Abstract
White adipose tissue is intimately involved in the regulation of immunity and inflammation. We reported that human mesenteric preadipocytes express the substance P (SP)-mediated neurokinin-1 receptor (NK-1R), which signals proinflammatory responses. Here we tested the hypothesis that SP promotes proliferation and survival of human mesenteric preadipocytes and investigated responsible mechanism(s). Preadipocytes were isolated from mesenteric fat biopsies during gastric bypass surgery. Proliferative and antiapoptotic responses were delineated in 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), bromodeoxyuridine (BrdU), caspase-3, and TUNEL assays, as well as Western immunoanalysis. SP (10−7 M) increased MTS and proliferation (BrdU) and time dependently (15–30 min) induced Akt, EGF receptor, IGF receptor, integrin αVβ3, phosphatidylinositol 3-kinase, and PKC-θ phosphorylation. Furthermore, pharmacological antagonism of Akt and PKC-θ activation significantly attenuated SP-induced preadipocyte proliferation. Exposure of preadipocytes to the proapoptotic Fas ligand (FasL, 100 μM) resulted in nuclear DNA fragmentation (TUNEL assay), as well as increased cleaved poly (ADP-ribose) polymerase, cleaved caspase-7, and caspase-3 expression. Cotreatment with SP almost completely abolished these responses in a NK-1R-dependent fashion. SP (10−7 M) also time dependently stimulated expression 4E binding protein 1 and phosphorylation of p70 S6 kinase, which increased protein translation efficiency. SP increases preadipocyte viability, reduces apoptosis, and stimulates proliferation, possibly via cell cycle upregulation and increased protein translation efficiency. SP-induced proliferative and antiapoptotic pathways in fat depots may contribute to development of the creeping fat and inflammation characteristic of Crohn's disease.
Keywords: neuropeptide, creeping fat, Crohn's disease, adipocyte, proliferation, protein kinase C-θ, Akt
the involvement of white adipose tissue (WAT) in the regulation of immune, inflammatory, and endocrine functions is now well established (43). Participation of WAT in these processes, together with epidemiological data, supports an important role for fat mass and body fat distribution in several chronic inflammatory conditions, including obesity, atherosclerosis, and inflammatory bowel disease (IBD) (20, 38, 43). However, despite evidence indicating the significance of adipocyte tissue enlargement in inflammatory states, the processes underlying changes in fat tissue size in relation to inflammation remain largely unknown (25).
Each adipose depot consists of a combination of preadipocytes, mature adipocytes, blood vessels, lymph nodes, macrophages, and nerves with the main cellular components being adipocytes (14, 30). The cellular changes associated with adipose tissue growth involve fat depot hypertrophy (increased cell size) and hyperplasia (increased cell number) (5). Increases in fat cell size lead to the production of paracrine factors that increase preadipocyte proliferation (25). Hyperplastic fat expansion, however, has more important consequences than hypertrophic expansion because hyperplastic adipose tissue is associated with the most severe forms of obesity and the poorest prognosis for treatment (6). Regarding the intestine, fat wrapping and hypertrophy of the bowel represent hallmarks of Crohn's disease (CD). Indeed, fat tissue hyperplasia is well correlated with the extent of transmural inflammation (1, 26, 31) and is used as a guide to identify inflamed bowel segments radiographically and during surgery.
Although not extensively studied, several factors appear to influence preadipocyte proliferation (46). Present evidence suggests that proliferation of cells within adipose tissue might be influenced both by circulating factors and neuronal inputs as well as by paracrine/autocrine factors secreted from the various cell types within adipose tissue (25). Neural inputs to fat cells, which vary from site to site, may contribute to regional variation in the metabolic and developmental characteristics of different fat depots (25). Adipose tissue has both sensory and sympathetic innervation. Sensory innervation was initially suggested by the identification of the neuropeptide substance P (SP) in fat depots (21, 22, 25) and has been suggested to play a role in trophic responses of brown adipose tissue (12, 13). Desensitization of sensory neurons induced by systemic administration of capsaicin results in reduced adiposity, reflected as decreased epididymal and retroperitoneal fat pad mass and cell number (11). Whether this trophic influence is attributable to direct effects of SP, a major constituent of capsaicin-sensitive sensory neurons, on adipocyte-related proliferative pathways has never been studied.
SP, an 11-amino-acid neuropeptide, is released from enteric nerves, sensory neurons, and inflammatory cells of the lamina propria during intestinal inflammation (34). Conversely, SP activates nerves, epithelial cells, and a variety of immune and inflammatory cells, leading to the release of cytokines, chemokines, and other neuropeptides that modulate diarrhea, inflammation, and motility changes associated with several intestinal disease states, including IBD (34). SP mediates the development and progression of intestinal inflammation by binding to its high-affinity receptor, the neurokinin 1 receptor (NK-1R) (9, 44). Our laboratory has shown that SP-NK-1R interactions play a dual role in the development and maintenance of colonic inflammation. Thus NK-1R-deficient mice are protected during the acute phase of colitis yet exhibit an enhanced inflammatory response during chronic colitis (8). SP was also recently shown to rescue colonic cells from apoptosis, preserving colonic tissue integrity in a murine DSS colitis model (36).
SP may mediate inflammatory changes in mesenteric fat depots during the course of colitis. We recently showed that mucosal inflammatory changes in the acute phase of experimental trinitrobenzidine sulfonic acid-induced colitis in mice are coupled with profound inflammatory changes in mesenteric fat. This is associated with increased expression of proinflammatory cytokines as well as the NK-1R (29). We also found that human mesenteric preadipocytes express a functional NK-1R that is linked to NF-κB activation and release of the proinflammatory chemokine, IL-8, upon SP exposure (29). Together, these results indicate that mesenteric depots may participate in intestinal inflammatory responses via SP-NK-1R-related pathways (29).
The potential relationship between SP and preadipocyte proliferation as it relates to fat tissue growth has not been reported. In this study, we tested the hypothesis that SP directly enhances proliferation of human mesenteric preadipocytes and investigated mechanisms underlying this response. Our results indicate that SP increases viability, reduces apoptosis, and stimulates proliferation of human mesenteric preadipocytes, possibly through cell cycle upregulation and increases in the efficiency of protein translation.
MATERIALS AND METHODS
Isolation of preadipocytes from human subjects and treatments.
Fat tissue was resected during gastric bypass surgery for the management of obesity from subjects who had given informed consent. The study was approved by the Internal Review Board at Boston University. Mesenteric preadipocytes were isolated as previously described by our laboratory (29). Cells were subcultured five to six times to ensure removal of macrophages. In mesenteric preadipocytes cultured this way, the macrophage marker, F4/80, was essentially undetectable by real-time PCR (50).
Cell treatments.
