Abstract
Although the inducible heat shock protein 70 (Hsp70) is essential for maintaining intestinal homeostasis in colitis, it is translationally downregulated in inflamed colonic mucosa, paradoxically rendering the gut more susceptible to injury. We examined the basis for this process by analyzing the role of untranslated regions (UTR) of Hsp70 mRNA in inflammation-associated downregulation in vitro and in vivo. Using luciferase-reporter assays in young adult mouse intestinal epithelial cells, we determined that cytokine-induced translational inhibition of Hsp70 mRNA was mediated by the 3′UTR, but not 5′UTR. In vivo, dextran sodium sulfate (DSS) colitis was induced in wild-type (WT) and villin-promoter regulated “UTR-less” Hsp70 transgenic (TG) mice, the latter exhibiting intestinal epithelial-specific transgene expression. Progressive downregulation of colonic Hsp70 protein expression was observed in WT, but not in TG, mice with increasing severity of mucosal inflammation, confirming the essential role of the 3′UTR in mediating inflammation-associated downregulation of Hsp70. Hsp70 TG mice demonstrated significantly lower endoscopic and histological inflammation scores in DSS-induced colitis than WT. In conclusion, downregulation of Hsp70 expression in inflamed mucosa is mediated by translational inhibition requiring the 3′UTR, resulting in increased mucosal injury. By forcing intestinal epithelial-specific Hsp70 expression in vivo, the severity of experimentally induced colitis was significantly reduced.
Keywords: 3′ untranslated region, dextran sodium sulfate, heat shock protein 70
heat shock proteins (Hsps) are highly conserved, multifunctional proteins, falling into two major categories, constitutive Hsps, such as Hsc70 and Hsp60, and inducible Hsps, such as Hsp70 and Hsp25. Inducible Hsps are preferentially synthesized under stress conditions, conferring cellular protection through their ability to stabilize critical cellular proteins, thereby preventing denaturation and preserving function (12, 13, 15, 27). The major induced Hsp, Hsp70, is primarily, but not exclusively, expressed in the colon by surface colonocytes. Its expression in this region is maintained by cues largely derived from enteric microflora, through Toll-like receptor signals, quorum-sensing molecules, such as organic cation/carnitine transporter OCTN2, and short-chain fatty acids (1, 7, 15, 22, 26). Colonic Hsp70 expression is important to maintain colonic homeostasis, rendering mucosal cells less susceptible to injury from stresses and pathogens and enhancing preservation of mucosal functions. Among its actions, Hsp70 binds and prevents stress-induced denaturation of critical proteins involved in barrier (tight junction) function, transport, cytoskeleton, immune modulation, and apoptosis (14, 19, 28, 33, 34). We recently showed that Hsp70-null mice treated with azoxymethane/dextran sodium sulfate (DSS) develop chronic colitis and multifocal flat dysplasia and invasive cancer similar to ulcerative colitis (33). Thus, in the absence of Hsp70, a normally transient injury is converted to a self-sustaining severe colitis, and complicating neoplastic transformation is greatly enhanced.
Prior studies from our laboratory (9) revealed that colonic epithelial Hsp70 is downregulated in inflamed colonic tissue in inflammatory bowel disease (IBD) and in chronic experimental colitis in IL-10−/−-deficient mice compared with noninflamed controls. In inflamed colonic mucosa, elevated levels of proinflammatory cytokines, especially IFN-γ and TNF-α, selectively inhibit Hsp70 translation, in part, through stimulation of dsRNA-dependent protein kinase (PKR), causing inhibitory phosphorylation of eukaryotic initiation factor 2α (eIF-2α). These actions interfere with polyribosomal engagement of mRNA in protein translation both in vivo and in vitro (9). However, the possibility that the 5′ and/or 3′ untranslated regions (UTR) of the Hsp70 mRNA are involved in inflammation-associated downregulation of Hsp70 has not been explored.
In this study, we explored the role of 5′- and 3′UTRs of Hsp70 mRNA in inflammation-induced downregulation of colonic Hsp70 in epithelial colonic cells and in transgenic (TG) mice engineered to express intestinal epithelial-specific Hsp70 using a villin-promoter-driven Hsp70 transgene that lacked Hsp70–3′UTR. Moreover, we examined the physiological consequences of forced Hsp70 expression in TG mice in the DSS-induced colitis model. Our long-term objective was a more complete understanding of the translational regulation of Hsp70, which may provide a basis for preventing Hsp70 downregulation and restoring intestinal homeostasis.
MATERIAL AND METHODS
Cell culture.
Conditionally immortalized young adult mouse colonic epithelial cells (YAMC) were used for in vitro experiments, which were generously provided by Dr. Robert Whitehead, Vanderbilt University, Nashville, TN. YAMC cells were grown at 33°C as previously described (36). Cells were fed IFN-γ-free medium and switched to the nonpermissive conditions of 37°C for 16–24 h. During this time, SV40 large T antigen is no longer produced because of a temperature-sensitive mutation at amino acid 58 (tsA). After treatments, cells were rinsed two times and scraped into ice-cold PBS. Cells were pelleted (14,000 g for 20 s) and lysed for RNA and protein extraction as described (9).
Animal.
Wildtype (WT), Hsp70 knockout (KO), and Hsp70 TG mice on a C57BL/6 background were used in the study. All mice were maintained under specific pathogen-free (SPF) conditions that are required for maintaining our mouse colonies. Under these conditions, the course of acute colitis induced by DSS is not affected and not different from that reported in mice from a non-SPF environment (33). All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Chicago and were consistent with NIH guidelines for humane care of animals.
Hsp70 KO mice (Hsp 70.1−/− and 70.3−/−) on a mixed 129S/C57BL/6J background (8, 10) were bred back 12 generations to a pure C57BL/6 background through the speed congenic services of Jackson Laboratory (Bar Harbor, ME). Mice heterozygous for Hsp70 deletion (Hsp 70.1+/− and 70.3+/−) were interbred, and WT and double Hsp70 KO littermates were used for experiments (33).
