Abstract
Background
Arterial grafts have patency rates superior to venous grafts in patients undergoing coronary bypass grafting surgery. Natriuretic peptides play a major role in vascular homeostasis. We hypothesized that natriuretic peptides might have different effects on arterial and venous conduits.
Methods
The relaxation responses and tissue levels of cyclic guanosine monophosphate (cGMP) after exposure to atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide were assessed in segments of internal mammary artery, radial artery, and saphenous vein obtained from the same patients at the time of bypass surgery (n = 12). Natriuretic peptide receptor (NPR) expression was assessed using immunohistochemistry and Western blotting.
Results
Relaxation of the internal mammary artery and radial artery to all the natriuretic peptides were similar, and greater than that of saphenous vein, correlating with increased tissue levels of cGMP in both arterial conduits. Relaxation responses to all three natriuretic peptides were nearly abolished in the presence of LY83583, an inhibitor of guanylyl cyclase. Exposure of the conduits to N(G)-Nitro-L-arginine methyl ester (nitric oxide synthase inhibitor) resulted in a modest but significant blunting of the relaxation responses. Expression of NPRA, NPRB and NPRC was strong in the endothelium and vascular smooth muscle layer of the internal mammary artery and radial artery, and was significantly less in saphenous vein.
Conclusions
Natriuretic peptides are potent vasodilators of the internal mammary artery and radial artery but not the saphenous vein. The relaxation response is mediated through guanylyl cyclase and nitric oxide synthase. These observations may provide additional insight into the mechanisms that account for superior patency of arterial conduits.
Long-term patency of arterial conduits used for coronary artery bypass surgery is superior to venous conduits, translating into reduced cardiacrelated events and improved survival [1, 2]. Graft thrombosis, intimal hyperplasia, and accelerated atherosclerosis are the principal mechanisms underlying saphenous graft failure [3, 4]. Natriuretic peptides are a family of endogenous hormones with numerous targets, including vascular smooth muscle and endothelial cells, and play a major role in controlling vascular homeostasis [5, 6].
Natriuretic peptides exert their effects by high-affinity binding to natriuretic protein receptors (NPRs) [7, 8]. Both atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) bind to NPRA receptor while C-type natriuretic peptide (CNP) binds selectively to NPRB. In vascular smooth muscle cells, interaction with either NPRA or B activates guanylyl cyclase, leading to increased levels of cyclic guanosine monophosphate (cGMP), which, in turn, causes relaxation and suppresses proliferation. The NPRC, also called the clearance receptor, shares extracellular domains similar to the other NPRs but has no catalytic domain. The primary function of NPRC is to capture and degrade natriuretic peptides, binding to all three natriuretic peptides with high affinity [7, 9]. Evidence in animal models suggests that activation of natriuretic peptides and their receptors results in a series of events including inhibition of adenylyl cyclase, mitogen-activated protein kinase, and inhibition of vascular smooth muscle cell proliferation [8, 10]. Reduced levels of natriuretic peptides and their receptors were shown to be associated with increased atherosclerotic plaque load [11]. In addition, natriuretic peptides secreted from endothelial cells have been shown to stimulate phospholipase C, as well as to suppress expression of P-selectin and endothelial nitric oxide (NO) synthase, and inhibit leukocyte adhesion and platelet aggregation [8, 12].
Given their importance as inhibitors of intimal proliferation, thrombosis, and other processes that contribute to graft failure, we hypothesized that natriuretic peptides might have lesser effects on venous conduits compared with arterial conduits. In the present study, we sought to characterize the effects of ANP, BNP, and CNP and the expression of NPRs in commonly used coronary artery bypass conduits, including internal mammary artery, radial artery, and saphenous vein.
Patients and Methods
Patients
Viable segments of internal mammary artery, radial artery, and saphenous vein were obtained from the same patients (n = 12) undergoing first-time, non-emergent, coronary artery bypass graft operation without concomitant valve surgery. The study was approved by the Boston Medical Center Institutional Review Board and written informed consent was obtained from all patients.
Specimen Collection
To preserve the optimal viability of conduits, extra care was taken to maintain the vessel integrity. Segments of the freshly dissected conduits were immediately placed in ice-cold Krebs-Henseleit (KH) solution containing (in mmol/L) NaCl (118.3), KCl (4.0), MgSO4.7H20 (1.2), KH2PO4 (1.2), Na2EDTA.2H2O (0.3), CaCl2.2H2O (2.5), NaHCO3 (25.0), and D-(+)-Glucose (11.1). Distention of the conduits, especially the saphenous vein was avoided. To ensure the viability of each conduit, particularly the saphenous vein given its fragile endothelial layer and thin smooth muscle layer, we tested every conduit for its viability with repeated cycles of contraction and relaxation response as detailed below.
