Abstract
In the age of stem cell engineering, it is critical to understand how stem cell activity is regulated during regeneration. Hairs are mini-organs that undergo cyclic regeneration throughout adult life,1 and are an important model for organ regeneration. Hair stem cells located in the follicle bulge2 are regulated by the surrounding micro-environment, or niche3. The activation of such stem cells is cyclic, involving periodic β-catenin activity4–7. In adult mouse, regeneration occurs in waves in a follicle population, implying coordination among adjacent follicles and the extra-follicular environment. Here we show unexpected periodic expression of Bmp2/4 in the dermis regulates this process. This Bmp cycle is out-of-phase with the Wnt/β-catenin cycle, thus dividing the conventional telogen into new functional phases: one refractory and the other competent for hair regeneration; characterized by high and low Bmp signaling respectively. Over-expression of noggin, a Bmp antagonist, in mouse skin resulted in a dramatically shortened refractory phase and faster propagation of the regenerative wave. Transplantation of skin from this mutant onto a wild type host showed that follicles in donor and host can affect their cycling behaviors mutually, with the outcome depending on the equilibrium of Bmp activity in the dermis. Administration of BMP protein caused the competent region to become refractory. These results show that Bmps may be the long-sought “chalone” inhibitors of hair growth postulated by classical experiments. Taken together, results presented in this study provide an example of hierarchical regulation of local organ stem cell homeostasis by inter-organ macro-environment. The expression of Bmp2 in subcutaneous adipocytes suggests physiological integration between these two thermo-regulatory organs. Our findings have practical importance to those using mouse skin as a model for carcinogenesis, intra-cutaneous drug delivery and stem cell engineering studies, as they highlight the acute need to differentiate supportive versus inhibitory regions in the host skin.
Keywords: biological rhythm, hair cycle, dermis, refractory, stem cell niche
Mammalian skin contains thousands of hair follicles, each undergoing continuous regenerative cycling. A hair follicle cycles through anagen (growth), catagen (involution) and telogen (resting) phases, and then re-enters anagen. At the base of this cycle is the ability of hair follicle stem cells to briefly exit their quiescent status to generate transient amplifying (TA) progeny, but maintain a cluster of stem cells. It is generally believed that a niche microenvironment is important in the control of stem cell homeostasis in various systems8. Within a single hair follicle, periodic activation of β-catenin in bulge stem cells is responsible for their cyclic activity3. However, how these stem cell activation events are coordinated among neighboring hairs remains unclear. It is possible that a population of hair follicles could cycle simultaneously, randomly, or in coordinated waves. We recently observed a “cyclic alopecia” phenotype in Msx2 null mice, which in essence represents coordinated hair regenerative activity in a population of follicles and is manifest as traversing hair waves9–11 (Supplementary Fig. S1).
Classical works have documented hair growth waves in rats, mice, and other mammals12,13. Opinions differ as to whether the hair growth pattern is controlled by local inherent rhythms, systemic factors or both. Since there is a period following anagen during which “the systemic stimulus is unable to exert an effect”, the concept of “telogen refractivity” was conceived14. A substance, termed a “chalone”, which can inhibit anagen development, was proposed to explain this phenomenon15. However, despite efforts to identify the “chalone”16,17, its molecular nature has remained elusive for the past 50 years.
Intrigued by these dynamic, complex hair growth patterns (Supplementary Fig. S1), we set out to find the underlying molecular mechanisms. A hair cycle domain is a region of skin which contains a population of hair follicles cycling in coordination. That such domains form implies the existence of signals that serve to spread and stop waves of hair growth. This prompted the suggestion that skin regions in telogen can be in either of the two functional phases: competent telogen which allows the anagen re-entry wave to propagate, and refractory telogen which arrests the wave (Fig. 1a, 1b). We analyzed the cycling behavior of domains in more than 30 living mice (older than 2 months) for up to 1 year (Supplementary Fig. S1), and consistently found that there is a minimal 28-days-long telogen phase (defined as the early telogen). Following this phase, telogen can either end right away (0 days) or persist for any number of days up to about 60 days. This phase (defined as the late telogen) contributes to the apparently highly variable telogen length (Fig. 1c).
Figure 1. Defining refractory and competent telogen.
