Abstract
The heat shock protein 70 family members Hsc70 and Hsp70 are known to play a protective role against the onset of experimental pancreatitis, yet their molecular function in acini is unclear. Cysteine string protein (CSP-α) is a zymogen granule (ZG) membrane protein characterized by an NH2-terminal “J domain” and a central palmitoylated string of cysteine residues. The J domain functions as a cochaperone by modulating the activity of Hsc70/Hsp70 family members. A role for CSP-α in regulating digestive enzyme exocytosis from pancreas was investigated by introducing CSP-α truncations into isolated acini following their permeabilization with Perfringolysin O. Incubation of acini with CSP-α1-82, containing the J domain, significantly augmented Ca2+-stimulated amylase secretion. Effects of CSP-α1-82 were concentration dependent, with a maximum 80% increase occurring at 200 μg/ml of protein. Although CSP-α1-82 had no effects on basal secretion measured in the presence of ≤10 nM free Ca2+, it did significantly augment GTP-γS-induced secretion under basal Ca2+ conditions by ∼25%. Mutation of the J domain to abolish its cochaperone activity failed to augment Ca2+-stimulated secretion, implicating the CSP-α/Hsc70 cochaperone system as a regulatory component of the secretory pathway. CSP-α physically associates with vesicle-associated membrane protein 8 (VAMP 8) on ZGs, and the CSP-α-VAMP 8 interaction was dependent on amino acids 83-112 of CSP-α. Immunofluorescence analysis of acinar lobules or purified ZGs confirmed the CSP-α colocalization with VAMP 8. These data establish a role for CSP-α in regulating digestive enzyme secretion and suggest that CSP-α and Hsc70 modulate specific soluble N-ethylmaleimide-sensitive attachment receptor interactions necessary for exocytosis.
Keywords: heat shock protein 70, zymogen granules
zymogen granules (ZGs) of exocrine pancreatic acinar cells are secretory organelles responsible for the regulated secretion of digestive enzymes. In response to a meal, secretagogs including cholecystokinin and acetylcholine activate G protein-coupled receptor signaling pathways to generate inositol 3,4,5-trisphosphate (IP3) and diacylglycerol, which, in turn, cause the release of Ca2+ from intracellular stores and activation of protein kinase signaling pathways (reviewed in 49). Although multiple signaling pathways are activated by secretagogs, analysis of IP3 receptor knockout mice have conclusively demonstrated that the acute elevation of cytosolic Ca2+ is an essential messenger to trigger ZG exocytosis and digestive enzyme secretion into the pancreatic duct (19).
The constitutively expressed cognate heat shock protein 70 (Hsc70) and stress-inducible Hsp70 are chaperone proteins that are expressed in acinar cells and, in the case of Hsp70, highly induced in response to various stressors both in isolated acini and in vivo (2, 44). Induction of Hsp70 expression before initiating acute experimental pancreatitis by treatment with high supraphysiological concentrations of cholecystokinin or its analog cerulein was shown to provide a protective role against the onset of acinar damage marking the earliest stages of pancreatitis (2, 44). The earliest events that accompany hyperstimulation of acini are the inhibition of normal apical secretion, basolateral exocytosis of ZGs (15), premature activation of trypsinogen, and formation of large cytoplasmic vacuoles (reviewed in 27). Perhaps not surprisingly given the highly differentiated secretory function of the acinar cell, these aberrant alterations in acinar function occur largely within the secretory pathway and, therefore, infer that the Hsp70-mediated protective effects are somehow targeted to regulatory proteins important for this process.
Cysteine string protein (CSP-α) is a ZG membrane protein bearing a signature “J domain” and a cysteine-rich string region (5). In addition to its presence on ZGs, CSP-α is found on synaptic vesicles (30), clathrin-coated vesicles (3), endocrine granules (8, 50), and neuroendocrine granules (9, 26). CSP-α associates with and stimulates the ATPase of heat shock protein 70 (Hsp70) family members including Hsc70 (6, 39) and Hsp70 (10). Over 40 human proteins contain a J domain and an ∼70-amino-acid signature domain that activates the ATPase activity of the Hsc70 family to mediate conformational changes in a number of cellular processes (reviewed in Refs. 36 and 52). Aside from CSP-α, which is one of the better characterized J proteins, clear functional roles have been described for only a few J proteins. They are generally thought to provide specificity to cellular conformational work by targeting client proteins to Hsc70 and stimulating the ATPase of Hsc70 to positively modulate its chaperone activity. Although CSP-α is anchored to secretory vesicles and thereby targets Hsc70 activity to the secretory pathway in cells, CSP-α and Hsc70 are reported to undergo dynamic associations with several accessory proteins including small glutamine-rich tetratricopeptide repeat domain protein (43), Hsp70 organizing protein (36), and Hsp70 interacting protein (36), which also likely regulate chaperone activity. The ATPase activity of Hsc70 is coupled to a wide range of cellular folding processes that include folding of newly synthesized proteins, refolding of misfolded proteins, as well as conformational changes of components in signal transduction and apoptotic pathways (reviewed in 7). How different members of the J protein family exploit Hsc70 for distinct conformational work in the cell is a major biological question to be addressed.
Identification of the cellular target(s) for the CSP-α/Hsc70 conformational work in neurons and endocrine cells has been controversial. Independent reports have emerged supporting a role for CSP-α in facilitating conformational changes in soluble N-ethylmaleimide-sensitive attachment receptor (SNARE) proteins necessary for exocytosis (13, 39), a role for CSP-α in the regulation of signaling through heterotrimeric GTP binding proteins (29, 31, 32), as well as a role for CSP-α in the regulation of transmembrane calcium flux (14, 23). Client proteins for CSP-α and Hsc70 chaperone activity in acinar cells have not been identified. Although Hsc70 is likely coupled to numerous folding processes in acinar cells, CSP-α almost certainly serves to anchor chaperone activity to ZGs and thereby targets Hsc70 and its stress-induced homolog Hsp70 to the secretory pathway.
In this study, we begin to address the hypothesis that the CSP-α/Hsc70 chaperone system is essential for maintaining the high fidelity of dynamic protein interactions necessary for the pancreatic acinar secretory response. As a first step toward testing this hypothesis, we evaluated the effects of introducing purified recombinant CSP-α truncation constructs into permeabilized acinar cells. Our findings demonstrate that CSP-α1-82 assembles with Hsc70 or Hsp70 and significantly augments the Ca2+-stimulated secretion of amylase from acinar cells. Furthermore, we demonstrate that, via residues 83-112, CSP-α associates with the vesicle associated membrane protein 8 (VAMP 8) present on ZG membranes. Our results demonstrate a role for CSP-α in the secretory pathway and implicate select acinar SNAREs as client proteins for the CSP-α/Hsc70 chaperone system.
MATERIALS AND METHODS
Antibodies.
Polyclonal anti-VAMP 8 and anti-VAMP 2 monoclonal antibody c169.1, were purchased from Synaptic Systems. Chicken polyclonal VAMP 2 antibody was from Chemicon. Anti-Hsc70 and -Hsp70 monoclonal antibodies were purchased from StressGen. Alexa-conjugated secondary antibodies were purchased from Molecular Probes. Peroxidase-conjugated sheep anti-mouse IgG and donkey anti-rabbit IgG were purchased from GE Healthcare. Anti-VAMP 8 polyclonal antibodies were generous gifts from T. Weimbs (28) and W. Hong (46). A polyclonal antibody to the cytosolic domain of recombinant human VAMP 8 was also produced in rabbits. All VAMP 8 antibodies gave essentially identical results for immunoblotting and immunofluorescence experiments. Two different anti-CSP polyclonal antibodies were utilized, a peptide antibody raised against the sequence CTQLTADSHPSYHTDGFN corresponding to amino acids 182-198 of rat CSP (5) and a second antibody raised against full-length recombinant CSP protein (8).
Other reagents.
Soybean trypsin inhibitor, benzamidine, phenylmethanesulfonyl fluoride, GTP-γS, pronase, and Triton X-100 were purchased from Sigma, essential amino acid solution from GIBCO, and a protease inhibitor cocktail containing AEBSF, aprotinin, EDTA, leupeptin, and E64 from Calbiochem. Protein A beads were from Pierce, Phadebas amylase assay kit from Fisher, and protein determination reagent from Bio-Rad. Percoll was from GE Healthcare. The Perfringolysin O (PFO) bacterial expression plasmid was a kind gift from A. Johnson and A. P. Heuk at the University of Texas and University of Massachusetts-Amherst, respectively (24). The anti-GP-3 polyclonal antibodies were a kind gift from John A. Williams at the University of Michigan (45).