Organ Chamber Methodology
Vessels were divided into 3- to 4-mm segments that were suspended between two stainless steel stirrups in 25-mL organ chamber baths (Radnoti, Monrovia, CA) filled with KH solution (pH 7.4, 37°C) and constantly aerated with 5% CO2/95% O2 to allow measurement of vessel contractile and relaxation response. After a 20-minute adaptation period, each vessel segment was stretched to approximately 20% of optimal resting tension, to produce a maximal contractile response to 2.0 M KCl. The time frame was based on repeated experiments to confirm that they were no longer spontaneously contracting or relaxing. Vessels were washed thrice to completely remove the KCl. After 30 minutes, vessels were precontracted with U46619 (a thromboxane-A2 analog) to achieve 60% to 70% of maximal KCl-induced contraction and exposed to increasing doses of the three natriuretic peptides to create dose-response relaxation curves. All experiments were performed in the presence of 10 μmol/L indomethacin to inhibit prostanoid synthesis. Dose-response curves to human ANP, BNP, or CNP (human ANP [1-28] and BNP [1-3] was purchased from Calbiochem, San Diego, CA; human CNP was purchased from Scy Tek Lab, Logan, UT) were performed in the presence and absence of the NO synthase inhibitor N(G)-Nitro-L-arginine methyl ester (L-NAME [1-6 M]) and the guanylyl-cyclase inhibitor LY-83583 (10-6 M [Calbiochem]).
Tissue Levels of cGMP
Vessel segments were exposed to submaximal doses of ANP, BNP, and CNP (10-7 M) and snap frozen in liquid N2. Samples were then homogenized using a Polytron homogenizer (Kinematica, Lucerne, Switzerland) in ice-cold 5% trichloroacetic acid solution. Supernatant was subjected to cGMP measurement using the Cyclic GMP ELISA kit, according to the manufacture’s protocol (Cayman Chemicals, Ann Arbor, MI).
Immunohistochemistry
Five-micron-thick sections were cut from formalin-fixed, paraffin-embedded vessel tissue, placed on slides, and dried for 1 hour at 60°C. Tissue samples were then deparaffinized using two changes of xylene for 2 minutes each followed by hydration in 100% and 95% ethyl alcohol (two changes of 1 minute each), and then washed in tap water for 1 minute. Slides were placed in hydrogen peroxide/methanol solution for 15 minutes at room temperature to quench endogenous peroxidases, and then washed for 1 minute in tap water. Tissue was then washed in deionized water for 1 minute. Antigen retrieval was accomplished by microwaving the slides in citrate buffer. The slides were then placed in a Biogenix I-6000 machine (Biogenix, Ramsey, MN) for incubation with the primary anti-NPRA, NPRB, and NPRC antibodies. One slide from each sample was used for staining each primary antibody. Each primary antibody was diluted in antibody dilutent at a concentration of 1:200; tissue plus antibody slides were incubated at room temperature for 30 minutes. To achieve secondary antibody staining, slides were washed in phosphate-buffered saline (PBS) and incubated at room temperature with the StrAvi-Gen Multilink Biotinylated Kit (Biogenix). The staining was revealed using goat anti-rat biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) at a dilution of 1:200 with an incubation time of 1 hour at 37°C. After 1X PBS wash, sections were incubated with ABC reagent (Vector Laboratories) for 30 minutes at 37°C; Vector DAB substrate was used to develop the brown positive signal by incubating the sections for 5 minutes. Sections were counterstained with Shandon Gill 3 Hematoxylin (Thermo Electron Corp, Waltham, MA). Intensity of staining was assessed using Image-Pro software (Media Cybernetics, Bethesda, MD).
Western Blot Analysis
Vessels were collected in radioimmunoprecipitation buffer, homogenized on ice for 5 minutes, vortexed, and cleared by centrifugation in a microcentrifuge at 14,000 rpm for 8 minutes at 4°C. Proteins were subjected to Western blot analysis (100 μg/lane) as previously described [13]. To avoid excessive background, a polyvinylidene difluroide membrane was used and blocked in 7% milk (Carnation nonfat dry milk) for at least 1 hour at room temperature or overnight at 4°C if antibodies yielded a high background. To reprobe the membrane, mild striping of antibodies was performed by shaking the membrane in 1X PBS containing 10% Tween. Expression of NPR was examined with monoclonal anti-NPRA, anti-NPRB, and Anti-NPRC immunoglobulin G antibodies (Santa Cruz Biotech, Santa Cruz, CA).
Statistical Analysis
Data are expressed as mean ± SEM. Dose-response curves to each natriuretic peptide on each vessel were constructed. Maximal contractile response (Emax) and effective drug concentration yielding 50% relaxation (EC50) were derived from dose-response curves using commercially available software (Origin version 7.5; Northampton, MA).
