Abstract
Helper T cells are known to mediate hepatic ischemia/reperfusion (I/R) injury. However, the precise mechanisms and subsets of CD4+ T cells that contribute to this injury are still controversial. Therefore, we sought to determine the contributions of different CD4+ T cell subsets during hepatic I/R injury. Wild-type, OT-II, or T cell receptor (TCR)-δ-deficient mice were subjected to 90 min of partial hepatic ischemia followed by 8 h of reperfusion. Additionally, wild-type mice were pretreated with anti-CD1d, -NK1.1, or -IL-2R-α antibodies before I/R injury. OT-II mice had diminished liver injury compared with wild-type mice, implicating that antigen-dependent activation of CD4+ T cells through TCRs is involved in hepatic I/R injury. TCR-δ knockout mice had decreased hepatic neutrophil accumulation, suggesting that γδ T cells regulate neutrophil recruitment. We found that natural killer T (NKT) cells, but not NK cells, contribute to hepatic I/R injury via CD1d-dependent activation of their TCRs, as depletion of NKT cells by anti-CD1d antibody or depletion of both NKT cells and NK cells by anti-NK1.1 attenuated liver injury. Although regulatory T cells (Treg) are known to suppress T cell-dependent inflammation, depletion of Treg cells had little effect on hepatic I/R injury. The data suggest that antigen-dependent activation of CD4+ T cells contributes to hepatic I/R injury. Among the subsets of CD4+ T cells, it appears that γδ T cells contribute to neutrophil recruitment and that NKT cells directly injure the liver. In contrast, NK cells and Treg have little effects on hepatic I/R injury.
Keywords: T cell receptor, γδ T cell, natural killer T cell, OT-II
ischemia/reperfusion (I/R) injury of the liver is a primary complication of liver resection surgery, transplantation, and trauma (27). This insult can lead to hepatocellular damage and organ dysfunction through the initiation of a biphasic inflammatory response (14). The initial phase of this response is characterized by activation of Kupffer cells and their subsequent production and release of reactive oxygen species, leading to mild hepatocellular injury (15, 16). Activated Kupffer cells also induce activation of redox-sensitive transcription factors such as NF-κB, which results in release of many proinflammatory mediators (28, 42). Proximal cytokines, such as IL-12, TNF-α, and IL-1, are critically involved in promoting the second phase of liver injury by inducing the hepatic expression of neutrophil-attracting CXC chemokines and endothelial cell adhesion molecules (6, 19, 29). The cooperative effects of CXC chemokines and adhesion molecules result in adherence of neutrophils in the hepatic microcirculation and their subsequent transmigration into the hepatic parenchyma (18). These activated neutrophils then directly injure hepatocytes and vascular endothelial cells through their release of oxidants and proteases (17).
In addition to neutrophil-dependent injury mechanisms, other studies have demonstrated that T cells also contribute to hepatic I/R injury. Several reports have shown that CD4+, but not CD8+, T cells are recruited into the postischemic liver within 1 h of reperfusion and regulate hepatic inflammation (20, 44). These studies demonstrated that depletion or deficiency of CD4+ T cells attenuates liver injury, hepatic neutrophil infiltration, and platelet-endothelial cell interaction. We have also reported that CD4+ T cells are an important regulator of hepatic neutrophil recruitment during I/R via their release of IL-17 (4).
The majority of CD4+ T cells possess the αβ T cell receptor (TCR), whereas γδ T cells constitute only a small proportion of CD4+ T cells in the circulating blood and peripheral organs (5, 10). Antigen recognition by γδ T cells is limited, and the repertoire of natural ligands is not well developed (12, 34). Previous studies have shown that γδ T cells play a critical role in protection against bacterial infection (8, 39). In addition, we have demonstrated that neutrophil recruitment to the site of inflammation is mediated by γδ T cells in the murine model of sepsis induced by cecal ligation and puncture (39). However, little is known about the role of γδ T cells during hepatic I/R injury although several studies have shown that γδ T cells mediate renal I/R injury (13, 36).