Isolation of pancreatic lobules and dispersed acini.
The University of Wisconsin Committee on Use and Care of Animals approved all studies involving animals. Pancreatic lobules were prepared by microdissection of an adult male Sprague-Dawley rat pancreas that had been injected with incubation buffer consisting of (in mM) 10 HEPES, 137 NaCl, 4.7 KCl, 0.56 MgCl2, 1.28 CaCl2, 0.6 Na2HPO4, 5.5 d-glucose, 2 l-glutamine, and an essential amino acid solution. The buffer was supplemented with 0.1 mg/ml soybean trypsin inhibitor and 1 mg/ml BSA, gassed with 100% O2, and adjusted to pH 7.48. Dispersed cultures of pancreatic acinar cells were isolated from adult male Sprague-Dawley rats by collagenase digestion as described previously (41). Acini were suspended in incubation buffer, and cells were maintained at 37°C for 1 h before performing assays. To induce Hsp70 expression, freshly harvested cells suspended in incubation buffer were incubated at 42°C for 30 min followed by a 2-h recovery at 37°C.
Immunofluorescence microscopy.
Lobules were gently pelleted and fixed in 4% paraformaldehyde in PBS. Immunofluorescence microscopy was conducted on 9-μm-thick cryostat sections as detailed previously (42). VAMP 8 was detected with rabbit polyclonal antibodies raised against the cytosolic domain VAMP 8 from T. Weimbs (28), W. Hong (46), or by our laboratory (1:50). CSP-α was detected with rabbit polyclonal antibody raised against a CH2-terminal peptide of the protein (1:50). VAMP 8 and CSP-α antibodies were detected using an Alexa 488-conjugated and Alexa 546-conjugated Zenon secondary detection kit, respectively. VAMP 2 was analyzed with a chicken polyclonal antibody (AB5625, 1:100) and an Alexa 546-conjugated anti-chicken IgG (1:250). When analyzed with VAMP 2, the CSP-α antibody (1:250) was detected with Alexa 488-conjugated anti-rabbit IgG (1:250). Fluorescence and differential interference contrast images were captured using a Nikon Eclipse TE2000 microscope, a PlanApo ×100 oil objective with a numerical aperture of 1.4, and a Hamamatsu Orca camera. Stacks of Z-series images were deconvolved using Volocity software and processed using Volocity or Photoshop software. Calculations of fluorophore overlay were determined using Image J software.
Acinar cell permeabilization.
Acini were suspended in a permeabilization buffer containing (in mM) 20 PIPES (pH 6.6), 139 K+-glutamate, 4 EGTA, 1.78 MgCl2, 2 Mg-ATP, 0.1 mg/ml soybean trypsin inhibitor, 1 mg/ml bovine serum albumin, and 35 pM PFO. PFO is a cholesterol-dependent cytolysin that assembles to create large aqueous pores in cell membranes (24). PFO was allowed to bind to intact cells on ice for 15 min, and excess unbound PFO was then removed by being washed at 4°C in the same buffer without PFO. Acini were aliquoted into prechilled microcentrifuge tubes (200 μl/tube) containing the indicated amounts of recombinant proteins. The cell suspension was then diluted with an equal volume of the same buffer containing enough CaCl2 to create the desired final concentration of free Ca2+. The quantity of CaCl2 added to the buffer was calculated on the basis of dissociation constants using a computer program as previously described (41). Cell suspensions were immersed in a 37°C water bath and incubated with gentle mixing for the indicated times. To measure basal secretion, permeabilized cells were incubated with indicated amounts of recombinant proteins and/or 100 μM GTP-γS in the presence of ≤10 nM of free Ca2+. Following incubation, cells were cooled in an ice bath and then centrifuged at 12,000 g for 1 min. The content of amylase in the medium was determined using a Phadebas assay kit. Data were calculated as the percent of total cellular amylase present in an equal amount of cells measured at the start of the experiment.
Preparation of ZGs.
Rat pancreases were minced in 5 vol of a buffer containing (in mM) 10 MOPS, pH 6.8, 250 sucrose, 0.1 MgCl2, 0.1 PMSF, and 1 benzamidine. Tissue was homogenized by 10 strokes of a motor-driven homogenizer (500 revolution/min) using a Teflon pestle with 0.5–1.0-mm clearance. A postnuclear supernatant was prepared by centrifugation at 1,000 g for 10 min and then further centrifuged at 3,200 g for 10 min to produce a white pellet enriched in ZGs overlaid by a brown pellet enriched in mitochondria. The remaining supernatant was centrifuged at 100,000 g for 1 h to separate microsomal and cytosolic fractions. ZGs were further purified by Percoll gradient centrifugation (42) and then lysed by sonication in buffer consisting of (in mM) 50 Tris (pH 7.4), 100 NaCl, 5 EDTA, 25 NaF, 10 Na pyrophosphate, and protease inhibitors. ZG membranes were then separated from content by 100,000 g centrifugation for 30 min. To remove peripherally associated proteins, ZG membranes were incubated in 0.1 M Na2Co3 (pH 11) for 30 min at 4°C and then recovered by centrifugation at 100,000 g for 1 h.
Pronase digestion of ZG proteins.
To digest ZG surface proteins, 200-μl aliquots of intact Percoll-purified ZGs containing 1 mg protein were further diluted in 200 μl of buffer containing 50 mM MES, pH 5.5, 250 mM sucrose, 0.1 mM MgSO4 with or without 35 μg/ml of pronase. Following 10-min incubation on ice, 200 μl of a 100× protease inhibitor cocktail (Calbiochem Cat. No. 539131) containing AEBSF, aprotinin, E-64, EDTA, and leupeptin was added followed by dilution in SDS-PAGE buffer and boiling. Digestion of proteins on the interior of ZGs was conducted in the same manner except that ZGs were initially diluted in buffer containing both pronase and 1% Triton X-100 and then immediately sonicated before incubation on ice.
Glutathione S-transferase.
Preparation of glutathione S-transferase (GST) fusion proteins of CSP and CSP truncation constructs were previously described (31). In all cases, GST was placed at the NH2 terminus of CSP. Likewise, the GST-VAMP 8 construct contains GST at the NH2 terminus . Fusion proteins were purified by glutathione affinity chromatography and either eluted in buffer containing 10 mM glutathione or released by thrombin cleavage as previously described (22).
GST pull-down assay and immunoprecipitations.
Cell cytosol fractions or membrane fractions prepared from purified ZGs, solubilized in lysis buffer containing 1% TX-100, were incubated with 15 μg of GST alone or the recombinant GST-tagged proteins, together with 20 μl of glutathione-Sepharose beads at 4°C. After 1-h incubation at 4°C, the beads were washed extensively and fusion proteins eluted with SDS sample buffer. Binding proteins were analyzed by immunoblotting. For immunoprecipitations, detergent solubilized membrane fractions containing 1% Triton X-100 were incubated with indicated antibodies overnight at 4°C. Antibodies were then precipitated with Protein A-Sepharose beads for 1 h, washed extensively in lysis buffer, and analyzed by SDS-PAGE and immunoblotting.
RESULTS
CSP-α is present on the outer leaflet of ZGs in acinar cells.
