Abstract
Extracts of bovine corneal stroma have been shown to activate keratocytes in culture to proliferate. We fractionated stromal extract on a column of Sephacryl S-300 and tested the fractions for mitogenic activity using cell culture and for the presence of IGF-II and its binding protein IGFBP-2 by Western blot. We found that the mitogenic activity in the extract separated into major and minor peaks and that immunologically detectable IGF-II and IGFBP-2 co-eluted with the minor peak. We also compared the effects of 10 ng IGF-II/ml on keratocytes in culture to that of 2 ng TGF-β/ml over a 7-day culture period. We found that IGF-II and TGF-β, alone or combined, increased both 3H-thymidine incorporation and DNA content of the cultures. The phenotype of the cells was determined by using antibodies to α-SM (smooth muscle) actin, fibronectin, SPARC, lumican and keratocan in Western blots of cell layers of media. Keratocytes cultured in IGF-II expressed no α-SM actin or fibronectin, low levels of SPARC and high levels of lumican and keratocan, indicating a native phenotype. Keratocytes in TGF-β expressed α-SM actin, fibronectin, SPARC and lumican, and expressed no or low levels of keratocan, indicating a myofibroblast phenotype. Keratocytes cultured in IGF-II plus TGF-β, however, expressed α-SM actin. fibronectin. SPARC, lumican, and keratocan by day 7 of culture. The results of this study show that IGF-II to be present in the corneal stroma, to stimulate keratocyte proliferation while maintaining native phenotype and to override the TGF-β mediated down regulation of keratocan production. The IGF-II in the stroma may serve as a mechanism to immediately activate keratocytes upon wounding and to ameliorate the scarring effects of TGF-β.
Keywords: keratocyte, myofibroblast, IGF-II, TGF-β, proliferation, activation, cell culture
1. Introduction
The corneal stroma contains an extensive extracellular matrix consisting of alternating layers of collagen lamellae interspersed with keratocytes. The matrix consists primarily of collagen types I, III, IV, V, and IV (Schmut 1977; Birk et al. 1986; Cintron and Hong 1988; Cintron et al. 1988; Guerriero et al. 2007), and four leucine-rich proteoglycans: decorin (Li et al. 1992), which bears chondroitin sulfate chains, and lumican (Blochberger et al. 1992) keratocan, (Corpuz et al. 1996) and osteoglycin/mimecan (Funderburgh et al. 1997), which have keratan sulfate chains. These matrix components are found in other connective tissues but the cornea stroma is unusually rich in keratan sulfate proteoglycans. The keratocytes have a dendritic morphology (Muller et al. 1995) and high levels of crystalline proteins in their cytoplasm (Jester et al. 1999). During periods of homeostasis, they are relatively quiescent. When a corneal wound occurs, the keratocytes adjacent to the wounded site undergo apoptosis (Helena et al. 1998; Zieske et al. 2001), and some of the remaining keratocytes are activated to proliferate (Hanna et al. 1989; Del Pero et al. 1990; Zieske et al. 2001). The keratocytes become quiescent again when the cell void has been repopulated and the wound healed.
Keratocytes isolated from the stroma by collagenase digestion and cultured in serum free media retain their dendritic morphology and their quiescence (Jester et al. 1996; Beales et al. 1999) but can be activated to proliferate by a number of growth factors including, FGF(fibroblast growth factor)-2, PDGF(platelet derived growth factor), TGF(transforming growth factor)- β, IL(interleukin)-1-α, IGF(insulin like growth factor)-I and insulin (Long et al. 2000; Jester et al. 2002; Jester and Ho-Chang 2003; Musselmann et al. 2005). Antibodies to TGF-β have been shown to reduce keratocyte activation in corneal wounds (Jester et al. 1997) and this suggests that TGF-β is present in wounds and activates keratocytes in vivo as well.
We previously showed an extract of corneal stroma stimulated proliferation of keratocytes in culture and proposed that growth factors may normally be present in the stroma to activate the keratocytes that, upon corneal wounding, had lost cell-cell contact inhibition due to apoptosis of adjacent keratocytes (Musselmann et al. 2003). Arnold and colleagues (Arnold et al. 1993) have shown IGF-II and IGFBP(insulin like growth factor binding protein)-2 to be in the aqueous humor, a fluid immediately posterior to the cornea and that extracts of the cornea exhibit a 100 fold higher binding capacity for IGF-II than that of the iris/ciliary. In this report we show that antibodies to IGF-II and IGFBP-2 react by western blot with the stromal extract and with fractions of stromal extract containing mitogenic activity and that IGF-II stimulates keratocyte proliferation in vitro.
