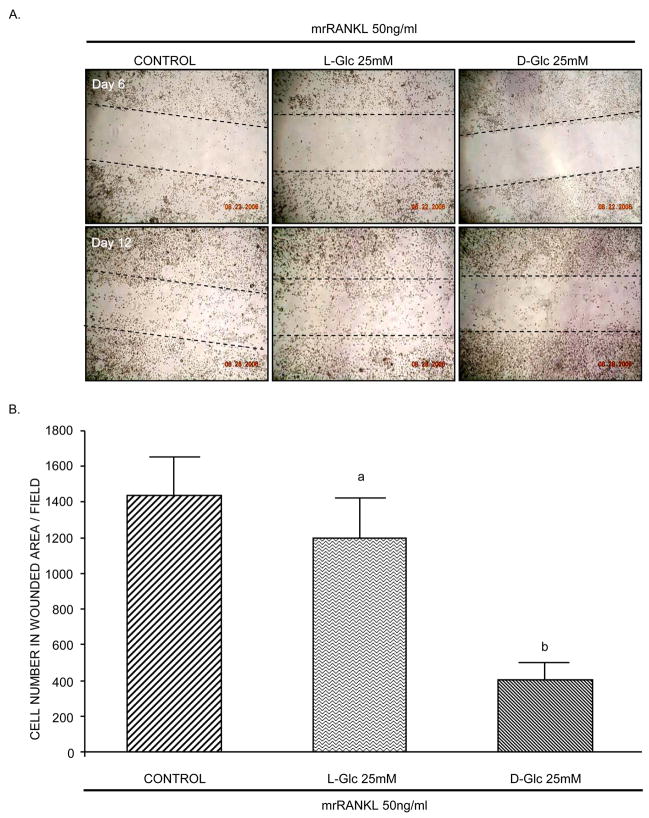
High D-Glc reduces cell migration
The effect of high glucose on cellular function was analyzed using a migration assay. RAW264.7 cells were incubated under differentiation conditions and tested for their ability to migrate into a wounded area (Figure 5A). In control cultures, cells migrated into and covered the site within 6 days after wounding. In high D-Glc-treated cultures, however, fewer cells migrated into the wound site and the number of cells covering the wound was decreased by approximately 65% compared to control cultures. Migration was not significantly altered by high L-Glc. Cells were also increased in the unwounded area in high D-Glc cultures, likely due to their inability to migrate.
Figure 5. Effect of glucose on cell migration.
A) RAW264.7 cells were seeded at a density of 1×104 cells/cm2 and incubated with mrRANKL (50ng/ml) for 12 days in the presence or absence of high L-Glc or high D-Glc. The medium was changed every 2 days. On day 6, cell monolayers were wounded, washed three times with serum-free α-MEM medium and continued for 6 additional days. Photomicrographs are representative of three independent experiments. B) Cells present in wounded area were counted on day 12. Values are means +/− S.E. of three independent experiments. a (p)>0.05 vs control; b (p)<0.001 vs control or L-Glc-treated cells.
DISCUSSION
The present study provides the first evidence that high D-Glc inhibits RANKL-mediated signaling events that lead to osteoclast differentiation and function. Incubation of osteoclast progenitor cells with high D-Glc inhibited TRACP activity and CTR expression in a dose-dependent manner. These findings correlated with inhibition of multinucleated osteoclast cell formation as demonstrated by decreased TRACP staining, cathepsin K, MMP-9 and MMP-14 expression. This effect occurred with high D-Glc, but not with the osmotic control (high L-Glc), and was associated with a decrease in ROS production, NF-κB transcriptional activity, caspase-3 and migration capacity. These findings are novel and contribute to define a mechanism for the altered bone turnover in diabetes that, in turn, may protect patients from early bone loss or lead to increased bone mass in type 2 diabetes.
Bone mass is tightly regulated by osteoclastic and osteoblastic bone remodeling. The contribution of glucose to bone remodeling in vivo is poorly understood. It is also unclear whether hyperglycemia has a role in maintaining normal bone mass in some cases of diabetes, and may protect against bone loss during early stages of the disease [9]. Although the role of glucose in osteoblast differentiation and mineralization has been examined [10–14], little is known regarding its effect on osteoclast differentiation and function. Our findings indicate that high D-Glc inhibits osteoclastogenesis, likely by preventing the formation of pre-osteoclast cells capable of fusing into multinucleated osteoclasts. RAW264.7 cultures incubated with high D-Glc showed a profound reduction in TRACP activity and TRACP-positive multinucleated osteoclast-like cells compared to controls. RT-PCR analysis of high D-Glc-treated cultures confirmed a substantial decrease in CTR and cathepsin K mRNAs, markers of the mature osteoclast phenotype.