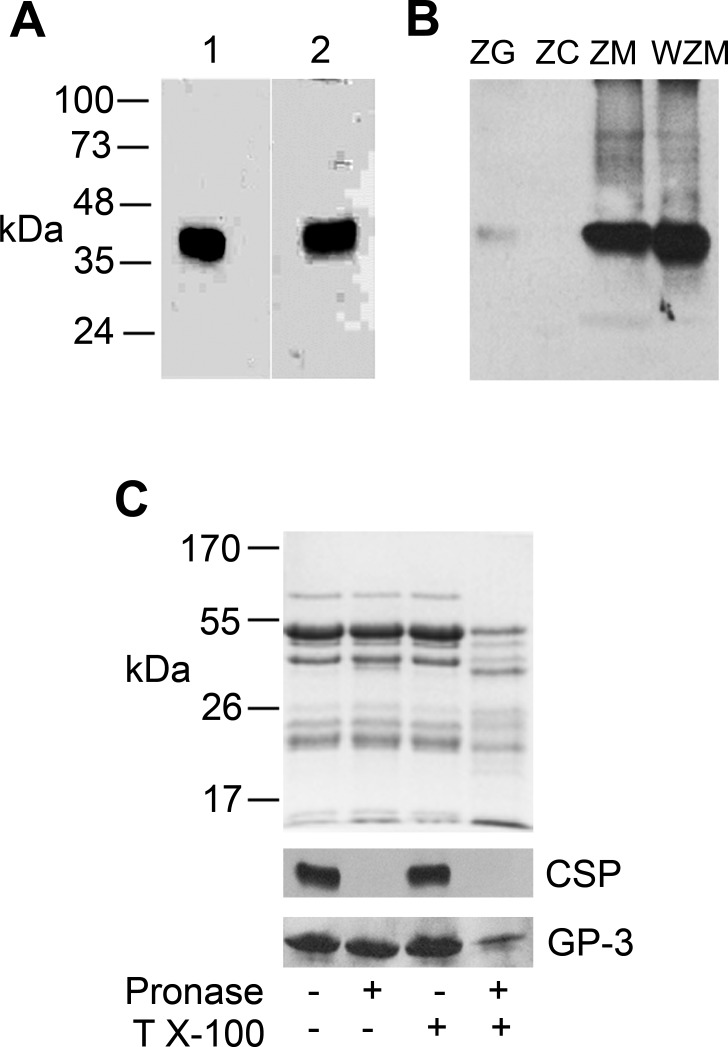
Three CSP genes have been identified; CSP-α, CSP-β and CSP-γ (18). CSP-β and CSP-γ are testis specific (18), whereas CSP-α, which is highly expressed in brain, was reported to undergo alternate splicing generating a truncated isoform in some cell lines (9). The expression of CSP-α in acini was demonstrated using two different polyclonal antibodies raised against either full-length rat CSP-α1-198 (8) or a C-terminal sequence unique to full-length CSP-α (5). In accordance with previous work (5), both antibodies detected a diffuse 35-kDa CSP-α signal in acini attributable to multiple palmitoylation sites within the cysteine string region demonstrating that full-length CSP-α is the predominant form present in acinar cells (Fig. 1A).
Fig. 1.
Cysteine string protein (CSP-α) is present on the outer surface of zymogen granule (ZG) membranes. A: acinar cell lysates (60 μg) were analyzed by immunoblotting with antibodies raised against full-length recombinant CSP-α (1:1,000) (lane 1) and a CH2-terminal peptide-specific CSP-α (1:5,000) (lane 2). B: ZG were purified by Percoll-density gradient centrifugation and then lysed by sonication to further separate ZG content (ZC) from ZG membranes (ZM) by centrifugation. The ZG membranes were further treated with 0.1 M Na2CO3 (pH 11) to remove peripherally associated proteins (WZM). Proteins from each fraction (30 μg) were separated by SDS-PAGE and analyzed by immunoblotting with anti-full-length CSP-α (1:1,000). Note the faint CSP-α signal in intact ZG was due to the extremely short exposure times necessary to detect the protein in ZM and WZM fractions. Also note that CSP-α is not removed by Na2CO3 washing. C: purified ZGs were treated with or without pronase and Triton X-100 (T X-100) and either left intact or lysed by sonication to allow access of pronase to intragranular proteins. Proteins (35 μg/lane) from each fraction were separated by SDS-PAGE and analyzed by Coomassie staining (top) or immunoblotting with antibodies to full-length CSP-α (1:1,000) or GP-3 (1:1,000). Note that CSP-α is completely degraded by pronase treatment of intact granules, whereas GP-3, a protein on the inner surface of granules (45), remains intact, indicating that CSP-α is present on the cytoplasmic surface of ZG.
Confirming previous studies (5, 42), tissue fractionation and immunoblotting demonstrated that CSP-α immunoreactivity was present in Percoll-purified ZG fractions of pancreas (Fig. 1B). Further fractionation of ZGs into ZG membranes and ZG content revealed a pronounced enrichment of CSP-α in the ZG membranes but no signal in ZG content. Moreover, Na2CO3 (pH 11) washing of ZG membranes to remove peripherally associated proteins further enriched the CSP-α signal, confirming that the molecule is embedded in the ZG membrane. Note that the faint CSP-α signal shown in the intact ZG fraction is due to the extremely short exposure time necessary to detect CSP-α immunoreactivity in ZG membrane and washed ZG membrane fractions. To evaluate whether CSP-α is present on the outer leaflet of ZGs and therefore oriented toward the cytoplasm, intact ZGs were treated with pronase to digest proteins present on the ZG surface, resulting in a complete loss of the CSP-α signal (Fig. 1C). As a control, the ZG membrane protein GP-3, which is present on the inner surface of granules (45), was unaltered by pronase treatment of intact ZGs. Conversely, GP-3 was significantly degraded when ZGs were lysed before protease treatment. For comparison, a Coomassie-stained gel showing total ZG proteins in each fraction is included, demonstrating that pronase treatment of lysed ZGs resulted in a massive degradation of ZG cargo proteins. Collectively, these data demonstrate that CSP-α is an integral ZG membrane protein that projects into the acinar cytoplasm and is therefore capable of interacting with regulatory proteins important for the secretory process.
CSP-α interacts with acinar cell Hsc70 and Hsp70.
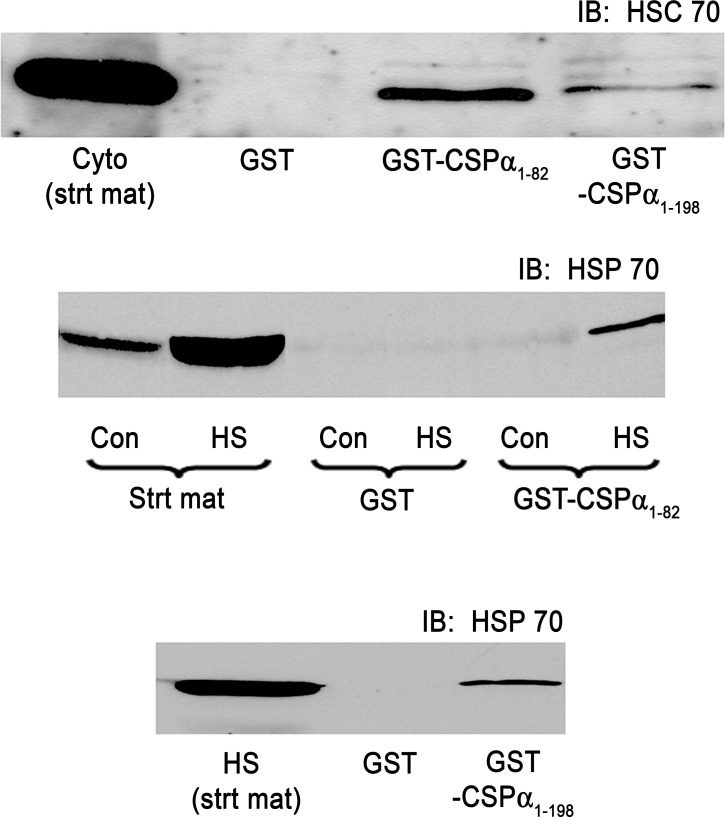
To begin to evaluate a role for the CSP-α cochaperone system in acinar function, pull-down assays were conducted using GST-CSP-α1-198 representing full-length CSP-α or the truncated GST-CSP-α1-82 containing the J domain (see Fig. 6 for a diagram of the protein), and the assembly of CSP-α/Hsc70 and CSP-α/Hsp70 complexes in acinar lysates was evaluated (Fig. 2). Consistent with previous studies (44), acinar cells were found to constitutively express Hsc70 under normal conditions (Fig. 2, top), as well as to undergo an approximate 10-fold increase in the expression of stress-inducible Hsp70 in response to a 30-min heat shock at 42°C followed by a 2-h recovery (Fig. 2, middle and bottom). Both full-length CSP-α1-198 and CSP-α1-82 precipitated Hsc70 or Hsp70 from acinar lysates. Quantitatively, each construct precipitated less than 10% of total Hsc70 or Hsp70 in the lysate, which likely reflects the transient nature of this molecular interaction. These results are in agreement with previous reports using yeast two-hybrid and ATPase assay analysis (10, 39) demonstrating Hsp70 and Hsc70 association with CSP-α via the J domain and indicate that, in acinar cells, CSP-α/Hsc70 and CSP-α/Hsp70 may form functional chaperone complexes.
Fig. 2.
The CSP-α J domain interacts with acinar cell Hsc70 and Hsp70. Isolated acini were incubated under control conditions at 37°C (top) or heat shocked at 42°C for 30 min (middle and bottom) and then returned to 37°C for an additional 120 min to induce Hsp70 expression. Lysates from control (Con) or heat-shocked (HS) acini were incubated with purified glutathione S-transferase (GST)-, GST-CSP-α1-82 containing the J domain, or full-length GST-CSP-α1-198 as indicated. Bound proteins were analyzed by immunoblotting (IB) using Hsc70 (1:600) or Hsp70 (1:300) specific antibodies. Note the large induction of Hsp70 by heat shock. Also note that the CSP-α1-82 and CSP-α1-198 coprecipitate both Hsc70 and Hsp70.
