Abstract
Dysregulation of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) has been implicated in the pathogenesis of alcoholic liver injury. Sirtuin 1 (SIRT1) is an NAD+-dependent class III protein deacetylase that is known to be involved in regulating production of proinflammatory cytokines including TNF-α. In the present study, we examined the role of SIRT1 signaling in TNF-α generation stimulated by either lipopolysaccharide (LPS), acetaldehyde (AcH), or acetate (two major metabolites of ethanol) in two cultured macrophage cell lines. In both rat Kupffer cell line 1 (RKC1) and murine RAW 264.7 macrophages, treatment with either LPS, AcH, or acetate caused significant decreases in SIRT1 transcription, translation, and activation, which essentially demonstrated an inverse relationship with TNF-α levels. LPS, AcH, and acetate each provoked the release of TNF-α from RKC1 cells, whereas coincubation with resveratrol (a potent SIRT1 agonist) inhibited this effect. Conversely, addition of sirtinol (a known SIRT1 inhibitor) or knocking down SIRT1 by the small silencing SIRT1 plasmid (SIRT1shRNA) augmented TNF-α release, suggesting that impairment of SIRT1 may contribute to TNF-α secretion. Further mechanistic studies revealed that inhibition of SIRT1 by LPS, AcH, or acetate was associated with a marked increase in the acetylation of the RelA/p65 subunit of nuclear transcription factor (NF-κB) and promotion of NF-κB transcriptional activity. Taken together, our findings suggest that SIRT1-NF-κB signaling is involved in regulating LPS- and metabolites-of-ethanol-mediated TNF-α production in rat Kupffer cells and in murine macrophages. Our study provides new insights into understanding the molecular mechanisms underlying the development of alcoholic steatohepatitis.
Keywords: inflammation, adiponectin, signal transduction
alcoholic liver disease (ALD) is clinically associated with the development of steatosis, inflammation, and eventually fibrosis and cirrhosis. The underlying cellular and molecular mechanisms by which alcohol causes liver injury are complex and incompletely understood.
Hepatic ethanol metabolism has long been implicated in the development of ALD (22, 23). Metabolic byproducts of ethanol, such as acetaldehyde (AcH) and acetate, have been presumed agents of such damage. Additionally, lipopolysaccharide (LPS), one of the components of the outer wall of gram-negative bacteria, has been thought to participate in the etiology (10, 31). Chronic ethanol exposure alters gut microflora and permeability, resulting in elevated release of LPS, which enters the liver through the portal vein and activates resident macrophages (Kupffer cells), which release proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), which, in turn, promote liver damage. Meanwhile, a corollary process occurs in which chronic ethanol feeding sensitizes Kupffer cells to LPS signaling, enhancing LPS-stimulated TNF-α production in rodents (31).
Sirtuin 1 (SIRT1) is an NAD+-dependent class III protein deacetylase that exerts anti-inflammatory effects by deacetylation of modified lysine residues on transcriptional regulators, particularly nuclear transcription factor-κB (NF-κB), a master transcription factor involved in regulation of proinflammatory cytokines (29). SIRT1 physically interacts with and deacetylates the RelA/p65 subunit of NF-κB at lysine 310 and subsequently inhibits NF-κB transcriptional activity (6, 36, 38, 39). The anti-inflammatory action of SIRT1 is further supported by a recent study demonstrating that activation of SIRT1 protects mice from high-fat diet-induced hepatic inflammation through decreasing the NF-κB-mediated induction of inflammatory cytokines including TNF-α (27).
Adiponectin is an adipocyte-derived protein that has potent anti-inflammatory properties. ALD is associated with reduced circulating adiponectin levels and impaired hepatic adiponectin signaling (28). The anti-inflammatory properties of adiponectin have been implicated in the protective action of adiponectin against development of alcoholic liver injury (25). However, the underlying molecular mechanisms by which adiponectin exerts its protective effects are still not fully understood.
In the present study, we used immortalized rat Kupffer cell line 1 (RKC1) and murine RAW 264.7 macrophages to test the hypothesis that SIRT1 signaling is involved in regulating the TNF-α production that is stimulated by three putative inducers of ALD [LPS and two major metabolites of ethanol (AcH and acetate)]. Furthermore, we investigated the corollary hypothesis, that SIRT1 plays a role in adiponectin's suppression of these compounds' abilities to induce TNF-α.
MATERIALS AND METHODS
Materials.
Most chemicals and supplies were purchased from Sigma Chemical, Schleicher and Schuell, GIBCO-BRL, and Dupont NEN Research Products. Acetate, AcH, LPS (tissue culture-tested, L-2654), and resveratrol were obtained from Sigma. Sirtinol and splitomicin were purchased from Calbiochem (Gibbstown, NJ). Recombinant human globular adiponectin (gAcrp) was purchased from PeproTech (Rockyhill, NJ).
Plasmids.