2. Materials and Methods
Reagents
All chemicals and growth factors were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. 3H-thymidine was obtained from Perkin Elmer (Boston, MA), polyacrylamide gels, electrophoresis solutions, nitrocellulose, Cyquant, and DMEM/F12 from Invitrogen (Carlsbad, CA), cell culture plates from Coring-Coster (Cambridge, MA), 10,000 MWCO spin concentrators from Amicon (Millipore Corp, Bedford, MA), endo-beta-galactosidase from Seikagaku Associates of Cape Cod (E> Falmouth, MA) and Sephacryl S-300 from GE healthcare (Piscataway, NJ).
Stromal extract preparation
The extract was prepared in similar fashion to the previously described method (Musselmann et al. 2003). In brief, epithelium and endothelium were removed from the corneas and the resulting stromas were frozen in liquid nitrogen. Frozen stromas were pulverized in a Waring blender pre-cooled to liquid nitrogen temperatures. Frozen stromal powder was weighed, added to DMEM/F12 (5mls/gram powder) and extracted by stirring at 4 degrees C for 4 hours. Insoluble material was removed by centrifugation in a Beckman 50.2 Ti rotor at 33,000 rpm at 4 degrees C for one hour. The resulting extract was filter-sterilized and stored at −80 degrees C as 100% extract.
Extract fractionation
Eighty ml of 100% extract was concentrated to twenty ml using centrifugual concentrators and applied to a column (5×40 cm) of Sephacryl S-300 that was equilibrated and eluted with DMEM/F12 at 5mls/minute. The eluant was monitored for absorbance at 280 nm, and 25 ml fractions were collected. Each fraction was filter-sterilized, and aliquots were either diluted to 10% with DMEM/F12 for use as culture medium or spun concentrated to one-tenth volume for analysis by SDS/PAGE.
Keratocyte isolation and culture
Eyes were obtained from one year old cows from Pel Freeze (Rogers, AR) and keratocytes were isolated from the corneas by using two sequential collagenase digestions as previously described (Berryhill et al. 2001). Cells were plated on day zero in DMEM/F12 in 6 well plates at 20,000-cells/square cm using 2 ml media/well. The cells were allowed to attach overnight at 37 degrees C in a humidified atmosphere containing 5% CO2. The medium was changed on days 1 and 4 to DMEM/F12 or to DMEM/F12 containing IGF-II (10ng/ml), TGF-beta (2ng/ml) or IGF-II and TGF-beta combined. The DMEM/F12 was supplemented with antibiotics and 1 mM 2-phospho-L-ascorbic acid. Cultures were harvested on days 1,4, and 7. Medium was removed from each well, cell layers were rinsed in PBS and the media and plates were stored frozen at −80C.
DNA measurements
The DNA content of the cells in each well was determined using a CyQuant kit according to the vendor’s (Invitrogen) instructions. DNA synthesis was determined in cultures that were radiolabeled with 740,000 Bq 3H-thymidine/ml of medium for 72 hours beginning on day 4. The incorporation of the radiolabel into DNA was determined as previously described (Musselmann et al. 2005).
Western Blot
Media harvested from four cultures was combined and spun concentrated to one twentieth of the original volume. The cells in each well were solublized in 150 microliters of 1x Sample Buffer (from invitrogen) and the extract from four wells were combined. Aliquots of media were digested with endo-beta-galactosidase according to the vendor’s (Cape Cod Associates) instructions for detection of lumican and keratocan core proteins by Western blot. The volume of the aliquots taken from the media at the day 4 and day 7 harvests of each of the four different culture conditions (i.e., control, IGF-II, TGF-beta, and IGF-II plus TGF-beta) was normalized to the DNA content obtained for the cell layers of those cultures or in the case of extracts of cell layers, the DNA content of parallel cultures. In this way it was possible to directly compare the amount of a particular protein produced by the same number of cells. SDS/PAGE of samples from the pooled media and cell layer extracts were run reduced as previously described (Musselmann et al. 2005) and Western blots were performed using the ECL Western blotting analysis system (GE Healthcare, Piscataway, NJ). Primary antibodies included: mouse monoclonal anti-α-SM actin (clone asm-1, Roche Molecular Biochemicals), rabbit anti-ALDH (provided by R. Lindahl, University of South Dakota), mouse monoclonal anti-fibronectin (clone TV-1, Millipore), rabbit anti-SPARC (provided by L. Fisher, NIDCR, NIH), rabbit anti-IGF-I (PAA1, Cell Sciences), rabbit anti-IGF-II (PA0382, Cell Sciences), rabbit anti-IGFBP-2 (US Biologicals). Rabbit antisera to bovine lumican and to bovine keratocan were previously described (Berryhill et al. 2001).