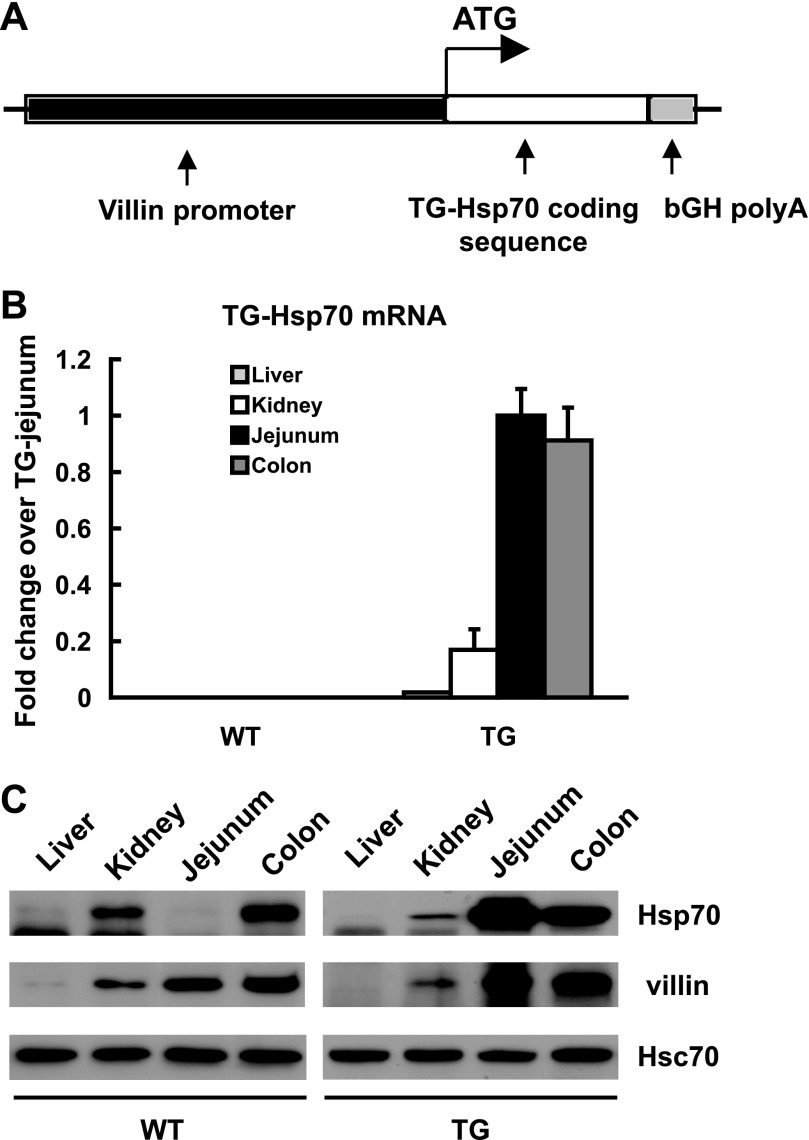
To study the role of the Hsp70 UTR in translation regulation in vivo, a tissue-specific “UTR-less” Hsp70 TG mouse was developed. The villin-Hsp70 TG expression construct was engineered by fusing the full-length coding sequence of human Hsp70 cDNA cloned at the initiation codon of mouse 9 kb villin promoter (24), as shown in schematic Fig. 4A. A termination signal and bovine growth hormone polyA signal were added downstream. The construct was linearized and injected into fertilized oocytes of C57BL/6 mice. High copy and low copy lines were obtained and bred on C57BL/6 background. TG animals were identified by Southern blotting and PCR analysis using tail genomic DNA. Under nonstressed conditions, the transgenic mice lines displayed no detectable phenotypic differences from WT littermate controls. Therefore, the high copy line was arbitrarily chosen for further characterization and analysis of colonic Hsp70 expression. The Hsp70 transgene mRNA can be differentiated from endogenous murine Hsp70 mRNA by real-time PCR with the use of the specific primers listed below.
Fig. 4.
Intestine-specific Hsp70 expression from “UTR-less” Hsp70 mRNA in villin-Hsp70 transgenic (TG) mice. A: schematic of targeting transgene construct. The 11.1-kbp fragment, comprising the villin promoter followed by the human Hsp70 protein coding sequence and bovine growth hormone polyA signal, was used to generate TG mice on C57BL/6 background. B: expression of TG Hsp70 mRNA (TG-Hsp70) in liver, kidney, jejunum, and transverse colon of WT and TG mice. C: Hsp70, villin, and Hsc70 were analyzed by Western blot. Images shown are representative of 4 individual experiments.
Induction of DSS-colitis.
To induce colitis, 8–10-wk-old mice were given 2% or 3% DSS (ICN Chemicals, Costa Mesa, CA) in drinking water for 7 days. Mice were monitored for weight changes, diarrhea, bloody stool, and overall health (4, 5). Mice were euthanized with sevoflurane at days 2, 3, 4, 5, 6, and 7 after DSS treatment. For Western blot, real-time PCR, and myeloperoxidase (MPO) assays, intestinal segments were opened along the longitudinal axis and mucosa scraped off with glass slides. Adjacent tissues were also fixed in 10% neutral buffered formalin for hematoxylin and eosin staining. MPO activity was measured as an indicator of neutrophil accumulation in colonic mucosa with the use of an MPO chlorination assay (EnzChek MPO Activity Assay Kit; Molecular Probes, Eugene, OR). Histological grading of intestinal inflammation was performed by a pathologist blinded to the treatment conditions and Hsp70 genotype using a previously validated scoring system previously described: no inflammation was scored as 0; modest numbers of infiltrating cells in the lamina propria was scored as 1; infiltration of mononuclear cells leading to separation of crypts and mild mucosal hyperplasia was scored as 2; massive infiltration with inflammatory cells accompanied by disrupted mucosal architecture, loss of goblet cells, and marked mucosal hyperplasia was scored as 3; and the presence of features in 1–3 plus crypt abscesses or ulceration were scored as 4 (16, 30).
Western blot analysis.
Mouse mucosal samples from transverse colon or pelleted YAMC cells were homogenized in 10 mM Tris, pH 7.4, 5 mM MgCl2, complete protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN), 50 U/ml DNAse (Amersham, Piscataway, NJ), and 50 U/ml RNAse (Ambion, Austin, TX). An aliquot (10 μl) was removed for protein analysis with the bicinchoninic acid method. To the remainder, 3× Laemmli stop solution was added and samples heated to 65°C for 10 min.
Twenty micrograms of protein were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes in 25 mM Tris, pH 8.8, 192 mM glycine, and 15% (vol/vol) methanol. Membranes were blocked with 5% (wt/vol) nonfat dry milk in Tween-Tris-buffered saline (TTBS). Blots were incubated overnight at 4°C in primary antibodies for Hsp70 (SPA810; StressGen/Assay Designs, Ann Arbor, MI), Hsc70 (SPA815, StressGen), and cytokeratin 18 (18–0158; Zymed Laboratory, South San Francisco, CA). Membranes were washed with TTBS, incubated with horseradish peroxidase-conjugated species-appropriate secondary antibodies (Jackson Immunoresearch, West Grove, PA) for 1 h at room temperature, and developed using an enhanced chemiluminescence system (Supersignaling; Pierce, Rockford, IL).
Quantification of images was performed by scanning densitometry using NIH Image J 1.54 software (National Institutes of Health, Bethesda, MD).
Quantitation of Hsp70 mRNA with real-time PCR.