Another important subset of CD4+ T cells is CD4+ NK1.1+ natural killer T (NKT) cells, which coexpress the invariant TCR, Vα14Jα18, but also express a variety of molecular markers, typically associated with NK cells, such as NK1.1 (9). The liver is one of the most abundant sources of NKT cells among the immune organs, which account for ∼50% of total αβ T cells in the liver (11). NKT cells are activated by recognizing glycolipid presentation to TCRs by CD1d, a member of the CD1 family of antigen-presenting molecules (22). Originally, it was thought that NKT cells could not play a role in postischemic tissue injury because of the absence of foreign antigen. However, recent published data have suggested that NKT cell activation by CD1d-dependent antigen presentation to TCR might be a key early event in the inflammation cascade during hepatic I/R injury (25, 37). Furthermore, the generation of self-antigens has been described in models of gut and hindlimb I/R (43).
Other lymphoid cells such as NK cells and regulatory T cells (Treg) have also been linked to inflammatory responses (1, 41); however, the roles of these cells in hepatic I/R injury is unclear. In the present studies, we sought to determine the contributions of specific CD4+ T cell subsets to hepatic I/R injury.
MATERIALS AND METHODS
Animals.
Wild-type, OT-II [C57BL/6-Tg(TcraTcrb)425Cbn/J] and TCR-δ-deficient (B6.129P2-Tcrdtm1Mom/J) mice on a C57BL/6J background were purchased from the Jackson Laboratory (Bar Harbor, ME). All mutant mice were backcrossed to the C57BL/6J strain at least 12 times. This project was approved by the University of Cincinnati Animal Care and Use Committee and was in compliance with the National Institutes of Health guidelines.
Hepatic I/R injury model.
Male mice between 7 and 10 wk of age weighing 20–26 g were used in these experiments. The animals underwent either sham surgery or I/R. Partial hepatic ischemia was induced as described previously (28). Briefly, mice were anesthetized with pentobarbital sodium (60 mg/kg ip). A midline laparotomy was performed, and an atraumatic clip was used to interrupt blood supply to the left lateral and median lobes of the liver. The caudal lobes retained intact portal and arterial inflow and venous outflow, preventing intestinal venous congestion. After 90 min of partial hepatic ischemia, the clip was removed to initiate hepatic reperfusion. Some wild-type mice were injected intraperitoneally with 100 μg of anti-CD1d antibody (BD Pharmingen, San Diego, CA) 4 h before operation, 100 μg of PK136 (kindly donated by Dr. Jorge A. Bezerra, Cincinnati Children's Hospital) 48 h before operation, or 250 μg of PC61 (kindly donated by Dr. Yasmine Belkaid, Cincinnati Children's Hospital) 48 h before operation. Saline was used as controls of anti-CD1d antibody and PK136. GL113 was used as a control of PC61. Mice were euthanized after 8 h of reperfusion, and blood and samples of the left lateral lobe were taken for analysis.
Blood and tissue analysis.
Blood was obtained by cardiac puncture for analysis of serum alanine aminotransferase (ALT) as an index of hepatocellular injury. Measurements of serum ALT were made using a diagnosis kit by bioassay (Wiener Laboratories, Rosario, Argentina). Liver tissues were fixed in 10% neutral-buffered formalin, processed, and then embedded in paraffin for light microscopy. Sections were stained with hematoxylin and eosin for histological examination.
Liver neutrophil accumulation.
Liver myeloperoxidase (MPO) content was assessed by methods described elsewhere (28). Briefly, liver tissue (100 mg) was homogenized in 2 ml of buffer A (3.4 mmol/l KH2HPO4, 16 mmol/l Na2HPO4, pH 7.4). After centrifugation for 20 min at 10,000 g, the pellet was resuspended in 10 volumes of buffer B (43.2 mmol/l KH2HPO4, 6.5 mmol/l Na2HPO4, 10 mmol/l EDTA, 0.5% hexadecyltrimethylammonium, pH 6.0) and sonicated for 10 s. After being heated for 2 h at 60°C, the supernatant was reacted with 3,3′,3,5′-tetramethylbenzidine (Sigma, St. Louis, MO), and the optical density was read at 655 nm.
Statistical analysis.
All data are expressed as means ± SE. Data were analyzed with a one-way analysis of variance with subsequent Student-Newman-Keuls test. Differences were considered significant when P < 0.05.
RESULTS
Activation of CD4+ T cells during hepatic I/R injury is partially antigen dependent.
