Abstract
The Anaphase-Promoting Complex (APC/C) orchestrates progression through mitosis by decorating cell cycle regulators with ubiquitin chains. To nucleate chain formation, the APC/C links ubiquitin to a lysine in substrates, but it elongates chains by modifying lysine residues in attached ubiquitin moieties. The mechanism enabling the APC/C, and ubiquitin ligases in general, to switch from lysine residues in substrates to specific ones in ubiquitin remains poorly understood. Here, we determine the topology and the mechanism of assembly for the ubiquitin chains mediating functions of the human APC/C. We find that APC/C triggers the degradation of substrates by assembling K11-linked ubiquitin chains, the efficient formation of which depends on a surface of ubiquitin, the TEK-box. Strikingly, homologous TEK-boxes are found in APC/C-substrates, where they facilitate chain nucleation. We propose that recognition of similar motifs in substrates and ubiquitin enables the APC/C to assemble ubiquitin chains with the specificity and efficiency required for tight cell cycle control.
Introduction
In eukaryotes, the posttranslational modification of key regulators with ubiquitin chains plays a crucial role in almost every process (Kerscher et al., 2006). Ubiquitination can trigger the re-organization of protein complexes, changes in localization, or degradation. The fate of ubiquitinated proteins is determined by adaptors, which recognize ubiquitin chains and deliver the modified substrates to effectors, such as the 26S proteasome (Hicke et al., 2005). To understand how ubiquitination gains its versatility in signaling, it is pivotal to dissect the mechanisms underlying ubiquitin chain assembly.
The formation of ubiquitin chains is carried out by an enzymatic cascade (Dye and Schulman, 2007). It is initiated by the generation of a thioester between the carboxy-terminus of ubiquitin and a cysteine in ubiquitin-activating enzymes (E1). This ubiquitin is transferred to the active site of ubiquitin-conjugating enzymes (E2), which deliver it to ubiquitin ligases (E3). E3s are classified depending on their catalytic domain: HECT-E3s possess an active-site cysteine, and receive ubiquitin from E2s before modifying the substrate. By contrast, RING-E3s simultaneously bind to E2s and substrates, and facilitate ubiquitin transfer directly from the E2.
All E3s nucleate chain formation by attaching the carboxy-terminus of the first ubiquitin to the ε-amino group of a substrate lysine. The subsequent chain elongation requires the modification of specific lysine residues in consecutive ubiquitin moieties. In yeast, all seven lysine residues of ubiquitin are used for chain assembly, resulting in chains of different topology (Peng et al., 2003). However, only the functions of chains linked through K48 or K63 of ubiquitin have been firmly established. While K48-linked chains trigger proteasomal degradation, K63-linked chains recruit binding partners during inflammation or DNA repair (Kerscher et al., 2006). Several E3s can assemble specific ubiquitin chains in a single substrate binding event (Petroski and Deshaies, 2005; Rape et al., 2006). It is not understood how this is accomplished, as lysine residues in substrates and ubiquitin are in different chemical environments and at different positions within the growing chain.
The Anaphase-Promoting Complex (APC/C) has served as a model for the analysis of RING-finger-dependent chain formation (Rape et al., 2006; Thornton et al., 2006; Rodrigo-Brenni and Morgan, 2007). Ubiquitin chain formation by the APC/C can trigger protein degradation to control cell cycle progression (reviewed in Peters, 2006), quiescence (Wirth et al., 2004), and differentiation (Lasorella et al., 2006), but it can also induce the non-proteolytic disassembly of spindle checkpoint complexes (Reddy et al., 2007). The APC/C can assemble chains on substrates, such as securin and cyclin B1, rapidly and with high processivity (Carroll and Morgan, 2002; Rape et al., 2006). An in vitro study using frog APC/C suggested that these chains can be linked through K11, K48 or K63 of ubiquitin (Kirkpatrick et al., 2006). However, the topology of the ubiquitin chains mediating the diverse functions of APC/C has remained unknown, complicating the analysis of APC/C-dependent chain formation and cell cycle control.
Here, we determine the topology of the ubiquitin chains that mediate functions of the human APC/C. We find that APC/C and its E2 UbcH10 trigger protein degradation preferentially by assembling K11-linked, rather than K48-linked chains. K11-linked ubiquitin chains act as efficient proteasomal targeting signals in vitro and in vivo. We identify a surface of ubiquitin, the TEK-box, which is necessary for the elongation of K11-linked chains. Strikingly, similar TEK-boxes are found in APC/C-substrates, where they facilitate the transfer of the first ubiquitin to a substrate lysine. We propose a mechanism in which recognition of a TEK-box first aligns a substrate lysine and later K11 of ubiquitin with the active site of UbcH10 to allow the rapid formation of a K11-linked chain by the APC/C.
Results
APC/C functions by assembling K11-linked ubiquitin chains
To determine the topology of the ubiquitin chains that mediate functions of the human APC/C, we tested recombinant ubiquitin mutants in in vitro assays recapitulating APC/C-activity. We employed mutants that had a single lysine replaced with arginine, such as ubiquitin-K48R (ubi-R48). Alternatively, all lysine residues were mutated except for one, as in ubiquitin with K48 as its only lysine (ubi-K48). Together, these mutants allowed us to assess whether chains of a specific topology are required or sufficient for APC/C-functions.
We first assayed the ubiquitin mutants for their capacity to support the degradation of a mitotic APC/C-substrate, cyclin B1. Addition of UbcH10 and p31comet to extracts of mitotic cells with an activated spindle checkpoint (CP-extracts) triggers the APC/C-dependent disassembly of Cdc20/Mad2 complexes (Reddy et al., 2007; Stegmeier et al., 2007). This leads to full activation of APC/CCdc20 and, consequently, cyclin B1 ubiquitination and degradation. As reported previously, cyclin B1 is efficiently degraded in UbcH10/p31comet-treated CP-extracts containing wt-ubiquitin (Fig. 1A). Strikingly, cyclin B1 is also turned over in a proteasome-dependent manner, when CP-extracts are supplemented with a ubiquitin mutant that has K11 as its only lysine (ubi-K11; Fig. 1A, B). By contrast, mutation of K11 of ubiquitin (ubi-R11) interferes with cyclin B1-degradation, and also with disassembly of Cdc20/Mad2-complexes (Fig. 1C, Supp. Fig. 1A). No single-lysine ubiquitin mutant other than ubi-K11, including ubi-K48, supports degradation of cyclin B1, while no mutation other than that of K11 stabilizes cyclin B1. These results suggest that in CP-extracts APC/CCdc20 achieves cyclin B1-degradation by decorating it with K11-linked chains.
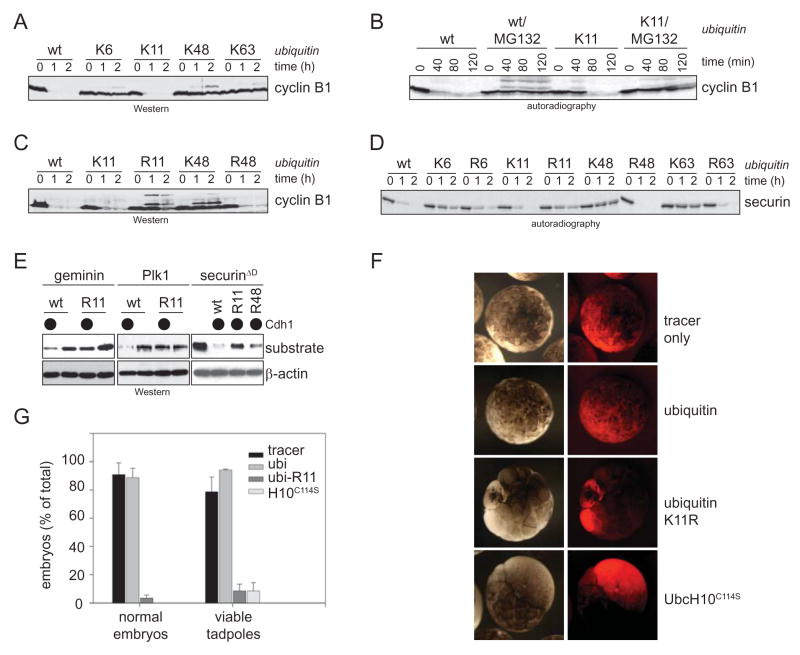
Figure 1. K11-linked ubiquitin chains mediate APC/C-functions.