Total RNA was extracted from mouse transverse colonic mucosa or pelleted YAMC cells by Trizol (Invitrogen, Grand Island, NY) according to the manufacturer's instructions. Complementary DNA was synthesized using SuperScript II (Invitrogen) and random hexanucleotide primers. The forward and reverse primers used were as follows: mouse endogenous Hsp70, 5′-GGCTGATCGGCCGCAAGTT-3′ and 5′-GGAAGGGCCAGTGCTTCAT-3′; TG human Hsp70, 5′-ACTGCCCTGATCAAGCGC-3′ and 5′-CGGGTTGGTTGTCGGAGTAG-3′; mouse GAPDH, 5′-GGCAAATTCAACGGCACAGT-3′ and 5′-AGATGGTGATGGGCTTCCC-3′; Firefly luciferase, 5′-AAGATTCAAAGTGCGCTGCTGGTG-3′ and 5′-TTGCCTGATACCTGGCAGATGGAA-3′; Renilla luciferase, 5′-CAGTGGTGGGCCAGATGTAAACAA-3′ and 5′-TAAGAAGAGGCCGCGTTACCATGT-3′. Real-time PCR was performed in an iCycler (Bio-Rad, Hercules, CA) using iQSYBR Green PCR Supermix (Bio-Rad). A two-step quantification cycling protocol was used. The Ct value is defined as the cycle number at which the fluorescence crosses a fixed threshold above the baseline. As a relative quantitation, fold changes were measured using the ΔΔCt method. For each sample, the Ct value of Hsp70 mRNA was normalized to the GAPDH endogenous control as ΔCt, (ΔCt = CtHsp − CtGAPDH). The fold change of Hsp70 mRNA in the experimental sample relative to control sample was determined by 2−ΔΔCt, where ΔΔCt = ΔCtExperimental − ΔCtControl (31).
Luciferase reporter assay.
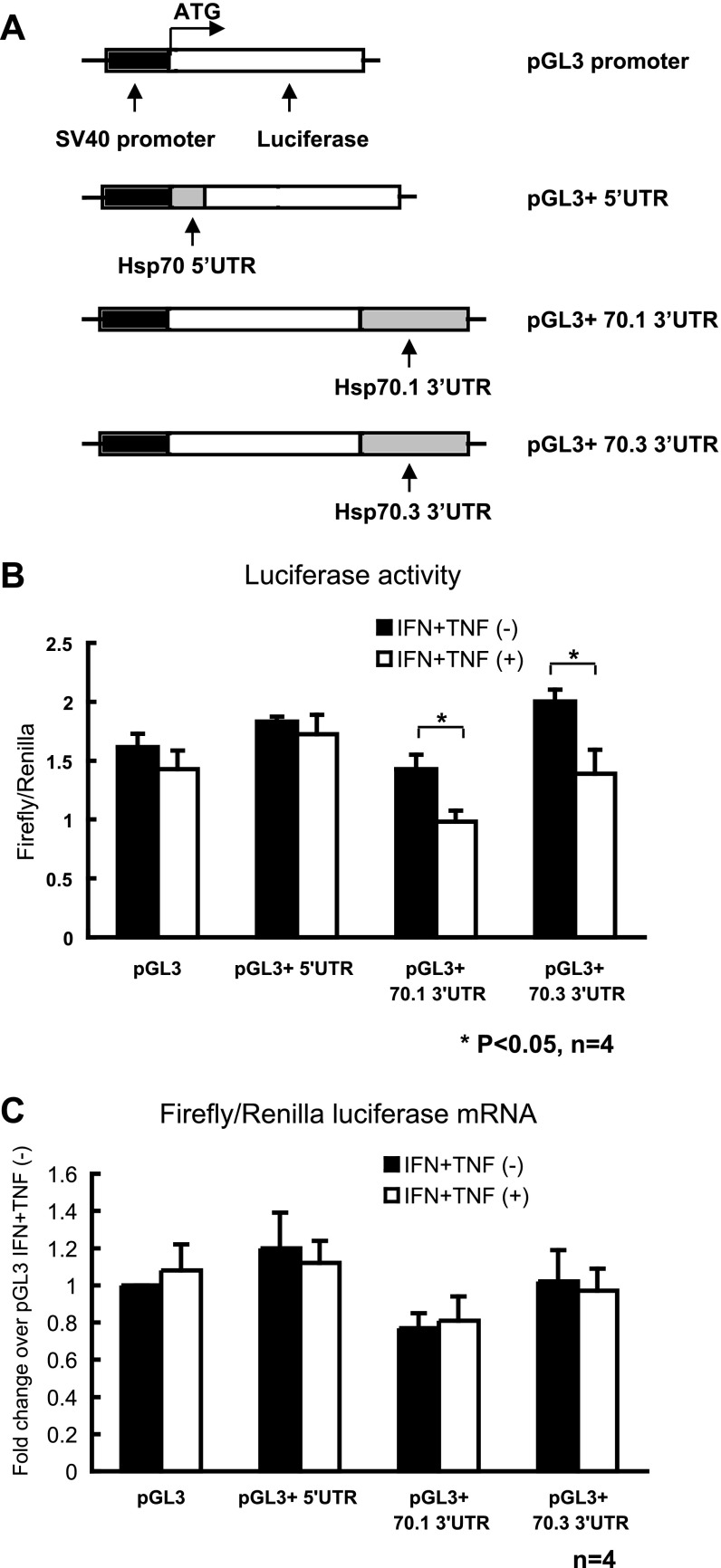
YAMC cells were transiently transformed with pGL3-promoter vector (E1761; Promega, Madison, WI) and pRL-TK plasmids (Renilla luciferase driven by thymidine kinase promoter, E2241, Promega) using TransIT LT-1 (Mirus, Madison, WI) transfection reagent according to the manufacturer's recommendations. Modified pGL3-promoter vectors were constructed with additional 5′UTR or 3′UTR of mouse Hsp70 mRNAs downstream to luciferase coding sequence (Fig. 3A). Two copies of Hsp70 gene are expressed in mature mouse colonic cells, Hsp70.1 and Hsp70.3, which have identical 5′UTR and coding sequences but different 3′UTR. Sixteen hours after transfection, cells were treated with a combination of murine IFN-γ (200 U/ml) and murine TNF-α (100 ng/ml) for 8 h before heat shock at 42°C for 23 min, as previously described (9). Cells were harvested 2 h after heat shock in 500 μl active lysis buffer (Promega). Firefly and Renilla luciferase activity in the lysate were determined with a Dual-Luciferase Reporter assay system, according to the manufacturer's instructions (Promega). Triplicate samples were assayed for the Firefly luciferase activities and normalized to Renilla luciferase activity. Firefly and Renilla luciferase mRNA levels were measured using real-time PCR. Firefly luciferase mRNA was normalized to the Renilla luciferase mRNA, which served as the transfection efficiency control.
Fig. 3.
Hsp70 3′ untranslated regions (UTRs) participate in translational inhibition induced by IFN-γ and TNF-α in young adult mouse colonic epithelial cells (YAMC) cells. A: schematic of constructs expressing chimeric luciferase transcripts containing 5′- or 3′UTR of Hsp70. All constructs derived from pGL3-promoter contain the Firefly luciferase-coding region as a reporter gene and full-length 5′UTR or 3′UTR of mouse Hsp 70.1 and 70.3. Mouse Hsp 70.1 and 70.3 mRNAs have identical 5′UTR nucleotide sequences. Transcription of the luciferase-coding region is under the control of the SV40 promoter. Luciferase vectors, pGL3 and pRL-TK, were transfected into YAMC cells. 16 h later, cells were treated with IFN-γ (200 U/ml) and TNF-α (100 ng/ml) for 8 h and then subjected to heat shock. B: firefly luciferase activity normalized with Renilla luciferase activity. C: firefly luciferase mRNA was analyzed by real-time PCR and normalized to Renilla luciferase mRNA as the transfection efficiency control. Results are means ± SE; n = 4. *P < 0.05 compared IFN + TNF(−) and IFN + TNF(+) by t-test.
