Abstract
There is a glaring lack of knowledge on mouse colonic motility in vivo, primarily due to unavailability of adequate recording methods. Using a noninvasive miniature catheter pressure transducer inserted into the distal colon, we assessed changes in colonic motility in conscious mice induced by various acute or chronic stressors and determined the neurotransmitters mediating these changes. Mice exposed to restraint stress (RS) for 60 min displayed distal colonic phasic contractions including high-amplitude giant migrating contractions (GMCs), which had peak amplitudes >25 mmHg and occurred at a rate of 15–25 h−1 of which over 50% were aborally propagative. Responses during the first 20-min of RS were characterized by high-frequency and high-amplitude contractions that were correlated with defecation. RS-induced GMCs and fecal pellet output were blocked by atropine (0.5 mg/kg ip) or the corticotrophin releasing factor (CRF) receptor antagonist astressin-B (100 μg/kg ip). RS activated colonic myenteric neurons as shown by Fos immunoreactivity. In mice previously exposed to repeated RS (60 min/day, 14 days), or in transgenic mice that overexpress CRF, the duration of stimulation of phasic colonic contractions was significantly shorter (10 vs. 20 min). In contrast to RS, abdominal surgery abolished colonic contractions including GMCs. These findings provide the first evidence for the presence of frequent cholinergic-dependent GMCs in the distal colon of conscious mice and their modulation by acute and chronic stressors. Noninvasive colonic manometry opens new venues to investigate colonic motor function in genetically modified mice relevant to diseases that involve colonic motility alterations.
Keywords: colon, mice, giant migrating contractions, manometry, stress, corticotropin releasing factor-overexpressing
the mammalian colon plays important roles in the absorption, storage, and propulsion of luminal contents as part of the physiology of digestion (54). Appropriate motor activity is required to subserve these functions, as shown by altered colonic propulsive activity in several intestinal disorders including fecal incontinence, constipation, irritable bowel syndrome (IBS), and diverticular diseases, as well as in pseudo-obstruction, diarrhea, inflammatory bowel disease (IBD), or diabetes (6, 9, 39, 57). However, the mechanisms underlying altered colonic motility and their contribution to symptom development are not well understood. These mechanisms are the subject of intensive clinical investigation using a variety of tools. The most common approaches employ endoscopic placement of side-hole manometry catheters and/or electrodes into a prepared colon (11, 23, 57). In experimental animals, colonic motility has been investigated typically by using EMG electrodes or strain gauges implanted in or on the colonic wall, respectively (14, 25, 41). Most other studies of colonic motor function in rats and particularly in mice have measured the effects of altered colonic motility, i.e., marker transit, bead expulsion, or defecation (2, 38, 46) as proxies of colonic motility. Although all methods used in experimental animals are highly valuable, either they are invasive and thus have inherent confounding factors due to surgery or, in transit assays, they do not provide adequate information on the spatiotemporal profile of colonic motility. The methodological constraints are more apparent in mice, where to date little is known about colonic motility in conscious state. Characterization of mouse colonic movement is mainly derived from either in vitro or isolated colon preparations (12, 33, 47, 48, 51). In particular, in the mouse isolated colon, the spontaneous motor activity comprises characteristic contractions, termed colonic migrating motor complex (CMMC) that are rhythmic, neurogenic, and propagative (12, 47, 58). However, the type, pattern, frequency, magnitude, and regulation of colonic motor activity in conscious mice are still not known. This contrasts with the vast knowledge of mouse colonic enteric neuron physiology (16, 44, 45, 53) and the availability of large and growing number of genetically modified mice with great potential as tools to help understand colonic motility and its impacts in health and disease states.
The colonic motility pattern of several species, in conscious state, is characterized by the appearance of phasic spike potentials and spontaneous high-amplitude migrating contractions termed long or short spike bursts (13, 15, 54) and giant migrating contractions (GMC) in conscious humans (55), dogs (21, 31), cats (22), and rats (25, 42). The form and pattern of colonic phasic contractions in these species vary depending on physiological status (feeding, fasting) and are altered by stress, colonic inflammation, and other diseases (4, 25, 56).
In the present study, we developed a novel noninvasive method to study distal colonic motility, using miniature catheter pressure transducer solid-state manometry, and characterized the patterns of distal colonic contractions in conscious mice. Since psychological stress either acute or chronic is known to alter colonic motility in experimental animals and humans (20, 61), we assessed the distal colon contractile pattern in response to an acute restraint stress (RS) in naive or in chronically stressed mice, i.e., mice exposed to repeated RS or in corticotropin releasing factor (CRF)-overexpressing (OE) mice, a genetic model of chronic stress (8). Cholinergic and nitrergic pathways are known to be involved in the occurrence of rat colonic GMC (25), whereas both central and peripheral CRF receptors play role in RS-induced stimulation of colonic motility in rats (60). We therefore investigated the role of nitrergic, cholinergic, and CRF receptors in colonic contractility response to RS using pharmacological and neurofunctional approaches in conscious mice. The latter consisted of Fos immunohistochemistry of the colonic longitudinal muscle myenteric plexus (LMMP) whole mount preparation as in our previous studies (63). We further validated the miniature pressure transducer solid-state manometry in conscious mice by assessing the time course of changes in the pattern of colonic contractions associated with abdominal surgery known to cause colonic ileus in mice (40).
MATERIAL AND METHODS
Animals
All experiments were conducted in nonfasted adult male mice (20–25 g, C57Bl/6; Harlan San Diego, CA) or male CRF-OE and wild-type (WT) mice (28–35 g) except otherwise stated. CRF-OE and their WT littermates (Center for Neurobiology of Stress, Animal Models Core, University of California Los Angeles) were generated as previously described (59). The chimeric CRF transgene was composed of the rat genomic CRF gene replacing the 5′ regulatory region by a mouse metallothionein-1 promoter and microinjected into the male pronucleus of fertilized eggs (C57Bl/6 × SJL). Animals were housed (3–4/cage) and maintained under temperature- (20–24°C) and light- (12 h light-12 h dark) controlled environment. Animals were fed ad libitum with standard rodent chow (Prolab RMH 2500-5P14; Purina LabDiet, St. Louis, MO) and tap water. The experimental protocols were approved by the Veterans Administration Animal Component of Research Protocols 9906-820 and 08047-05. All animal experiments were conducted in accordance with principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Substances and Treatments
Astressin B (Peptide Biology Laboratory, The Salk Institute, La Jolla, CA) was synthesized by the solid-phase approach and the Boc strategy as previously described (50) and stored in powder form at −80°C. l-NAME (NG-nitro-l-arginine methyl ester) and atropine sulfate (Sigma Chemical, St. Louis, MO) were kept in powder form at −4°C and room temperature, respectively. Immediately before use, astressin B was dissolved in distilled water and l-NAME and atropine were dissolved in saline (NaCl 0.9%). Intraperitoneal (ip) injections were made in a volume of 0.1 ml/mice.
Stress Models
Acute restraint.
Restraint consisted of placing each mouse, for a 60-min period, in a Falcon plastic tube (2.7 cm diameter, 7–10 cm long, BD, Franklin Lakes, NJ) with several holes on the side and front for adequate ventilation (partial restraint tube device) as previously described (28, 35). The dimensions of the tube prevented the mice from turning around and limited their ability to move forward or backward.
Acute abdominal surgery.
Under brief isoflurane anesthesia and aseptic condition, the mouse abdomen was incised, and the cecum was identified and palpated for 60 s with saline wet gauze as in our previous study (26). The control group (sham group) consisted of brief isoflurane anesthesia without performance of the surgical procedure.
Repeated restraint.
Repeated RS consisted of performing the RS procedure described above for 1 h each day for 14 consecutive days.
Chronic CRF-OE mice.
CRF-OE mice have been characterized as a genetic model of chronic stress in various behavioral, autonomic, and visceral responses (8).
Assessment of Colonic Motor Function
Intracolonic pressure recording procedure.
Mice were briefly anesthetized with isoflurane (3% in O2), and a miniaturized pressure transducer catheter (SPR-524 Mikro-Tip catheter; Millar Instruments, Houston, TX) lubricated with medical grade lubricant was introduced into the colon such that the middle of the pressure sensor (3.5 F) was 2 cm proximal to the anus. The catheter was then secured to the tail with tape, and colonic contractions were recorded in conscious mice immediately after their placement in the restraint tube. When required, two pressure transducer catheters were inserted at 2 and 4 cm proximal to the anus by the same procedure. Each pressure transducer catheter was connected to a preamplifier (model 600; Millar Instruments). The signal was then amplified by using a transducer amplifier in differential mode (TBM4, World Precision Instruments, Boca Raton, FL), acquired via a Micro1401 analog-to-digital interface (Cambridge Electronic Design) at 100 samples/s, and recorded with Spike 2 version 5 data acquisition software (Fig. 1, A and D). The system was calibrated by using known pressures at 0, 20, 40, and 60 mmHg at the start of each experiment to convert voltage output to intraluminal pressure. Abdominal contractions and breathing artifacts were excluded by smoothing the original trace with a time constant of 2 s (Fig. 1, B and E).
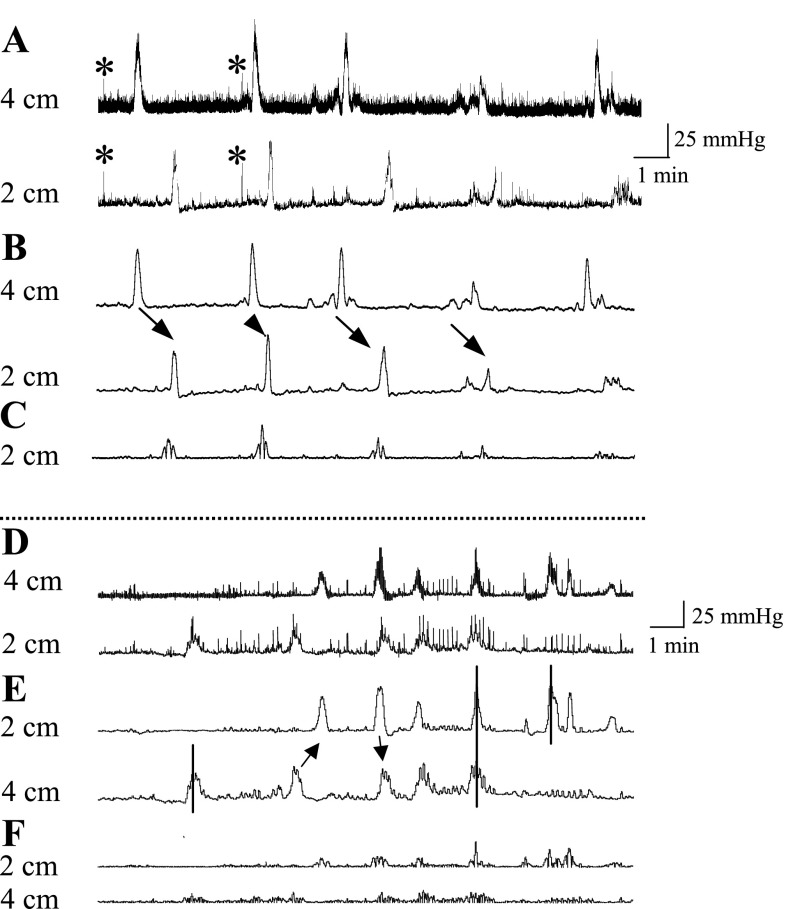
Fig. 1.