Dose-response curves were compared using two-way repeated measures analysis of variance. One-way analysis of variance was used to compare Emax and EC50 among vessels or natriuretic peptides as well as tissue cGMP levels and NPR expression. A p value of less than 0.05 was considered significant. Statistical analyses were performed using SigmaStat version 3.1 (Point Richmond, CA) software. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Study Subjects
The clinical profile of the enrolled study subjects is depicted in Table 1. By definition, all patients had coronary artery disease, and as expected, there was a high prevalence of cardiovascular disease risk factors.
Table 1.
Baseline Patient Profile
| Characteristics | n = 12 |
|---|---|
| Age, years | 67.5 ± 5.3 |
| Male (%) | 7 (58) |
| Body mass index, kg/m2 | 29.7 ± 6.2 |
| Prior myocardial infarction (%) | 7 (58) |
| Family history of coronary artery disease (%) | 7 (58) |
| Hypertension (%) | 9 (75) |
| Hypercholesterolemia (%) | 11 (92) |
| Diabetes mellitus (%) | 7 (58) |
| History of cerebrovascular disease (%) | 1 (8) |
| Smoking (%) | 5 (42) |
| Chronic obstructive pulmonary disease (%) | 3 (25) |
| Chronic renal failure (%) | 2 (16) |
| Peripheral vascular disease (%) | 2 (16) |
| Total cholesterol (mg/dL) | 129.7 ± 36.8 |
| High-density lipoprotein (mg/dL) | 34.8 ± 9.7 |
| Low-density lipoprotein (mg/dL) | 71.7 ± 13.4 |
| Triglycerides (mg/dL) | 127.6 ± 35.5 |
| Aspirin (%) | 10 (83) |
| Statins (%) | 10 (83) |
| Angiotensin-converting enzyme inhibitor (%) | 5 (42) |
Relaxation Response of Internal Mammary Artery, Radial Artery, and Saphenous Vein to the Three Natriuretic Peptides
After the viability of each conduit was confirmed by their contractile and relaxation responses, we proceeded to evaluating their response to natriuretic peptides. The overall relaxation response of the internal mammary artery, radial artery, and saphenous vein to the three natriuretic peptides is depicted in Figure 1, and maximal responses and EC50 values are displayed in Table 2. As shown, relaxation responses of the internal mammary artery and radial artery to natriuretic peptides were similar, and both were significantly greater than that of saphenous vein. To validate the underlying signaling pathway in the NP-mediated relaxation, we preincubated the conduits with LY-83583 (guanylyl-cyclase inhibitor) or L-NAME for 20 minutes before treating them with NPs. Natriuretic peptides-induced relaxation of all three vessels was markedly inhibited by a maximal dose of the guanylyl-cyclase inhibitor LY-83583 (Fig 2). A lesser (yet significant) degree inhibition of natriuretic peptide-induced vessel relaxation was observed in the presence of the NO synthase inhibitor L-NAME (Fig 3).
Fig 1.
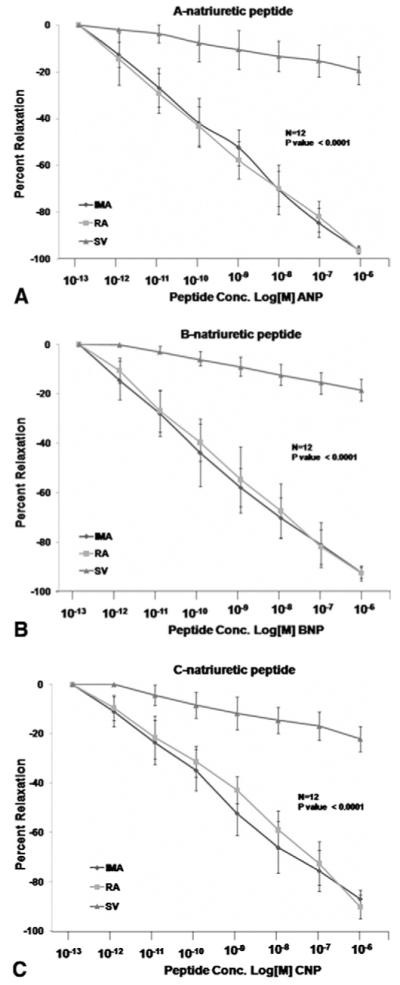
Effect of natriuretic peptides (NP) on conduit vasomotor tone. Segments of internal mammary artery, radial artery, and saphenous vein were precontracted with U46619 and exposed to the indicated concentrations of (A) atrial NP (ANP), (B) brain NP (BNP), and (C) C-type NP (CNP). Relaxation response of the internal mammary artery and radial artery are similar and significantly greater than that of saphenous vein.