Cells were grown in α-MEM plus 10% FBS until 95% confluence. Cells were washed with sterile PBS before SP treatment, and then the appropriate amount of SP was added for the required time (see results). In the experiments where the NK-1R antagonist, CJ 012,255 (Pfizer, New York, NY), was used, cells were pretreated with the antagonist 40 min before adding SP. Compound CJ 012,255 is an analog of the parent compound CJ-11974 (Ezlopitant) shown to be highly specific for inhibiting the binding of [3H]SP to the human NK-1R, with little to no affinity for the NK-2 or NK-3 receptors (29). Cells were also pretreated with Akt inhibitor V (40 μM) (Calbiochem, San Diego, CA), control IgG, or anti-PKC-θ (Upstate, Albany, NY) 30–40 min before SP or trifluoracetic acid (control vehicle) exposure.
Western immunoblotting.
For immunoblots, proteins (20–30 μg) were separated by electrophoresis in a 12.5% polyacrylamide gel. Protein samples were mixed with DTT (3×; Cell Signaling Technology, Danvers, MA) and denatured by boiling. Samples were electrophoresed at 100–150 V for 1.5 h. The separating gel was equilibrated in transfer buffer (20 mM Tris·HCl, 150 mM glycine, 20% methanol, 0.1% SDS) for 10 min. The proteins were then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA) at 4°C. All membrane incubations were carried out at room temperature with rocking. The membranes were blocked for 1 h at room temperature in blocking buffer [Tris-buffered saline, 5% nonfat dry milk (Bio-Rad, Hercules, CA), 0.1% Tween 20] and then incubated with primary antibodies against cleaved caspase-7, cleaved poly (ADP-ribose) polymerase (PARP), phosphor-Akt (Ser 473), p70 S6 kinase, 4E binding protein 1 (4E-BP1), PKC-θ, phosphatidylinositol 3 (PI3)-kinase, EGF receptor (EGFR), IGF (Cell Signaling Technology), or integrin αVβ3 (Y759) (Santa Cruz Biotechnology, Santa Cruz, CA) in blocking buffer overnight at 4°C. Horseradish peroxidase-conjugated secondary antibodies in blocking buffer were used (Santa Cruz Biotechnology). Protein bands were visualized using SuperSignal West Pico chemiluminescent substrate (Pierce, Madison, WI). The membranes were exposed to X-ray films from 5 s to 10 min. In some experiments, Western blot bands were quantified by densitometry using Scion image analysis software with normalization of the phosphorylated protein to the corresponding band of control signal (GAPDH) from the same samples.
Cell viability (MTS) assay.
Overnight serum-starved human mesenteric preadipocytes were seeded on 96-well plates and pretreated with CJ 012,255 with or without SP (10−7 M). After 2, 4, 8, and 24 h, 20 μl of CellTiter Aqueous One Solution [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) tetrazolium compound; Promega, Madison, WI] was added to each well and incubated at 37°C for 1 h. Absorbance at 490 nm (indicating cell viability) was measured using a 96-well plate reader.
Bromodeoxyuridine assay.
The bromodeoxyuridine (BrdU) assay incorporates BrdU into newly synthesized DNA strands of actively proliferating cells. After incorporation of BrdU, DNA is partially denatured and BrdU is detected immunochemically. Human mesenteric preadipocytes were cultured as described above to 70–80% confluence. Cells were first pretreated (40 min at 37°C) with either the Akt inhibitor V (20 μM), the PKC-θ pseudosubstrate inhibitor Myr-LHQRRGAIKQAKVHHVKC-NH2 (10 μM), or CJ 012,255 (10−6 M), and then exposed to SP (10−7) for 8 h. BrdU assay was conducted according to manufacturer's instructions (Cell Proliferation Elisa, BrdU; Roche Diagnostics, Indianapolis, IN). Briefly, 10 μl/ml BrdU was added to all groups and allowed to incubate at 37°C for 4, 8, 12, or 24 h. At the end of the incubations, medium was removed and the cells were fixed for 30 min at room temperature. Anti-BrdU antibody was then added for 90 min, and cells were washed with washing solution. Substrate solution was added for 30 min, and then H2SO4 was added to complete the reaction. After 5 min, absorbance of the cells was read at 690 nm.
In situ detection of apoptosis in cultured preadipocytes.
Human mesenteric preadipocytes were seeded on chamber slides (no. 3544104; BD Falcon, Bedford, MA) until 50% confluence, pretreated with CJ 012,255 (10−6) for 40 min, and then exposed to Fas ligand (FasL) (1 μM) in the presence or absence of SP (10−7) for 6 h at 37°C. Cells were then fixed in 4% paraformaldehyde for 25 min and then permeabilized with a 0.2% Triton X-100 solution in PBS for 5 min. Slides were processed for TUNEL assay (Promega) as described below.
In situ detection of apoptosis by colorimetric TUNEL assay.
Deparaffinized slides were fixed in 4% formaldehyde and treated with proteinase K to permeabilize the tissues. The slides were incubated with labeling reaction mix for 1 h at 37°C according to the manufacturer's instructions (DeadEnd Calorimetric TUNEL System no. G7130, Promega). The reaction was stopped by 2× SSC. Slides were incubated with streptavidin horseradish peroxidase (1:500 dilution in PBS) followed by apoptosis localization with enhanced diaminobenzidine solution included in the TUNEL assay kit. The slides were then observed for apoptosis (brown nuclei) by light microscopy.
Caspase-3 assay.
The EnzChek caspase-3 assay kit (Molecular Probes, Eugene, OR) assesses increases in caspase-3 by evaluating the amount of a 7-amino-4-methylcoumarin-derived substrate Z-DEVD-AMC, which yields a bright blue fluorescent product upon proteolytic cleavage. After treatment with FasL for the induction of apoptosis, human mesenteric preadipocytes were harvested and washed with PBS. Cells were then lysed with lysis buffer working solution by subjecting cells to a freeze-thaw cycle. They were centrifuged for 5 min to pellet the cellular debris. A portion (50 μl) of supernatant from each sample was transferred to individual microplate wells, and 50 μl of substrate working solution was added to each sample and control. Finally, cells were incubated for 30 min, and fluorescence was measured (excitation/emission 342/441).
TNF nuclear index assay.
Confluent human mesenteric preadipocytes plated on 96-well plates were pretreated with TNF-α (10 or 50 nM) 4 h prior and with SP 40 min prior to treatments. Following pretreatments, all cells were incubated for 24 h at 37°C with 0.8 mM oxalacetic acid. Cells were washed twice with PBS and permeabilized using 100 μl 0.1% Saponin (no. S-7900; Sigma, St. Louis, MO;) in PBS for 5–10 min. Cells were then washed twice in PBS, and 100 μl Hoechst solution (bisbenzimide 33258; Sigma no. B-2883) (2.5 μg/ml) was added for 5 min in the dark. Cells were washed a final time with PBS, and 10 fields were photographed by fluorescence microscopy. Observers who were not aware of the treatments the cells had received counted apoptotic nuclei in the photographs. Cells were counted as number of apoptotic cells per 100 cells. Apoptotic cell numbers for all 10 slides in each group were averaged.