Conscious mouse colon contractile pressure changes and signal analysis. Miniature pressure transducer catheters were placed in the colon of mice (2 and 4 cm proximal to the anus) under brief isoflurane anesthesia. Upon recovery from anesthesia, mice were placed in a partial restraint tube and colonic pressures were simultaneously recorded. A: representative colon pressure raw traces at 4 and 2 cm. Note the distinctive signature of the sharp rising and short-duration pressure excursions that simultaneously occur at the 2- and 4-cm sites (*) owing to abdominal muscle contractions and the slow and long-duration pressure excursions that occur owing to colonic contractions. B: colon traces smoothed with 2-s time constant. Pressure changes due to abdominal contractions and breathing are removed by the 2-s smoothing whereas the colonic pressure changes are not (arrows show aboral propagation). C: real-time area under the curve (AUC) of contraction of the colon. Numbers are distance (cm) of the pressure sensor from the anal verge. D–F: arrows show propagative (aboral or oral) contractions, and solid lines show simultaneously occurring contractions or those that were seen only at 1 site.
Intracolonic pressure data analysis.
Colonic contractile pressure changes were quantified by measuring the area under the curve of the phasic component of the intraluminal pressure trace (pAUC) for every minute. The phasic component of intracolonic pressure was extracted from the original trace as previously reported (17, 18) by removing the direct current (DC) component with a time constant of 10 s from the 2-s smoothed original trace (Fig. 1, C and F). Since the main contractile colonic pressure occurred usually during the first 20 min, the mean pAUC was calculated for the time periods of 0–10, 10–20, and 20–60 min. Results are expressed as 1) the time course of the phasic contraction, by calculating the mean pAUC for every minute (pAUCm) from the rolling average of pAUC for the period between 2 min before and 2 min after each minute's pAUC (i. e., pAUCm = average of pAUCm±2 min) or 2) the mean pAUC over a time period of 0–10, 10–20, and 20–60 min. In addition, frequency and amplitude as well as duration of contractions were determined for a total of 737 contractions obtained from 34 mice. The first 20 min of contractions were compared with the 20- to 60-min time period. In rats, the high-amplitude migrating distal colon contractions have pressure rise over 15 mmHg (10). Since in the present study we could not reliably attribute pressures below the 10 mmHg level to colonic contractile pressures, we took the 10 mmHg as the detectable threshold and added the 15 mmHg rise, as defined by Croci et al. (10), and considered pressures over 25 mmHg as the threshold for defining an excursion in the pressure trace as a GMC. Although several studies identified and described CMMC as the main contractile pattern in isolated mouse colon (12, 47), there is so far only one study that reported colonic contractility in awake mice (40). In contrast, GMC has been described to be the main propulsive contraction in the colon of several species in conscious state, including in rats and humans (25, 55). Because the biochemical codes of GMCs described in conscious rat distal colon resemble that observed in the present conscious mice distal colon high-amplitude migrating contractions, we adopted the term GMC in the analysis and description of these contractions in the present study.
Propagation of GMCs.
In a separate study, propagation of GMCs was investigated in 14 mice by using two pressure probes placed at 2 and 4 cm proximal to the anus. The propagative nature of contractions >25 mmHg was evaluated by determining the order of appearance of the peak of the distal intraluminal pressure wave as described previously (47). Four different types of propagative and nonpropagative contractions were identified (Fig. 1, D–F). Contractions that propagate proximal to distal in sequence from the 4-cm to the 2-cm site were classified as aborally propagating contractions whereas contractions that propagate distal to proximal from the 2-cm to the 4-cm site were classified as orally propagating contractions. These propagative contractions usually display similar shapes although their amplitude may vary. Nonpropagative contractions are those that appear only at the 4- or 2-cm site. Lastly, contractions that appear simultaneously at both the 4- and 2-cm sites are considered as other types of contractions. The aborally propagated contractions were considered as propagated when detected at the level of 2 cm during an interval between 2 and 120 s after detection at 4 cm. The time of occurrence of such a contraction was set at the peak of its detection at the 4-cm site. The speed of propagation in mm/s was obtained by dividing the distance between the two pressure sensors (20 mm) by the time (s) elapsed between the peaks of the contractions at 4- and 2-cm sites. Of the 14 mice, 6 did not expel a fecal pellet during the 1-h recording period and displayed a subocclusive-like motility pattern, probably because of the presence of the two pressure probes. Thus the two probes study was only used to investigate propagative properties of GMCs that were analyzed on a total of 165 contractions from 8 recordings (1/mouse).
Fecal pellet output.
In nonfasted conscious mice, defecation was monitored as described previously by counting the number of fecal pellets excreted for 1 h (35), along with colonic contractions recording. Correlation with colonic motility was determined by comparing the number of fecal pellets output to the normalized first 20-min colonic motility. Normalized colonic motility was calculated by dividing the mean pAUC of the first 20 min by the mean pAUC of the last 40 min. This provided a representative value of the initial activation of colonic motility in response to acute stress and thus allows intraindividual comparison among mice. The reasons for making a correlation study between the 60-min fecal pellet output and the first 20 min of normalized colonic contractions are that 1) because the presence of catheters in the colon in the RS conscious mice, fecal pellet output can only be measured at the end of the stress session (60 min); 2) several studies have shown that more than 80% of fecal pellet output induced by RS in rats and mice occurred in the first 30 min of the stress session (28, 34, 35); and 3) the present data show that the robust colonic contractile response to RS occurs in the first 20-min period, in agreement with the second point above. Thus to validate the use of miniature pressure transducer solid-state manometry to monitor propulsive colonic motility, we assessed whether there was a correlation between the first 20 min of colonic contractions with the classic colonic propulsive assay, the 60-min fecal pellet output.
Fos Immunohistochemistry
Mice were euthanized by cervical dislocation, and the abdominal cavity was opened. The distal colon was dissected out and opened longitudinally along the mesenteric border, and longitudinal muscle myenteric preparation was prepared and processed for Fos immunohistochemistry as detailed previously (63). The presence of Fos immunoreactivity (IR) was revealed as a dark brown precipitate located in neuronal nuclei in a microscope (Zeiss, Axiocope II). Fos-IR cells were counted in 20 ganglia of each mouse LMMP preparation from the distal colon.
Experimental Protocols
Effect of RS on colonic motility in conscious mice.
ACUTE RS IN NAIVE MICE PRETREATED OR NOT WITH CRF ANTAGONIST.
Immediately after transducer placement, naive mice were injected ip with saline (n = 12) or astressin B (100 μg/kg, n = 6) and 5 min later were placed in the restraint tube and left undisturbed whereas colonic contractions were recorded for a 1-h period. Dose of astressin B was based on our previous studies (29).
ACUTE RS IN MICE EXPOSED TO REPEATED RS.
Mice (n = 6) exposed to daily RS (1 h/day × 14 days) were placed on day 15 in the restraint tube immediately after transducer placement, and colonic contractions were monitored as described above for 1 h.
ACUTE RS IN CRF-OE MICE.
CRF-OE (n = 10) and WT (n = 4) were placed in the restraint tube immediately after transducer placement, and colonic contractions were monitored as described above for 1 h. Because WT littermate and the C57Bl/6 WT mice (n = 6) had similar contractions patterns, data were pooled together and used as control WT group.
EFFECTS OF CHOLINERGIC AND NITRERGIC BLOCKADE ON ACUTE RS.
Mice were injected ip with saline (0.1 ml), atropine (0.5 mg/kg), or l-NAME (10 mg/kg) during anesthesia, 3–5 min before being placed in the restraint tube, and colonic contractions were recorded for 60 min after mice placement in the tube. Doses of atropine and l-NAME were adopted from our previous studies in rats (27).
EFFECTS OF ACUTE RS ON FOS EXPRESSION IN THE MOUSE DISTAL COLONIC MYENTERIC PLEXUS.
Mice were placed in a restraint tube or kept in their home cage and were euthanized 60 min later. Distal colons were then collected for immunohistochemical staining of Fos in the LMMP neurons.
Effects of abdominal surgery.
Conscious mice were first placed in the partial restraint stress device and baseline colonic contraction was monitored for 60 min. A group of mice (n = 6) were briefly anesthetized with isoflurane (3% in O2) and a laparotomy plus a 60-s cecal palpation was performed. The sham surgery group (n = 6) underwent only a brief anesthesia without laparotomy and cecal palpation. Mice were then put back into the restraint device and colonic contraction was recorded for another 60 min.
Data Analysis
Values are presented as means ± SE. Influence of treatment was calculated by ANOVA followed by a Student-Newman-Keuls post hoc test, and the time course effect in each group was analyzed by ANOVA for repeated measures followed by a Student-Newman-Keuls post hoc test. Intraindividual pairwise comparisons (baseline vs. response) were performed with a nonparametric Wilcoxon's test whereas interindividual comparisons (control vs. treated) were investigated by running a nonparametric Mann-Whitney test. Correlation between colonic motility and fecal pellet output was done by Spearman rank-order correlation. Mean values of Fos IR in the distal colon LMMP were compared by Student's t-test. A P value <0.05 was considered significant.
