Abstract
1,2,4-Triazole anion has been identified as an active acyl transfer catalyst suitable for the aminolysis and transesterification of esters.
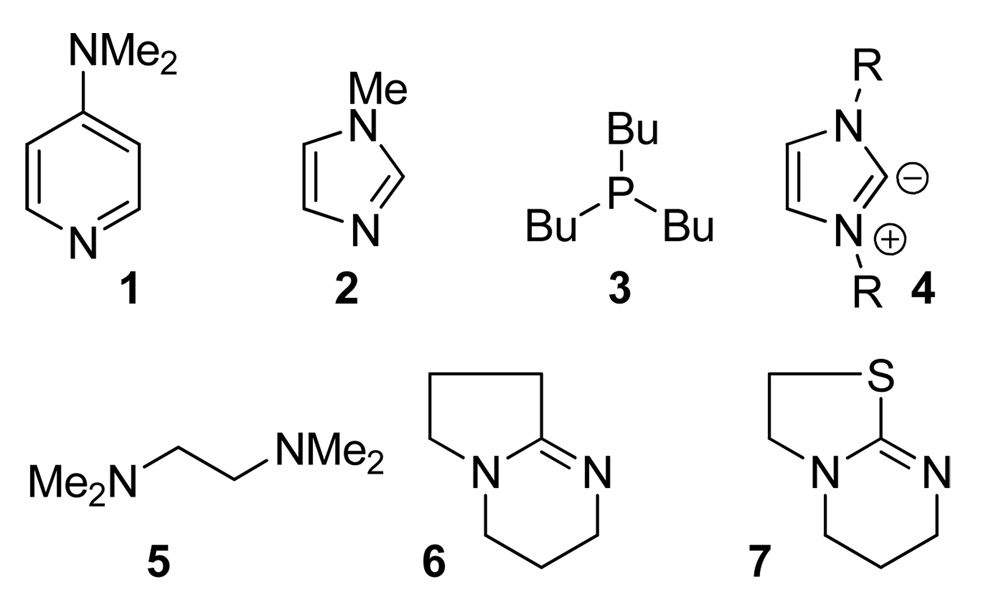
Neutral nucleophiles (Figure 1), such as 4-dialkylaminopyridines (1),1 N-alkylimidazoles (2),2 phosphines (3),3 imidazolylidene carbenes (4),4 1,2-diamines (5),5 and the recently introduced bicyclic amidines (6) and isothioureas (7),6 have proved to be effective acyl transfer catalysts. As such, they have found a variety of applications in organic synthesis.7 Their chiral derivatives have demonstrated considerable utility in catalyzing enantioselective transformations.8 By contrast, the potential of anionic nucleophiles in acyl transfer catalysis remains much less explored.
Figure 1.
Achiral acyl transfer catalysts
In the course of our studies on enantioselective acyl transfer catalysis we became interested in achieving catalytic acylation of amines using easily accessible achiral acyl donors.9 Carboxylic esters would be especially attractive in this regard, since their uncatalyzed reaction with amines is typically very slow at ambient temperature. However, the aforementioned neutral Lewis base catalysts,7 which successfully promote acylations with carboxylic anhydrides (1–3, 6 and 7) or acyl chlorides (5), can attack only highly activated esters. Imidazolylidene carbenes 4, which have gained popularity as transesterification catalysts,4 have also proved to be ineffective in promoting ester aminolysis.10 Recently, Mioskowski et al. reported that a variety of unactivated esters undergo efficient aminolysis under solvent-free conditions in the presence of TBD 8 (Figure 2), which was proposed to act as a bifunctional Lewis base catalyst.11 Its catalytic activity, however, is only moderate, requiring a high catalyst loading (30 mol%).
Figure 2.