A. K11-linked chains are sufficient for degradation of cyclin B1 in mitotic extracts. CP-extracts were supplemented with wt-ubi or single-lysine mutants. APC/C was activated by addition of UbcH10 and p31comet, and degradation of cyclin B1 was monitored by Western blotting. B. Degradation of APC/C-substrates by K11-linked chains is proteasome-dependent. Degradation of radiolabeled cyclin B1 in CP-extracts was triggered by addition of p31comet/UbcH10 in the presence wt-ubi or ubi-K11. The proteasome inhibitor MG132 was added when indicated. C. K11 is required for rapid degradation of cyclin B1 in mitotic extracts. CP-extracts were supplemented with ubiquitin mutants and treated as described above. Degradation of cyclin B1 was monitored by Western blotting. D. K11-linkages are required for full activity of APC/CCdh1 in G1. The degradation of radiolabeled securin was monitored by autoradiography in G1-extracts in the presence of ubiquitin mutants. E. K11-linked chains target APC/CCdh1-substrates for degradation in vivo. The APC/C-dependent degradation of geminin, Plk1, and securinΔD was triggered in 293T cells in the presence of indicated ubiquitin mutants (wt-ubi, ubi-R11, ubi-R48) by co-expression of Cdh1. The expression levels were analyzed by Western blotting. F. K11-linkages are required for rapid cell cycle progression in embryos of Xenopus tropicalis. One cell of X. tropicalis embryos at the two-cell stage was injected with recombinant wt-ubi or ubi-R11 and a fluorescent tracer. Injected cells were followed by fluorescence microscopy, and cell division was monitored by phase microscopy. G. K11-linkages are required for X. tropicalis development. Injected embryos were allowed to develop to the tadpole stage. The percentage of embryos without developmental aberrations (“normal”) and that of viable embryos was determined.
From anaphase until late in G1, Cdc20 is replaced by a homologous co-activator, Cdh1 (Peters, 2006). To determine whether the co-activator or cell cycle stage influence the topology of APC/C-dependent chains, we tested our ubiquitin mutants in degradation assays using extracts with active APC/CCdh1. Consistent with our experiments in mitotic extracts, the APC/C-substrate securin is rapidly degraded by the 26S proteasome in G1-extracts supplemented with ubi-K11, but it is stabilized if K11 of ubiquitin is absent, such as in ubi-R11 or methyl-ubiquitin (Fig. 1D; Supp. Fig. 1B). No single-lysine mutant other than ubi-K11 fully supports the degradation of securin in G1-extracts. Ubi-K11 allows the degradation of multiple APC/C-substrates (Supp. Fig. 1C) in extracts prepared from cells in G1 or in quiescence, when APC/CCdh1 is also active (Supp. Fig. 1D). By contrast, inhibiting the formation of K11-linked chains does not impair the ubiquitination or degradation of the SCF-substrate Emi1 (Supp. Fig. 1E). These findings provide evidence that in extracts both APC/CCdc20 and APC/CCdh1 function by decorating substrates with K11-linked chains.
To determine the importance of K11-linked chains in mediating APC/C-functions in vivo, we overexpressed ubi-R11 in human cells or injected recombinant ubi-R11 into Xenopus tropicalis embryos at the two cell stage. The overexpression of ubi-R11 in human 293T cells impedes the Cdh1-dependent degradation of the APC/C-substrates geminin, Plk1, and securinΔD (Fig. 1E). Moreover, injection of ubi-R11 into X. tropicalis embryos delays early cell divisions and results in death of injected embryos before gastrulation (Fig. 1F, G). These phenotypes are less dramatic, but similar to those observed after injection of a dominant-negative mutant of the APC/C-specific E2, UbcH10C114S. By contrast, overexpression or injection of wild-type ubiquitin does not affect the degradation of APC/C-substrates, progression through the cell cycle, or development of embryos. Thus, interfering with the formation of K11-linked chains stabilizes APC/C-substrates and impairs cell cycle progression and development in vivo, attesting to the importance of K11-linked chains for APC/C-activity.
UbcH10 provides specificity for the assembly of K11-linked chains
E2s often contribute to the specificity of ubiquitin chain formation (Dye and Schulman, 2007). Human APC/C has been reported to cooperate with three E2s, the specific UbcH10 and the more promiscuous UbcH5 and E2-25K. To dissect the mechanism underlying the formation of K11-linked chains, we purified these E2s and tested their specificity in APC/CCdh1-dependent chain assembly. Strikingly, APC/CCdh1 and its specific E2 UbcH10 form long ubiquitin chains only in the presence of ubi-K11, but not with other single-lysine mutants. The same strong preference for formation of K11-linked chains is observed with the distributive substrate cyclin A (Fig. 2A), the processive substrate securin (Fig. 2B), and for UbcH10-autoubiquitination (Fig. 2C). The mutation of K11 in ubiquitin delays chain formation by APC/CCdh1 and UbcH10 (Fig. 2D). Furthermore, as shown below, ubiquitin chains assembled by APC/CCdh1 and UbcH10 using ubi-R11 are not efficiently recognized by proteasomal receptors. These results indicate that UbcH10 endows the APC/C with specificity for assembling functional K11-linked chains.
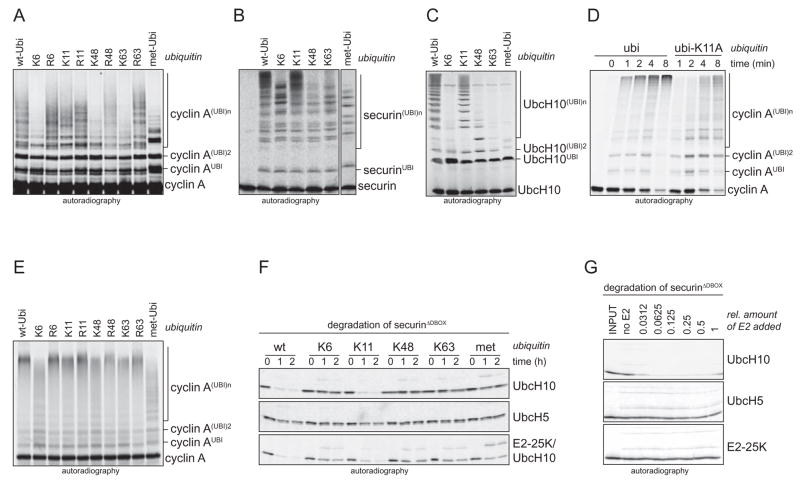
Figure 2. APC/CCdh1 and UbcH10 preferentially assemble K11-linked chains in vitro.
A. APC/CCdh1 and UbcH10 assemble chains on cyclin A using ubi-K11, but no other single-lysine mutant. APC/CCdh1 and UbcH10 were incubated with E1 and ubiquitin mutants. B. APC/CCdh1 and UbcH10 assemble chains on the processive substrate securin using ubi-K11, but no other tested single-lysine mutant. C. UbcH10-autoubiquitination by APC/CCdh1 is supported by ubi-K11 while other single-lysine mutants are much less efficient. D. The formation of ubiquitin chains by APC/CCdh1 and UbcH10 on cyclin A is delayed, if K11 of ubiquitin is mutated. The kinetics of chain formation on cyclin A by APC/CCdh1 and UbcH10 in the presence of wt-ubi and ubi-K11A was analyzed by autoradiography. E. APC/CCdh1 and UbcH5c form ubiquitin chains on cyclin A linked through K11, K48, and K63. APC/CCdh1 and UbcH5c were incubated with E1 and ubiquitin mutants. Similar results were obtained with UbcH5a. F. UbcH5 is less efficient in promoting the degradation of securinΔD in G1-extracts. G1-extracts were supplemented with ubiquitin mutants and UbcH10 (upper panel), UbcH5c (lower panel), and a combination of UbcH10 and E2-25K (lower panel). Degradation of radiolabeled securinΔD was monitored by autoradiography. G. UbcH10 is more potent in triggering degradation of securinΔD in G1-extracts over a wide range of concentrations. Three E2s, UbcH10, UbcH5c, and E2-25K, were titrated in G1-extracts. Reactions were analyzed after 60 min for securinΔD-levels by autoradiography.
In contrast to UbcH10, UbcH5a and UbcH5c can use ubi-K11, ubi-K48, and ubi-K63 to catalyze the ubiquitination of APC/CCdh1-substrates (Fig. 2E; data not shown), and thus, allow the formation of chains linked through lysine residues other than K11. E2-25K assembles chains very inefficiently, and earlier experiments indicated that these chains are linked through K48 (Supp. Fig. 2A, B; Rodrigo-Brenni and Morgan, 2007). Consistent with the importance of K11-linked chains for APC/C-activity, the specific UbcH10 is more potent in promoting the degradation of the APC/CCdh1-substrate securinΔD in G1-extracts than UbcH5 or E2-25K, as observed over a wide range of E2 concentrations (Fig. 2F, G). These results further suggest that UbcH10, but not UbcH5 or E2-25K, provide the APC/C with specificity for assembling functional K11-linked chains.