Previous studies have suggested that T cells contribute to hepatic I/R injury independently of the antigen presentation (20). However, other studies have shown that self-antigens are generated in postischemic tissues and recognized as targets for circulating IgM (43), suggesting that antigen-dependent mechanisms may be involved in I/R injury. To determine whether antigen-dependent mechanisms of injury are relevant to hepatic I/R injury, we employed OT-II mice. These mice have CD4+ T cells that express a TCR that only recognizes ovalbumin (7). Hepatic I/R injury was attenuated in OT-II mice after both 4 and 8 h of reperfusion, as measured by serum ALT levels, compared with wild-type mice (Fig. 1A). Hepatic neutrophil accumulation, as determined by liver MPO content, was similar between wild-type and OT-II mice at both time points (Fig. 1B). These findings were confirmed by histological examination, since hepatocellular necrosis was markedly lower in OT-II mice compared with wild-type mice after 8 h of reperfusion (Fig. 1C). Therefore, it appeared that hepatic I/R injury is mediated in part by antigen-dependent CD4+ T cells activation through TCRs.
Fig. 1.
Response of OT-II mice to hepatic ischemia/reperfusion (I/R). A: liver injury, assessed by serum levels of alanine aminotransferase (ALT), was determined after 90 min of ischemia and 4 and 8 h of reperfusion. Data are means ± SE with n = 5 per group. *P < 0.05, compared with wild-type mice. B: neutrophil accumulation after 4 and 8 h of reperfusion was determined by liver content of myeloperoxidase (MPO). Data are means ± SE with n = 5 per group. C: liver histology. Normal hepatic architecture was observed in sham-operated wild-type (WT) mice. Sham-operated OT-II mice also showed normal hepatic architecture. After 8 h of reperfusion, livers from wild-type mice had large areas of necrosis, whereas livers from OT-II mice had less evidence of necrosis. Original magnification was ×50.
Hepatic neutrophil recruitment during I/R is regulated by γδ T cells.
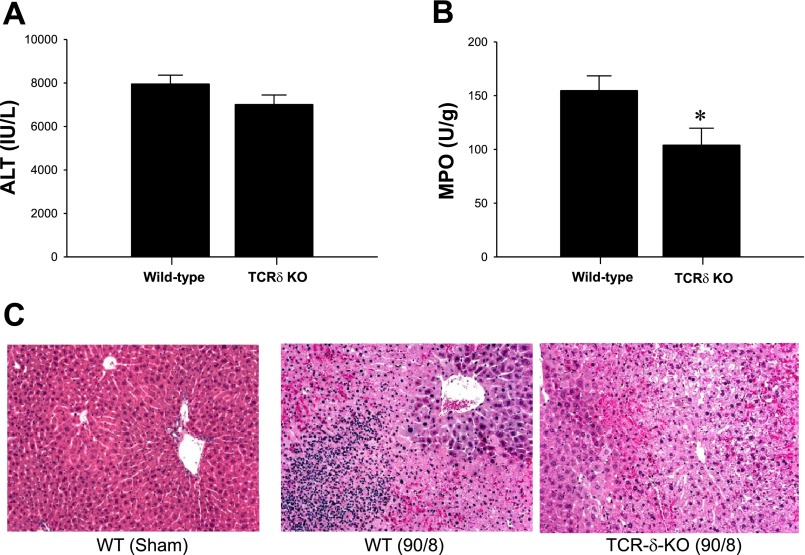
γδ T cells have been reported to regulate the inflammatory response induced by bacterial infection (8, 39). Moreover, several reports have demonstrated that γδ T cells mediate renal I/R injury, since TCR-δ-deficient mice show less renal injury during I/R compared with wild-type mice (13, 36). To determine the effects of TCR-dependent γδ T cell activation on hepatic I/R injury, TCR-δ-deficient mice and their wild-type controls were subjected to I/R. There were no detectable differences in liver injury between wild-type and TCR-δ-deficient mice (Fig. 2A). However, liver MPO content was significantly lower in TCR-δ-deficient mice compared with wild-type mice after 8 h of reperfusion (Fig. 2B), suggesting that γδ T cells regulate hepatic neutrophil recruitment during I/R injury. Histopathological analysis of liver sections showed that wild-type mice subjected to ischemia and 8 h of reperfusion had substantial hepatocellular necrosis and neutrophilic infiltrates, whereas livers from TCR-δ-deficient mice showed less evidence of neutrophil accumulation with similar degree of necrosis (Fig. 2C). These results were supported by our previous findings that revealed the contribution of γδ T cells to neutrophil recruitment into the site of inflammation in sepsis model (39).
