Abstract
Y2@C79N and Tb2@C79N have been prepared by conducting the Krätschmer-Huffman electric arc-process under 20 torr of N2 and 280 torr of He with metal oxide doped graphite rods. These new heterofullerenes were separated from the resulting mixture of empty cage fullerenes and endohedral fullerenes by chemical separation and a two stage chromatographic process. Crystallographic data for Tb2@C79N • Ni(OEP) • 2C6H6 demonstrate the presence of an 80-atom cage with idealized Ih symmetry and two, widely separated Tb atoms inside with a Tb---Tb separation of 3.9020(10) Å for the major terbium sites. The EPR spectrum of the odd-electron Y2@C79N indicates that the spin density largely resides on the two, equivalent yttrium ions. Computational studies on Y2@C79N suggest that the nitrogen atom resides at a 665 ring junction in the equator on the fullerene cage and that the unpaired electron is localized in a bonding orbital between the two yttrium ions of this stable radical. Thus, the Tb-Tb bond length of the single-electron bond is the longest metal-metal bond reported so far.
Introduction
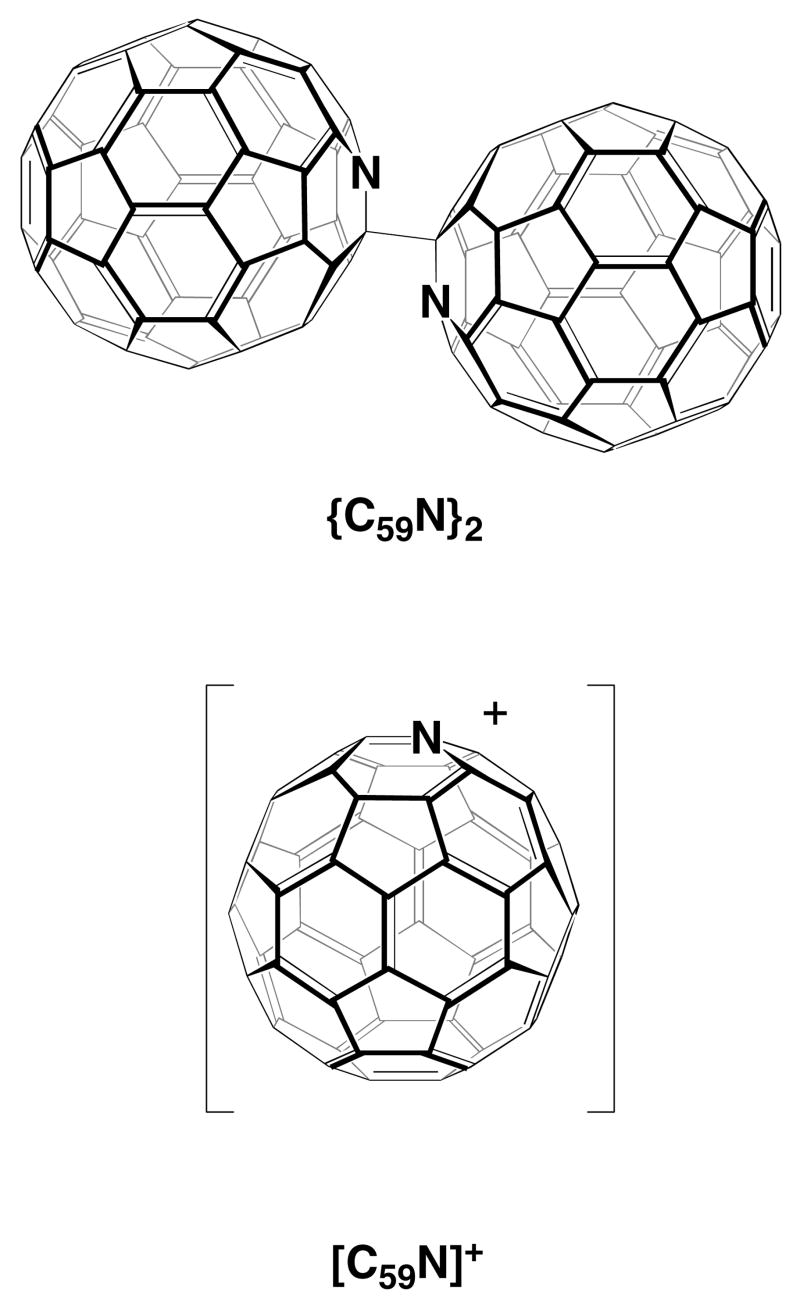
A number of reports of the detection and isolation of heterofullerenes, closed cages comprised of carbon atoms along with one or more non-carbon atoms arranged into five- and six-membered rings, have appeared.1–3 Of these heterofullerenes, the mono-azafullerene derived from C60 by substituting a nitrogen atom for one of the carbon atoms, is the most thoroughly characterized.4 Since the hypothetical C59N is an odd electron species, the neutral form of this mono-azofullerene is a dimer {C59N}2 with a single C-C bond connecting the two cages as seen in Scheme 1.5 The corresponding monomeric cation, [C59N]+, is isoelectronic with C60 and is a stable species that has been isolated in crystalline form as salts with various counter anions.6
Scheme 1.
Azafullerenes
Fullerenes themselves can act as hosts that encapsulate other atoms, molecules or atomic clusters. The resulting endohedral fullerenes have attracted considerable attention because the entrapped atoms bring with them an array of useful physical properties. For example, endohedral fullerenes with paramagnetic metal ions firmly trapped inside have been considered promising candidates for the next generation of contrast agents for magnetic resonance imaging (MRI).7–10 Similarly, endohedrals may be used to deliver radioactive atoms for applications in nuclear medicine.11,12
There has been only a limited amount of consideration given to the possibility that heterofullerenes could encapsulate other atoms or molecules. Theoretical work has considered the electronic structural properties that would result from substitution of carbon atoms in known endohedrals by heteroatoms like nitrogen or boron.13,14 Experimental observations on such heterofullerene endohedrals appears to be limited to a single report that describes the formation of species purported to be [La@C81N]+ and [La2@C79N]+, that were formed by fast atom bombardment mass fragmentation of the adducts, La@C82NCH2Ph and La2@C80NCH2Ph.15 This report also contained the results of electronic structure calculations on the corresponding neutral molecules that supported the formulation of these heteroendohedrals. In particular these computations suggested that the neutral molecules, La@C81N and La2@C79N, would exist as monomers and that the added electron that results from the nitrogen substitution would be transferred to the metal centers within the cage.
