Abstract
Gastric electrical stimulation (GES) has been suggested as a therapy for patients with gastric motility disorders or morbid obesity. However, it is unclear whether GES also affects intestinal sensory and motor functions. Furthermore, little is known about intraspinal visceroreceptive transmission and processing for duodenal afferent information. The aims of this study were to characterize responses of thoracic spinal neurons to duodenal distension, to determine the afferent pathway, and to examine the effects of GES on activity of these neurons. Extracellular potentials of single T9-T10 spinal neurons were recorded in pentobarbital anesthetized, paralyzed, ventilated male rats (n=19). Graded duodenal distension (DD, 0.2–0.6 ml, 20 s) was produced by water inflation of a latex balloon surgically placed into the duodenum. One pair of platinum electrodes (1.0–1.5 cm apart) was sutured onto the serosal surface of the lesser curvature of the stomach. GES with four sets of parameters was applied for one minute: GES-A (6 mA, 0.3 ms, 40 Hz, 2s on, 3s off), GES-B (6 mA, 0.3 ms, 14 Hz, 0.1s on, 5s off), GES-C (6 mA, 3 ms, 40 Hz, 2s on, 3s off), GES-D (6 mA, 200 ms, 12 pulses/min). Results showed that 33/117 (28%) spinal neurons responded to noxious DD (0.4 ml, 20s). Of these, 7 (6%) neurons had low-threshold responses to DD (≤0.2 ml) and 26 (22%) had high-threshold responses to DD (≥0.4 ml). DD-responsive spinal neurons were encountered more frequently in deeper (depth: 0.3–1.2 mm) than in superficial laminae (depth: <0.3 mm) of the dorsal horn (24/67 vs 9/50, P<0.05). DD excited all 9 superficial neurons. In contrast, 20 deeper neurons were excited and 4 neurons were inhibited by DD. Activity of DD-responsive neurons was affected more frequently with GES-C (13/15, 87%) than GES-A (6/16, 38%), -B (3/15, 20%), -D (5/14, 36%), (P<0.01). Bilateral cervical vagotomy did not significantly alter the effects of DD and GES on 5/5 neurons. Resiniferatoxin (2.0 μg/kg, i.v.), an ultrapotent agonist of transient receptor potential vanilloid receptor-1 (TRPV1), abolished DD responses and GES effects on all neurons examined in vagotomized rats. Additionally, 29/33 (88%) DD-responsive neurons received inputs from somatic receptive fields on the back, flank, and medial/lateral abdominal areas. It was concluded that GES mainly exerted an excitatory effect on T9-T10 spinal neurons with duodenal input transmitted by sympathetic afferent fibers expressing TRPV1; spinal neuronal responses to GES were strengthened with an increased pulse width and/or frequency of stimulation; T9-T10 spinal neurons processed input from the duodenum and might mediate effects of GES on duodenal sensation and motility.
Keywords: Duodenal afferents, sympathetic afferents, vagal afferents, vanilloid receptor-1, spinal cord
1. Introduction
Although visceral pain originating from the duodenum is a common manifestation in patients with peptic ulcer, inflammation or cancer of the gastrointestinal tract, clinical and basic research of duodenal pain is relatively less in comparison to other visceral pains such as irritable bowel syndrome. Early clinical studies (Bloomfield and Polland 1931) show that inflation of a balloon in the duodenum produces an intense pain described as unpleasant pressure, fullness, burning, aching and colic. Sites of duodenal pain are predominantly located between the epigastric and periumbilical areas, although it is most frequently referred to the right upper quadrant of abdomen. In animal models, duodenal distension often is used for studying visceral pain and its neurohormonal mechanisms. In conscious and freely moving rats, DD can induce passive avoidance behaviour in addition to altered postures (arching, squashing, grooming, stretching and writhing), visceromotor reflex responses (an increase in abdominal EMG), and cardiovascular responses (changes in arterial blood pressures and heart rate) (Colburn et al. 1989; DeLeo et al. 1991; Feng et al. 1998; Nijsen et al. 2003; Stam et al. 2004). These pseudoaffective pain-like responses can be reduced by morphine, suggesting that DD is perceived as a noxious aversive stimulus under these situations (Colburn et al. 1989; Moss and Sanger 1990; Nijsen et al. 2003). However, little is known about the neural mechanisms underlying duodenal nociception. It is well known that the duodenum is richly innervated with vagal (parasympathetic) and splanchnic (sympathetic) nerve afferent fibers. In general, it is believed that vagal afferents from the duodenum may play a role in conveying digestive information, such as absorption, secretion, and storage; whereas, nociceptive information mainly travels via the splanchnic sympathetic afferent nerves to the spinal cord. Anatomic studies have shown that splanchnic afferent fibers from the duodenum mainly project to the caudal thoracic and upper lumbar spinal cord in various species (Cottrell and Greenhorn 1987; DeLeo et al. 1991; El Ouazzani and Mei, 1978; Hazarika et al. 1964; Khurana and Petras 1991; Quinson et al. 2001). However, to the best of our knowledge, no electrophysiological study has been done to examine the activity of spinal neurons receiving duodenal afferent input.
Gastric electrical stimulation (GES) is a direct delivery of a small electrical current with varying parameters to the stomach to modulate gastric sensory and motor functions. It has been shown to be effective in normalizing gastric dysrhythmia, accelerating gastric emptying, decreasing nausea and vomiting, and treating gastroparesis as well as obesity (see review, Zhang and Chen 2006). However, previous basic and clinical studies have only investigated the effects of GES on gastric functions and diseases (Abell and Minocha 2002; D’Argent et al. 2002; Greenstein and Belachew 2002; Lin and Chen 2002; Liu et al. 2006; Zhang and Chen 2006). We hypothesize that GES not only modulates gastric motility but also affects the functions of other segments of the gastrointestinal tract, such as the intestine. In support of this hypothesis, physiological and anatomical data indicate that a large degree of overlap occurs in the thoracolumbar spinal ganglia and spinal segments that supply both the stomach and duodenum in various species (Hazarika et al. 1964; Khurana and Petras 1991; McSwinney and Suffolk 1938). Both the duodenum and the stomach receive dual innervations by vagal (parasympathetic) and splanchnic (sympathetic) nerves in humans and animals, which play an important role in the regulation of gastric function. Effects of GES on gastric motility have been shown to involve vagal afferent and/or efferent pathways in dogs (Chen et al. 2003; Grundfest-Bronaltowski et al. 1990; Liu et al. 2004; Ouyang et al. 2003). In rats, GES can activate vagal afferent fibers innervating the stomach (Peles et al. 2003) and modulate activity of neurons in the nucleus tractus solitarii receiving gastric vagal afferents (Qin et al. 2005). A few recent studies further suggest that effects of GES with varying parameters on gastric motility involve the sympathetic alpha- and beta-adrenergic sympathetic efferent pathways system (Zhu and Chen 2005; Ouyang et al. 2005). The spinal sympathetic afferent pathways and the intraspinal neuronal activity relevant to GES effects also have been characterized recently in rats (Qin et al. 2007). The aims of this study in rats were to: 1) characterize thoracic (T9-T10) spinal neurons responding to duodenal distension (DD); 2) examine the effect of GES with different parameters on the activity of neurons excited by DD; 3) determine afferent pathways of duodenal input to spinal neurons and GES effects on activity of these neurons; 4) elucidate the involvement of spinal afferent fibers expressing transient receptor potential vanilloid receptor-1 (TRPV1) in DD responses and GES effects. Preliminary results of this study have been presented previously in an abstract (Qin et al. 2006).
2. Results
Duodenal distension (DD, ≥ 0. 4 ml, 20s) changed the activity of 33/117 (28%) spinal neurons that were recorded in T9 (n=16) and T10 (n=17) segments. Twenty DD-responsive neurons were recorded from the left side and 13 neurons from the right side of the spinal cord. Electrolytic lesions of the recording sites were verified histologically. The neurons excited by DD were primarily located in laminae I–III, V, VII and X, whereas the spinal neurons inhibited by DD were found in laminae V and VII (Fig. 1A, B). The DD-responsive neurons were encountered more frequently in deeper (depth: 0.3–1.2 mm) laminae than in superficial laminae (depth: <0.3 mm) of the dorsal horn (24/67 vs 9/50, P<0.05).