Immunohistochemical staining for Hsp70.
Sections of mouse tissues were fixed in 10% (vol/vol) neutral buffered formalin and embedded in paraffin, and 4-μm cross-sections were cut. Sections were stained for mouse Hsp70 using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions, modified by using the citrate-microwave antigen recapture procedure, as previously described (12). The primary antibodies used for Western blots (SPA810; StressGen) were also employed for immunostaining.
Colonoscopic assessment of colitis.
Colonic mucosal damage attributable to the DSS treatment was assessed in vivo using the Coloview (Karl Storz, Tuttlingen, Germany) experimental colonoscopy system, as previously described (20, 35). Mice undergoing colonoscopic examination were placed under anesthesia using 100 μg ketamine and 10 μg xylazine per gram of body weight in accordance with an approved IACUC protocol. The colitis severity was determined by using a previously described murine endoscopic intestinal colitis scoring system: the evaluation of colon translucency (0–3 points), presence of fibrin attached to the bowel wall (0–3 points), granular features of the mucosa (0–3 points), morphology of the vascular pattern (0–3 points), and presence of loose stool (0–3 points), with range of 0–15 points (20, 35).
Statistical analysis.
Results are presented as the means ± SE for the indicated number of experiments. The results of multiple experiments were analyzed by using Student's t-test or analysis of variance using a Bonferroni correction for multiple comparisons.
RESULTS
Colonic Hsp70 is downregulated in DSS-induced colitis.
To determine whether murine colonic Hsp70 levels are altered by inflammation, C57BL/6 mice were treated with 3% DSS in their drinking water for 7 days. This is a well-established murine model of acute colitis, characterized by a lag time of 3–4 days, then gradual weight loss, appearance of bloody stools, and onset of colonic inflammation (4, 5). Following DSS treatment, intestinal inflammation (Fig. 1B), MPO activity (Fig. 1C), weight loss, and appearance of bloody stool were evident by day 4 and peaked at about day 7. As tissue inflammation increased, colonic Hsp70 levels decreased (Fig. 1A). A significant reduction in Hsp70 expression was evident by day 5 of DSS treatment, decreasing even further by day 7. In contrast, expression of constitutive Hsc70 and epithelial-specific cytokeratin 18 was unchanged over the course of DSS treatment. No significant changes in Hsp70 mRNA levels were detected in DSS-treated mouse colon at days 4 and 7 (Fig. 1D), suggesting that posttranscriptional processing of Hsp70 was involved. Decreased Hsp70 expression was also evident by immunohistochemistry analysis, shown in Fig. 5A.
Fig. 1.
Colonic heat shock protein 70 (Hsp70) decreases in dextran sodium sulfate (DSS)-induced inflammation. C57BL/6 mice were given 3% wt/vol DSS for up to 7 days in their drinking water. On days 0, 2, 3, 4, 5, 6, and 7, DSS-treated and control mice were euthanized. Mucosa from transverse colon were harvested for the following: Western blot of Hsp70, Hsc70 and cytokeratin-18 (A); histological grading of intestinal inflammation (B); and myeloperoxidase (MPO) activity (μg/g) (C). D: real-time PCR analysis of mRNA abundance of Hsp70 in control and DSS-treated mouse colonic mucosa on days 4 and 7. Changes in Hsp mRNA relative to GAPDH were determined by 2−ΔΔCt as fold change over control. Results are means ± SE; n = 4.
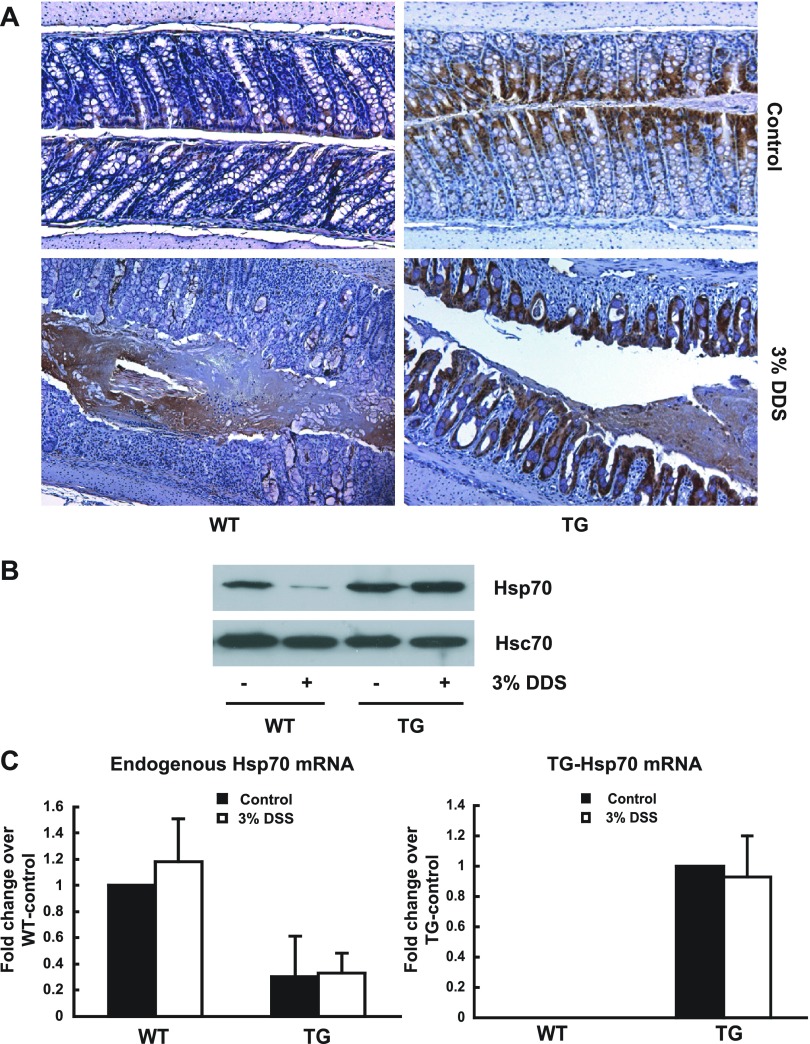
Fig. 5.
Protein expression of UTR-less Hsp70 mRNA is resistant to DSS-induced inflammation. WT and Hsp70 UTR-less TG mice were given 3% DSS for 7 days and then euthanized. Mice given normal tap water were euthanized as controls. Colonic mucosa of transverse colon was harvested for the following: immunohistochemistry staining of Hsp70 (A), Western blot of Hsp70 and Hsc70 (B), and real-time PCR of endogenous Hsp70 mRNA and UTR-less TG-Hsp70 mRNA (C).
Compromised intestinal homeostasis in Hsp70 KO mice with DSS-induced colitis.
