Abstract
Diffuse brain injury is a leading cause of mortality in infants and children under 4 years of age and results in cognitive deficits in survivors. The anatomic basis for these behavioral deficits may be traumatic axonal injury (TAI), which manifests as impaired axonal transport (IAT) and neurofilament compaction (NFC), and may occur as a result of glutamate receptor activation. The extent of IAT and NFC was evaluated at 6, 24 and 72 hours following non-contusive brain trauma in the 17 day-old rat to examine the causal relationship between these two pathologic entities; in addition, the effect of antagonists to the ionotropic glutamate receptors on TAI was evaluated. At 6 hours post-injury, NFC was observed primarily in the cingulum, and appeared as swollen axons and terminal bulbs. By 24 hours, swollen axons were additionally present in the corpus callosum and lateral white matter tracts, and appeared to increase in diameter. At 72 hours, the extent of axonal swellings exhibiting compacted neurofilaments appeared to decrease, and was accompanied by punctate immunoreactivity within axon tracts suggestive of axonal degeneration. Although NFC was present in the same anatomical locations where axonal accumulation of amyloid precursor protein (APP) has been observed, double-label immunohistochemistry revealed no evidence of colocalization of compacted neurofilament and APP. Pre-injury treatment with either the NMDA receptor antagonist, ifenprodil, or the AMPA receptor antagonist, NBQX, had no significant effect on the extent of TAI, suggesting that excitotoxicity may not be a primary mechanism underlying TAI. Importantly, these data are indicative of the heterogeneity of mechanisms underlying TAI in the traumatically-injured immature brain.
Traumatic brain injury (TBI) is a leading cause of death and disability in the pediatric population, with infants and children less than 4 years of age exhibiting higher morbidity and mortality when compared to older children and adults (Langlois et al., 2003; Langlois et al., 2005; Levin et al., 1992). The pathophysiologic hallmark of pediatric TBI is diffuse axonal injury (DAI), and recent data using susceptibility-weighted imaging revealed that the severity of DAI positively correlates with negative outcome (Ashwal et al., 2006). In TBI, a limited number of axons undergo primary axotomy directly resulting from impact, while the majority of injured axons degenerate via secondary axotomy (Gennarelli, 1996; Maxwell et al., 1997). Secondary axotomy may occur due to a combination of focal alterations of the axolemmal structure and function, focal loss of the microtubular network, ionic dysregulation, proteolytic activity, impaired axonal transport (IAT), and neurofilament compaction (NFC) (Buki et al., 1999; Okonkwo et al., 1998; Pettus and Povlishock, 1996; Povlishock et al., 1983; Saatman et al., 1996; Stone et al., 2001).
Traumatic axonal injury (TAI), the pathologic manifestation of DAI, has been reported following diffuse brain trauma in the 3–5 day old piglet (Raghupathi and Margulies, 2002; Raghupathi et al., 2004) and 17 day-old rat (Adelson et al., 2001; Raghupathi and Huh, 2007). In all cases, TAI was first observed as swellings in contiguous axons which progressed to terminal bulbs (secondary axotomy) in multiple subcortical white matter tracts and brainstem (Adelson et al., 2001; Raghupathi and Margulies, 2002). Both the 68kDa and 200kDa neurofilament subunits have been observed in these focal axonal swellings (Raghupathi and Margulies, 2002; Raghupathi et al., 2004). Neurofilaments are heteropolymers comprised of the light neurofilament subunit (NF-L, 68kDa) with either the medium (NF-M, 160kDa) or the heavy (NF-H, 200kDa) subunit, all of which are differentially expressed throughout development (Lariviere and Julien, 2004). Axonal NF-H does not reach adult levels until several weeks following birth and has more phosphorylation sites than NF-M (Carden et al., 1987; Lariviere and Julien, 2004). It has been suggested NFC results from a conformational change in NF-M and NF-H subunits related to either dephosphorylation (de Waegh et al., 1992) or proteolysis (Hall and Lee, 1995). It is currently unknown whether alterations in neurofilaments impair axonal transport, which we have demonstrated in the 17 day-old rat (Huh and Raghupathi, 2007; Huh et al., 2008; Raghupathi and Huh, 2007). In adult models of diffuse brain trauma, IAT and NFC are reported to occur in separate populations of injured axons (Marmarou et al., 2005; Stone et al., 2001). In the present study, a co-labeling strategy was employed to investigate the spatial and temporal relationships between IAT and NFC in the traumatically-injured immature brain, and to test the hypothesis that NFC may have a causal relationship to IAT.
Despite growing attention, the mechanisms underlying traumatic pathology in the immature brain remain unknown. Although a role for calcium in IAT has not been identified, both calpains (calcium-activated proteases) and calcineurin (calcium-dependent phosphatase) have been implicated in NFC following TBI in the adult rat (Buki and Povlishock, 2006). In the 17 day-old rat, concussive brain trauma resulted in an acute increase in intracellular calcium concentrations which returned to control levels by 4 days post-injury (Osteen et al., 2001). It has been suggested that this rise in intracellular calcium may be causally related to a decrease in the expression of the NR2A subunit of the N-methyl-D-aspartate receptor (NMDAR) complex thereby increasing the relative expression of the NR2B subunit (Giza et al., 2006). The latter subunit is more sensitive to glutamate, has higher peak currents, and has a longer inactivation time (Scheetz and Constantine-Paton, 1994). Furthermore, ifenprodil, the NMDAR antagonist which selectively inhibits the NR2B subunit, was found to attenuate the TBI-induced calcium uptake observed in the adult animal following injury (Osteen et al., 2004). Both competitive (CPP) and non-competitive (MK-801) NMDAR antagonists, but not the non-competitive alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) antagonist 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzol[f]quinoxaline-7-sulfonamide (NBQX), reduced excitotoxic cell death but increased apoptotic cell death following contusive brain trauma in the neonate rat (Ikonomidou and Turski, 1996; Pohl et al., 1999). Antagonists to the AMPAR are effective in reducing axonal damage and white matter injury following traumatic spinal cord injury (Park et al., 2004), and IAT following diffuse brain injury (Goda et al., 2002). Therefore, we hypothesized that the AMPAR antagonist, but not the NMDAR antagonist, may attenuate TAI in the immature brain.