To determine the molecular basis underlying the specificity of UbcH10, we compared mutants of UbcH10 and UbcH5 in APC/C-dependent assays. The interaction of E2s with the RING-finger of E3s requires an aromatic side chain in loop 1 of the E2 (Zheng et al., 2000). As expected, mutation of the respective residue in UbcH10 and UbcH5, UbcH10Y91D and UbcH5F62D, inactivates both E2s in degradation and ubiquitination assays dependent on APC/CCdh1 (Supp. Fig. 2C–E). When added to G1-extracts, UbcH10Y91A and UbcH10Y91D, but not UbcH5cF62D, impair degradation of the APC/C-substrate securin, and thus, behave as dominant negative mutants (Supp. Fig. 2D). Consistent with this observation in extracts, injection of UbcH10Y91D into X. tropicalis embryos delays cell cycle progression (Supp. Fig. 2F). UbcH10Y91D does not interfere with proteasomal degradation, as the SCF-substrate Emi1 is ubiquitinated and degraded in its presence (Supp. Fig. 2G). Therefore, despite a defective RING-finger-interaction, UbcH10Y91D can bind the APC/C and compete with endogenous E2s in the extracts, suggesting that UbcH10 contains additional APC/C-binding motifs.
A likely candidate for a second APC/C-binding site in UbcH10 is helix-1 of its UBC-domain, which in other E2s participates in E3-binding (Reverter and Lima, 2005; Zheng et al., 2000) and is not conserved between UbcH10 and UbcH5. Indeed, mutations in or close to helix-1 (UbcH10K33D and UbcH10D47K) significantly reduce the activity of UbcH10 in degradation and ubiquitination assays (Supp. Fig. 2H, I). In contrast to UbcH10Y91D, UbcH10K33D and UbcH10D47K do not act as dominant-negatives, indicating their binding to APC/C is disturbed. UbcH10K33D is also less efficiently charged by E1, which is consistent with findings that E1 and E3-binding sites in E2s overlap (Eletr et al., 2005). These results imply that residues in or close to helix-1 constitute part of a second APC/C-binding motif in UbcH10. We suggest that the simultaneous engagement of two binding motifs stabilizes UbcH10 binding to APC/C to orient it in the optimal position for assembling K11-linked chains.
Importantly, the assembly of homogenous K11-linked chains by APC/C and UbcH10 allowed us to determine whether these chains function as proteasomal targeting signals. Indeed, APC/C-substrates decorated with K11-linked chains are recognized by the proteasomal substrate receptors Rad23 (Fig. 3A) and S5a in vitro (Fig. 3B). Consequently, they are efficiently degraded by 26S proteasomes that co-purify with APC/C (Fig. 3E; Verma et al., 2000). APC-substrates modified with K11-linked chains are also rapidly turned over by purified 26S proteasomes from human embryonic kidney cells that were added subsequent to the ubiquitination (Fig. 3F). Securin can be modified with K11-linked chains and captured by Rad23 also in 293T cells (Fig. 3C, D). These findings provide strong evidence that K11-linked ubiquitin chains function as efficient proteasomal targeting signals.
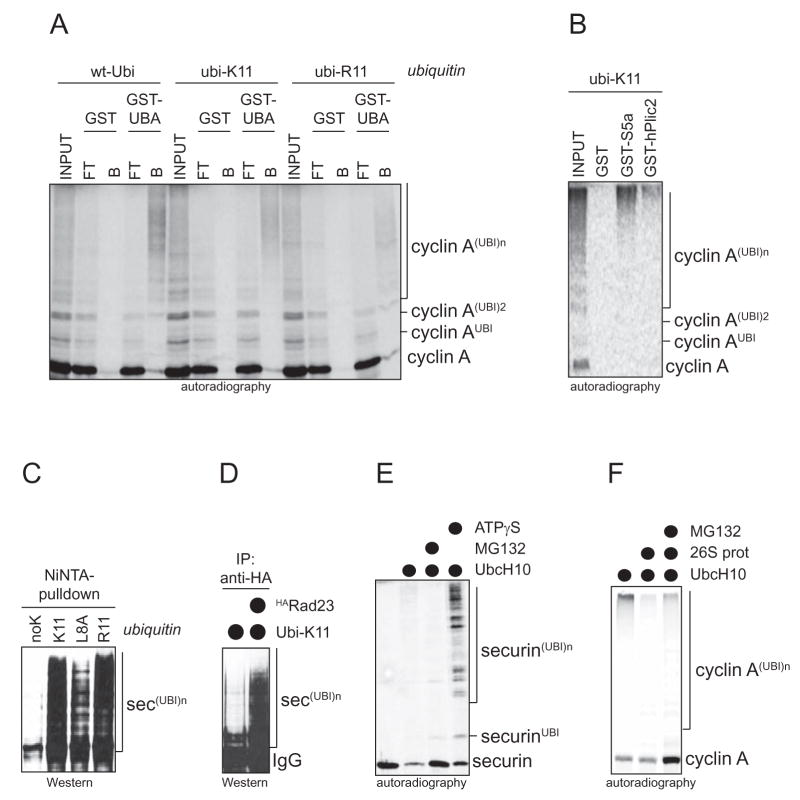
Figure 3. K11-linked ubiquitin chains are a proteasomal targeting signal.
A. K11-linked chains are recognized by the proteasomal receptor Rad23. The UBA-domains of Rad23 were immobilized on beads and incubated with cyclin A that was ubiquitinated in the presence of wt-ubi, ubi-K11, and ubi-R11. Proteins retained by the UBA-domains are shown in “B” (bound). B. APC/C-substrates modified with K11-linked chains are recognized by proteasomal receptors. Cyclin A was ubiquitinated by APC/CCdh1 and UbcH10 in the presence of ubi-K11, and tested for binding to Gst (negative control), Gst-S5a, and hPlic2. Bound proteins were analyzed by autoradiography. C. APC/C-substrates can be modified with K11-linked chains in cells. Securin was co-expressed in 293T cells with His6-ubiquitin mutants. Conjugates were purified on NiNTA-agarose under denaturing conditions. Ubiquitin without lysine residues (“noK”) is not incorporated into chains, showing that ubi-K11-expression leads to the decoration of securin with K11-linked chains. D. K11-linked chains are recognized by proteasomal receptors in vivo. HARad23 was purified from 293T cells expressing securin and ubi-K11 by anti-HA affinity chromatography. Ubiquitinated securin co-eluted with Rad23-, but not control immunoprecipitations, as detected by Western blotting. E. K11-linked chains target an APC/C-substrate for proteasomal degradation in a semi-purified system. APC/C was purified under conditions allowing the co-purification of active proteasomes (Verma et al., 2000), and used for ubiquitination/degradation of securin. When indicated, MG132 or ATPγS (which inhibits the proteasome, but also deubiquitination by Rpn11) were added. F. K11-linked chains target APC/C-substrates for degradation. Cyclin A was ubiquitinated by APC/CCdh1 and UbcH10, and subsequently incubated with purified human 26S proteasomes. MG132 was added when indicated.
As described above, APC/C and UbcH10 are able to modify substrates with ubiquitin chains also in the absence of K11, but this occurs with delayed kinetics. In addition, the affinity of APC/C-substrates to Rad23 is reduced, if chains are assembled by UbcH10 using ubi-R11 (Fig. 3A), and these chains are less sensitive to proteasome activity in cells (Supp. Fig. 3A). K11 is not part of the surface of ubiquitin that is recognized by Rad23, as determined by structural analysis (Veradan et al., 2005), and substrates modified with ubi-R11 by E2s other than UbcH10 are efficiently retained by Rad23 (Supp. Fig. 3B). This indicates that mutation of K11 alters the structure of ubiquitin chains, which are formed by APC/CCdh1 and UbcH10, thereby impeding recognition by Rad23. We conclude that the APC/C and UbcH10 function by preferentially assembling K11-linked chains, which, as shown here, are efficient proteasomal targeting signals.
The TEK-box in ubiquitin is required for assembly of K11-linked chains
In addition to the proper orientation of UbcH10, formation of K11-linked chains by the APC/C requires the alignment of K11 in the acceptor ubiquitin relative to the active site of UbcH10. To identify residues in ubiquitin that help present K11, we mutated surface-exposed amino acids to alanine, and monitored the capacity of these mutants to support APC/C-activity in extracts.