Protic nucleophiles
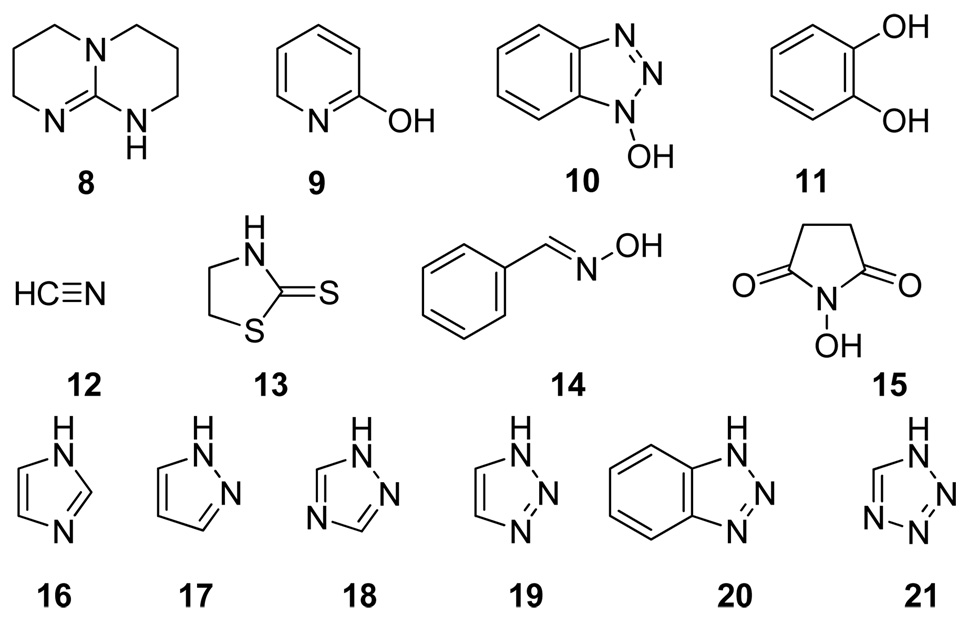
We considered the possibility of catalyzing ester aminolysis with anionic nucleophiles, which might be expected a priori to be more nucleophilic than their neutral counterparts, and therefore better able to attack the ester group. Although the anions of protic nucleophiles 9–12 have been reported in the literature to promote this reaction, their catalytic activities were usually rather modest.12
In an effort to identify more active anionic acyl transfer catalysts potentially suitable for asymmetric catalyst design, we tested the aforementioned 9–12 and several other commercially available protic nucleophiles (8, 13–21) for their ability to promote the reaction of phenyl acetate with isopropylamine in the presence of stoichiometric amounts of DBU (Table 1). For comparison, we also included DMAP 1, a powerful aprotic Lewis base acylation catalyst. Most compounds tested showed only negligible effect. Among the previously reported catalysts, only catechol and cyanide anions effected significant rate acceleration (entries 6 and 7). The most remarkable results were obtained in the azole series (entries 11–16). 1,2,4-Triazole 18 displayed by far the greatest activity among all the nucleophiles tested. Diminished activity was also found in the case of its close structural analogues with higher or lower pKa values, 1,2,3-triazole 19 and pyrazole 17.13
Table 1.
Catalytic activity test
 | ||
|---|---|---|
| entry | additive | t½ |
| 1 | none | ND (6%/3 days) |
| 2 | DMAP 1 | ND (7%/3 days) |
| 3 | TBD 8 | ND (38%/3 days) |
| 4 | 2-hydroxypyridine 9 | 66 h |
| 5 | HOBt 10 | ND (13%/3 days) |
| 6 | Catechol 11 | 5.7 h |
| 7 | Bu4NCN (12) | 2.2 h |
| 8 | Thiazolidine-2-thione 13 | 24 h |
| 9 | Benzaldoxime 14 | 57 h |
| 10 | N-Hydroxysuccinimide 15 | 3 days |
| 11 | Imidazole 16 | 9 h |
| 12 | Pyrazole 17 | 2.2 h |
| 13 | 1,2,4-Triazole 18 | 8 min |
| 14 | 1,2,3-Triazole 19 | 40 min |
| 15 | Benzotriazole 20 | 4.3 h |
| 16 | Tetrazole 21 | ND (11%/3 days) |
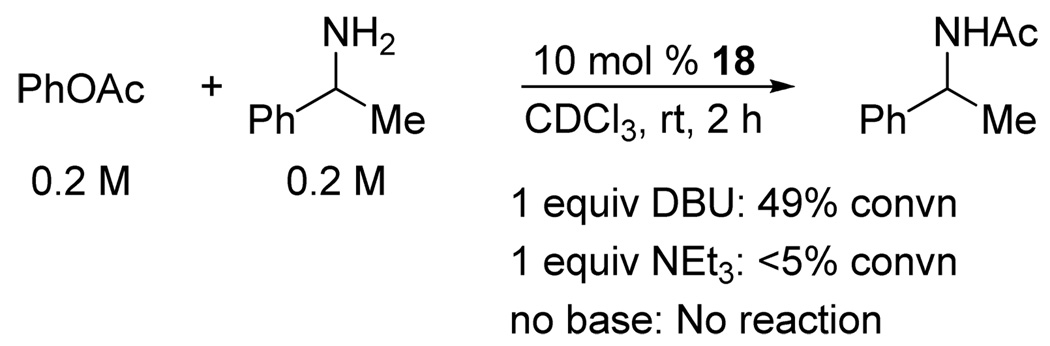
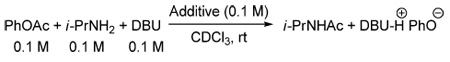
Although 1,2,4-triazole and pyrazole were shown to catalyze aminolysis of nitrophenyl, thiocresyl and cyanomethyl esters decades ago,14 they were believed to act as bifunctional catalysts and accordingly were used in the absence of base. To differentiate between the anionic and the bifunctional modes of catalysis in our case, we examined the effect of substituting DBU with a weaker base, triethylamine, or not adding any base at all (Figure 3). Very little reaction was observed in the absence of DBU, which supports the anionic mode of catalysis. Salts of azoles 17–20 have been recently described in the patent literature as catalysts for isocyanate oligomerization and polyisourethane production.15 To the best of our knowledge, they have not been utilized in other types of acyl transfer reactions.