Table 2.
Conduit Relaxation Response to Natriuretic Peptides (n = 12)
| Maximal Relaxation (%) | ||||
|---|---|---|---|---|
| Internal Mammary Artery | Radial Artery | Saphenous Vein | p Value | |
| ANP | 96.1 ± 1.6 | 96.5 ± 1.4 | 9.5 ± 3.4 | < 0.0001 |
| ANP + LY83583 | 11.9 ± 2.4 | 16.3 ± 5.0 | 5.6 ± 2.0 | < 0.0001 |
| ANP + L-NAME | 46.6 ± 2.4 | 45.5 ± 6.0 | 11.9 ± 2.0 | < 0.0005 |
| BNP | 92.4 ± 2.2 | 92.5 ± 2.9 | 18.6 ± 4.3 | < 0.0001 |
| BNP + LY83583 | 13.0 ± 4.0 | 11.7 ± 2.2 | 7.0 ± 3.1 | < 0.0005 |
| BNP + L-NAME | 55.9 ± 4.0 | 42.6 ± 2.2 | 11.9 ± 3.6 | < 0.0001 |
| CNP | 86.9 ± 3.5 | 90.1 ± 4.7 | 22.1 ± 5.0 | < 0.0001 |
| CNP + LY83583 | 10.6 ± 2.9 | 12.4 ± 3.8 | 5.9 ± 1.8 | < 0.0005 |
| CNP + L-NAME | 43.1 ± 2.9 | 40.0 ± 3.8 | 11.6 ± 1.7 | < 0.0005 |
| EC50(log[M]) | ||||
| ANP | -8.9 ± 0.8 | -9.9 ± 0.1 | -9.9 ± 0.5 | < 0.005 |
| BNP | -8.6 ± 0.6 | -9.2 ± 0.5 | -9.8 ± 0.7 | < 0.001 |
| CNP | -8.8 ± 0.5 | -9.6 ± 0.4 | -9.7 ± 0.8 | < 0.005 |
ANP = atrial natriuretic peptide; B = brain natriuretic peptide; C = C-type natriuretic peptide; EC50 = drug concentration producing 50% of maximal relaxation; L-NAME = N(G)-Nitro-L-arginine methyl ester.
Fig 2.
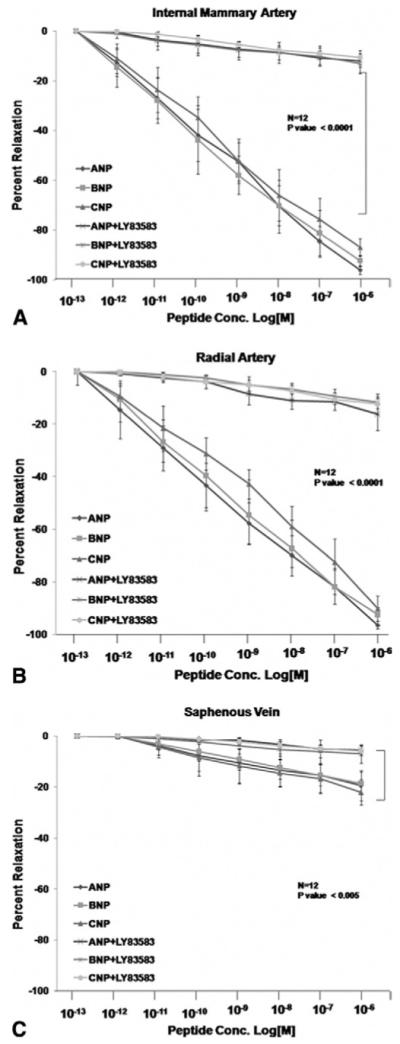
Contribution of cyclic guanosine monophosphate (cGMP) to the natriuretic peptide (NP) response. Segments of internal mammary artery (A), radial artery (B), and saphenous vein (C) were precontracted with U46619 and exposed to the indicated concentrations of ANP, BNP, and CNP in the presence of the guanylyl cyclase inhibitor LY-83583. Relaxation response of all three vessels to the three natriuretic peptides were nearly abolished by LY-83583.
Fig 3.