Subsequent to the report describing [La@C81N]+ and [La2@C79N]+, it has become clear that a number of different types of clusters of atoms can be found inside fullerene cages. Thus, there are large families of endohedrals built about generally planar M3N units,16–18 of which the prototype is Sc3N@Ih-C80.19 Additionally there is a class of endohedrals that involves an MxC2(x = 2 ~ 3) unit encased in a fullerene cage. Examples of these carbide containing endohedrals include Sc2C2@C8420 and Sc3C2@Ih-C80.21 As a result, it is possible that a molecule having the composition La2C79N could have any of the following structures: La2NC@C78, La2C2@C77N, or La2@C79N.
Here we report the preparation and isolation of sufficient quantities of M2@C79N (M = Y, Tb) to allow structural characterization.
Results
Preparation and Isolation
Samples of the dimetallic aza[80]fullerenes, M2@C79N (M = Tb or Y) were prepared utilizing the well established Krätschmer-Huffman (K-H) electric arc-process under 20 torr of N2 and 280 torr of He to vaporize cored graphite rods that were packed with Y2O3 or Tb4O7.16 The toluene-soluble extract from the electric-arc generator was separated first based on chemical reactivity differences using a cyclopentadiene-functionalized Merrifield peptide resin (CPDE-MPR) column and then further purified by two-stage HPLC chromatography as previously described.16,22,23 There were seven fractions from the 5PBB column at the first-stage of HPLC. The initially eluting fraction contained M2@C79N and C84. M2@C79N was further separated from C84 on a 5PYE column in the second-stage of HPLC. The HPLC chromatograms and mass spectra establish the identity of the purified samples of Y2@C79N and Tb2@C79N and the presence of a nitrogen atom in Tb2@C79N was confirmed by the MS spectrum of 15N-labeled sample (see supporting information).
The close correspondence between the UV/vis spectra of Y2@C79N and Tb2@C79N and the similar chromatographic retention behavior of Y2@C79N and Tb2@C79N suggests close correspondence of the cage electronic polarizabilities that are not significantly influenced by the nature of the internal M2 cluster. It is also well recognized that the chromatographic capacity factor is proportional to the fullerene cage polarizability and that it has a simple linear relationship with the fullerene cage size or carbon number. The retention mechanism is generally proportional to the fullerene cage polarizability and is dominated by π-π interactions with the stationary phase.24–26 Thus, the elution profile (co-elution with empty cage C84,, see SI) of M2@C79N suggests charge transfer to the cage surface representing to a first approximation 84–85 π-electrons. This surface charge is consistent with the computational model of [M2]5+@[C79N]5− (M = Y or Tb) (vide infra).
Crystallographic Studies
Black prisms of Tb2@C79N • Ni(OEP) • 2C6H6 were obtained by cocrystallization of Tb2@C79N and Ni(OEP) (OEP is the dianion of octaethylporphyrin) from a benzene solution of the components. Data were collected at a synchrotron source and refined by standard procedures. A drawing of the molecule obtained from the crystallographic data is shown in Figure 4. The crystallographic data are completely consistent with the Tb2@C79N formulation for the molecule and definitively rule out the alternative possibilities that the molecule could be Tb2CN@C78 or Tb2C2@C77N. The results demonstrate the presence of an 80-atom cage with idealized Ih symmetry and two, widely separated Tb atoms inside with a Tb-Tb separation of 3.9020(10) Å for the major terbium sites. Each Tb atom coordinates with an adjacent hexagon of the cage in η6-fashion. The Tb-C distances range from 2.366(10) to 2.523(11) Å with the Tb to ring centroid distance of 1.969(10) Å. The placement of the Tb atoms above the hexagons is remarkably similar to the location of the three Tb atoms in Tb3N@C80-Ih, where the Tb-C distances range from 2.404(3) to 2.518(3) Å and the Tb to ring centroid distance is 1.975(3) Å.16
Figure 4.
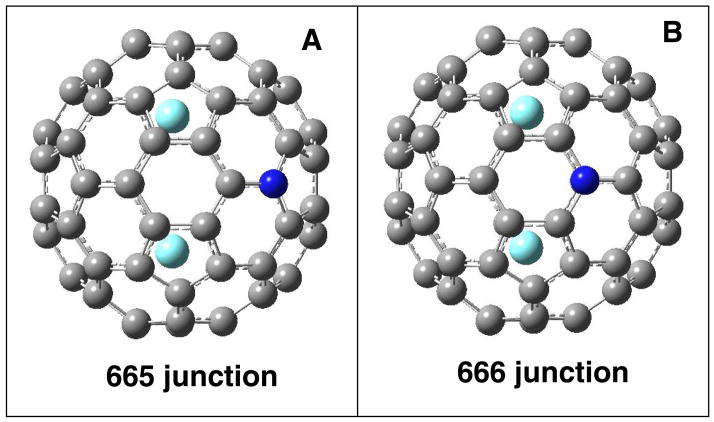
Illustrations of the computed structures of Y2@C79N with the nitrogen atom replacing: (A) a carbon atom in a pentagon (a 665 junction) and (B) a carbon atom that is not in a pentagon (a 666 junction).