Out of a total of 17 ubiquitin mutants, substituting K6, L8, T9, E34, and I36 with alanine strongly stabilizes securin in extracts (Fig. 4A). Accordingly, overexpression of ubi-K6A and ubi-L8A in 293T cells interferes with the degradation of the APC/CCdh1-substrate securinΔD to a similar extent as overexpression of ubi-R11 (Fig. 4B). Ubiquitination reactions using purified APC/CCdh1 and UbcH10 revealed that the stabilization of APC/C-substrates is a consequence of impaired chain formation in the presence of these mutants (Fig. 4C–E). Overexpression of ubi-L8A reduced the modification of securin also in cells (Fig. 3C). Interestingly, if the positive charge at position 6 is maintained, as in ubi-R6, neither degradation nor ubiquitination of APC/C-substrates is strongly affected (Fig. 1D). This suggests that K6 contributes to binding, but is unlikely to be ubiquitinated itself. These experiments identify the ubiquitin residues K6, L8, T9, E34, and I36 to be required for the efficient formation of K11-linked chains by APC/C and UbcH10. Importantly, these residues form a cluster surrounding K11, which we refer to as the TEK-box of ubiquitin (Fig. 4F).
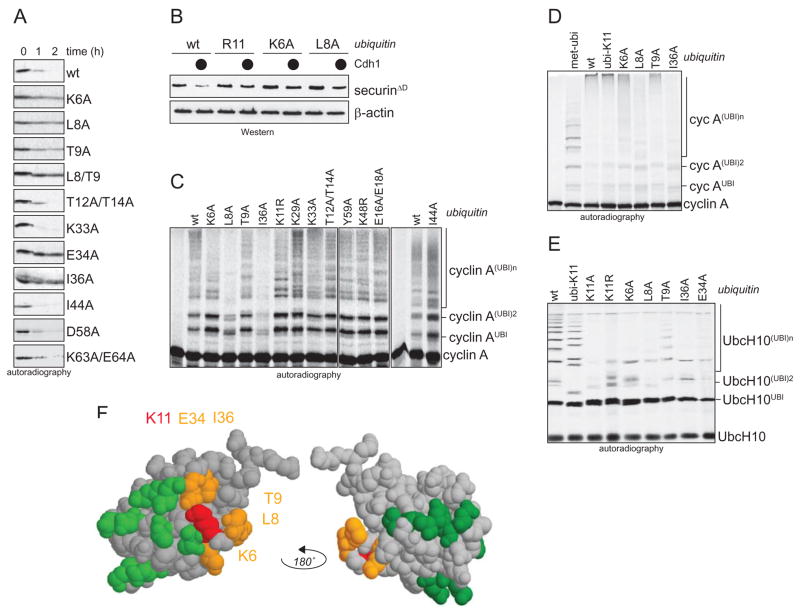
Figure 4. The TEK-box in ubiquitin is required for UbcH10-dependent chain formation.
A. Degradation of the APC/C-substrate securin in G1-extracts in the presence of ubiquitin mutants, as monitored by autoradiography. B. Degradation of the APC/CCdh1-substrate securinΔD in 293T cells is inhibited by overexpression of ubi-R11, ubi-K6A, and ubi-L8A. Cdh1 was co-expressed where indicated (●), and the levels of securinΔD and β-actin were monitored by Western blotting. C. In vitro ubiquitination of cyclin A by purified APC/CCdh1 and UbcH10 is impaired by mutation of K6, L8, T9, K11, and I36. Other ubiquitin mutants had less severe effects on cyclin A ubiquitination. D. Mutation of K6, L8, T9, and I36 in ubi-K11 impairs the assembly of K11-linked chains on cyclin A. Ubiquitination was catalyzed by APC/CCdh1 and UbcH10. E. Mutation of K6, L8, T9, K11, I36, and E34 interferes with autoubiquitination of radiolabeled UbcH10 by APC/CCdh1. F. Localization of mutations that affect APC/C-activity in G1 on the surface of ubiquitin. K11 is marked in red; mutants of ubiquitin interfering with APC/C-activity are labeled orange; mutants that didn’t affect APC/C-activity are marked in green.
In contrast to mutating the TEK-box, altering several other positions of ubiquitin does not affect ubiquitination or degradation of APC/C-substrates. This includes residues shown to support the formation of K29-linkages by a HECT-E3 (E16A/E18A), the formation of K48- and K63-linkages by several E3s (I44A; K48R; Y59A; K63A/E64A), and ubiquitin recognition (I44A, D58A). Moreover, when UbcH5c is used as E2, mutations in the TEK-box inhibit the APC/C-dependent chain formation less severely (Supp. Fig. 4A). Only ubi-L8A, and to a lesser extent ubi-I36A, are deficient in supporting chain formation by APC/CCdh1 and UbcH5c. None of the TEK-box residues of ubiquitin is important for the monoubiquitination of an unrelated protein (UEV1A) or for the formation of K63-linked ubiquitin dimers by Ube2N/UEV1A (Supp. Fig. 4B). All ubiquitin mutants are soluble at high concentrations and, with the exception of the slightly impaired E34A-mutant, efficiently loaded onto the active site of UbcH10 (data not shown). These experiments underscore the specific importance of the TEK-box of ubiquitin for UbcH10-dependent chain formation. We conclude that a cluster of residues surrounding K11 of ubiquitin, the TEK-box, is required for the efficient formation of K11-linked chains by APC/C and UbcH10.
The TEK-box is found in APC/C-substrates
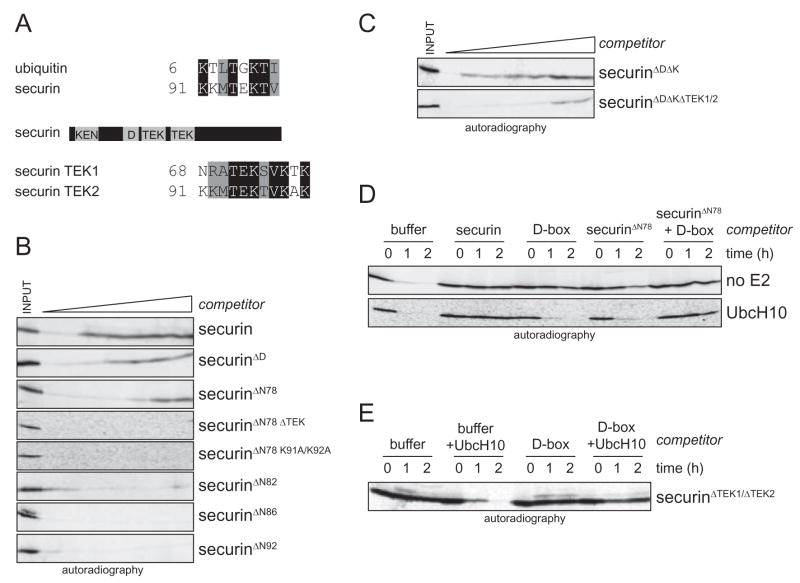
Strikingly, we found sequences closely related to the TEK-box of ubiquitin in the APC/C-substrate securin. The two TEK-boxes in securin are located immediately downstream of its D-box, which is an APC/C-binding motif responsible for its processive ubiquitination (Fig. 5A; Burton et al., 2005; Kraft et al., 2005; Rape et al., 2006). Especially the second TEK-box of securin is well conserved (Supp. Fig. 5A). In analogy to the TEK-box in ubiquitin, TEK-boxes in substrates could facilitate the modification of a substrate lysine, thereby nucleating ubiquitin chain formation.
Figure 5. TEK-boxes in securin contribute to APC/C-binding.
A. Identification of a motif in securin, which is highly related to the TEK-box in ubiquitin. B. The TEK-boxes contribute to binding of securin to APC/C. The degradation of a radiolabeled APC/C-substrate in G1-extracts was monitored in the presence of increasing concentrations of recombinant securin mutants. C. Deletion of TEK-boxes in securinΔDΔK reduces the affinity of securin to APC/CCdh1. The degradation of a radiolabeled APC/C-substrate in G1-extracts was monitored in the presence of increasing concentrations of securinΔDΔK or securinΔDΔKΔTEK1/2. D. The D-box and the TEK-boxes are recognized by two independent binding sites on APC/C and/or UbcH10. Degradation of radiolabeled securin in early G1-extracts was monitored by autoradiography. Addition of a D-box- or a TEK-box-peptide (securinΔN78) stabilized securin in early G1-extracts (upper panel). Addition of recombinant UbcH10 could overcome this competition (lower panel). If both the D-box- and the TEK-box-binding site are blocked by addition of both peptides, UbcH10 is unable to overcome the competitive inhibition. E. The saturation of the D-box binding site is sufficient to stabilize a TEK-box-mutant of securin. The degradation of securinΔTEK1/2 was monitored in G1-extracts in the presence of a D-box peptide.