ROS including superoxide anion and hydrogen peroxide (H2O2) have been recognized as major intermediaries in the formation and activation of osteoclasts in vitro and in vivo [21,22,28]. Recent studies indicate that RANKL-mediated ROS production serves to regulate RANKL signaling pathways required for osteoclast differentiation [24,27]. In RAW264.7 and BMM precursor cells, RANKL increases ROS, whereas expression of catalase in BMM cells blocks RANKL-induced ROS production and inhibits the formation of TRACP+ osteoclasts [28]. Our findings show that RANKL-treated cultures have abundant DCF fluorescence intensity, indicating oxidation by intracellular ROS and, in particular, peroxide such as H2O2. In contrast, high D-Glc markedly inhibited ROS generation. Similar results were obtained with DHE, an indicator of intracellular superoxide generation. These effects correlated with the decreased formation of multinucleated osteoclast-like cells in the TRACP assays and likely contributed to the inability of these cells to differentiate in response to RANKL. Interestingly, in cultures differentiated with RANKL for 6 days, ROS generation in mature osteoclasts was not altered by the addition of high D-Glc. These findings suggest that, perhaps, D-Glc may affect osteoclast precursors undergoing differentiation to a greater extent than mature osteoclasts. In diabetes, high glucose enhances oxidative stress within cells and tissues [2,38,39]. It is intriguing that in osteoclasts, high D-Glc functions as an antioxidant factor by suppressing ROS production rather than triggering oxidant production. To the best of our knowledge this is the first example of such antioxidative action of D-Glc.
Previous studies have shown that ROS act as mediators in RANKL-induced signaling pathways that involve NF-κB. For example, pretreatment of osteoclasts with antioxidants reduced RANKL-induced NF-κB, Akt/Protein Kinase B and ERK activation [27]. In our studies, RAW264.7 cells transfected with a vector containing NF-κB response elements showed the expected induction of reporter gene activity in response to H2O2 and RANKL. Incubation of the transfected cells with H2O2 directly stimulated reporter activity, demonstrating that the activity of the transcription factor is redox-sensitive. In high D-Glc-treated cells, RANKL-induced NF-κB responsiveness declined to near basal levels observed in untreated cultures, whereas little effect was seen in L-Glc-treated cultures. Taken together, these data suggest that the decline in RANKL-induced NF-κB activity by high D-Glc may be due, in part, to the reduction in ROS production observed under high D-Glc conditions.
Recent studies have shown that caspase-3 is required for RANKL-mediated osteoclast differentiation [29]. RANKL induces cleavage of procaspase-3 to caspase-3 and increases caspase-3 activity that, in turn, activates NF-κB. Szymczyk et al.[29] showed that RAW264.7 procaspase-3 knockdown cells fail to form mature TRACP+ osteoclasts and remain mononuclear. We observed that high D-Glc inhibited caspase-3 activity in response to RANKL in RAW264.7 and BMM cells and this correlated with a predominance of mononuclear cells in cultures and decreased activation of NF-κB. The combined decreased of caspase-3 and ROS by high D-Glc, both of which converge on NF-κB, may contribute to the high D-Glc-mediated inhibition of RANKL-induced NF-κB activity.
MMPs are important for normal bone development and remodeling. MMP-9 expression by pre-OCs is obligatory for their migration to the developing bone marrow cavity [40]. During differentiation, RANKL increases MMP-9 that facilitates migration of osteoclastic cells toward the bone surface and initiation of bone resorption [41,42]. Cell culture and animal studies have shown that high glucose is a potent inhibitor of MMPs [43]. Our findings indicate that RANKL increases MMP-9 [44] and MMP-14 mRNA expression in RAW264.7 cultures during osteoclast formation and that high D-Glc but not high L-Glc inhibited this effect. Inhibition of MMPs by high D-Glc correlated with its effect on cell migration. In RAW264.7 cultures incubated with RANKL or RANKL plus high L-Glc, cells migrated into the wounded area, whereas cells incubated with RANKL plus high D-Glc showed impaired migration. In addition, cell proliferation was enhanced by D-Glc whereas it was slightly reduced by L-Glu (data not shown), indicating that the inhibitory effect of D-Glc on migration is not related to an inhibition of cell proliferation. It is tempting to speculate that high levels of extracellular glucose in diabetes increases a pool of defective osteoclast progenitors with decreased MMP expression that, in turn, prevents their migration within the marrow to sites for osteoclast differentiation and bone resorption.