Results
Injured Axons Exhibit Neurofilament Compaction
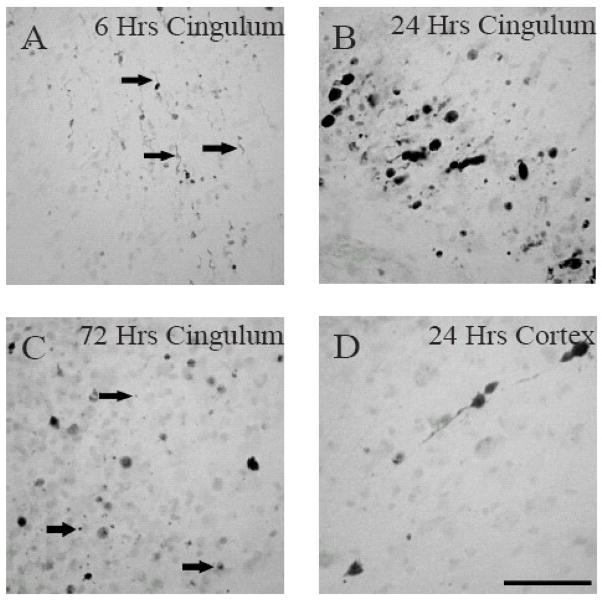
Neurofilament compaction (NFC), a marker of traumatic axonal injury (TAI), was observed in multiple subcortical white matter tracts in both hemispheres of the traumatically-injured immature rat brain (Fig. 1). There was no evidence of RM014 immunoreactivity in sham-injured animals (Fig. 1A–C). As early as 6 hours post-injury (Fig. 1D–F), RM014 immunoreactivity was extensive in the cingulum (Cg, Fig. 1E), but was not observed in the corpus callosum (CC, Fig. 1D) or lateral white matter tracts (LWM) between the Cg and rhinal fissure (Fig. 1F). By 24 hours, injured axons exhibiting RM014 were visible in the CC on both sides of the midline (Fig. 1G), the Cg (Fig. 1H) and LWM (Fig. 1I). By 3 days, the extent of staining had decreased in the Cg (Fig 1K) and LWM (Fig 1L). In the CC (Fig 1J), RM014 (+) labeling was present throughout the entire tract with smaller punctate patterns observed in the most dorsal aspect. Finally, sporadic axons and cell bodies exhibited RM014 immunoreactivity in the cortex adjacent to the midline at 6 and 24 hours post-injury (data not shown). Spatially, immunoreactivity extended from bregma through to 5.6mm posterior to bregma. The temporal pattern of RM014-labeled axons in the different white matter tracts was accompanied by a morphological progression of the RM014-labeled injured axons (Fig. 2). Immunoreactivity for RM014 at 6 hours post-injury in the Cg (Fig. 2A) and 24 hours in the cortex (Fig. 2D) manifested as small processes attached to axonal swellings as well as elongated axons; the swellings in the Cg appeared to progress to terminal bulbs by 24 hours (Fig. 2B). By 72 hours, in addition to staining in terminal bulbs, RM014 immunoreactivity was observed as punctate labeling, suggestive of axonal degeneration (Fig. 2C).
Figure 1. Intra-axonal RM014 immunoreactivity following diffuse brain injury in immature rats.
Representative photomicrographs of RM014-labeled axons in sham-injured animals (A–C), at 6 hours (D–F), 24 hours (G–I), and 72 hours (J–L) post-injury in the corpus callosum (A, D, G, J), cingulum (B, E, H, K), and lateral white matter tracts (C, F, I, L). The dashed lines represent the separation of the lateral aspect of corpus callosum from the cingulum. Scale bar=100μm for all panels.
Figure 2. Morphological appearance of injured axons exhibiting RM014 immunoreactivity.
Representative photomicrographs demonstrate RM014-labeled swollen axons at 6 hours (A, arrows) and 24 hours (D), and terminal bulbs at 24 hours (B). (C) Note the presence of punctate RM014 immunoreactivity (arrows) indicative of axonal degeneration at 72 hours. Scale bar=50μm for all panels.