Statistical analysis.
Results were analyzed using the Prism professional statistics software program (GraphPad Software, San Diego, CA). ANOVA and t-tests (for comparisons between two groups) were used for intergroup comparisons. P < 0.05 was considered significant.
RESULTS
SP increases viability of human mesenteric preadipocytes.
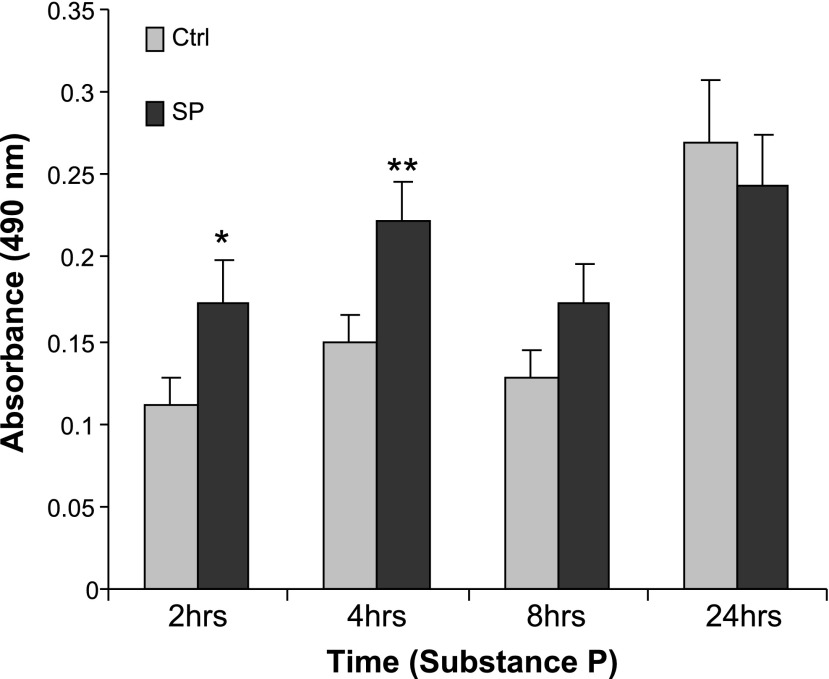
Previous results indicated that SP increases viability in human colonocytes stably transfected with NK-1R (36). To examine whether SP increases preadipocyte numbers, human mesenteric preadipocytes were exposed to SP (10−8 M) for 2, 4, and 8 h, and cell viability was tested by MTS assay. Compared with vehicle-exposed monolayers, SP significantly increased cell viability, indicated by an absorbance reading at 490 nm, after 2 and 4 h of exposure, with the most significant difference seen at 4 h (Fig. 1, P < 0.05 2 h, P < 0.01 4 h, n = 6).
Fig. 1.
Substance P (SP) increases human mesenteric preadipocyte viability. Serum-starved human mesenteric preadipocytes were exposed to SP (10−8 M) or trifluoracetic acid (TFA) (vehicle) for up to 24 h. During the last hour of exposure, 20 μl of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) tetrazolium compound solution was added to the media (100 μl per well), and color changes were recorded by absorbance at 490 nm. Data are expressed as means ± SE and are representative of 4 independent samples. *P < 0.05; **P < 0.01 vs. control.
SP increases preadipocyte proliferation by Akt- and PKC-θ-dependent mechanisms.
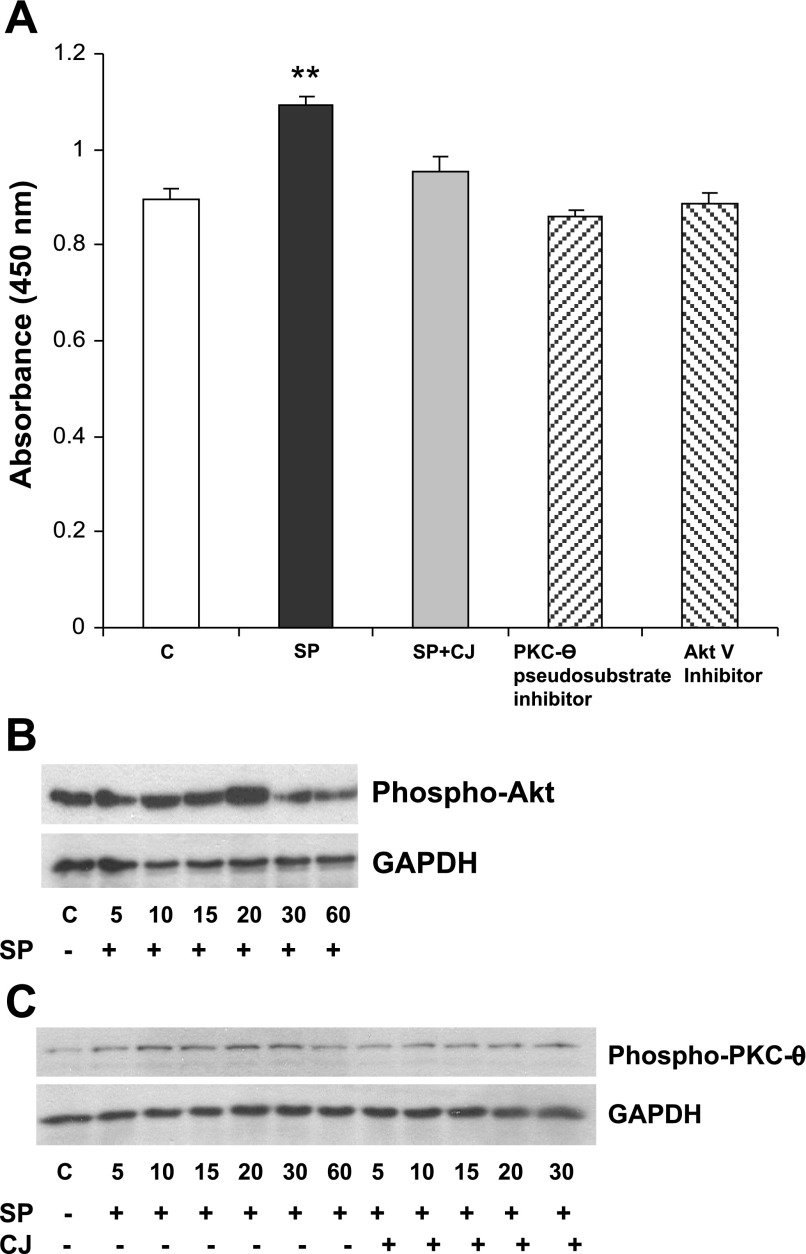
The MTS assay shown in Fig. 1 does not delineate between proliferative and antiapoptotic mechanisms. Therefore, we examined whether SP stimulates proliferation of human preadipocytes using the BrdU assay and studied the mechanism of this response. Time-course analyses have shown the highest effect of SP on human mesenteric preadipocyte proliferation to be at 8 h of treatment (data not shown). Treatment of preadipocytes for 8 h with SP (10−7 M) increased proliferation (P < 0.01, n = 6), and this affect was completely blocked by preincubating preadipocytes with CJ 012,255 for 40 min before SP administration (Fig. 2A). This suggests that SP increases human preadipocyte proliferation by activating the NK-1R. Previous studies indicated that the PKC protein family and the antiapoptotic molecule Akt are major regulators of proliferative responses in other cell types (7, 15). Moreover, SP activates both PKC (33) and Akt in other cell types, whereas colitis mouse models that had PKC-θ knocked out provided the strongest evidence for its importance in such disease states (2). To test whether PKC and Akt are involved in SP-induced cell proliferation in human preadipocytes, preadipocyte monolayers were preincubated with either a specific PKC-θ pseudosubstrate inhibitor or Akt inhibitor V, followed by SP (10−7 M) exposure. As shown in Fig. 2A, SP-induced cell proliferation was fully inhibited by both inhibitors, suggesting that PKC-θ and Akt are involved in this SP-induced response.