To test this hypothesis, we first determined whether the TEK-boxes in securin contribute to APC/C-binding. We used a competition assay, in which the ubiquitination and degradation of a radiolabeled APC/C-substrate is competitively inhibited by addition of recombinant securin mutants. As expected, wild-type securin is an efficient competitor of APC/C-dependent degradation in G1-extracts, i.e. it binds well to APC/C (Fig. 5B; Supp. Fig. 5B, C). Even if both the D-box and a redundant motif, the KEN-box, are deleted (securinΔDΔK), the securin-mutant inhibits APC/C, albeit with reduced efficiency. The same is observed if the D-box, KEN-box and the first TEK-box of securin are removed by deleting the amino-terminal 78 amino acids (securinΔN78), suggesting that the remaining TEK-box in securinΔN78 is able to mediate APC/C-binding. Indeed, the deletion (securinΔN78ΔTEK) or mutation (securinΔN78K91A/K92A) of this TEK-box abolishes competition by securinΔN78. Moreover, when both TEK-boxes are deleted in a securinΔDΔK-background, the binding of securin to APC/CCdh1 is strongly impaired (Fig. 5C). If more than 78 residues are deleted at the amino-terminus, binding of securin to APC/CCdh1 is also lost, but we cannot exclude that this is caused by misfolding of the truncated proteins. Together, these experiments strongly suggest that just like the D-box, TEK-boxes contribute to the binding of securin to APC/CCdh1.
To test whether APC/C recognizes D-boxes and TEK-boxes by using distinct sites, we employed D-box- and TEK-box-peptides in our competition assay. As expected, the addition of a D-box-peptide to G1-extracts stabilizes the labeled APC/C-substrate (Fig. 5D). This competition can be overcome by increasing the concentration of UbcH10 in the extracts, which allows the APC/C to ubiquitinate weakly bound substrates (Rape et al., 2006; Fig. 5D). In a similar manner, competition by the TEK-box peptide securinΔN78 is overcome by addition of UbcH10 (Fig. 5D; Supp. Fig. 5D). In striking contrast, when both the D-box- and TEK-box-binding sites are saturated by the simultaneous addition of the two peptides, even high concentrations of UbcH10 are unable to bypass the competitive inhibition of APC/C. If the labeled substrate itself does not contain TEK-boxes (securinΔTEK1/ΔTEK2), the D-box-peptide alone inhibits APC/C in the presence of high UbcH10-concentrations (Fig. 5E). These results indicate that D-boxes and TEK-boxes are recognized by two non-identical sites on APC/C and/or UbcH10.
Substrate TEK-boxes promote the nucleation of ubiquitin chains
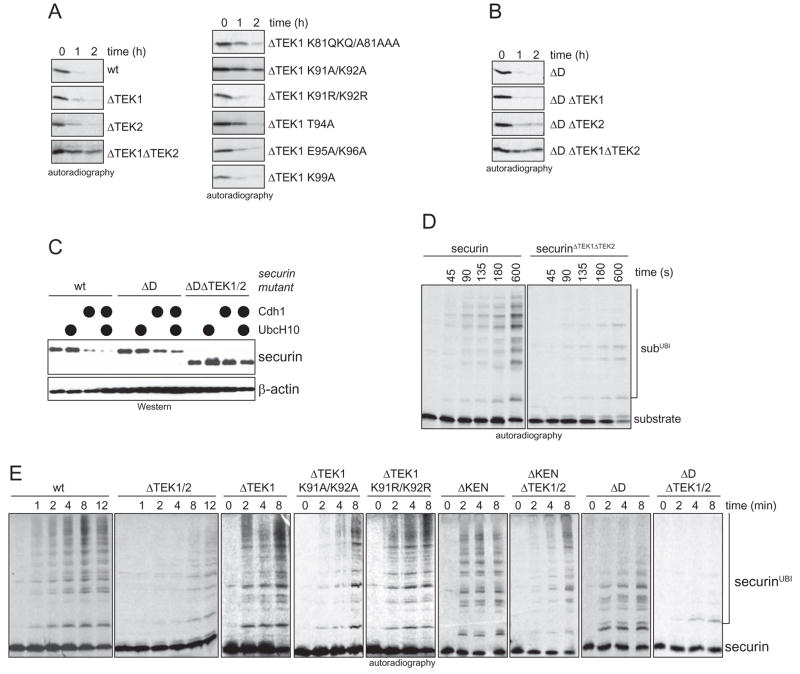
To determine how TEK-box-binding affects the UbcH10-dependent degradation of securin, we monitored securin turnover in extracts of quiescent T24-cells (G0-extracts). These extracts have very low levels of UbcH10, and APC/C-substrates are degraded rapidly only after recombinant UbcH10 has been added. As expected, wild-type securin is degraded in G0-extracts supplemented with UbcH10 (Fig. 6A). The deletion of both TEK-boxes (securinΔTEK1/ΔTEK2), but not of each TEK-box alone, strongly stabilizes securin under these conditions. If the first TEK-box is deleted, mutation of K91/K92 in the second TEK-box to alanine (securinΔTEK1/K91A/K92A) is sufficient to stabilize securin in G0-extracts. If K91/K92 are replaced by arginine, securin degradation is not affected, indicating that, reminiscent of K6 of ubiquitin, K91/K92 of securin serve as binding, but not as ubiquitination site. A similar dependency on TEK-boxes is observed in G1-extracts, when we measured the degradation of securinΔD after addition of UbcH10. Again, simultaneous deletion of both TEK-boxes results in stabilization of the substrate in the presence of UbcH10 (Fig. 6B). Finally, deletion of both the D-box and the TEK-boxes, but not deletion of either motif alone, strongly stabilizes securin against APC/CCdh1-dependent degradation in intact cells (Fig. 6C). The TEK-boxes in securin are therefore important for its APC/C-dependent degradation in extracts and cells.
Figure 6. The TEK-box in securin is required for efficient UbcH10-dependent ubiquitination and degradation.
A. Deletion of both TEK-boxes stabilizes securin in a UbcH10-dependent degradation assay. Degradation of indicated securin-mutants in extracts of quiescent T24 cells supplemented with UbcH10 was monitored by autoradiography. B. Deletion of both TEK-boxes impairs the UbcH10-dependent degradation of securinΔD. Degradation of securinΔD-mutants in G1-extracts supplemented with UbcH10, as monitored by autoradiography. C. Deletion of both TEK-boxes stabilizes the APC/CCdh1-substrate securinΔD in cells. The APC/C-dependent degradation of the indicated securin mutants was triggered in 293T cells by co-expression of Cdh1, mycUbcH10, or both (●). Expression levels were analyzed by Western blotting. D. Deletion of both TEK-boxes delays modification of securin lysine residues. The kinetics of monoubiquitination of radiolabeled securin or securinΔTEK1/2 by APC/CCdh1 and UbcH10 was monitored in the presence of methylubiquitin by autoradiography. E. Deletion of both TEK-boxes delays the onset of chain formation on securin by APC/CCdh1 and UbcH10. Radiolabeled securin and the indicated mutants were incubated with APC/CCdh1 and UbcH10 in the presence of ubiquitin, and analyzed by autoradiography.
Since the similarity to the TEK-box in ubiquitin implied that the TEK-boxes in securin promote the modification of a securin lysine, we monitored ubiquitination kinetics in the presence of methyl-ubiquitin, which is unable to form chains. As reported previously (Rape et al., 2006), APC/CCdh1 and UbcH10 rapidly modify wild-type securin on several lysine residues (Fig. 6D). By contrast, the deletion of both TEK-boxes strongly delays the monoubiquitination of securin and reduces the number of modified lysine residues. A similar reduction in the number of modified lysine residues is observed, when the TEK-box-peptide securinΔN78 is added to block the TEK-box-binding site (Supp. Fig. 6A). We conclude that the TEK-boxes in securin are required for efficient modification of substrate lysine residues.
As expected from the impaired monoubiquitination of securinΔTEK1/ΔTEK2, the onset of the UbcH10-dependent multiubiquitination of securinΔTEK1/ΔTEK2 is strongly delayed (Fig. 6E). However, following the initial delay, ubiquitin chains approaching full length are rapidly assembled. The same delayed onset of ubiquitin chain formation is observed upon mutation of K91/K92 in securinΔTEK1 to alanine (securinΔTEK1/K91A/K92A), but not when these residues are replaced by arginine (securinΔTEK1/K91R/K92R). By contrast, the deletion of the D-box of securin does not delay chain formation, but results in reduced chain length. Consistent with the cooperation between D-box and TEK-boxes, the deletion of both motifs almost completely abrogates securin ubiquitination. The deletion of the TEK-boxes in securin has less severe effects on chain formation by APC/CCdh1 and UbcH5c (Supp. Fig. 6B, C). These findings all suggest that the TEK-boxes in securin promote the nucleation of ubiquitin chains, especially if UbcH10 is the E2.