Fig. 2.
Effects of T cell receptor (TCR)-δ deficiency on hepatic I/R. A: liver injury, assessed by serum levels of ALT, was determined after 90 min of ischemia and 8 h of reperfusion. Data are means ± SE with n = 12–13 per group. KO, knockout. B: neutrophil accumulation after 8 h of reperfusion was determined by liver content of MPO. Data are means ± SE with n = 12–14 per group. *P < 0.05, compared with wild-type mice. C: liver histology. Normal hepatic architecture was observed in sham-operated wild-type mice and TCR-δ-deficient mice. After 8 h of reperfusion, livers from wild-type mice had large areas of necrosis with marked neutrophilic infiltrates, whereas TCR-δ-deficient mice had severe necrotic liver with reduced neutrophilic infiltrates. Original magnification was ×50.
CD1d-dependent activation of NKT cells contributes to hepatic I/R injury.
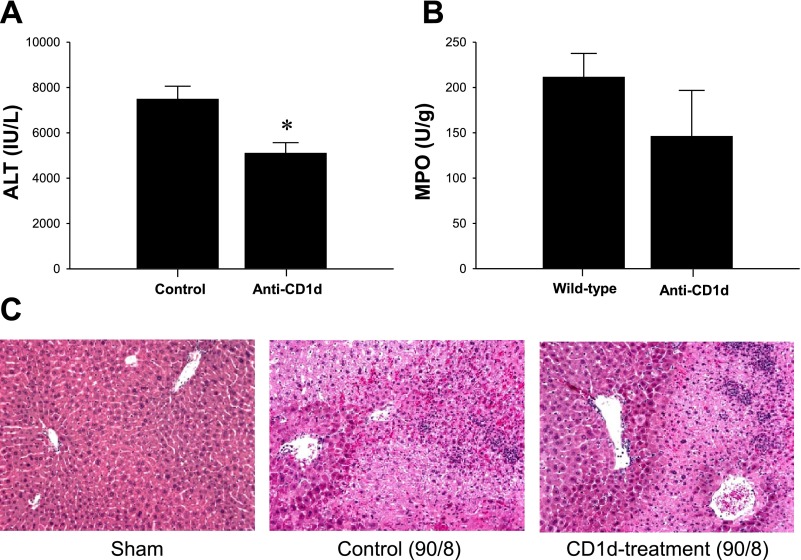
We have previously shown that NKT cells are rapidly recruited to the liver after I/R (4). Other reports indicate that NKT cells contribute to liver injury induced by I/R (25, 37). Therefore, we examined whether inhibition of NKT cell function by blocking its CD1d-dependent activation showed any effects on hepatic I/R injury. Treatment of mice with anti-CD1d antibody resulted in ∼30% less liver injury compared with mice treated with vehicle (Fig. 3A). However, this decrease in injury was not associated with reduced neutrophil accumulation (Fig. 3B), suggesting that NKT cells may be directly injurious to liver parenchyma. Histopathology revealed similar results because control mice subjected to ischemia and 8 h of reperfusion had substantial hepatocellular necrosis and neutrophilic infiltrates, whereas livers from mice pretreated with anti-CD1d antibody showed reduced areas of necrosis but high levels of neutrophil infiltration (Fig. 3C).
Fig. 3.
Effects of depletion of natural killer T (NKT) cells on hepatic I/R. Wild-type mice were injected intraperitoneally with saline (control) or 100 μg of anti-CD1d antibody 4 h before operation. A: liver injury, assessed by serum levels of ALT, was determined after 90 min of ischemia and 8 h of reperfusion. Data are means ± SE with n = 5 per group. *P < 0.05, compared with control mice. B: neutrophil accumulation after 8 h of reperfusion was determined by liver content of MPO. Data are means ± SE with n = 5 per group. C: liver histology. Sham-operated wild-type mice had normal hepatic architecture. After 8 h of reperfusion, livers from wild-type mice had large areas of necrosis, whereas mice treated with anti-CD1d had less evidence of necrosis. Original magnification was ×50.