Figure 3.
Anionic vs. bifunctional mode of catalysis
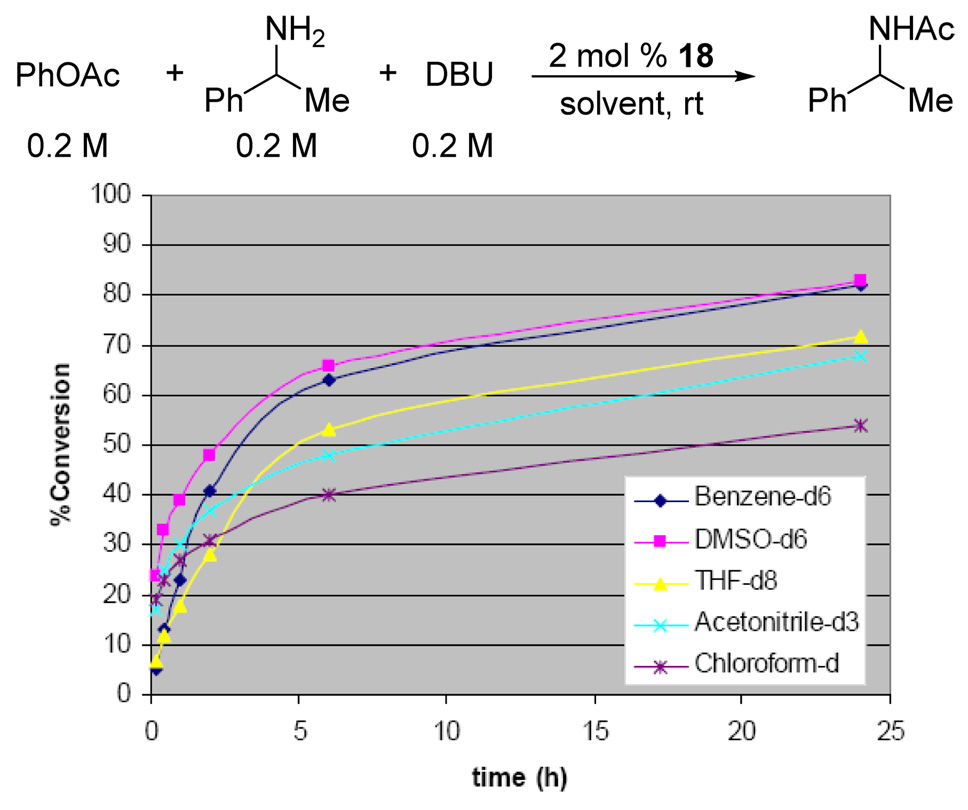
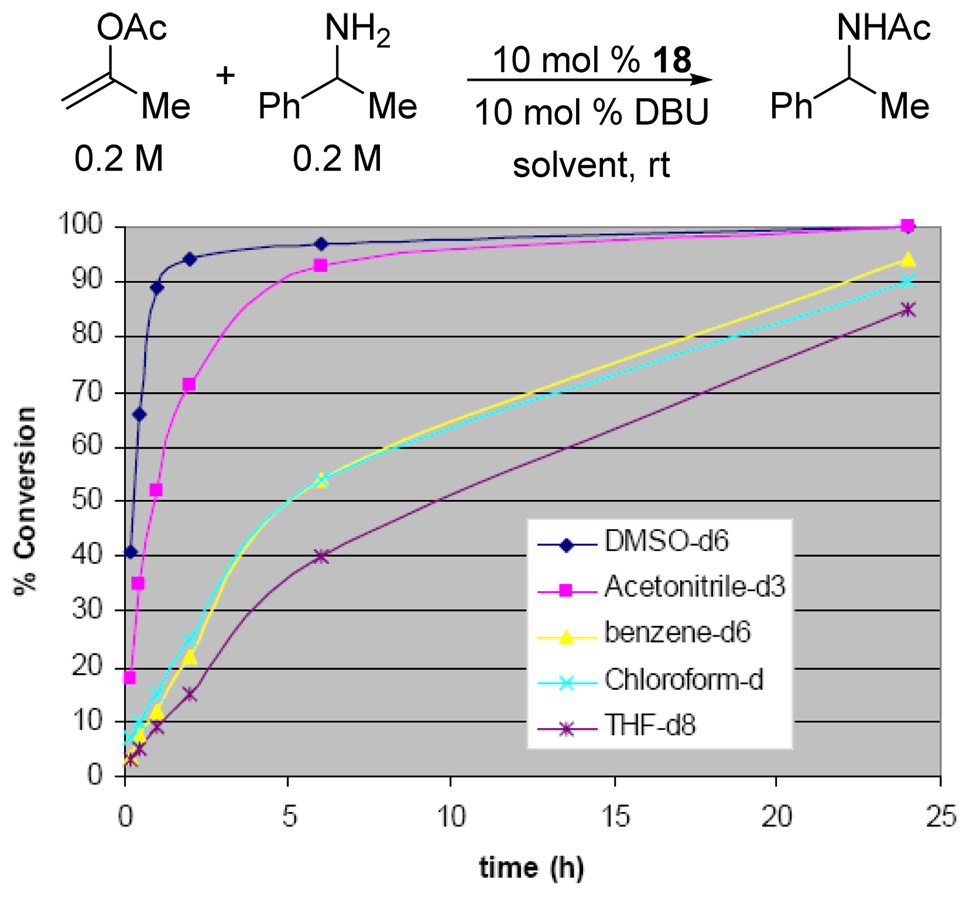
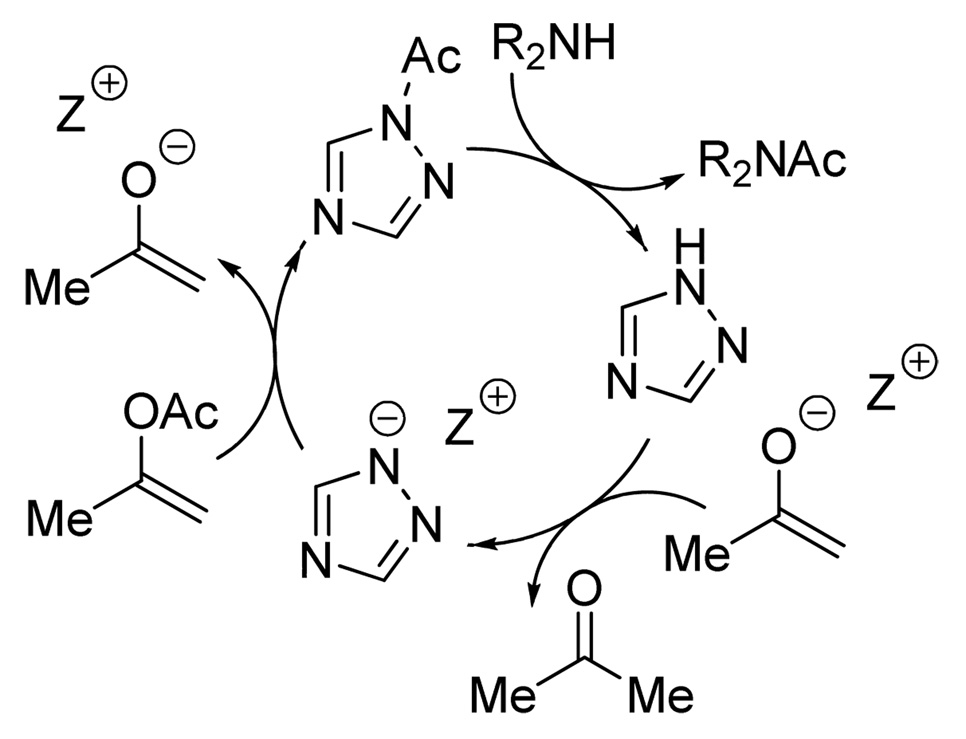
Optimization of reaction conditions was undertaken next. Solvent effects were examined using 2 mol % loading of 1,2,4-triazole and α̃-phenethylamine as a test substrate (Figure 4). The reaction rates diminished significantly at high conversions, suggesting that accumulation of the phenolate anion and/or depletion of available base led to less effective catalysis. Although the highest initial rates were observed in polar solvents, such as MeCN and DMSO, benzene was deemed to be more practical, as it displayed less of a rate drop-off. Replacing phenyl acetate with isopropenyl