Fig. 2.
SP increases preadipocyte proliferation via Akt and PKC-θ-dependent mechanisms. A: serum-starved human mesenteric preadipocytes were exposed to TFA (vehicle) or a specific PKC-θ inhibitor, Akt inhibitor V, or the neurokinin-1 receptor (NK-1R) antagonist CJ 012,255 (CJ) with SP (10−7 M) for 8 h. Cells were then incubated with bromodeoxyuridine, and color changes were recorded at an absorbance of 450 nm. Data are expressed as means ± SE. **P < 0.01 vs. all other groups. C, control. B and C: SP induces Akt and PKC-θ phosphorylation in human mesenteric preadipocytes. Serum-starved human mesenteric preadipocytes were exposed to SP (10−7 M) or SP + CJ 012,255 for the indicated times. Cells were lysed, and equal amounts of protein were fractionated by SDS/12.5% PAGE to determine the levels of phospho-Akt (B), phospho-PKC-θ (C), and GAPDH (B and C).
To confirm that SP activates PKC-θ and Akt in human mesenteric preadipocytes, we assayed the phosphorylated forms of these proteins. SP (10−7 M) induced Akt phosphorylation starting at 15 min and beginning to decrease by 30 min (Fig. 2B, P < 0.05 at 15 and 30 min, n = 3). SP also induced phosphorylation of PKC-θ that peaked at 20 min and began to decrease by 60 min. Semiquantification of the bands at 20 min of exposure indicated a statistically significant increase, (P < 0.05; data not shown). Moreover, CJ 012,255 attenuated SP-induced PKC-θ phosphorylation (Fig. 2C), suggesting the NK-1R dependency of this response.
SP phosphorylates integrin αVβ3 in human mesenteric preadipocytes.
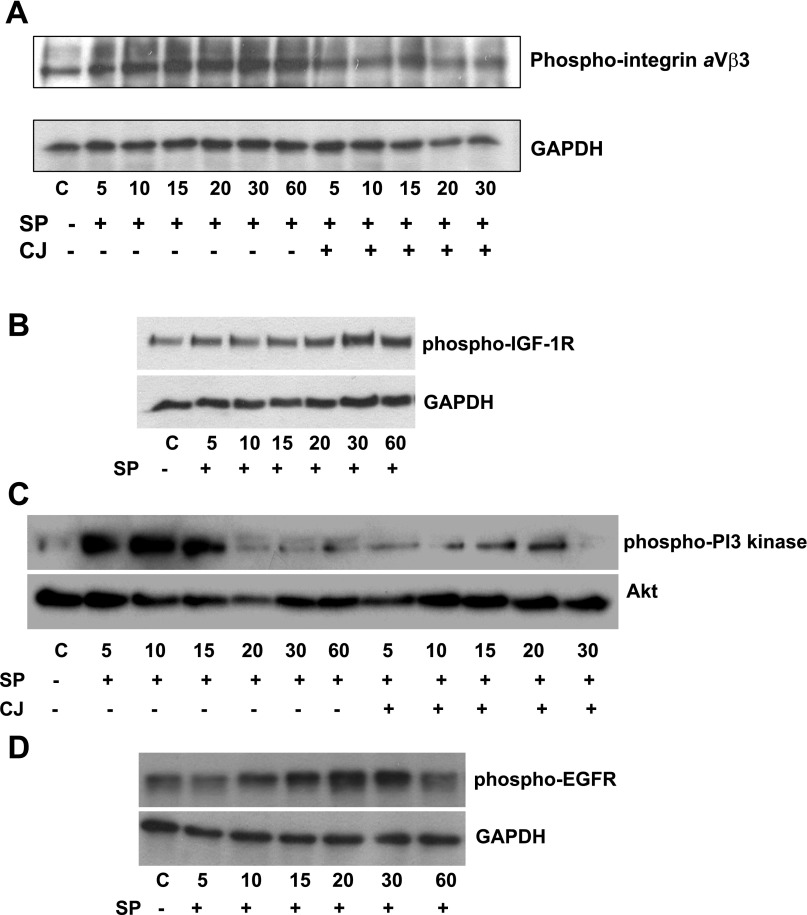
Integrins, a family of receptors that bind to extracellular matrix proteins (27), have been associated with the Akt pathway in various cell types (10), including adipocytes (24). Although SP signals through integrin αVβ3 phosphorylation in colonocytes (36), a similar role in SP-mediated signaling in preadipocytes has not been examined to our knowledge. Our results demonstrate that SP induces integrin αVβ3 phosphorylation in a time-dependent manner. This was evident at 15 min and achieved a peak at 30 min (Fig. 3A, P < 0.01 after 15 min, n = 4). Blocking NK-1R with CJ 012,255 diminished SP-induced integrin αVβ3 activation (Fig. 3A).
Fig. 3.