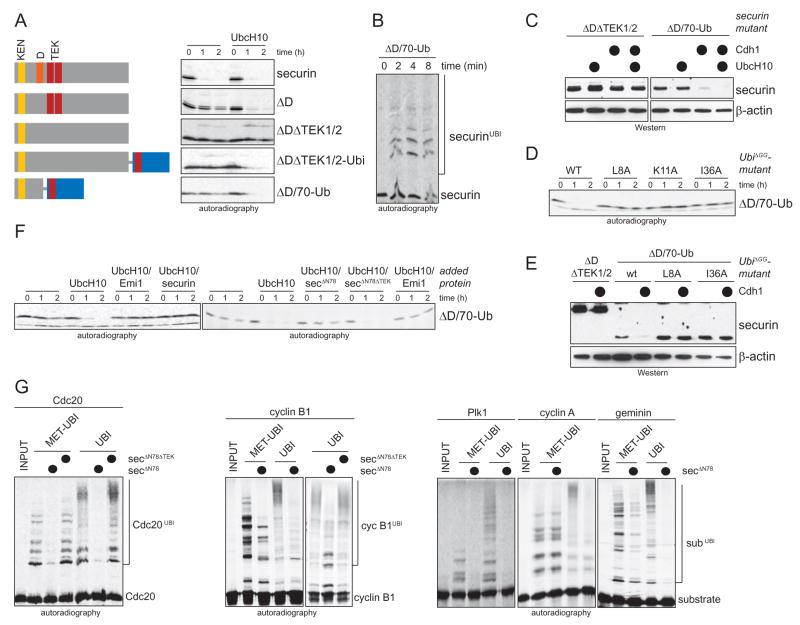
If the sole function of TEK-boxes in substrates is to promote ubiquitin chain nucleation, they should be required only for the addition of the first ubiquitin. By contrast, the D-box of substrates should remain important throughout the reaction. To test this hypothesis, we bypassed chain nucleation in D-box- and TEK-box-mutants by fusing ubiquitin to securinΔDΔTEK1/2 (securinΔDΔTEK1/2-UbiΔGG), or by replacing the carboxy-terminus of securinΔD, including both TEK-boxes, with ubiquitin (ΔD/70-Ub). Intriguingly, despite the lack of TEK-boxes, both ubiquitin fusions are degraded in G1-extracts in an APC/C-dependent manner (Fig. 7A, F), and ubiquitinated by purified APC/CCdh1 (Fig. 7B). The fused ubiquitin is only functional, if neither its TEK-box nor K11 are mutated (Fig. 7D). The degradation of the ubiquitin-fusions is inhibited by the TEK-box peptide securinΔN78, indicating that the TEK-box in the fused ubiquitin recognizes the same site as the TEK-box in securin (Fig. 7F). All fusions are degraded only after the extracts are supplemented with UbcH10, which suggests that addition of the first ubiquitin overcomes the lack of TEK-boxes, but not the lack of a D-box in securin. These findings can be reproduced in cells, where the fusion ΔD/70-Ub, but not securinΔDΔTEK1/2, is degraded in an APC/CCdh1-dependent manner (Fig. 7C). As in extracts, mutation of the TEK-box in ubiquitin interferes with the APC/C-dependent degradation of the fusion in cells (Fig. 7E). Thus, both in extracts and cells, addition of the first ubiquitin eliminates the requirement for TEK-boxes, but not for the D-box in securin. We conclude that the TEK-boxes in securin function primarily in ubiquitin chain nucleation, while the D-box is recognized throughout the ubiquitination reaction.
Figure 7. The TEK-boxes in securin function in ubiquitin chain nucleation.
A. APC/C-independent addition of the first ubiquitin to securin obviates the requirement for TEK-boxes. Degradation of securin mutants in G1-extracts with or without additional UbcH10 was analyzed by autoradiography. Chain nucleation was bypassed by fusing ubiquitin to securinΔDΔTEK1/2 or by replacing the carboxy-terminus of securin with ubiquitin (ΔD/70-Ub). B. Addition of the first ubiquitin restores APC/C-dependent ubiquitination. Ubiquitination of ΔD/70-Ub by APC/CCdh1 and UbcH10 was monitored by autoradiography. C. Bypassing chain nucleation rescues APC/CCdh1-dependent degradation of TEK-box mutants in cells. SecurinΔDΔTEK1/2 and ΔD/70-Ub were co-expressed with Cdh1 and mycUbcH10 in 293T cells as indicated. Expression levels were determined by Western blotting. D. Bypassing chain nucleation rescues degradation of TEK-box deleted securin only if the fused ubiquitin contains a TEK-box and K11. Mutants of ΔD/70-Ub were analyzed for degradation in G1-extracts supplemented with UbcH10. E. Ubiquitin-fusions are degraded in cells only if the fused ubiquitin contains a TEK-box. The respective mutants were co-expressed with Cdh1 where indicated (●) and expression levels were determined by Western blotting. F. Bypassing chain nucleation does not obviate the requirement for a D-box, the APC/C, or TEK-box recognition. Degradation of ΔD/70-Ub was analyzed by autoradiography in G1-extracts supplemented with UbcH10, the APC/C-inhibitor Emi1, an excess of the APC/C-substrate securin, the TEK-box peptide securinΔN78, or mutant securinΔN78ΔTEK. G. The TEK-box-binding site on APC/CCdh1 is required for the ubiquitination of several APC/C-substrates. The APC/CCdh1-substrates Cdc20, cyclin B1, cyclin A, Plk1, and geminin were incubated with APC/CCdh1 and UbcH10 in the presence of methylubiquitin (to measure nucleation) and ubiquitin (to monitor elongation). Reactions were challenged with the TEK-box peptide securinΔN78 or the securinΔN78ΔTEK mutant, and ubiquitinated species were visualized by autoradiography.
Several APC/C-substrates, including cyclin B1 and geminin, contain TEK-box-like sequences downstream of their D-box (Supp. Fig. 6D). To test whether TEK-boxes are recognized during the modification of other APC/C-substrates, we monitored their ubiquitination after the TEK-box binding site had been saturated with the TEK-box-peptide securinΔN78 (Fig. 7G). With the exception of cyclin A, the monoubiquitination of all APC/C-substrates analyzed in this assay is impaired by securinΔN78, but not by securinΔN78ΔTEK. In addition, the multiubiquitination of all APC/C-substrates tested, including cyclin A, is inhibited by securinΔN78, but not securinΔN78ΔTEK. Accordingly, addition of securinΔN78 to G1-extracts stabilizes all examined APC/C-substrates, including cyclin A (Supp. Fig. 6E). Thus, saturation of the TEK-box binding site interferes with the ubiquitination and degradation of several APC/C-substrates. Based on the results presented in this study, we propose that TEK-boxes in substrates facilitate the nucleation of ubiquitin chains, while the TEK-box in ubiquitin promotes the elongation of the K11-linked chains mediating APC/C-dependent reactions.
Discussion
The modification of proteins with ubiquitin chains is a crucial regulatory event in eukaryotes. This process is nucleated by the modification of a substrate lysine, but it proceeds by targeting of lysine residues in each following ubiquitin. Often, specific lysine residues in ubiquitin are preferred for chain formation, resulting in chains of distinct topology and function. Despite the importance of ubiquitination for signaling, little is known about how lysine residues are selected in substrates and ubiquitin, nor is it understood how E3s can both nucleate and elongate ubiquitin chains of specific topologies.
Here, we have determined the topology of the ubiquitin chains mediating functions of the human APC/C, and dissected the mechanism underlying their assembly. Surprisingly, we find that APC/C and its E2 UbcH10 target substrates for degradation by decorating them with K11-linked instead of canonical K48-linked ubiquitin chains. The assembly of K11-linked chains depends on a cluster of amino acids, the TEK-box, which is present in substrates and ubiquitin. In substrates, the TEK-box facilitates the transfer of the first ubiquitin to a substrate lysine, and thus, chain nucleation. The TEK-box of ubiquitin is required for the modification of K11 in ubiquitin, and thus, chain elongation. The recognition of similar sequences in substrates and ubiquitin empowers the APC/C to rapidly decorate substrates with K11-linked chains, which is critical for its central regulatory role in mitosis.
K11-linked ubiquitin chains as a signaling entity in cell cycle control
In yeast, linkages involving all lysine residues of ubiquitin, including K11, have been observed, but no function has been attributed to the modification of cellular substrates with K11-linked chains (Peng et al., 2003). Xenopus laevis APC/C is able to catalyze the formation of K11-, K48-, and K63-linkages, but the relevance of the different linkages for the functions of the APC/C has not been addressed (Kirkpatrick et al., 2006). By using the degradation of cell cycle regulators as a readout, we show that the human APC/C and UbcH10 preferentially function by assembling K11-linked chains. APC/C-substrates modified with K11-linked chains, are recognized by proteasomal receptors, and consequently, degraded by the 26S proteasome. This strongly suggests that K11-linked ubiquitin chains serve as proteasomal targeting signals, and thus, represent a novel signaling entity important for cell cycle regulation.