As is typical with structures of this sort,16,19,23 there is disorder in the cage orientation and the locations of the Tb atoms inside. Only the major orientation is shown in Figure 1, but there are two orientations of the cage and ten other partially occupied sites for the terbium atoms. Another cage orientation and the locations of all terbium sites are shown in Figure 2. The multiplicity of terbium sites within the cage suggest that there is a low barrier to re-orientation of the Tb2 unit with regard to the cage itself. Similarly the Sc3N unit within Sc3N@C80 was found to possess a low barrier for re-orientation.27 As a result of the disorder, the crystallographic data do not identify specifically the site of the nitrogen atom in the cage. Because of the similar sizes and scattering power of carbon and nitrogen, differentiation between these two atoms is challenging in such a high symmetry environment. The X-ray crystal structure of the carbocation, (C59N)+, also displays disorder in the position of the nitrogen atom.6
Figure 1.
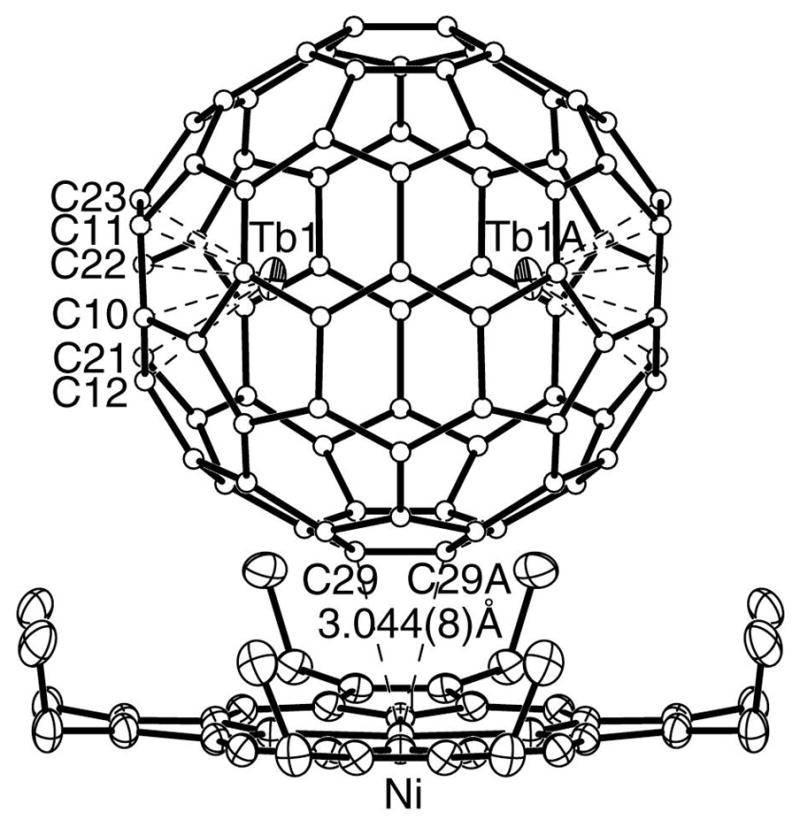
A drawing of the crystallographically determined structure of Tb2@C79N, Only the symmetrical molecular site with 0.050 fractional occupancy is shown. At this site there is a crystallographic mirror plane which lies perpendicular to the plane of the paper and bisects the line between the two terbium atoms. The Tb1-Tb1A separation in 3.9020(10) and the occupancy of Tb1 corresponds to 0.43 of the total Tb content.
Figure 2.
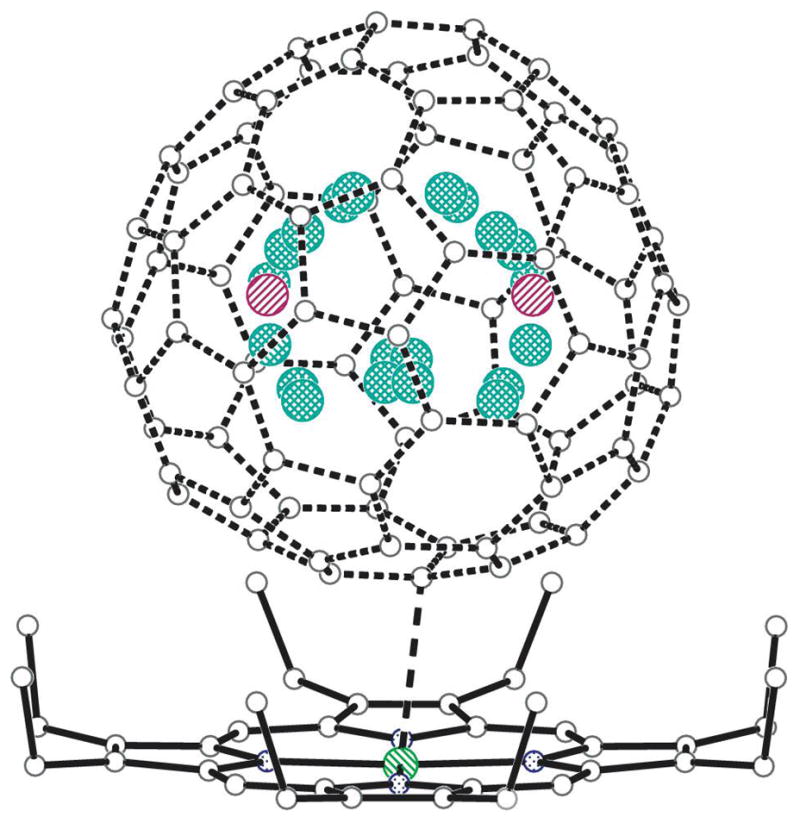
A drawing of the crystallographically determined structure of Tb2@C79N showing the unsymmetrical orientation of the heterofullerene cage. The orientation shown here has 0.25 fractional occupancy and a symmetry-related orientation generated by the crystallographic mirror plane has 0.25 occupancy. All of the terbium atom positions are shown along with those generated by the crystallographic mirror plane. The sites Tb2 through Tb11 have fractional occupancies that range from 0.102 to 0.026. When summed, these represent 0.57 of the total terbium content, while Tb1 (shown in red and hidden behind another terbium site) represents 0.43 of the terbium content in the asymmetric unit. The shortest contact (2.805(3) Å) between a cage atom and the nickel ion in the porphyrin is indicated by a dashed line.