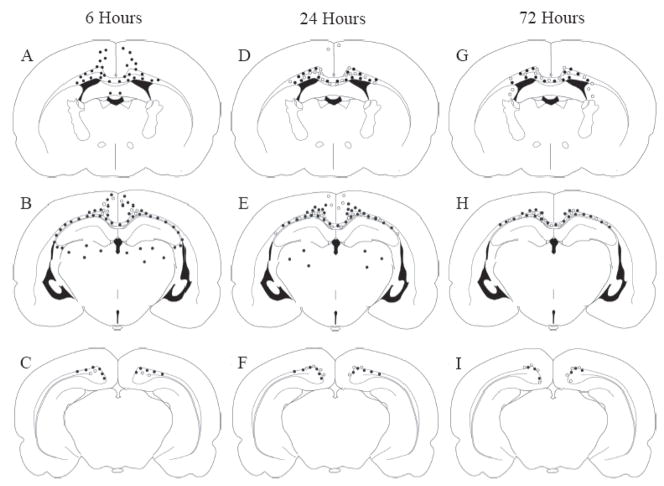
Figure 3 illustrates, in schematic form, the spatial distribution and temporal pattern of intra-axonal accumulations of APP (filled circles; Huh et al., 2008), indicative of impaired axonal transport (IAT), along with injured axons exhibiting RM014 immunoreactivity (open circles). Both IAT and NFC were observed in the same anatomical locations such as the CC, Cg, LWM, and in the cortex directly under the site of impact (Fig. 3). At all times post-injury, in all injured regions, there appeared to be a greater abundance of IAT when compared to NFC. In most white matter tracts, axonal APP accumulation was observed at an earlier time point (6 hours, panels A–C) than the appearance of RM014 immunoreactivity (24 hours, panels D–F). Moreover, IAT was unaccompanied by NFC in the thalamus and fimbria at 6 (Fig. 3A–C) and 24 hours (Fig. 3D–F) post-injury, suggesting that IAT may occur independently of NFC.
Figure 3. Anatomical and temporal distribution of axons exhibiting APP or RM014 immunoreactivity.
APP- (filled circles) and RM014-labeled (open circles) injured axonal profiles were located in the corpus callosum, cingulum, and lateral white matter tracts at 6 (A–C), 24 (D–F), and 72 hours (G–I) following diffuse brain injury. Note the greater extent of APP(+) immunoreactivity compared to RM014(+) axons in all brain regions.
Neurofilament Compaction Does Not Co-localize With APP Accumulation
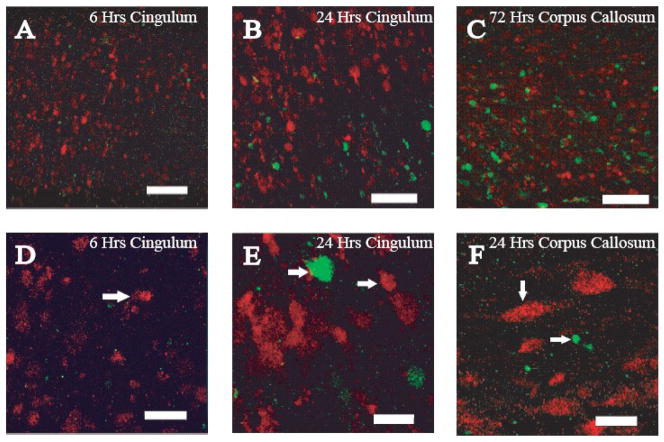
Single-labeling immunohistochemistry revealed IAT and NFC localized to axons in the same anatomical locations (Fig 3). To investigate whether these two pathologic entities occurred in the same axons, we performed double-label immunohistochemical analysis. Sham-injured animals revealed no evidence of IAT or NFC (data not shown). As observed with the single-labeling technique, a greater extent of APP(+) injured axons was noted compared to axons exhibiting RM014(+) axons (Fig 4). Importantly, intra-axonal RM014 immunoreactivity in the Cg did not co-localize with APP(+) axonal swellings at either 6 (Fig. 4A) or 24 (Fig. 4B) hours post-injury. In the CC at 72 hours post-injury, RM014(+) and APP(+) axons were intermingled, but were observed as independent immunoreactive axons (Fig. 4C). Morphological differences were observed between APP(+) and RM014(+) immunoreactive axons (Figures 4D–4F). At 6 hours, APP(+) and RMO14(+) immunoreactivities appeared as small-diameter swellings (Fig. 4D), and by 24 hours, both APP(+) and RM014(+) swellings were increased in diameter (Fig. 4E, 4F). At all times, in all regions, APP(+) axonal swellings were typically larger in diameter than RM014(+) swellings (Fig. 4E, 4F). It must be noted that the extent of RM014 immunoreactivity in these fluorescent images appears less than that observed in figure 1, and must be considered in light of the differences in sensitivity of detection between single (ABC-DAB) and double-labeling (directly conjugated fluorescent secondary antibodies) immunohistochemical techniques.
Figure 4. Double-label immunofluorescence for intra-axonal APP accumulation and RM014 immunoreactivity.
Axons containing accumulations of APP (red) were present to a greater extent than RM014-labeled axonal swellings (green) at 6 (A), 24, (B), and 72 hours (C) in the cingulum (A,B) and corpus callosum (C). (D) APP accumulation manifested as terminal bulbs (arrows) was observed in the cingulum at 6 hours post-injury. (E) APP(+) and RM014(+) terminal bulbs (arrows) were observed at 24 hours post-injury in the cingulum. (F) Terminal bulbs (arrow) can be detected in the corpus callosum at 24 hours post-injury. Scale bars represent 47μm for panels A–C, and 11μm for panels D–F.