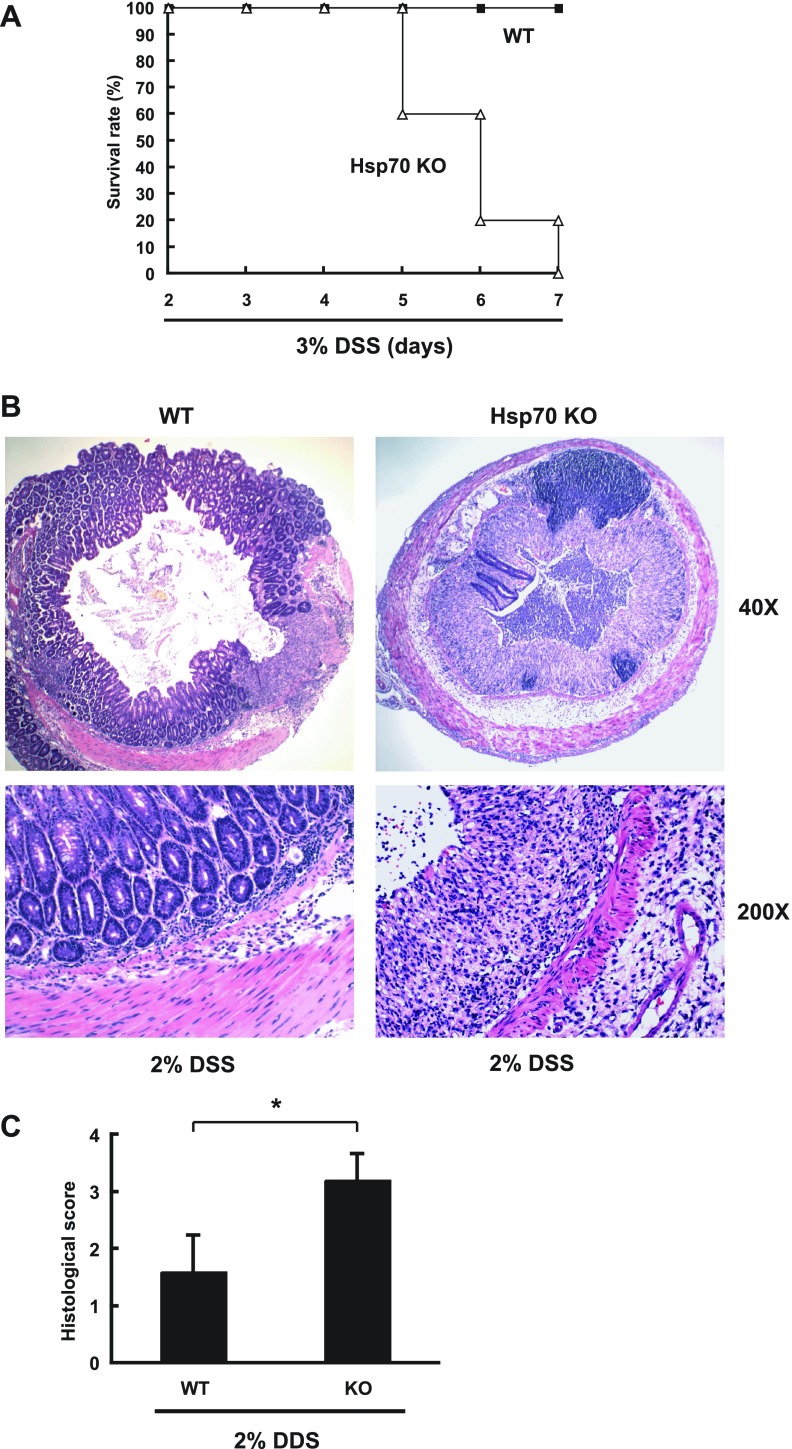
To determine whether intestinal homeostasis is compromised by loss of Hsp70 expression, Hsp70 KO mice and littermate WT controls were treated DSS. As shown in Fig. 2A, Hsp70 KO mice did not survive a 7-day-course of 3% DSS, whereas WT mice were all alive. To assess the histological change between WT and Hsp70 KO mice in colitis, DSS treatment was adjusted to a lower 2% dose for 5 days and mice euthanized 5 days after DSS cessation. All WT and Hsp70 KO mice survived 2% DSS treatment. Hsp70 KO mice demonstrated severe colitis with luminal necrotic debris, whereas WT mice showed almost complete recovery with limited residual ulceration (Fig. 2B). Significantly higher histological inflammation scores (P < 0.05) were observed in 2% DSS-treated Hsp70 KO mice compared with WT (Fig. 2C), indicating that absence of Hsp70 renders KO mice significantly more susceptible to DSS injury.
Fig. 2.
Hsp70 knockout (KO) mice are more susceptible to DSS treatment than wild-type (WT) mice. Hsp70 KO mice (Hsp 70.1−/− and 70.3−/−) and WT littermates were given DSS in drinking water. A: survival rate of WT and Hsp70 KO mice given 3% DSS for 7 days. B: WT and Hsp70 KO mice were treated with 2% DSS for 5 days and euthanized 5 days after treatment. The transverse colon was harvested for hematoxylin and eosin staining. Images shown are representative of 5 individual experiments. C: histological grading of intestinal inflammation in WT and Hsp70 KO mice treated with 2% DSS. *P < 0.05 compared WT and KO mice; n = 5.
The 3′UTR of Hsp70 mediates cytokine inhibition of reporter gene translation in YAMC cells.
To investigate mechanisms responsible for the inflammation-induced reduction in colonic Hsp70 expression, YAMC cells were transfected with different luciferase reporters vectors containing regulatory 5′- or 3′UTR from Hsp70. Measurement of firefly luciferase activity served as a measure of Hsp70 mRNA translation as described (18). Previously, we reported that the proinflammatory cytokines, IFN-γ and TNF-α, contribute to decreases in colonic Hsp70 expression in intestinal inflammation (9). As shown in Fig. 3A, Hsp70 5′UTR was fused upstream of the luciferase gene, Hsp70.1 and Hsp70.3 3′UTR downstream (Hsp70.1 and Hsp70.3 mRNA have identical 5′UTR sequences). The pRL-TK vector expressing Renilla luciferase driven by thymidine kinase promoter was transfected simultaneously to normalize for transfection efficiency. Cells were treated with IFN-γ and TNF-α and then heat shocked as described in the materials and methods. There was significant luciferase reporter activity after transfection under all conditions (Fig. 3B). IFN-γ and TNF-α treatment did not change the luciferase expression in cells transfected with UTR-less reporter vectors or vectors fused with Hsp70 5′UTR. However, in cells pretreated with IFN-γ and TNF-α, the presence of either Hsp70.1 or Hsp70.3 3′UTR significantly decreased the translation of chimeric luciferase mRNAs (Fig. 3B). Firefly luciferase mRNA levels were also measured in these cells and normalized by Renilla luciferase mRNA levels that severed as the transfection efficiency control. As shown in Fig. 3C, IFN-γ and TNF-α treatment did not change the ratio of luciferase mRNAs in cells transfected with the reporter vectors. These findings suggested that the 3′UTRs from Hsp70.1 and Hsp70.3 mRNAs mediate the inhibitory effects of IFN-γ and TNF-α on translation of these Hsp70 isoforms.