The possibility that there is a specific interaction between the nitrogen atom of the cage and the Ni(OEP) molecule has also been examined and excluded. The closest contacts of atoms of the cage with the nickel atom are 3.044(8) and 2.805(3) Å for the two cage orientations. These distances are within the range found for numerous analogous structures with all-carbon cages.2
Electron Paramagnetic Resonance Studies
Y2@C79N contains an odd number of electrons and the paramagnetic character of Y2@C79N was confirmed by X-band EPR solution measurements at 298 K as illustrated in Figure 3. For a dilute sample in toluene solution three symmetric lines with a 1:2:1 intensity ratio were observed. This pattern is consistent with hyperfine splitting due to two equivalent 89Y nuclides (100 % natural abundance, nuclear spin of ½). The observed g factor, g = 1.9740 and large observed yttrium coupling of |81.23| G indicates that there is significant unpaired spin density localized on the yttrium centers. No hyperfine coupling was observed due to the nitrogen atom. The isotropic yttrium coupling of |81.23| G for Y2@C79N is larger than the anisotropic coupling constants obtained for Y3 clusters in frozen argon matrices by Knight and coworkers.28 The spectra of the Y3 cluster revealed two equivalent yttrium centers with a|| = 27.7 G and a⊥ = 14.5 G and a second yttrium center with a|| = 27.7 G and a|| = 19.7 G. The yttrium coupling for Y2@C79N has a greater magnitude than those observed for [Y3N@Ih-C80C4H9N]−, which had an isotropic hyperfine splitting of |6.26| G for two equivalent yttrium centers and an isotropic hyperfine splitting of |1.35| G for a third inequivalent yttrium center.29 The isotropic g factors for Y2@C79N (1.9740) and [Y3N@Ih-C80C4H9N]−(1.998915) and the anisotropic g factors for the Y3 cluster (g|| = 1.9603 and g⊥ = 1.9578) are all below 2 and are indicative of significant spin density on the yttrium centers in each of these clusters. These results are in sharp contrast to the isotropic g factor and hyperfine coupling reported for the metallofullerene Y@C82 (a = 0.49 G, g = 2.0006) where the unpaired electron spin density is mainly delocalized on the carbon cage.30
Figure 3.
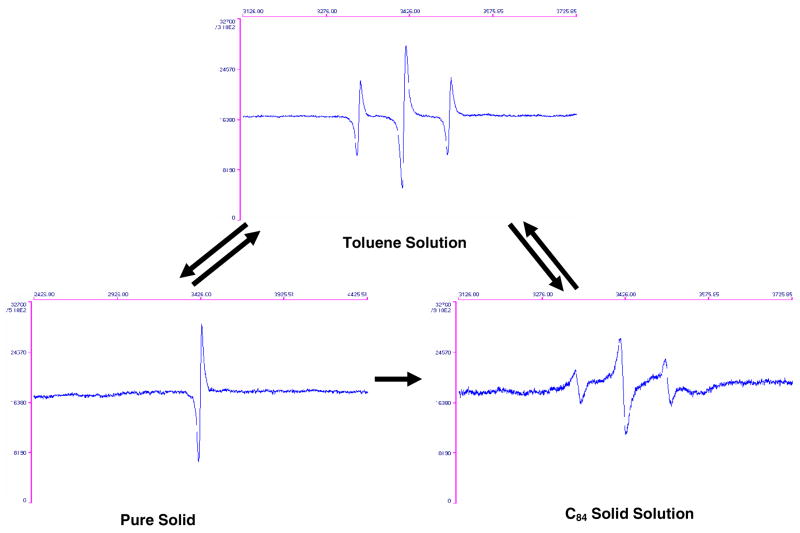
ESR spectra of Y2@C79N samples in toluene solution, as a solid, and as a solid solution with C84.
A solid sample of Y2@C79N exhibits an EPR spectrum consisting of a single line, which is broadened due to Heisenberg exchange. The effect of Heisenberg exchange was confirmed by a solid-state dilution experiment where the Y2@C79N sample was mixed with an empty cage fullerene, C84, of similar size. In this experiment, a set of three symmetric resonances in a 1:2:1 intensity ratio was observed as illustrated in Figure 3. The hyperfine coupling and g factor were similar to the data obtained from a toluene solution. The appearance of the EPR spectrum of the mixed solid solution suggests nearly isotropic motion of the Y2@C79N molecule and/or Y-Y cluster motional averaging in the C84 solid matrix. It is also important to note that the EPR spectrum for the Y2@C79N sample was unchanged after 6 months (even when exposed to O2). Thus, this paramagnetic molecule possesses considerable chemical stability.
An EPR spectrum for the Tb2@C79N sample was not observed. The lack of a readily detected EPR spectrum is consistent with the well-recognized, short electron relaxation time for the Tb+3 ion.31,32
Computational Studies
In order to augment the experimental observations and assist in explaining some of the physical properties of these new azafullerene endohedrals, density functional theory (DFT) computations using the spin-unrestricted B3LYP functional as defined in the Gaussian 0333 program package34–36 were performed for Y2@C79N as a suitable prototype. In the Ih-C80 cage there are two types of carbon atoms: 60 carbon atoms reside in pentagons at a 665 junction, while the remaining 20 are not part of pentagons but reside at the junctions of three hexagons (a 666 junction) as can be seen in Figure 4. In order to define further the location of the nitrogen atom in the C79N cage, UB3LYP geometry optimizations using the DZVP basis set for Y37 and the 6-31G* basis set for C and N38 were carried out with the nitrogen atom located at the two possible sites on the cage surface that are shown in Figure 4. Each isomer was demonstrated to be a minimum on the potential energy hypersurface via analytic second derivative (harmonic vibrational frequency) calculations. Our computational results for Y2@C79N predict that the 665 isomer shown in Part A of Figure 4 is 13.3 kcal/mol more stable than the 666 isomer. The HOMO-LUMO gaps are relatively large for both isomers at ca. 2.4 eV. 39-42These values are similar to the HOMO-LUMO gaps for the well known M3N@Ih-C80 class.39–42 similar computational approach for the empty [C79N]5− cage also predicts significantly higher thermodynamic and kinetic stability for the (665) isomer in comparison with the (666) isomer.