NK cells are not involved in hepatic I/R injury.
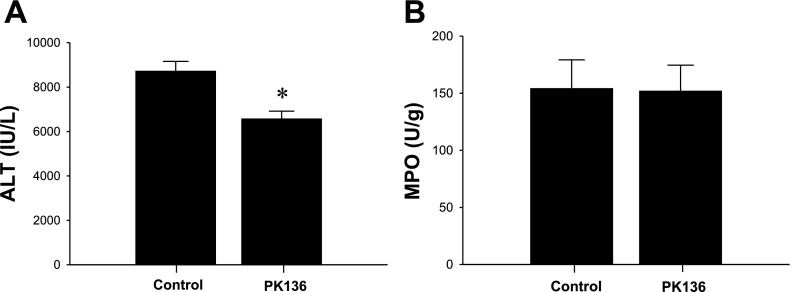
Because NK cells are also known to increase at the site of inflammation and mediate the inflammatory responses in several inflammation models, we next sought to reveal the effects of NK cells on hepatic I/R injury. NK1.1 is the C type lectin receptor and known to be expressed on both NK cells and NKT cells (31). Since treatment with PK136, an anti-NK1.1 antibody, 48 h before operation is reported to deplete NK cells and NKT cells in the liver (25), we pretreated mice with PK136 or saline (control) 48 h before hepatic I/R. Mice treated with PK136 had lower serum ALT levels after 8 h of reperfusion compared with control mice (Fig. 4A). However, the impact of PK136 treatment on decrease in serum ALT levels was similar to anti-CD1d antibody treatment, as ∼25% reduction compared with control mice. Similar to anti-CD1d antibody treatment, PK136 treatment did not change the liver MPO content after 8 h of reperfusion (Fig. 4B). These findings suggest that NKT cells, and not NK cells, contribute to hepatic I/R injury.
Fig. 4.
Effects of depletion of NK and NKT cells on hepatic I/R. Wild-type mice were injected intraperitoneally with saline (control) or 100 μg of PK136 48 h before operation. A: liver injury, assessed by serum levels of ALT, was determined after 90 min of ischemia and 8 h of reperfusion. Data are means ± SE with n = 10 per group. *P < 0.05, compared with control mice. B: neutrophil accumulation after 8 h of reperfusion was determined by liver content of MPO. Data are means ± SE with n = 10 per group.
As reported in previous studies, CD4+ CD25+ Treg are known to regulate inflammatory responses by suppressing CD4+ T cell functions upon TCR stimulation (23, 38). To determine whether depletion of CD4+ CD25+ Treg had any regulatory effects on hepatic I/R injury, mice were pretreated with PC61, an anti-CD25+ antibody that was reported to reduce ∼70% of CD4+ CD25+ Treg population at this time point (33). We found no significant differences in serum ALT levels or liver MPO content between mice treated with PC61 or the vehicle control (data not shown). These results suggest that CD4+ CD25+ Treg do not suppress CD4+ T cell-mediated liver injury induced by I/R.
Response of OT-II and wild-type mice depleted of NK T cells on hepatic I/R.
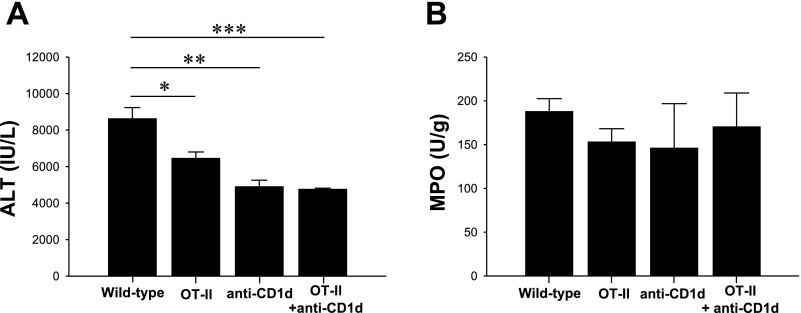
Our data suggest that hepatic I/R injury is attenuated in OT-II mice as well as treatment that resulted in the depletion of NKT cells. Therefore, we wanted to determine the contribution of NKT cells to residual liver injury in OT-II mice following liver I/R. We found that pretreatment OT-II mice with anti-CD1d antibody significantly decreased ALT compared with untreated wild-type mice (Fig. 5A). However, we did not observe significant differences in liver injury between the untreated OT-II, and anti-CD1d-treated wild-type and OT-II mice. Additionally, the tested mice did not exhibit altered MPO content (Fig. 5B). Thus it was not possible to abrogate residual liver injury in OT-II mice post-I/R by anti-CD1d antibody treatment.