RESULTS
Characterization of Colonic Motility and GMCs in Acutely Stressed Conscious Mice
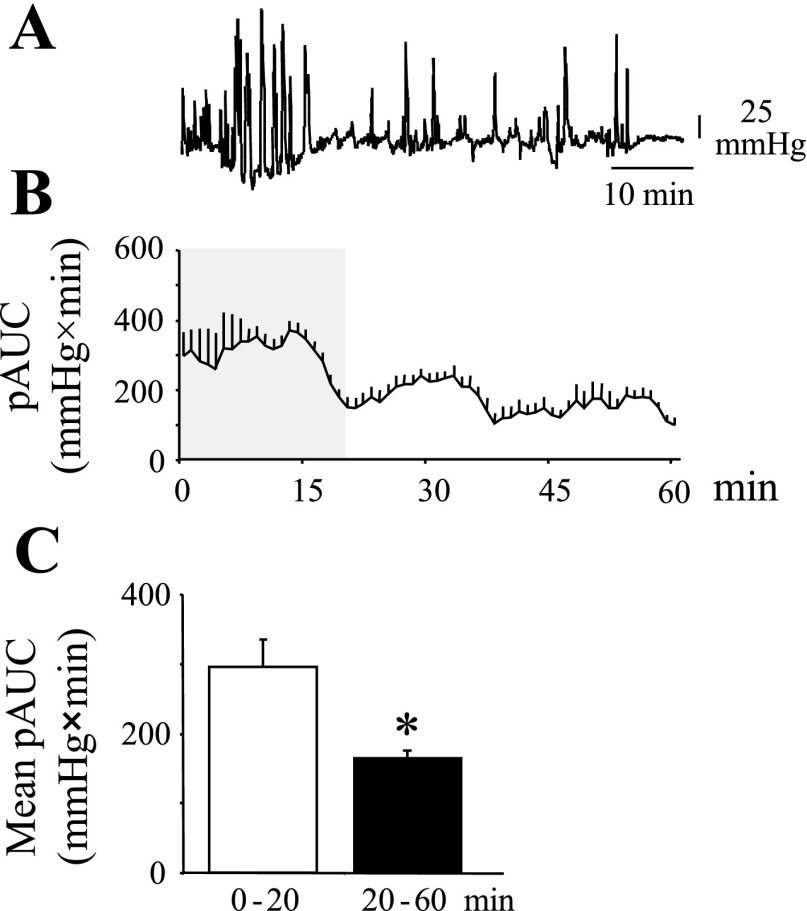
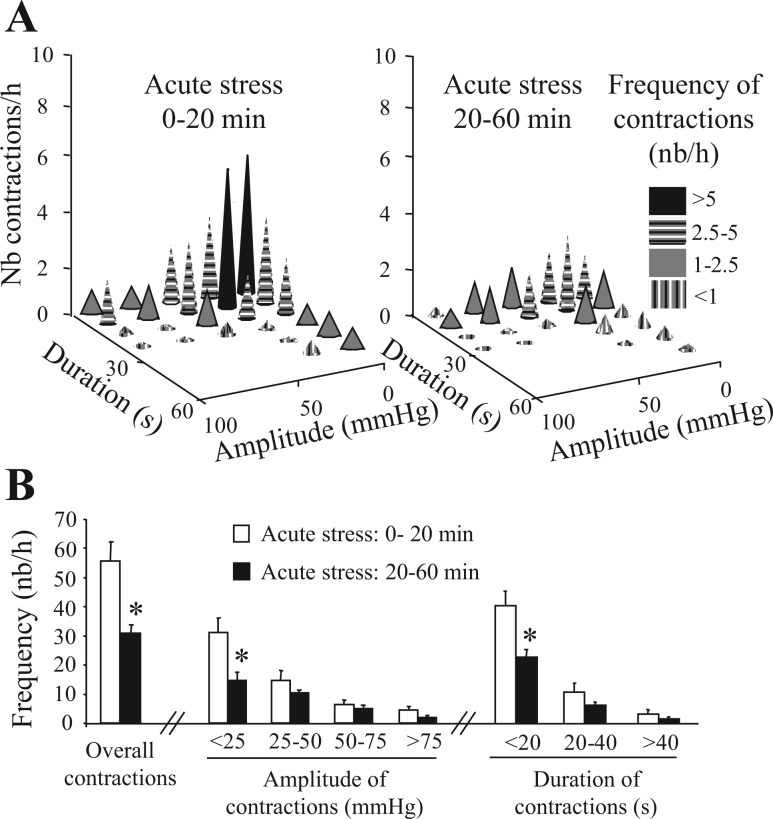
Mice placed in the restraint tube displayed mainly low- (<25 mmHg) and high-amplitude (>25 mmHg) colonic contractions. Further analysis showed that the first 20 min, after mice were placed in the restraint tube, were marked by a robust transient activation of colonic contractions (Fig. 2, A–C). The mean pAUC for the 0- to 20-min time period was significantly increased compared with the subsequent 20- to 60-min period (P < 0.05; Fig. 2C). The overall transient increase in colonic contractions was associated with an increase in frequency of >10 mmHg contractions compared with the 20- to 60-min time period (55.8 ± 6.6 vs. 31.1 ± 2.7 contractions/h, respectively; P < 0.0001). In addition, during the first 20 min, there was a significant increase in low-amplitude (<25 mmHg) (P < 0.01) (Fig. 3, A and B) and short-duration (<20 s) (P < 0.01) (Fig. 3, A and B) contractions compared with the 20- to 60-min period. Recordings from the mouse distal colon showed high-amplitude (>25 mmHg) contractions, referred as GMC (Fig. 1; Figs. 2A and 3A) similar to that reported in other species (10, 25). The frequency of GMC as well was increased during the first 20 min compared with those in the 20- to 60-min period (25.2 ± 3.3 vs. 16.8 ± 2.6 contractions/h; P < 0.05). GMCs represented 54.3% and 45.3% of the total contractions of the 0- to 20-min and 20- to 60-min time periods, respectively. Phasic contractions with amplitude less than 10 mmHg were often not detectable. When detectable, their mean frequency was 4.8 ± 0.3 contractions/min with a range of 3.6–6.0 contractions/min.
Fig. 2.
Distal colon contractions pattern in conscious mouse placed in partial restraint tube. A miniature pressure transducer catheter was placed in the distal colon of mice (2 cm proximal to the anus) under brief isoflurane anesthesia. A: representative raw distal colon pressure trace. Note the enhanced contractile response during the first 20 min of placement of mice in the tube that becomes stable and low after 20 min. B: time course of AUC (mmHg × min) over a 60-min period. C: mean AUC of the phasic component of the intraluminal pressure trace (pAUC; mmHg × min) of the first 20 min (0–20) vs. the mean pAUC (mmHg*min) of the subsequent 40 min (20–60); n = 12/group. *P < 0.05 vs. 0–20 min; ANOVA.
Fig. 3.
Distal colonic contraction profile (amplitude, duration, and frequency of contractions) in acutely stressed conscious mice. A miniature pressure transducer catheter was placed in the distal colon of mice (2 cm proximal to the anus) under brief isoflurane anesthesia. A: plots showing distal colonic contractions profile [number (Nb), duration, and amplitude] during the first 20 min and during the 20- to 60-min stress period. B: frequency of contractions as a function of amplitude or duration of contractions; 732 contractions from n = 12 mice were analyzed. *P < 0.05 vs. the corresponding 0–20 min time period; ANOVA.
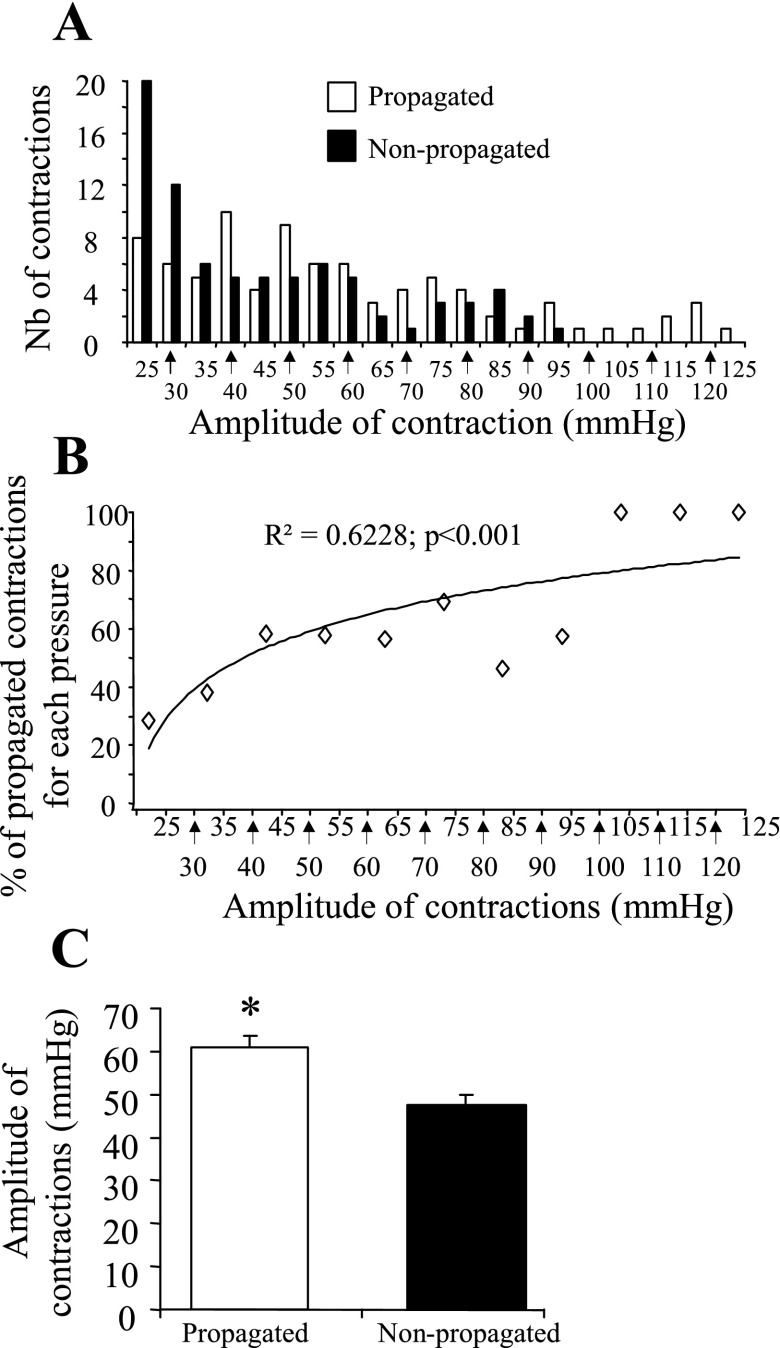
Study on propagation of GMCs showed that the overall frequency of high-amplitude contractions (>25 mmHg) detected at the 4- and 2-cm site was 20.5 ± 1.5 and 20.6 ± 3.2 h−1, respectively. Of these the number of contractions that propagated aborally from 4-cm site and identified at the 2-cm site had a frequency of 10.6 ± 1.9 h−1, representing 51.8% of the GMCs (Fig. 1). The propagated GMCs were of higher amplitude compared with the nonpropagated GMCs (60.9 ± 2.9 vs. 47.6 ± 2.3 mmHg, respectively; P < 0.001; Fig. 4, A–C). The colonic phasic contraction pressure at which 50% of the contractions would propagate aborally was 30 mmHg (Fig. 4, A and B). In addition, the likelihood for a contraction to be propagated aborally was positively correlated with its amplitude (P < 0.001) yielding 59.7% and 92.3% propagation rate for contractions over 30 and 90 mmHg, respectively (Fig. 4, A and B). The mean velocity of aborally and orally propagated GMCs were 1.0 ± 0.1 and 1.1 ± 0.2 mm/s, respectively and were not correlated with the amplitude of the GMCs (P = 0.60). The orally propagating GMCs had a frequency of 1.0 ± 0.3 h−1 whereas the number of contractions that did not propagate to the 2-cm site and those contractions that occurred at both sites simultaneously were 7.8 ± 1.6 and 1.1 ± 0.4 h−1, respectively.