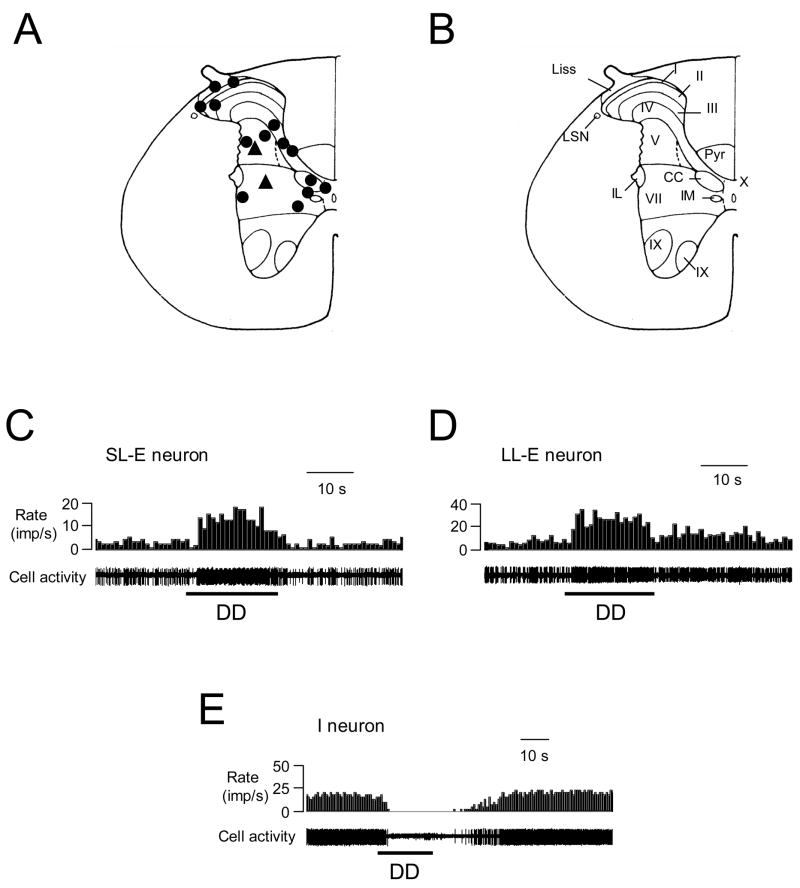
Fig. 1.
Recording sites and response patterns of low thoracic (T9-T10) spinal neurons to duodenal distension (DD, 0.4 ml, 20s). A: Locations of spinal neurons responding to DD. The black circles represent neurons excited by DD. The black triangles represent neurons inhibited by DD. B: Schematic drawing of the T10 spinal segment (Molander et al. 1984). I–X indicates laminae; Liss, Liss’s tract; LSN, lateral spinal nucleus; Pyr, pyramidal tract; IL, intermediolateral nucleus. IM, intermediomedial nucleus. CC, column of Clarke. C: Short-lasting excitatory (SL-E) response to DD. D: Long-lasting excitatory (LL-E) response to DD. E: Inhibitory (I) response to DD.
2.1. Response patterns to DD
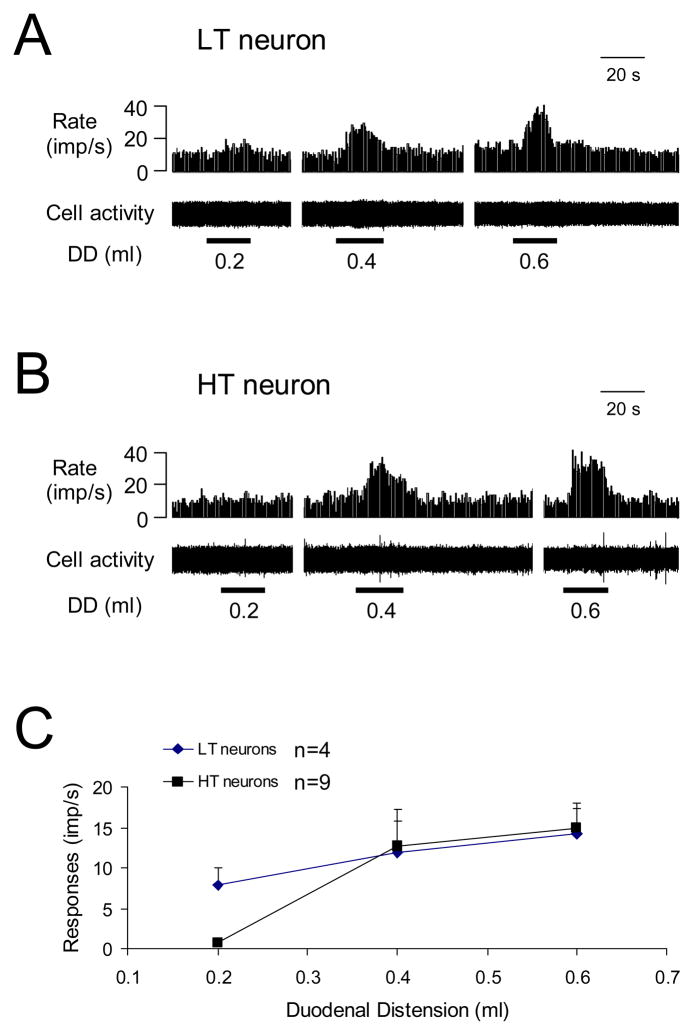
Of the DD-responsive neurons, all 9 superficial neurons were excited by DD, whereas 20 of the 24 deeper neurons were excited and four were inhibited. Examples of these neuronal responses are shown in Fig. 1C–E. Quantitative analyses of spontaneous activity and responses of the spinal neurons to noxious DD (0.4 ml, 20s) are shown in Table 1. Based on neuronal responses to graded DD, neurons with excitatory responses were divided into the following two subgroups: 7 low threshold (LT) neurons started responding to ≤ 0.2 ml DD; 26 high threshold (HT) neurons only responded to ≥ 0.4 ml DD. Of these, 13 neurons were fully characterized for the entire range of graded DD (0.2, 0.4, 0.6 ml, 20s), including 4 LT and 9 HT neurons. Examples and stimulus-response curves of these neurons are shown in Fig. 2. Based on the recovery time of neuronal activity to control level after termination of DD (0.4 ml, 20 s), neurons with excitatory responses to DD were further divided into the following two subgroups: 11 neurons with a recovery time of ≤ 10 s were classified as short-lasting excitatory (SL-E, Fig. 1C), and 18 neurons with a recovery time of >10 s were classified as long-lasting excitatory (LL-E, Fig. 1D). Quantitative analyses of the response characteristics of SL-E and LL-E neurons to DD are shown in Table 2. Spontaneous activity was significantly higher and the response duration of LL-E neurons was significantly longer than those of SL-E neurons (P<0.05).
Table 1.
Response patterns and characteristics of T9-T10 spinal neurons to noxious duodenal distension (DD, 0.4 ml, 20s).
| Responses to DD | n | Spontaneous activity (imp/s) | Latency (s) | E-Response (imp/s) | I-Responses (imp/s) | Duration (s) |
|---|---|---|---|---|---|---|
| Excitation (E) | 29 | 8.7±1.1 | 2.9±0.3 | 13.6±1.8 | / | 35.5±3.4 |
| Inhibition (I) | 4 | 10.9±1.6 | 3.9±0.9 | / | 7.4±0.9 | 41.0±7.3 |
Fig. 2.
Thoracic (T9-T10) spinal neuronal responses to graded duodenal distension (DD). A: Low-threshold (LT) excitatory response to DD. B: High-threshold (HT) excitatory response to DD. C: Summary for excitatory responses to graded DD.