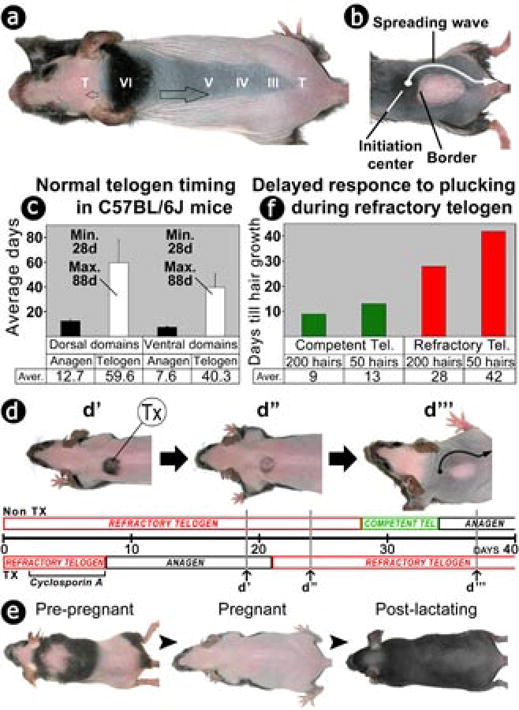
(a) Propagation (blank arrow) of hair regenerative waves is seen in Msx2 null mice (also see supplementary Fig. S1). Similar patterns can be seen in normal black mice after hair clipping. Roman characters, anagen stages; T, telogen.
(b) Under physiological conditions, some domains can become refractory to the spreading wave.
(c) The durations of anagen and telogen were measured in 22 hair cycle domains from dorsal and ventral skin.
(d) Experimental induction of refractory telogen with cyclosporine A (cyclA). X coordinate represents time scale (in days) when experiments began in the early telogen of the non-treated skin region. CyclA was applied to a localized region (treated, Tx) during early telogen and induced new anagen at about 8 days later. The surrounding non-treated refractory telogen skin (Non Tx) remained in telogen. When the non-treated skin was at day 19 of their telogen, treated Tx skin already proceeded to the late stage of its induced new anagen (panel d′, day 19). When non-treated skin was at day 24 of their telogen, cyclosporine treated region had finished its induced new anagen phase and entered new telogen (panel d″, day 24). Soon the non-treated skin progressed into the competent telogen. At day 34, non-Tx region entered its natural anagen. The regenerative wave spread but can not enter Tx region because it is still in its refractory telogen period (panel d‴, day 37). Black, anagen; green, competent telogen; red, refractory telogen.
(e) In female mice, multiple hair cycle domains were reset into one after pregnancy/lactation.
(f) Hair plucking/regeneration was used to gauge competent and refractory telogen status (n=16). The minimum time (shown in days) represents the time required for new pigmented hair filaments to be visible. This time is shorter when more hairs were plucked or when the same number of hairs were plucked in competent period.
This suggests the first 28 days of telogen are essential for the hair cycle and may represent the refractory phase. To test this idea, we used club hair plucking, which can induce hair regeneration. We gauged responses by the time required for regeneration to start after hairs are plucked (see Supplementary Methods). When 50 hairs were plucked from early telogen skin, a longer time was required for hair growth than was the case when a comparable number of hairs were plucked during late telogen (requiring 42 vs 13 days). When 200 hairs were plucked, the time required for hairs to re-grow became shorter but still differed between early and late telogen (28 vs 9 days; Fig. 1f, Supplementary Fig. S2). Thus the functional status of a particular skin region can be determined by the hair plucking/regeneration assay. In the follicles we studied, early (up to 28 days) and late (after 28 days) telogen periods correlate well with refractory and competent telogen phases.
If the refractory and competent states of hair cycle domains are transient, then offsetting the timing of hair cycling in a localized region should lead to the formation of new hair cycle domains. We tested this by local application of cyclosporine A (a powerful anagen inducing agent which can overcome refractory telogen18) to skin region about 10mm in diameter which was in telogen day 1. Eight days later, the treated region was in the induced new anagen, whilst the surrounding skin continued its progression through refractory telogen. Soon after the treated region completed anagen and re-entered early (new) telogen, the surrounding skin had progressed into late (competent) telogen. When a new hair growth wave approached, it propagated without obstruction over the untreated competent skin, but met resistance in the treated refractory region, thus forming a new hair cycle domain (Fig. 1d).