Fig. 4.
Propagative characteristics of conscious mouse distal colonic contractions. A miniature pressure transducer catheters were placed in the distal colon of mice (2 and 4 cm proximal to the anus) under brief isoflurane anesthesia. A: number of propagated and nonpropagated contractions. B: correlation between the amplitude of contractions and their chance to be propagated. C: mean amplitude of propagated vs. nonpropagated contractions; n = 8 mice. *P < 0.05 vs. nonpropagated contractions.
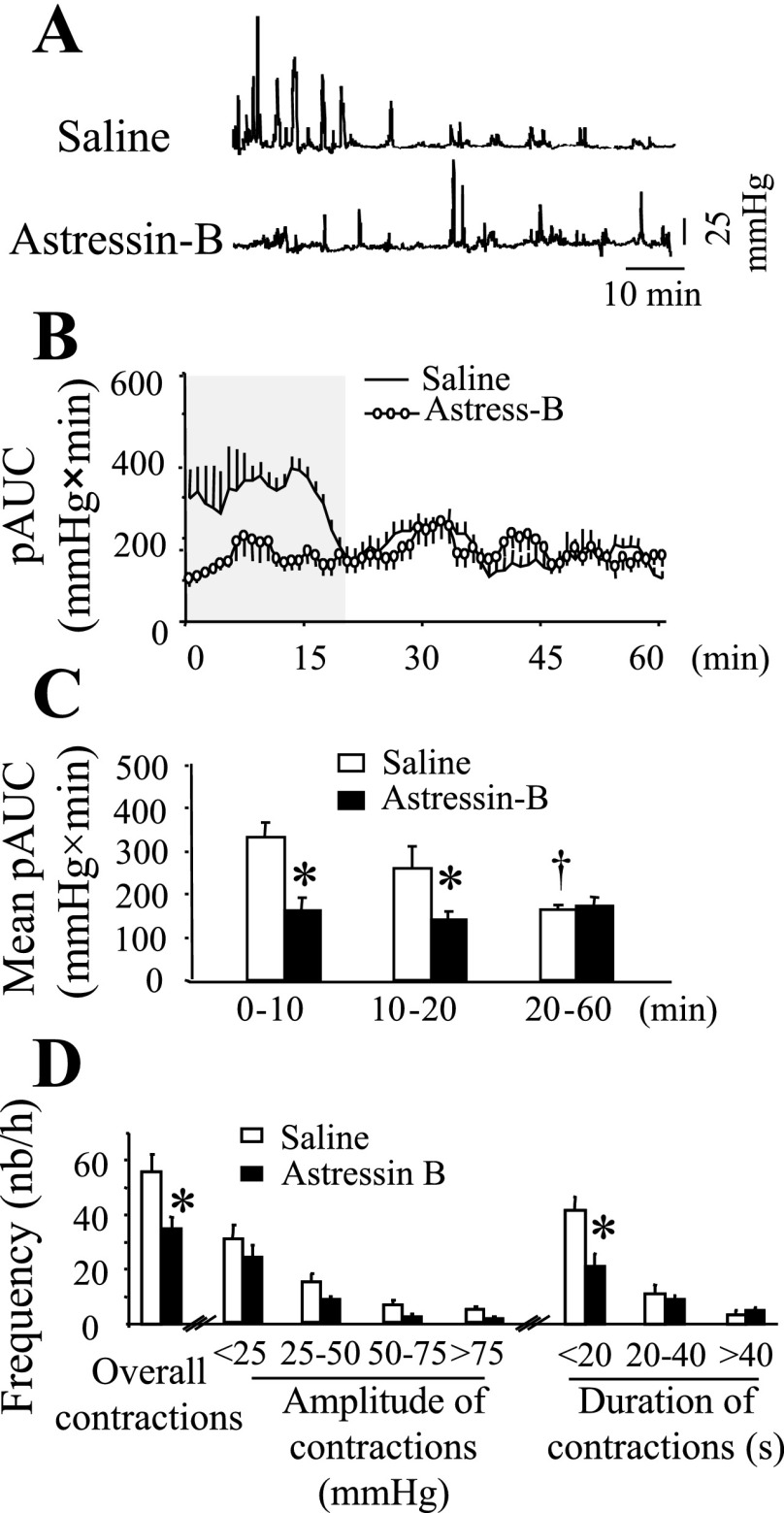
CRF Receptor Blockade Prevents Acute RS-Induced Distal Colonic Contractions and Fecal Pellet Expulsion
Pretreatment with astressin-B (100 μg/kg ip in 0.1 ml), a CRF receptor subtype 1 (CRF1) and CRF receptor subtype 2 (CRF2) antagonist, prevented the transient increase in colonic contractions compared with the saline (0.1 ml ip)-treated group (Fig. 5, A–C) as evidenced by the significant decrease in mean pAUC for the 0–10 min and 10–20 min time periods (Fig. 5C). This was mainly due to the decrease in the frequency of all contractions (55.7 ± 6.6 vs. 35.0 ± 4.1 contractions/h; P < 0.05), and more specifically of the short-duration (<20 s) contractions (41.5 ± 4.6 vs. 21.0 ± 3.6 contractions/h; P < 0.05) (Fig. 5D) and of GMCs (8.4 ± 1.1 vs. 3.8 ± 0.7 contractions/h; P < 0.05). Similarly, astressin B attenuated the fecal pellet output induced by RS (9.5 ± 1.6 vs. saline 13.7 ± 1.4 pellets/h, P < 0.05).
Fig. 5.
Corticotropin releasing factor (CRF) receptor antagonist astressin-B blocked acute restraint stress-induced distal colonic contraction in conscious mice. A miniature pressure transducer catheter was placed in the distal colon of mice (2 cm proximal to the anus) under brief isoflurane anesthesia. Mice were pretreated (−3–5 min) with either saline (0.1 ml ip) or astressin-B (100 μg/kg ip) and placed for 60 min in a partial restraint tube for distal colonic pressure recording. A: representative raw trace of distal colon pressure changes of saline- (top) or astressin-B- (bottom) pretreated mice. B: time course (during 60 min) AUC of the distal colon. C: AUC of distal colon pressure changes at different time intervals during the 60-min recording period. D: frequency of contractions as a function of amplitude or duration of contractions. *P < 0.05 vs. the corresponding saline; saline n = 12, astressin-B n = 8; ANOVA.
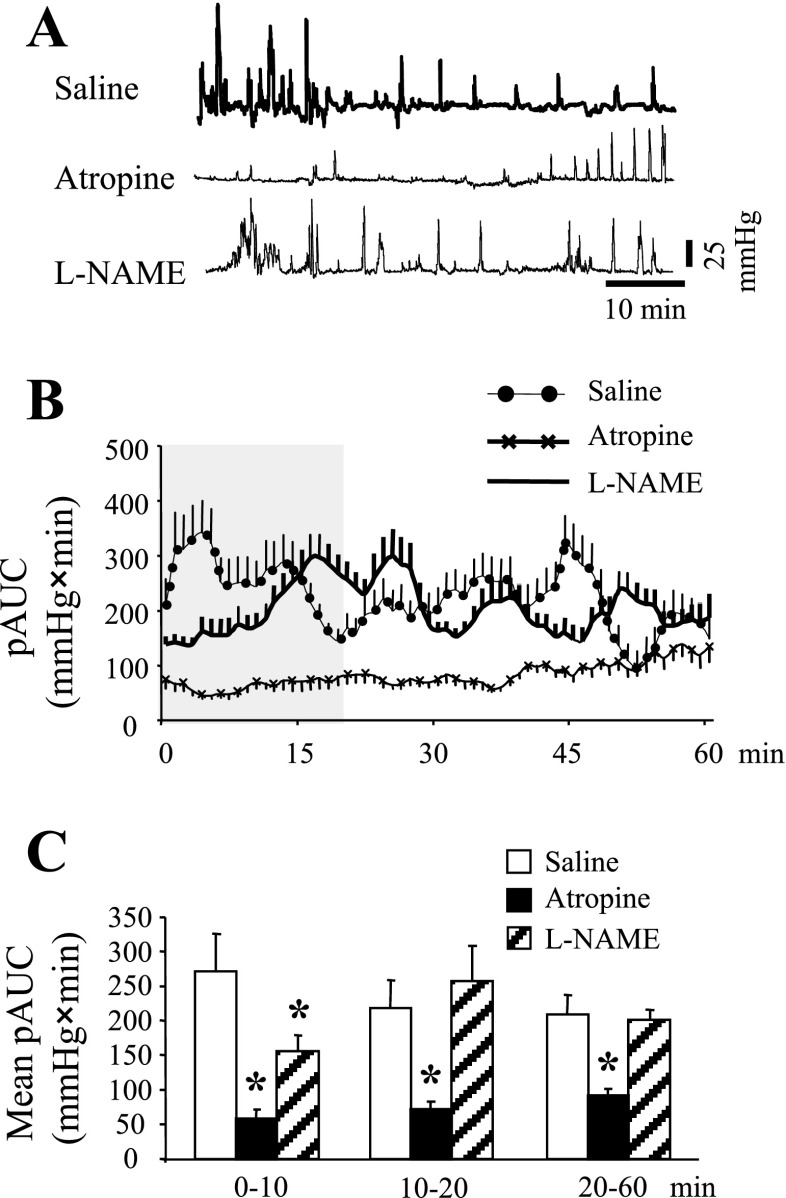
Atropine Blocks Acute RS-Induced Distal Colonic Contractions and Fecal Pellet Output
In saline-pretreated mice, in the first 20 min colonic contractile response was higher than in the subsequent 40 min (Fig. 6, A–C). The overall 60 min pAUC was 222 ± 24 mmHg × min. This was decreased to 81 ± 10 mmHg × min in mice pretreated with atropine (0.5 mg/kg ip). Similarly atropine, compared with saline, reduced the 60-min pellet output (11 ± 0.4 vs. 4 ± 1.4 pellets/60 min; P < 0.05). However, pretreatment with l-NAME (10 mg/kg ip) only decreased significantly the pAUC for the first 0–10-min period (Fig. 6, A–C) and had no significant effect on the 60 min AUC (202 ± 17 mmHg × min) or fecal pellet output (8.5 ± 1.3 h−1).