SP activates cell surface receptors as well as downstream regulators involved in the promotion of preadipocyte proliferation. A: SP increases integrin αV-β3 phosphorylation. Serum-starved human mesenteric preadipocytes were exposed to SP (10−7 M) or SP + CJ 012,255 (10−6) for the indicated times. Cells were lysed, and equal amounts of protein were fractionated by SDS/12.5% PAGE to determine the levels of phospho-integrin αVβ3 (A), IGF receptor 1 (IGF-1R) (B), phosphatidylinositol 3 (PI3)-kinase (C), and EGF receptor (EGFR) (D).
SP activates IGF receptor, EGFR, and PI3-kinase in human mesenteric preadipocytes.
Both IGF receptor (IGFR) and EGFR have been associated with proliferative responses in preadipocytes (32, 52). Recent results also indicate that SP-NK-1R interactions transactivate the EGFR (35). However, to our knowledge there are no studies indicating interaction between SP and the IGFR in any cell type. We exposed human mesenteric preadipocytes to SP (10−7 M) and assayed EGFR and IGFR phosphorylation using specific antibodies. SP induced EGFR phosphorylation within 15 min, peaking at 15 min and decreasing by 60 min (Fig. 3D). Interestingly, SP (10−7 M) induced IGFR phosphorylation that peaked at 30 min and remained evident after 60 min (Fig. 3B, P < 0.01 after 15 min, n = 3). Use of an antibody against the nonphosphorylated forms of these receptors showed that their levels did not change after SP treatment (data not shown, P ≥ 0.05). IGF-1R is known to elicit antiapoptotic responses involving the activation of PI3-kinase. Consequently, we also assayed PI3-kinase phosphorylation using a specific antibody. SP (10−7 M) induced PI3-kinase phosphorylation [the p55 (Tyr199), P < 0.001] that was evident at 5 min after treatment (Fig. 3C).
SP rescues human mesenteric preadipocytes from FasL-induced apoptosis.
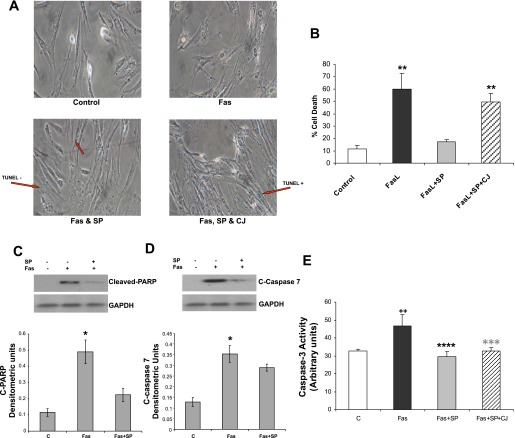
After we established the effects of SP on human mesenteric preadipocyte proliferation, we sought to determine whether SP also protects these cells from apoptosis. We exposed human mesenteric preadipocytes to FasL (100 μM), a potent inducer of apoptosis in adipocytes (51), for 0, 6, 12, and 24 h in the presence or absence of SP and assayed DNA fragmentation by TUNEL assay. Nontreated cells were also used as controls. As expected, FasL increased the number of TUNEL-positive cells at 6 h, compared with untreated controls (11.5% control vs. 60.1% FasL vs. 17.4% FasL + SP vs. 49.6% FasL + SP + CJ 012,255, P < 0.001; Fig. 4B), whereas SP treatment reversed FasL-induced effects on apoptosis (Fig. 4A). As shown in the same figure, the effects of SP on FasL-induced apoptosis were abolished in the presence of the NK-1R antagonist, CJ 012,255 (Fig. 4A). Addition of CJ 012,255 alone had no significant apoptotic effect, whereas addition of CJ 012,255 in Fas-exposed cells did not alter Fas-induced apoptosis (data not shown).
Fig. 4.
SP has an antiapoptotic effect in human mesenteric preadipocytes. A: serum-starved human mesenteric preadipocytes were pretreated with CJ 012,255 or DMSO (vehicle) for 30 min and then exposed to Fas ligand (FasL) (10−4 M) or TFA (vehicle) for 6 h in the absence or presence of SP (10−7 M) on chamber slides. Cell apoptosis (presented as condensed blue nuclei) was then measured by TUNEL assay. Arrows indicate either TUNEL-positive or TUNEL-negative cells. B: quantification of these data showed a significant antiapoptotic effect of SP. C and D: SP may contribute to reduced Fas ligand (FasL)-induced apoptosis via downregulation of cleaved caspase-7 and cleaved poly (ADP-ribose) polymerase (C-PARP). Serum-starved human mesenteric preadipocytes were exposed to FasL (10−4 M) for 6 h, with or without SP (10−7 M). Cells were lysed, and equal amounts of protein were fractionated by SDS/12.5% PAGE to determine the levels of cleaved PARP (C) and cleaved caspase-7 (D). E: SP increases caspase-3 activity in human mesenteric preadipocytes. Serum-starved human mesenteric preadipocytes were pretreated with the NK-1R antagonist CJ 012,255 for 30 min and then exposed to FasL (10−4 M) for 6 h, with or without SP (10−7 M). Cells were lysed, and 50 μl of substrate working solution was added to all samples. Fluorescence of cells was then measured (excitation/emission 342/441). *P < 0.05; **P < 0.01; ****P < 0.001; ***P < 0.01; all relative to FasL alone. ++P < 0.01 relative to control.
To investigate the antiapoptotic effects of SP further, we exposed human mesenteric preadipocytes to FasL for 6 h in the presence or absence of SP and determined expression of cleaved PARP and cleaved caspase-7 by Western blot analysis and caspase-3 activity by a fluorescence assay. As expected, FasL increased expression of cleaved PARP (Fig. 4C, P < 0.01, n = 3) and cleaved caspase-7 (Fig. 4D, P < 0.01, n = 3). This effect was almost completely abolished by coincubation of FasL with SP (P < 0.05 for both PARP and caspase-7). We also found that FasL increased caspase-3 expression, which was completely abolished by the presence of SP in the incubation medium (Fig. 4E, P < 0.01, n = 6). These results further support the notion that SP has an important antiapoptotic effect in human mesenteric preadipocytes.
SP rescues human mesenteric preadipocytes from TNF-α-mediated apoptosis.
Because TNF-α is known to cause apoptosis in preadipocytes (45), we sought to determine whether SP protects human mesenteric preadipocytes from TNF-α-induced apoptosis using the TNF-α nuclear index assay. A small percent (10.8%) of medium-exposed preadipocytes (control) were apoptotic, and this number was increased upon TNF-α exposure (18.8%, P < 0.05). In contrast, coadministration of SP (10−7 M) with TNF-α decreased the number of apoptotic cells to control levels (12.7%, P < 0.05 vs. TNF-α alone).