Expression of Hsp70 in UTR-less TG mice.
On the basis of the in vitro luciferase reporter assays (Fig. 3), we investigated the role of Hsp70 3′UTR in downregulation of this chaperone in vivo in experimental colitis. A TG mouse was developed that expressed Hsp70 transgene lacking 3′UTR under the control of the intestinal-epithelial specific villin promoter (Fig. 4A). TG-Hsp70 mRNA levels were measured in different organs, including liver, kidney, jejunum, and transverse colon. In WT mice, as expected, TG-Hsp70 mRNA was not detected in any tissues. In TG mice, high levels of TG-Hsp70 mRNA were present in jejunum and colonic mucosa, lower levels in kidney, and minimum levels in liver tissue (Fig. 4B). Similarly, high Hsp70 protein expression was found in the mature epithelium of intestinal mucosa of TG mice by Western blot analysis (Fig. 4C), corresponding to regions where villin expression would be anticipated. As might be expected with constitutive transgenic Hsp70 protein expression, endogenous Hsp70 mRNA was significantly inhibited in the colonic mucosa of TG mice (Fig. 5C).
DSS-induced colitis is associated with inhibited Hsp70 protein expression in WT but not in Hsp70 TG mice.
Hsp70 expression was measured in the transverse colonic mucosa of DSS-treated WT and TG mice. As assessed by immunohistochemistry, colonic tissues from both mice demonstrated significant Hsp70 expression in epithelia under basal condition (Fig. 5A). Colonic specimens from DSS-treated WT mice showed greatly reduced Hsp70 staining, even in areas with relatively mild colitis. In contrast, colonic tissues from DSS-treated TG-mice continued to express abundant Hsp70 in the actively inflamed areas with inflammatory infiltration and mucosal destruction (Fig. 5A). Western blot analysis was performed on mucosal samples to provide a more quantitative assessment of Hsp70 protein expression (Fig. 5B). In WT mice, Hsp70 protein expression was significantly reduced in the actively inflamed specimens after DSS treatment compared with that in tissues from control, non-DSS treated mice. In TG mice, colonic Hsp70 levels were not reduced after DSS treatment. Constitutive Hsc70 was expressed at the same level in all samples. Endogenous and UTR-less TG-Hsp70 mRNA were analyzed in colon tissues from the same mice by real-time PCR using specific primers (Fig. 5C). Although endogenous Hsp70 mRNA was expressed in colonic mucosa of WT, it was decreased by about 60% in TG mouse. TG-Hsp70 mRNA was not detectable in WT mouse but highly expressed in TG mouse. In both WT and TG mouse, DSS treatment did not decrease either endogenous or TG Hsp70 mRNA. These in vivo results thus confirm that the 3′UTR mediates the translational downregulation of Hsp70 in intestinal inflammation.
Reduced inflammation process in TG mice after DSS treatment.
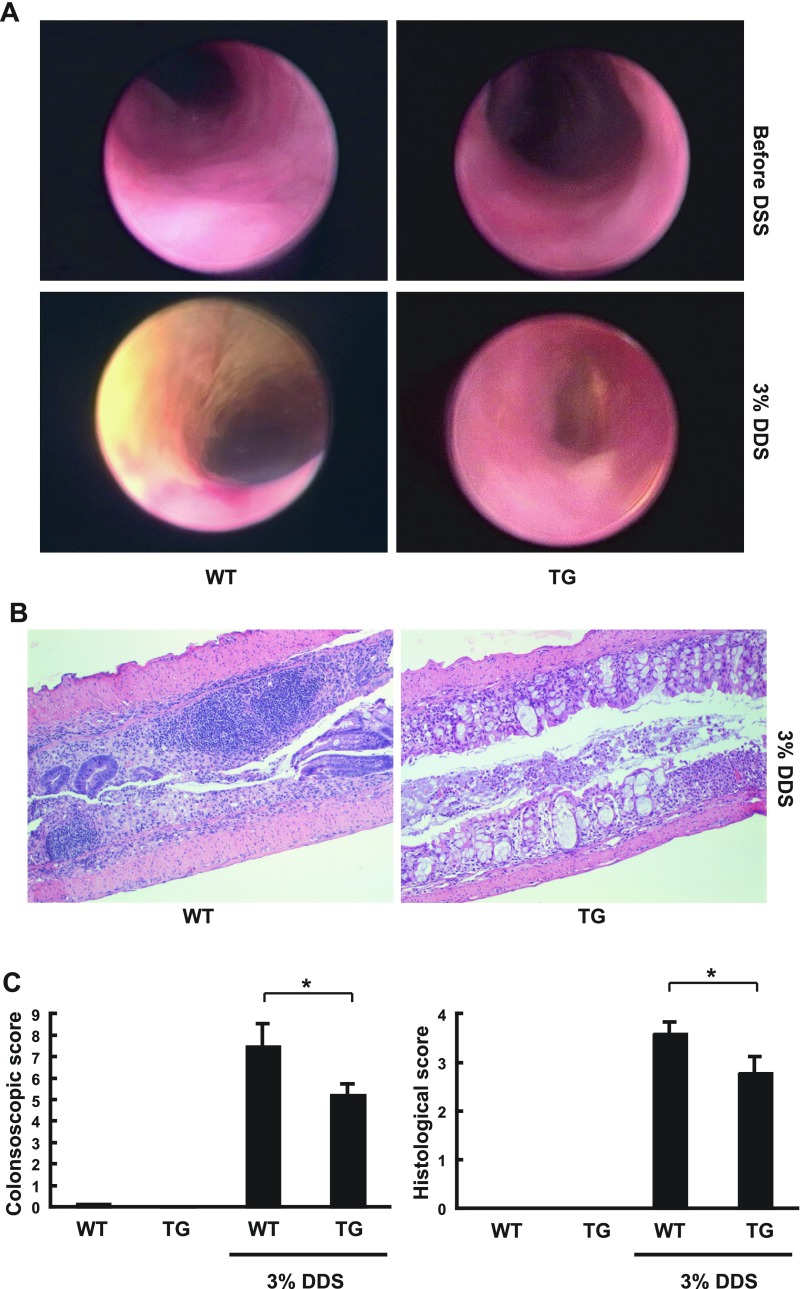
To analyze the effects of constitutive colonic epithelial Hsp70 on the development of experimental colitis, both WT and TG mice were treated with 3% DSS for 7 days to induce colitis and then changed back to tap water for another 3 days. Endoscopic and histological grading of distal and transverse colon mucosa were performed at day 10, a time when severe degree of clinical colitis was consistently detected in both WT and TG mice (Fig. 6). Before DSS treatment, healthy mucosa was observed with minimum lesions and no differences in endoscopic and histological inflammation scores between WT and TG mice. After DSS treatment, WT mice developed severe colitis with high endoscopic and histological inflammation scores as expected. In contrast, significantly lower endoscopic and histological inflammation scores (P < 0.05) were observed in DSS-treated TG mice. Overall, there was less mucosal destruction with more intact crypts and significantly fewer lymphoid aggregates.