Fig. 6.
Differential effects of cholinergic or nitrergic blockade on conscious mouse distal colon contractions pattern. A miniature pressure transducer catheter was placed in the distal colon of mice (2 cm proximal to the anus) under brief isoflurane anesthesia. Mice were pretreated (3–5 min) with either saline (0.1 ml ip), atropine (0.5 mg/kg ip) or l-NAME (10 mg/kg ip) and placed in a partial restraint tube for distal colonic pressure recording for 60 min. A: representative raw traces of distal colonic contractions. B: time course of colonic pAUC (mmHg × min) during the 60-min period. C: mean colonic pAUC (mmHg × min) of the 0- to 10-, 10- to 20-, and 20- to 60-min periods. *P < 0.05 vs. the respective saline group; n = 6/group; ANOVA.
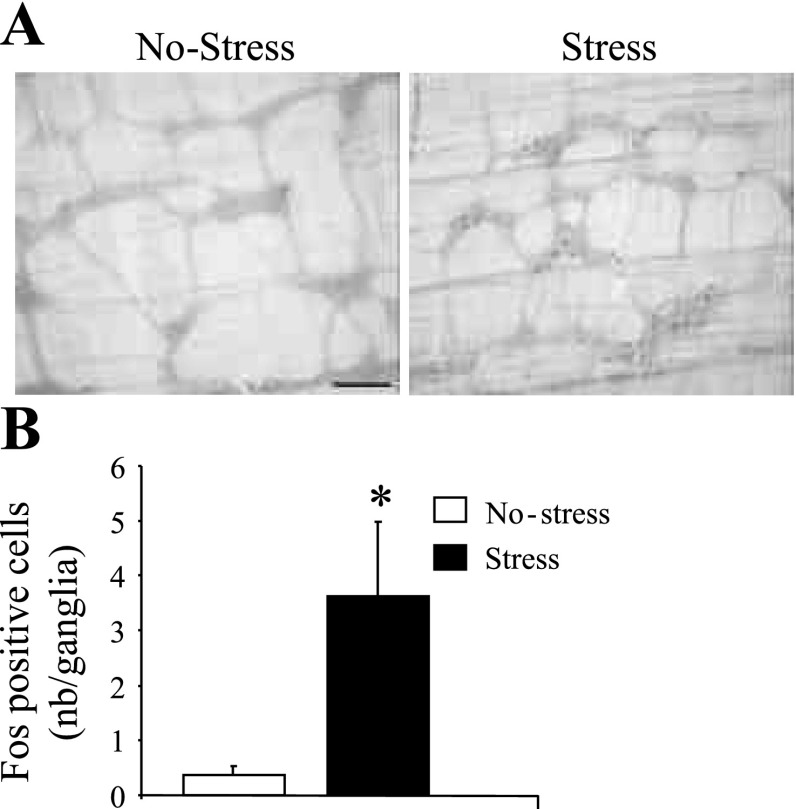
Acute RS Induces Fos Expression in Distal Colonic Myenteric Ganglia
Distal colon LMMP from control mice showed no or few Fos-expressing neurons (0.36 ± 0.17 cells/ganglion, n = 4, Fig. 7, A and B). However, acute RS (60 min) induced Fos-IR in distal colonic myenteric ganglia neurons (3.63 ± 1.35 cells/ganglion, n = 6) (Fig. 7, A and B). The presence of Fos IR was restricted to cells located within the myenteric ganglia, and no Fos-IR was observed in smooth muscle cells (Fig. 7A).
Fig. 7.
Acute partial restraint stress activates distal colonic myenteric neurons. Distal colon longitudinal muscle myenteric plexus (LMMP) whole mount tissue from control and mice submitted to 60 min of acute partial restraint stress were processed for Fos immunohistochemistry. A: photomicrographs of distal colon LMMP from control (no stress) and acute stress mice. B: number of Fos immunoreactive LMMP neurons (nb/ganglia) in control and stressed mice (n = 6/group). *P < 0.05 vs. no-stress (control). Scale bar = 100 μm.
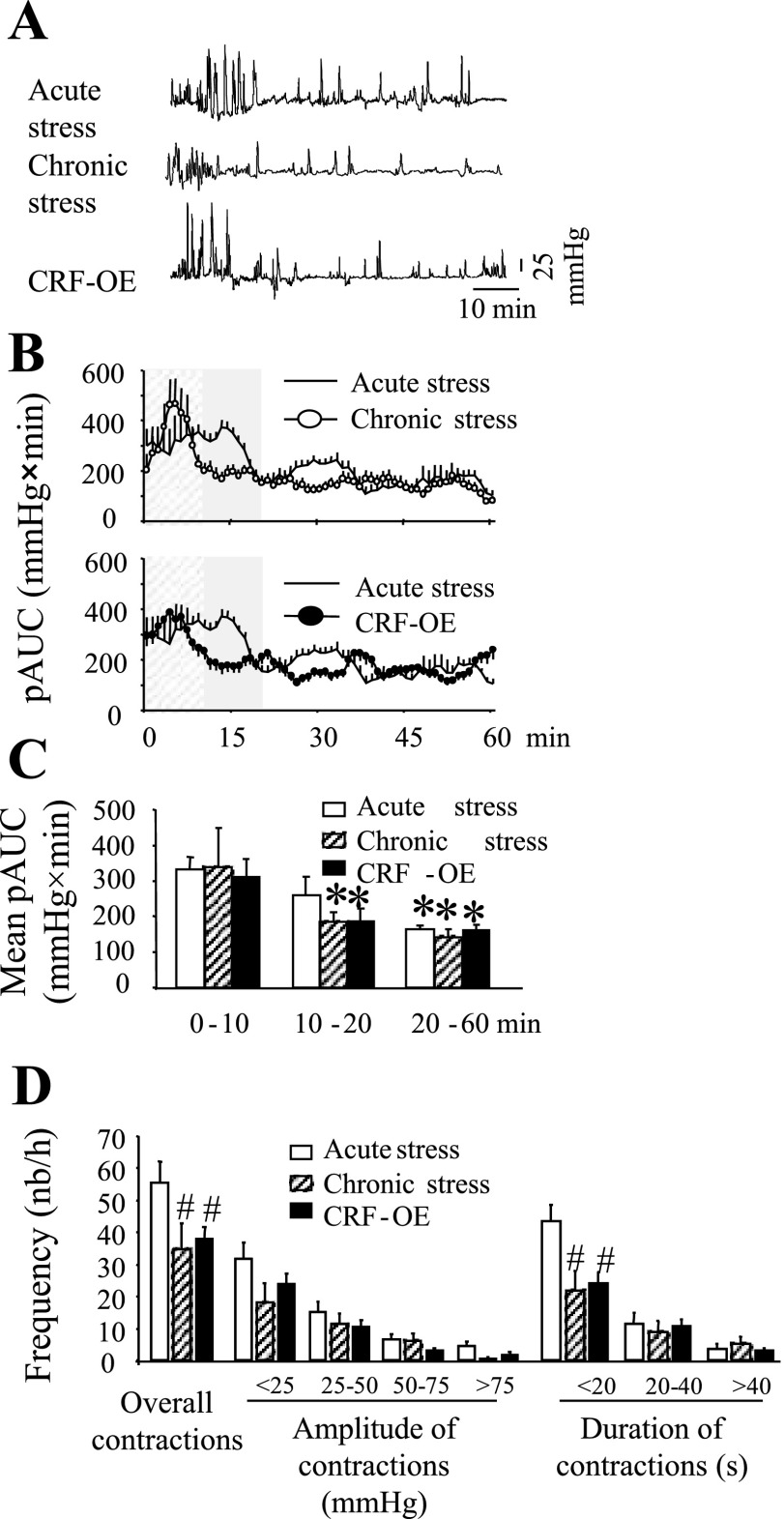
Acute and Chronic Stresses Differentially Affect Colonic Motility in Conscious Mice
Mice exposed to an acute RS, 24 h after chronic intermittent RS (1 h/day for 14 days), had a robust immediate stimulation of phasic colonic contraction that was shorter in duration (first 10 min) than the initial stimulation observed in naive mice that were subjected to a single RS (first 20 min) (Fig. 8, A–C). The relatively short-lasting activation of the colon in the chronically stressed mice was associated with a significant decrease in the number of short-duration (<20 s) contractions but not of GMCs (>25 mmHg amplitude) (Fig. 8D). In the genetic model of chronic stress, CRF-OE mice exposed to a single RS exhibited a colonic motor pattern similar to that of mice subjected to chronic intermittent RS. In CRF-OE mice, the stimulated colonic response lasted only for the first 10 min (Fig. 8, A–C) and was associated with a decrease in the frequency of short-duration contractions, but not that of GMCs (Fig. 8D). Fecal pellet expulsion was similar in chronic RS mice or in CRF-OE mice compared with controls under acute RS [12.3 ± 1.0 and 9.8 ± 1.9 pellets/h, respectively; P > 0.05 compared with acute RS (13.7 ± 1.4 pellets/h)]. When detectable, phasic contractions below 10 mmHg had a similar frequency in both chronic RS and CRF-OE mice (5.0 ± 0.4 and 6.2 ± 0.4 contractions/min, respectively; P > 0.05 compared with acute RS).
Fig. 8.
Effect of chronic stress on distal colonic contractions in conscious mice. A miniature pressure transducer catheter was placed in the distal colon of mice (2 cm proximal to the anus) under brief isoflurane anesthesia. CRF-overexpressing (OE) mice (n = 10), a genetic model of chronic stress or control (wild-type littermates and C57Bl/6) mice submitted to 15 consecutive sessions of partial restraint stress (60 min/day, for 15 days, colon contractions monitored on the 15th day, n = 6) or control mice (no prior stress, n = 12) were placed in the partial restraint stress tube and distal colonic contractions recorded. A: representative raw trace of distal colon in mice submitted to a single acute stress (top), mice submitted to chronic stress [15 consecutive sessions of acute partial restraint stress (60 min·h−1·day−1) (middle), and CRF-OE mice (bottom)]. B: time course (during 60 min) AUC of the distal colon in the acute vs. the chronic stress groups. C: AUC of distal colonic at different time intervals during the 60-min recording period. D: frequency of contractions as a function of amplitude or duration of contractions. *P < 0.05 vs. the corresponding 0- to 10-min time period, #P < 0.05 vs. the corresponding acute stress; ANOVA.