acetate, however, proved to be even more convenient. Even though the latter displayed lower reactivity, which necessitated a higher loading of triazole, it generated only acetone as byproduct, thus obviating the need for stoichiometric amounts of DBU (Figure 5). In this case, the use of polar solvents was clearly advantageous. At this point, we wished to ascertain whether the conjugate acid of DBU was essential for the aminolysis reaction. In fact, both tetrabutylammonium and sodium triazolides were even more active than the DBU salt.16 These results suggest that, although the cation may play an important role in the reaction, the presence of a hydrogen bond donor is not required. Overall, these observations are consistent with the mechanism outlined in Figure 6.
Figure 4.
Solvent effect on aminolysis of phenyl acetate
Figure 5.
Solvent effect on aminolysis of isopropenyl acetate
Figure 6.
Catalytic cycle with 1,2,4-triazole anion
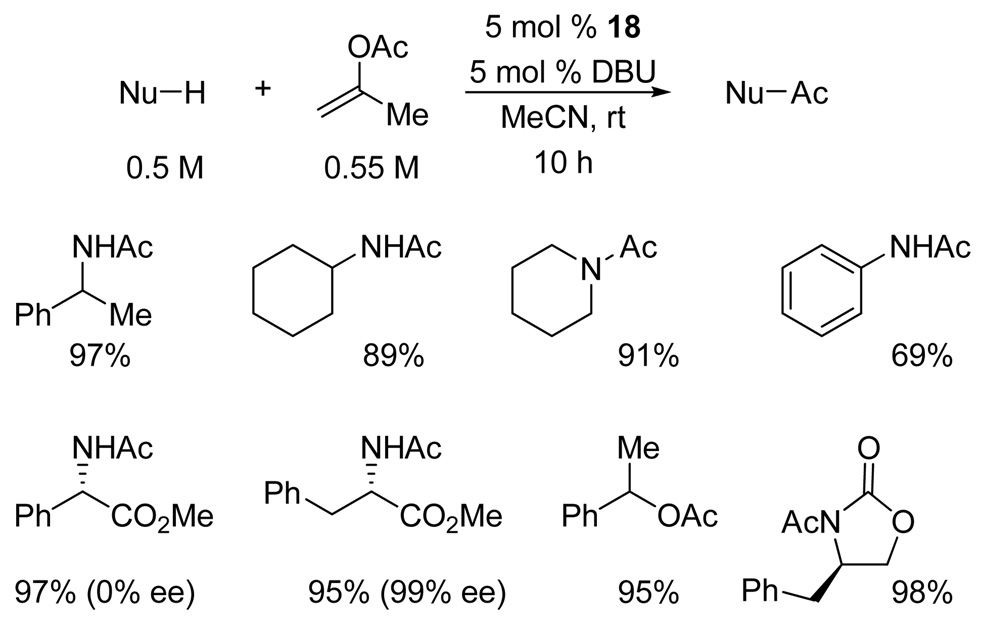
Several representative substrates were acetylated on a preparative scale using slight excess of isopropenyl acetate and 5 mol % of each DBU and triazole (Figure 7). In addition to amines, the new procedure proved effective for the transesterification of alcohols and acylation of oxazolidinones. Under the reaction conditions, the stereochemical integrity of methyl L-phenylalaninate was fully preserved under the reaction conditions, whereas the more base-sensitive methyl D-phenylglycinate underwent complete racemization.
Figure 7.