CSP-α1-82 augments acinar secretion.
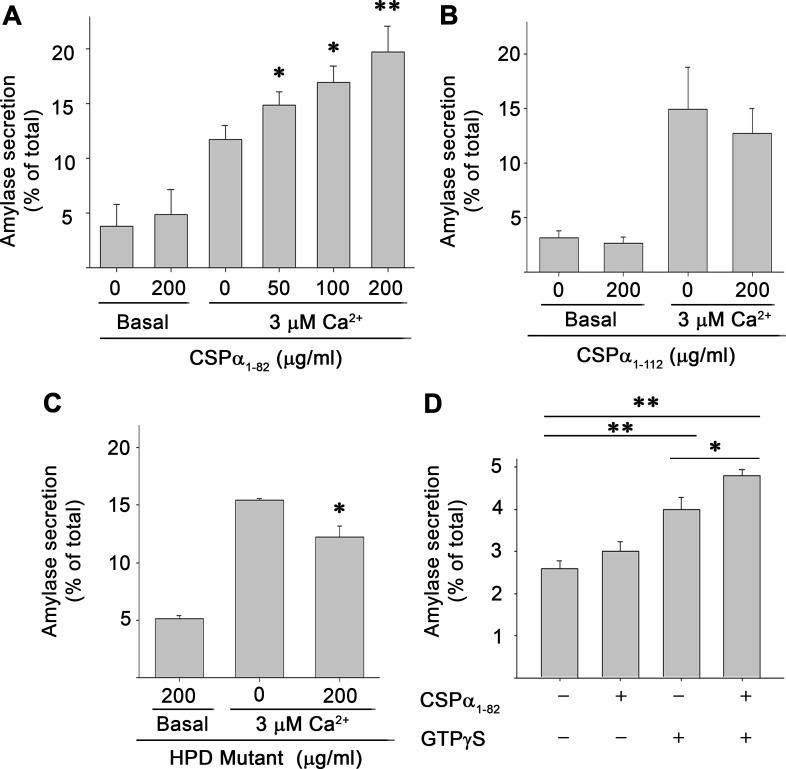
Next the effect of introducing recombinant J domain (CSP-α1-82) into PFO-permeabilized acinar cells on Ca2+-stimulated amylase secretion was evaluated (Fig. 3A). Maximum amylase secretion from permeabilized cells was achieved at 3 μM free Ca2+ (data not shown). Thus isolated cells were permeabilized and incubated with the indicated concentrations of CSP-α1-82 and 3 μM Ca2+ for 30 min, and the release of amylase into the media was determined. CSP-α1-82 strongly enhanced Ca2+-stimulated secretion in a concentration-dependant manner with a maximal 180% of basal levels achieved at 200 μg/ml of protein. No further increase in secretion was detected at higher concentrations of CSP-α1-82 or with longer incubation times (data not shown). CSP-α1-82 had no effects on basal secretion, measured at ≤10 nM Ca2+. Unexpectedly, a CSP-α1-112 construct containing both the J domain and adjacent linker region had little or no effect on Ca2+-stimulated secretion when incubated at concentrations as high as 200 μg/ml (Fig. 3B). Similarly, introduction of full-length CSP-α1-198 also had no effects on Ca2+-stimulated secretion (data not shown), findings that potentially reflect the pronounced aggregating properties of the linker region of the molecule (see discussion). These results indicate that the NH2-terminal region of CSP-α, which encodes the J-domain, is able to modulate Ca2+-dependent secretion of digestive enzymes and further suggest that the mechanism of this effect involves Hsc70 chaperone function.
Fig. 3.
CSP-α J domain augments Ca2+- and GTP-γS-stimulated amylase secretion. Acini were permeabilized with Perfringolysin O (PFO) and incubated in the presence or absence of indicated concentrations of CSP-α1-82 (A), CSP-α1-112 (B), or a CSP-α1-82 histidine, proline, aspartic acid (HPD)-mutant of the J domain to abolish Hsc70 coactivation (C). Amylase secreted into the media over 30 min was measured in response to basal (≤10 nM) or stimulatory (3 μM) concentrations of free Ca2+. D: isolated acini were permeabilized with PFO in the presence of 100 μM GTP-γS and/or 200 μg/ml CSP-α1-82, and amylase secretion was determined in the presence of basal Ca2+ (≤10 nM) following a 30-min incubation. Secretion is expressed as a percentage of total cellular amylase measured at the start of the experiment. Data in A–C are the means ± SD and in D are the means ± SE of 3 independent experiments, each performed in triplicate or quadruplicate. *P < 0.05, **P < 0.01.
The J domain of CSP-α is comprised of four helices with a tripeptide of histidine, proline, and aspartic acid (HPD motif) located between helices II and III that is essential for activation of Hsc70 (52). If our hypothesis that CSP-α anchors chaperone work to zymogen granules and that this conformational work is critical to digestive enzyme secretion is correct, then mutation of the HPD motif to abolish its Hsc70 activating ability would be expected to eliminate the secretory effects of CSP-α1-82 when introduced into permeabilized acini. The histidine-proline-aspartate (HPD) sequence (amino acids 43-45) was mutated to glutamine-proline-alanine and introduced into the acinar cells (Fig. 3C). Consistent with our hypothesis, introduction of high concentrations (200 μg/ml) of CSP-α1-82HPD-QPA failed to augment Ca2+-stimulated secretion and modestly inhibited secretion by ∼20%. These data support the concept that Hsc70 activation is essential in mediating the effects of CSP-α1-82 on Ca2+-stimulated secretion.
Similar to the present findings, overexpression of CSP-α in PC12 cells was previously shown to augment Ca2+-dependent secretion in permeabilized cells, indicating that CSP-α acts at a late step in the secretory pathway (11). Interestingly, this same study demonstrated that treatment of permeabilized cells overexpressing CSP-α with GTP-γS significantly enhanced basal secretion independent of elevated Ca2+. Incubation of permeabilized acini with GTP-γS enhanced basal secretion (in the presence of ≤10 nM free Ca2+) by ∼155% of control values (Fig. 3D). Similar to results in PC12 cells (11), coincubation of permeabilized acini with both CSP-α1-82 and GTP-γS further elevated basal secretion by 125% of that seen for GTP-γS alone. These results suggest that CSP-α may play a role in regulating the activity of G proteins that are important for the secretory response.
CSP-α colocalizes with VAMP proteins in the secretory pathway of acinar cells.