Fig. 6.
Endoscopic and histological grading of DSS colitis in WT and Hsp70 TG mice. WT and Hsp70 UTR-less TG mice were given 3% DSS for 7 days and then given tap water for another 3 days before analysis. A: colonoscopy was performed before and after DSS treatment in WT and TG mice under anesthesia. An endoscopic score was calculated. Images shown are representative of 4 sets of experiments. B: after colonoscopy, WT and TG mice were euthanized for hematoxylin and eosin staining and histological grading of colitis. C: endoscopic score and histological score of various mouse groups. *P < 0.05 compared WT and TG mice after DSS treatment; n = 4.
DISCUSSION
Intestinal homeostasis is the essential and dynamic equilibrium of factors that maintain normal mucosal function, integrity, self-renewal and host defense. Inducible Hsp70, which is constitutively expressed in colonic mucosa, has multiple functions that are important for maintaining intestinal homeostasis, especially under stress conditions (14, 19, 28, 33, 34). In the Hsp70-null mice, no gross histological colitis can be detected under basal condition, but elevated levels of proinflammatory chemokines and cytokines, indicative of a shift in immune homeostasis, can be detected (33). We believe that other pathways compensate for the loss of Hsp70, e.g., soluble TNF-α receptor. When the colon is stressed further, e.g., DSS, the balance is disturbed. As shown in Fig. 2, Hsp70 KO mice are more susceptible to DSS-induced colitis compared with WT mice, demonstrating that Hsp70 plays a critical role in protecting the colonic mucosa from this colitis-inducing stress.
We previously reported that Hsp70 is downregulated in human IBD and in a model of chronic experimental colitis (IL-10−/− mice, 9). This report shows that the phenomenon is not specific to either of these conditions and can also occur as a direct consequence of acute DSS-induced injury (Fig. 1). On the basis of the finding from Hsp70-null mouse, we would predict that the downregulation of Hsp70 would compromise intestinal homeostasis and promote the intensity and duration of inflammation-associated mucosal injury.
Hsp70 is downregulated in colitis through translational inhibition by proinflammatory cytokines. Previous studies from our laboratory revealed that the mechanisms involve activation of PKR, causing phosphorylation of eIF-2α, preventing its engagement of the polyribosome. However, this effect would inhibit global mRNA translation and does not explain the selective downregulation of Hsp70 by inflammation and proinflammatory mediators. In contrast, gene-specific translational regulation is commonly mediated by regulatory elements in the flanking untranslated regions of mRNA (6, 11, 29). Our in vitro findings suggest that the 3′UTR of Hsp70 mRNA plays a key role in the downregulation of Hsp70 during inflammation (Fig. 3). This region in mouse and human Hsp70.1 and 70.3 contains alanine and uridine (AU)-rich elements and other candidate binding sites for regulatory factors, such as RNA binding proteins and miRNAs (11, 17, 18). These factors are likely induced or activated by proinflammatory cytokines in IBD and experimental colitis to cause downregulation of Hsp70 (5, 21). This notion is further supported by the fact that the UTR-less Hsp70 transgene is constitutively expressed in inflamed colonic mucosa, despite downregulation of the endogenous Hsp70 in WT mice (Figs. 4 and 5). Interestingly, endogenous Hsp70 mRNA was decreased by about 60% in TG mouse (Fig. 5C), likely resulting from feedback transcriptional downregulation as previously described (15). As Hsp70 protein expression increases, it binds the transcription factor, heat shock factor-1, preventing transcription of endogenous Hsp70 genes.
Our study is significant in that Hsp70 protein expression was specifically forced in colonic epithelial cells with the use of a transgene driven by villin promoter (Fig. 4). Forced expressed Hsp70 in the villin-expressed mature colonocytes protected colonic mucosa in DSS-induced colitis, resulting in significantly lower endoscopic and histological scores of mucosal damage in TG mice compared with WT mice (Figs. 5 and 6). These data demonstrate a direct contribution of colonic epithelial Hsp70 to the maintenance of intestinal homeostasis when challenged with colitis-inducing chemicals. The role of Hsp70 in nonepithelial cells, however, remains to be determined and should not be minimized. In innate immune cells, for example, Hsp70 mediates potent anti-inflammatory effects (2).
Tanaka et al. (32) had also shown that DSS-induced colitis was less severe in the universal Hsp70 and heat shock factor-1 TG mouse lines, as assessed by lower disease index and reduced inflammatory mediators, such as MPO and thiobarbituric acid reactive substances in response to DSS. Although DSS treatment and clinical outcomes in Tanaka's studies were similar to ours, there were several important differences that are difficult to reconcile. Tanaka analyzed Hsp70-protective effects in Hsp70 TG mice where the human Hsp70 transgene was derived from a cDNA transcript (PAT-HSP70) containing both 5′- and 3′UTR, driven by globally expressed β-actin promoter (25, 37). The 350-bp 3′UTR in this transgene contains AU-rich elements and potential miRNA binding sites that are likely involved in inflammation-associated downregulation of Hsp70. Therefore, we should predict that the Hsp70 transgene expression would very likely be inhibited under conditions of colitis, similar to the endogenous Hsp70 mRNA. For this reason, assessment of Hsp70 expression levels would be critical in interpreting the results of Tanaka's TG mouse study. Unfortunately, there was no confirmation of Hsp70 protein levels aside from immunohistochemistry, which appeared to stain noncellular stromal elements and a few lamina propria cells. Surprisingly, Hsp70 was not seen in colonic epithelium under even basal conditions. Since a β-actin promoter was used to drive transgene expression, Hsp70 expression should have been observed in many cell types, including the intestinal epithelium. The details of the primary antibody used in the study were not described either and might account for some of the differences with our study.
We believe that the downregulation of colonic epithelial Hsp70 in human IBD and experimental colitis contributes to the severity and chronicity of the disease because of the importance of Hsp70 in maintaining intestinal homeostasis. The loss of critical protection from epithelial Hsp70 might have multiple profound negative consequences, rendering the mucosa highly susceptible to inflammatory and immune stresses. Furthermore, therapies to induce Hsp70 in colonic mucosa in the presence of inflammation, e.g., probiotics, mesalamine, glucocorticoids, and short-chain fatty acids, are not likely to be useful because translation of endogenous Hsp70 mRNA would be inhibited (3, 22, 23, 34). Future strategies to induce Hsp70 might therefore be directed at forcing Hsp70 translation, or reducing translational inhibition, e.g., siRNA against critical miRNA or regulatory proteins involved in Hsp70 translational blockage (17).
GRANTS
This work was supported by NIH Grants DK-47722 (E. Chang) and DK-38510 (E. Chang), the Digestive Disease Research Core Center DK-42086 of the University of Chicago, Senior Research Award (M. Bissonnette), Research Training Awards (S. Hu) from the Crohn's and Colitis Foundation of America, and Moshe Goldgraber Advanced IBD Fellowship (L. Lichtenstein).