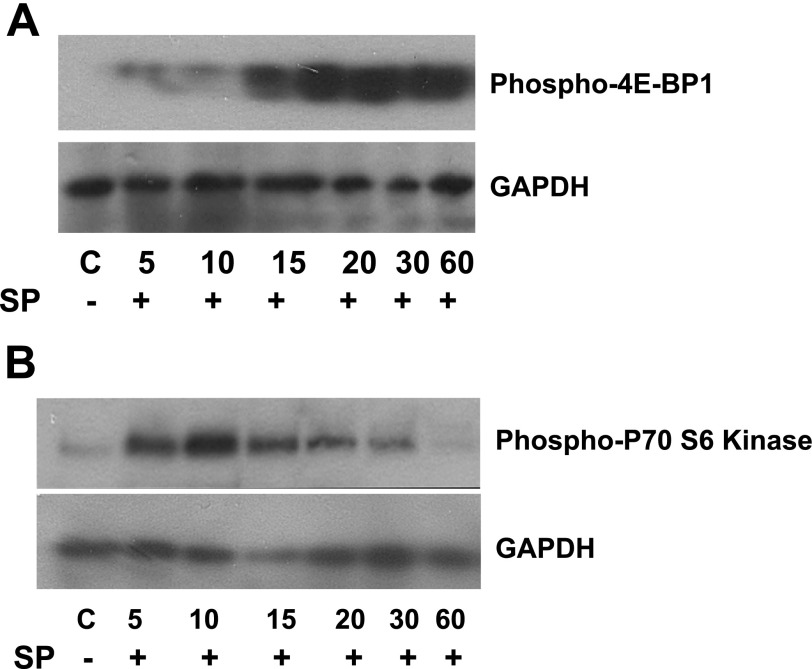
SP promotes cell cycle entry through promotion of protein synthesis.
Progression through the cell cycle requires increased protein synthesis. Human eukaryotic translation initiation factor 4E (EIF4E) is an important modulator of cell growth and proliferation. Unphosphorylated 4E-BP binds to EIF4E and inhibits adipocyte proliferation and initiation of translation (41). Treatment of human mesenteric preadipocytes with SP leads to 4E-BP1 phosphorylation 20 to 30 min posttreatment (Fig. 5A, P < 0.05 after 20 min and P < 0.01 at 60 min, n = 3), potentially increasing the amount of free EIF4E available for the promotion of protein translation. Phosphorylated-p70 S6 kinase increases the translational efficiency of adipocytes entering the cell cycle via phosphorylation of the S6 40S ribosomal subunit (47). We found that SP treatment increased p70 S6 kinase phosphorylation in human mesenteric preadipocytes, with a peak at 10 min (Fig. 5B, P < 0.05 at 10 and 15 min, n = 4).
Fig. 5.
SP increases the expression of molecules that are involved in cell cycle progression. SP increases the efficiency of protein translation. Serum-starved human mesenteric preadipocytes were exposed to SP (10−7 M) or SP + CJ 012,255 for the indicated times. Cells were lysed, and equal amounts of protein were fractionated by SDS/12.5% PAGE to determine levels of phospho-4E binding protein 1 (4E-BP1) (A), phospho-p70 S6 kinase (B), and GAPDH.
DISCUSSION
We recently reported that human mesenteric preadipocytes express NK-1R and that binding of SP to this receptor signals proinflammatory, NF-κB-dependent gene expression (29). Here we show that SP increases viability, reduces apoptosis, and stimulates proliferation of human mesenteric preadipocytes. Our results also indicate that SP-induced cell proliferation might be accomplished through cell cycle promotion and an increase in the efficiency of protein translation. Together, these results identify SP as a potential regulator of mesenteric fat depot hyperplasia.
SP treatment of human mesenteric preadipocytes led to PKC-θ activation. Inhibition of this signaling molecule resulted in reduced proliferation in response to SP in an NK-1R-dependent fashion (Fig. 2A). Previous results indicate that SP activates NF-κB via PKC-θ activation (33), whereas studies with PKC-θ-deficient mice indicate that PKC-θ is important in the development of experimental colitis (2). Although the role of PKC-θ in adipocyte proliferation has not been documented, a relationship between PKC-θ and AKT/PKB isotypes in proliferation and survival of other cell types is clearly established (3). Additionally, inhibition of PKC activity inhibits G1/S progression and reduces cyclin E protein levels (23). Our results demonstrate that SP activates p70 S6 kinase and 4E-BP1 (Fig. 5, A and B), indicating increased translational efficiency, a process that is required for cell cycle progression. Interestingly both p70 S6 kinase and 4E-BP1 have been implicated in insulin signaling and adipogenesis in mouse 3T3-L1 preadipocytes (17).
Akt is a transcription factor involved in controlling the balance between cell survival and apoptosis (18, 19). We have recently shown that SP activates Akt and rescues colonic epithelial cells from FasL-induced apoptosis (36). Consistent with these observations, we show here that stimulation of human mesenteric preadipocytes with SP resulted in Akt activation (Fig. 2A), whereas Akt inhibition significantly decreased the effects of SP on human mesenteric preadipocyte proliferation (Fig. 2A).
We have previously reported that binding of SP to NK-1R in colonic epithelial cells transactivates the EGFR, via release of metalloproteinases (35). EGF also stimulates replication of primary human preadipocytes (32), potentially via inhibition of adipocyte differentiation (4). Our results show that EGFR is phosphorylated by SP in mesenteric WAT (Fig. 3C), implicating it as a potential regulator of mesenteric hypertrophy.
The importance of IGF-1R signaling in adipocyte proliferation has been well documented (28, 40, 52). We found that SP exposure of human preadipocytes leads to IGF-1R activation (Fig. 3B). SP and IGF-1 act synergistically to facilitate corneal epithelial wound healing in vitro and in vivo, potentially by inducing DNA synthesis, cellular proliferation, and entry into the cell cycle (37). Of note, IGF-1R is remarkably efficacious in protecting cells from apoptosis via the intrinsic cell death pathway, which involves activation of PI3-kinase and Akt/protein kinase B (42). In concert with this, we show here that SP activates PI3-kinase, consistent with our prior observation in human colonocytes (36). This is the first account, to our knowledge, for a SP-IGF-1R communication that might be involved in human mesenteric preadipocyte proliferation. Along these lines we also show that SP treatment phosphorylates integrin αVβ3 (Fig. 3A), a cell surface receptor that signals cell growth, division, survival, differentiation, apoptosis, and wound repair (10). Koon et al. (36) also reported that integrin αVβ3 activation mediates SP-associated proliferative and antiapoptotic responses in NCM460 colonocytes.