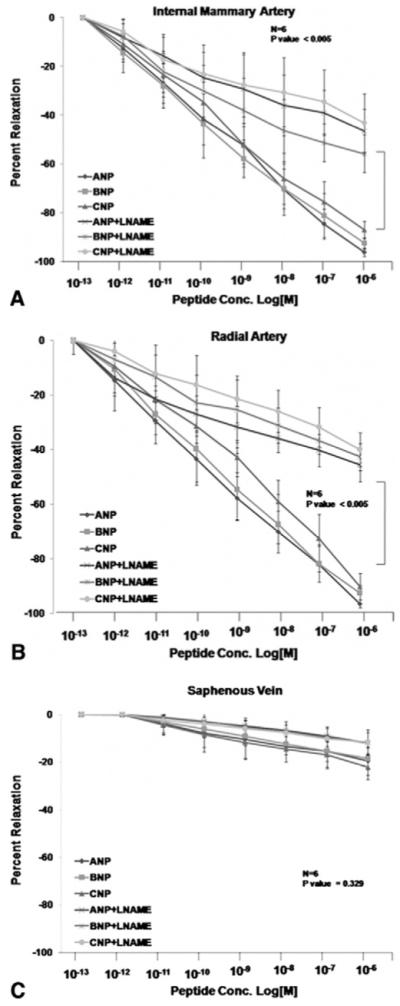
Contribution of nitric oxide synthase to the natriuretic protein response. Segments of internal mammary artery (A), radial artery (B), and saphenous vein (C) were precontracted with U46619 and exposed to the indicated concentrations of natriuretic peptides A, B, or CNP in the presence of the nitric oxide synthase inhibitor L-nitro arginine methyl ester (L-NAME). L-NAME induced modest (yet significant) inhibition of the relaxation response to natriuretic peptides.
Tissue Levels of cGMP in Response to Natriuretic Peptides
To examine the mechanism of natriuretic peptide-induced vessel relaxation, we measured the tissue levels of cGMP in response to submaximal doses of ANP, BNP, and CNP (10-7 M). Tissue levels of cGMP were significantly higher in internal mammary artery and radial artery compared with saphenous vein (Table 3).
Table 3.
Effect of Natriuretic Peptides on Tissue Cyclic Guanosine Monophosphate (cGMP) Levels (n = 8) and Natriuretic Peptide Receptor (NPR) Expression (n = 6)
| Internal Mammary Artery | Radial Artery | Saphenous Vein | p Value | |
|---|---|---|---|---|
| cGMP level, pmole/ug protein |
||||
| ANP | 2.5 | 4.9 | 0.6 | < 0.0001 |
| BNP | 2.7 | 2.5 | 0.7 | < 0.0001 |
| CNP | 1.5 | 1.8 | 0.3 | 0.0098 |
| NPR expression, normalized arbitrary unit |
||||
| NPRA | 92.4 | 73.2 | 4.8 | < 0.0001 |
| NPRB | 10.4 | 23.1 | 20.5 | < 0.0001 |
| NPRC | 86.9 | 57.7 | 3.3 | < 0.0001 |
Cyclic GMP levels were quantified directly from tissue homogenates. Equal segments of internal mammary artery, radial artery, and saphenous vein were obtained from the same patient, immersed in Krebs-Henseleit solution, aerated with 5% CO2/95% O2, and precontracted with U46619 in organ chamber for 30 minutes. Vessels were then exposed to a submaximal dose (10-7 M) of atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP) inducing 60% to 70% of maximal relaxation. Expression of NPR was assessed by quantitative immunohistochemistry as described.
Immunohistochemistry of NPRs in Internal Mammary Artery, Radial Artery, and Saphenous Vein>
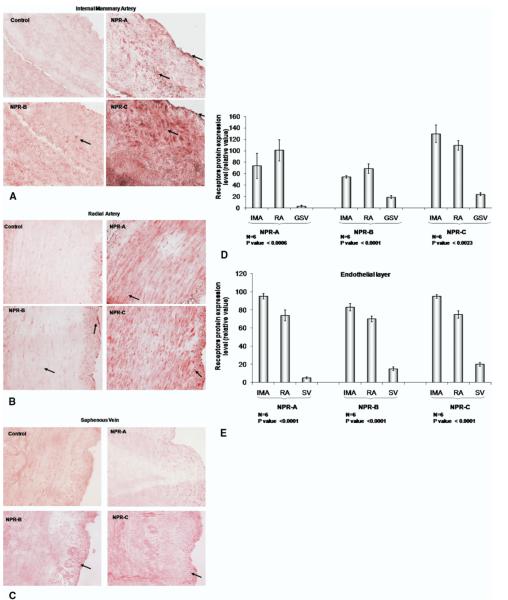
Expression of NPRA, NPRB, and NPRC was readily detected in the vascular smooth muscle layer and the endothelium of the internal mammary artery and the radial artery (Fig 4A and B), and very weakly detected in saphenous vein (Fig 4C). Quantitative assessment of NPRs in the endothelium and smooth muscle layers of vessels using densitometry is depicted in Figure 4, panel D and E. As shown, NPR expression was significantly greater in both the endothelium and smooth muscle layers of the arterial conduits compared with saphenous vein.
Fig 4.