Statistics
Statistical analysis was performed with four determined values for each point using Statview (SAS Institute, Gary, NC). Data is expressed as the mean +/− standard error. Significant differences were determined by paired t-test. Western blots were obtained from three separate experiments and a representative one is shown.
3. Results
Stromal extract was concentrated to 1/10 volume and examined by SDS/PAGE Western blot with antibodies to IGF-II and IGFBP2 (Figure 1, lanes 3 and 5, respectively). An immunoreactive band was detected in the extract with each antiserum that corresponded to the migration position of purified IGF-II (lane 4) and IGFBP2 (lane 6) used as positive standards.
Fig. 1.
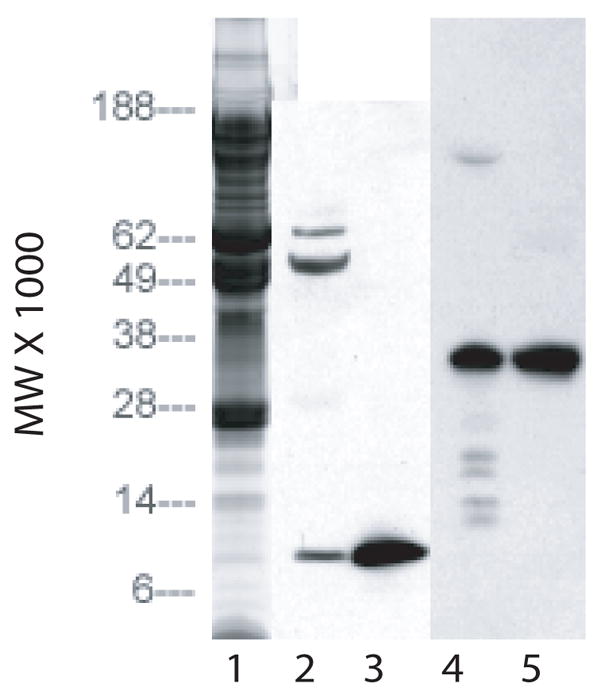
SDS/PAGE of concentrated stromal extract on 4–12% gels. Lane 1: Coomassie blue stain of 20 micro liters of extract. Lane 2: Western blot of 20 micro liters of extract using antibodies to IGF-II. Lane 3: Western blot of 2 micrograms of IGF-II using antibodies to IGF-II. Lane 4: Western blot of 20 micro liters of extract using antibodies to IGFBP. Lane 5: Western blot of 2 micrograms of IGFBP using antibodies to IGFBP.
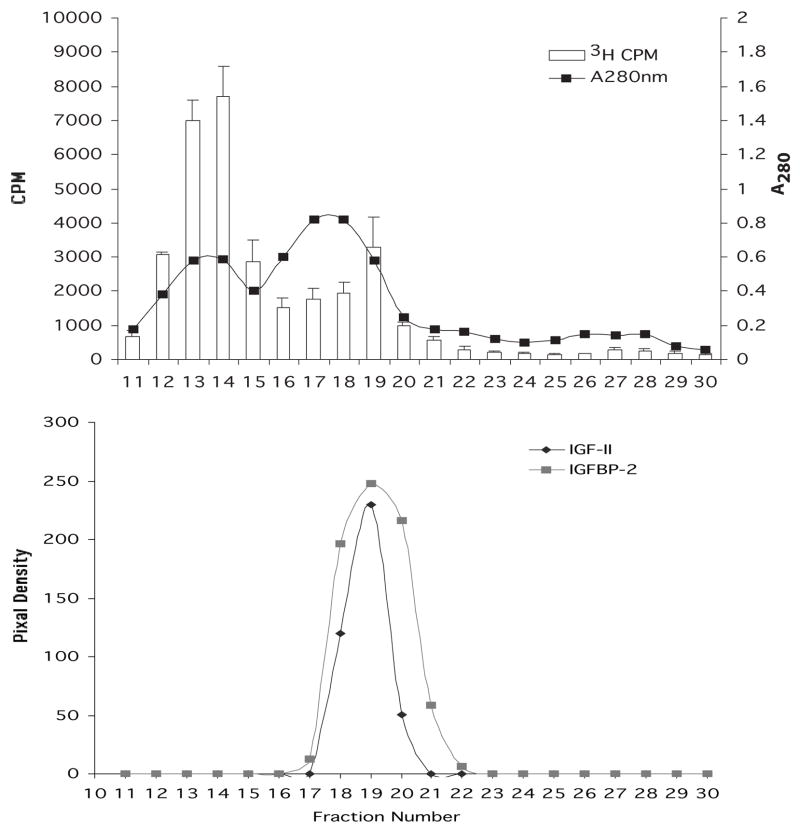
Chromatography of the extract on a column of Sephacryl S-300 had previously shown that the mitogenic activity of the extract eluted just after the void volume (Musselmann et al. 2003). The longer column of S-300 column used in this report resolved the mitogenic activity of the extract into two distinct peaks eluting just after the void volume (Figure 2A): a major peak (fractions 12–15) and a second, minor peak (fractions 17–20). Fractions 11–30 were individually concentrated to 1/10 volume and examined by Western blot using both the IGF-II and the IGFBP2 antibodies (data not shown). The intensity of the IGF-II and IGFBP2 bands was measured using a Bio-Rad GS710 calibrated imaging densitometer. The resulting pixel density values were plotted (Figure 2B). Both IGF-II and IGFBP2 were present in fractions 18–20, which corresponded to the elution position of the minor peak of mitogenic activity and were not detected in any of the other fractions. Antibodies to IGF-I, a growth factor related to IGF-II, readily detected the purified IGF-I used as a positive standard but did not detect any IGF-I in the extract or in the fractions from S-300 by Western blot (data not shown)
Fig. 2.
