Abstract
Cyclin-dependent kinase 5 (cdk5) is a ubiquitous protein activated by neuron-specific activators, p35 and p39. Cdk5 regulates neuronal migration, differentiation, axonogenesis, synaptic transmission and apoptosis. However, its role in primary neurogenesis remains unexplored. Here, we have cloned and characterized the zebrafish cdk5 ortholog. Zebrafish cdk5 is 96% identical to its human counterpart. In situ hybridization analyses demonstrated that zebrafish cdk5 transcripts are ubiquitously expressed as early as the blastula stage. At 11.5 hours of development, cdk5 transcripts were present in the neural plate at the domains where primary neurons begin to be specified. RT-PCR analyses showed equal levels of cdk5 transcripts up to 72 h of development. SiRNA-mediated cdk5 knockdown resulted in a reduction in primary sensory neurons of the trigeminal ganglia of the peripheral nervous system, suggesting that cdk5 plays a crucial role in the development of the peripheral nervous system.
Keywords: neurogenesis, protein kinase, siRNA, zebrafish
Cyclin-dependent kinase 5 (cdk5) is a member of the cyclin-dependent kinase family [13]. Although cdk5 is found in mitotic cells [4], it is not involved in the cell cycle and cdk5 kinase activity is not stimulated by an associated cyclin but by neuron-specific activators, p35 and p39. The major activator, p35 is expressed predominantly in postmitotic neurons of the central nervous system [11, 22, 25].
Cdk5 knockout mice exhibit defects in organization of the cortex and cerebellum and are embryonically lethal [16], whereas mice lacking p35 reveal cortical lamination defects [1]. Overexpression of cdk5 and p35 promotes neurite outgrowth of cultured cortical neurons while a dominant-negative mutant of cdk5 inhibits neurite outgrowth [15]. In Xenopus, cdk5 plays a role in eye and muscle development and has two p35 activators [6, 17, 18].
Neural development of vertebrates is regulated by a precise coordination of cell proliferation and differentiation. Neural precursor cells are generated through a series of steps comprising neural induction, patterning, and neurogenesis [7, 19]. After neural induction and regional patterning, neurogenic domains are established at the late gastrula or neurula stages.
In mammals, cdk5’s role during early neural development is not known. Previously, we have shown that cdk5 activity varies during early development in zebrafish and the spinal Rohon-Beard neuron survival is dependent on cdk5 [9]. To further explore cdk5 function during early development, we cloned, characterized and determined the expression pattern of the zebrafish ortholog of mammalian cdk5. We used cdk5 siRNA to knockdown cdk5 expression in the embryos. In the siRNA-mediated cdk5 knockdown embryos, there was a marked decrease in the primary sensory neurons in the peripheral nervous systems, such as, the trigeminal ganglion neurons.
Zebrafish (Danio rerio) were kept and raised essentially according to standard procedures (NICHD, NIH facility). Fertilization was achieved by natural spawning and embryos were raised at 28.5° C and staged [10].
Mouse monoclonal anti-acetylated α-tubulin - antibody was purchased from Sigma (St. Louis, MO). Rabbit polyclonal antibodies against mammalian cdk5 (C-8) and β-actin were purchased from Santa Cruz Biotech (Santa Cruz, CA). Mouse monoclonal antibody against zebrafish islet-1 was purchased from the Developmental Studies Hybridoma Bank, University of Iowa (product # 39.4D5).
A 24 hpf (hours post fertilization) zebrafish embryo cDNA library was purchased from Stratagene (La Jolla, CA). The zebrafish cdk5 clones were obtained by screening 6 × 105 pfu at low stringency with a cDNA probe made from a 0.9 kb fragment from human cdk5. Positive clones were identified and sequenced.
Whole-mount in situ hybridization was carried out as described [24] by using digoxigenin-11-UTP-labeled probes (Roche Diagnostics Corp., Indianapolis, USA). The antisense probe for full-length zebrafish cdk5 was synthesized by linearizing the PCS2-Cdk5 plasmid with HindIII and using T3 RNA polymerase. The sense probe for cdk5 was synthesized by linearizing the PCS2-Cdk5 plasmid with Not1 and using SP6 RNA polymerase. Cross sections of the paraffin-embeded embryos, following whole mount in situ hybridization, were analyzed for tissue-specific expression of cdk5 mRNA.