Preparative scale acetylation of substrates
Aminolysis of unactivated esters catalyzed by the 1,2,4-triazole anion was investigated next (Table 2). In most cases, the reactions in the presence of DBU alone were extremely sluggish even at elevated temperatures under solvent-free conditions. Addition of 1,2,4-triazole uniformly resulted in a dramatic rate acceleration. It is particularly noteworthy that methyl L-phenylalaninate underwent smooth cyclocondensation to produce the corresponding diketopiperazine as a 95:5 mixture of cis- and trans-diastereomers. The uncatalyzed version of this reaction is well known, but usually requires prolonged heating well above 100 °C and gives only moderate yields.17 The present protocol may find utility in the synthesis of structurally complex diketopiperazines.18
Table 2.
Aminolysis of unactivated esters
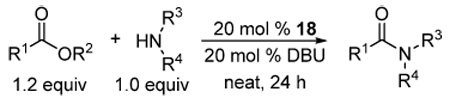 | ||||
|---|---|---|---|---|
| entry | ester | amine | Temp °C | % yielda |
| 1 | MeCO2Me | PhCH2NH2 | 23 | 83 (3) |
| 45 | 94 (4) | |||
| 2 | PhCO2Me | PhCH2NH2 | 23 | 26b (2) |
| 70 | 92 (3) | |||
| 3 | Me(CH2)6CO2Me | PhCH2NH2 | 23 | 43b (1) |
| 70 | 94 (2) | |||
| 4 | i-PrCO2Me | PhCH2NH2 | 23 | 23b (nd) |
| 70 | 62 (2) | |||
| 5 | MeCH(OH)CO2Me | PhCH2NH2 | 23c | 52b (8) |
| 23 | 84 (50) | |||
| 6 | MeCO2Et | PhCH2NH2 | 23 | 58 b (1) |
| 70 | 91 (7) | |||
| 7 | γ−butyrolactone | PhCH2NH2 | 23c | 84 (5) |
| 8 | Me(CH2)6CO2Me | piperidine | 23 | 2b (nd) |
| 95 | 77 (2) | |||
| 9 | Me(CH2)6CO2Me | PhCH(Me)NH2 | 23 | 1 b (nd) |
| 95 | 71 (5) | |||
| 10 | Me(CH2)6CO2Me | c-C6H11NH2 | 23 | 3b (nd) |
| 95 | 84 (2) | |||
| 11 | (L)-Phe-OMe | 90 | 79 (7) | |
Isolated yield is given, unless specified otherwise. % Conversion by 1H NMR in the absence of 18 is given in parentheses.
Yield estimated by 1H NMR
Carried out in CDCl3 at 1.0 M of amine
In summary, we have demonstrated that the 1,2,4-triazole anion serves as an effective acyl transfer catalyst in both aminolysis and transesterification reactions. Further investigation of the synthetic utility of azole anions and their potential in asymmetric catalysis are under active investigation in our laboratory.
Supplementary Material
Experimental procedures and 1H NMR spectra of compounds. These materials are available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
Financial support of this study by NIGMS (R01 GM072682) is gratefully acknowledged.
References
- 1.DMAP 1: Höfle G, Steglich W, Vorbruggen A. Angew. Chem. Int. Ed. Engl. 1978;17:569.
- 2.NMI 2: Connors KA, Pandit NK. Anal. Chem. 1978;50:1542.
- 3.PBu3 3: Vedejs E, Diver ST. J. Am. Chem. Soc. 1993;115:3358.
- 4.Imidazolylidene carbenes 4: Grasa GA, Kissling RM, Nolan SP. Org. Lett. 2002;4:3583. doi: 10.1021/ol0264760.Nyce GW, Lamboy JA, Connor EF, Waymouth RM, Hedrick JL. Org. Lett. 2002;4:3587. doi: 10.1021/ol0267228.
- 5.TMEDA 5: Sano T, Ohashi K, Oriyama T. Synthesis. 1999:1141.
- 6.DBN 6 and THTP 7: Birman VB, Li X, Han Z. Org. Lett. 2007;9:37. doi: 10.1021/ol0623419.
- 7.For a recent review, see: Denmark SE, Beutner GL. Angew. Chem. Int. Ed. 2008;47:1560. doi: 10.1002/anie.200604943.