Hair cycle domains are different from regionally specific domains specified differently in development (e.g., footpad vs dorsal paw). The exact domain boundaries can shift from cycle to cycle and the domain patterns become more complex as the mouse matures (Supplementary Fig. S1). These complex hair cycle domains can be affected by systemic factors. For example, during pregnancy and lactation, female mouse hairs which enter telogen are unable to re-enter anagen. Thus multiple hair cycle domains are reset into one single domain after pregnancy/lactation19 (Fig. 1e). Estrogen and prolactin have been implicated inhibition of anagen initiation (Supplementary Reference 1, 2).
We then wanted to know the molecular mechanisms which constitute this refractoriness. Using in situ hybridization and several lacZ reporter mice (including Bmp4-lacZ, Noggin-lacZ, TOPGAL reporter), we searched for cyclic molecular expressions which correlate with refractory and competent telogen. In longitudinal sections of a hair cycle domain, the hair wave is “frozen” and successive temporal hair cycle stages are laid out in a spatial order11, thus facilitating molecular analyses. We observed Wnt signaling and Msx2, amongst others, to be expressed in different hair follicle compartments and to fluctuate with hair cycling as reported (Supplementary Fig. S6). Unexpectedly, we observed the expression dynamics of inter-follicular Bmp2 to be out of phase with the dynamics of Wnt signaling (Fig. 2a–b, Supplementary Fig. S5a–e). Bmp2 expression is absent in early anagen and gradually intensifies to reach its peak level in anagen V–VI. Bmp2 expression remains high in early telogen, but becomes absent in late telogen (Fig. 2a–b, Supplementary Fig. S5c–e). A long skin strip spanning two hair cycle domains shows two Bmp2-expressing segments (Fig. 2c). Bmp4 exhibits similar on and off expression dynamics as shown by semi-quantitative RT-PCR, in situ hybridization (Fig. 2d–e) and Bmp4-lacZ expression (Supplementary Fig. S3). On the other hand, Noggin-lacZ expression shows that on and off dynamics of mesenchymal noggin (including dermal papilla and dermal sheath; Supplementary Fig. S4)17,20 coincides with the hair cycle rhythm. Since BMP activity can be modulated by multiple factors (different ligands, antagonists, and receptors), we measured BMP signaling output by immunostaining for p-SMAD 1/5/8. The results showed distinct periods of nuclear pSMAD activity, high in early/refractory and absent in late/competent telogen hair follicles (Fig. 2f).
Figure 2. Periodic Bmp signaling in the dermis and subcutaneous adipose tissue.
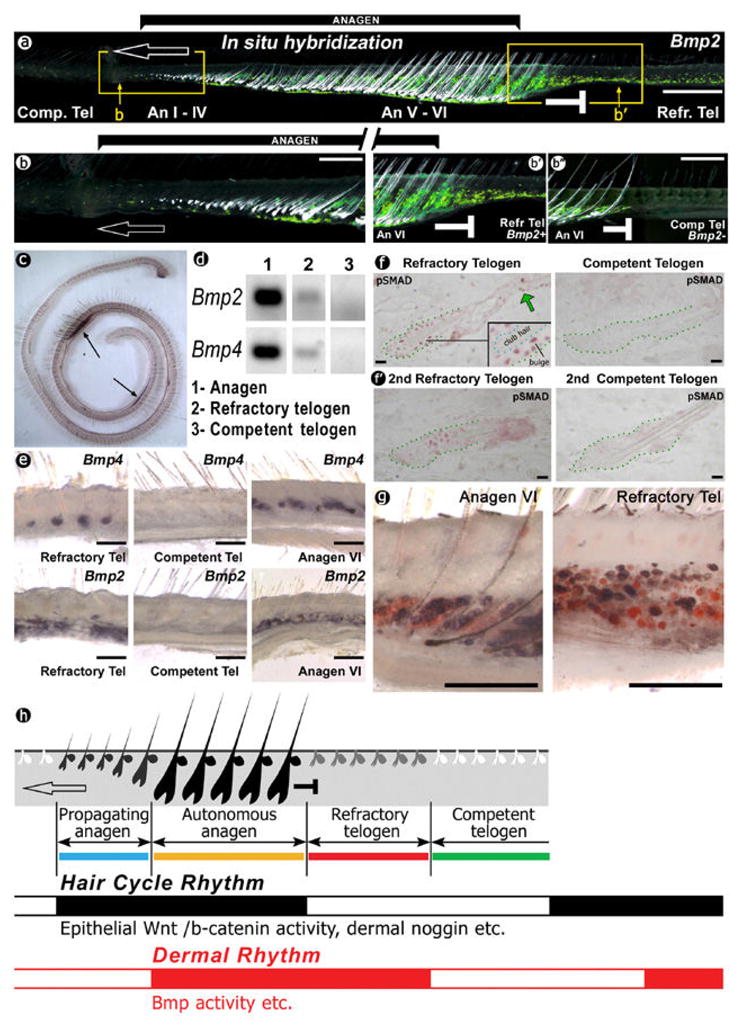
(a) Different temporal stages are spatially laid across the skin strip. The dark field illumination shows hair follicles (white) and Bmp2 in situ hybridization (green). Note the two are out of phase.