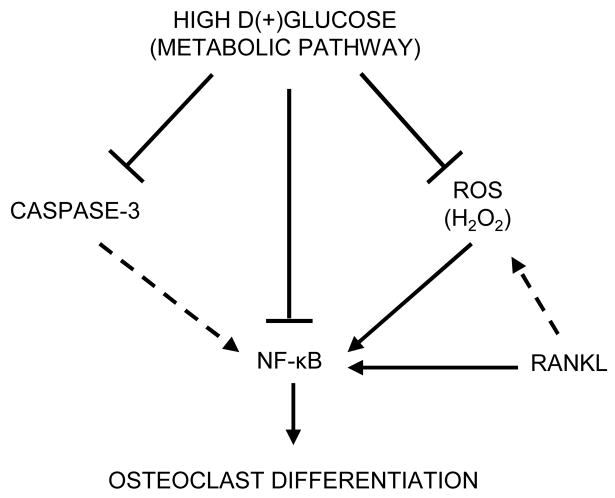
In summary, this study indicates for the first time, that high D-Glc acts through a metabolic pathway to inhibit RANKL-mediated osteoclast differentiation and function (Figure 6). Our data suggest that high D-Glc interferes with RANKL signaling by reducing ROS production, caspase-3 and NF-κB activation. Recently, advanced glycation end products (AGES) that accumulate with aging in bone tissues of diabetics have been identified as inhibitors of osteoclast formation and resorption [45]. Taken together with our findings, it is possible that high glucose and AGES may act in concert to prevent excessive osteoclast-mediated bone loss in some cases of diabetes. The precise factors that participate in the development of bone loss in diabetes requires further study. Our data provide new insight into the biologic effects of glucose on osteoclastogenesis and may suggest novel therapeutic strategies designed to enhance skeletal integrity in diabetes.
Figure 6. Hypothesis of potential mechanism(s) involved in high D(+) glucose-mediated inhibition of osteoclastogenesis.
—— Data from our study – – - Data from literature
Acknowledgments
This work was supported in part by funding from NIH (AR-42306, S.W.) and Veteran’s Administration Merit Award (S.W.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Y Wittrant, Email: wittrant@uthscsa.edu.
Y Gorin, Email: gorin@uthscsa.edu.
K Woodruff, Email: woodruff@uthscsa.edu.
D Horn, Email: hornd@uthscsa.edu.
HE Abboud, Email: abboud@uthscsa.edu.
S Mohan, Email: mohan@uthscsa.edu.
SL Abboud-Werner, Email: abboudwerner@uthscsa.edu.