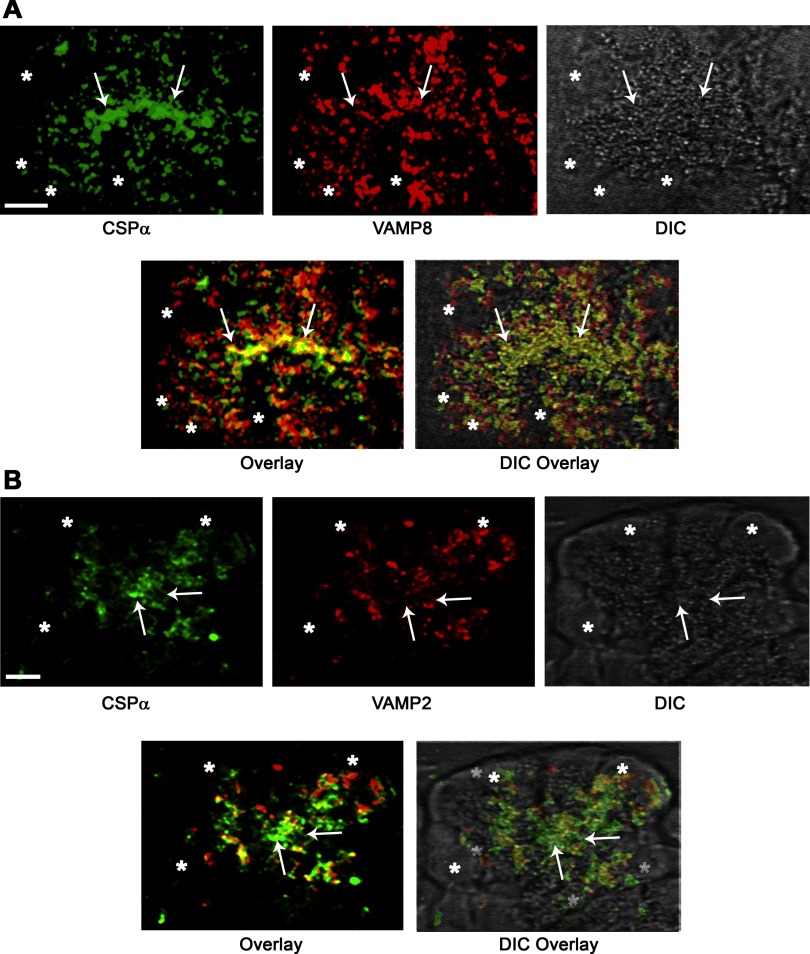
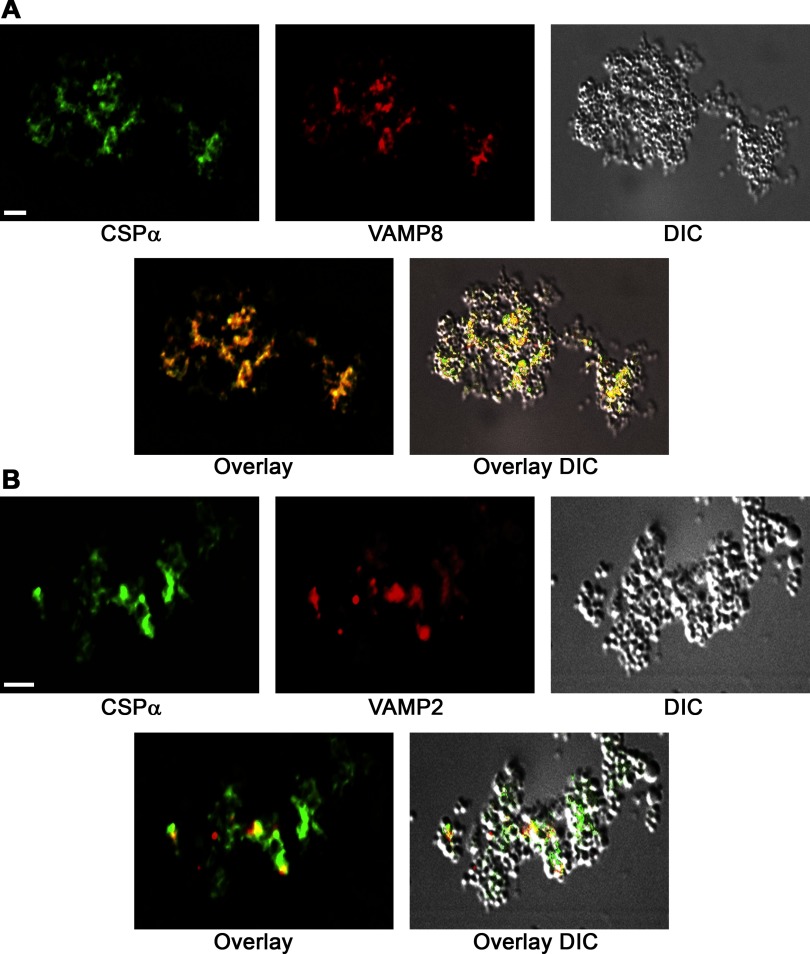
To further evaluate the subcellular localization of CSP-α in acini, immunofluorescence analysis was conducted on cryostat sections of pancreatic lobules (Fig. 4), confirming that the protein is abundant in apical ZG-rich regions of the cytoplasm. We recently reported that acinar cells of the pancreas contain at least 2 populations of ZGs on the basis of their expression of VAMP 2 or VAMP 8 (48). Thus dual immunofluorescence and quantitative analysis of CSP-α and VAMP proteins was conducted to determine the VAMP-specific localization of CSP-α. Results indicated a 52.1 ± 5.0% (n = 10) colocalization of CSP-α with VAMP 8 in apical regions of acini. In contrast, CSP-α showed a more modest 36.2 + 2.8% (n = 10) colocalization with VAMP 2. Similarly, immunofluorescence analysis of cryostat sections prepared from Percoll-purified ZG granules supported these findings demonstrating a more extensive colocalization of CSP-α with VAMP 8 compared with VAMP 2 (Fig. 5). These data confirm the tissue fractionation experiments localizing CSP-α to ZGs (5, 42) and further show that CSP-α significantly colocalizes with VAMP 8-containing ZGs.
Fig. 4.
CSP-α colocalizes with vesicle-associated membrane protein (VAMP) 8 on ZG in the secretory pathway of acinar cells. CSP-α was analyzed together with VAMP 8 (A) or VAMP 2 (B) by immunofluorescence microscopy of pancreatic lobules. In the representative image shown, VAMP 8 was analyzed with a rabbit polyclonal antibody raised in our laboratory against the cytosolic domain of VAMP 8 and CSP-α with an anti-peptide rabbit polyclonal antibody. The same results were achieved using 3 separate VAMP 8 polyclonal antibodies. Consistent with tissue fractionation studies (5, 42), CSP-α immunofluorescence was localized to the ZG-rich apical pole of acini. Note the more pronounced overlap of CSP-α with VAMP 8 and comparatively modest overlap with VAMP 2. The arrows denote the apical plasma membrane, and asterisks denote nuclei (Bar = 2.5 μm). DIC, differential interference contrast.
Fig. 5.
CSP-α colocalizes with VAMP-8 on purified ZGs. ZGs were isolated by Percoll-density centrifugation and then fixed in 4%-formaldehyde. Cryostat sections were analyzed as in Fig 3. Note that CSP-α is more highly colocalized with VAMP 8 compared with VAMP 2.
CSP-α-SNARE interactions in acinar cells.
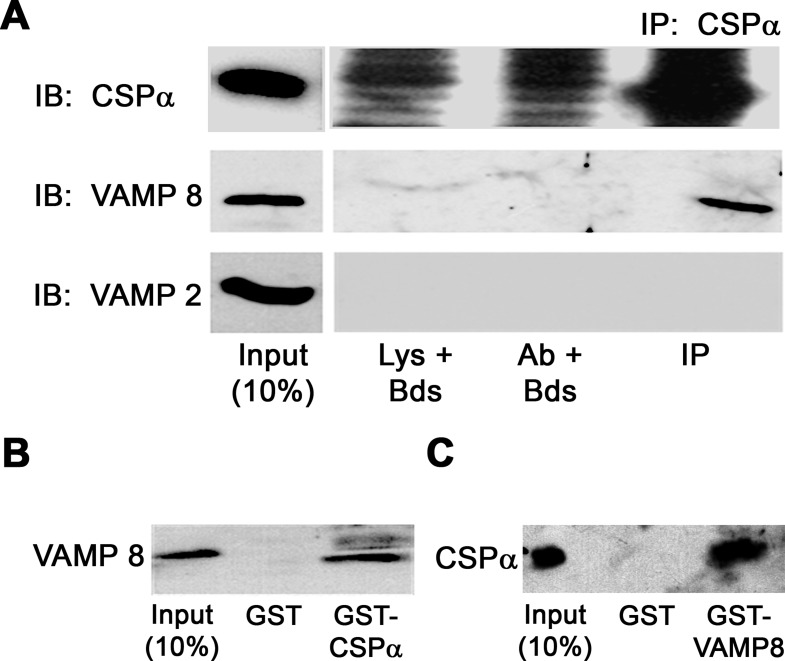
CSP-α has been shown to interact with a number of SNARE proteins including syntaxins 1A (34) and 4 (12), synaptotagmin 1 (16), as well as some isoforms of VAMPs (4). We were unable to detect interactions between CSP-α and the plasma membrane SNARE proteins SNAP 23, syntaxin 2, or syntaxin 4 when analyzed by coimmunoprecipitation from microsomal fractions of acini (data not shown). However, it has been our experience that coimmunoprecipitation of SNARE proteins in acini yield extremely small quantities of mature SNAREpin complexes, making these difficult to detect (48). As an alternative, potential CSP-α interactions with various SNARE proteins expressed on ZGs were examined in Triton X-100-solubilized ZG membrane fractions by coimmunoprecipitation with anti-CSP-α antibodies (Fig. 6A). Consistent with the colocalization of CSP-α and VAMP 8 in acini and ZG fractions, these proteins coimmunoprecipitated from the ZG membrane fraction. Conversely, neither VAMP 2 nor syntaxin 3, which are also expressed on ZGs (20), was detected with CSP-α precipitated protein (syntaxin 3 is not shown). The interaction between CSP-α and VAMP 8 was further confirmed by pull-down assays where GST-VAMP 8 precipitated CSP-α (Fig. 6B) and GST-CSP-α precipitated VAMP 8 (Fig. 6C) from ZG membranes.
Fig. 6.