An, anagen; Comp, competent; Refr, refractory; Tel, telogen. Open arrows, the direction of the spreading waves; --| sign, boundary between anagen and refractory telogen.
(b, b′) Enlarged regions from boxed region of a. It shows details of Bmp2 expression during anagen spreading (b) and on the border of anagen VI – refractory telogen (b′). (b″), While later refractory telogen region becomes competent, anagen VI follicles still do not propagate.
(c) A long skin strip includes two segments of dermal Bmp on and off expression.
(d, e) Bmp2 and Bmp4 expressions are detected by semi-quantitative RT-PCR and in situ.
(f) pSMAD immuno-staining is present in follicular epithelium, including bulge area (insert) and adjoining infundibulum (green arrow). (f′) Since skin in its second telogen phase (45–70 day after birth) is usually used in hair follicle and carcinogenesis studies, we show results at this stage, which are consistent with older mice.
(g) Bmp2 expression (blue) co-localized within some Sudan red-positive adipocytes (red).
(h) Schematic summary of the hair cycle rhythm (black) and newly identified dermal rhythm (red). Together, they define 4 new functional stages. Catagen is omitted for simplification. Scale bars: a: 1 mm; b: 500 um; e–g: 200 um.
We noted that the ability to propagate anagen induction is limited to early anagen follicles. A wave front is halted when it faces a refractory telogen region. By the time this refractory telogen region progresses into competent telogen, the previously propagating anagen follicles have progressed into late anagen, and propagation does not resume (Fig. 2b′, b″; Supplementary Fig. S5c, d). Although the surrounding environment is now competent, late anagen follicles are unable to propagate. In this way, the traditional anagen period can be divided into early (anagen I–IV) propagating and late (anagen V, VI) autonomous anagen, with low and high Bmp2 expression respectively in these phases (Fig. 2a, b, Supplementary Fig. S3, 5). We summarize the rhythms of marker genes expression in Fig. 2h. and Supplementary Fig. S7.
Where are the Bmp producing cells? Interestingly, much of the periodically expressed Bmp2 transcripts are produced by subcutaneous adipocytes, as judged by double staining with Sudan Red (Fig. 2g). Periodic expression of Bmp4 is seen in the intra-follicular epithelium, secondary hair germ, dermal papilla and adjacent extra-follicular dermal fibroblasts (Supplementary Fig. S3). Collectively, we define the extra-follicular sources of periodic Bmp2/4 expression as the dermal macro-environment. On the other hand, Bmp-antagonizing noggin appears to be constitutively expressed in bulge stem cells (Supplementary Fig. S4). The macro-environmental Bmps may have major additive effect on strength of intrafollicular (micro-environmental) Bmp6 and Bmp4 signaling21,25 in regulating quiescence of pSMAD–positive bulge stem cells, although the specific mechanisms of this regulation remain to be investigated. Since the eventual anagen initiation requires activation of Wnt/β-catenin3,4, there is competitive equilibrium between Bmp and Wnt signaling21. Stem cells have to integrate the multiple signaling inputs from both the micro- and macro- environments in order to make the decision of activation.