References
- 1.Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 2.Brownlee M. Biochemistry and molecular cell biology diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 3.Hui SL, Epstein S, Johnston CC., Jr A prospective study of bone mass in patients with type I diabetes. J Clin Endocrinol Metab. 1985;60:74–80. doi: 10.1210/jcem-60-1-74. [DOI] [PubMed] [Google Scholar]
- 4.Ziegler R. Diabetes mellitus and bone metabolism. Horm Metab Res Suppl. 1992;26:90–4. [PubMed] [Google Scholar]
- 5.Kemink SA, Hermus AR, Swinkels LM, Lutterman JA, Smals AG. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J Endocrinol Invest. 2000;23:295–303. doi: 10.1007/BF03343726. [DOI] [PubMed] [Google Scholar]
- 6.Thrailkill KM, Lumpkin CK Jr, Bunn RC, Kemp SF, Fowlkes JL. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab. 2005;289:E735–45. doi: 10.1152/ajpendo.00159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes meta-analysis. Osteoporos Int. 2007;4:427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 8.Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. 2007;148:198–205. doi: 10.1210/en.2006-1006. [DOI] [PubMed] [Google Scholar]
- 9.Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. 2005;146:3622–31. doi: 10.1210/en.2004-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serrano S, Marinoso ML, Nacher M, Torres A, Cuevas X, Loreta J, Munne A, Diez A. Modulation of osteoblast activity by serum from diabetic and non-diabetic patients on hemodialysis: a three-dimensional culture study. J Nephrol. 2004;17:369–76. [PubMed] [Google Scholar]
- 11.Balint E, Szabo P, Marshall CF, Sprague SM. Glucose-induced inhibition of in vitro bone mineralization. Bone. 2001;28:21–8. doi: 10.1016/s8756-3282(00)00426-9. [DOI] [PubMed] [Google Scholar]
- 12.Terada M, Inaba M, Yano Y, Hasuma T, Nishizawa Y, Morii H, Otani S. Growth-inhibitory effect of a high glucose concentration on osteoblast-like cells. Bone. 1998;22:17–23. doi: 10.1016/s8756-3282(97)00220-2. [DOI] [PubMed] [Google Scholar]
- 13.Zayzafoon M, Stell C, Irwin R, McCabe LR. Extracellular glucose influences osteoblast differentiation and c-Jun expression. J Cell Biochem. 2000;79:301–10. doi: 10.1002/1097-4644(20001101)79:2<301::aid-jcb130>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Botolin S, McCabe LR. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem. 2006;99:411–24. doi: 10.1002/jcb.20842. [DOI] [PubMed] [Google Scholar]
- 15.Williams JP, Blair HC, McDonald JM, McKenna MA, Jordan SE, Williford J, Hardy RW. Regulation of osteoclastic bone resorption by glucose. Biochem Biophys Res Commun. 1997;235:646–51. doi: 10.1006/bbrc.1997.6795. [DOI] [PubMed] [Google Scholar]
- 16.Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13:66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- 17.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;402:304–9. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 18.Burgess TL, Qian Y, Kaufman S, Ring RD, Van G, Capparelli C, Kelley M, Hsu H, Boyle WJ, Dunstan C, Hsu S, Lacey DL. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol. 1999;145:527–38. doi: 10.1083/jcb.145.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess TL, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 20.Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XY, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A. 1999;96:3540–5. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990;85:632–639. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suda N, Morita I, Kuroda T, Murota S. Participation of oxidative stress in the process of osteoclast differentiation. Biochim Biophys Acta. 1993;1157:318–23. doi: 10.1016/0304-4165(93)90116-p. [DOI] [PubMed] [Google Scholar]
- 23.Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou ZP, Zeng WS, Cheng BL, Luo SQ. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J Biol Chem. 2005;280:17497–506. doi: 10.1074/jbc.M409332200. [DOI] [PubMed] [Google Scholar]
- 24.Koh JM, Lee YS, Kim YS, Kim DJ, Kim HH, Park JY, Lee KU, Kim GS. Homocysteine enhances bone resorption by stimulation of osteoclast formation and activity through increased intracellular ROS generation. J Bone Miner Res. 2006;21:1003–11. doi: 10.1359/jbmr.060406. [DOI] [PubMed] [Google Scholar]
- 25.Garg AK, Aggarwal BB. Reactive oxygen intermediates in TNF signaling. Mol Immunol. 2002;39:509–17. doi: 10.1016/s0161-5890(02)00207-9. [DOI] [PubMed] [Google Scholar]