Chromatography of stromal extract on a column of Sephacryl S-300. Upper panel: fractions 11–30 were monitored for absorbance at 280 nm and for the ability to stimulate 3H thymidine incorporation in keratocytes in culture. Lower panel: fractions 11–30 were analyzed by Western blot using antibodies to IGF-II and to IGFBP. The pixel density of the IGF-II and IGFPB bands was measured and graphed.
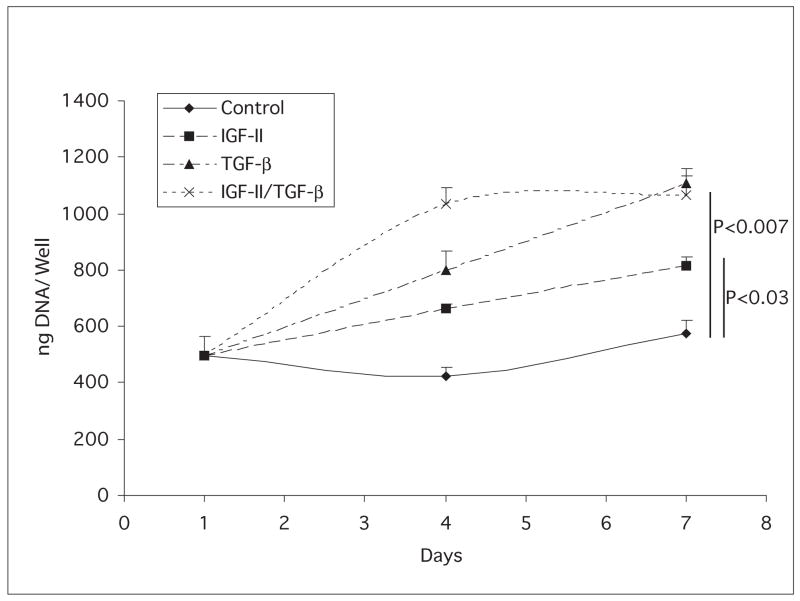
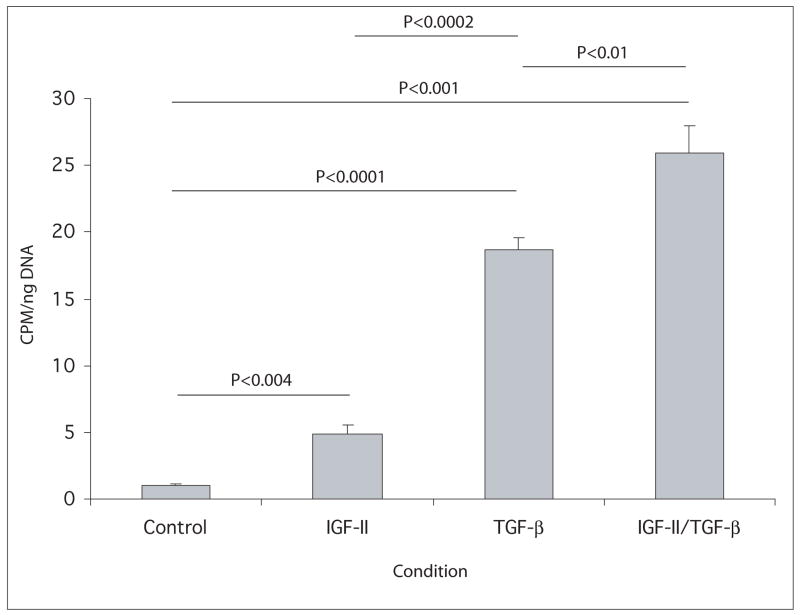
The ability of IGF-II to stimulate proliferation of keratocytes in culture was compared to that of the TGF-β1 isoform (TGF-β), a mitogen previously shown to stimulate keratocyte proliferation and to be present in the stroma during wound healing (Jester et al. 1997). Keratocytes were cultured in medium containing IGF-II, TGF-β or IGF-II plus TGF-β beginning on day 1. DNA content of the cultures was determined on days 4 and 7 (Figure 3). Compared to the DNA content on day 1, only those cultures receiving the growth factors had increased their DNA contents significantly by day 7. Cultures in medium supplemented with IGF-II showed a 66% increase in DNA content by day 7 and cultures treated with TGF-β alone or with IGF-II plus TGF-β had increased their DNA content by 1.8 fold by day 7. Cultures were also incubated with media containing 3H-thymidine during days 4 to 7 of culture, and the incorporation of the radiolabel into DNA was determined (Figure 4). Thymidine incorporation by keratocytes increased 4.5 fold when cultured in IGF-II, by 18 fold in TGF-β, and by 26 fold in IGF-I plus TGF-β.