It should be noted that the first telogen (around postnatal day 19) is very short and new anagen initiates quickly without detectable refractory telogen. Dermis acquires telogen refractivity with maturation and 2nd telogen (postnatal day 45–70; Fig. 2f′) does have it.
These findings lead us to hypothesize that: 1) in the “BMP ON” phase, the macro-environment prevents microenvironment-based activation of bulge stem cells (via Wnt signaling), resulting in refractory telogen; 2) in the “BMP OFF” phase, the macroenvironmental block is removed and the threshold for microenvironment-based activation of stem cells is low. This results in competent telogen; hair follicles are free to enter new anagen either by stochastic self-activation or upon facilitation by adjacent early anagen follicles. We tested both parts of this hypothesis by means of transgenic perturbation of Bmp signaling, skin transplantation, and administration of exogenous BMP4.
If Bmps play a causative role in conferring refractory status, we should be able to reduce the period of refractory telogen by down-regulating Bmp signaling. We did this by over-expressing noggin under the control of a keratin 14 promoter in KRT14-NOG mice22. The minimal length of telogen was reduced down to 6 days, and the maximal length down to 11 days (Fig. 3b). As a result, these mice display continuous propagation of hair regenerative waves and have highly simplified patterns of hair cycle domains (Fig. 3a). We further tested the response of KRT14-NOG hair follicles to hair plucking. The differences in response that we observed in WT mice in early vs. late telogen were eliminated in KRT14-NOG mice. In all cases, plucked KRT14-NOG hair follicles required only approximately 6 days to re-enter anagen (Figure 3c). Recently, the importance of Bmp activity in suppressing stem cell activity has also been shown by tissue specific deletion of BMP receptors21,23.
Figure 3. Altered hair regenerative wave dynamics in KRT14-NOG mice and non-autonomous interactions with normal cycling host skin after transplantation.
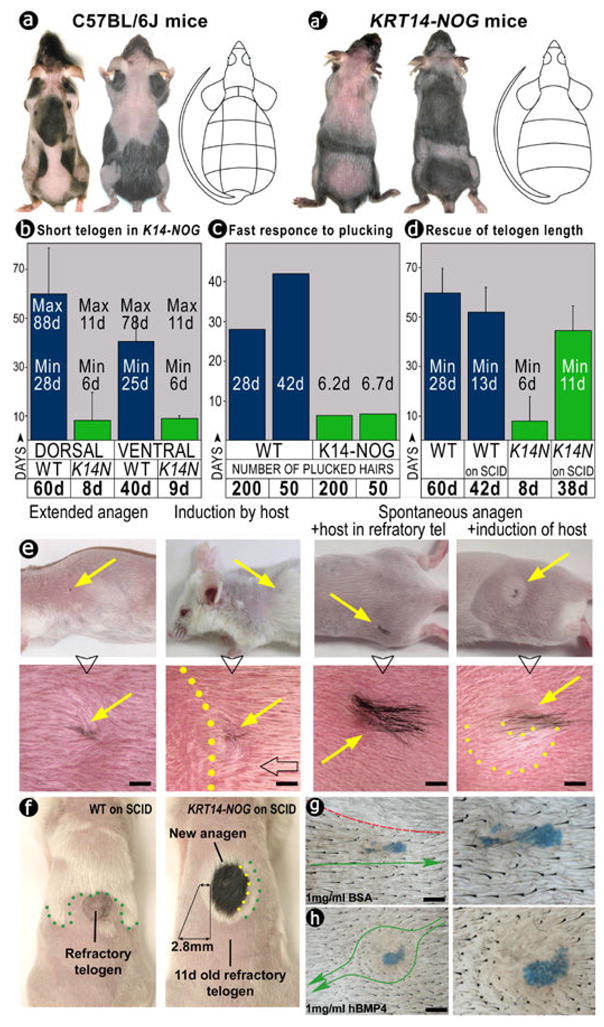
(a) Control and (a′) KRT14-NOG mice. Hair cycle domains in two different stages are shown, together with schematic domain boundaries generated by similar analysis used in supplementary Fig. S1.