- 26.Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J Clin Invest. 2003;111:769–78. doi: 10.1172/JCI18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim HH, Lee ZH. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res. 2004;301:119–27. doi: 10.1016/j.yexcr.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–9. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 29.Szymczyk KH, Freeman TA, Adams CS, Srinivas V, Steinbeck MJ. Active caspase-3 is required for osteoclast differentiation. J Cell Physiol. 2006;209:836–44. doi: 10.1002/jcp.20770. [DOI] [PubMed] [Google Scholar]
- 30.Tezuka K, Tezuka Y, Maejima A, Sato T, Nemoto K, Kamioka H, Hakeda Y, Kumegawa M. Molecular cloning of a possible cysteine proteinase predominantly expressed in osteoclasts. J Biol Chem. 1994;269:1106–9. [PubMed] [Google Scholar]
- 31.Delaissé JM, Andersen TL, Engsig MT, Henriksen K, Troen T, Blavier L. Matrix metalloproteinases (MMP) and cathepsin K contribute differently to osteoclastic activities. Microsc Res Tech. 2003;61:504–13. doi: 10.1002/jemt.10374. [DOI] [PubMed] [Google Scholar]
- 32.Sato T, Foged NT, Delaisse JM. The migration of purified osteoclasts through collagen is inhibited by matrix metalloproteinase inhibitors. J Bone Miner Res. 1998;13:59–66. doi: 10.1359/jbmr.1998.13.1.59. [DOI] [PubMed] [Google Scholar]
- 33.Wittrant Y, Theoleyre S, Couillaud S, Dunstan C, Heymann D, Redini F. Relevance of an in vitro osteoclastogenesis system to study receptor activator of NF-kB ligand and osteoprotegerin biological activities. Exp Cell Res. 2004;293:292–301. doi: 10.1016/j.yexcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Ek-Rylander B, Barkhem T, Ljusberg J, Ohman L, Andersson KK, Andersson G. Comparative studies of rat recombinant purple acid phosphatase and bone tartrate-resistant acid phosphatase. Biochem J. 1997;15:305–11. doi: 10.1042/bj3210305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorin Y, Kim NH, Feliers D, Bhandari B, Choudhury GG, Abboud HE. Angiotensin II activates Akt/protein kinase B by an arachidonic acid/redox-dependent pathway and independent of phosphoinositide 3-kinase. FASEB J. 2001;15:1909–20. doi: 10.1096/fj..01-0165com. [DOI] [PubMed] [Google Scholar]
- 36.Block K, Ricono JM, Lee D-Y, Bhandari B, Choudhury GG, Abboud HE, Gorin Y. Arachidonic acid-dependent activation of a p22phox-based NAD(P)H oxidase mediates angiotensin II-induced mesangial cell protein synthesis and fibronectin expression via Akt/PKB. Antioxid Redox Signal. 2006;8:1497–1508. doi: 10.1089/ars.2006.8.1497. [DOI] [PubMed] [Google Scholar]
- 37.Mohan S, Hamuro M, Sorescu GP, Koyoma K, Sprague EA, Jo H, Valente AJ, Prihoda TJ, Natarajan M. IkappaBalpha-dependent regulation of low-shear flow-induced NF-kappa B activity: role of nitric oxide. Am J Physiol Cell Physiol. 2003;284:1039–47. doi: 10.1152/ajpcell.00464.2001. [DOI] [PubMed] [Google Scholar]
- 38.Callaghan MJ, Ceradini DJ, Gurtner GC. Hyperglycemia-induced reactive oxygen species and impaired endothelial progenitor cell function. Antioxid Redox Signal. 2005;7:1476–82. doi: 10.1089/ars.2005.7.1476. [DOI] [PubMed] [Google Scholar]
- 39.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–26. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 40.Blavier L, Delaisse JM. Matrix metalloproteinases are obligatory for the migration of preosteoclasts to the developing marrow cavity of primitive long bones. J Cell Sci. 1995;108:3649–59. doi: 10.1242/jcs.108.12.3649. [DOI] [PubMed] [Google Scholar]
- 41.Yu X, Huang Y, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J Bone Miner Res. 2003;18:1404–18. doi: 10.1359/jbmr.2003.18.8.1404. [DOI] [PubMed] [Google Scholar]
- 42.Ishibashi O, Niwa S, Kadoyama K, Inui T. MMP-9 antisense oligodeoxynucleotide exerts an inhibitory effect on osteoclastic bone resorption by suppressing cell migration. Life Sciences. 2006;79:1657–60. doi: 10.1016/j.lfs.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 43.McLennan SV, Fisher E, Martell SY, Death AK, Williams PF, Lyons JG, Yue DK. Effects of glucose on matrix metalloproteinase and plasmin activities in mesangial cells: possible role in diabetic nephropathy. Kidney Int. 2000;77:S81–87. doi: 10.1046/j.1523-1755.2000.07713.x. [DOI] [PubMed] [Google Scholar]
- 44.Wittrant Y, Theoleyre S, Couillaud S, Dunstan C, Heymann D, Redini F. Regulation of osteoclast protease expression by RANKL. Biochem Biophys Res Commun. 2003;310:774–8. doi: 10.1016/j.bbrc.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 45.Valcourt U, Merle B, Gineyts E, Viguet-Carrin S, Delmas PD, Garnero P. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J Biol Chem. 2007;282:5691–5703. doi: 10.1074/jbc.M610536200. [DOI] [PubMed] [Google Scholar]