Fig. 3.
DNA content of keratocyte cultures. Keratocytes were cultured in media without (diamonds) or with IGF-II (squares), TGF-β (triangles) or IGF-II plus TGF-β (X). Compared to the DNA content of control day 1 cultures, the day 7 IGF-I, the day 7 TGF-β, and the day 7 IGF-II plus TGF-β cultures were significantly higher. P values are listed on figure.
Fig. 4.
Incorporation of 3H thymidine by keratocyte cultures. Keratocytes were cultured in media without (control) or with IGF-II, TGF-β or IGF-II plus TGF-β from days 1 to 7. The media contained 3H thymidine from days 4 to 7. Compared to the control, incorporation of 3H thymidine by cells cultured in IGF-II, TGF-β, and IGF-II plus TGF-β were significantly higher. Cultures in TGF-β were also significantly higher than cultures in IGF-II and significantly lower than cultures in IGF-II plus TGF-β. P values are listed on figure.
Keratocytes cultured in the presence of TGF-β have been shown to undergo a myofibroblastic differentiation (Jester et al. 1996; Jester et al. 2002; Jester and Ho-Chang 2003). A cytosolic marker for the myofibroblastic phenotype is α-SM actin, a protein that aids in wound contraction (Jester et al. 1996). Extracts of keratocytes cultured in media with and without the growth factors were examined by Western blot using an antibody to α-SM actin (Figure 5). By day 4, only the keratocytes cultured in IGF-II plus TGF-β treatment were positive for α-SM actin. However, by day 7, both the keratocytes cultured in IGF-II plus TGF-β were strongly positive for α-SM actin with lower levels in keratocytes cultured in TGF-β alone. In contrast, α-SM actin was not detected in keratocytes cultured in IGF-II. ALDH, a cytoplasmic protein that is absent in keratocytes cultured in media containing fetal bovine serum (Berryhill et al. 2002) was readily detected in all cultures, but at slightly lower levels in the day 7 cultures treated with either TFG-β alone or with IGF-II plus TGF-β. These results indicate that culture in IGF-II stimulates proliferation but does not cause a myofibroblastic differentiation of the cells.
Fig. 5.
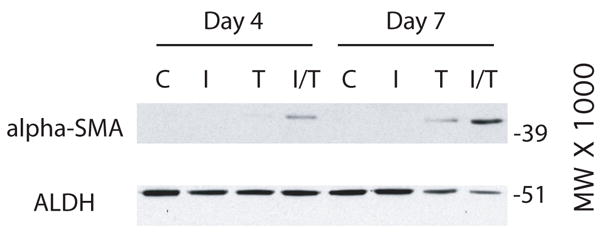
Western blots of extracts of cells after four (Day 4) and seven (Day 7) days of culture. Keratocytes were cultured in media without (C) or with IGF-II (I), TGF-β (T), or IGF-I plus TGF-β (I/T). Blots were probed with antibodies to α-SM actin (alpha-SMA) or to aldehyde dehydrogenase (ALDH).
Keratocytes that become myofibroblastic are also known to produce increased levels of fibronectin (Jester et al. 1996) and SPARC (Berryhill et al. 2003) as well as having reduced or undetectable levels of keratocan (Funderburgh et al. 2003). Western blots of media from days 4 and 7 of culture using an antibody to SPARC show SPARC was detected in the medium of keratocytes cultured in TGF-β alone and in IGF-II plus TGF-β with only trace amounts of SPARC in media from cultures receiving IGF-II alone (Figure 6). A similar result was obtained with an antibody to fibronectin, although in contrast to SPARC, fibronectin was not detected in media of keratocytes cultured in IGF-II.