(b) Measurements show both refractory and competent telogen are shortened in KRT14-NOG mice (green bars).
(c) Plucking/regenerative response in KRT14-NOG (green bars) is about 5 times faster.
(e) When a small KRT14-NOG skin graft was transplanted into SCID skin, hair growth (e) and duration of refractory telogen (d) were partially rescued.
(f) When a large KRT14-NOG skin graft (>10mm) was transplanted, it caused reduction of refractory telogen by inducing a rim of white hair from the host.
(g, h) hBMP4-soaked beads caused hair propagation wave (green arrowed curve) to go around them, creating a new telogen domain. Albumin does not have this effect. Red broken line, domain border. Scale bars: e, g, h: 1 mm.
The currently-held concept of the stem cell microenvironment implies only autonomous regulation: thus the activation of stem cells depends only on signaling inputs from components intrinsic to the organ (here the hair follicle itself3). To test directly whether the activation of stem cells is indeed subjected to non-autonomous regulation, we transplanted skin grafts from pigmented KRT14-NOG mice onto albino SCID host mice. If the control of stem cells activation is intrinsic to the follicles, hair cycling behavior should remain the same for both donor and host. Instead, we observed donor-host interactions, reflecting a non-autonomous relationship, with the outcome dependent on the size of the transplanted skin graft. When a small graft of KRT14-NOG skin (~1mm in diameter) was transplanted, the donor skin remained in telogen longer and could respond to an anagen activating wave originating from the host (Fig. 3e). Thus we achieved functional rescue of KRT14-NOG phenotypes. On the other hand, when a large skin graft (>10mm in diameter) was transplanted, the graft exhibited a greater degree of autonomous control. Within the graft, hair follicles continued to re-enter anagen rapidly. Providing evidence of a donor effect, host telogen hair follicles surrounding the perimeter of KRT14-NOG skin graft acquired early competence, after only 11 days in telogen (vs. 28 days), and re-entered anagen (visible as a rim of white hairs) together with pigmented donor hairs (Fig. 3f).
Classical experiments using skin graft transplantation to ask whether hair growth patterns are controlled intrinsically or systemically have produced different results14. We have repeated autologous skin graft transplantation experiments and observed that hair growth patterns are initially intrinsic to the donor, but are gradually entrained to the host rhythm after several hair cycles (not shown). Consequently the discrepancy amongst classical experiments may be due to the size of the graft and the time chosen for readout. At the molecular level, our results demonstrate involvement of the Bmp pathway in the non-autonomous interactions among follicle populations. It remains to be investigated whether the process depends on the direct diffusion of Bmps and Bmp antagonists or is indirectly mediated by other mechanisms24.
Finally, we tested whether a direct local delivery of BMP protein can convert competent telogen status to refractory in normal mice. hBMP4-soaked beads were implanted into competent telogen skin ahead of an anagen spreading wave (see Supplementary Methods17). 12 days later, hBMP4, but not control BSA, prevented the propagation of the wave around the beads (Fig. 3g, h). Thus the level of BMP activity can indeed explain the functional status (refractory vs competent) of a skin region.
The results presented here add new dimensions to our understanding of skin biology. Firstly, these findings demonstrate that in addition to short distance microenvironmental control17,25, the activation of stem cells within large groups of neighboring hair follicles is subject to long distance macroenvironmental control from the surrounding dermis (Fig. 4). This concept is readily applicable to other organs. For example, while Bmp4 is constantly expressed in the mesenchyme of intestinal microvilli, bursts of noggin expression in the villi stem cell niche may act to transiently lower BMP signaling, thus allowing stem cells to proliferate for epithelial renewal26. Secondly, extrafollicular periodically expressed Bmp2/4 appear to fulfill the criteria of the elegant but elusive “chalone” proposed to explain patterned hair growth14,15,17, thus solving a 50 year old puzzle. Thirdly, the dynamic expression of Bmp2 in dermal adipocytes suggests a link between two skin organ systems. Since subcutaneous fat, like hairs, has a thermo-regulatory function and leptin is present in the dermal papilla of hair follicles27, periodically expressed Bmp2 may coordinate the function of these two organs in response to the external environment, and may have implications for the evolution of integuments28. Fourth, the asynchronous cyclic expression of Bmps and β-catenin in the dermis and hair follicle provide a platform for mutual modulations of these “clocks” in the skin. They also imply that stem cell regeneration is subject to the control of biological rhythms.