To study the expression of cdk5 mRNA in embryos at various developmental stages, RT-PCR was performed with the following pairs of oligonucleotide primer sequences: 5’-373 CGTGACCTTAAACCACAAAACC 394 –3’ and 5’-660 TCCCAGCAATCGAAAGATCC 641-3’. Beta-actin was amplified as a control using the following primers: 5’-22 TGAATCCCAAAGCCAACAGAG 42-3’ and 5’-171 TCACACCATCACCAGAGTCC 152 –3’. One μg total RNA from embryos at various developmental stages was employed in making first strand cDNA with avian myeloblastosis virus-reverse transcriptase (Roche Diagnostics Corp., Indianapolis, USA); 1/10th of the resulting cDNA was used for PCR reactions. The reaction extended through 15 cycles and was in the linear range.
Embryos were collected at the specified time point and were homogenized in 120 μl of lysis buffer (10 mM Tris-HCl, pH 7.5, 1% sodium deoxycholate, 1% Nonidet P-40, 150 mM NaCl, protease and phosphatase inhibitors). Homogenates were then sonicated and centrifuged for 5 min at 14,000 × g. Immunoprecipitations of extracts containing 200 μg of total protein were performed by adding the cdk5 antibody (C-8) and incubating overnight at 4° C with constant rotation. Thirty microliters of a 50% slurry of Protein A-Sepharose beads (Amersham Pharmacia Biotech) were then added followed by incubation under the same conditions for an additional 4 hr. Immunocomplexes were centrifuged for 2 min at 14,000-× g and washed twice in lysis buffer, twice in kinase assay buffer (25 mM Tris–HCl, pH 7.5, 5 mM b-glycerol phosphate, 0.1 mM sodium orthovanadate, 2 mM dithiothreitol, 10 mM magnesium chloride). Kinase activity assays were performed as described earlier [12].
Extracts were prepared from embryos microinjected with either the control or the cdk5 siRNA. Fifty micrograms of total protein were separated by 10-20% SDS-PAGE and immunoblotted to PVDF membrane. Cdk5 was detected using an antibody against mammalian cdk5. The immunoblots were developed for signal visualization by enhanced chemiluminescence (ECL) (Amersham, Chicago, IL).
Control and cdk5 siRNAs were designed and purchased from Xeragon, Switzerland. Control siRNA (non-silencing) sense and antisense sequences were: 5’-r(UUUUCCGAACGUGUCACGU)d(TT)-3’ and 5’-r(ACGUGACACGUUCGGAAAA)d(TT)-3’, respectively. Cdk5 siRNA (silencing) sequences were: 5’-r(AAGCCGUACCCGAUGUAUC)d(TT)-3’ and 5’-r(GAUACAUCGGGUACGGCUU)d(TT)-3’, respectively. The sense and antisense strands were annealed to create the double-stranded siRNA at a 10μM concentration. Control siRNA and cdk5 siRNA were dissolved in Danieu’s buffer [14] before injection. SiRNAs were injected into each egg at one- to two-cell stage (2 nl of a 10 μM stock solution per embryo). Rescue experiments were performed by coinjecting the siRNA mixed with 200 pg of in vitro transcribed mRNA obtained from the zebrafish cdk5 cDNA cloned into the Hind III/Xba I sites of the PCS2 vector using the SP6 RNA polymerase (Ambion Inc., Austin, TX).
For whole-mount immunohistochemistry, embryos were fixed in 4% paraformaldehyde overnight, blocked in 5% sheep serum and 2 mg/ml BSA in PBS, and incubated overnight (4°C) in the monoclonal antibodies against either acetylated α-tubulin (1:2000) or islet-1 (1:100). Embryos were then incubated in HRP-conjugated anti-mouse antibody at 4° C overnight. Color development for positive signals was performed using the Vectastatin ABC kit (Vector Laboratories).
To identify the zebrafish homolog of cyclin-dependent kinase 5 (cdk5), we screened a 24h zebrafish embryo cDNA library at low stringency with partial human cdk5 cDNA corresponding to the N-terminal 700 bp. Three positive clones were obtained by screening the 600,000 phage plaques. One of these clones contained a 2.9-kb insert encoding a complete open reading frame (ORF) that displayed a high degree of homology to human cdk5 (Genbank database accession number: AF 203736). The predicted 293-amino-acid residue- ORF of zebrafish cdk5 shows an overall 96.5% identity to human and Xenopus cdk5 and most other vertebrate cdk5s (Fig. 1).
Figure 1.