REFERENCES
- 1.Arvans DL, Vavricka SR, Ren H, Musch MW, Kang L, Rocha FG, Lucioni A, Turner JR, Alverdy J, Chang EB. Luminal bacterial flora determines physiological expression of intestinal epithelial cytoprotective heat shock proteins 25 and 72. Am J Physiol Gastrointest Liver Physiol 288: G696–G704, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Bendz H, Ruhland SC, Pandya MJ, Hainzl O, Riegelsberger S, Brauchle C, Mayer MP, Buchner J, Issels RD, Noessner E. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J Biol Chem 282: 31688–31702, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Burress GC, Musch MW, Jurivich DA, Welk J, Chang EB. Effects of mesalamine on the hsp72 stress response in rat IEC-18 intestinal epithelial cells. Gastroenterology 113: 1474–1479, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238–249, 1993. [PubMed] [Google Scholar]
- 5.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SGM, van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114: 385–391, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duttagupta R, Vasudevan S, Wilusz CJ, Peltz SW. A yeast homologue of Hsp70, Ssa1p, regulates turnover of the MFA2 transcript through its AU-rich 3′ untranslated region. Mol Cell Biol 23: 2623–2632, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujiya M, Musch MW, Nakagawa Y, Hu S, Alverdy J, Kohgo Y, Schneewind O, Jabri B, Chang EB. The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe 1: 299–308, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Hampton CR, Shimamoto A, Rothnie CL, Griscavage-Ennis J, Chong A, Dix DJ, Verrier ED, Pohlman TH. HSP70.1 and -70.3 are required for late-phase protection induced by ischemic preconditioning of mouse hearts. Am J Physiol Heart Circ Physiol 285: H866–H874, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Hu S, Ciancio MJ, Lahav M, Fujiya M, Lichtenstein L, Anant S, Musch MW, Chang EB. Translational inhibition of colonic epithelial heat shock proteins by interferon-gamma and TNF-alpha in intestinal inflammation. Gastroenterology 133: 1894–1904, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt CR, Dix DJ, Sharma GG, Pandita RK, Gupta A, Funk M, Pandita TK. Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp703-deficient mice. Mol Cell Biol 24: 899–911, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di Padova F, Lin SC, Gram H, Han J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 120: 623–634, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Kojima K, Musch MW, Ren H, Boone DL, Hendrickson BA, Ma A, Chang EB. Enteric flora and lymphocyte-derived cytokines determine expression of heat shock proteins in mouse colonic epithelial cells. Gastroenterology 124: 1395–1407, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Kojima K, Musch MW, Ropeleski MJ, Boone DL, Ma A, Chang EB. Escherichia coli LPS induces heat shock protein 25 in intestinal epithelial cells through MAP kinase activation. Am J Physiol Gastrointest Liver Physiol 286: G645–G652, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Liu TS, Musch MW, Sugi K, Walsh-Reitz MM, Ropeleski MJ, Hendrickson BA, Pothoulakis C, Lamont JT, Chang EB. Protective role of HSP72 against Clostridium difficile toxin A-induced intestinal epithelial cell dysfunction. Am J Physiol Cell Physiol 284: C1073–C1082, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Malago JJ, Koninkx FJG, van Dijk JE. The heat shock response and cytoprotection of the intestinal epithelium. Cell Stress Chaperones 7: 191–199, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCall RD, Haskill S, Zimmermann EM, Lund PK, Thompson RC, Sartor RB. Tissue interleukin 1 and interleukin-1 receptor antagonist expression in enterocolitis in resistant and susceptible rat. Gastroenterology 106: 960–972, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Meister G, Landthaler M, Dorsett Y, Tuschl T. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA 10: 544–550, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhopadhyay D, Houchen CW, Kennedy S, Dieckgraefe BK, Anant S. Coupled mRNA stabilization and translational silencing of cyclooxygenase-2 by a novel RNA binding protein, CUGBP2. Mol Cell 11: 113–126, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Musch MW, Kaplan B, Chang EB. Role of increased basal expression of heat shock protein 72 in colonic epithelial c2BBE adenocarcinoma cells. Cell Growth Differ 12: 419–426, 2001. [PubMed] [Google Scholar]
- 20.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, Gumucio D, Neurath MF, Pasparakis M. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446: 557–561, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med 51: 289–298, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Parhar K, Baer KA, Parker K, Ropeleski MJ. Short-chain fatty acid mediated phosphorylation of heat shock protein 25: effects on camptothecin-induced apoptosis. Am J Physiol Gastrointest Liver Physiol 291: G178–G188, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology 127: 1474–1487, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Pinto D, Robine S, Jaisser F, El Marjou FE, Louvard D. Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J Biol Chem 274: 6476–6482, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Plumier JC, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest 95: 1854–1860, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by Toll-Like receptors is required for intestinal homeostasis. Cell 118: 229–241, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Ropeleski MJ, Tang J, Walsh-Reitz MM, Musch MW, Chang EB. Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology 124: 1358–1368, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Ropeleski MJ, Riehm J, Baer KA, Musch MW, Chang EB. Anti-apoptotic effects of l-glutamine-mediated transcriptional modulation of the heat shock protein 72 during heat shock. Gastroenterology 129: 170–184, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Rubtsova MP, Sizova DV, Dmitriev SE, Ivanov DS, Prassolov VS, Shatsky IN. Distinctive properties of the 5′-untranslated region of human hsp70 mRNA. J Biol Chem 278: 22350–22356, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Sartor RB, Cromartie WJ, Powell DW, Schwab JH. Granuloamatous enterocolitis induced in rats by purified bacterial cell wall fragment. Gastroenterology 89: 587–596, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription polymerase chain reaction to study mRNA decay: comparison of end point and real-time methods. Anal Biochem 285: 194–204, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka K, Namba T, Arai Y, Fujimoto M, Adachi H, Sobue G, Takeuchi K, Nakai A, Mizushima T. Genetic evidence for a protective role for heat shock factor 1 and heat shock protein 70 against colitis. J Biol Chem 282: 23240–23252, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Tao Y, Hart J, Lichtenstein L, Joseph L, Ciancio MJ, Hu S, Chang EB, Bissonnette M. Inducible heat shock protein70 prevents multifocal flat dysplastic lesions and invasive tumors in an inflammatory model of colon cancer. Carcinogenesis 30: 175–182, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urayama S, Musch MW, Retsky J, Madonna MB, Straus D, Chang EB. Dexamethasone protection of rat intestinal epithelial cells against oxidant injury is mediated by induction of heat shock protein 72. J Clin Invest 102: 1860–1865, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vetrano S, Rescigno M, Cera MR, Correalelow C, Rumiolow C, Donilow A, Frantinishort M, Sturm A, Borronilow E, Repicilow A, Lacatilow M, Malescilow A, Dejana E, Daneselow S. Unique role of junctional adhesion molecule-A in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology 135: 173–184, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci USA 90: 587–591, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu B, Hunt C, Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol 5: 330–341, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]