Glutamate Receptor Antagonists Do Not Affect Traumatic Axonal Injury
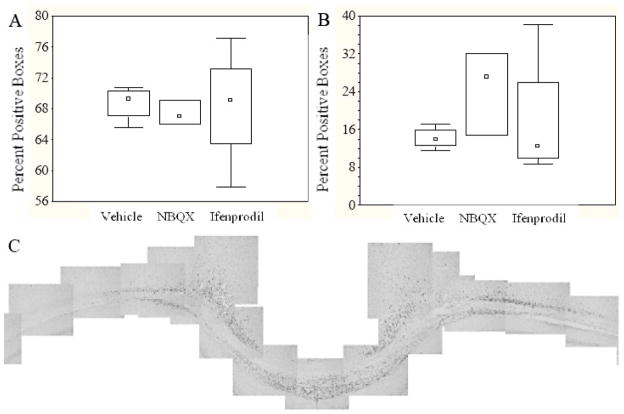
Similar to the observations in the first study (vide supra), brain-injured animals in the second study, irrespective of treatment, exhibited intra-axonal accumulations of APP and RM014 (+) swellings in the CC, Cg and LWM at 24 hours post-injury (data not shown). Moreover, administration of either ifenprodil or NBQX prior to injury did not affect the morphologic appearance of APP(+) and RMO14(+) injured axonal profiles, compared to vehicle-treated rats. Quantification of the extent of APP and RM014 immunoreactivity in the subcortical white matter tracts below the site of impact did not reveal statistically significant differences between the vehicle- and drug-treated animals (Fig. 5A: APP, H (2,11)=0.67); Fig. 5B: RM014, H (2,11)=2.71).
Figure 5. Quantification of APP(+) and RM014(+) profiles in the subcortical white matter tracts.
Panel A illustrates the average number of boxes containing APP(+) profiles, while Panel B illustrates the average number of RM014(+) profiles. Box-and-whisker plots denote the median (squares), 25th and 75th quartiles (rectangle), and range (whiskers) of the data. (C) A representative montage of the subcortical white matter region at 3.60mm posterior to bregma used to quantify the immunoreactivity of APP (illustrated) and RM014.
Discussion
The current study illustrates the temporal and spatial progression of neurofilament compaction (NFC) following non-contusive (diffuse) head injury in the immature rat. Compaction was observed in the corpus callosum, cingulum, and lateral white matter tracts, and was more prevalent at 24 and 72 hours post-injury. Axons exhibiting NFC progressed from swellings to terminal bulbs to a punctate appearance, indicative of progressive axonal degeneration. Although axons with compacted neurofilaments were intermingled in the same white matter tracts where axonal APP accumulation was detected, confocal microscopic analysis did not reveal colocalization. Despite the evidence of a role for excitotoxicity in pediatric TBI, the glutamate receptor antagonists ifenprodil (NMDA receptor), and NBQX (AMPA receptor), had no detectable effect on the extent of traumatic axonal injury (TAI) in the immature brain.
This is the first evidence of NFC following traumatic brain injury in the immature rat. Following diffuse brain trauma in adult rats, RM014(+) staining, indicative of NFC, was observed in thin, elongated axons as early as 30 minutes post-injury and did not progress morphologically through 6 hours post-injury (Stone et al., 2001). Our observations revealed that by 6 hours post-injury (the earliest time point evaluated in the present study), RM014 immunoreactivity was present in both axonal swellings and thin, elongated axons. These pathologic appearances progressed to terminal bulbs before undergoing degeneration revealed by punctate staining patterns. It is also important to recognize that evaluation of NFC in the adult brain was restricted to the long white matter tracts of the brainstem; in the immature brain, we report incidence of NFC in the subcortical white matter tracts because we did not observe evidence of NFC in the brainstem (data not shown). These differences may be related to a combination of the age and the mechanism of the primary injury (weight-drop in the adult versus indentor-impact in the immature rat). In the traumatically-injured adult rat brain, electron microscopy and tract-tracing studies revealed evidence of NFC, which was accompanied by axolemmal folding and permeabilization, and mitochondrial swelling (Pettus and Povlishock, 1996). Axolemmal permeability may lead to an increase in intracellular calcium, thereby activating both calpains and calcineurin (Buki and Povlishock, 2006). Compaction may subsequently result from calcium-mediated dephosphorylation (via calcineurin) of the neurofilament side-arm domains of either NF-M or NF-H which decreases the side arm angle reducing the electro-repulsion between neighboring neurofilaments (de Waegh et al., 1992; Nixon et al., 1994). Alternatively, side-arm domains can undergo proteolysis leading to compaction of neurofilaments (Hall and Lee, 1995). In this regard, the calpain inhibitor MDL28170, but not the calcineurin inhibitor FK506, attenuated NFC in the brainstem of adult rats following diffuse brain injury (Buki et al., 2003; Marmarou and Povlishock, 2006). Because the immature brain has a higher ratio of NF-M to NF-H when compared to the adult animal (Carden et al., 1987), our observations suggest that conformational changes to NF-M alone - dephosphorylation or proteolysis - may lead to compaction.