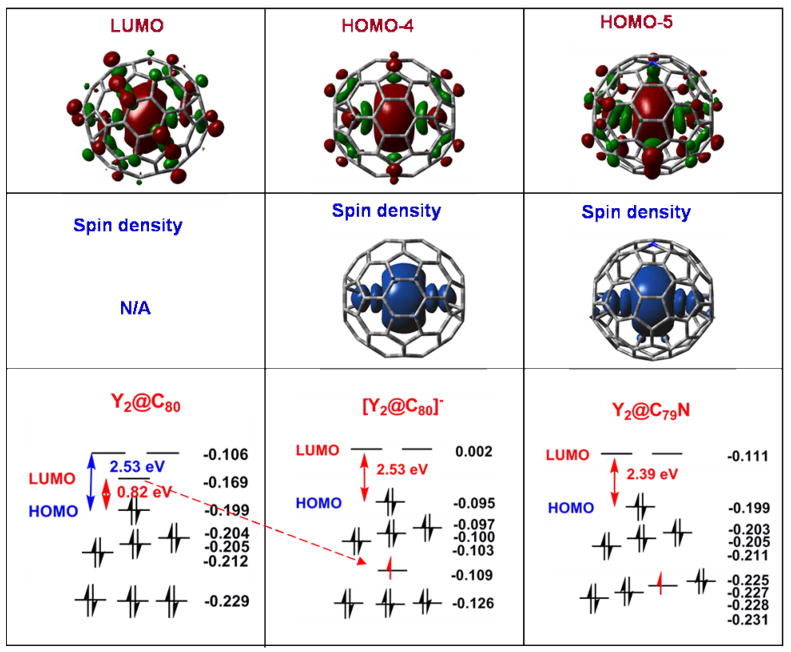
Figure 5 shows the UB3LYP molecular orbital energies for the orbitals near the HOMO-LUMO gap for three related species: even-electron Y2@Ih-C80, and odd-electron [Y2@Ih-C80]− obtained by one-electron reduction of Y2@Ih-C80, and Y2@C79N. The latter two are isoelectronic. The computational results for Y2@Ih-C80 indicate that it is a small band-gap material with a HOMO-LUMO gap of 0.82 eV. This small gap suggests this endohedral will have low stability, a result that is consistent with the paucity of observed experimental quantities of the Y2@Ih-C80.
Figure 5.
Kohn-Sham UB3LYP/DZVP(Y)+6-31G*(C,N) molecular orbitals for the optimized structures of Y2@ Ih-C80, [Y2@ Ih-C80]−, and Y2@C79N molecules. Orbital energies are shown at the bottom (energies of beta LUMO of [Y2@ Ih-C80]− and Y2@C79N molecules are omitted), while drawings of the critical yttrium based orbitals that contains the free spin in the paramagnetic molecules are shown at the top along with drawings of the distribution of the spin density in [Y2@ Ih-C80]− and Y2@C79N.
In contrast, iso-electronic [Y2@Ih-C80]− and Y2@C79Nare predicted by the spin-unrestricted B3LYP approach to be large band-gap materials with the electron spins residing in orbitals that are localized between the yttrium ions. By comparing the shapes of the UB3LYP molecular orbitals, it is recognized that the low-lying HOMO-4 orbital of [Y2@Ih-C80]− originates from the LUMO of Y2@Ih-C80. Upon accepting one electron, the LUMO in Y2@Ih-C80 becomes the HOMO-4 orbital of [Y2@Ih-C80]−. The small HOMO-LUMO gap (0.82 eV) of Y2@Ih-C80evolves as the large HOMO-LUMO gap (2.53 eV) of [Y2@Ih-C80]− as shown in Figure 5. As such, this system violates the Aufbau principle. However, there are precedents in spin-unrestricted calculations where spin-polarized orbitals reside at lower energy than the HOMO.43,44 Additionally, photoelectron spectroscopy has provided experimental evidence for the occurrence of a spin-containing orbital that resides at lower energy than the HOMO.45 (We note that, in contrast to the above, spin-restricted B3LYP calculations place the singly occupied orbital higher in energy than all other occupied orbitals. However, both the RB3LYP and UB3LYP models agree as to the size of the HOMO-LUMO gap and the shapes of the relevant valence MOs.)
The B3LYP/DZVP(Y)+6-31G*(C,N) model produces a calculated yttrium hyperfine coupling of −62.4 G for the 665 isomer (−61.2 G for the 666 isomer), which is consistent with the large α spin density observed between the two yttrium atoms in the cluster and compares well with the observed yttrium coupling constant of |81.23| G. The lack of an observed 14N hyperfine coupling for the cage nitrogen atom in the observed EPR spectrum is consistent with the small calculated value for the nitrogen hyperfine coupling of 0.01 G.
The computed Y-Y separation for Y2@C79N is 3.994 Å. This is unusually long for a molecule in which computations suggest that a one-electron bond connects the two yttrium ions. However, this distance is rather similar to the Tb-Tb distance of 3.9020(10) Å for the major terbium site in Tb2@C79N. Electrostatic repulsion within these M25+ units is likely to play a major role in determining the M-M separations.