NF-κB-responsive reporter-3xκB luciferase plasmid, wild-type SIRT1 (SIRT1wt) or deacetylase-defective SIRT1(H363Y) mutant expression plasmids were kind gifts from Dr. Marty W. Mayo (University of Virginia, Charlottesville, VA) (38). The small silencing SIRT1 plasmid (SIRT1shRNA) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture and treatment.
Immortalized rat Kupffer cell line 1 (RKC1) was established as described previously (26). The murine RAW264.7 macrophages were obtained from the American Type Culture Collection (Manassas, VA). All cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 μg/ml streptomycin, and 63 μg/ml penicillin G. For treatments, cells were plated onto six-well plates. At 80–90% confluence, the cells were incubated for 16 h in serum-free RPMI 1640 medium. The cells were treated with various reagents for 18 h. For AcH treatment, the culture plates were sealed to prevent the loss of AcH by evaporation.
TNF-α assay.
TNF-α concentration in cell culture media of RKC1 or RAW 264.7 macrophages was measured by using a mouse TNF-α ELISA kit (eBioscience, San Diego, CA) according to manufacturer's protocol.
Total RNA isolation and qRT-PCR.
Total RNA was prepared from cells by use of an RNAeasy Total RNA kit (QIAGEN). Reverse transcription of 2 μg of total RNA to cDNA was performed by using the StrataScript QPCR cDNA Synthesis Kit (Stratagene). Real-time quantitative polymerase chain reaction (qRT-PCR) amplification was performed in a iCycler Spectrofluorometric thermal cycler (Bio-Rad Laboratories, Hercules, CA) with use of RT2 SYBR Green qPCR Master Mix. The following primer sets were purchased from SuperArray Bioscience (Frederick, MD) and used: SIRT1 (PPM05054A) and GAPDH (PPM02946E). The relative amount of target mRNA was calculated by the comparative cycle threshold (Ct) method by normalizing target mRNA Ct to those for GAPDH.
SIRT1 deacetylase activity.
Sirtuin activity was measured by a SIRT1 fluorometric kit (AK-555, Biomol, Plymouth Meeting, PA) (14, 38). Briefly, the assays were performed by incubation with recombinant human SIRT1 and substrates, including a fluorogenic acetylated Lys382 p53 peptide (50 μM) and NAD (100 μM) at 37°C for 30 min. Fluorescent intensity was measured at 460 nm by using a fluorescence plate reader. Resveratrol (200 μM) was used as a positive control, and 1 mM nicotinamide or splitomicin (120 μM) served as negative controls. A standard calibration curve was prepared with a known amount of the deacetylated standard included in the kit.
Small interfering RNA transfection.
The day before transfection, plates were inoculated with an appropriate number of RKC1 cells in serum-containing medium to ensure 50–70% confluence in the following day. Control shRNA or SIRT1shRNA mixed with siLentFect (Bio-Rad) was added to the cells at a concentration of 10 nM. At 48 h after transfection, the cells were treated with various regents in serum-free RPMI 1640 medium.
Immunoprecipitation and Western blots.
For immunoprecipitation assays (42), primary antibodies were mixed with precleared lysates and 20 μl of protein agarose A/G (Santa Cruz Biotechnology), and reactions were tumbled overnight at 4°C. The agarose beads were washed, and the protein was eluted and then subjected to Western blotting analysis. Western blotting analyses were performed by using 8- to 15-μg whole cell extract separated by electrophoresis in a 10% SDS-polyacrylamide gel and transferred to nitrocellulose filters. SIRT1 antibody was from Upstate Biotechnology (Lake Placid, NY). GAPDH antibody (Santa Cruz Biotechnology) was used to normalize the signal obtained for total protein extracts. The protein bands were quantified on a PhosphorImager and ImageQuant (Amersham Biosciences) software analysis.
RelA/p65 acetylation assays.
RelA/p65 protein was immunoprecipitated from whole cell extracts by a RelA/p65 antibody (Santa Cruz Biotechnology). Acetylation and protein levels of RelA/p65 were detected by use of antibodies for acetyl-lysine (Cell Signaling) and RelA/p65, respectively.
Transfections and luciferase assays.
Cell transfection assays were carried out as described previously (40, 42). Briefly, plasmids were transiently transfected into cells by use of Lipofectamine reagent (Invitrogen). Luciferase assays were carried out using cell extracts, and the results were averaged to represent a single data point for each transfection. β-Galactosidase was used as an internal control to correct for transfection efficiency.
Statistical analysis.
Data are presented as means ± SE. Multiple comparisons were evaluated by ANOVA followed by Tukey's multiple-comparison procedure with P < 0.05 being considered significant.
RESULTS
SIRT1's mRNA, protein, and enzymatic activity were reduced by LPS, AcH, or acetate in RKC1 and RAW 264.7 macrophages.
Both RKC1 and murine RAW 264.7 macrophages display many characteristics similar to Kupffer cells, particularly their pathways regulating LPS-induced production of TNF-α (26, 24). Furthermore, they each express ample amounts of SIRT1 mRNA and protein (Fig. 1). Hence, these two cell lines were used to investigate the effects of LPS, AcH, and acetate on SIRT1 signaling.