Our analysis revealed the APC/C-specific E2 UbcH10 as a key player conveying specificity for K11-linked chains. UbcH10 can use ubi-K11, but no other single-lysine mutant for efficient chain formation. If K11 is mutated, but other lysine residues are still present (ubi-R11), UbcH10 is able to assemble chains, but these are formed slowly and recognized less efficiently by proteasomal acceptors. We suspect that when K11 is missing, UbcH10 modifies the remaining lysine residues non-specifically, which is likely to result in the formation of short, branched and non-functional ubiquitin chains.
In contrast to UbcH10, the E2 UbcH5 is less specific and promotes APC/C-dependent chain formation in the presence of ubi-K11, ubi-K48, and ubi-K63. This is consistent with a previous study showing that ubiquitin conjugates formed by APC/C and UbcH5 contain equal amounts of K11-, K48-, and K63-linkages (Kirkpatrick et al., 2006). Being more promiscuous coincides with the reduced activity of UbcH5 in several E2-dependent assays. Our findings are reminiscent of the lower activity of UbcH5 compared to UbcH10 in promoting the degradation of cyclin A in G1 (Rape and Kirschner, 2004). Consistent with this, APC/C inactivation in G1 involves the degradation of its specific E2 UbcH10, which assembles K11-linked chains, whereas the levels of UbcH5 are not altered (Rape and Kirschner, 2004).
Why do APC/C and UbcH10 assemble K11-linked chains rather than canonical K48-linked chains? We suspect that this is related to the regulation of the APC/C by deubiquitinating enzymes (DUBs). DUBs regulate the timing of APC/C-substrate ubiquitination and protect cells against premature APC/C-dependent inactivation of the spindle checkpoint (Rape et al., 2006; Stegmeier et al., 2007). Many DUBs recognize substrates based on their ubiquitin chain and display a preference for chains of a certain topology (Nijman et al., 2005). K11-linked chains could identify substrates ubiquitinated by the APC/C, and DUBs could play their role in cell cycle regulation without interfering with the degradation of substrates ubiquitinated by other E3s in mitosis.
Nucleation and elongation of ubiquitin chains by the APC/C
Ubiquitin chain formation requires E3s to nucleate chains by modifying a substrate lysine, but to elongate chains by targeting lysine residues in ubiquitin. E3s have evolved distinct strategies to accomplish this difficult reaction. The SCF can nucleate and elongate ubiquitin chains using a single E2, Cdc34, but these reactions occur with strikingly different kinetics (Petroski and Deshaies, 2005). In the UFD-pathway, two distinct enzymes, the E3 Ufd4 and the E4 Ufd2, act in succession to mediate chain nucleation and elongation (Koegl et al., 1999). Yeast APC/C employs one E2, Ubc4, to modify a substrate lysine, whereas a second E2, Ubc1, elongates K48-linked chains (Rodrigo-Brenni and Morgan, 2007). Some of these mechanistic differences could be related to the observation that some E2s transfer pre-formed ubiquitin chains (Li et al., 2007; Ravid and Hochstrasser, 2007), but they could also reflect complex ways of regulation.
In contrast to the aforementioned enzymes, human APC/C and its E2 UbcH10 are able to nucleate and elongate chains in a single binding event (Rape et al., 2006). This processive multiubiquitination is critical for the rapid degradation of securin and cyclin B at the transition from metaphase to anaphase. As we show here, ubiquitin chain formation by the APC/C relies on a sequence motif, the TEK-box, which is present in substrates and ubiquitin (Supp. Fig. 7). TEK-boxes in substrates promote the modification of a substrate lysine with ubiquitin, while the TEK-box in ubiquitin supports elongation of the K11-linked chain.
Our data show that TEK-boxes promote the association of substrates with the APC/C. In mediating substrate binding, TEK-boxes collaborate with the D-box, an APC/C-binding motif that determines the processivity of ubiquitination (Burton et al., 2005; Kraft et al., 2005). The simultaneous recognition of the D-box by Cdh1 and the TEK-box by APC/C or UbcH10 could explain the increased stability of the complex between APC/C, Cdh1, and substrate compared to dimeric sub-complexes (Burton et al., 2005). Following the transfer of ubiquitin to a lysine within or in proximity to the TEK-box, the substrate-TEK-box is likely to be replaced by the TEK-box of the attached ubiquitin. Importantly, as substrates remain bound to the APC/C by their D-box, elongation of K11-linked ubiquitin chains still occurs only on APC/C-substrates. It is tempting to speculate that the recognition of the D-box is a prerequisite for the engagement of the TEK-box with its cognate site on APC/C or UbcH10. In fact, it may be coupled to conformational changes that were observed on APC/C following substrate-binding (Dube et al., 2005).
In addition, TEK-boxes may play a pivotal role during catalysis by aligning the acceptor lysine with the thioester of UbcH10, and by providing an electrostatic environment that allows the acceptor lysine to act as nucleophile. This hypothesis is supported by the observation that a cluster of charged amino acids surrounds the active site of UbcH10, but not UbcH5 (Lin et al., 2002; Tolbert et al., 2005). A function of the TEK-box in catalysis would be reminiscent of a motif with similar charge distribution, the sumoylation motif ΦKxE recognized by the E2 Ubc9 (Yunus and Lima, 2006). We propose that motifs such as the TEK-box or the ΦKxE-motif are common elements required for the modification with ubiquitin or ubiquitin-like proteins.
TEK-boxes are present in several substrates, but we could not detect them in all APC/C-substrates. However, the TEK-box is not necessarily a linear sequence motif and could be generated by the three-dimensional orientation of charged amino acids. If substrates do not contain TEK-boxes, their APC/C-dependent degradation might depend on a combination of E2s, as suggested for yeast APC/C, or on an E2 different from UbcH10 that recognizes different surfaces in substrates or ubiquitin. As noted previously (Kirkpatrick et al., 2006), a combination of different E2s might result in several nucleation events before chains are formed. Such a mechanism could increase the probability of deubiquitinating enzymes acting on these substrates, thereby delaying their degradation and improving substrate discrimination by kinetic proofreading.
TEK-boxes are not found in budding yeast, but yeast APC/C assembles conventional K48-linked instead of K11-linked chains. Strikingly, yeast APC/C cannot form chains by using a single E2, but rather employs a nucleating E2 (Ubc4) and an elongating E2 (Ubc1;Rodrigo-Benni and Morgan, 2007). As budding yeast also does not have a UbcH10 homolog, these observations suggest that TEK-boxes and cognate E2s, such as UbcH10, arose later in evolution to cope with increased demands of regulating cell cycle progression. It is an exciting hypothesis that TEK-boxes in substrates were favored by evolution because they allowed the rapid formation of ubiquitin chains on cell cycle regulators, and thus, tighter cell cycle control. It will be important to determine whether similar surfaces in substrates and ubiquitin are a specific feature of the APC/C, or whether they are a more general hallmark of ubiquitin chain formation in humans.
Material and Methods
Plasmids and Antibodies
Human securin, geminin, cyclin A, cyclin B1, Plk1, and Cdc20 were cloned into pCS2 for IVT/T, and into pET28 for purification. Deletions of the TEK-boxes in securin encompassed R79ATEKSVK (TEK1) or K91KMTEKVK (TEK2). The securin-ubiquitin fusions contained securinΔDΔTEK1/2 or the first 70 amino acids of securinΔD, followed by a Gly/Ser-rich linker, and ubiquitinΔGG. Rad23, S5a, and hPlic2 were cloned into pGEX4T1 for purification, and into pCS2-HA for immunoprecipitations. His6-tagged Ubiquitin was cloned into pET28 for purfication. Ubiquitin was cloned into pCS2 for expression in cells. Antibodies were purchased for detection of Cdc27, Mad2, securin, geminin, and cyclin B1 (Santa Cruz), Plk1 (Upstate), securin (MBL), and β-actin (Abcam).
Peptides and Proteins
His6-tagged proteins were expressed in BL21 (RIL) cells and purified by NiNTA-agarose. Human HisE1 was purified from baculovirus-infected SF9 cells by NiNTA-purification. The securin D-box-peptide (aa 51–70) was purchased from Elim. Rad23, its UBA domains, S5a, and hPlic2 were purified as GST-fusions using glutathione sepharose. Purified human 26S proteasomes were purchased from Boston Biochem.
Degradation assays
Degradation assays were performed as described (Rape et al., 2006). Concentrated extracts of mitotic HeLa S3 cells arrested by nocodazole were supplemented with UbcH10 (5 μM) and p31comet (1 μM) to activate APC/C. Recombinant ubiquitin or mutants (~50 μM) were added. Reactions were analyzed for degradation of endogenous cyclin B1 by Western blotting.