Deduced amino acid sequence of zebrafish cdk5 is aligned with that of human, mouse, and Xenopus. The sequence has been submitted to Genbank database (Accession number: AF 203736). The underlined sequence (KPYPMYP) represents the region targeted by cdk5 siRNA.
To investigate both spatial and temporal expression of zebrafish cdk5, we performed whole mount in situ hybridization using an antisense probe to the coding region of the zebrafish gene (Fig. 2). At 2.5 hours after fertilization (hpf), before mid-blastula transition, all the blastomeres express cdk5 transcript (Fig. 2A a). During the end of gastrulation at 11.5 hpf, at the neural plate stage, cdk5 mRNA is also expressed ubiquitously in the embryos, with highest expression in the neural plate (Fig. 2A b), especially at the regions where primary neurons begin to be specified. At 24 hpf, the anterior region including the whole brain and eyes abundantly express cdk5 mRNA (Fig. 2A c, d). A posterior region as demarcated in Fig. 2A c, upon magnification, demonstrates a ubiquitous expression of cdk5 mRNA including the neural tissues (Fig. 2A e). In cross sections of 24 hpf embryos, cdk5 mRNA expression appears to be ubiquitous, its presence being detected in the brain and the eyes (Fig. 2A f). However, a relatively increased expression is observed in the eye, especially in the lens capsule. A cross sectional area of the posterior region of the 24 hpf embryo shows cdk5 expression in the spinal cord and muscles (Fig. 2A g). In situ hybridization results of blastula (2.5 hpf) and 24 hpf embryo using the cdk5 sense probe are shown to indicate background signals (Fig. 2A h, i).
Figure 2.
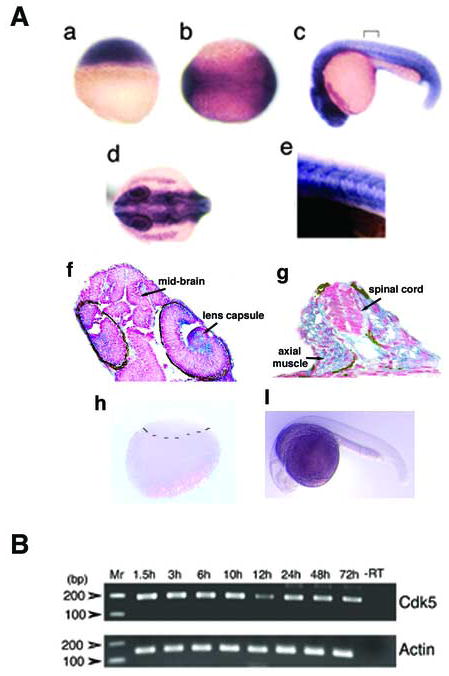
(A) In situ hybridization shows cdk5 expression at 2.5 h after fertilization (hpf) (a), neurula stage at 11.5 hpf (b); 24 h larva, lateral (c) and dorsal views (d). A demarcated region as shown in ‘c’ is magnified 40X (e). A longitudinal section through the anterior region of the 24 hpf embryo shows cdk5 mRNA expression (f) (300 × magnification); and a cross section through the posterior region of the 24 hpf embryo shows cdk5 expression in the spinal cord and axial muscle (g) (400 × magnification). Arrows indicate brain and lens capsule. In situ hybridization assays of blastula (h) and 24 hpf embryo (i) using the cdk5 sense RNA probe are shown. The demarcation in ‘h’ separates the upper blastomeres and lower yolk sac. (B) RT-PCR analyses of cdk5 (upper panel) and β-actin (lower panel) from cDNAs synthesized from the embryos at various stages of development. Molecular markers are shown and the amplification product containing the mRNA from the 72 hpf embryos but no reverse transcriptase is shown (-RT) as a negative control.
To examine the developmental expression pattern of cdk5 mRNA, RT-PCR was performed. Amplicons obtained semi-quantitatively from the total RNA isolated from stages, 1.5, 3, 6, 10, 12, 24, 48 and 72 hpf embryos showed no significant difference in cdk5 mRNA expression (Fig. 2B, upper panel). A simultaneous amplification product for β-actin is shown for comparison (Fig. 2B, lower panel). The early expression of cdk5 mRNA in the egg and during cleavage is in marked contrast to Xenopus where cdk5 mRNA is first expressed between the midblastula transition and gastrulation (Gervasi and Szaro, 1995).