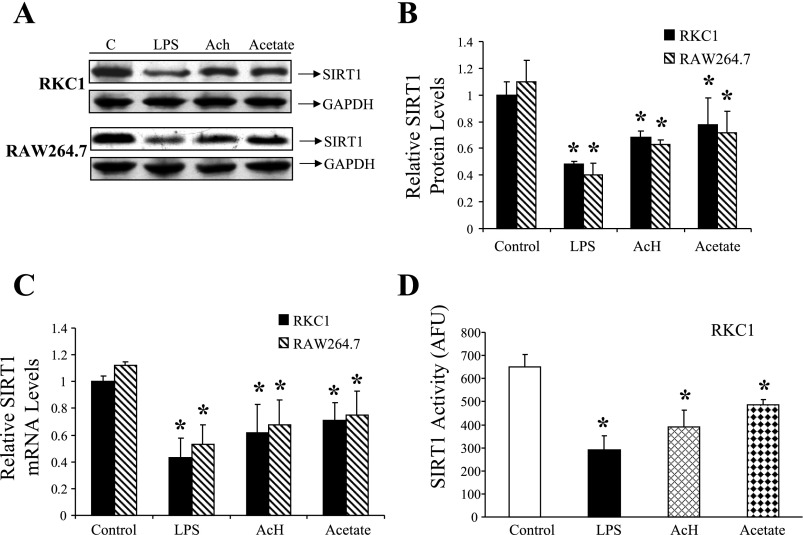
Fig. 1.
Effects of LPS, acetaldehyde (AcH), or acetate on sirtuin 1 (SIRT1) mRNA, protein, and activity in RKC1 or RAW 264.7 macrophages. RKC1 or RAW 264.7 macrophages were maintained in serum-free DMEM for 16 h and incubated for 18 h without or with LPS (100 ng/ml), AcH, (100 μM), or acetate (20 mM). A: representative Western blots of SIR1 protein levels. B: relative SIRT1 protein levels. C: relative SIRT1 mRNA levels. D: relative SIRT1 deacetylase activity in RKC1 cells. Fluor de Lys fluorescence deacetylase assays were performed as described in materials and methods. SIRT1 deacetylase activity is represented as arbitrary fluorescence units (AFU). Data are means ± SE for 3 or 4 replications. *P < 0.05 compared with controls by 1-way ANOVA.
We initially sought to determine the effect of each molecule on the expression and activity of SIRT1. Cells were exposed to various concentrations of LPS, AcH, or acetate for 18 h and were then harvested. SIRT1 protein expression levels were determined by utilizing Western blotting techniques. In each cell line, treatment with either LPS, AcH, or acetate significantly reduced SIRT1 protein levels, with an optimal effect at 100 ng/ml for LPS, 100 μM for AcH, and 20 mM for acetate (Fig. 1, A and B). These concentrations did not adversely affect cell viability and were thus used in subsequent experiments. Next, utilizing qRT-PCR, we determined that treatment with each compound significantly suppressed SIRT1 mRNA expression in both cell types (Fig. 1C). Similarly, SIRT1 deacetylase activity was determined fluorimetrically and shown to be significantly reduced in RKC1 cells following 18-h incubation with either LPS, AcH, or acetate (Fig. 1D). Taken together, these results provided evidence that LPS, AcH, and acetate each inhibit SIRT1's transcription, translation, and activation in cultured RKC1 and RAW 264.7 macrophages.
Pharmacological or genetic modulation of SIRT1 regulates TNF-α secretion stimulated by LPS, AcH, or acetate in RKC1.
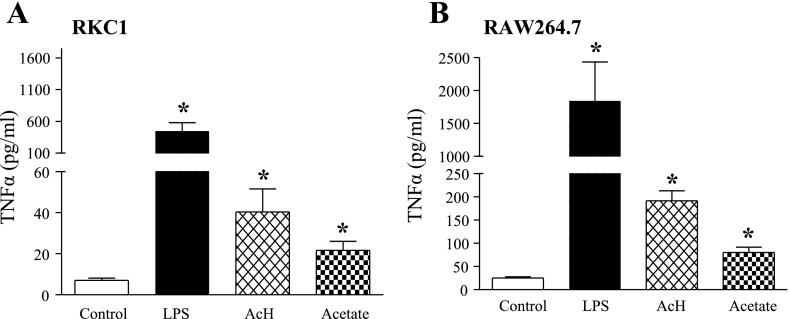
We utilized the same treatment protocol to determine the effect of these molecules on TNF-α induction. As shown in Fig. 2, incubation with LPS, AcH, or acetate caused a robust rise in TNF-α secretion in RKC1 or RAW 264.7 macrophages.
Fig. 2.