Adipocyte apoptosis has been shown to involve the FasL-mediated death receptor pathway (43). Although the effects of SP on apoptosis in intestinal inflammation at the colonic epithelial cell level have been previously reported (39), we are not aware of studies evaluating the potential SP-induced effects on adipocyte apoptosis. We demonstrate that SP rescues FasL-induced preadipocyte apoptosis, potentially via PARP and caspase-7 cleavage (Fig. 4, A–C) and by reducing caspase-3 activity (Fig. 4D). Our observation that SP diminishes TNF-α-induced apoptosis in preadipocytes might be of clinical importance. TNF-α is a major proapoptotic stimulus in adipocytes (45), and its expression is increased in mesenteric fat of patients with Crohn's disease (16). Thus diminished TNF-α-induced apoptosis in preadipocytes in response to SP might be involved in the pathophysiology of mesenteric hypertrophy seen in Crohn's disease.
Although the changes in SP-induced viability and survival in our study were over 50%, the difference in proliferation in response to SP was relatively small (∼20–30%) although statistically significant. However, the cumulative effect of changes in both apoptosis and proliferation would be anticipated to translate into large changes in a chronic inflammatory condition such as Crohn's disease over the long term.
It is known that NK-1R is the main receptor through which SP exerts its biological actions. However, numerous studies have demonstrated that SP can also signal through interactions with the other two neurokinin receptors, namely NK-2R and NK-3R (48, 49). Such interactions may also explain the inability of CJ 012,255 to inhibit SP action in some of our experiments (e.g., inhibition of caspase and PARP activation, Akt activation).
As increased adipose tissue increases the risk for many chronic inflammatory conditions, it is important to better understand the mechanisms involved in increases in fat mass. The results of this study, in conjunction with the apparent proinflammatory effects of SP in experimental colitis in mesenteric WAT (29), prompt the speculation that SP-induced proliferative and antiapoptotic pathways in fat depots may contribute to the creeping fat phenotype and the inflammation characteristic of CD.
Importantly, as the results of this study point out, SP antagonists, or antagonists of the signaling pathways activated by this peptide may also prove beneficial at the adipocyte level. As such, modulators of mesenteric adipose tissue function might be of potential therapeutic benefit, especially for diseases with an inflammatory component. It should be noted that, to date, there is no evidence for expression of SP in human fat depots. However, our results indicating a high level of expression of functional SP, NK-1R in human preadipocytes in this and our previous study (29) indicate the likely presence of SP in human fat depots. Additionally, our findings suggest that SP may be a hitherto unappreciated means through which the nervous system regulates fat mass, inflammation, and possibly insulin responsiveness. However, additional studies are required to elucidate the importance of SP in inflammatory bowel disease-associated increases of visceral fat mass in vivo. This is challenging at the moment because of the central role of SP signaling in the generation of intestinal inflammation in the animal models tested to date. More extensive investigation in the potential mechanisms of such actions is also required for the identification of molecules that may participate in these processes along with SP.
GRANTS
This work was supported by National Institutes of Health Grants R01-DK47343 (to C. Pothoulakis), F32-DK076412 (to K. Gross), and R01–23960 (to J. Kirkland) and by a Research Fellowship from the Crohn's and Colitis Foundation of America (to I. Karagiannides).
REFERENCES
- 1.Aaronson SA Growth factors and cancer. Science 254: 1146–1153, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Anderson K, Fitzgerald M, Dupont M, Wang T, Paz N, Dorsch M, Healy A, Xu Y, Ocain T, Schopf L, Jaffee B, Picarella D. Mice deficient in PKC theta demonstrate impaired in vivo T cell activation and protection from T cell-mediated inflammatory diseases. Autoimmunity 39: 469–478, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bauer B, Baier G. Protein kinase C and AKT/protein kinase B in CD4+ T-lymphocytes: new partners in TCR/CD28 signal integration. Mol Immunol 38: 1087–1099, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya I, Ullrich A. Endothelin-1 inhibits adipogenesis: role of phosphorylation of Akt and ERK1/2. FEBS Lett 580: 5765–5771, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bjorntorp P Size, number and function of adipose tissue cells in human obesity. Horm Metab Res Suppl 4: 77–83, 1974. [PubMed] [Google Scholar]
- 6.Bjorntorp P, Karlsson M, Pettersson P. Expansion of adipose tissue storage capacity at different ages in rats. Metabolism 31: 366–373, 1982. [DOI] [PubMed] [Google Scholar]
- 7.Brazil DP, Park J, Hemmings BA. PKB binding proteins: getting in on the Akt. Cell 111: 293–303, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Castagliuolo I, Morteau O, Keates AC, Valenick L, Wang CC, Zacks J, Lu B, Gerard NP, Pothoulakis C. Protective effects of neurokinin-1 receptor during colitis in mice: role of the epidermal growth factor receptor. Br J Pharmacol 136: 271–279, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castagliuolo I, Riegler M, Pasha A, Nikulasson S, Lu B, Gerard C, Gerard NP, Pothoulakis C. Neurokinin-1 (NK-1) receptor is required in Clostridium difficile-induced enteritis. J Clin Invest 101: 1547–1550, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemmons DR, Maile LA. Minireview: Integral membrane proteins that function coordinately with the insulin-like growth factor I receptor to regulate intracellular signaling. Endocrinology 144: 1664–1670, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Cui J, Himms-Hagen J. Long-term decrease in body fat and in brown adipose tissue in capsaicin-desensitized rats. Am J Physiol Regul Integr Comp Physiol 262: R568–R573, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Cui J, Zaror-Behrens G, Himms-Hagen J. Capsaicin desensitization induces atrophy of brown adipose tissue in rats. Am J Physiol Regul Integr Comp Physiol 259: R324–R332, 1990. [DOI] [PubMed] [Google Scholar]
- 13.De Matteis R, Ricquier D, Cinti S. TH-, NPY-, SP-, and CGRP-immunoreactive nerves in interscapular brown adipose tissue of adult rats acclimated at different temperatures: an immunohistochemical study. J Neurocytol 27: 877–886, 1998. [DOI] [PubMed] [Google Scholar]
- 14.DeMartinis FD, Francendese A. Very small fat cell populations: mammalian occurrence and effect of age. J Lipid Res 23: 1107–1120, 1982. [PubMed] [Google Scholar]
- 15.Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol 279: L429–L438, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Desreumaux P, Ernst O, Geboes K, Gambiez L, Berrebi D, Muller-Alouf H, Hafraoui S, Emilie D, Ectors N, Peuchmaur M, Cortot A, Capron M, Auwerx J, Colombel JF. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology 117: 73–81, 1999. [DOI] [PubMed] [Google Scholar]