Expression of cdk5 mRNA at early stages suggests an early developmental role. However, cdk5 activity during these early stages is negligible until 24 hpf. To explore the role of cdk5 in zebrafish development, we carried out loss-of-function experiments by injecting siRNA into the one- to two-cell embryos (2 nl of a 20 μM stock solution per embryo) designed specifically against the zebrafish cdk5. When compared to the uninjected (Fig. 3A) and control siRNA-injected embryos (Fig. 3B), cdk5 siRNA-injected embryos exhibited no gross morphological changes, as shown for 78 hpf embryos (Fig. 3C). The low residual levels of activity may have been sufficient to sustain development without any gross morphological abnormalities in the eye and body axis. However, we examined in more detail, the effect of cdk5 siRNA on the primary sensory neuron development since cdk5 is known to have multiple roles in nervous system development.
Figure 3.
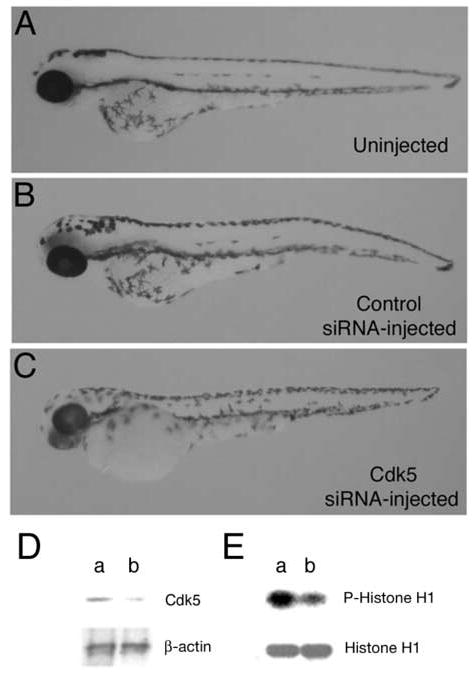
Morphology of 78 hpf zebrafish embryos, (A) uninjected, (B) injected with 2nl of 20 μM control siRNA, and (C) 2 nl of 20 μM cdk5 siRNA. Immunoblot of extracts prepared from 10 h embryos after injecting the siRNAs was probed with anti-cdk5 and anti-actin antibodies (D) Cdk5 protein levels are shown in the control siRNA-injected (lane a) and cdk5 siRNA-injected (lane b) embryos. Lower panel shows β-actin levels. (F) Control siRNA-injected (lane a) and cdk5 siRNA-injected (lane b) embryos (24 hpf) were used for determining the cdk5 kinase activity. A representative autoradiograph of three separate experiments shows phosphorylated histone H1 (upper panel) and Coomassie Blue-stained histone H1 substrate (lower panel).
To determine whether cdk5 has been reduced in the cdk5 siRNA-injected embryos, total protein extracts were prepared from injected embryos at 10 hpf. Twenty μg total proteins were used for immunoblot analysis using an antibody against cdk5. In the control siRNA-injected embryos, there was an ~ 33 kD polypeptide corresponding to the size of cdk5 protein in the extracts (Fig. 3D, lane a; upper panel). The results demonstrated an ~ 80% loss of the cdk5 protein in the cdk5 siRNA-injected embryos at 10 hpf (Fig. 3D, lane b; upper panel). Duplicate immunoblots were probed with an antibody against β–actin (Fig. 3D, lower panels) confirming the comparable amount of total protein load in the two lanes. In order to determine the effect of the cdk5 protein knockdown on its kinase activity, extracts from 24 hpf embryos were used for kinase assays, since the activity is noticeably higher at 24 hpf, as compared to the earlier stages [9]. Control siRNA-injected embryos showed cdk5 activity (Fig. 3E, lane a; upper panel) that was drastically reduced in cdk5 siRNA-injected embryos (Fig. 3E, lane b; upper panel). The amount of histone H1 used as the substrate for the kinase activity assays is shown at the bottom panel (Fig. 3E).
To determine whether cdk5 knockdown has an effect on neurogenesis, we analyzed the trigeminal ganglia that are comprised of early born primary sensory neurons of the peripheral nervous system. Whole mount immunohistochemistry was performed using an antibody to islet-1 that is a marker for primary neurons. Islet-1 is a LIM-homeodomain (LIM-HD) protein expressed at the earliest stage of neural differentiation, in motor and sensory neurons in zebrafish [8]. In addition, an anti-acetylated α-tubulin antibody was used to immunostain the neurons in the trigeminal ganglia. Immunostaining data revealed that in the control siRNA-injected embryos (n= 50), the trigeminal ganglion was well developed (Fig. 4A, E). However, the siRNA-mediated cdk5 knockdown embryos (25 out of 40) showed a smaller trigeminal ganglion (Fig. 4B, F). The difference in the number of the islet-1 positive cells between the control siRNA-injected and cdk siRNA-injected embryos was statistically significant (P < 0.005).