- 8.Chiral DMAP derivatives: Wurz RP. Chem. Rev. 2007;107:5570. doi: 10.1021/cr068370e.Chiral NMI derivatives: Miller SJ. Acc. Chem. Res. 2004;37:601. doi: 10.1021/ar030061c.Ishihara K, Kosugi Y, Akakura M. J. Am. Chem. Soc. 2004;126:12212. doi: 10.1021/ja045850j.Chiral phosphines: Vedejs E, Daugulis O. J. Am. Chem. Soc. 2003;125:4166. doi: 10.1021/ja021224f.Chiral N-heterocyclic carbenes: Enders D, Niemeier O, Henseler A. Chem. Rev. 2007;107:5606. doi: 10.1021/cr068372z.Marion N, Díez-González S, Nolan SP. Angew. Chem. Int. Ed. 2007;46:2988. doi: 10.1002/anie.200603380.Chiral vic-diamines: Oriyama T, Imai K, Sano T, Hosoya T. Tetrahedron Lett. 1998;39:3529.Chiral bicyclic amidines: Birman VB, Uffman EW, Jiang H, Li X, Kilbane CJ. J. Am. Chem. Soc. 2004;126:12226. doi: 10.1021/ja0491477.Chiral bicyclic isothioureas: Birman VB, Li X. Org. Lett. 2006;8:1351. doi: 10.1021/ol060065s.Birman VB, Li X. Org. Lett. 2008;10:1115. doi: 10.1021/ol703119n.
- 9.Previously, catalytic kinetic resolution of amines has only been achieved using substituted 5-acyloxyoxazoles as stoichiometric acyl donors: Arai S, Bellemin-Laponnaz S, Fu GC. Angew. Chem. Int. Ed. 2001;40:234.Arp FO, Fu GC. J. Am. Chem. Soc. 2006;128:14264. doi: 10.1021/ja0657859.
- 10.(a) Movassaghi M, Schmidt MA. Org. Lett. 2005;7:2453. doi: 10.1021/ol050773y. [DOI] [PubMed] [Google Scholar]; (b) Vora HU, Rovis T. J. Am. Chem. Soc. 2007;129:13796. doi: 10.1021/ja0764052. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bode JW, Sohn SS. J. Am. Chem. Soc. 2007;129:13798. doi: 10.1021/ja0768136. [DOI] [PubMed] [Google Scholar]
- 11.Sabot C, Kumar KA, Meunier S, Mioskowski C. Tetrahedron Lett. 2007;48:3863. [Google Scholar]
- 12.2-pyridinolate anion: Nakamizo N. Bull. Chem. Soc. Jpn. 1971:2006.cyanide anion: Hoegberg T, Stroem P, Ebner M, Raemsby S. J. Org. Chem. 1987;52:2033.Kabouche Z, Bruneau C, Dixneuf PH. Tetrahedron Lett. 1991;32:5359.HOBt anion: Horiki K, Murakami A. Heterocycles. 1989;28:615.Catechol monoanion: Ivanova G, Bratovanova E, Petkov D. J. Peptide Sci. 2002;8:8. doi: 10.1002/psc.362.
- 13.Calculated values pka (17) = 14.0, pka (18) = 10.2, pka (19) = 8.7 were obtained through SciFinder.
- 14.(a) Beyerman HC, Maasen van den Brink W. Proc. Chem. Soc. (London) 1963:266. [Google Scholar]; (b) Wieland T, Kahle W. Chem. Ber. 1966;691:212. [Google Scholar]
- 15.(a) Koecher J, Richter F, Laas H-J, Wintermantel M. PCT Int. Appl. 2002 WO 2002092658 A1. [Google Scholar]; (b) Kometani H, Tamano Y. PCT Int. Appl. 2008 WO 2008018601 A1. [Google Scholar]
- 16.t½ = 4 h (Bu4N+) and 5.5 h (DBU-H+) in CDCl3 at 10 mol % catalyst loading; t½ = 12 min (Na+) and 45 min (DBU-H+), in DMSO at 5 mol % catalyst loading under conditions similar to those in Figure 5. Despite its lower activity, DBU triazolide, which is easily prepared in situ and soluble in common organic solvents, was used for the remainder of this study.
- 17.Schöllkopf U, Busse U, Kilger R, Lehr P. Synthesis. 1984;271 [Google Scholar]
- 18.Schkeryantz JM, Woo JCG, Siliphaivanh P, Depew KM, Danishefsky SJ. J. Am. Chem. Soc. 1999;121:11964. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures and 1H NMR spectra of compounds. These materials are available free of charge via the Internet at http://pubs.acs.org.