Effects of LPS, AcH, or acetate on TNF-α generation in RKC1 or RAW 264.7 macrophages. RKC1 (A) or RAW 264.7 (B) macrophages were starved in serum-free DMEM for 16 h and incubated for 18 h without or with LPS (100 ng/ml), AcH, (100 μM) or acetate (20 mM). Cell culture media samples were collected, and TNF-α was quantified by ELISA. All data are means ± SE for 3 or 4 replications. *P < 0.05 compared with controls by 1-way ANOVA.
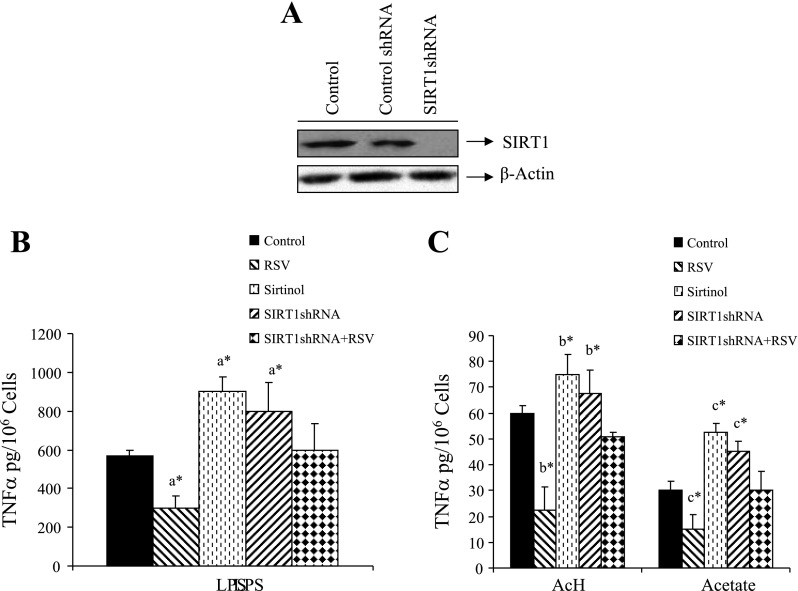
We then employed pharmacological and genetic manipulations of SIRT1 to study its role in mediating TNF-α levels. Pretreatment of RKC1 cells with 10 μM resveratrol (a potent SIRT1 activator) for 2 h, followed by coincubation with LPS, AcH, or acetate for 18 h significantly attenuated elevations in TNF-α (Fig. 3, B and C). In a complementary fashion, SIRT1 antagonists were utilized. Inhibition of SIRT1 was mediated respectively by sirtinol (a known SIRT 1 inhibitor) or the small silencing SIRT1 plasmid (SIRT1shRNA). Functionality of SIRT1 shRNA as an effective inhibitor of SIRT1 expression was demonstrated in RKC1 cells via Western blot analysis (Fig. 3A). Knocking down SIRT1 by SIRT1shRNA largely blunted the inhibitory effect of resveratrol on the TNF-α release stimulated by LPS, AcH, or acetate, suggesting that SIRT1 activity is integral to this effect. (Fig. 3, B and C). Interestingly, in the absence of resveratrol treatment, inhibition of SIRT1 resulted in a modest but significant increase in TNF-α, suggesting that the basal activity of SIRT1 lightly moderates release of the cytokine. In the absence of LPS, AcH, or acetate, sirtinol and SIRT1shRNA had negligible effect on TNF-α generation (data not shown). Similar effects were also obtained in the RAW 264.7 macrophage cell line (data not shown). Taken together, our results suggest that, in cultured RKC1 or RAW 264.7 macrophages, TNF-α release induced by treatment with LPS, AcH, or acetate may be mediated, at least in part, through inhibition of SIRT1.
Fig. 3.
Effects of pharmacological or genetic modulation of SIRT1 on TNF-α secretion stimulated by LPS, AcH, or acetate in RKC1. A: Western blots of RKC1 cells transfected with control small silencing (shRNA) or SIRT1shRNA plasmids. SIRT1 was detected by Western blots with an anti-SIRT1 antibody. B and C: RKC1 were transfected with SIRT1shRNA plasmid or pretreated with SIRT1 activator resveratrol (RSV, 10 μM) or SIRT1 inhibitor (sirtinol, 10 μM) for 2 h and then incubated for 18 h without or with LPS (100 ng/ml), AcH, (100 μM) or acetate (20 mM). TNF-α in cell culture media was measured by ELISA. Data are means ± SE for 3 or 4 replications. *P < 0.05 by 1-way ANOVA. aSignificantly different compared with LPS-treated control group. bSignificantly different compared with AcH-treated control group. cSignificantly different compared with acetate-treated control group.
SIRT1 signaling regulates LPS, AcH, or acetate-induced NF-κB transcriptional activity.