Immunohistochemistry staining for natriuretic protein receptors (NPRs). Panels A, B, and C show representative segments of internal mammary artery (IMA), radial artery (RA), and saphenous vein (SV) obtained from the same patient and stained for NPRs. Arrows indicate NPR staining. Panels D and E show quantification analysis of NPRs in the whole section and in the endothelial layer, respectively.
Western Blot Analysis of NPRs in Internal Mammary Artery, Radial Artery, and Saphenous Vein
Two representative examples of Western blot analysis of the NPRs in each vessel are depicted in Figure 5. The receptors NPRA and NPRB were detected at approximately 130 to 180 kDa whereas NPRC was detected as a monomer around 66 kDa. The receptors NPRAand NPRC were highly expressed in the internal mammary artery and radial artery, and much less in saphenous vein. The receptor NPRB was less abundant in all three vessels.
Fig 5.
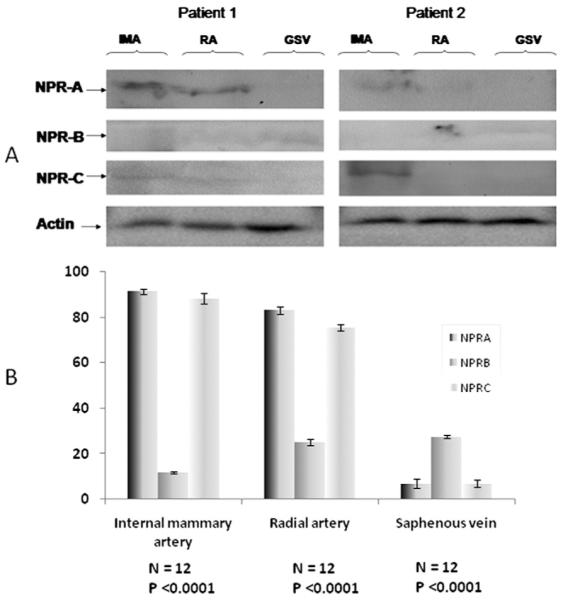
Western blotting analysis (A) and densitometer reading (B) of natriuretic protein receptor (NPR) expression in the vessels wall, in 2 representative patients. (GSV = greater saphenous vein; IMA = internal mammary artery; NPR-A = dark shaded bars; NPR-B = medium shaded bars; NPR-C = light shaded bars; RA = radial artery.)
Comment
The long-term patency rate of saphenous vein is significantly inferior to that of internal mammary artery and radial artery due to thrombosis, neointimal hyperplasia, and accelerated atherosclerosis [14]. The mechanisms underlying these processes are only partially understood. Recent animal and human studies show an association between natriuretic peptide expression and reduced smooth muscle cell proliferation, neointima formation, and atherosclerosis plaque formation [15-17]. Thus, we hypothesized that the superior patency rates of arterial coronary bypass conduits compared with saphenous vein may be related to a different profile of the NPRs as well as to varied responses to natriuretic peptides among the conduits.
Data on the effects of natriuretic peptides and NPRs on human internal mammary artery and saphenous vein are limited, and our study has assessed the natriuretic peptides effect on the radial artery [18, 19]. To avoid many confounding effects, we studied only patients from whom all three vessels were obtained. Thus, each patient served as his or her own control. We demonstrated that the relaxation response of the internal mammary artery and radial artery to the three natriuretic peptides was similar, and significantly greater than that of saphenous vein. These responses were associated with higher tissue levels of cGMP in the arterial conduits compared with saphenous vein. Furthermore, immunohistochemistry and Western blot analysis showed that NPRs were highly expressed in both arterial conduits and significantly less in saphenous vein. Higher NPR levels were detected both in the endothelium and smooth muscle cell layers.
The role of natriuretic peptides and their receptors in the pathophysiology of atherosclerosis has been recently investigated by several authors [11, 16]. The common theme is that natriuretic peptides are protective against atherosclerosis affecting multiple vascular events [12, 20]. For example, Qian and colleagues [20] reported that local expression of CNP in balloon-injured carotid arteries suppresses inflammation, eliminates shear-stress-induced thrombosis, and prevents neointima formation. Alexander and colleagues [16] reported that lack of NPRA increases blood pressure, atherosclerotic lesion size, and aortic medial area in apolipoprotein E deficient mice. Thus, it is possible that higher expression of the NPR and augmented response to the natriuretic peptides in arterial conduits observed in our study could be one of the protective mechanisms against graft atherosclerosis. Finally, these observations could provide the basis for possible therapeutic options. The effect of transfection of saphenous vein with genes coding for CNP or other natriuretic peptides merits further investigation [15, 20].