Association of Colonic Contraction and Fecal Pellet Output
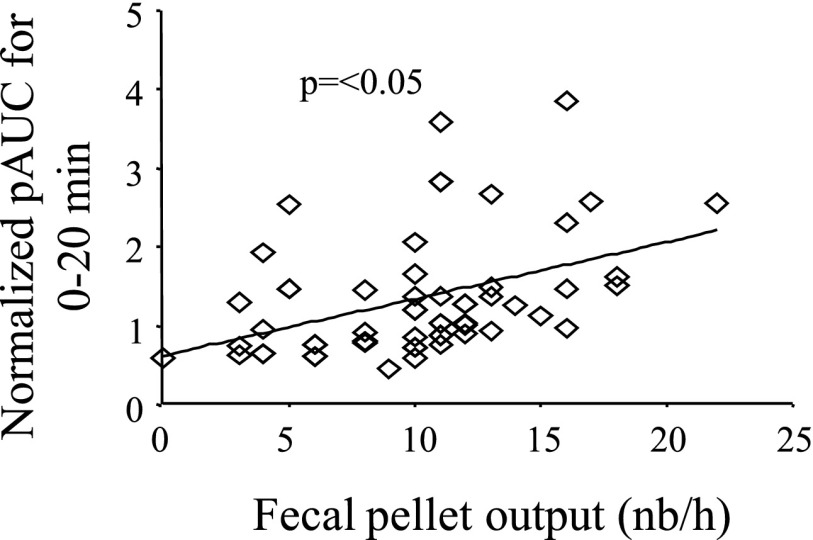
Previous studies have shown that over 80% of the fecal pellet output response to acute stress occurs in the first 30 min of the stress sessions (28, 34, 35). In the present study setting, colonic contractile response was continuously recorded for 60 min. In acutely RS-nontreated mice the initial 20-min colonic contractile response and the overall 60-min fecal pellet output were significantly higher than in the atropine- or astressin B-treated mice (see Figs. 5 and 6). However, because of the presence of catheter in the colon, fecal pellet output could only be counted at the end of the 60-min RS. Data from a total of 60 mice showed that the fecal pellet expulsion and normalized first 20 min distal colonic contractions were significantly correlated (r = 0.43, P < 0.05) (Fig. 9).
Fig. 9.
Correlation of distal colonic contractions and fecal pellet output. A miniature pressure transducer catheter was placed in the distal colon of mice (2 cm proximal to the anus) under brief isoflurane anesthesia and mice were placed for 60 min in a partial restraint tube. Distal colonic pressure changes as well as the 60-min fecal pellet output were monitored. Distal colon contractility during the first 20 min is positively correlated with the 60-min fecal pellet expulsion in conscious mice (n = 60, r = 0.43, P < 0.05).
Postoperative Ileus Reduces Colonic Contraction in Mice
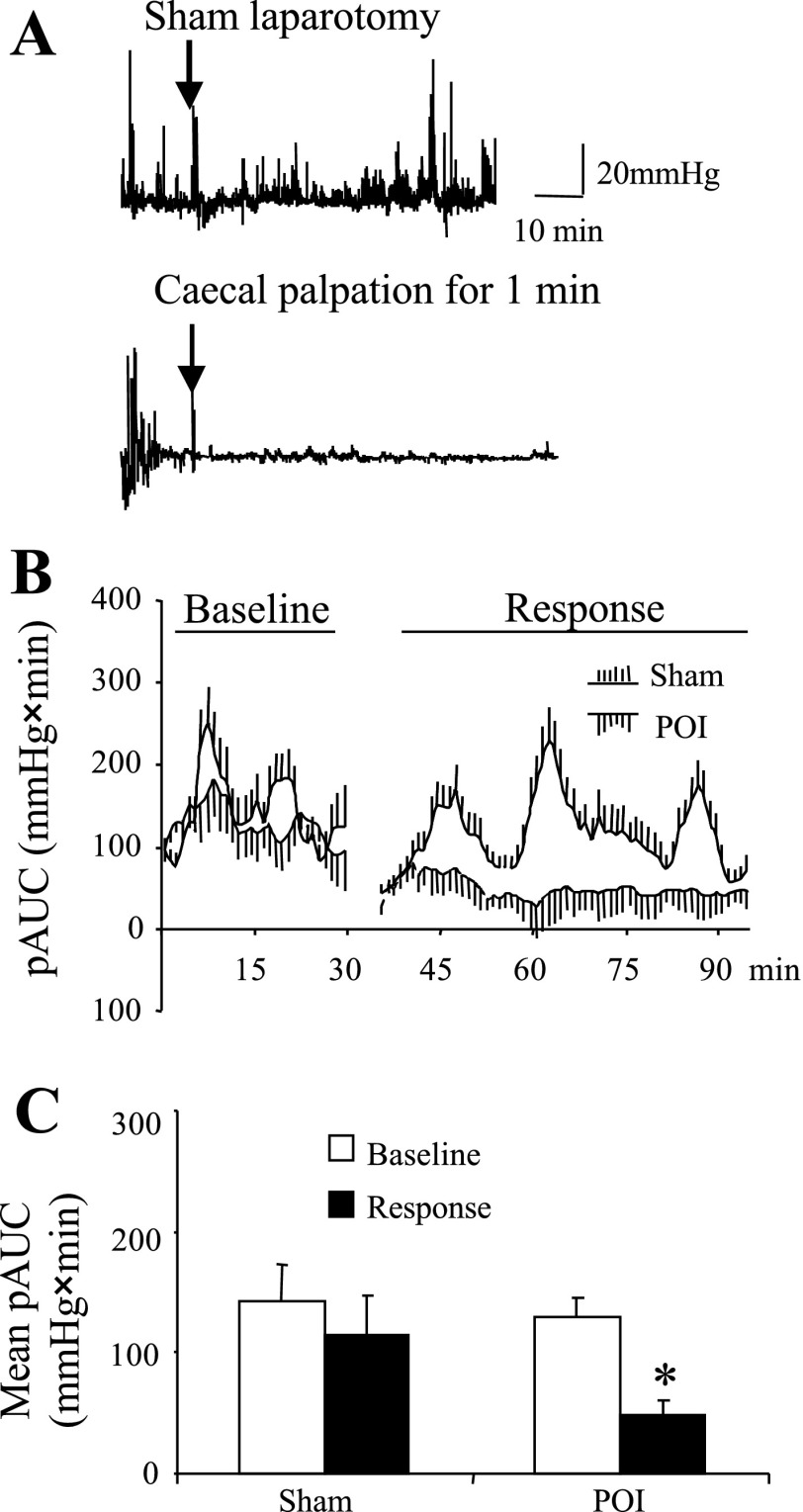
Colonic contractions recorded for the 30-min baseline in both groups were comparable (mean pAUC for 30 min baseline: 142 ± 30 and 129 ± 34 mmHg × min for sham and postoperative ileus groups, respectively; P > 0.05; Fig. 10, A–C). Sham procedure (anesthesia only) tends to decrease the 1-h mean pAUC, but this effect was not significant compared with baseline (114 ± 18 mmHg × min; P > 0.05; Fig. 10, A–C). By contrast, a marked reduction of the 1-h mean pAUC was achieved by laparotomy plus 1-min cecal palpation (49 ± 12 mmHg × min; P < 0.05; Fig. 10, A–C).
Fig. 10.
Effect of interoceptive stress, abdominal surgery, on distal colonic contractions in conscious mice. Under brief isoflurane anesthesia mice underwent either sham treatment (only anesthesia) or abdominal surgery (abdominal incision, opening of the peritoneum, and palpation of the cecum for 1 min) and suturing of the incision. Colonic contractions before the surgery (30 min) and after surgery (60 min) were recorded and compared. A: representative raw trace of distal colonic contractions in sham (top) and surgery mice (bottom). B: time course of the baseline and postsurgery period AUC in sham and surgery groups. C: total baseline and postsurgery AUC in sham and surgery groups. *P < 0.05 vs. baseline and sham; n = 6/group; ANOVA.
DISCUSSION
The present study, using a novel noninvasive miniature pressure transducer manometry, characterized the pattern, frequency, amplitude, and speed of propagation of distal colonic contractions in conscious mice under various experimental conditions. The distal colonic contractions of naive mice exposed to acute RS is characterized by the apparition of low-amplitude (<25 mmHg) phasic contractions and high-amplitude (>25 mmHg) GMC that is correlated with fecal pellet output. These contractions are blocked by atropine but not by l-NAME, suggesting a primary role of cholinergic pathway in distal colon motor activity in conscious mice. Acute RS-induced stimulation of colonic motor activity in conscious mice was also prevented by the CRF receptor antagonist astressin B injected peripherally, indicating the role of CRF receptors in the response. In chronically stressed mice, either in a genetic model of CRF-OE mice or in mice exposed to daily 1-h RS for 14 days, the colonic phasic motor activity to RS, compared with control mice, is characterized by a shorter duration of the initial increase in colonic phasic contractions. The noninvasive miniature pressure transducer manometry allows us to detect the hitherto unidentified alterations of colonic contractions and/or motility patterns in response to various treatments in conscious mice.
Distal Colonic Motor Profile in Conscious Mice
Using the novel noninvasive manometry method, we showed that, in conscious mice placed in the restraint tube, distal colon exhibits primarily low-amplitude (<25 mmHg) phasic and high-amplitude (>25 mmHg) contractions throughout the recording period. The high-amplitude contractions in the distal colon constitute over 50% of the contractions and occur at a frequency of 15–25 h−1, comparable to that described in the isolated mouse colon (one contraction every 2–5 min) (12, 47) and the distal colon of conscious rats (17 h−1) (25). However, they are much higher than the distal colonic GMCs reported in dogs (3 h−1) (21) and the 1–2 day−1 high-amplitude propagative contractions in human colon. Although only half of the overall mice distal colon GMCs propagates aborally, the proportion of such contractions that propagate distally increases as the contraction pressure rises. Most of the contractions with >75 mmHg propagate distally at a speed of about 1.0 ± 0.1 mm/s. The relatively higher number of GMCs in mice compared with large animals including humans is likely to be related to the type of stool as well as the increased frequency and number of fecal pellets expelled in mice. The higher number of propagative GMCs in mice (reaching over 50% of the 15–25 contractions/h), than in rats (∼5 h−1) (10, 25) could also be related to the increased number of fecal pellets expelled in mice than rats per unit of time. However, the presence of two probes may have contributed to the increased rate of propagative GMCs by acting as a semiobstructive body that triggers expulsive contractions.