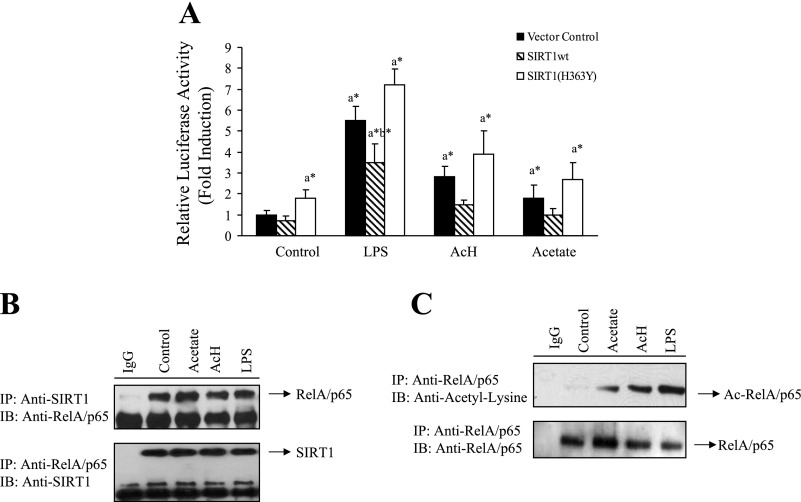
SIRT1-NF-κB axis is known to be involved in regulating production of proinflammatory cytokines such as TNF-α (29). We investigated the role of SIRT1 in LPS- or ethanol metabolite (AcH- or acetate)-mediated NF-κB transcriptional activity in murine RAW 264.7 macrophages. Cells were transfected with an NF-κB-responsive reporter (a 3xκB luciferase) alone or jointly with a plasmid for either wild-type SIRT1 (SIRT1wt) or a dominant-negative, deacetylase-defective SIRT1 [SIRT1(H363Y)] (38). Treatment of vector control-transfected cells with LPS, AcH, or acetate significantly increased NF-κB transcriptional activity by ∼5.5-, 2.9-, and 1.8-fold, respectively, (Fig. 4A). However, the cells that were transfected with SIRT1wt displayed significant reduction in the NF-κB transcriptional activity induced by LPS, AcH, or acetate. Conversely, cotransfection with the deacetylase-defective SIRT1(H363Y) plasmid modestly increased the effects of LPS, AcH, or acetate on NF-κB activity (Fig. 4A). Collectively, these results suggest that the NF-κB transcriptional activity that is induced by LPS, AcH, or acetate treatments may be, at least in part, mediated through downregulating SIRT1 signaling.
Fig. 4.
LPS-, AcH-, or acetate-mediated inhibition of SIRT1 was associated with increased acetylation of RelA/p65 and stimulated NF-κB transcriptional activity. A: RKC1 cells were transiently transfected with a NF-κB-responsive reporter-3xκB luciferase (10 μg) and expression plasmids (5 μg/each) of SIRT1wt or SIRT1(H363Y) and β-galactosidase (2 μg; internal control). LPS (100 μM), AcH, (100 μM), or acetate (20 mM) was added for 18 h. At 48 h after transfection, cells were harvested, and luciferase and β-galactosidase activities were determined as described in materials and methods. B: RKC1 cells were starved in serum-free DMEM overnight and treated with LPS (100 ng/ml), AcH, (100 μM) or acetate (20 mM) for 18 h. Cell extracts were immunoprecipitated (IP) with an antibody to SIRT1 or RelA/65 and immunoblotted (IB) with an anti-SIRT1 or an anti-RelA/65 antibody. C: RKC1 cells were treated as described in A. Immunoprecipitations were performed with an antibody to RelA/p65 and immunoblotted with an acetyl-lysine or a RelA/p65 antibody. All data are means ± SE for 3 or 4 replications. *P < 0.05 by 1-way ANOVA. aSignificantly different compared with control group. bSignificantly different compared with LPS-treated vector control group.
LPS, AcH, or acetate-mediated inhibition of SIRT1 signaling was associated with increased acetylation of RelA/p65 and enhanced NF-κB transcriptional activity.
SIRT1 is capable of inhibiting NF-κB transcriptional activity by deacetylating RelA/p65 (6, 36, 38). Therefore, we determined whether LPS- or ethanol metabolites (AcH or acetate)-mediated inhibition of SIRT1 results in hyperacetylation of RelA/p65. We first examined the physical association of SIRT1 with RelA/p65 of NF-κB by performing coimmunoprecipitation assays in RKC1 cells. In agreement with reported findings (6, 36), an antibody to SIRT1 coprecipitated RelA/p65 and an antibody to RelA/p65 coprecipitated SIRT1 from RKC1 cells, suggesting that SIRT1 was physically associated with RelA/p65 (Fig. 4B). To determine the effect of LPS, AcH, or acetate on acetylation of Rel/p65, RKC1 cells were treated with LPS, AcH, or acetate for 18 h, and whole cell lysates were prepared and immunoprecipitated with an anti-RelA/p65 antibody and then immunoblotted with an anti-acetyl-lysine antibody. As shown in Fig. 4C, treatment with either LPS, AcH, or acetate substantially induced acetylation of RelA/p65.