The relaxation response of all three vessels to natriuretic peptides was nearly abolished by the guanylyl-cyclase inhibitor LY83583, confirming that the primary effect of natriuretic peptides is exerted by production cGMP as a second messenger [6, 8]. However, we also showed a clear interaction between the NO and the natriuretic peptides systems. Addition of the NO synthase inhibitor resulted in a modest but significant inhibition of vessel response to natriuretic peptides. These findings are consistent with prior studies suggesting cross-talk between natriuretic peptides, NPRs activation, and NO synthase activity.20-23 For example, Qian and colleagues [20] demonstrated that the antithrombotic and antiatherosclerotic effects of CNP were blocked by inhibition of NO synthase. Marumo and colleagues [23] showed that natriuretic peptides augment transcriptional activation of inducible NO synthase induced by inflammatory cytokines (a combination of interleukin-1 and tumor necrosis factor-alpha), and hence augment the production of NO.
The interaction between the natriuretic peptides and NO systems may be particularly important in the pathophysiology of accelerated graft atherosclerosis. Endothelial NO acts as a strong vasodilator; it inhibits platelet aggregation, leukocyte adhesion, and vascular smooth muscle proliferation and migration. As such, NO is a strong antithrombotic and antiatherosclerotic agent. Previous studies demonstrated enhanced NO production by arterial coronary bypass conduits compared with saphenous vein, and enhanced NO production has been hypothesized to be a major factor explaining the superior patency rates of arterial conduits compared with saphenous vein grafts [24, 25].
In summary, we demonstrate that natriuretic peptides are potent vasodilators of the internal mammary artery and radial artery but not the saphenous vein. Relaxation response to all three conduits is primarily mediated by NPRs and the cGMP pathway, but also involves activation of the NO system. The powerful vasodilating effects of ANP and BNP appear to be related, at least in part, to the high expression of NPR in the vascular smooth muscle and endothelial layers of the internal mammary artery and radial artery. These observations may provide additional insight to the observed greater patency rates of arterial conduits compared with saphenous vein, and may serve as potential therapeutic interventions.
Acknowledgments
This work was supported by a Boston University M. Yanai grant. Chey Collura was supported in part by the Boston University School of Medicine, Department of Graduate Medical Sciences. Doctor Vita is supported by grants from the National Institutes of Health (HL083269, HL083801, HL801587, HL78795) and the Boston University Leadership Program in Vascular Medicine (K12 HL083781). We thank Dr John Keaney and Tina Wu for technical support and insightful discussion.
Abbreviations and Acronyms
- ANP
atrial natriuretic peptide
- BNP
brain natriuretic peptide
- cGMP
cyclic guanosine monophosphate
- CNP
C-type natriuretic peptide
- EC50
effective drug concentration yielding 50% laxation
- Emax
maximal contractile response
- L-NAME
N(G)-Nitro-L-arginine methyl ester
- NO
nitric oxide
- NPR
natriuretic protein receptor
References
- 1.Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 2.Guru V, Fremes SE, Tu JV. How many arterial grafts are enough? A population-based study of midterm outcomes. J Thorac Cardiovasc Surg. 2006;131:1021–8. doi: 10.1016/j.jtcvs.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 3.Cox JL, Chiasson DA, Gotlieb AI. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34:45–68. doi: 10.1016/0033-0620(91)90019-i. [DOI] [PubMed] [Google Scholar]
- 4.Lie JT, Lawrie GM, Morris GC. Aortocoronary bypass saphenous vein graft atherosclerosis: anatomic study of 99 vein grafts from normal and hyperlipoproteinemic patients up to 75 months postoperatively. Am J Cardiol. 1977;40:906–14. doi: 10.1016/0002-9149(77)90041-8. [DOI] [PubMed] [Google Scholar]
- 5.D’Souza SP, Davis M, Baxter GF. Autocrine and paracrine actions of natriuretic peptides in the heart. Pharmacol Ther. 2004;101:113–29. doi: 10.1016/j.pharmthera.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Potter LO, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocrinol Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 7.Gardner DG, Chen S, Glenn DJ, et al. Molecular biology of the natriuretic peptide system: implications for physiology and hypertension. Hypertension. 2007;49:419–26. doi: 10.1161/01.HYP.0000258532.07418.fa. [DOI] [PubMed] [Google Scholar]