Table 2.
Short- and long-lasting excitatory responses of thoracic (T9-T10) spinal neurons to duodenal distension (0.4 ml, 20s).
| Groups | n | Spontaneous activity (imp/s) | Latency (s) | Responses (imp/s) | Duration (s) |
|---|---|---|---|---|---|
| Short-lasting | 11 | 6.0±1.6 | 2.5±0.4 | 14.3±3.6 | 20.2±1.3 |
| Long-lasting | 18 | 10.3±1.4* | 3.2±0.4 | 13.2±2.0 | 45.0±4.1* |
P<0.05 compared to duration of short-lasting responses.
2.2. GES modulation of neuronal activity
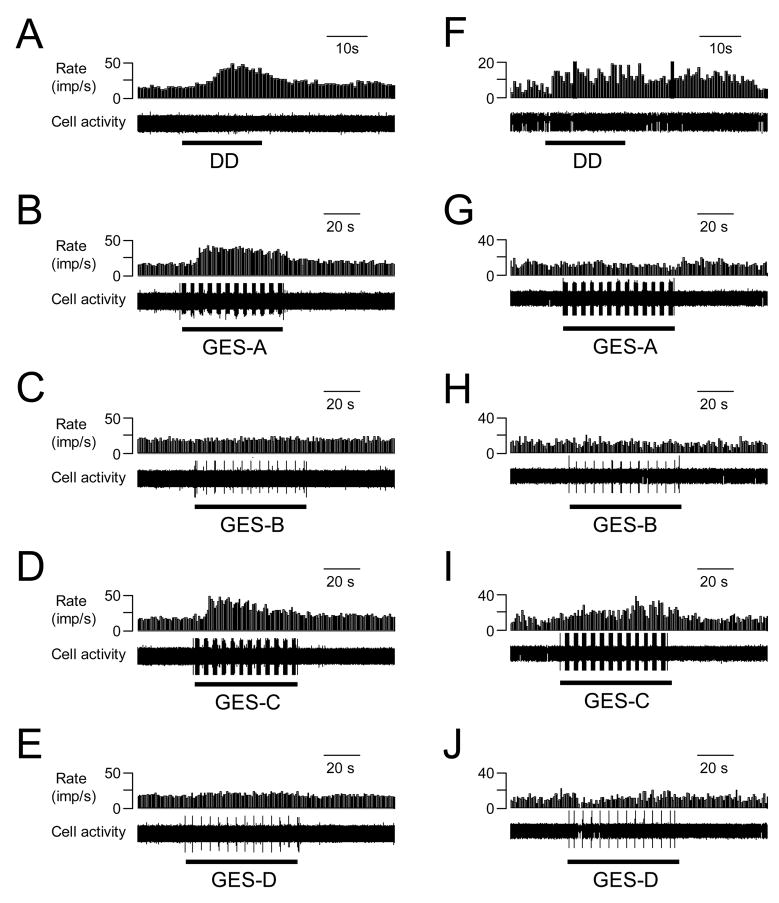
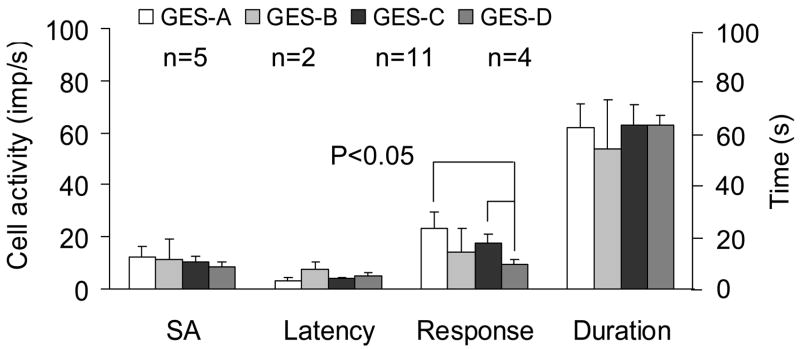
GES-A, -B, -C and –D changed the activity of 6/16 (38%), 3/15 (20%), 13/15 (87%) and 5/14 (36%) spinal neurons excited by DD, respectively (Table 3). Spinal neuronal activity was affected more frequently with GES-C (6 mA, 3 ms, 40 Hz, 2 s-on and 3 s-off), i.e. an increased pulse width of 3 ms, than with other GES parameters (P<0.05). Gastric electrical stimulation primarily increased activity of most spinal neurons with duodenal input, and a few neurons were inhibited (Table 3). Figure 3A–E shows an example of a spinal neuron with excitatory responses to DD and to GES-A and GES-C. A few neurons exhibited different responses to different parameters of GES. Fig. 3F–J shows an example of a spinal neuron with LL-E response to DD, which was excited by GES-C, inhibited by GES-D, and unaffected by GES-A and -B. Fig. 4 is a summary of the effects of GES with different parameters on spinal neurons excited by DD. Mean excitatory responses to GES-A (23.3±6.2 imp/s) and GES-C (17.8±3.4 imp/s) were significantly greater than responses to GES-D (9.4±2.7 imp/s) (P<0.05). In addition, GES changed the activity of spinal neurons with high-threshold responses to DD and also with low-threshold responses to DD (Table 4).
Table 3.
Effects of gastric electrical stimulation (GES) with different parameters on the activity of T9-T10 spinal neurons responding to duodenal distension.
| GES-A | GES-B | GES-C | GES-D | |||||
|---|---|---|---|---|---|---|---|---|
| E | I | E | I | E | I | E | I | |
| Response (R) | 5 | 1 | 2 | 1 | 11 | 2 | 4 | 1 |
| No response | 10 | 12 | 2 | 9 | ||||
|
| ||||||||
| Total (R/tested) | 6/16 (38%) | 3/15 (20%) | 13/15* (87%) | 5/14 (36%) | ||||
P< 0.05 compared to responsive neurons with other GES parameters.
Fig. 3.
Examples of the effects of GES with different parameters on activity of 2 different spinal neurons excited by duodenal distension (DD). A–E: GES-A, -C increased and GES-B, D did not affect activity of a spinal neuron excited by DD (0.4 ml, 20s). F–J. GES-A, B did not affect, GES-C increased, and GES-D inhibited activity of another spinal neuron excited by DD.
Fig. 4.
A summary for the effects of GES with different parameters on activity of thoracic (T9-T10) spinal neurons excited by duodenal distension (DD). SA, spontaneous activity.
Table 4.
Effects of gastric electrical stimulation (GES) with different parameters on the activity of low- and high-threshold (LT, HT) spinal neurons excited by duodenal distensions.