Together, these data demonstrate that SIRT1 does physically interact with the RelA/p65 subunit of NF-κB and that inhibition of SIRT1 by LPS or ethanol metabolites (AcH and acetate) leads to hyperacetylation of RelA/p65, activation of NF-κB, and potentially increased release of TNF-α.
Adiponectin attenuated LPS- or acetate-induced TNF-α production through upregulation of SIRT1 in RKC1 and RAW 264.7 macrophages.
The protective effect of adiponectin against ALD has been partially attributed to its ability to suppress LPS-stimulated TNF-α production in Kupffer cells (24, 25, 31). Hence, we investigated the involvement of SIRT1 signaling in the inhibitory effect of adiponectin on TNF-α secretion stimulated by LPS or ethanol metabolites.
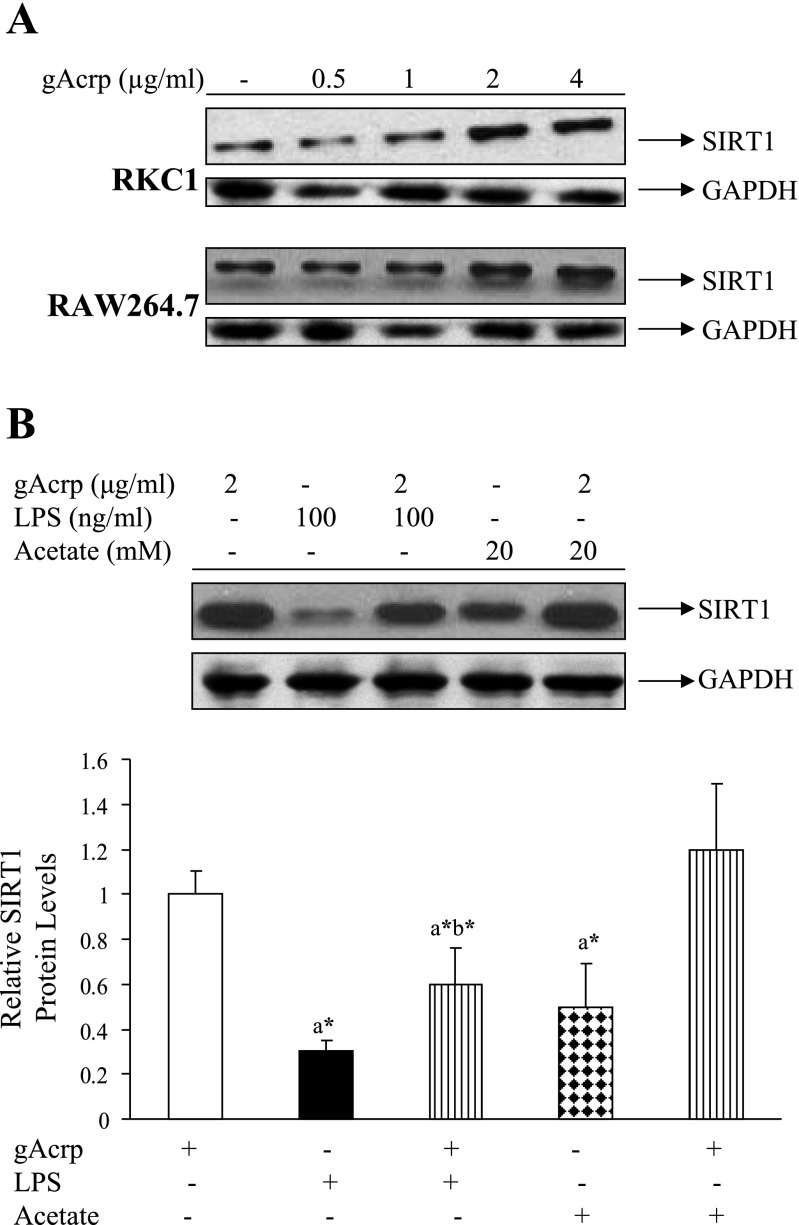
Both RKC1 and RAW 264.7 macrophages are more sensitive to globular adiponectin (gAcrp) than to full-length adiponectin (data not shown). Thus all experiments were carried out with gAcrp. The effect of gAcrp on SIRT1 protein expression was tested in both RKC1 and RAW 264.7 cell lines by treating cells with various concentrations of gAcrp for 18 h. As shown in Fig. 5A, in both cell lines, treatment with gAcrp significantly increased SIRT1 protein levels in a dose-dependent manner with a maximal effect at 2 μg/ml. Although SIRT1 protein levels were significantly reduced by treatment with LPS (100 ng/ml) or acetate (20 mM), preincubation with gAcrp (2 μg/ml) for 1 h partially or completely relieved the inhibition of SIRT1 protein expression caused by LPS or acetate in RKC1 cells (Fig. 5B).
Fig. 5.