In order to ensure that the DZVP basis set for Y used in the above calculations is sufficiently complete to provide a realistic description of the fullerene, we carried out a re-contraction of the correlation-consistent triple-zeta (cc-pVTZ) basis set using the exponents developed by Peterson and co-workers for Douglas-Kroll relativistic calculations.46 Using diffuse functions taken from the aug-cc-pVTZ-DK published by Peterson et al. to define an aug-cc-pVTZ basis for Y, we have carried out single-point calculations at the B3LYP/aug-cc-pVTZ(Y)+6-31G*(C,N) level of theory. We find that the results are essentially identical to those reported above using the DZVP basis set for Y, including the energy difference between the 665 and 666 isomers of Y2@C79N (13.2 kcal/mol), the HOMO-LUMO gap, and the yttrium hyperfine coupling (−59.7 G).
Discussion and Conclusions
The results presented here demonstrate that significant quantities of Y2@C79N and Tb2@C79N were formed along with a range of endohedrals of the M3N@C2n class and empty cage fullerenes when the Krätschmer-Huffman electric arc-process was conducted under 20 torr of N2 and 280 torr of He. Y2@C79N and Tb2@C79N are soluble species that can be separated from other fullerene and endohedral fullerene molecules by chromatographic means. The crystallographic data for Tb2@C79N clearly reveal the presence of a monomeric endohedral. Tb2@C79N consists of a cage of 80 atoms built with an atomic arrangement similar to that of Ih-C80 with two widely separated (3.9020(10) Å) terbium ions inside. While the crystallographic data do not locate the specific position of the nitrogen atom on the cage, our computational studies indicate that the nitrogen atom is probably located within a pentagon at a 665 site in the equatorial mirror plane that is orthogonal to the line between the two terbium ions as seen in Figure 4. Similarly, computational studies on La2@C79N also came to the conclusion that the nitrogen atom would reside at a 665 site symmetrically displaced between the two metal ions.15 Although we did not isolate the significant amount of pure La2@C79N for characterization, our high resolution LD-TOF mass spectrum clearly shows the existence of La2@C79N, along with La@C81N (see supporting information).
The EPR spectrum of Y2@C79N are consistent with a stable heterofullerene radical with a g-factor and yttrium hyperfine coupling that indicate that there is a significant amount of spin density localized on the two equivalent yttrium ions. The computational studies are consistent with these observations and indicate that the odd electron resides in an unusual bonding orbital that is localized between the two yttrium ions. The combination of our crystallographic and EPR data as well as theoretical calculation results demonstrates that the single-electron bond between two metal ions inside the [C79N]5− heterofullerene cage is the longest metal-metal bond reported so far.47–49
Experimental
Synthesis of Y2@C79N and Tb2@C79N
Core-drilled graphite rods (6.4 mm in diameter by152 mm in length) were packed with a mixture of Y2O3 or Tb4O7, graphite powder and iron nitride (FexN, x = 2 ~ 4). The total M:C molar ratios were about 3:100. The packed rods were pre-heated to about 1000°C under a flow of dinitrogen for about 10 hours to remove air and moisture. The rods were then vaporized in a Krätschmer -Huffman arc-discharge fullerene generator filled with a mixture of 20 Torr dinitrogen and 280 Torr helium gases. For 15N-labelled sample, 15N2 was used instead of natural dinitrogen gas.The raw soot produced in the reactor was extracted in a Soxhlet extractor using toluene as solvent for approximately 20 hours. The resulting extract was initially separated utilizing a CPDE-MPR column as previously described.16 With chemical separation, most of the empty cages and reactive classical endofullerenes are retained on the CPDE-MPR column and thus cannot be eluted. The HPLC chromatograms of the yttrium and terbium samples before and after chemical separation are shown in the supporting information.
In the case of yttrium, seven fractions were separated. The initially eluting fraction, Y1, contained Y2@C79N and C84. After chemical separation, the terbium-based sample had a similar HPLC trace, and also was separated into seven fractions. The first fraction, Tb1, contained Tb2@C79N and C84. Fractions Y1 and Tb1 were collected and were further separated using a 5PYE column. C84 was easily separated from Y2@C79N (or Tb2@C79N) since their retention times on a 5PYE column were very different. The HPLC chromatograms of the pure Y2@C79N and Tb2@C79N samples are shown in Figure 1.
X-ray Crystallography and Data Collection
The crystals were removed from the glass tubes in which they were grown together with a small amount of mother liquor, placed on a microscope slide together with a small amount of mother liquor, and immediately coated with a hydrocarbon oil. Suitable crystals were mounted on glass fibers with silicone grease. Data were collected at beamline 11.3.1 at the Advanced Light Source with the use of 0.7749 Å synchrotron radiation and a Bruker Platinum 200 goniometer. All data sets were integrated with the Bruker SAINT (v.7.16) program. Crystal data are reported below. A semi-empirical absorption correction utilizing equivalents was employed.50 The structure was solved by direct methods and refined using all data (based on F2) using the software of SHELXTL 5.1. Hydrogen atoms were located in a difference map, added geometrically, and refined with a riding model.
Crystal data
Tb2@C80N·Ni(OEP) ·2benzene, black prism (0.07 × 0.12 × 0.15 mm) of C128H56N4NiTb2, Mw = 2026.32, monoclinic, space group C2/m, a = 25.1848(7) Å, b = 15.1160(5) Å, c = 19.7406(7) Å, β = 95.063(1)°, V = 7485.8(4) Å3 at 90(2) K, γ = 0.77490 Å, ∝= 2.727 mm−1, Z = 4. Refinement of 10012 reflections, 568 parameters and 1 restraint yielded wR2 = 0.243 for all data and a conventional R1 of 0.0844 based on 8915 reflections with I > 2σ(I).