| GES-A | GES-B | GES-C | GES-D | ||||
|---|---|---|---|---|---|---|---|
| LT | HT | LT | HT | LT | HT | LT | HT |
| 2 | 3 | 0 | 2 | 4 | 7 | 1 | 3 |
2.3. Viscerosomatic convergence
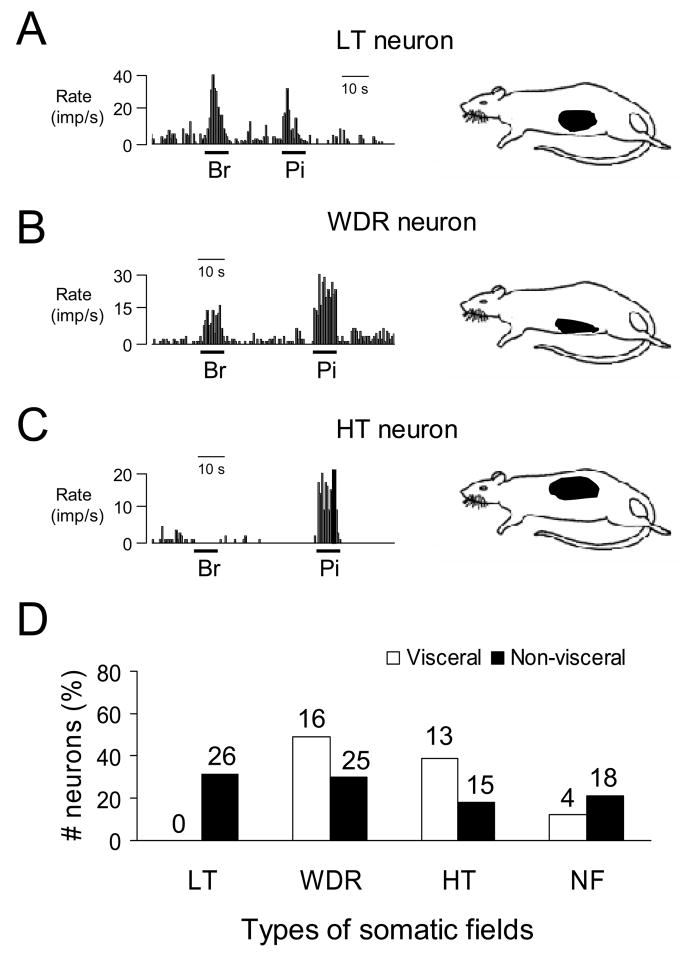
Of 33 DD-responsive neurons examined for somatic mechanical stimulation, 29 (88%) neurons received convergent somatic inputs, which was not statistically different from the proportion (66/84, 79%) of neurons that did not respond to DD. Of the DD-responsive spinal neurons with viscerosomatic inputs, 16 neurons were classified as WDR, 13 neurons were HT, and no LT neurons were found. For neurons that did not respond to DD; however, 26 LT, 25 WDR and 15 HT neurons with somatic input were identified. Somatic receptive fields of DD-responsive spinal neurons were located on the low back, flank, and medial/lateral abdominal areas. Fig. 5 shows examples and a summary of the different properties of somatic receptive fields of spinal neurons with or without duodenal input.
Fig. 5.
Characteristics of somatic receptive fields of lower thoracic (T9-T10) spinal neurons. A: Responses of a low threshold (LT) neuron to brush (Br) and pinch (Pi) of somatic fields and location of somatic receptive field (right panel). B: A spinal neuron with wide dynamic range (WDR) responses to somatic stimulation and location of somatic receptive field. C: A spinal neuron with high-threshold (HT) responses to somatic stimulation and location of somatic receptive field. D: Summary for somatic field properties of spinal neurons with duodenal or somatic only inputs. NF, somatic field not found.
2.4. Effects of cervical vagotomy
Bilateral cervical vagotomy reduced the excitatory responses to noxious DD (0.4 ml, 20s) in 3 neurons but did not affect the other 2 neurons. Taken together, the mean excitatory DD-responses (15.4±4.1 imp/s, n=5) after vagotomy were not statistically different from responses before vagotomy (20.0±4.4 imp/s). For 5 neurons excited by GES-C (n=4) and GES-A (n=1), bilateral cervical vagotomy increased responses to GES in one neuron, decreased responses to GES in one neuron and did not change the responses in the other 3 neurons. The mean excitatory responses to GES after vagotomy (20.0±4.2 imp/s) were similar to responses before vagotomy (20.4±6.2 imp/s). Fig. 6 shows an example of the excitatory responses of a spinal neuron to DD and GES-C before (A) and after (B) vagotomy.
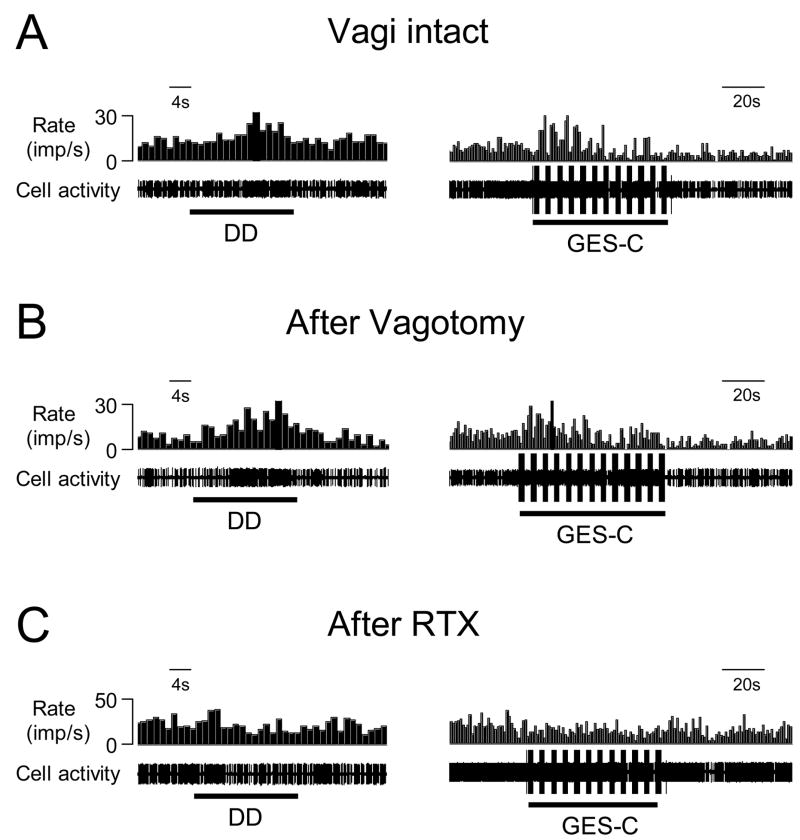
Fig. 6.
The effects of cervical vagotomy and intravenous RTX on the responses to DD and GES in one T9 spinal neuron. A: Excitatory responses to DD (0.4 ml, 20s) and GES-C (right panel) in an animal with intact vagi. B: Excitatory responses of neuron A to DD and GES-C after bilateral cervical vagotomy. C: Intravenous RTX (2.0 μg/kg) eliminated excitatory responses to both DD and GES-C in the same neuron.
2.5. Effects of RTX
To determine whether TRPV1 receptors play a role in spinal neuronal responses to duodenal mechanical stimulation and GES, intravenous RTX was used as a pharmacological tool to desensitize capsaicin-sensitive afferent fibers containing TRPV1. Excitatory responses of spinal neurons to DD and GES were examined 20 min after RTX in 4 vagotomized rats. Intravenous RTX (2.0 μg/kg) initially increased the activity in 3 neurons and reduced the activity in one neuron responding to DD and GES. Twenty minutes later, RTX abolished the excitatory responses to DD in 4/4 spinal neurons and GES-C in 3/3 neurons. Fig. 6B and C show the elimination of excitatory responses of the spinal neuron to DD and GES-C by RTX administration.
3. Discussion
To the best of our knowledge, this is the first study using electrophysiological technique to characterize spinal neurons receiving visceral input from the duodenum in any species. Results showed that innocuous and/or noxious DD changed activity in 28% of T9-T10 spinal neurons. The majority of these responsive neurons (88%) were excited by DD, and the remaining neurons were inhibited. Most spinal neurons responding to DD had nociceptive somatic fields on the low back, flank, and medial/lateral abdominal areas. Furthermore, GES with different parameters mainly exerted an excitatory effect on activity of 20–87% T9-T10 spinal neurons responding to DD. The degree of neuronal excitation with GES depended upon stimulation parameters, i.e. effects of GES on neuronal activity were enhanced with stimulation at an increased pulse width and frequency. Bilateral cervical vagotomy did not significantly affect excitatory responses of T9-T10 spinal neurons to DD and/or GES. In contrast, intravenous RTX abolished all spinal neuronal responses to DD and GES in animals with vagotomy. These data indicated that spinal neuronal activation by DD and GES depended on afferent impulses traveling in thoracic spinal (sympathetic) fibers expressing TRPV1.