Adiponectin increased SIRT1 protein expression levels and reversed the inhibition of SIRT1 by LPS or acetate in RKC1 or RAW 264.7 macrophages. A: RKC1 cells were starved in serum-free DMEM overnight, and globular adiponectin (gAcrp) was added at the indicated concentrations for 18 h. SIRT1 protein levels were detected by Western blots with an anti-SIRT1 antibody. B: RKC1 cells were starved in serum-free DMEM overnight, and pretreated with gAcrp (2 μg/ml) for 2 h followed by addition of LPS (100 ng/ml) or acetate (20 mM) for 18 h. SIRT1 protein expression levels were detected by Western blots with an anti-SIRT1 antibody. Data are means ± SE for 3 or 4 replications. *P < 0.05 by 1-way ANOVA. aSignificantly different compared with gAcrp-alone group. bSignificantly different compared with LPS-alone group.
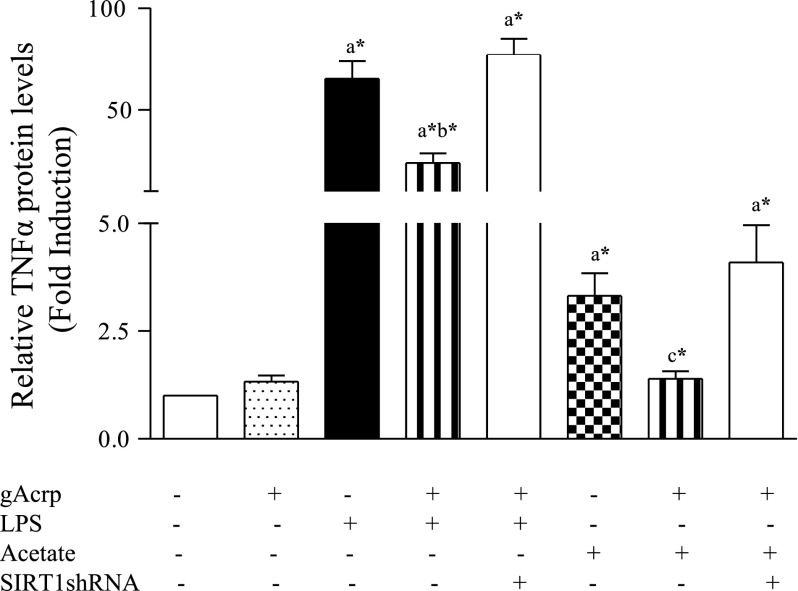
To examine the effect of adiponectin on LPS- or acetate-induced TNF-α secretion, RKC1 cells were pretreated with gAcrp (2 μg/ml) for 1 h, followed by stimulation with LPS (100 ng/ml) or acetate (20 mM) for 18 h. As shown in Fig. 6, marked increases in TNF-α were produced by exposure of RKC1 to LPS or acetate. Pretreatment with adiponectin partially, but significantly, attenuated TNF-α production induced by LPS and completely blocked TNF-α secretion stimulated by acetate. More importantly, inhibition of SIRT1 by overexpression of SIRT1shRNA plasmid abolished the inhibitory effect of adiponectin on TNF-α, suggesting that the ability of adiponectin to regulate downstream TNF-α production stimulated by LPS or acetate may occur, at least in part, through stimulating SIRT1 signaling (Fig. 6).
Fig. 6.
Adiponectin attenuated LPS- or acetate-induced TNF-α production in RKC1. RKC1 cells or RKC1 cells transfected with SIRT1shRNA plasmid were pretreated with gAcrp (2 μg/ml) for 2 h and followed by addition of LPS (100 μM) or acetate (20 mM) for 18 h. TNF-α concentrations in cell culture media were measured by ELISA. Data are means ± SE for 3 or 4 replications. *P < 0.05 by 1-way ANOVA. aSignificantly different compared with control. bSignificantly different compared with LPS-alone group. cSignificantly different compared with acetate-alone group.
Similar results were generated in RAW 264.7 macrophages (data not shown). Moreover, we attempted to measure the effect of gAcrp on AcH-mediated SIRT1 reduction and TNF-α production, but the data did not reach statistical significance (data not shown).
DISCUSSION
In the present study, using cultured macrophage cell lines, we investigated the involvement of SIRT1 in the generation of TNF-α induced by three putative agents of ALD: LPS, and two major metabolites of ethanol (AcH and acetate). Using RKC1 and RAW 264.7 macrophages, we demonstrated that LPS, AcH, and acetate each significantly inhibited the transcription, translation, and activation of SIRT1. The inhibition of SIRT1 coincided with significantly increased release of TNF-α from both cell lines. Pretreatment with resveratrol, a known agonist of SIRT1, largely prevented the induction of TNF-α in RKC1 cells. In contrast, cotreatment with sirtinol (a known SIRT1 inhibitor) or knocking down SIRT1 with SIRT1shRNA augmented the LPS-, AcH-, or acetate-induced TNF-α release compared with the controls. Furthermore, inhibition of SIRT1 by LPS, AcH, or acetate was accompanied by significantly increased acetylation of RelA/p65 and enhanced NF-κB transcriptional activity. In addition, we demonstrated that adiponectin, an adipocyte-derived adipokine, attenuated LPS- or acetate-induced TNF-α secretion in RKC1 through upregulation of SIRT1. These findings, for the first time, suggest that three compounds, which have all been implicated in the development of ALD, may mediate TNF-α release from Kupffer cells, at least in part, by impairing SIRT1-NF-κB signaling.