Qualitative assessment of our data did not reveal any evidence of injured axons containing both APP accumulation and RM014 immunoreactivity in any subcortical white matter region of the injured immature brain. Intra-axonal accumulation of APP along with other organelles leads to axonal swellings and is the result of impaired axonal transport (IAT) (Maxwell et al., 1997). It was originally hypothesized that structural changes in the axon such as axolemmal disruption and/or NFC could be causative mechanisms for IAT (Maxwell and Graham, 1997). Although APP accumulation in injured axons did not co-localize with those demonstrating axolemmal disruption (Stone et al., 2004) or with RM014 immunoreactivity in pyramidal and corticospinal tracts (Stone et al., 2001), quantitative analysis revealed a small subset (25%) of injured axons in the medial lemniscus of the brain stem of the adult rat did possess both APP accumulation and RM014-labeled axons (Marmarou et al., 2005). Post-injury administration of the calcineurin inhibitor, FK506, decreased the extent of IAT (Marmarou and Povlishock, 2006; Reeves et al., 2007), but did not affect NFC (Marmarou and Povlishock, 2006). Taken together it would appear that IAT and NFC lack a causal relationship in both the adult and pediatric models of diffuse brain injury.
Our data, albeit preliminary, suggest that neither ifenprodil nor NBQX significantly affected TAI in traumatically-injured immature rats. Whereas AMPAR antagonists have been effective in reducing white matter tissue loss and functional deficits in axons following traumatic spinal cord injury (Park et al., 2004), few studies examine a link between excitotoxicity and white matter damage in the traumatically-injured adult or immature brain. In the adult, the AMPAR antagonist, NBQX, reduced the extent of IAT in the brainstem following diffuse brain injury (Goda et al., 2002), but did not affect post-traumatic cell death in the neonate rat (Ikonomidou and Turski, 1996), suggestive of an age-dependent role for AMPARs in closed head injury. Similarly, our observations suggest that calcium entry via AMPARs may not be associated with post-traumatic neurofilament alterations. However, it must be emphasized that only one dose - albeit a dose that was effective in previous studies (Rosenberg et al., 1999) - was tested in the present study. Increases in cellular calcium flux have been observed in the cortex and thalamus after concussive brain trauma in the immature rat (Osteen et al., 2001), which has been causally related to an increase in the relative expression of the NR2B subunit of the NMDA receptor complex (Giza et al., 2006). Contusive brain trauma in the immature rat resulted in excitotoxic damage to the cortex, which was decreased by NMDAR antagonists (Ikonomidou and Turski, 1996; Pohl et al., 1999). Again, our observations must be cautiously interpreted in light of a single-dose administration of ifenprodil (10mg/Kg) and analysis at one survival time (24 hours), although this dose of ifenprodil has been demonstrated to attenuate acute damage following contusive brain trauma in the adult rat (Dempsey et al., 2000). Finally, we must emphasize that our negative observations may not be indicative of a lack of an effect of the two receptor antagonists used; rather, our data suggest that the effect may not be observed with the sample sizes used in the present study.
The mechanisms behind impaired axonal transport and neurofilament compaction are still not clearly understood, notwithstanding the observations that the mechanisms of IAT and NFC are separate and distinct following diffuse brain injury in the immature rat. These data provide further evidence for the heterogeneity of traumatically induced axonal injury in the developing brain.
Experimental Procedure
Brain Injury
The animals used in the present analysis were part of a larger study evaluating neurodegeneration, impaired axonal transport, blood brain barrier breakdown, reactive astrocytosis, acute and chronic behavioral deficits as well as histopathological alterations following diffuse head injury in immature rats (Huh et al., 2008). Brain injuries were induced using the electronically-driven controlled cortical impact (eCCI) device (Custom Design and Fabrication, Richmond, VA), a modification of the pneumatic CCI previously described (Dixon et al., 1991; Huh and Raghupathi, 2007). The metal indentor was convex, measured 5mm in diameter and was driven with a velocity of 5m/sec with a dwell time of 100msec. Seventeen day old male and female Sprague-Dawley rat pups (Charles River Laboratories, Wilmington, MA; 33 ± 4g, mean ± SD) were anesthetized with isoflurane (5%) using a nose cone, and, once a loss of a tail-pinch reflex was observed, a midline incision was made to expose the skull. The periosteum was reflected and the animal was placed in a standard mouse restrainer (Braintree Scientific, Braintree, MA); the head was supported by a soft foam pad in order to make it level with the body. The restrainer was positioned under the CCI device, the nose cone was removed, and the zero-point for the indenter was made on the skull over the midline suture, midway between the lambda and bregma. At about 45 seconds after removal of anesthesia (by which time the animals began to exhibit a pain reflex, as evidenced by a toe pinch and retraction of the limb), rats were subjected to an impact, where the indentor traveled a distance of 3mm into the skull. Sham-injured animals were anesthetized with isoflurane (5%) via a nose cone, their scalps were opened, they were placed in the restrainer, the nose cone was removed, and the impactor tip was zeroed on the skull. The total time from initiation of anesthesia to removal of the nose cone prior to the zeroing of the impactor tip to the surface of the skull was 4 minutes. Once animals regained normal breathing, they were re-anesthetized, the scalp was sutured shut, and the pups were returned to the dam. Animals were placed on a heating pad maintained at 37°C to maintain body temperature throughout the procedures and recovery. One group of 18 animals were euthanized at 6, 24, and 72 hours (N=4 injured and 2 shams per time point). A second group of 12 animals received intraperitoneal injections of either saline (1ml/Kg, N=4), ifenprodil (10mg/Kg/injection, N=4, Sigma-Aldrich, St. Louis, MO) or NBQX (10mg/Kg/injection, N=4, Sigma-Aldrich) 10 minutes prior to and 10 minutes following injury, and were euthanized at 24 hours post-injury. The doses of these two compounds were chosen based on their efficacy in limiting acute damage following brain (Dempsey et al., 2000) or spinal cord injury (Rosenberg et al., 1999). All surgical procedures were done in accordance with the rules and regulations of the Institutional Animal Care and Use Committee of Drexel University College of Medicine and were in compliance with the Guide for the Care and Use of Animals.