CSP-α interacts with VAMP 8 on ZG membranes. A: ZG membrane proteins were solubilized in 1% Triton X-100 and immunoprecipitated (IP) using anti-full length CSP-α antibody and then immunoblotted (IB) with CSP-α-, VAMP 8-, or VAMP 2-specific antibodies. Note the high background in the lysate + beads (Lys + Bds) and antibody + beads (Ab + Bds) lanes for CSP-α caused by using the same antibody for both IP and IB. B and C: solubilized ZG membranes were incubated with purified GST, GST-VAMP 8, or GST-CSP and pulled down with glutathione-agarose beads. Bound proteins were washed, separated by SDS-PAGE, and analyzed by immunoblotting using CSP-α- or VAMP 8-specific antibodies.
The CSP-α linker region is required for VAMP 8 interactions.
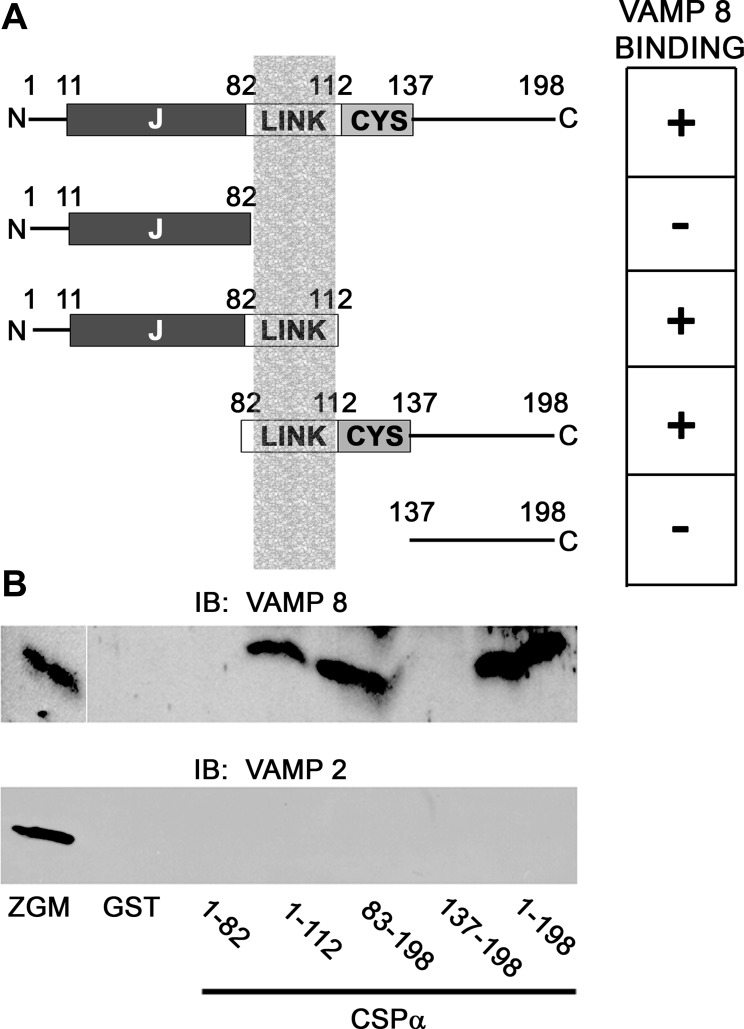
The molecular interaction between CSP-α and VAMP 8 was characterized using GST-CSP-α truncation constructs (Fig. 7). Neither CSP-α1-82 nor CSP-α137-198 was found to interact with VAMP 8 in Triton X-100-soluble ZG membrane fractions. In contrast, CSP-α1-112 and CSP-α83-198 interacted with VAMP 8. Compared with full-length CSP-α1-198, which pulled down 12.5 ± 7.8% of the total VAMP 8 in the fraction, the CSP-α1-82 and CSP-α137-198 pulled down 5.1 ± 1.6% and 6.0 ± 0.4% of total VAMP 8, respectively; however, these differences were not statistically significant (n = 3). None of the CSP-α constructs tested interacted with VAMP 2. Because CSP-α 1-112 bound to VAMP 8, whereas CSP-α1-82 did not, these data indicate that the linker region (amino acids 83-112) is capable of VAMP 8 binding. Results that the COOH-terminal construct CSP-α137-198 showed no binding activity, whereas CSP-α83-198 bound to VAMP 8, confirmed the significance of the linker region in binding; however, the overall importance of the cysteine string region (amino acids 113-137) in VAMP 8 binding was not determined.
Fig. 7.
Mapping of the CSP-α/VAMP 8 interaction. A: indicated CSP-α truncation constructs were utilized for GST pull-down assays of VAMP 8 from purified zymogen granule membrane (ZGM) fractions. J, J-domain; LINK, linker domain; CYS, cysteine string domain. The table on the right indicates which of the CSP-α truncation constructs bound to VAMP 8. The shaded column in the figure depicts the VAMP 8 binding region of CSP-α. B: representative immunoblot from GST-CSP pull-down assays using the construct depicted above. Bound proteins were immunoblotted using anti-VAMP 8- (1:500) or anti-VAMP 2- (1:1,000) specific antibodies. Note that CSP-α interaction with VAMP 8 requires a minimum of the CSP-α linker region (amino acids 82-112) because binding occurs with CSP-α1-112 but does not with CSP-α1-82.
DISCUSSION
The present study establishes that CSP-α1-82 encoding the signature J domain augments Ca2+-induced digestive enzyme secretion when introduced into permeabilized acini. CSP-α1-82 is soluble, and we anticipate that cytosolic CSP-α1-82 when introduced into permeabilized cells is able to mimic acinar folding events carried out by native ZG-anchored CSP-α. A major question that remains is whether the action of CSP-α1-82 is interchangeable with respect to other J domain proteins that may be expressed in acinar cells. Sequence analysis has revealed over 40 J domain proteins; however, to date, functional roles for only a few of these molecules have been demonstrated (52). The specificity of the J domain-Hsc/Hsp70 protein folding apparatus appears to be based largely on the select subcellular distribution of J proteins that act to target Hsc/Hsp70 chaperone activity in the cell. Clearly, our demonstration that CSP-α localizes to the outer leaflet of ZGs and undergoes a direct interaction with VAMP 8 strongly supports that this chaperone complex acts to promote conformational changes in the exocytic protein machinery of the acinar cell necessary for maintaining a robust secretory response.
A functional role for CSP-α in regulated secretion has been demonstrated in neuronal, neuroendocrine, and endocrine cell types revealing several potential mechanisms for the protein including modulation of voltage-regulated Ca2+ channels, neurotransmitter synthesis, vesicle filling, regulation of heterotrimeric G proteins, and phosphorylation-dependent conformational changes in exocytic SNARE proteins (reviewed in 17, 18). Drosophila CSP-α-null mutants exhibit temperature-sensitive paralysis, and most flies die as larvae or within days of adulthood (53). Likewise, deletion of CSP-α in mice causes blindness and progressive neurodegeneration with no survival beyond 4 mo (18, 37). Compared with neural and endocrine secretory models, the acinar cell has a number of unique features. Secretion does not involve voltage-dependent Ca2+ influx but is mediated via G protein-coupled receptors and the IP3-dependent release of Ca2+ from internal stores (49). Moreover, secretion involves the sequential compound exocytosis of ZGs following an initial round of ZG fusion to the apical plasma membrane (33), and this process is regulated by unique SNARE protein isoforms (46, 48 and reviewed in 49). Thus it is probable that CSP-α function in acini may vary with respect to its effects on Ca2+ signaling and SNARE isoform interactions seen in neural and endocrine systems.
Results that CSP-α1-82 modulates Ca2+-stimulated exocytosis in permeabilized acini independent of hormone/neurotransmitter activation are clearly in line with the demonstrated role for CSP-α in other secretory models, indicating that the protein acts at a late stage in the exocytic process (reviewed in 17). Similar to our findings, Chamberlin et al. (11) stably overexpressed CSP-α in PC12 cells, demonstrating that it augmented Ca2+-stimulated exocytosis from permeabilized cells. When overexpressed, CSP-α was shown to localize correctly within the cells and had no effects on cell morphology, granule distribution, and number or intracellular Ca2+-signaling, indicating that the protein was acting at a late stage in the secretory pathway. Conversely, transient overexpression of CSP-α in β-cells (8, 50), HIT-T15 cells (51), or chromaffin cells (21) inhibited secretion. Interestingly, Boal et al. (4) confirmed that CSP-α overexpression in HIT-T15 cells inhibited insulin secretion from intact cells, whereas CSP-α1-82 containing only the J domain had no inhibitory effects. Rather, the inhibitory effects of CSP-α with transient overexpression were mapped to the central linker and COOH-terminal regions of the molecule. These findings confirmed earlier results from Zhang et al. (51) demonstrating that mutation of glutamate 93 to valine within the linker region significantly reduced the inhibitory effects of the molecule on secretion. Finally, and in contrast to the present results, overexpression of the CSP J domain in Xenopus oocytes inhibited phorbol-ester-induced cortical granule exocytosis, and this effect was abolished by mutation of the J domain to interfere with Hsc70 binding (39). The wide variability of these secretory effects caused by overexpressing CSP-α in cells is no doubt related to the experimental conditions utilized in each study including cell type, transient or stable transfections, permeabilized vs. intact cells, and the nature of the secretory stimulus.