Extracts of HeLa S3 cells in G1 or T24 and T98G cells in quiescence were prepared as described (Rape and Kirschner, 2004). Degradation assays were supplemented with recombinant ubiquitin mutants (~50 μM) and radiolabeled securin-mutants. The radiolabeled substrates were synthesized by IVT/T using TnT-system (Promega). Reactions were analyzed for substrate degradation by autoradiography.
Competition assays
Competition assays were performed in G1-extracts. The degradation of radiolabeled securin after 60 min at 23°C was monitored by autoradiography. Increasing concentrations of recombinant competitors were added, and the effect on degradation of securin was measured.
In vitro ubiquitination reactions
In vitro ubiquitination reactions were performed as described (Rape et al., 2006). APC/C was purified from 1.5 ml G1-extracts using 75 μl monoclonal αCdc27-antibodies and 100 μl Protein G-agarose (Roche). Washed beads were incubated with 50 nM E1, 100 nM E2, 1 mg/ml ubiquitin, energy mix (20 mM ATP, 15 mM creatine phosphate, creatine phosphokinase), 1 mM DTT at 23°C. Reactions were analyzed by autoradiography.
Purification of ubiquitin conjugates from cells
293T cells expressing securin and Hisubiquitin mutants were lysed by TCA. Precipitates were resuspended in 6M GdHCl and purified by NiNTA agarose. Ubiquitinated securin was detected by Western blotting using securin antibodies. For binding assays, 293T cells expressing securin, ubi, and HARad23 were lysed by detergent. Cleared lysates were added to anti-HA agarose (Roche), incubated at 4°C, and probed for co-purifying securin by Western blotting.
In vivo degradation assays
Most in vivo degradation assays were performed in 293T cells. APC/C-substrates and Cdh1 were co-expressed for 20–24h in the presence of Hisubiquitin or respective mutants. Cells were lysed and probed for the levels of the APC/C-substrates by Western blotting.
In vitro fertilization and injection of Xenopus tropicalis embryos
Females were primed with 10 U hGC and males boosted with 100 U hGC. The next day, females were boosted with 100 U hGC. Males were anesthetized in 0.05% benzocaine and testes were isolated. Sperm and eggs were gently mixed. 30 minutes after activation, media is changed to 3% cysteine for 15min, and then to 1/9 MR solution containing 3% ficoll. One cell of two-cell stage embryo is injected with 32 ng of protein premixed with miniRed tracer. Injected embryos were selected by fluorescence, and the phenotypes at different developmental stages were analyzed and quantified.
Supplementary Material
Acknowledgments
We thank Julia Schaletzky, Ling Song, Hermann-Josef Meyer, and James Walker for help and advice, and JS for critically reading the manuscript. We are indebted to Michelle Yasukawa and Ann Fischer for tissue culture support, and to Tao Wu and Marc Kirschner for sharing results prior to publication. Lingyan Jin is funded in part by a Tang scholarship. This work was funded by the NIH Director’s New Innovator Award and the Pew Scholar Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burton JL, Tsakraklides V, Solomon MJ. Assembly of an APC-Cdh1-substrate complex is stimulated by engagement of a destruction box. Mol Cell. 2005;18:533–542. doi: 10.1016/j.molcel.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Carroll CW, Morgan DO. The Doc1 subunit is a processivity factor for the anaphase-promoting complex. Nat Cell Biol. 2002;4:880–887. doi: 10.1038/ncb871. [DOI] [PubMed] [Google Scholar]
- Dube P, Herzog F, Gieffers C, Sander B, Riedel D, Muller SA, Engel A, Peters JM, Stark H. Localization of the coactivator Cdh1 and the cullin subunit Apc2 in a cryo-electron microscopy model of vertebrate APC/C. Mol Cell. 2005;20:867–879. doi: 10.1016/j.molcel.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Dye BT, Schulman BA. Structural mechanisms underlying posttranslational modification by ubiquitin-like proteins. Annu Rev Biophys Biomol Struct. 2007;36:131–150. doi: 10.1146/annurev.biophys.36.040306.132820. [DOI] [PubMed] [Google Scholar]
- Eletr ZM, Huang DT, Duda DM, Schulman BA, Kuhlman B. E2 conjugating enzymes must disengage from their E1 enzymes before E3-dependent ubiquitin and ubiquitin-like transfer . Nature Struct Mol Biol. 2005;12:933–934. doi: 10.1038/nsmb984. [DOI] [PubMed] [Google Scholar]
- Hicke L, Schubert HL, Hill CP. Ubiquitin-binding domains. Nature Rev Mol Cell Biol. 2005;6:610–621. doi: 10.1038/nrm1701. [DOI] [PubMed] [Google Scholar]
- Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick DS, Hathaway NA, Hanna J, Elsasser S, Rush J, Finley D, King RW, Gygi SP. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nature Cell Biol. 2006;8:700–710. doi: 10.1038/ncb1436. [DOI] [PubMed] [Google Scholar]
- Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–644. doi: 10.1016/s0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- Kraft C, Vodermaier HC, Maurer-Stroh S, Eisenhaber F, Peters JM. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol Cell. 2005;18:543–553. doi: 10.1016/j.molcel.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Lasorella A, et al. Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature. 2006;442:471–474. doi: 10.1038/nature04895. [DOI] [PubMed] [Google Scholar]
- Li W, Tu D, Brunger AT, Ye Y. A ubiquitin ligase transfers preformed polyubiquitin chains from a conjugating enzyme to a substrate. Nature. 2007;446:333–337. doi: 10.1038/nature05542. [DOI] [PubMed] [Google Scholar]
- Lin Y, Hwang WC, Basavappa R. Structural and functional analysis of the human mitotic-specific ubiquitin-conjugating enzyme, UbcH10. J Biol Chem. 2002;277:21913–21921. doi: 10.1074/jbc.M109398200. [DOI] [PubMed] [Google Scholar]
- Nijman SM, et al. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Peng J, et al. A proteomics approach to understanding protein ubiquitination. Nature Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- Peters JM. The anaphase-promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ. Mechanism of lysine 48-linked ubiquitin-chain synthesis by the cullin-RING ubiquitin-ligase complex SCF-Cdc34. Cell. 2005;123:1107–1120. doi: 10.1016/j.cell.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Rape M, Kirschner MW. Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature. 2004;432:588–595. doi: 10.1038/nature03023. [DOI] [PubMed] [Google Scholar]
- Rape M, Reddy SK, Kirschner MW. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell. 2006;124:89–103. doi: 10.1016/j.cell.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Ravid T, Hochstrasser M. Autoregulation of an E2 enzyme by ubiquitin-chain assembly on its catalytic residue. Nature Cell Biol. 2007;9:422–427. doi: 10.1038/ncb1558. [DOI] [PubMed] [Google Scholar]
- Reddy SK, Rape M, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- Reverter D, Lima CD. Insights into E3 ligase activity revealed by a SUMO-RanGAP1-Ubc9-Nup358 complex. Nature. 2005;435:687–692. doi: 10.1038/nature03588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo-Brenni MC, Morgan DO. Sequential E2s drive polyubiquitin chain assembly on APC targets. Cell. 2007;130:127–139. doi: 10.1016/j.cell.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Stegmeier F, et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 2007;446:876–881. doi: 10.1038/nature05694. [DOI] [PubMed] [Google Scholar]
- Thornton BR, Ng TM, Matyskiela ME, Carroll CW, Morgan DO, Toczyski DP. An architectural map of the anaphase-promoting complex. Genes Dev. 2006;20:449–460. doi: 10.1101/gad.1396906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert BS, et al. The active site cysteine of ubiquitin-conjugating enzymes has a significantly elevated pKa: functional implications. Biochemistry. 2005;44:16385–16391. doi: 10.1021/bi0514459. [DOI] [PubMed] [Google Scholar]
- Varadan R, Assfalg M, Raasi S, Pickart C, Fushman D. Structural determinants for selective recognition of a Lys48-linked polyubiquitin chain by a UBA domain. Mol Cell. 2005;18:687–698. doi: 10.1016/j.molcel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Verma R, et al. Proteasomal proteomics: identification of nucleotide-sensitive proteasome-interacting proteins by mass spectrometric analysis of affinity-purified proteasomes. Mol Cell Biol. 2000;11:3425–39. doi: 10.1091/mbc.11.10.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth KG, et al. Loss of the anaphase-promoting complex in quiescent cells causes unscheduled hepatocyte proliferation. Genes Dev. 2004;18:88–98. doi: 10.1101/gad.285404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunus AA, Lima CD. Lysine activation and functional analysis of E2-mediated conjugation in the SUMO pathway. Nat Struct Mol Biol. 2006;13:491–499. doi: 10.1038/nsmb1104. [DOI] [PubMed] [Google Scholar]
- Zheng N, Wang P, Jeffrey PD, Pavletich NP. Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell. 2000;102:533–539. doi: 10.1016/s0092-8674(00)00057-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.