Figure 4. Functional phases of the hair cycle.
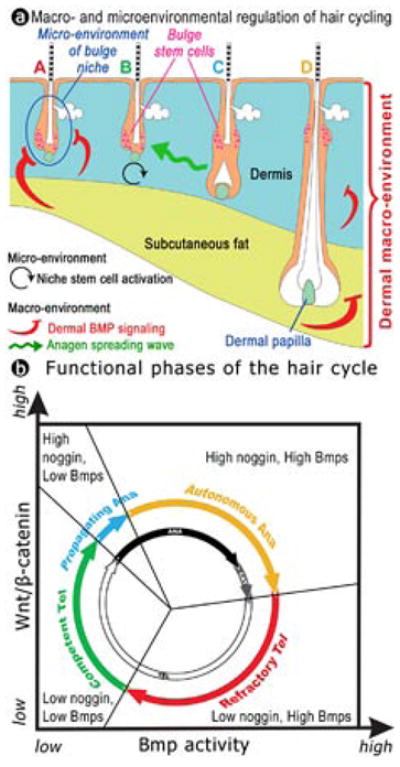
(a) Illustration of the bulge niche microenvironment and inter-follicular dermal macroenvironment, including dermis, subcutaneous fat and adjacent follicles. Anagen stimulating (black and green) or inhibitory (red) activities are depicted with colored arrows. Follicles are in different stages: A, refractory telogen; B, competent telogen; C, propagating anagen; D, autonomous anagen follicles. Blue circle in A, intra-follicular micro-environment. Color coded similar to panel b.
(b) New functional phases (colored outer circle) mapped against classical hair cycle stages (black and white inner circle). Based on the growth-inducing ability of follicles, anagen is divided into propagating (inducing, blue) and autonomous (non-inducing, yellow) phases. Based on ability to respond to regenerative signals, telogen is divided into refractory telogen (red) and competent (green) phases.
Finally, we note that mouse skin has been used extensively as a model in studies of carcinogenesis, intra-cutaneous drug delivery, and stem cell biology29,30. Such studies are usually designed on the assumption that the skin is a stable and largely uniform medium. Our novel findings show clearly that this assumption is rarely if ever justified.
Methods Summary
Animals
C75BL/6J, Crl:CD1(ICR), C3H/HeJ and SCID mice were used in this study. Msx2 null (C.Cg-Msx2tm1Rilm/Mmcd), KRT14-NOG (B6, CBA-Tg(KRT14-NOG)), Bmp4-lacZ (129S-Bmp4lacZneo), NOG-lacZ (129S-Nogtm1Amc/J) and TOPGAL (STOCK Tg(Fos-lacZ)34Efu/J) transgenic mice were also used.
Hair cycle observation
Progression of hair growth patterns was monitored in mice for various intervals of time, up to 1 year. Changes were documented every 48 hours and pictures were taken. The fur of all wild type mice was periodically clipped for recording. Hair clipping was selected over plucking or shaving to avoid wounding that can potentially interfere with normal hair growth13,15.
Animal procedures
All procedures were performed on anaesthetized animals with protocols approved by USC vivaria. For skin transplantation, surgical procedures were performed when both donor and recipient skins were in early telogen. This was done to ensure that wounded skin is healed by the beginning of the next anagen phase and that the impact of wound healing on the hair cycle is minimal. SCID mice were used as recipients.
Histology and detection of molecular expressions
Tissues were collected, fixed, and processed for histology as described22. Whole mount in situ hybridization on thin slices of adult mouse skin were performed using the InsituPro automated in situ detection module (Intavis AG, Koeln, Germany). Analysis was performed according to the standard whole mount in situ protocol. Whole mount beta-galactosidase detection was performed on adult skin from Bmp4-lacZ, NOG-lacZ and TOPGAL mice. Staining was performed according to the standard X-gal staining protocol.