It is to be noted that the present conscious mouse distal colon contractions share similarities with those observed in the isolated mouse colon because both involved neural activation or have a cholinergic component; both have similar frequencies, are propagative, and are associated with defecation or “ejection” of luminal content (58). These features of contractions are also shared by the GMCs in the conscious rat colon (25). In the present study the term GMC has been adopted to describe the conscious mice distal colon contractions for the following reasons. One, in the isolated mouse colon, NOS inhibitors increase the frequency of CMMCs and associated terminal pressure waves and volume ejections (3, 47, 51) whereas l-NAME has no effect on distal colonic GMC, in both conscious mice and rats (present study; Ref. 25). Two, the in vitro mouse colonic tissue CMMCs are reported to be of low amplitude (∼10 mmHg) (47) that is below GMCs that could be recorded in the colon of conscious mice and rats (present study; Refs. 25, 42). Three, migrating motor complex (MMC) is a term usually used to describe the cyclical fasted state intestinal contractions that is composed of three, and sometimes four, distinct phases that are defined in terms of the amount and regularity of contractile or electrical spiking activity. In our in vivo study, colonic contractions appeared in a random manner with no apparent MMC pattern. Four, the term GMC has been used for physiologically comparable colonic contractions in several species including conscious rat (25, 42), dogs (21, 31), cats (22), and humans (55). Given that the in vitro colonic tissue is devoid of extrinsic innervation and lacks the natural milieu of abdominal cavity pressure, difference between the in vitro and the conscious mouse colon motility pattern is not surprising. Similar differences have also been reported between in vivo vs. in vitro rat colon motor pattern and neuromediators roles on colonic motility (25). Further studies are needed to dissect out the chemical codes and neuronal pathway similarities and differences of colonic contractions in mice in vitro and in vivo (awake and anesthetized) to accurately characterize the mouse colon contractions.
Validity of Solid-State Manometry for Conscious Mouse colon Contraction Measurement
Appropriate methods to measure colonic motility pattern should be able to detect colonic motor events comparable to or better than the existing methods and be able to detect effects of inhibitory or excitatory stimuli known to modulate colonic motor function. The simultaneous measurement of colonic motor response by a classical method of fecal pellet output and the present manometry based colonic contractions show a positive correlation between the two methods. Similarly, the robust initial (first 20 min) colonic contractions detected by manometry in the present study is in line with the time course of fecal pellet output in rodents where over 85–90% of fecal expulsion in response to acute stressors occurs during the first 15 min (34). Contractions after the first 20 min are decreased to low levels with the overall colonic activity remaining the same for 40 min and even beyond the 60 min in few of the recordings made (data not shown). Another condition known to influence colonic motility is abdominal surgery that induces a postoperative ileus in humans and experimental animals including in mice (40). Postoperative colonic ileus in mice has been studied primarily by using gastrointestinal transit assays (43) and in one study by recording intracolonic pressure in awake restrained mice (40). We provide here evidence that both low-amplitude phasic contractions and high-amplitude GMCs are inhibited following abdominal surgery and 1-min cecal palpation in mice. This is in agreement with the reduction of large pressure waves of short duration, reported in awake mice (40). The above points argue in favor of a strong face validity of colonic contractions measured by the present miniature pressure transducer manometry in response to various stimuli known to influence colonic transit in conscious mice.
In addition, the present data show that colonic contractions including GMCs are atropine sensitive, suggesting a role of muscarinic pathway in the occurrence of colonic GMCs in conscious mice. Cholinergic involvement in the distal colon motor activity has also been shown in rats (25). However, l-NAME had no effect on the overall 60-min distal colonic contractility and only mildly attenuated the initial 10-min response. Given that NO is one of the inhibitory neurotransmitters in the gastrointestinal tract (5, 30), l-NAME was expected to cause contractions. The lack of stimulatory effects of l-NAME on conscious mouse distal colonic contraction may relate to 1) RS-induced mouse colon contractions may primarily involve excitatory neurotransmitters such as acetylcholine, as shown by the blocking effect of atropine, whose effect is not affected by l-NAME, and 2) NO in the distal colon may trigger cell signaling that does not involve cyclic GMP, such as extracellular Ca2+ influx through L-type Ca2+ channels that could result in contraction rather than relaxation (24). The latter could also explain the moderate inhibitory effect of l-NAME seen in the first 10 min of the colonic response. Several studies have also shown a region specific lack of l-NAME stimulatory effect in the gastrointestinal tract (25), particularly when the colon is under a stimulated state (19). It is nevertheless possible for NO to be involved in the propagation of the high-amplitude waves and transit by facilitating descending relaxation as has previously been described in rats (37). The present data on l-NAME provide also a basis to further study whether and how NO exerts different effects on basal vs. stress-induced colonic contractions.
In rodents, it has been shown that acute stress-induced stimulation of colonic motor function involves activation of central and peripheral CRF signaling in both rats and mice (60). The present study shows that increased distal colonic contractions are blocked by the peripheral administration of CRF1 and CRF2 receptor antagonist astressin B, in agreement with evidence that peripheral CRF receptor play a role in colonic response to exteroceptive stress (62, 64). Taken together, the successful use of still manometry to characterize colonic contractions in conscious mice placed in restraint tubes using astressin B as well as atropine and l-NAME lends support to the construct validity of the still manometry to measure conscious mouse colonic contractions reliably. We also showed that acute RS activates colonic myenteric neurons, as evidenced by Fos expression. It is likely that the activated enteric neurons represent a population of cholinergic motor neurons that may be activated through CRF pathways. We reported in the rat colon that intraperitoneal CRF peptide administration activates cholinergic myenteric neurons through CRF1-dependent receptors located on myenteric neurons (32, 64). Of significance is also that the colonic contractile response to restraint stress is characterized by an initial higher response (the first 20 min) followed by a lower and stable activity (20- to 60-min recording period). Astressin B selectively blocked the initial robust stress-induced response without affecting the later lower response. Similar blockade by astressin B of the initial high colonic motor response to stress, without affecting the subsequent basal like response, are well documented (28, 35). The evidence that astressin does not have effect on basal colonic motor activity (28, 34–36), coupled with the present data in which astressin B blocked the initial robust response without affecting the subsequent 20- to 60-min period response, suggests that the later non-astressin B-sensitive low and stable contractile pattern may represent a basal type of activity. A definitive description of conscious mice distal colon baseline contraction pattern, however, requires recordings from freely moving mice, in the presence or not of a catheter, a method that is not yet available.
Chronic Stress and Colonic Motor Pattern in Conscious Mice
Although chronic stress is the most relevant event in stress-related gut function alterations, to date the influence of chronic stressors on colonic motility in conscious mice as well as its underlying pathways have never been investigated. In addition, the few existing reports on chronic stress-related colonic fecal pellet output in rats are inconsistent in that an increase (52), decrease (1), or absence of effect (35) have been reported. Such variability is likely to be due to the use of different stress protocols and strains of animals along with the fact that fecal pellet output does not provide spatiotemporal information of motor events occurring in the colon. In the present study, fecal pellet output response was similar in the acute stress as well as the chronic stress groups, i.e., chronic repeated RS or in CRF-OE mice. However, we found a significant decrease in the duration of the initial colonic contraction in chronically stressed mice (10 min) compared with the acute stress controls (20 min). Similar pellet output between mice pretreated with l-NAME or saline and submitted to acute RS was also observed despite the short initial colonic response in the l-NAME-pretreated mice, suggesting that initial stimulation as short as 10 min is enough to cause maximal pellet output response to RS. Therefore, solid-phase colonic manometry method provides additional information on the motility pattern that could not been detected in the classical transit measurement methods and could be of great value to provide a physiological basis for chronic stress-related symptoms of colonic motor function alterations. Although CRF pathway was not explored in the chronically stressed mice, the striking similarity between the colonic contraction patterns of the chronic partial restraint stress and the CRF-OE mice with regards to the time and space organization of contractions suggests that such modifications may be related to CRF pathway. For instance, CRF2 signaling has been shown to play a stress-coping role to attenuate the magnitude and duration of the endocrine and defecation responses to stress in mice (7, 35, 49). Whether such a mechanism develops in chronically stressed mice is not known and requires further studies.
In summary, conscious mice distal colonic contraction involves rhythmic apparition of high- and low-amplitude phasic contractions. Mice display a higher number of high-amplitude propagative contractions than that reported in other mammalian species, consistent with the elevated number of fecal pellets expelled per unit time in mice. The study provides also evidence that acute RS-induced distal colonic contractility in conscious mice involves cholinergic and CRF signaling pathways and activates colonic myenteric neurons. Chronic stress does not affect the fecal pellet output response to an acute RS in mice, but leads to different colonic contractility pattern. Solid-state colonic manometry is a noninvasive and reliable method to monitor colonic motility in conscious mice and opens a new venue to measure subtle and discrete colonic motility changes. It is of note however, that whereas the new tool is easily and reliably used for a single site recording, simultaneous recording form several colonic regions without compromising the potency of the colon for luminal flow necessitates incorporation of multiple miniature transducers on a single probe. In view of the availability of several genetically modified mice and absence of tools to assess colonic motility in awake mice, solid-state manometry will offer a valuable alternative to study the hitherto unknown awake mice's normal and altered colonic motor activity and is poised to help better understand the pathophysiological basis for stress-related gut dysfunction.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R21 DK-068155 and RO1 DK078676 (M. Million), DK-57238; Veterans Affairs Career Scientist Award and Veterans Affairs Merit Award (Y. Taché), the French Foreign Office (Egide program) and the French Society of Gastroenterology (S.N.F.G.E.) (G. Gourcerol).
Acknowledgments
We thank Dr. J. Rivier (The Salk Institute, Peptide Biology Laboratories) for the synthesis and gift of astressin-B, Dr. M. Fanselow (R24AT00268) for the CRF-OE mice, and Honghui Liang for skillful technical assistance.
REFERENCES
- 1.Bonaz B, Rivest S. Effect of a chronic stress on CRF neuronal activity and expression of its type 1 receptor in the rat brain. Am J Physiol Regul Integr Comp Physiol 275: R1438–R1449, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Bonaz B, Tache Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res 641: 21–28, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Brierley SM, Nichols K, Grasby DJ, Waterman SA. Neural mechanisms underlying migrating motor complex formation in mouse isolated colon. Br J Pharmacol 132: 507–517, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bueno L, Collins S, Junien JL. Stress and Digestive Motility. Montrouge, France: John Libbey Eurotext, 1989.