3.1. Locations of neurons
Viscerotopic organization of the spinal sensory innervation of the duodenum is slightly different in various animal species, but in general the innervation extends from segments T2-L3, with the peak innervation occurring between segments T9 and L1 (Khurana and Petras, 1991; El Ouazzani and Mei, 1978; Hazarika et al. 1964; Cottrell and Greenhorn, 1987; DeLeo et al., 1991; Quinson et al. 2001). Furthermore, anatomical studies indicate variable degrees of overlap in sensory projections from both the stomach and duodenum to spinal ganglia and segments. For example, a large overlap of spinal ganglia innervation for the stomach and duodenum is in T6-L1 segments in cats (Hazarika et al. 1964; Khurana and Petras 1991; McSwinney and Suffolk 1938). Because one of the purposes of the present study was to determine whether GES modulated activity of spinal neurons with duodenal input, T9-T10 spinal segments were selected to search for spinal neurons responding to DD. These segments receive primary sympathetic afferent fibers innervating the duodenum and also receive afferent input from the stomach in rats (Holzer et al. 2005; Neuhuber and Niedrle 1979; Ozaki and Gebhart 2001; Qin et al. 2007; Schicho et al. 2005; Schuligoi et al. 1996; Sugiura et al. 2005). Therefore, this method provided an opportunity to determine the effects of GES on spinal neurons with duodenal input. The intraspinal regional sites of DD-responsive neurons were located in laminae I, II, III, V, VII and X in the present study. Neurons excited by DD were located in both the superficial and deeper laminae, whereas the neurons inhibited by DD were found in deeper laminae. These observations generally agreed with a previous report, in which repeated noxious DD induce intense neuronal staining of c-fos-like immunoreactive neurons bilaterally in laminae I–VI, IX and X predominately at T6-T9 segments in rats (DeLeo et al. 1991).
3.2. Neuronal responses to DD
Twenty-eight percent of T9-T10 spinal neurons responded to noxious DD in the present study, which was lower than 39% of spinal neurons in the same segments that responded to noxious gastric distension (Qin et al. 2007). Furthermore, 88% of T9-T10 spinal neurons responding to DD were excited and the remaining neurons were inhibited by DD in the present study. In contrast, 70% of T9-T10 spinal neurons responding to gastric distension were excited; 19% were inhibited and the other neurons had a biphasic pattern of responses (Qin et al. 2007). These differences might represent visceral organ-specific processing of afferent information in the spinal cord, although the same spinal segments have been examined for both gastric and duodenal afferent inputs traveling through the splanchnic nerve (Ozaki and Gebhart 2001; Schloithe et al. 2006). In Australian possums, only 12/99 (12%) splanchnic nerve afferent fibers respond to DD (Schloithe et al. 2006), which is significantly lower than 28% of T9-T10 spinal neurons that responded to DD in the present study. One possible explanation for this small number of afferent fibers activating a larger population of spinal neurons may be due to branches of the sympathetic afferent fibers that distribute rostrocaudally within the spinal gray matter and trans-segmentally innervate spinal neurons in different levels of the spinal cord (Sugiura et al. 1989).
In the present study the neurons were classified as LT or HT based on their excitatory responses to DD. A few studies in rats have shown that DD induce volume-dependent increases in passive avoidance behavior, pseudoaffective cardiovascular responses and visceromotor responses (Colburn et al. 1989; Feng et al. 1998; Nijsen et al. 2003; Stam et al. 2004). For example, a lower volume (0.2 ml) of graded distension of the duodenum induces shaking and exploring behavior that may be perceived as non-noxious, whereas higher volumes (0.4–0.6 ml) cause grooming and stretching behavior, suggestive of pain in conscious and freely moving rats (Nijsen et al. 2003). Another study in rats shows that writhing-like responses to DD ≤ 0.3 ml vary but become more frequent to DD>0.4 ml (Feng et al. 1998). The mean threshold volume of DD to elicit a writhing-like response is 0.23 ± 0.06 ml in conscious rats (Feng et al. 1998). Pseudoaffective cardiovascular (arterial blood pressure and heart rate) and visceromotor responses (abdominal contraction EMG) to DD exhibit similar volume-dependent changes (Feng et al. 1998; Nijsen et al. 2003; Stam et al. 2004). These physiological and behavioral responses to DD can be inhibited by the administration of morphine in a dose dependent manner, suggesting that visceral pain or nociception originates in the duodenum (Colburn et al. 1989; Nijsen et al. 2003). Presumably, HT spinal neurons likely play an important role in intraspinal processing associated with duodenal nociception, whereas LT spinal neurons might be relevant to nonpainful sensations such as fullness and nausea. Thus, some spinal neurons recorded in the present study likely processed innocuous duodenal input, although the majority of neurons primarily were relevant to duodenal nociception.
In the present study, excitatory neuronal responses also were divided into two categories of spinal neurons with noxious duodenal input, i.e. 38% short-lasting (SL) or abrupt-responsive neurons and 62% long-lasting (LL) or sustained-responsive neurons. One explanation for these two categories is that SL and LL responses might be associated with afferent activity of spinal visceral A-delta and C fibers from the duodenum (Floyd and Morrison 1974). Another possibility is that these characteristics represent rapid- and slow-adapting mechanical duodenal receptors, similar to vagal afferents originating in the duodenum (Cottrell and Iggo 1984). However, studies to elucidate a direct relationship between spinal visceral afferent fiber activity and spinal neuronal responses encoding duodenal stimulation are not available in the literature for any species. A recent study in Australian possums shows that all (12/12) splanchnic afferent fibers responding to DD are slow-adapting and activity persists throughout sustained distension (Schloithe et al. 2006). These data are difficult to reconcile with the observations of the present study because of the different criteria used for responses to DD. However, the characteristics of SL and LL neuronal responses found in this study might be involved in different aspects of visceral sensory processing for duodenal inputs. For example, the SL or abrupt-responsive neurons are more likely to be involved in the localization of painful events, whereas LL or sustained-responsive neurons are more likely related to the clinical phenomenon of poor localization of visceral pain (Ness and Gebhart 2001).
3.3. Effects of GES
GES with various parameters have been applied in both human and animals for inducing gastric sensory and motor effects (Abell and Minocha 2002; Familoni et al. 1997; Forster et al. 2001; Lin et al. 2002; McCallum et al. 1998; Sobocki et al. 2003). Different stimulus parameters, such as frequency, pulse width and amplitude of GES, are designed to achieve the different effects, and typical stimuli include short-pulses, long-pulses, and trains of pulses (Zhang and Chen 2006). In the present study, we used four sets of stimulation parameters: “standard” pulse trains (GES-A), pulse trains with decreased frequency (GES-B), pulse trains with increased pulse width (GES-C) and single long pulses (GES-D). The four sets of GES parameters mainly induced excitatory responses in most spinal neurons with duodenal input. GES-C, with pulse trains of increased pulse width, was most effective, whereas GES-B, with decreased frequency, was least effective in eliciting neuronal responses in the spinal cord. Moreover, GES-D, with single long pulses, elicited smaller excitatory responses than those produced by GES-A and GES-C, which used shorter pulses at higher frequency than GES-D. These results suggested that both the frequency and width of the stimulation pulse play an important role in the modulation of thoracic spinal neuronal activity. In general, these data are similar to previous observations on the effects of GES on the activity of thoracic spinal neurons with gastric input (Qin et al. 2007), and on neuronal activity in the nucleus of the solitary tract and in the paraventricular nucleus of the hypothalamus in rats (Qin et al. 2005; Tang et al. 2006). Because a large overlap of spinal innervation for the stomach and duodenum occurs in the lower thoracic segments (Hazarika et al. 1964; Khurana and Petras 1991), it is likely that spinal afferent impulses evoked by GES activated spinal neurons that also received primary input from the duodenum. Recently, a preliminary report has showed that some T9-T10 neurons received convergent mechanical receptive inputs from both stomach and duodenum (Qin et al. 2007).