- 8.Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides. 2005;26:1044–59. doi: 10.1016/j.peptides.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Sandow SL, Tare M. C-type natriuretic peptide: a new endothelium-derived hyperpolarizing factor? Trends Pharmacol Sci. 2007;28:61–7. doi: 10.1016/j.tips.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Itoh H, Pratt RE, Dzau VJ. Atrial natriuretic polypeptide inhibits hypertrophy of vascular smooth muscle cells. J Clin Invest. 1990;86:1690–7. doi: 10.1172/JCI114893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casco VH, Veinot JP, Kuroski de Bold ML, Masters RG, Stevenson MM, de Bold AJ. Natriuretic peptide system gene expression in human coronary arteries. J Hystochem Cytochem. 2002;50:799–809. doi: 10.1177/002215540205000606. [DOI] [PubMed] [Google Scholar]
- 12.Scotland RS, Cohen M, Foster P, et al. C-type natriuretic peptide inhibits leukocyte recruitment and platelet-leukocyte interactions via suppression of P-selectin expression. Proc Natl Acad Sci USA. 2005;102:14452–7. doi: 10.1073/pnas.0504961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen HG, Chinnappan D, Urano T, et al. Mechanism of aurora-B degradation and its dependency on intact KEN and A-boxes: identification of an aneuploidy-promoting property. Mol Cell Biol. 2005;25:4977–92. doi: 10.1128/MCB.25.12.4977-4992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motawni JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–31. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda S, Kanna M, Sakuragi S, et al. Local delivery of single low-dose C-type natriuretic peptide, an endogenous vascular modulator, inhibits neointimal hyperplasia in a balloon-injured rabbit iliac artery model. J Cardiovasc Pharmacol. 2002;39:784–8. doi: 10.1097/00005344-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Alexander MR, Knowles JW, Nishikimi T, Maeda N. Increased atherosclerosis and smooth muscle cell hypertrophy in natriuretic peptide receptor A-/- apolipoprotein E-/- mice. Arterioscler Thromb Vasc Biol. 2003;23:1077–82. doi: 10.1161/01.ATV.0000071702.45741.2E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naruko T, Itoh A, Haze K, et al. C-type natriuretic peptide and natriuretic peptide receptors are expressed by smooth muscle cells in the neointima after percutaneous coronary intervention. Atherosclerosis. 2005;181:241–50. doi: 10.1016/j.atherosclerosis.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Bonatti J, Dichtl W, Dworzak EA, et al. Atrial natriuretic peptide-induced release of cyclic guanosine monophosphate by coronary bypass grafts. Ann Thorac Surg. 1998;65:1621–4. doi: 10.1016/s0003-4975(98)00267-7. [DOI] [PubMed] [Google Scholar]
- 19.Hammerer-Lercher A, Fersterer J, Holzmann H, et al. Direct comparison of relaxation and cGMP production in human coronary by-pass grafts in response to stimulation with natriuretic peptides and a nitric oxide donor. Clin Sci (Lond) 2006;111:225–31. doi: 10.1042/CS20060034. [DOI] [PubMed] [Google Scholar]
- 20.Qian JY, Haruno A, Asada Y, et al. Local expression of C-type natriuretic peptide suppresses inflammation, eliminates shear stress-induced thrombosis, and prevents neointima formation through enhanced nitric oxide production in rabbit injured carotid arteries. Circ Res. 2002;91:1063–9. doi: 10.1161/01.res.0000043631.25915.e6. [DOI] [PubMed] [Google Scholar]
- 21.Leskinen H, Vuolteenaho O, Leppäluoto J, Ruskoaho H. Role of nitric oxide on cardiac hormone secretion: effect of NG-nitro-L-arginine methyl ester on atrial natriuretic peptide and brain natriuretic peptide release. Endocrinology. 1995;136:1241–9. doi: 10.1210/endo.136.3.7867578. [DOI] [PubMed] [Google Scholar]
- 22.Brown C, Pan X, Hassid A. Nitric oxide and C-type atrial natriuretic peptide stimulate primary aortic smooth muscle cell migration via a cGMP-dependent mechanism: relationship to microfilament dissociation and altered cell morphology. Circ Res. 1999;84:655–67. doi: 10.1161/01.res.84.6.655. [DOI] [PubMed] [Google Scholar]
- 23.Marumo T, Nakaki T, Hishikawa K, et al. Natriuretic peptide-augmented induction of nitric oxide synthase through cyclic guanosine 3′,5′-monophosphate elevation in vascular smooth muscle cells. Endocrinol. 1995:2135–42. doi: 10.1210/endo.136.5.7536663. [DOI] [PubMed] [Google Scholar]
- 24.Shapira OM, Xu A, Aldea GS, Vita JA, Shenin RJ, Keaney JF. Enhanced nitric oxide-mediated vascular relaxation in radial artery compared with internal mammary artery or saphenous vein. Circulation. 1999;100(Suppl 2):322–7. doi: 10.1161/01.cir.100.suppl_2.ii-322. [DOI] [PubMed] [Google Scholar]
- 25.Luscher TF, Diederich D, Siebenmann R, et al. Difference between endothelium-dependent relaxation in arterial and venous coronary bypass grafts. N Engl J Med. 1988;319:462–7. doi: 10.1056/NEJM198808253190802. [DOI] [PubMed] [Google Scholar]