Figure 4.
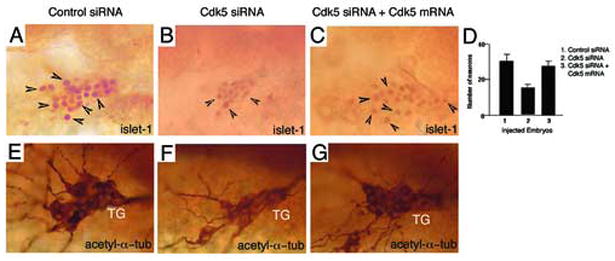
Cdk5 is required for primary sensory neurogenesis. Immunostaining of 24 hpf embryos with an antibody against islet-1 marks (lateral views of the anterior regions of the embryos) primary neurons in (A) control siRNA-injected, (B) cdk5 siRNA-injected, and (C) cdk5 siRNA with cdk5 mRNA (200 pg)-injected embryos. Arrowheads indicate some of the neurons. (D) Quantitative analyses of islet-1 positive neurons are presented. Acetylated α-tubulin (acetyl α-tub) staining reveals the primary sensory neurons in the trigeminal ganglia of the zebrafish (lateral views) injected with control siRNA (E), cdk5 siRNA (F) and cdk5 siRNA plus cdk5 mRNA (G).
In order to confirm that the effect was specific to cdk5, we performed rescue experiments by coinjecting the specified amount of cdk5 siRNA (2 nl of 20μM stock per embryo) along with 200 pg of in vitro synthesized cdk5 mRNA. The results demonstrated a rescue of the neuronal phenotype (50 out of 50, P < 0.001) in the cdk5 siRNA and cdk5 mRNA co-injected embryos (Fig. 4C, G). The average numbers of neurons in the control siRNA-injected, cdk5 siRNA-injected and cdk5 siRNA with cdk5 mRNA co-injected embryos were 30, 16 and 26, respectively, implying that cdk5 is necessary for the proper development of trigeminal ganglia with a full complement of neurons (Fig. 4D).
In this study, we cloned the zebrafish ortholog of human cdk5. The zebrafish cdk5 is strikingly homologous (at ~ 97%) to all vertebrate cdk5s, a conservation consistent with the view that this kinase plays a major role in development and function of the vertebrate nervous systems. Compared to other vertebrates, particularly closely related Xenopus [17, 18, 21], cdk5 mRNA is expressed very early before the midblastula transition its expression remaining relatively constant up to 72 hrs of development in zebrafish. The presence of cdk5 mRNA in zebrafish embryos much earlier than muscle or neuronal differentiation in contrast to its late expression in Xenopus [17] implies a function devoted to early developmental processes including a possible role in neurogenesis. Subsequent ubiquitous expression of cdk5 mRNA in the brain, eyes and body axis is consistent with its well-established function in neuromuscular development and differentiation [5, 17, 18, 23]. However, in spite of the reduction in cdk5 protein level in the cdk5 siRNA-injected embryos, no delays in development or gross morphological abnormalities were observed as compared to the control embryos.
Previous reports indicate that cdk5 affects neurite outgrowth, axonogenesis and neuronal differentiation in mammals and Drosophila [2, 3, 15, 16, 20, 23]. We demonstrate unequivocal defects in sensory neuron numbers in the peripheral nervous systems of the siRNA-mediated cdk5 knockdown embryos. Furthermore, the specificity of the effects could be shown by restoring the neuron numbers to a higher level after coinjection of cdk5 mRNA along with the cdk5 siRNA.
The early reduction of the trigeminal ganglion neuron could be attributed to a role of cdk5 in neuronal cell development, differentiation, and apoptosis. Whether cdk5 regulates the expression of proneural genes, such as, ngn1 remains to be explored. These results provide evidence that cdk5 plays a critical role in the peripheral nervous system development in zebrafish.