Fig. 6.
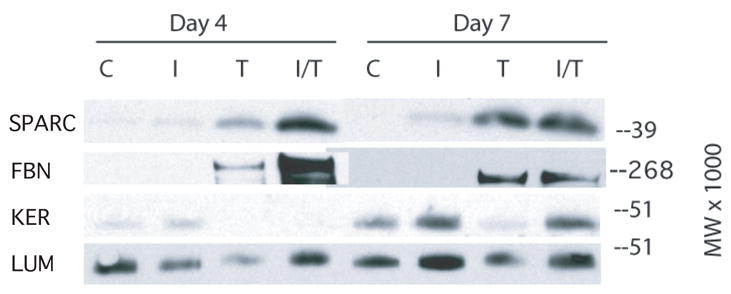
Western blot of culture media collected days 1–4 (Day 4) and days 4–7 (Day 7). Keratocytes were cultured in media without (C) or with IGF-II (I), TGF-β (T), or IGF-I plus TGF-β (I/T). Blots were probed with antibodies to fibronectin (FBN), to SPARC (SPARC), to keratocan (KER), or to lumican (LUM).
Media from the day 4 and 7 time points was also digested with endo beta galactosidase to remove keratan sulfate chains on lumican and keratocan prior to SDS/PAGE, enabling detection of their core proteins by Western blot. Analysis of digested day 4 media by using an antibody to keratocan core protein shows keratocan to be present in media from keratocytes cultured in IGF-II and not detected in media of keratocytes cultured in TGF-β or in IGF-II plus TGF-β (figure 6). Media harvested on day 7 from keratocytes cultured in IGF-II or in IGF-II plus TGF-β contained readily detectable levels of keratocan but only trace amounts were dected in media from keratocytes cultured in TGF-β. In contrast, analysis of digested media using antibodies to lumican core protein shows lumican to be present in media from all culture conditions and both time points although there was slightly less lumican in the media from keratocytes cultured in TGF-β.
4. Discussion
This study shows that an extract of the cornea stroma contains immunologically detectable IGF-II that co-elutes on S-300 with the fractions containing the minor peak of mitogenic activity in the extract. This suggests that there may be other growth factors in the stroma that elute with the major, earlier eluting peak of mitogenic activity. The extract also contained immunologically detectable IGFBP-2, and although IGFBP-2 is 4–5 times larger than IGF-II, they co-eluted on S-300. This suggests that the IGF-II and the IGFBP-2 in the extract are bound to each other. Previous studies have shown the cornea to have high levels of IGFBP-2 mRNA (Arnold et al. 1993) and this suggests that the IGFBP-2 detected in extracts of the stroma may be a product of corneal cells.
The results of this study also show that IGF-II stimulated the proliferation of keratocytes in culture, as determined by increased radio labeled thymidine incorporation into the keratocytes and the increase in DNA content of the cell cultures. However, growth in IGF-II was less than that in TGF-β, a growth factor known to cause the keratocytes to become myofibroblasts (Jester et al. 1997). This is consistent with previous work (Musselmann et al. 2003) that showed growth in extract is less than that in fetal bovine serum, another agent known to cause keratocytes to become fibroblasts/myofibroblasts.
While keratocytes cultured in IGF-II proliferated, they produced no detectable levels of α-SM actin or fibronectin, only trace amounts of SPARC and high levels of keratocan. This indicates that keratocytes cultured in IGF-II can proliferate and maintain their native phenotype similar to that shown for keratocytes cultured in 10 micrograms of insulin/ml (Musselmann et al. 2005; Musselmann et al. 2006; Guerriero et al. 2007). IGF-II and high concentrations of insulin can bind IGF-IR, the receptor for IGF-I, to activate cell proliferation [for review see (Lelbach et al. 2005)].
Keratocytes cultured in TGF-β contained α-SM actin on day 7, produced fibronectin and SPARC on days 4 and 7, and produced no detectable keratocan on day 4 with only trace amounts of keratocan on day 7, all markers of myofibroblasts. Keratocytes cultured in IGF-II plus TGF-β, however, contained α-SM actin and produced fibronectin and SPARC on days 4 and 7, three markers of myofibroblasts, but also produced high levels of keratocan on day 7, a marker of the native phenotype. This suggests that IGF-II can override the TGF-β mediated down regulation of keratocan synthesis. Since keratocan participates in the regulation of collagen fibril assembly (Hassell et al. 1983; Rada et al. 1993; Liu et al. 2003), keratocytes that are activated by both IGF-II and TGF-β may be able to produce a more normal extracellular matrix while preserving wound contractility.