- 5.Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 345: 346–347, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri M, Lee JS, Viramontes B, Bharucha AE, Tangalos EG. Insights into the pathophysiology and mechanisms of constipation, irritable bowel syndrome, and diverticulosis in older people. J Am Geriatr Soc 48: 1142–1150, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet 24: 403–409, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Coste SC, Murray SE, Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides 22: 733–741, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Coulie B, Camilleri M, Bharucha AE, Sandborn WJ, Burton D. Colonic motility in chronic ulcerative proctosigmoiditis and the effects of nicotine on colonic motility in patients and healthy subjects. Aliment Pharmacol Ther 15: 653–663, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Croci T, Basilisco G, Bassani A, Manara L. Manometric patterns of rat colonic motor activity and defecation. Effect of selective 5HT1A agonist 8-OH-DPAT. Dig Dis Sci 39: 1968–1973, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Dinning PG, Szczesniak MM, Cook IJ. Proximal colonic propagating pressure waves sequences and their relationship with movements of content in the proximal human colon. Neurogastroenterol Motil 20: 512–520, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Fida R, Lyster DJ, Bywater RA, Taylor GS. Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol Motil 9: 99–107, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Fioramonti J, Bueno L, Frexinos J. [An intraluminal probe for recording myoelectrical activity of the human colon (author's translation)]. Gastroenterol Clin Biol 4: 546–550, 1980. [PubMed] [Google Scholar]
- 14.Fioramonti J, Dupuy C, Bueno L. In vivo motility of rat colon chronically pretreated with sennosides. Pharmacology 47, Suppl 1: 155–161, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Fioramonti J, Garcia-Villar R, Bueno L, Ruckebusch Y. Colonic myoelectrical activity and propulsion in the dog. Dig Dis Sci 25: 641–646, 1980. [DOI] [PubMed] [Google Scholar]
- 16.Furness JB, Robbins HL, Xiao J, Stebbing MJ, Nurgali K. Projections and chemistry of Dogiel type II neurons in the mouse colon. Cell Tissue Res 317: 1–12, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Gourcerol G, Coskun T, Craft LS, Mayer JP, Heiman ML, Wang L, Million M, St Pierre DH, Tache Y. Preproghrelin-derived peptide, obestatin, fails to influence food intake in lean or obese rodents. Obesity (Silver Spring) 15: 2643–2652, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Gourcerol G, Million M, Adelson DW, Wang Y, Wang L, Rivier J, St Pierre DH, Tache Y. Lack of interaction between peripheral injection of CCK and obestatin in the regulation of gastric satiety signaling in rodents. Peptides 27: 2811–2819, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Gursoy N, Durmus N, Bagcivan I, Sarac B, Parlak A, Yildirim S, Kaya T. Investigation of acute effects of aflatoxin on rat proximal and distal colon spontaneous contractions. Food Chem Toxicol 46: 2876–2880, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Halpert A, Drossman D. Biopsychosocial issues in irritable bowel syndrome. J Clin Gastroenterol 39: 665–669, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Karaus M, Sarna SK. Giant migrating contractions during defecation in the dog colon. Gastroenterology 92: 925–933, 1987. [DOI] [PubMed] [Google Scholar]
- 22.Lang IM, Sarna SK, Shaker R. Gastrointestinal motor and myoelectric correlates of motion sickness. Am J Physiol Gastrointest Liver Physiol 277: G642–G652, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Law NM, Bharucha AE, Undale AS, Zinsmeister AR. Cholinergic stimulation enhances colonic motor activity, transit, and sensation in humans. Am J Physiol Gastrointest Liver Physiol 281: G1228–G1237, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Lefebvre RA, Bartho L. Mechanism of nitric oxide-induced contraction in the rat isolated small intestine. Br J Pharmacol 120: 975–981, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Johnson CP, Adams MB, Sarna SK. Cholinergic and nitrergic regulation of in vivo giant migrating contractions in rat colon. Am J Physiol Gastrointest Liver Physiol 283: G544–G552, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Luckey A, Wang L, Jamieson PM, Basa NR, Million M, Czimmer J, Vale W, Tache Y. Corticotropin-releasing factor receptor 1-deficient mice do not develop postoperative gastric ileus. Gastroenterology 125: 654–659, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Martinez V, Barrachina MD, Ohning G, Tache Y. Cephalic phase of acid secretion involves activation of medullary TRH receptor subtype 1 in rats. Am J Physiol Gastrointest Liver Physiol 283: G1310–G1319, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Martinez V, Wang L, Rivier J, Grigoriadis D, Tache Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol 556: 221–234, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez V, Wang L, Rivier JE, Vale W, Tache Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther 301: 611–617, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Mashimo H, He XD, Huang PL, Fishman MC, Goyal RK. Neuronal constitutive nitric oxide synthase is involved in murine enteric inhibitory neurotransmission. J Clin Invest 98: 8–13, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsushima Y [Studies on colonic motor correlates of spontaneous defecation in conscious dogs]. Nippon Heikatsukin Gakkai Zasshi 25: 137–146, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Miampamba M, Million M, Yuan PQ, Larauche M, Tache Y. Water avoidance stress activates colonic myenteric neurons in female rats. Neuroreport 18: 679–682, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller SM, Szurszewski JH. Relationship between colonic motility and cholinergic mechanosensory afferent synaptic input to mouse superior mesenteric ganglion. Neurogastroenterol Motil 14: 339–348, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Million M, Wang L, Martinez V, Tache Y. Differential Fos expression in the paraventricular nucleus of the hypothalamus, sacral parasympathetic nucleus and colonic motor response to water avoidance stress in Fischer and Lewis rats. Brain Res 877: 345–353, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Million M, Wang L, Stenzel-Poore MP, Coste SC, Yuan PQ, Lamy C, Rivier J, Buffington T, Tache Y. Enhanced pelvic responses to stressors in female CRF-overexpressing mice. Am J Physiol Regul Integr Comp Physiol 292: R1429–R1438, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Million M, Wang L, Stenzel-Poore MP, Coste SC, Yuan PQ, Lamy C, Rivier J, Buffington T, Tache Y. Enhanced pelvic responses to stressors in female CRF-overexpressing mice. Am J Physiol Regul Integr Comp Physiol 292: R1429–R1438, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizuta Y, Takahashi T, Owyang C. Nitrergic regulation of colonic transit in rats. Am J Physiol Gastrointest Liver Physiol 277: G275–G279, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Monnikes H, Schmidt BG, Tebbe J, Bauer C, Tache Y. Microinfusion of corticotropin releasing factor into the locus coeruleus/subcoeruleus nuclei stimulates colonic motor function in rats. Brain Res 644: 101–108, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Monnikes H, Tebbe JJ, Hildebrandt M, Arck P, Osmanoglou E, Rose M, Klapp B, Wiedenmann B, Heymann-Monnikes I. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig Dis 19: 201–211, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Moore BA, Turler A, Pezzone MA, Dyer K, Grandis J, Bauer AJ. Tyrphostin AG 126 inhibits development of postoperative ileus induced by surgical manipulation of murine colon. Am J Physiol Gastrointest Liver Physiol 286: G214–G224, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Morrow NS, Garrick T. Effects of intermittent tail shock or water avoidance on proximal colonic motor contractility in rats. Physiol Behav 62: 233–239, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Nakade Y, Fukuda H, Iwa M, Tsukamoto K, Yanagi H, Yamamura T, Mantyh C, Pappas TN, Takahashi T. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol 292: G1037–G1044, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Nakao A, Schmidt J, Harada T, Tsung A, Stoffels B, Cruz RJ Jr, Kohmoto J, Peng X, Tomiyama K, Murase N, Bauer AJ, Fink MP. A single intraperitoneal dose of carbon monoxide-saturated ringer's lactate solution ameliorates postoperative ileus in mice. J Pharmacol Exp Ther 319: 1265–1275, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Neal KB, Parry LJ, Bornstein JC. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J Physiol 587: 567–586, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nurgali K, Stebbing MJ, Furness JB. Correlation of electrophysiological and morphological characteristics of enteric neurons in the mouse colon. J Comp Neurol 468: 112–124, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Pinto L, Izzo AA, Cascio MG, Bisogno T, Hospodar-Scott K, Brown DR, Mascolo N, Di Marzo V, Capasso F. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology 123: 227–234, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Powell AK, Fida R, Bywater RA. Motility in the isolated mouse colon: migrating motor complexes, myoelectric complexes and pressure waves. Neurogastroenterol Motil 15: 257–266, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Powell AK, O'brien SD, Fida R, Bywater RA. Neural integrity is essential for the propagation of colonic migrating motor complexes in the mouse. Neurogastroenterol Motil 14: 495–504, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Rivier CL, Grigoriadis DE, Rivier JE. Role of corticotropin-releasing factor receptors type 1 and 2 in modulating the rat adrenocorticotropin response to stressors. Endocrinology 144: 2396–2403, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Rivier JE, Kirby DA, Lahrichi SL, Corrigan A, Vale WW, Rivier CL. Constrained corticotropin releasing factor antagonists (astressin analogues) with long duration of action in the rat. J Med Chem 42: 3175–3182, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Roberts RR, Bornstein JC, Bergner AJ, Young HM. Disturbances of colonic motility in mouse models of Hirschsprung's disease. Am J Physiol Gastrointest Liver Physiol 294: G996–G1008, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Saito K, Kasai T, Nagura Y, Ito H, Kanazawa M, Fukudo S. Corticotropin-releasing hormone receptor 1 antagonist blocks brain-gut activation induced by colonic distention in rats. Gastroenterology 129: 1533–1543, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Sang Q, Young HM. The identification and chemical coding of cholinergic neurons in the small and large intestine of the mouse. Anat Rec 251: 185–199, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Sarna SK Physiology and pathophysiology of colonic motor activity (1). Dig Dis Sci 36: 827–862, 1991. [DOI] [PubMed] [Google Scholar]
- 55.Sarna SK Molecular, functional, and pharmacological targets for the development of gut promotility drugs. Am J Physiol Gastrointest Liver Physiol 291: G545–G555, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Sarna SK, Lang IM. Colonic motor response to a meal in dogs. Am J Physiol Gastrointest Liver Physiol 257: G830–G835, 1989. [DOI] [PubMed] [Google Scholar]
- 57.Scott SM Manometric techniques for the evaluation of colonic motor activity: current status. Neurogastroenterol Motil 15: 483–513, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Spencer NJ Control of migrating motor activity in the colon. Curr Opin Pharmacol 1: 604–610, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci 14: 2579–2584, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest 117: 33–40, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taché Y, Million M. Central corticotropin-releasing factor and the hypothalamic-pituitary-adrenal axis in gastrointestinal physiology, In: Physiology of the Gastrointestinal Tract, edited by Johnson LR and Wood J. Burlington, MA: Elsevier Academic, 2006, p. 791–816.
- 62.Taché Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol 141: 1321–1330, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, Martinez V, Kimura H, Tache Y. 5-Hydroxytryptophan activates colonic myenteric neurons and propulsive motor function through 5-HT4 receptors in conscious mice. Am J Physiol Gastrointest Liver Physiol 292: G419–G428, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Yuan PQ, Million M, Wu SV, Rivier J, Tache Y. Peripheral corticotropin releasing factor (CRF) and a novel CRF1 receptor agonist, stressin1-A activate CRF1 receptor expressing cholinergic and nitrergic myenteric neurons selectively in the colon of conscious rats. Neurogastroenterol Motil 19: 923–936, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]