Acknowledgments
We thank Dr. Ajay Chitnis (NICHD, NIH) for critical discussions and Dr. Paul Liu (NHGRI, NIH) for his comments on the manuscript. This work was supported by NIH (NINDS) intramural funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- 2.Cicero S, Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J Neurosci. 2005;25:9658–68. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connell-Crowley L, Le Gall M, Vo DJ, Giniger E. The cyclin-dependent kinase Cdk5 controls multiple aspects of axon patterning in vivo. Curr Biol. 2000;10:599–602. doi: 10.1016/s0960-9822(00)00487-5. [DOI] [PubMed] [Google Scholar]
- 4.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–59. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 5.Fu AK, Fu WY, Ng AK, Chien WW, Ng YP, Wang JH, Ip NY. Cyclin-dependent kinase 5 phosphorylates signal transducer and activator of transcription 3 and regulates its transcriptional activity. Proc Natl Acad Sci U S A. 2004;101:6728–33. doi: 10.1073/pnas.0307606100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gervasi C, Szaro BG. The Xenopus laevis homologue to the neuronal cyclin-dependent kinase (cdk5) is expressed in embryos by gastrulation. Brain Res Mol Brain Res. 1995;33:192–200. doi: 10.1016/0169-328x(95)00109-6. [DOI] [PubMed] [Google Scholar]
- 7.Hartenstein V. Early neurogenesis in Xenopus: the spatio-temporal pattern of proliferation and cell lineages in the embryonic spinal cord. Neuron. 1989;3:399–411. doi: 10.1016/0896-6273(89)90200-6. [DOI] [PubMed] [Google Scholar]
- 8.Inoue A, Takahashi M, Hatta K, Hotta Y, Okamoto H. Developmental regulation of islet-1 mRNA expression during neuronal differentiation in embryonic zebrafish. Dev Dyn. 1994;199:1–11. doi: 10.1002/aja.1001990102. [DOI] [PubMed] [Google Scholar]
- 9.Kanungo J, Li BS, Zheng Y, Pant HC. Cyclin-dependent kinase 5 influences Rohon-Beard neuron survival in zebrafish. J Neurochem. 2006 doi: 10.1111/j.1471-4159.2006.04114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 11.Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–6. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 12.Li BS, Zhang L, Gu J, Amin ND, Pant HC. Integrin alpha(1) beta(1)-mediated activation of cyclin-dependent kinase 5 activity is involved in neurite outgrowth and human neurofilament protein H Lys-Ser-Pro tail domain phosphorylation. J Neurosci. 2000;20:6055–62. doi: 10.1523/JNEUROSCI.20-16-06055.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyerson M, Enders GH, Wu CL, Su LK, Gorka C, Nelson C, Harlow E, Tsai LH. A family of human cdc2-related protein kinases. Embo J. 1992;11:2909–17. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–20. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 15.Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–25. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- 16.Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93:11173–8. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philpott A, Porro EB, Kirschner MW, Tsai LH. The role of cyclin-dependent kinase 5 and a novel regulatory subunit in regulating muscle differentiation and patterning. Genes Dev. 1997;11:1409–21. doi: 10.1101/gad.11.11.1409. [DOI] [PubMed] [Google Scholar]
- 18.Philpott A, Tsai L, Kirschner MW. Neuronal differentiation and patterning in Xenopus: the role of cdk5 and a novel activator xp35.2. Dev Biol. 1999;207:119–32. doi: 10.1006/dbio.1998.9146. [DOI] [PubMed] [Google Scholar]
- 19.Sasai Y, De Robertis EM. Ectodermal patterning in vertebrate embryos. Dev Biol. 1997;182:5–20. doi: 10.1006/dbio.1996.8445. [DOI] [PubMed] [Google Scholar]
- 20.Smith D. Cdk5 in neuroskeletal dynamics. Neurosignals. 2003;12:239–51. doi: 10.1159/000074626. [DOI] [PubMed] [Google Scholar]
- 21.Szaro BG, Lee VM, Gainer H. Spatial and temporal expression of phosphorylated and non-phosphorylated forms of neurofilament proteins in the developing nervous system of Xenopus laevis. Brain Res Dev Brain Res. 1989;48:87–103. doi: 10.1016/0165-3806(89)90095-3. [DOI] [PubMed] [Google Scholar]
- 22.Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–23. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 23.Tsai LH, Takahashi T, Caviness VS, Jr, Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993;119:1029–40. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–73. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- 25.Zheng M, Leung CL, Liem RK. Region-specific expression of cyclin-dependent kinase 5 (cdk5) and its activators, p35 and p39, in the developing and adult rat central nervous system. J Neurobiol. 1998;35:141–59. doi: 10.1002/(sici)1097-4695(199805)35:2<141::aid-neu2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]