Consistent with results in permeabilized PC12 cells overexpressing CSP-α (11), treatment of acini with GTP-γS in the presence of basal Ca2+ levels significantly enhanced amylase secretion by ∼125% of that seen for GTP-γS alone (Fig. 3D). This nonhydrolyzable analog of GTP will activate heterotrimeric proteins as well as small G proteins, both of which are involved in multiple aspects of the secretory response, making it difficult to assign specificity to this effect. CSP-α has been shown to act as a guanine nucleotide exchange factor for heterotrimeric G protein α-subunits in PC12 cells (32) and to bind to both Gα- and Gβγ-subunits in modulating N-type Ca2+ channels (29, 31). Ohnishi et al. (35) previously demonstrated that G-αq/11 and Gβ-subunits are present on ZGs. Moreover, introduction of an inhibitory peptide into permeabilized acini to antagonize G-αq/11 activity significantly inhibited Ca2+-stimulated amylase secretion. We were unable to demonstrate a direct interaction between CSP-α and G-αq/11 by either coimmunoprecipitation or GST-CSP-α pull-down assays in acinar and ZG lysates (data not shown), suggesting that CSP-α may regulate the activity of other G proteins important for secretion.
The present findings that neither full-length CSP-α nor CSP-α1-112 containing the J domain and adjacent linker region significantly altered amylase secretion in permeabilized cells may reflect the apparent inhibitory role of the linker region on secretion when transiently overexpressed in intact cells (4, 51). Nie et al. (34) demonstrated that CSP overexpression in Drosophila impaired fly development and viability, and this phenotype was rescued by coexpressing syntaxin 1A, which directly interacts with CSP. These results suggest that, when overexpressed in cells for prolonged periods, the linker region may interact with or otherwise sequester important components of the SNARE apparatus, thereby inhibiting exocytosis. These interactions may not be as extensive when the molecule is acutely introduced into permeabilized cells during Ca2+ stimulation. Under these conditions the linker region may be unable to acutely interact with regulatory molecules (including SNARE proteins) when ZGs are docked and/or poised for secretion. A second possibility is that recombinant CSP-α is known to form high molecular mass SDS-resistant oligomers and aggregates following purification from bacterial lysates (1, 40). Moreover, this self-association of CSP-α was mapped to the linker and cysteine string domains (amino acids 83-136) of the protein (40). Thus it is conceivable that the high molecular mass CSP-α oligomers that form during purification are unable to gain sufficient access into PFO-permeabilized cells compared with the highly soluble CSP-α1-82 J domain. Whether or not this aggregation property of CSP-α plays a role in inhibiting secretion when the protein is overexpressed at high levels in cells is not certain.
Coimmunoprecipitation and GST pull-down assays have established that CSP-α directly interacts with syntaxin 1A (34), syntaxin 4 (12), synaptotagmin 1 (16), VAMP 2, and VAMP 7, but not VAMP 3 (4). Interestingly, the interaction of GST-CSP-α with VAMP 2 was dependent on the presence of Ca2+ and appeared to require the C-terminal amino acids of the protein because truncation of these residues (representing the alternatively spliced isoform CSP2) showed no binding activity (4). In the present study, no interaction between CSP-α and VAMP 2 was detected in Triton X-100 solubilized ZG membrane fractions. Furthermore, inclusion of Ca2+ in the buffer did not promote a VAMP 2 interaction with CSP-α or enhance its association with VAMP 8 (data not shown). A lack of VAMP 2 binding may potentially reflect the low levels of CSP-α and VAMP 2 colocalization in acini; rather, our results indicate that CSP-α interacts with VAMP 8 via the linker region, whereas the C-terminal construct (CSP137-198) showed no binding activity (see Fig. 6). The differences for these results are uncertain; however, the lack of CSP-α association with VAMP 2 is clearly supported by the more modest colocalization of these molecules compared with VAMP 8.
Prior induction of Hsp70 in isolated cells and rodents has been shown to provide a substantial protective effect against the onset of acute pancreatitis (2, 44), and Hsp70-deficient mice display increased sensitivity to the disease (25). The cascade of events that underlie acute pancreatitis and the protective effects of Hsp70 remain to be identified but appear to involve aberrant alterations in the acinar secretory pathway (27). Acute pancreatitis is experimentally induced by administering supraphysiological levels of secretagogs to rodents or isolated acinar cells. The earliest events seen within minutes of stimulation involve the inhibition of apical secretion, exocytosis of ZGs at the basolateral membrane, premature activation of trypsinogen within cells, and the formation of large cytoplasmic vacuoles (reviewed in 27). Further evidence implicating the acinar secretory pathway in the pathogenesis of acute pancreatitis comes from the finding that genetic deletion of the ZG protein VAMP 8 significantly reduces the severity of experimental pancreatitis in rodents (15, 46). Thus it is tempting to speculate that, just as CSP-α has been implicated as an important factor in attenuating neurodegenerative disease (18), it may also play a significant role in maintaining the overall integrity of the secretory pathway and, in the presence of high levels of Hsp70, protect against the onset of acute pancreatitis. Clearly further investigation of a potential role for CSP-α in mediating the protective effects of Hsp70 against acute pancreatitis may provide valuable mechanistic insight into the pathophysiology of this disease as well as potential targets for therapeutic intervention.
GRANTS
This work was supported by NIH grant DK07088 and a USDA HATCH grant to G. E. Groblewski and Canadian Institute of Health Research grant MOP47413 to J. E. A. Braun. M. Baumler was supported in part by NIH T32 DK007665.