Fig. 5.
Response of OT-II and wild-type mice depleted of NKT cells on hepatic I/R. Wild-type on OT-II mice were injected intraperitoneally with 100 μg of PK136 48 h before operation. A: liver injury, assessed by serum levels of ALT, was determined after 90 min of ischemia and 8 h of reperfusion. Data are means ± SE with n = 5 per group. B: neutrophil accumulation after 8 h of reperfusion was determined by liver content of MPO. Data are means ± SE with n = 5 per group. *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
Our understanding of the functions of T lymphocytes in hepatic pathophysiology is rapidly evolving. Accumulating data suggest that CD4+ T cells mediate liver neutrophil recruitment and liver injury (4, 20, 44). However, the precise mechanisms by which subsets of T cells contribute to hepatic I/R injury are not fully understood. Similarly, involvement of antigens in T cell activation during hepatic I/R injury has not been rigorously examined. Our present studies show that liver I/R injury was attenuated in OT-II mice. These results suggest that antigen-dependent activation of CD4+ T cells contributes to I/R injury in the liver. Previous studies have suggested that activation of CD4+ T cells during I/R was antigen-independent because it was observed that blockade of major histocompatibility complex (MHC) class II by antibody had no effects on I/R-induced liver injury (20). It was proposed that cytokines and chemokines produced during I/R might directly activate CD4+ T cells since T cells were known to be activated by cytokines and chemokines such as IL-18 and RANTES in a manner independent of TCR engagement (2, 26). In contrast, other studies have demonstrated that dendritic cells are activated by Toll-like receptor-mediated pathway and increase their expression of MHC class II during hepatic I/R injury, leading to antigen-dependent activation of CD4+ T cells (30, 40). Moreover, blockade of TCR signaling with cyclosporine treatment is known to reduce hepatic I/R injury (24, 35). Recent studies have suggested that innate recognition of stress-induced self-antigens is involved in the induction of intestinal and skeletal muscle I/R injury (43). On the basis of our present data and the collective literature, it seems that activation of CD4+ T cells during hepatic I/R injury may be induced by both antigen-dependent and independent mechanisms.
The present study is the first to show that γδ T cells mediate the inflammatory response during hepatic I/R injury. We have previously shown that γδ T cells are recruited to the liver within 1 h of reperfusion, a time that precedes the recruitment of neutrophils (4). In the present study, we found that hepatic neutrophil recruitment was suppressed in TCR-δ-deficient mice after I/R compared with wild-type mice. We have previously reported that γδ T cells mediate intestinal neutrophil recruitment in a peritoneal sepsis model (39). Similar to those studies (39) and others in a renal I/R model (13, 36), we found no correlation between altered neutrophil recruitment and hepatic I/R injury in TCR-δ-deficient mice. Therefore, it appears that γδ T cells mediate neutrophil recruitment into the site of inflammation in a manner that is independent of the degree of tissue damage.
Interestingly, previous findings regarding renal I/R in TCR-δ-deficient mice have demonstrated that TCR-δ-deficient mice show decreased serum creatinine levels, improved pathological findings, and better survival rate after 72 h of reperfusion compared with wild-type mice (36). We have previously shown that after hepatic I/R, signals for liver recovery/regeneration do not occur until after 24 h of reperfusion (3, 22). Accordingly, although no differences were seen in the degree of liver injury as manifested by serum ALT levels and histology after 8 h of reperfusion in this study, TCR-δ deficiency may have effects on liver recovery and regeneration. This possibility, although beyond the scope of the present study, deserves further investigation.