3.4. Afferent pathways
The duodenum is richly innervated with afferent fibers of the vagal and splanchnic nerves, which convey chemical, osmotic, mechanical, and thermal information from duodenal receptors to the central nervous system. It has been shown that capsaicin-sensitive afferents expressing TRPV1 play an important role in the transmission of the duodenal sensory information to the central nervous system when the duodenum is distended or perfused with acid (Holzer and Raybould 1992; Takeuchi et al. 1991; Kagawa et al. 2003). It is believed that low volume DD activates capsaicin-sensitive vagal afferents, whereas high volume DD involves splanchnic capsaicin-sensitive afferent fibers (Holzer and Raybould 1992). Treatment with a large dose of capsaicin selectively destroys unmyelinated visceral afferent fibers without affecting unmyelinated efferent fibers in autonomic nerves (Cervero and McRitchie 1982). In the present study, all T9-T10 tested spinal neurons still responded to DD after bilateral vagotomy, but intravenous administration of RTX eliminated spinal neuronal responses to DD following vagotomy. These data indicated that splanchnic (sympathetic) afferent fibers expressing TRPV1 were essential for spinal neuronal responses to DD. It should be noted that vagotomy enhanced, reduced or did not affect responses to DD in different spinal neurons, although the vagal input was not necessary to initially elicit spinal neuronal responses to DD in the present study. This finding suggested that vagal afferents might modulate individually the activity of thoracic spinal neurons receiving duodenal inputs, although vagotomy did not affect average responses to DD in a population of spinal neurons. Similarly, vagal afferent modulation of spinal neuronal activity is often observed in responses to various visceral stimuli such as esophageal and gastric distensions (Qin et al. 2003; 2007). The present study also showed that T9-T10 spinal neurons encoded duodenal inputs at non-noxious and noxious ranges of graded intensities of DD. Thus, it is possible that, in addition to duodenal nociception, these spinal neurons also process non-painful mechanical sensations such as fullness and nausea.
The neural pathways involved in GES effects on gastric motility involve vagal- and sympathetic-mediated neural mechanisms(Zhang and Chen 2006). For example, GES increases the firing rate of single vagal gastric afferent fibers and mainly exerts excitatory effects on neuronal activity in the nucleus tractus solitarii in rats (Peles et al. 2003, Qin et al. 2005). Vagotomy eliminates the anti-emetic effects of short-pulse GES on nausea and vomiting induced by vasopressin (Chen et al. 2003). The assessment of vagal activity with an advanced spectral analysis of heart rate variability indicates that effects of GES with certain parameters are relevant to increased vagal activity and accelerated gastric emptying in dogs and rats (Liu et al. 2004; Ouyang et al. 2003). Furthermore, intravenous administration of an adrenergic blocker prevents the inhibitory effect of GES on antral motility and/or rectal tone, indicating an involvement of the sympathetic adrenergic nerve fibers (Zhu and Chen 2005; Liu et al. 2005; Ouyang et al. 2005). The present study showed that vagotomy did not significantly affect the effects of GES on spinal neuronal activity but intravenous RTX abolished responses to GES in rats with vagotomy. This observation is consistent with a previous study (Qin et al. 2007). These data indicated that spinal (sympathetic) afferent fibers expressing TRPV1 might play an important role in producing effects of GES on gastrointestinal function.
In conclusion, rat T9-T10 spinal neurons might play an important role intraspinal processing for innocuous and noxious mechanical input from the duodenum; GES with different parameters mainly exerted an excitatory effect on T9-T10 spinal neurons with duodenal input transmitted by sympathetic afferent fibers expressing TRPV1; spinal neuronal responses to GES were strengthened with an increased pulse width and/or frequency of stimulation. It is also suggested that GES might modulate duodenal sensory and motor functions, potentially as a treatment for duodenal disorders, in addition to its well studied applications for gastric disorders (Zhang and Chen 2006).
4. Experimental procedures
Nineteen male Sprague-Dawley rats (Charles River Inc. 350–450 g) were used for this study. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center. Animals were initially anesthetized with intraperitoneal injection of sodium pentobarbital (60 mg/kg). The right carotid artery and left jugular vein were cannulated for continuous blood pressure monitoring and for intravenous infusion of pentobarbital (15–25 mg/kg/hr) throughout the experiments, respectively. The trachea was cannulated through a tracheotomy for artificial ventilation using a constant-volume pump (55–60 strokes/min, 3.0–5.0 ml stroke volume). To provide and maintain muscle relaxation, pancuronium bromide (initial dose 0.4 mg/kg) was administered intravenously and animals were given supplemental doses (0.2 mg/kg) as needed throughout the experiments. During an experiment, the average blood pressure was kept at 80–100 mmHg. Rectal temperature was kept at 37±0.3°C by a servo-controlled heating blanket and overhead infrared lamps.
After midline laparotomy, a segment of duodenum 2–3 cm distal to the pylorus of the stomach was isolated and duodenal contents were removed through a small incision in the duodenal wall. A latex balloon (1.0 cm in length) attached to a polyethylene tubing (PE-240) with 2–3 small holes near the tip was inserted into the duodenal cavity through the incision and fixed at the edge of the incision with a ligature. Special care was taken not to damage blood vessels and nerve branches around the duodenum. To prevent stomach contents from emptying into the duodenum, gastric contents were gently removed through a small incision at the fundus. Duodenal distensions (DD) were produced by injecting warm water (0.2, 0.4, 0.6 ml) through a catheter over 2–5 seconds (s); distension was maintained for 20 s. This range selected for DD was based on previous studies, in which a DD volumes ≥ 0.4 ml cause significant passive avoidance behavior and pseudoaffective cardiovascular responses in rats and are believed to be noxious (Nijsen et al. 2003; Stam et al. 2004). Therefore, to find the maximal number of spinal neurons with duodenal input, noxious DD (0.4 ml, 20s) was used as a search stimulus. For delivering GES, one pair of platinum electrodes (32 GA) each made with 5–8 circles of a spring (0.5 cm long) was sutured onto the serosal surface (the electrodes were separated by 1.0 cm) of the lesser curvature of the stomach (Qin et al. 2005; 2007). Dental impression material was placed around the electrodes to isolate them from other visceral organs and the abdominal wall. Gastric electrical stimulations at four sets of parameters were applied for one minute: GES-A (pulse trains of standard parameters: 6 mA, 0.3 ms, 40 Hz, 2 s-on and 3 s-off), GES-B (pulse trains of decreased frequency: 6 mA, 0.3 ms, 14 Hz, 0.1 s-on and 5 s-off), GES-C (pulse trains of increased pulse width: 6 mA, 3 ms, 40 Hz, 2 s-on and 3 s-off), and GES-D (single long pulses: 6 mA, 200 ms, 12 pulses/min).
After laminectomy to expose the T9-T10 spinal segments, rats were mounted in a stereotaxic headholder and stabilized with clamps attached to L1-L2 and T5-T7 vertebral processes. Dura mater was carefully removed and the spinal cord was covered with warm agar (3–4% in saline) to improve recording stability. Carbon-filament glass microelectrodes were used for extracellular recordings of action potentials of single T9-T10 spinal neurons within 0–1.2 mm from the dorsal surface and 0.5–1.5 mm lateral to the midline in either the left or right side of the spinal cord. Superficial neurons were recorded within 0–0.3 mm and deeper neurons within 0.31–1.2 mm from the dorsal surface of the spinal cord. Extracellular potentials were transmitted to a window discriminator, displayed on an oscilloscope, and stored in a computer with Spike 3 data acquisition software (CED, Cambridge) for off-line analyses. The latency was determined by measuring the interval between the onset of the stimulus and the increase in cell activity. Neuronal activity was measured using rate histograms (1 s/bin). Spontaneous activity of neurons was determined by counting activity for 10 s before DD or GES to obtain impulses per second (imp/s). Neuronal responses (imp/s) during DD or GES were defined as increases or decreases (≥ 20%) in maximal activity compared to spontaneous activity. Raw tracings of neuronal responses to GES were processed by a Spike 3 digital filter to eliminate GES artifacts. Statistical comparisons were made using ANOVA followed by the Tukey’s test and the Chi-square analysis. Data are presented as means ± S.E and P < 0.05 was considered statistically significant.