Supplementary Material
Acknowledgments
We thank Dr. Widelitz for discussion. We are grateful to Drs. Hogan, Holland, and Bellusci for providing transgenic mice. This work is supported by Grants (AR42177, AR47364) from NIH to CMC. MVP is a postdoctoral scholar of California Institute of Regenerative Medicine. REB is supported by a Research Councils UK Fellowship and a Microsoft European Postdoctoral Research Fellowship.
References
- 1.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 2.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–7. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 4.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–45. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 5.Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 6.Lo Celso C, Prowse DM, Watt FM. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–99. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- 7.Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, et al. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–5. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 9.Ma L, Liu J, Wu T, Plikus M, Jiang TX, et al. ‘Cyclic alopecia’ in Msx2 mutants: defects in hair cycling and hair shaft differentiation. Development. 2003;130:379–89. doi: 10.1242/dev.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Militzer K. Hair growth pattern in nude mice. Cells Tissues Organs. 2001;168:285–94. doi: 10.1159/000047845. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki N, Hirata M, Kondo S. Traveling stripes on the skin of a mutant mouse. PNAS. 2003;100:9680–5. doi: 10.1073/pnas.1731184100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durward A, Rudall KM. Studies on hair growth in the rat. J Anat. 1949;83:325–35. [PMC free article] [PubMed] [Google Scholar]
- 13.Plikus MV, Chuong CM. Complex hair cycle domain patterns and regenerative hair waves in living rodents. J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5701180. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebling FJ, Johnson E. Systemic influence on activity of hair follicles in skin homografts. J Embryol Exp Morphol. 1961;9:285–93. [PubMed] [Google Scholar]
- 15.Chase H. Growth of the hair. Physiol Rev. 1954:113–126. doi: 10.1152/physrev.1954.34.1.113. [DOI] [PubMed] [Google Scholar]
- 16.Paus R, Stenn KS, Link RE. Telogen skin contains an inhibitor of hair growth. Br J Dermatol. 1990;122:777–84. doi: 10.1111/j.1365-2133.1990.tb06266.x. [DOI] [PubMed] [Google Scholar]
- 17.Botchkarev VA, Botchkareva NV, Nakamura M, Huber O, Funa K, et al. Noggin is required for induction of the hair follicle growth phase in postnatal skin. FASEB J. 2001;15:2205–14. doi: 10.1096/fj.01-0207com. [DOI] [PubMed] [Google Scholar]
- 18.Maurer M, Handjiski B, Paus R. Hair growth modulation by topical immunophilin ligands: induction of anagen, inhibition of massive catagen development, and relative protection from chemotherapy-induced alopecia. Am J Pathol. 1997;150:1433–41. [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson E. Quantitative studies of hair growth in the albino rat. II The effect of sex hormones. J Endocrinol. 1958;16:351–9. doi: 10.1677/joe.0.0160351. [DOI] [PubMed] [Google Scholar]
- 20.Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- 21.Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. PNAS. 2007;104:10063–8. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plikus M, Wang WP, Liu J, Wang X, Jiang TX, et al. Morpho-regulation of ectodermal organs: integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway. Am J Pathol. 2004;164:1099–114. doi: 10.1016/S0002-9440(10)63197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, He XC, Tong WG, Johnson T, Wiedemann LM, et al. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells. 2006;24:2826–39. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]
- 24.Oro AE, Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255:238–48. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 25.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–48. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 26.He XC, Zhang J, Tong WG, Tawfik O, Ross J, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–21. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 27.Iguchi M, Aiba S, Yoshino Y, Tagami H. Human follicular papilla cells carry out nonadipose tissue production of leptin. J Invest Dermatol. 2001;117:1349–56. doi: 10.1046/j.0022-202x.2001.01606.x. [DOI] [PubMed] [Google Scholar]
- 28.Wu P, Hou L, Plikus M, Hughes M, Scehnet J, et al. Evo-Devo of amniote integuments and appendages. Int J Dev Biol. 2004;48:249–70. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sausville EA, Burger AM. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 2006;66:3351–4. doi: 10.1158/0008-5472.CAN-05-3627. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Y, Du X, Wang W, Boucher M, Parimoo S, et al. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J Invest Dermatol. 2005;124:867–76. doi: 10.1111/j.0022-202X.2005.23716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