Two cell surface receptors bind IGF-II: IGF-IR and IGF-II/M6PR [for review see (Lelbach et al. 2005)]. It is the binding of IGF-II to IGF-IR, however, that causes the increased cell proliferation (Storckenfeldt et al. 1991). Mannose-6-phosphate (M6P) also binds to IGF-II/M6PR, and M6P has been shown to interfere with the IGF-II/M6PR mediated activation of latent TGF-β (Dennis and Rifkin 1991). Therefore, IGF-II could be overriding the TGF-β mediated down regulation of keratocan synthesis either through its binding to IGF-IR or IGF-II/M6PR individually or to both IGF-IR and IGF-II/M6PR.
We propose that the IGF-II present in the corneal stroma provides a reservoir mechanism in a non-vascular tissue to immediately activate keratocytes after corneal wounding and to ameliorate the scaring effects of TGF-β, a growth factor shown to be present in the cornea after wounding (Jester et al. 1997).
Acknowledgments
Supported by Grant EY08104 from the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold DR, Moshayedi P, Schoen TJ, Jones BE, Chader GJ, Waldbillig RJ. Distribution of IGF-I and -II, IGF binding proteins (IGFBPs) and IGFBP mRNA in ocular fluids and tissues: potential sites of synthesis of IGFBPs in aqueous and vitreous. Exp Eye Res. 1993;56:555–565. doi: 10.1006/exer.1993.1069. [DOI] [PubMed] [Google Scholar]
- Beales MP, Funderburgh JL, Jester JV, Hassell JR. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Invest Ophthalmol Vis Sci. 1999;40:1658–1663. [PubMed] [Google Scholar]
- Berryhill BL, Beales MP, Hassell JR. Production of prostaglandin D synthase as a keratan sulfate proteoglycan by cultured bovine keratocytes. Invest Ophthalmol Vis Sci. 2001;42:1201–1207. [PubMed] [Google Scholar]
- Berryhill BL, Kader R, Kane B, Birk DE, Feng J, Hassell JR. Partial restoration of the keratocyte phenotype to bovine keratocytes made fibroblastic by serum. Invest Ophthalmol Vis Sci. 2002;43:3416–3421. [PubMed] [Google Scholar]
- Berryhill BL, Kane B, Stramer BM, Fini ME, Hassell JR. Increased SPARC accumulation during corneal repair. Exp Eye Res. 2003;77:85–92. doi: 10.1016/s0014-4835(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Birk DE, Fitch JM, Linsenmayer TF. Organization of collagen types I and V in the embryonic chicken cornea. Invest Ophthalmol Vis Sci. 1986;27:1470–1477. [PubMed] [Google Scholar]
- Blochberger TC, Vergnes JP, Hempel J, Hassell JR. cDNA to chick lumican (corneal keratan sulfate proteoglycan) reveals homology to the small interstitial proteoglycan gene family and expression in muscle and intestine. J Biol Chem. 1992;267:347–352. [PubMed] [Google Scholar]
- Cintron C, Hong BS. Heterogeneity of collagens in rabbit cornea: type VI collagen. Invest Ophthalmol Vis Sci. 1988;29:760–766. [PubMed] [Google Scholar]
- Cintron C, Hong BS, Covington HI, Macarak EJ. Heterogeneity of collagens in rabbit cornea: type III collagen. Invest Ophthalmol Vis Sci. 1988;29:767–775. [PubMed] [Google Scholar]
- Corpuz LM, Funderburgh JL, Funderburgh ML, Bottomley GS, Prakash S, Conrad GW. Molecular cloning and tissue distribution of keratocan. Bovine corneal keratan sulfate proteoglycan 37A. J Biol Chem. 1996;271:9759–9763. doi: 10.1074/jbc.271.16.9759. [DOI] [PubMed] [Google Scholar]
- Del Pero RA, Gigstad JE, Roberts AD, Klintworth GK, Martin CA, L’Esperance FA, Jr, Taylor DM. A refractive and histopathologic study of excimer laser keratectomy in primates. Am J Ophthalmol. 1990;109:419–429. doi: 10.1016/s0002-9394(14)74608-2. [DOI] [PubMed] [Google Scholar]
- Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci U S A. 1991;88:580–584. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburgh JL, Corpuz LM, Roth MR, Funderburgh ML, Tasheva ES, Conrad GW. Mimecan, the 25-kDa corneal keratan sulfate proteoglycan, is a product of the gene producing osteoglycin. J Biol Chem. 1997;272:28089–28095. doi: 10.1074/jbc.272.44.28089. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero E, Chen J, Sado Y, Mohan RR, Wilson SE, Funderburgh JL, Sundarraj N. Loss of alpha3(IV) collagen expression associated with corneal keratocyte activation. Invest Ophthalmol Vis Sci. 2007;48:627–635. doi: 10.1167/iovs.06-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna KD, Pouliquen Y, Waring GO, 3rd, Savoldelli M, Cotter J, Morton K, Menasche M. Corneal stromal wound healing in rabbits after 193-nm excimer laser surface ablation. Arch Ophthalmol. 1989;107:895–901. doi: 10.1001/archopht.1989.01070010917041. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Cintron C, Kublin C, Newsome DA. Proteoglycan changes during restoration of transparency in corneal scars. Arch Biochem Biophys. 1983;222:362–369. doi: 10.1016/0003-9861(83)90532-5. [DOI] [PubMed] [Google Scholar]
- Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998;39:276–283. [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505–516. [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Petroll WM, Olsen DR, Cavanagh HD. Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF beta in the rabbit. Cornea. 1997;16:177–187. [PubMed] [Google Scholar]
- Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- Jester JV, Huang J, Petroll WM, Cavanagh HD. TGFbeta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFbeta, PDGF and integrin signaling. Exp Eye Res. 2002;75:645–657. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. J Cell Sci. 1999;112 (Pt 5):613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- Lelbach A, Muzes G, Feher J. The insulin-like growth factor system: IGFs, IGF-binding proteins and IGFBP-proteases. Acta Physiol Hung. 2005;92:97–107. doi: 10.1556/APhysiol.92.2005.2.1. [DOI] [PubMed] [Google Scholar]
- Li W, Vergnes JP, Cornuet PK, Hassell JR. cDNA clone to chick corneal chondroitin/dermatan sulfate proteoglycan reveals identity to decorin. Arch Biochem Biophys. 1992;296:190–197. doi: 10.1016/0003-9861(92)90562-b. [DOI] [PubMed] [Google Scholar]
- Liu CY, Birk DE, Hassell JR, Kane B, Kao WW. Keratocan-deficient mice display alterations in corneal structure. J Biol Chem. 2003;278:21672–21677. doi: 10.1074/jbc.M301169200. [DOI] [PubMed] [Google Scholar]
- Long CJ, Roth MR, Tasheva ES, Funderburgh M, Smit R, Conrad GW, Funderburgh JL. Fibroblast growth factor-2 promotes keratan sulfate proteoglycan expression by keratocytes in vitro. J Biol Chem. 2000;275:13918–13923. doi: 10.1074/jbc.275.18.13918. [DOI] [PubMed] [Google Scholar]
- Muller LJ, Pels L, Vrensen GF. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995;36:2557–2567. [PubMed] [Google Scholar]
- Musselmann K, Alexandrou B, Kane B, Hassell JR. Maintenance of the keratocyte phenotype during cell proliferation stimulated by insulin. J Biol Chem. 2005;280:32634–32639. doi: 10.1074/jbc.M504724200. [DOI] [PubMed] [Google Scholar]
- Musselmann K, Kane B, Alexandrou B, Hassell JR. Stimulation of collagen synthesis by insulin and proteoglycan accumulation by ascorbate in bovine keratocytes in vitro. Invest Ophthalmol Vis Sci. 2006;47:5260–5266. doi: 10.1167/iovs.06-0612. [DOI] [PubMed] [Google Scholar]
- Musselmann K, Kane BP, Hassell JR. Isolation of a putative keratocyte activating factor from the corneal stroma. Exp Eye Res. 2003;77:273–279. doi: 10.1016/s0014-4835(03)00160-x. [DOI] [PubMed] [Google Scholar]
- Rada JA, Cornuet PK, Hassell JR. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp Eye Res. 1993;56:635–648. doi: 10.1006/exer.1993.1081. [DOI] [PubMed] [Google Scholar]
- Schmut O. The identification of type III collagen in calf and bovine cornea and sclera. Exp Eye Res. 1977;25:505–509. doi: 10.1016/0014-4835(77)90179-8. [DOI] [PubMed] [Google Scholar]
- Storckenfeldt L, Schofield PN, Engstrom W. Stimulatory effect of insulin like growth factor II on DNA synthesis in the human embryonic cornea. Cell Biol Int Rep. 1991;15:1217–1223. doi: 10.1016/0309-1651(91)90093-x. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Guimaraes SR, Hutcheon AE. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp Eye Res. 2001;72:33–39. doi: 10.1006/exer.2000.0926. [DOI] [PubMed] [Google Scholar]