Supplementary Material
The HPLC chromatograms for the initial yttrium and terbium soot extracts as well as the purified products, the mass spectrum of Y2@C79N, Tb2@C79N, and 15N labeled Tb2@C79N sample, and corresponding Uv-vis spectra and the initial separation of La2@C79N and La@C81N and their mass spectra, and X-ray crystallographic files in CIF format for Tb2@C79N • Ni||(OEP) • 2.5C6H6. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank the National Science Foundation [CHE-0413857 (A.L.B.), CHE-0443850 (H.C.D.), DMR-0507083 (H.C.D.), CHE-0715185 (T.D.C)] and the National Institute of Health [1R01-CA119371-01 (H.C.D.)] for support. The authors gratefully acknowledge the Advanced Light Source, Beamline 11.3.1, operated under the auspices of the Director, Office of Science, Office of Basic Energy Sciences of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. We thank Mr. Kim Harich, Ms Anne Campbell, and Dr. Keith Ray for the mass spectra. We are also grateful to Prof. Kirk Peterson of Washington State University for providing the re-contracted cc-pVTZ basis set for yttrium used in the computational studies.
References
- 1.Vostrowsky O, Hirsch A. Chem Rev. 2006;106:5191–5207. doi: 10.1021/cr050561e. [DOI] [PubMed] [Google Scholar]
- 2.Branz W, Billas IML, Malinowski N, Tast F, Heinebrodt M, Martin TP. J Chem Phys. 1998;109:3425. [Google Scholar]
- 3.Poblet JM, Winkler K, Cancilla M, Hayashi A, Lebrilla CB, Balch AL. Chem Comm. 1999:493. [Google Scholar]
- 4.Hirsch A, Nuber B. Accounts Chem Res. 1999;32:795. [Google Scholar]
- 5.Hummelen JC, Knight B, Pavlovich J, Gonzalez R, Wudl F. Science. 1995;269:1554–6. doi: 10.1126/science.269.5230.1554. [DOI] [PubMed] [Google Scholar]
- 6.Kim KC, Hauke F, Hirsch A, Boyd PDW, Carter E, Armstrong RS, Lay PA, Reed CA. J Am Chem Soc. 2003;125:4024. doi: 10.1021/ja034014r. [DOI] [PubMed] [Google Scholar]
- 7.Mikawa M, Kato H, Okumura M, Narazaki M, Kanazawa Y, Miwa N, Shinohara H. Bioconjugate Chem. 2001;12:510–514. doi: 10.1021/bc000136m. [DOI] [PubMed] [Google Scholar]
- 8.Bolskar RD, Benedetto AF, Husebo LO, Price RE, Jackson EF, Wallace S, Wilson LJ, Alford JM. J Am Chem Soc. 2003;125:5471–5478. doi: 10.1021/ja0340984. [DOI] [PubMed] [Google Scholar]
- 9.Tóth E, Bolskar RD, Borel A, González G, Helm LEMA, Sitharaman B, Wilson LJ. J Am Chem Soc. 2005;127:799–805. doi: 10.1021/ja044688h. [DOI] [PubMed] [Google Scholar]
- 10.Fatouros PP, Corwin FD, Chen ZJ, Broaddus WC, Tatum JL, Kettenmann B, Ge Z, Gibson HW, Russ JL, Leonard AP, Duchamp JC, Dorn HC. Radiology. 2006;240:756. doi: 10.1148/radiol.2403051341. [DOI] [PubMed] [Google Scholar]
- 11.Wilson LJ, Cagle DW, Thrash TP, Kennel SJ, Mirzadeh S, Alford JM, Ehrhardt GJ. Coord Chem Rev. 1999;192:199–207. [Google Scholar]
- 12.Li QN, Xiu Y, Zhang XD, Liu RL, Du QQ, Shun XG, Chen SL, Li WX. Nucl Med Biol. 2002;29:707–710. [Google Scholar]
- 13.Hou JQ, Kang HS. Chem Phys. 2007;334:29. [Google Scholar]
- 14.Hou JQ, Kang HS. J Phys Chem A. 2007;111:1111. doi: 10.1021/jp065097b. [DOI] [PubMed] [Google Scholar]
- 15.Akasaka T, Okubo S, Wakahara T, Yamamoto K, Kobayashi K, Nagase S, Kato T, Kako M, Nakadaira Y, Kitayama Y, Matsuura K. Chem Lett. 1999:945. [Google Scholar]
- 16.Zuo T, Beavers CM, Duchamp JC, Campbell A, Dorn HC, Olmstead MM, Balch AL. J Am Chem Soc. 2007;129:2035–2043. doi: 10.1021/ja066437+. [DOI] [PubMed] [Google Scholar]
- 17.Dunsch L, Yang S. Small. 2007;3:1298. doi: 10.1002/smll.200700036. [DOI] [PubMed] [Google Scholar]
- 18.Melin F, Chaur MN, Engmann S, Elliott B, Kumbhar A, Athans AJ, Echegoyen L. Angew Chem Int Ed. 2007;46:9032. doi: 10.1002/anie.200703489. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson S, Rice G, Glass T, Harlch K, Cromer F, Jordan MR, Craft J, Hadju E, Bible R, Olmstead MM, Maltra K, Fisher AJ, Balch AL, Dorn HC. Nature. 1999;401:55–57. [Google Scholar]
- 20.Wang CR, Kai T, Tomiyama T, Yoshida T, Kobayashi Y, Nishibori E, Takata M, Sakata M, Shinohara H. Angew Chem Int Ed. 2001;40:397. doi: 10.1002/1521-3773(20010119)40:2<397::AID-ANIE397>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Liduka Y, Wakahara T, Nakahodo T, Tsuchiya T, Sakuraba A, Maeda Y, Akasaka T, Yoza K, Horn E, Kato T, Liu MTH, Mizorogi N, Kobayashi K, Nagase S. J Am Chem Soc. 2005;127:12500. doi: 10.1021/ja054209u. [DOI] [PubMed] [Google Scholar]