To examine the effects of vagal afferent pathways on T9-T10 spinal neurons with duodenal input, the cervical vagus nerves were separated from the carotid arteries and silk suture was looped around each nerve trunk to prepare for transection. Vagus nerves were cut bilaterally with a scissors after gently pulling the ties around the nerves, so that spinal neuronal responses to DD or GES could be compared before and after vagotomy. To characterize the phenotype of afferent nerve fibers from the duodenum to spinal cord, resiniferatoxin (RTX), an ultrapotent analogue of capsaicin, was used to desensitize and inactivate capsaicin-sensitive afferent fibers containing TRPV1. Systemic administration of a low dose of RTX desensitizes TRPV1-expressing primary afferent fibers and eliminates spinal neuronal responses to mechanical and electrical gastric stimulation in adult rats (Qin et al. 2007). Therefore, RTX was used as a pharmacological tool to determine the role of TRPV1-containing nerve fibers in duodenal afferent pathways in the present study. A stock solution of RTX (1 mg, FW 628.7, Sigma) was dissolved in 0.5 ml ethanol and 0.5 ml Tween 80. The bottle was wrapped in aluminum foil and stored in a −80°C freezer. On the experimental day, 2.0 μl RTX was removed from the stock solution and diluted with 1 ml normal saline. The intravenous dose of RTX was 2.0 μg/kg. Duodenal distensions or GES were examined for spinal neuronal responses 20 min after RTX administration.
Somatic receptive fields of spinal neurons were also examined for responses to innocuous brushing with a camel-hair brush, pressure with a blunt wooden stick (diameter of 2.0 mm), and noxious pinching of skin with a blunt forceps. Neurons were classified as follows: low-threshold (LT) neurons responded to hair movement and/or pressure; high-threshold (HT) neurons responded only to noxious pinching of the somatic field; wide dynamic range (WDR) neurons responded to innocuous stimuli and also had greater responses to noxious pinch of somatic fields. Outlines and descriptions of receptive fields were recorded manually for all neurons examined. After a spinal neuron responsive to DD and/or GES was studied, an electrolytic lesion (50 μA DC, 20 s) was made to mark the recording site. At the end of experiments, the animals were euthanized with an intravenous euthanasia-5 solution or overdose of pentobarbital (200 mg/kg). The lower thoracic spinal cord was removed and placed in 10% buffered formalin solution. Frozen sections (55–60 μm) of the spinal cord were viewed to find lesion sites where the neuronal recordings had been made. Locations were drawn on cross sections from the cytoarchitectonic scheme of Molander et al. (1984).
Acknowledgments
The authors would like to thank Dr. M. J. Chandler for helpful comments and D. Holston for her excellent technical assistance. We also appreciate Dr. S. H. Liu for histological examination of recording sites in spinal cord. This study was partially supported by grants from National Institutes of Health (DK063733, Dr. J.D.Z. Chen; HL075524, Dr. R.D. Foreman).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abell TL, Minocha A. Gastroparesis and the gastric pacemaker: a revolutionary treatment for an old disease. J Miss State Med Assoc. 2002;43:369–375. [PubMed] [Google Scholar]
- Bloomfield AL, Polland WS. Experimental referred pain from the gastrointestinal tract. II. Stomach, duodenum and colon. J Clin Invest. 1931;10:453–473. doi: 10.1172/JCI100364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F, McRitchie HA. Neonatal capsaicin does not affect unmyelinated efferent fibers of the autonomic nervous system: functional evidence. Brain Res. 1982;239:283–288. doi: 10.1016/0006-8993(82)90853-8. [DOI] [PubMed] [Google Scholar]
- Chen JDZ, Qian L, Quyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterol. 2003;124:401–409. doi: 10.1053/gast.2003.50048. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Coombs DW, Degnan CC, Rogers LL. Mechanical visceral pain model: chronic intermittent intestinal distention in the rat. Physiol Behav. 1989;45:191–197. doi: 10.1016/0031-9384(89)90184-4. [DOI] [PubMed] [Google Scholar]
- Cottrell DF, Greenhorn JG. The vagal and spinal innervation of the gastro-duodenal junction of sheep. Q J Exp Physiol. 1987;72:513–524. doi: 10.1113/expphysiol.1987.sp003093. [DOI] [PubMed] [Google Scholar]
- Cottrell TF, Iggo A. Tension receptors with vagal afferent fibres in the proximal duodenum and pyloric sphincter of sheep. J Physiol. 1984;354:457–75. doi: 10.1113/jphysiol.1984.sp015388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argent J. Gastric electrical stimulation as therapy of morbid obesity: preliminary results from the French study. Obes Surg. 2002;12(Suppl 1):21S–25S. doi: 10.1381/096089202762552638. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Coombs DW, McCarthy LE. Differential c-fos-like protein expression in mechanically versus chemically induced visceral nociception. Brain Res Mol Brain Res. 1991;11:167–170. doi: 10.1016/0169-328x(91)90118-h. [DOI] [PubMed] [Google Scholar]
- El Ouazzani T, Mei N. Sensory innervation of the gastro-intestinal junction: new electrophysiological, histological and histochemical data. C R Seances Soc Biol Fil. 1978;172:283–288. [PubMed] [Google Scholar]
- Familoni BO, Abell TL, Nemoto D, Voeller G, Johnson B. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892–897. doi: 10.1023/a:1018804128695. [DOI] [PubMed] [Google Scholar]
- Feng Y, Cui M, Al-Chaer ED, Willis WD. Epigastric antinociception by cervical dorsal column lesions in rats. Anesthesiol. 1998;89:411–420. doi: 10.1097/00000542-199808000-00018. [DOI] [PubMed] [Google Scholar]
- Floyd K, Morrison JF. Splanchnic mechanoreceptors in the dog. Q J Exp Physiol Cogn Med Sci. 1974;59:361–366. doi: 10.1113/expphysiol.1974.sp002279. [DOI] [PubMed] [Google Scholar]
- Forster J, Sarosiek I, Delcore R, Lin Z, Raju GS, McCallum RW. Gastric pacing is a new surgical treatment for gastroparesis. Am J Surg. 2001;182:676–681. doi: 10.1016/s0002-9610(01)00802-9. [DOI] [PubMed] [Google Scholar]
- Green T, Dockray GJ. Characterization of the peptidergic afferent innervation of the stomach in the rat, mouse and guinea-pig. Neurosci. 1988;25:181–193. doi: 10.1016/0306-4522(88)90017-6. [DOI] [PubMed] [Google Scholar]
- Greenstein RJ, Belachew M. Implantable gastric stimulation (IGS) as therapy for human morbid obesity: report from the 2001 IFSO symposium in Crete. Obes Surg Suppl. 2002;1:3S–5S. doi: 10.1007/BF03342139. [DOI] [PubMed] [Google Scholar]
- Grundfest-Bronaltowski S, Davies CR, Olsen E. Electrical control of gastric emptying in denervated and reinnervated canine stomach: a pilot study. Artif Organs. 1990;14:254–259. doi: 10.1111/j.1525-1594.1990.tb02966.x. [DOI] [PubMed] [Google Scholar]
- Hazarika NH, Coote J, Downman CB. Gastrointestinal dorsal root viscerotomes in the cat. J Neurophysiol. 1964;27:107–116. doi: 10.1152/jn.1964.27.2.107. [DOI] [PubMed] [Google Scholar]
- Holzer HH, Raybould HE. Vagal and splanchnic sensory pathways mediate inhibition of gastric motility induced by duodenal distension. Am J Physiol. 1992;262:G603–608. doi: 10.1152/ajpgi.1992.262.4.G603. [DOI] [PubMed] [Google Scholar]