Tissue preparation for immunohistochemistry and immunofluorescence
Sham- and brain-injured animals were anesthetized (sodium pentobarbital, 60mg/Kg, i.p.) and euthanized by trans-cardial perfusion with 4% paraformaldehyde. Brains were post-fixed in the skull for 24 hours, then post-fixed outside the cranial cavity for an additional 24 hours, cryoprotected in 30% sucrose, and frozen in liquid isopentane at −35°C. Twelve sets of coronal (40 μm thick) sections were taken every 0.5 mm from 1mm anterior to bregma and 5.6 mm posterior to bregma; each “set” contained approximately 10 sections. One set of sections was evaluated for neurofilament compaction using the antibody RM014 (1:50,000; gift from John Trojanowski, University of Pennsylvania, Philadelphia, PA). This antibody binds to the rod domain of NF-M and NF-H, which is suggested to be exposed following structural alteration to the side-arm domain (Hall and Lee, 1995). This antibody has been used to demonstrate evidence of NFC in adult brain injured rats (Marmarou et al., 2005; Stone et al., 2001). Prior to incubation with RM014, antigen retrieval was effected by incubating sections in 10mM sodium citrate (pH 6.5) at 60°C for 20min. Primary antibody binding was detected using a biotinylated donkey anti-mouse IgG (1:500, Jackson ImmunoResearch, West Grove, PA) and was visualized using the ABC Elite system (Vector Laboratories, Burlingame, CA) with diaminobenzidine (DAB, Vector Laboratories) as chromogen. As a negative control, 1–2 sections from each animal were incubated with secondary antibody and the ABC reagent prior to exposure to DAB. Tissue sections were mounted on gelatin-coated slides and counterstained with hematoxylin (Gill-1, Thermo Shandon, Pittsburgh, PA).
For double-label immunofluorescent studies, sections underwent antigen retrieval as described above, and were incubated overnight with an antibody which recognizes the C-terminus of amyloid precursor protein (1:1000; Zymed Laboratories, San Fransisco, CA). Primary antibody detection was performed using Alexa 594-conjugated donkey anti-rabbit IgG antibody (1:300; Molecular Probes, Carlsbad, Ca). Sections were washed, incubated overnight in RM014 (1:5,000; Zymed Laboratories), followed by incubation with Alexa 488-conjugated donkey anti-mouse IgG (1:500; Molecular Probes). Sections were mounted on gelatin-coated slides and were coverslipped with Hardset Vectashield (Vector Laboratories). Negative control sections were incubated with both secondary antibodies. Slides were imaged using the Leica TCS SP2 microscope (a Laser Scanning Confocal Microscope) equipped with an argon laser for excitation at 488nm and a neon laser for excitation at 543nm.
Qualitative assessment of IAT and NFC distribution
A schematic of immunoreactivity for APP(+) and RMO(+) axons, intended to identify the anatomic locations of each pathologic entity at each of 3 coordinates relative to the bregma suture, was created for each brain as previously described (Huh et al., 2008). Then a single schematic for each coordinate was generated to represent the four brains used for analysis and illustrated in Figure 3. An open circle was placed in the specific anatomical location if an RM014(+) profile was present in at least 3 of the 4 brains analyzed. The anatomical locations of APP(+) axonal profiles were taken from Huh et al., (2008).
Quantification of APP(+) and RM014(+) axonal profiles
The extents of IAT and NFC following treatment with saline, ifenprodil or NBQX were quantified in 3 sections from each brain, taken from 2.80mm (section 1), 3.60 mm (section 2) and 4.3mm (section 3) posterior to bregma. These sections were chosen because they represented the rostral-caudal distribution of APP and RM014 immunoreactivity within axons. Digitized images of the subcortical white matter tract from 4.5mm lateral to the midline in each hemisphere in each of the 3 coronal brain section were taken at 10X magnification (objective: 10x/.25, WD10.5) and stitched together manually in Adobe Photoshop (version 7.0), to form a contiguous montage (Fig. 5C). A grid composed of 33.3μm × 33.3μm boxes (average number ± standard deviation of boxes for APP in section 1=2545±314, section 2=2742±547, section 3=2722±470; for RM014 in section 1=1769±170, section 2=1742±188, section 3=2360±466) was placed over the montage using Adobe Photoshop. A box was considered “positive” if it contained at least one immunoreactive profile, with borders on the bottom and left considered as exclusion lines. The percentage of positive boxes for each brain represented the average obtained from the 3 sections. Quantification was performed independently by two observers, blinded to the treatment status of the animal with an inter-rater reliability of 95%. Due to the non-parametric nature of the data, the groups were compared using the Kruskal-Wallis analysis of variance (Statistic version 7.1, Statsoft, Tulsa OK), and illustrated as box-and-whisker plots.
Acknowledgments
The authors acknowledge expert technical assistance from Ashley Widing (animal surgery), Ami Oristaglio-Wilson (blinded quantification) and Louise Bertrand (confocal microscopy). We are also grateful to Dr. John Q. Trojanowski (Center for Neurodegenerative Disease Research, University of Pennsylvania, Philadelphia PA) for the gift of the RM014 antibody. These studies were supported, in part, by The Endowed Chair of Critical Care Medicine, the Florence RC Murray grant from the Children’s Hospital of Philadelphia (JWH, RR), a Research Foundation grant from the University of Pennsylvania (JWH, RR), and NINDS grants K08-NS053651 (JWH) and R01-NS41561 (RR).