- 22.Ge Z, Duchamp JC, Cai T, Gibson HW, Dorn HC. J Am Chem Soc. 2005;127:16292. doi: 10.1021/ja055089t. [DOI] [PubMed] [Google Scholar]
- 23.Beavers CM, Zuo T, Duchamp JC, Harich K, Dorn HC, Olmstead MM, Balch AL. J Am Chem Soc. 2006;128:11352–11353. doi: 10.1021/ja063636k. [DOI] [PubMed] [Google Scholar]
- 24.Zuo TM, Olmstead MM, Beavers CM, Balch AL, Wang GB, Yee GT, Shu CY, Xu LS, Elliott B, Echegoyen L, Duchamp JC, Dorn HC. Inorganic Chemistry. 2008;47:5234–5244. doi: 10.1021/ic800227x. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs D, Rietschel H, Michel RH, Fischer A, Weis P, Kappes MM. Journal of Physical Chemistry. 1996;100:725–729. [Google Scholar]
- 26.Stevenson S, Burbank P, Harich K, Sun Z, Dorn HC, van Loosdrecht PHM, deVries MS, Salem JR, Kiang CH, Johnson RD, Bethune DS. Journal of Physical Chemistry A. 1998;102:2833–2837. [Google Scholar]
- 27.Campanera JM, Bo C, Olmstead MM, Balch AL, Poblet JM. J Phys Chem A. 2002;106:12356–12364. [Google Scholar]
- 28.Knight LB, Jr, Woodward RW, Van Zee RJ, Weltner W., Jr J Chem Phys. 1983;79:5820–7. [Google Scholar]
- 29.Echegoyen L, Chancellor CJ, Cardona CM, Elliot B, Rivera J, Olmstead MM, Balch AL. Chem Commun. 2006:2653. doi: 10.1039/b604011j. [DOI] [PubMed] [Google Scholar]
- 30.Kikuchi K, Nakao Y, Suzuki S, Achiba Y, Suzuki T, Maruyama Y. J Am Chem Soc. 1994;116:9367–8. [Google Scholar]
- 31.Wortman DE. Phys Rev. 1968;175:488–98. [Google Scholar]
- 32.Gafurov MR, Ivanshin VA, Kurkin IN, Rodionova MP, Keller H, Gutmann M, Staub U. J Magn Reson. 2003;161:210–4. doi: 10.1016/s1090-7807(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 33.Frisch MJ, et al. Gaussian, Inc; Wallingford, CT: 2004. [Google Scholar]
- 34.Becke AD. J Chem Phys. 1993;98:5648. [Google Scholar]
- 35.Lee C, Yang W, Parr RG. Phys Rev B. 1988;37:785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 36.Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ. J Phys Chem. 1994;98:11623. [Google Scholar]
- 37.Godbout N, Salahub DR, Andzelm J, Wimmer E. Can J Chem. 1992;70:560. [Google Scholar]
- 38.Hehre WJ, Ditchfield R, Pople JA. J Chem Phys. 1972;56:2257. [Google Scholar]
- 39.Cai T, Xu L, Anderson MR, Ge Z, Zuo T, Wang X, Olmstead MM, Balch AL, Gibson HW, Dorn HC. J Am Chem Soc. 2006;128:8581–8589. doi: 10.1021/ja0615573. [DOI] [PubMed] [Google Scholar]
- 40.Iiduka Y, Ikenaga O, Sakuraba A, Wakahara T, Tsuchiya T, Maeda Y, Nakahodo T, Akasaka T, Kako M, Mizorogi N, Nagase S. J Am Chem Soc. 2005;127:9956–9957. doi: 10.1021/ja052534b. [DOI] [PubMed] [Google Scholar]
- 41.Dunsch L, Krause M. ChemPhysChem. 2004;5:1445–1449. doi: 10.1002/cphc.200400085. [DOI] [PubMed] [Google Scholar]
- 42.Elliott B, Yu L, Echegoyen L. J Am Chem Soc. 2005;127:10885–10888. doi: 10.1021/ja052446r. [DOI] [PubMed] [Google Scholar]
- 43.Wu X, Lu X. J Am Chem Soc. 2007;129:2171–2177. doi: 10.1021/ja067281g. [DOI] [PubMed] [Google Scholar]
- 44.Cloke FGN, Green JC, Kalysoyannis N. Organometallics. 2004;23:832. [Google Scholar]
- 45.Westcott BL, Gruhn NE, Michelson LJ, Lichtenberger DL. J Am Chem Soc. 2000;122:8083. [Google Scholar]
- 46.Peterson KA, Figgen D, Dolg M, Stoll H. J Chem Phys. 2007;126:124101. doi: 10.1063/1.2647019. [DOI] [PubMed] [Google Scholar]
- 47.Cotton FA, Koch SA, Millar M. Inorg Chem. 1978;17:2084. [Google Scholar]
- 48.Kreisel KA, Yap GPA, Dmitrenko O, Landis CR, Theopold KH. J Am Chem Soc. 2007;129:14162. doi: 10.1021/ja076356t. [DOI] [PubMed] [Google Scholar]
- 49.Green SP, Jones C, Stasch A. Science. 2007;318:1754–1757. doi: 10.1126/science.1150856. [DOI] [PubMed] [Google Scholar]
- 50.Sheldrick GM. SADABS 2.10. Acta Crystallogr Sect A. 1995;A51:33. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The HPLC chromatograms for the initial yttrium and terbium soot extracts as well as the purified products, the mass spectrum of Y2@C79N, Tb2@C79N, and 15N labeled Tb2@C79N sample, and corresponding Uv-vis spectra and the initial separation of La2@C79N and La@C81N and their mass spectra, and X-ray crystallographic files in CIF format for Tb2@C79N • Ni||(OEP) • 2.5C6H6. This material is available free of charge via the Internet at http://pubs.acs.org.