- Holzer P, Painsipp E, Schuligoi R. Differential effects of intragastric acid and capsaicin on gastric emptying and afferent input to the rat spinal cord and brainstem. BMC Neurosci. 2005;6:60. doi: 10.1186/1471-2202-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa S, Aoi M, Kubo Y, Kotani T, Takeuchi K. Stimulation by capsaicin of duodenal HCO3(−) secretion via afferent neurons and vanilloid receptors in rats: comparison with acid-induced HCO3(−) response. Dig Dis Sci. 2003;48:1850–1856. doi: 10.1023/a:1025480003388. [DOI] [PubMed] [Google Scholar]
- Khurana RK, Petras JM. Sensory innervation of the canine esophagus, stomach, and duodenum. Am J Anat. 1991;192:293–306. doi: 10.1002/aja.1001920309. [DOI] [PubMed] [Google Scholar]
- Lin Z, Chen JD. Advances in gastrointestinal electrical stimulation. Crit Rev Biomed Eng. 2002;30:419–457. doi: 10.1615/critrevbiomedeng.v30.i456.70. [DOI] [PubMed] [Google Scholar]
- Liu J, Qiao X, Chen JDZ. Vagal afferents involved in short-pulse gastric electrical stimulation in rats. Dig Dis Sci. 2004;49:729–737. doi: 10.1023/b:ddas.0000030081.91006.86. [DOI] [PubMed] [Google Scholar]
- Liu J, Hou X, Song G, Cha H, Yang B, Chen JD. Gastric electrical stimulation using endoscopically placed mucosal electrodes reduces food intake in humans. Am J Gastroenterol. 2006;101:798–803. doi: 10.1111/j.1572-0241.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterol. 1998;114:456–461. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- McSwiney BA, Suffolk SF. Segmental distribution of certain visceral afferent neurones of the pupillo-dilator reflex in the cat. J Physiol. 1938;93:104–116. doi: 10.1113/jphysiol.1938.sp003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I The lower thoracic and lumbosacral cord. J Comp Neurol. 1984;230:133–141. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- Moss HE, Sanger GJ. The effects of granisetron, ICS 205–930 and ondansetron on the visceral pain reflex induced by duodenal distension. Br J Pharmacol. 1990;100:497–501. doi: 10.1111/j.1476-5381.1990.tb15836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Inflammation enhances reflex and spinal neuron responses to noxious visceral stimulation in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G649–657. doi: 10.1152/ajpgi.2001.280.4.G649. [DOI] [PubMed] [Google Scholar]
- Neuhuber W, Niederle B. Spinal ganglion cells innervating the stomach of the rat as demonstrated by somatopetal transport of horseradish peroxidase (HRP) Anat Embryol. 1979;155:355–362. doi: 10.1007/BF00317648. [DOI] [PubMed] [Google Scholar]
- Nijsen MJ, Ongenae NG, Coulie B, Meulemans AL. Telemetric animal model to evaluate visceral pain in the freely moving rat. Pain. 2003;105:115–123. doi: 10.1016/s0304-3959(03)00170-2. [DOI] [PubMed] [Google Scholar]
- Ouyang H, Yin J, Zhu H, Xu X, Chen JDZ. Effects of gastric electrical field stimulation with long pulses on gastric emptying in dogs. Neurogastroenterol Motil. 2003;15:109–416. doi: 10.1046/j.1365-2982.2003.00425.x. [DOI] [PubMed] [Google Scholar]
- Ouyang H, Xing J, Chen JD. Tachygastria induced by gastric electrical stimulation is mediated via alpha- and beta-adrenergic pathway and inhibits antral motility in dogs. Neurogastroenterol Motil. 2005;17:846–853. doi: 10.1111/j.1365-2982.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Gebhart GF. Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am J Physiol. 2001;281:G1449–G1459. doi: 10.1152/ajpgi.2001.281.6.G1449. [DOI] [PubMed] [Google Scholar]
- Peles S, Petersen J, Aviv R, Policker S, Abu-Hatoum O, Ben-Haim SA, Gutterman DD, Sengupta JN. Enhancement of antral contractions and vagal afferent signaling with synchronized electrical stimulation. Am J Physiol Gastrointest Liver Physiol. 2003;285:G577–585. doi: 10.1152/ajpgi.00109.2003. [DOI] [PubMed] [Google Scholar]
- Qin C, Chandler MJ, Foreman RD. Afferent pathways and responses of T3-T4 spinal neurons to cervical and thoracic esophageal distensions in rats. Auton Neurosci. 2003;109:10–20. doi: 10.1016/j.autneu.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Qin C, Sun Y, Chen JD, Foreman RD. Gastric electrical stimulation modulates neuronal activity in nucleus tractus solitarii in rats. Auton Neurosci. 2005;119:1–8. doi: 10.1016/j.autneu.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007;57:29–39. doi: 10.1016/j.neures.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Chen JD, Zhang J, Foreman RD. Characterization of T9-T10 spinal neurons with duodenal input and modulation by gastric electrical stimulation in rats. Neurogastroenterol Motili. 2006;18:721. doi: 10.1016/j.brainres.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinson N, Robbins HL, Clark MJ, Furness JB. Locations and innervation of cell bodies of sympathetic neurons projecting to the gastrointestinal tract in the rat. Arch Histol Cytol. 2001;64:281–294. doi: 10.1679/aohc.64.281. [DOI] [PubMed] [Google Scholar]
- Schicho R, Donnerer J, Liebmann I, Lippe IT. Nociceptive transmitter release in the dorsal spinal cord by capsaicin-sensitive fibers after noxious gastric stimulation. Brain Res. 2005;1039:108–115. doi: 10.1016/j.brainres.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Schloithe AC, Woods CM, Davison JS, Blackshaw LA, Toouli J, Saccone GT. Pancreatobiliary afferent recordings in the anaesthetised Australian possum. Auton Neurosci. 2006;126–127:292–298. doi: 10.1016/j.autneu.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Schuligoi R, Herzeg G, Wachter C, Jocic M, Holzer P. Differential expression of c-fos messenger RNA in the rat spinal cord after mucosal and serosal irritation of the stomach. Neurosci. 1996;72:535–544. doi: 10.1016/0306-4522(95)00552-8. [DOI] [PubMed] [Google Scholar]
- Sobocki J, Thor PJ, Krolczyk G. High frequency electrical stimulation of the stomach is more effective than low frequency pacing for the treatment of postoperative functional gastric stasis in humans. Neuromodulation. 2003;6:254–257. doi: 10.1046/j.1525-1403.2003.03033.x. [DOI] [PubMed] [Google Scholar]
- Stam R, van Laar TJ, Wiegant VM. Physiological and behavioural responses to duodenal pain in freely moving rats. Physiol Behav. 2004;81:163–169. doi: 10.1016/j.physbeh.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Terui N, Hosoya Y. Difference in distribution of central terminals between visceral and somatic unmyelinated (C) primary afferent fibers. J Neurophysiol. 1989;62:834–840. doi: 10.1152/jn.1989.62.4.834. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci. 2005;25:2617–2627. doi: 10.1523/JNEUROSCI.2894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Matsumoto J, Ueshima K, Okabe S. Role of capsaicin-sensitive afferent neurons in alkaline secretory response to luminal acid in the rat duodenum. Gastroenterol. 1991;101:954–961. doi: 10.1016/0016-5085(91)90721-v. [DOI] [PubMed] [Google Scholar]
- Tang M, Zhang J, Chen JD. Central mechanisms of gastric electrical stimulation involving neurons in the paraventricular nucleus of the hypothalamus in rats. Obes Surg. 2006;16:344–352. doi: 10.1381/096089206776116372. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen JD. Systematic review: applications and future of gastric electrical stimulation. Aliment Pharmacol Ther. 2006;24:991–1002. doi: 10.1111/j.1365-2036.2006.03087.x. [DOI] [PubMed] [Google Scholar]
- Zhu H, Chen JDZ. Implantable gastric stimulation inhibits gastric motility via sympathetic pathway in dogs. Obes Surg. 2005;15:95–100. doi: 10.1381/0960892052993549. [DOI] [PubMed] [Google Scholar]