Abbreviations
- AMPA
Alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- APP
Amyloid precursor protein
- CC
Corpus Callosum
- Cg
Cingulum
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- IAT
Impaired Axonal Transport
- LWM
Lateral White Matter Tracts
- NBQX
1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzol[f]quinoxaline-7-sulfonamide
- NFC
Neurofilament Compaction
- NMDA
N-methyl-D-aspartate
- TAI
Traumatic Axonal Injury
- TBI
Traumatic Brain Injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adelson PD, Jenkins LW, Hamilton RL, Robichaud P, Tran MP, Kochanek PM. Histopathologic response of the immature rat to diffuse traumatic brain injury. J Neurotrauma. 2001;18:967–76. doi: 10.1089/08977150152693674. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Babikian T, Gardner-Nichols J, Freier MC, Tong KA, Holshouser BA. Susceptibility-weighted imaging and proton magnetic resonance spectroscopy in assessment of outcome after pediatric traumatic brain injury. Arch Phys Med Rehabil. 2006;87:S50–8. doi: 10.1016/j.apmr.2006.07.275. [DOI] [PubMed] [Google Scholar]
- Buki A, Siman R, Trojanowski JQ, Povlishock JT. The role of calpain-mediated spectrin proteolysis in traumatically induced axonal injury. J Neuropathol Exp Neurol. 1999;58:365–75. doi: 10.1097/00005072-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Buki A, Farkas O, Doczi T, Povlishock JT. Preinjury administration of the calpain inhibitor MDL-28170 attenuates traumatically induced axonal injury. J Neurotrauma. 2003;20:261–8. doi: 10.1089/089771503321532842. [DOI] [PubMed] [Google Scholar]
- Buki A, Povlishock JT. All roads lead to disconnection?--Traumatic axonal injury revisited. Acta Neurochir (Wien) 2006;148:181–93. doi: 10.1007/s00701-005-0674-4. discussion 193-4. [DOI] [PubMed] [Google Scholar]
- Carden MJ, Trojanowski JQ, Schlaepfer WW, Lee VM. Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J Neurosci. 1987;7:3489–504. doi: 10.1523/JNEUROSCI.07-11-03489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waegh SM, Lee VM, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 1992;68:451–63. doi: 10.1016/0092-8674(92)90183-d. [DOI] [PubMed] [Google Scholar]
- Dempsey RJ, Baskaya MK, Dogan A. Attenuation of brain edema, blood-brain barrier breakdown, and injury volume by ifenprodil, a polyamine-site N-methyl-D-aspartate receptor antagonist, after experimental traumatic brain injury in rats. Neurosurgery. 2000;47:399–404. doi: 10.1097/00006123-200008000-00024. discussion 404-6. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–62. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Gennarelli TA. The spectrum of traumatic axonal injury. Neuropathol Appl Neurobiol. 1996;22:509–13. doi: 10.1111/j.1365-2990.1996.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Giza CC, Maria NS, Hovda DA. N-methyl-D-aspartate receptor subunit changes after traumatic injury to the developing brain. J Neurotrauma. 2006;23:950–61. doi: 10.1089/neu.2006.23.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda M, Isono M, Fujiki M, Kobayashi H. Both MK801 and NBQX reduce the neuronal damage after impact-acceleration brain injury. J Neurotrauma. 2002;19:1445–56. doi: 10.1089/089771502320914679. [DOI] [PubMed] [Google Scholar]
- Hall GF, Lee VM. Neurofilament sidearm proteolysis is a prominent early effect of axotomy in lamprey giant central neurons. J Comp Neurol. 1995;353:38–49. doi: 10.1002/cne.903530106. [DOI] [PubMed] [Google Scholar]
- Huh JW, Raghupathi R. Chronic cognitive deficits and long-term histopathological alterations following contusive brain injury in the immature rat. J Neurotrauma. 2007;24:1460–74. doi: 10.1089/neu.2006.3787. [DOI] [PubMed] [Google Scholar]
- Huh JW, Widing AG, Raghupathi R. Midline brain injury in the immature rat induces sustained cognitive deficits, bihemispheric axonal injury and neurodegeneration. Exp Neurol. 2008;213:84–92. doi: 10.1016/j.expneurol.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Turski L. Prevention of trauma-induced neurodegeneration in infant and adult rat brain: glutamate antagonists. Metab Brain Dis. 1996;11:125–41. doi: 10.1007/BF02069500. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Kegler SR, Butler JA, Gotsch KE, Johnson RL, Reichard AA, Webb KW, Coronado VG, Selassie AW, Thurman DJ. Traumatic brain injury-related hospital discharges. Results from a 14-state surveillance system, 1997. MMWR Surveill Summ. 2003;52:1–20. [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20:229–38. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Lariviere RC, Julien JP. Functions of intermediate filaments in neuronal development and disease. J Neurobiol. 2004;58:131–48. doi: 10.1002/neu.10270. [DOI] [PubMed] [Google Scholar]
- Levin HS, Aldrich EF, Saydjari C, Eisenberg HM, Foulkes MA, Bellefleur M, Luerssen TG, Jane JA, Marmarou A, Marshall LF, et al. Severe head injury in children: experience of the Traumatic Coma Data Bank. Neurosurgery. 1992;31:435–43. doi: 10.1227/00006123-199209000-00008. discussion 443-4. [DOI] [PubMed] [Google Scholar]