- 17.El-Chaar D, Gagnon A, Sorisky A. Inhibition of insulin signaling and adipogenesis by rapamycin: effect on phosphorylation of p70 S6 kinase vs eIF4E-BP1. Int J Obes Relat Metab Disord 28: 191–198, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275: 665–668, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81: 727–736, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Gautier JF, Mourier A, de Kerviler E, Tarentola A, Bigard AX, Villette JM, Guezennec CY, Cathelineau G. Evaluation of abdominal fat distribution in noninsulin-dependent diabetes mellitus: relationship to insulin resistance. J Clin Endocrinol Metab 83: 1306–1311, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Giordano A, Morroni M, Carle F, Gesuita R, Marchesi GF, Cinti S. Sensory nerves affect the recruitment and differentiation of rat periovarian brown adipocytes during cold acclimation. J Cell Sci 111: 2587–2594, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Giordano A, Morroni M, Santone G, Marchesi GF, Cinti S. Tyrosine hydroxylase, neuropeptide Y, substance P, calcitonin gene-related peptide and vasoactive intestinal peptide in nerves of rat periovarian adipose tissue: an immunohistochemical and ultrastructural investigation. J Neurocytol 25: 125–136, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Graham MA, Rawe I, Dartt DA, Joyce NC. Protein kinase C regulation of corneal endothelial cell proliferation and cell cycle. Invest Ophthalmol Vis Sci 41: 4124–4132, 2000. [PubMed] [Google Scholar]
- 24.Guilherme A, Torres K, Czech MP. Cross-talk between insulin receptor and integrin alpha5 beta1 signaling pathways. J Biol Chem 273: 22899–22903, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev 2: 239–254, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Herlinger H, Furth EE, Rubesin SE. Fibrofatty proliferation of the mesentery in Crohn disease. Abdom Imaging 23: 446–448, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Horton MA The alpha v beta 3 integrin “vitronectin receptor”. Int J Biochem Cell Biol 29: 721–725, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Kim SO, Yang N, Jiang J, Frank SJ. Physical and functional interaction of growth hormone and insulin-like growth factor-I signaling elements. Mol Endocrinol 18: 1471–1485, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Karagiannides I, Kokkotou E, Tansky M, Tchkonia T, Giorgadze N, O′Brien M, Leeman SE, Kirkland JL, Pothoulakis C. Induction of colitis causes inflammatory responses in fat depots: evidence for substance P pathways in human mesenteric preadipocytes. Proc Natl Acad Sci USA 103: 5207–5212, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirkland JL, Hollenberg CH, Kindler S, Gillon WS. Effects of age and anatomic site on preadipocyte number in rat fat depots. J Gerontol 49: B31–B35, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Knutson H, Lunderquist A. Vascular changes in Crohn's disease. Am J Roentgenol Radium Ther Nucl Med 103: 380–385, 1968. [DOI] [PubMed] [Google Scholar]
- 32.Koellensperger E, von Heimburg D, Markowicz M, Pallua N. Human serum from platelet-poor plasma for the culture of primary human preadipocytes. Stem Cells 24: 1218–1225, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Koon HW, Zhao D, Zhan Y, Simeonidis S, Moyer MP, Pothoulakis C. Substance P-stimulated interleukin-8 expression in human colonic epithelial cells involves protein kinase Cdelta activation. J Pharmacol Exp Ther 314: 1393–1400, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Koon HW, Pothoulakis C. Immunomodulatory properties of substance P: the gastrointestinal system as a model. Ann NY Acad Sci 1088: 23–40, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Koon HW, Zhao D, Na X, Moyer MP, Pothoulakis C. Metalloproteinases and transforming growth factor-alpha mediate substance P-induced mitogen-activated protein kinase activation and proliferation in human colonocytes. J Biol Chem 279: 45519–45527, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Koon HW, Zhao D, Zhan Y, Moyer MP, Pothoulakis C. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc Natl Acad Sci USA 104: 2013–2018, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorenzo M, Valverde AM, Teruel T, Benito M. IGF-I is a mitogen involved in differentiation-related gene expression in fetal rat brown adipocytes. J Cell Biol 123: 1567–1575, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montague CT, O'Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes 49: 883–888, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Mudter J, Neurath MF. Apoptosis of T cells and the control of inflammatory bowel disease: therapeutic implications. Gut 56: 293–303, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nougues J, Reyne Y, Barenton B, Chery T, Garandel V, Soriano J. Differentiation of adipocyte precursors in a serum-free medium is influenced by glucocorticoids and endogenously produced insulin-like growth factor-I. Int J Obes Relat Metab Disord 17: 159–167, 1993. [PubMed] [Google Scholar]
- 41.Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature 371: 762–767, 1994. [DOI] [PubMed] [Google Scholar]
- 42.Peruzzi F, Prisco M, Dews M, Salomoni P, Grassilli E, Romano G, Calabretta B, Baserga R. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol Cell Biol 19: 7203–7215, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peyrin-Biroulet L, Chamaillard M, Gonzalez F, Beclin E, Decourcelle C, Antunes L, Gay J, Neut C, Colombel JF, Desreumaux P. Mesenteric fat in Crohn's disease: a pathogenetic hallmark or an innocent bystander? Gut 56: 577–583, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pothoulakis C, Castagliuolo I, LaMont JT, Jaffer A, O'Keane JC, Snider RM, Leeman SE. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci USA 91: 947–951, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prins JB, Niesler CU, Winterford CM, Bright NA, Siddle K, O'Rahilly S, Walker NI, Cameron DP. Tumor necrosis factor-alpha induces apoptosis of human adipose cells. Diabetes 46: 1939–1944, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Prins JB, O'Rahilly S. Regulation of adipose cell number in man. Clin Sci (Lond) 92: 3–11, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett 410: 78–82, 1997. [DOI] [PubMed] [Google Scholar]
- 48.Silva MA, Jocham G, Barros M, Tomaz C, Muller CP. Neurokinin3 receptor modulation of the behavioral and neurochemical effects of cocaine in rats and monkeys. Rev Neurosci 19: 101–111, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Song IS, Bunnett NW, Olerud JE, Harten B, Steinhoff M, Brown JR, Sung KJ, Armstrong CA, Ansel JC. Substance P induction of murine keratinocyte PAM 212 interleukin 1 production is mediated by the neurokinin 2 receptor (NK-2R). Exp Dermatol 9: 42–52, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, Gerry N, Forse RA, Tchoukalova Y, Jensen MD, Pothoulakis C, Kirkland JL. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab 292: E298–E307, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Valverde AM, Mur C, Brownlee M, Benito M. Susceptibility to apoptosis in insulin-like growth factor-I receptor-deficient brown adipocytes. Mol Biol Cell 15: 5101–5117, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright JT, Hausman GJ. Insulinlike growth factor-1 (IGF-1)-induced stimulation of porcine preadipocyte replication. In Vitro Cell Dev Biol Anim 31: 404–408, 1995. [DOI] [PubMed] [Google Scholar]