REFERENCES
- 1.Bai L, Swayne LA, Braun JE. The CSPalpha/G protein complex in PC12 cells. Biochem Biophys Res Commun 352: 123–129, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bhagat L, Singh VP, Hietaranta AJ, Agrawal S, Steer ML, Saluja AK. Heat shock protein 70 prevents secretagogue-induced cell injury in the pancreas by preventing intracellular trypsinogen activation. J Clin Invest 106: 81–89, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blondeau F, Ritter B, Allaire PD, Wasiak S, Girard M, Hussain N, Angers A, Legendre-Guillemin V, Roy L, Boismenu D, Kearney RE, Bell AW, Bergeron JJ, McPherson PS. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc Natl Acad Sci USA 101: 3833–3838, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boal F, Zhang H, Tessier C, Scotti P, Lang J. The variable C-terminus of cysteine string proteins modulates exocytosis and protein-protein interactions. Biochemistry 43: 16212–16223, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Braun JE, Scheller RH. Cysteine string protein, a DnaJ family member, is present on diverse secretory vesicles. Neuropharmacology 34: 1361–1369, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Braun JE, Wilbanks SM, Scheller RH. The cysteine string secretory vesicle protein activates Hsc70 ATPase. J Biol Chem 271: 25989–25993, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky JL, Chiosis G. Hsp70 molecular chaperones: emerging roles in human disease and identification of small molecule modulators. Curr Top Med Chem 6: 1215–1225, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Brown H, Larsson O, Branstrom R, Yang S, Leibiger B, Leibiger I, Fried G, Moede T, Deeney JT, Brown GR, Jacobsson G, Rhodes CJ, Braun JE, Scheller RH, Corkey BE, Berggren P, Meister B. Cysteine string protein (CSP) is an insulin secretory granule-associated protein regulating beta-cell exocytosis. EMBO J 17: 5048–5058, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chamberlain LH, Burgoyne RD. Identification of a novel cysteine string protein variant and expression of cysteine string proteins in non-neuronal cells. J Biol Chem 271: 7320–7323, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain LH, Burgoyne RD. Activation of the ATPase activity of heat-shock proteins Hsc70/Hsp70 by cysteine-string protein. Biochem J 322: 853–858, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamberlain LH, Burgoyne RD. Cysteine string protein functions directly in regulated exocytosis. Mol Biol Cell 9: 2259–2267, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamberlain LH, Graham ME, Kane S, Jackson JL, Maier VH, Burgoyne RD, Gould GW. The synaptic vesicle protein, cysteine-string protein, is associated with the plasma membrane in 3T3-L1 adipocytes and interacts with syntaxin 4. J Cell Sci 114: 445–455, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 123: 383–396, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Zheng X, Schulze KL, Morris T, Bellen H, Stanley EF. Enhancement of presynaptic calcium current by cysteine string protein. J Physiol 538: 383–389, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosen-Binker LI, Binker MG, Wang CC, Hong W, Gaisano HY. VAMP8 is the v-SNARE that mediates basolateral exocytosis in a mouse model of alcoholic pancreatitis. J Clin Invest 118: 2535–2551, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans GJ, Morgan A. Phosphorylation-dependent interaction of the synaptic vesicle proteins cysteine string protein and synaptotagmin I. Biochem J 364: 343–347, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans GJ, Morgan A, Burgoyne RD. Tying everything together: the multiple roles of cysteine string protein (CSP) in regulated exocytosis. Traffic 4: 653–659, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Chacon R, Wolfel M, Nishimune H, Tabares L, Schmitz F, Castellano-Munoz M, Rosenmund C, Montesinos ML, Sanes JR, Schneggenburger R, Sudhof TC. The synaptic vesicle protein CSP alpha prevents presynaptic degeneration. Neuron 42: 237–251, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Futatsugi A, Takeshi Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science 309: 2232–2234, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Gaisano HY, Ghai M, Malkus PN, Sheu L, Bouquillon A, Bennett MK, Trimble WS. Distinct cellular locations of the syntaxin family of proteins in rat pancreatic acinar cells. Mol Biol Cell 7: 2019–2027, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham ME, Burgoyne RD. Comparison of Cysteine String Protein (Csp) and mutant alpha-SNAP overexpression reveals a role for Csp in late steps of membrane fusion in dense-core granule exocytosis in adrenal chromaffin cells. J Neurosci 20: 1281–1289, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groblewski GE, Wishart MJ, Yoshida M, Williams JA. Purification and identification of a 28-kDa calcium-regulated heat-stable protein. A novel secretagogue-regulated phosphoprotein in exocrine pancreas. J Biol Chem 271: 31502–31507, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Gundersen CB, Umbach JA. Suppression cloning of the cDNA for a candidate subunit of a presynaptic calcium channel. Neuron 9: 527–537, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Heuck AP, Tweten RK, Johnson AE. Beta-barrel pore-forming toxins: intriguing dimorphic proteins. Biochemistry 40: 9065–9073, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Hwang JH, Ryu JK, Yoon YB, Lee KH, Park YS, Kim JW, Kim N, Jeong JB, Seo JS, Kim YT. Spontaneous activation of pancreas trypsinogen in heat shock protein 70.1 knock-out mice. Pancreas 31: 332–336, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Kohan SA, Pescatori M, Brecha NC, Mastrogiacomo A, Umbach JA, Gundersen CB. Cysteine string protein immunoreactivity in the nervous system and adrenal gland of rat. J Neurosci 15: 6230–6238, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lerch MM, Gorelick FS. Early trypsinogen activation in acute pancreatitis. Med Clin North Am 84: 549–563, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Low SH, Li X, Miura M, Kudo N, Quiñones B, Weimbs T. Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Dev Cell 4: 753–759, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Magga JM, Jarvis SE, Arnot MI, Zamponi GW, Braun JE. Cysteine string protein regulates G-protein modulation of N-type calcium channels. Neuron 28: 195–204, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Mastrogiacomo A, Parsons SM, Zampighi GA, Jenden DJ, Umbach JA, Gundersen CB. Cysteine string proteins: a potential link between synaptic vesicles and presynaptic Ca2+ channels. Science 263: 981–982, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Miller LC, Swayne LA, Kay JG, Feng ZP, Jarvis SE, Zamponi GW, Braun JEA. Molecular determinants of cysteine string protein modulation of N-type calcium channels. J Cell Sci 116: 2967–2974, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Natochin M, Campbell TN, Barren B, Miller LC, Hameed S, Artemyev NO, Braun JE. Characterization of the G alpha(s) regulator cysteine string protein. J Biol Chem 280: 30236–30241, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Nemoto T, Kimura R, Ito K, Tachikawa A, Miyashita Y, Iino M, Kasai H. Sequential-replenishment mechanism of exocytosis in pancreatic acini. Nat Cell Biol 3: 253–258, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Nie Z, Ranjan R, Wenniger JJ, Hong SN, Bronk P, Zinsmaier KF. Overexpression of cysteine-string proteins in Drosophila reveals interactions with syntaxin. J Neurosci 19: 10270–10279, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohnishi H, Ernst SA, Yule DI, Baker CW, Williams JA. Heterotrimeric G-protein Gq/11 localized on pancreatic zymogen granule is involved in calcium-regulated amylase secretion. J Biol Chem 272: 16056–16061, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Rosales-Hernandez A, Beck KE, Zhao X, Braun AP, Braun JE. RDJ2 (DNAJA2) chaperones neural G protein signaling pathways. Cell Stress Chaperones 14: 71–82, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz F, Tabares L, Khimich D, Strenzke N, de La Villa-Pollo P, Castellano-Munoz M, Bulankina A, Moser T, Fernandez-Chacon R, Sudhof TC. CSPalpha-deficiency causes massive and rapid photoreceptor degeneration. Proc Natl Acad Sci USA 103: 2926–2931, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith GB, Umbach JA, Hirano A, Gundersen CB. Interaction between constitutively expressed heat shock protein, Hsc70, and cysteine string protein is important for cortical granule exocytosis in Xenopus oocytes. J Biol Chem 280: 32669–32675, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stahl B, Tobaben S, Südhof TC. Two distinct domains in hsc70 are essential for the interaction with the synaptic vesicle cysteine string protein. Eur J Cell Biol 78: 375–381, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Swayne LA, Beck KE, Braun JE. The cysteine string protein multimeric complex. Biochem Biophys Res Commun 348: 83–91, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Thomas DD, Taft WB, Kaspar KM, Groblewski GE. CRHSP-28 regulates Ca(2+)-stimulated secretion in permeabilized acinar cells. J Biol Chem 276: 28866–28872, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Thomas DD, Weng N, Groblewski GE. Secretagogue-induced translocation of CRHSP-28 within an early apical endosomal compartment in acinar cells. Am J Physiol Gastrointest Liver Physiol 287: G253–G263, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Tobaben S, Thakur P, Fernández-Chacón R, Südhof TC, Rettig J, Stahl B. A trimeric protein complex functions as a synaptic chaperone machine. Neuron 31: 987–999, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Wagner AC, Weber H, Jonas L, Nizze H, Strowski M, Fielder F, Printz H, Steffen H, Goke B. Hyperthermia induces heat shock protein expression and protection against cerulein-induced pancreatitis in rats. Gastroenterology 111: 1333–1342, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Wagner AC, Wishart MJ, Mulders SM, Blevins PM, Andrews PC, Lowe AW, Williams JA. GP-3 a newly characterized glyprotein on the inner surface of the zymogen granule membrane undergoes regulated secretion. J Biol Chem 269: 9099–9104, 1994. [PubMed] [Google Scholar]
- 46.Wang CC, Ng CP, Lu L, Atlashkin V, Zhang W, Seet LF, Hong W. A role of VAMP8/endobrevin in regulated exocytosis of pancreatic acinar cells. Dev Cell 7: 359–371, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Wasle B, Edwardson JM. The regulation of exocytosis in the pancreatic acinar cell. Cell Signal 14: 191–197, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Weng N, Thomas DD, Groblewski GE. Pancreatic acinar cells express vesicle-associated membrane protein 2- and 8-specific populations of zymogen granules with distinct and overlapping roles in secretion. J Biol Chem 282: 9635–9645, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Williams JA Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annu Rev Physiol 63: 77–97, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Kelley WL, Chamberlain LH, Burgoyne RD, Wollheim CB, Lang J. Cysteine-string proteins regulate exocytosis of insulin independent from transmembrane ion fluxes. FEBS Lett 437: 267–272, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Zhang H, Kelley WL, Chamberlain LH, Burgoyne RD, Lang J. Mutational analysis of cysteine string protein in insulin exocytosis. J Cell Sci 112: 1345–1351, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Zhao X, Braun AP, Braun JE. Biological roles of neural J proteins. Cell Mol Life Sci 65: 2385–2396, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zinsmaier KE, Eberle KK, Buchner E, Walter N, Benzer S. Paralysis and early death in cysteine string protein mutants of Drosophila. Science 263: 977–980, 1994. [DOI] [PubMed] [Google Scholar]