- Marmarou CR, Walker SA, Davis CL, Povlishock JT. Quantitative analysis of the relationship between intra- axonal neurofilament compaction and impaired axonal transport following diffuse traumatic brain injury. J Neurotrauma. 2005;22:1066–80. doi: 10.1089/neu.2005.22.1066. [DOI] [PubMed] [Google Scholar]
- Marmarou CR, Povlishock JT. Administration of the immunophilin ligand FK506 differentially attenuates neurofilament compaction and impaired axonal transport in injured axons following diffuse traumatic brain injury. Exp Neurol. 2006;197:353–62. doi: 10.1016/j.expneurol.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Graham DI. Loss of axonal microtubules and neurofilaments after stretch-injury to guinea pig optic nerve fibers. J Neurotrauma. 1997;14:603–14. doi: 10.1089/neu.1997.14.603. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma. 1997;14:419–40. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Paskevich PA, Sihag RK, Thayer CY. Phosphorylation on carboxyl terminus domains of neurofilament proteins in retinal ganglion cell neurons in vivo: influences on regional neurofilament accumulation, interneurofilament spacing, and axon caliber. J Cell Biol. 1994;126:1031–46. doi: 10.1083/jcb.126.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo DO, Pettus EH, Moroi J, Povlishock JT. Alteration of the neurofilament sidearm and its relation to neurofilament compaction occurring with traumatic axonal injury. Brain Res. 1998;784:1–6. doi: 10.1016/s0006-8993(97)01075-5. [DOI] [PubMed] [Google Scholar]
- Osteen CL, Moore AH, Prins ML, Hovda DA. Age-dependency of 45calcium accumulation following lateral fluid percussion: acute and delayed patterns. J Neurotrauma. 2001;18:141–62. doi: 10.1089/08977150150502587. [DOI] [PubMed] [Google Scholar]
- Osteen CL, Giza CC, Hovda DA. Injury-induced alterations in N-methyl-D-aspartate receptor subunit composition contribute to prolonged 45calcium accumulation following lateral fluid percussion. Neuroscience. 2004;128:305–22. doi: 10.1016/j.neuroscience.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–74. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- Pettus EH, Povlishock JT. Characterization of a distinct set of intra-axonal ultrastructural changes associated with traumatically induced alteration in axolemmal permeability. Brain Res. 1996;722:1–11. doi: 10.1016/0006-8993(96)00113-8. [DOI] [PubMed] [Google Scholar]
- Pohl D, Bittigau P, Ishimaru MJ, Stadthaus D, Hubner C, Olney JW, Turski L, Ikonomidou C. N-Methyl-D-aspartate antagonists and apoptotic cell death triggered by head trauma in developing rat brain. Proc Natl Acad Sci U S A. 1999;96:2508–13. doi: 10.1073/pnas.96.5.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock JT, Becker DP, Cheng CL, Vaughan GW. Axonal change in minor head injury. J Neuropathol Exp Neurol. 1983;42:225–42. doi: 10.1097/00005072-198305000-00002. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, Margulies SS. Traumatic axonal injury after closed head injury in the neonatal pig. J Neurotrauma. 2002;19:843–53. doi: 10.1089/08977150260190438. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, Mehr MF, Helfaer MA, Margulies SS. Traumatic axonal injury is exacerbated following repetitive closed head injury in the neonatal pig. J Neurotrauma. 2004;21:307–16. doi: 10.1089/089771504322972095. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, Huh JW. Diffuse brain injury in the immature rat: evidence for an age-at-injury effect on cognitive function and histopathologic damage. J Neurotrauma. 2007;24:1596–608. doi: 10.1089/neu.2007.3790. [DOI] [PubMed] [Google Scholar]
- Reeves TM, Phillips LL, Lee NN, Povlishock JT. Preferential neuroprotective effect of tacrolimus (FK506) on unmyelinated axons following traumatic brain injury. Brain Res. 2007;1154:225–36. doi: 10.1016/j.brainres.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg LJ, Teng YD, Wrathall JR. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. J Neurosci. 1999;19:464–75. doi: 10.1523/JNEUROSCI.19-01-00464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman KE, Bozyczko-Coyne D, Marcy V, Siman R, McIntosh TK. Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. J Neuropathol Exp Neurol. 1996;55:850–60. doi: 10.1097/00005072-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Scheetz AJ, Constantine-Paton M. Modulation of NMDA receptor function: implications for vertebrate neural development. FASEB J. 1994;8:745–52. doi: 10.1096/fasebj.8.10.8050674. [DOI] [PubMed] [Google Scholar]
- Stone JR, Singleton RH, Povlishock JT. Intra-axonal neurofilament compaction does not evoke local axonal swelling in all traumatically injured axons. Exp Neurol. 2001;172:320–31. doi: 10.1006/exnr.2001.7818. [DOI] [PubMed] [Google Scholar]
- Stone JR, Okonkwo DO, Dialo AO, Rubin DG, Mutlu LK, Povlishock JT, Helm GA. Impaired axonal transport and altered axolemmal permeability occur in distinct populations of damaged axons following traumatic brain injury. Exp Neurol. 2004;190:59–69. doi: 10.1016/j.expneurol.2004.05.022. [DOI] [PubMed] [Google Scholar]