Previous reports indicate that activation of NKT cells by CD1d glycolipid presentation to TCRs promotes the rapid release of IFN-γ (21), contributing to liver injury during I/R (25, 37). Because NK cells also have cytotoxic functions like NKT cells (41), we depleted both NKT cells and NK cells by PK136 during hepatic I/R injury. Interestingly, treatment with PK136 showed similar effects on the degree of reduction in serum ALT levels as treatment with anti-CD1d antibody (∼25–30% reduction compared with wild-type mice), suggesting that NK cells were not involved in hepatic I/R injury. These results were supported by our previous studies that showed that NKT cells, but not NK cells, were recruited to the postischemic liver during hepatic I/R injury (4). Although our present study does not define the precise mechanism by which NKT cells mediate hepatic I/R injury, the release of IFN-γ by recruited NKT cells might stimulate other proinflammatory cytokines such as TNF-α, leading to augmented liver injury.
Our study demonstrates no significant differences in neutrophil accumulation in mice lacking NK or NKT cells or mice with an invariant TCR on CD4 T cells. However, in all these cases, the liver injury as determined by ALT and histology is decreased. Although the precise mechanisms of these effects are unclear, we speculate that CD4 or NKT cell-specific cytokines could directly or indirectly regulate neutrophil function such that the same degree of liver injury could be induced by a lower number of neutrophils with a higher state of activation. This concept was validated in our previous studies, which demonstrated that neutrophils isolated from the livers of CD4 knockout mice had a higher oxidative burst than those from wild-type mice (4).
Similar to NK cells, depletion of CD4+ CD25+ Treg by PC61 had no effects on hepatic I/R injury. Our findings are consistent with a preliminary study in a model of renal I/R that reported that PC61 treatment had little impact on the early phase of injury (32). However, this study showed higher necrosis in PC61-treated mice after 3 days of reperfusion, suggesting delayed recovery from I/R injury in Treg-depleted animals.
In conclusion, the present study demonstrates that antigen-dependent activation of CD4+ T cells through their TCRs contributes to hepatic I/R injury. Among the subsets of CD4+ T cells, γδ T cells appear to facilitate neutrophil recruitment, and NKT cells directly mediate liver injury. In contrast, NK cells and Treg seem to have no role in the acute response to I/R.
GRANTS
This work was supported by National Institutes of Health grants AG025881, DK56029 and HL72552 to A. Lentsch.
REFERENCES
- 1.Askenasy N, Kaminitz A, Yarkoni S. Mechanisms of T regulatory cell function. Autoimmun Rev 7: 370–375, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Bacon KB, Premack BA, Gardner P, Schall TJ. Activation of dual T cell signaling pathways by the chemokine RANTES. Science 269: 1727–1730, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Barone S, Okaya T, Rudich S, Petrovic S, Tenrani K, Wang Z, Zahedi K, Casero RA, Lentsch AB, Soleimani M. Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia-reperfusion injury. Am J Physiol Cell Physiol 289: C826–C835, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, Lentsch AB. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol 289: G969–G976, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol 2: 336–345, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA Jr. Role of tumor necrosis factor-alpha in the pathophysiologic alternations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest 85: 1936–1943, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derkow K, Loddenkemper C, Mintern J, Kruse N, Klugewitz K, Berg T, Wiedenmann B, Ploegh HL, Schott E. Differential priming of CD8 and CD4 T-cells in animal models of autoimmune hepatitis and cholangitis. Hepatology 46: 1155–1165, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Emoto M, Miyamoto M, Emoto Y, Zerrahn J, Kaufmann SH. A critical role of T-cell receptor gamma/delta cells in antibacterial protection in mice early in life. Hepatology 33: 887–893, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol 4: 231–237, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol 11: 637–685, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Hammond KJ, Pellicci DG, Poulton LD, Naidenko OV, Scalzo AA, Baxter AG, Godfrey DI. CD1d-restricted NKT cells: an interstrain comparison. J Immunol 167: 1164–1173, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol 3: 233–242, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Hochegger K, Schätz T, Eller P, Tagwerker A, Heininger D, Mayer G, Rosenkranz AR. Role of alpha/beta and γ/δ T cells in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 293: F741–F747, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Jaeschke H Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol 284: G15–G26, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun 15: 277–284, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol Gastrointest Liver Physiol 260: G355–G362, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol 61: 647–653, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Jaeschke H, Smith CW, Clemens MG, Ganey PE, Roth RA. Mechanisms of inflammatory liver injury: adhesion molecules and cytotoxicity of neutrophils. Toxicol Appl Pharmacol 139: 213–226, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Kato A, Gabay C, Okaya T, Lentsch AB. Specific role of interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Pathol 161: 1797–1803, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khandoga A, Hanschen M, Kessler JS, Krombach F. CD4+ T cells contribute to postischemic liver injury in mice by interacting with sinusoidal endothelium and platelets. Hepatology 43: 306–315, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Kronenberg M Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 23: 877–900, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Kuboki S, Shin T, Huber N, Eismann T, Galloway E, Schuster R, Blanchard J, Edwards MJ, Lentsch AB. Hepatocyte signaling through CXCR2 is detrimental to liver recovery after ischemia/reperfusion in mice. Hepatology 48: 1213–1223, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuniyasu Y, Takahashi T, Itoh M, Shimizu J, Toda G, Sakaguchi S. Naturally anergic and suppressive CD4+CD25+ T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation. Int Immunol 12: 1145–1155, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Kurokawa T, Kobayashi H, Nonami T, Harada A, Nakao A, Sugiyama S, Ozawa T, Takagi H. Beneficial effects of cyclosporine on postischemic liver injury in rats. Transplantation 53: 308–311, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med 203: 2639–2648, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leite-De-Moraes MC, Hameg A, Arnould A, Machavoine F, Koezuka Y, Schneider E, Herbelin A, Dy M. A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement. J Immunol 163: 5871–5876, 1999. [PubMed] [Google Scholar]
- 27.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology 32: 169–173, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology 27: 1172–1177, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology 27: 1172–1177, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Loi P, Paulart F, Pajak B, Nagy N, Salmon I, Moser M, Goldman M, Flamand V. The fate of dendritic cells in a mouse model of liver ischemia/reperfusion injury. Transplant Proc 36: 1275–1279, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Mercer JC, Ragin MJ, August A. Natural killer T cells: rapid responders controlling immunity and disease. Int J Biochem Cell Biol 37: 1337–1343, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Midory R, Camara NOS, Cenedeze M, Rodrigues MM, Pacheco-Silva A. Regulatory T cells (CD4+ CD25+) in kidney ischemia-reperfusion injury (Abstract). Transplantation 82: 1042–1043, 2006.17060852 [Google Scholar]
- 33.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 59: 3128–3133, 1999. [PubMed] [Google Scholar]
- 34.Pennington DJ, Vermijlen D, Wise EL, Clarke SL, Tigelaar RE, Hayday AC. The integration of conventional and unconventional T cells that characterizes cell-mediated responses. Adv Immunol 87: 27–59, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Sakr MF, Abdel-Aal AN. Protective effect of cyclosporine A (CyA) against the hepatic injury associated with ischemia and reperfusion. Int Surg 81: 180–183, 1996. [PubMed] [Google Scholar]
- 36.Savransky V, Molls RR, Burne-Taney M, Chien CC, Racusen L, Rabb H. Role of the T-cell receptor in kidney ischemia-reperfusion injury. Kidney Int 69: 233–238, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Shimamura K, Kawamura H, Nagura T, Kato T, Naito T, Kameyama H, Hatakeyama K, Abo T. Association of NKT cells and granulocytes with liver injury after reperfusion of the portal vein. Cell Immunol 234: 31–38, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory cells is antigen nonspecific. J Immunol 164: 183–190, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Tschöp J, Martignoni A, Goetzman HS, Choi LG, Wang Q, Noel JG, Ogle CK, Pritts TA, Johannigman JA, Lentsch AB, Caldwell CC. Gammadelta T cells mitigate the organ injury and mortality of sepsis. J Leukoc Biol 83: 581–588, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsung A, Zheng N, Jeyabalan G, Izuishi K, Klune JR, Geller DA, Lotze MT, Lu L, Billiar TR. Increasing numbers of hepatic dendritic cells promote HMGB1-mediated ischemia-reperfusion injury. J Leukoc Biol 81: 119–128, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Vivier E, Toselo E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 9: 503–510, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Wanner GA, Ertel W, Müller P, Höfer Y, Leiderer R, Menger MD, Messmer K. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock 5: 34–40, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoka M, Chan R, Friend D, Mahmood U, Weissleder R, Moore FD, Carroll MC. Identification of the target self-antigens in reperfusion injury. J Exp Med 203: 141–152, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest 100: 279–289, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]