The precise mechanisms by which these molecules inhibit SIRT1 were not established in this study, but it is possible that they may all trigger a common molecular signaling cascade that ultimately impinges on SIRT1. If this is the case, then the reactive oxygen species (ROS) that are generated from Kupffer cells that are exposed to LPS or metabolites of ethanol are likely involved (3, 7, 32, 33). Moreover, studies have shown that ethanol exposure increases LPS-stimulated NADPH oxidase-dependent production of ROS in Kupffer cells (25, 32). Oxidative stress suppresses SIRT1 mRNA and protein expression levels (1, 35). It is likely that LPS or metabolites of ethanol may downregulate SIRT1 through excess production of ROS.
Studies have shown that Kupffer cells isolated from livers of chronically ethanol-fed animals were sensitized to LPS, responding with increased TNF-α generation (4, 12, 25, 31, 32). Although several factors, such as activation of transcription factor early-growth response factor-1 (Egr-1), have been attributed to this effect, our present findings may provide additional explanations (17, 30). Chronic ethanol administration inhibits activities of hepatic SIRT1 in mice and rats (2, 19, 20, 41, 42). It is logical to speculate that, in chronically ethanol-fed animals, impaired hepatic SIRT1 signaling may prime and sensitize Kupffer cells to increase TNF-α production in response to LPS.
In addition to NF-κB, activator protein-1 (AP-1) has also been shown to be involved in regulating TNF-α expression in response to LPS (37). SIRT1 has been shown to directly inhibit the transcriptional activity of AP-1 by targeting c-Jun, a major component of AP-1 (11). It should be quite interesting to investigate whether inhibition of SIRT1-AP-1 signaling also contributes to increased TNF-α production by LPS or ethanol metabolites in Kupffer cells.
Alcoholic liver injury is associated with a global change in gene expression. For example, exposure of Kupffer cells to ethanol or LPS increased Toll-like receptors (TLRs) gene expression, particular Toll-like receptor 4 (TLR4) (13). TLR4 is a major receptor on Kupffer cells that binds LPS, stimulating TNF-α transcription, and plays a critical role in development of ALD (34). SIRT1 is known to directly regulate gene expression through its activity on histones and is generally associated with gene silencing (8). We demonstrated that both LPS and ethanol metabolites inhibit SIRT1 activity. This raises the possibility that such inhibition of SIRT1 may result in increased gene expression of TLR4.
The ability of resveratrol (a naturally occurring phenolic phytoalexin) to inhibit TNF-α production in macrophages has been demonstrated by several studies (9, 18, 21). Our data shows that knocking down SIRT1 largely blocks the inhibition of resveratrol on TNF-α, suggesting that the inhibitory effect of SIRT1 on TNF-α may be, at least in part, mediated through activation of SIRT1. Several lines of evidence have suggested that activation of SIRT1 by resveratrol blocked the upregulation of NF-κB signaling by stimulators such as TNF-α or amyloid-β (6, 29, 36, 38). These studies further support our present findings that SIRT1 signaling is involved in the protective effects of resveratrol. Furthermore, the action of resveratrol against TNF-α generation stimulated by LPS or ethanol metabolites may explain its beneficial effects on ALD (2, 15, 16).
There is accumulating evidence that adiponectin suppresses LPS-stimulated TNF-α production in Kupffer cells and in murine macrophages (24, 25). Both transcriptional and posttranscriptional mechanisms have been suggested to mediate the action of adiponectin on LPS-stimulated TNF-α release (24). The present study suggests that adiponectin-SIRT1 signaling is also involved in these specific effects. Moreover, our observation that gAcrp treatment significantly increases SIRT1 protein expression levels in Kupffer cells is consistent with a report that gAcrp directly upregulates SIRT1 in primary human myotubes (5). Future studies are needed to elucidate a detailed mechanism of action of adiponectin on SIRT1 signaling.
Elevated levels of LPS and ethanol metabolites such as acetate and acetaldehyde are commonly associated with ethanol consumption and alcoholic liver injury (10, 22, 23, 31). Our present findings for the first time demonstrate that inhibition of SIRT1 signaling by LPS or major metabolites of ethanol may be, at least in part, responsible for the activation of NF-κB pathways and subsequent generation of TNF-α in Kupffer cells and macrophages. Interestingly, we found that ethanol exposure to RKC1 cells only modestly reduced SIRT1 protein expression without statistical significance. Kupffer cells may have a relatively low capacity to metabolize ethanol compared with other liver cell types such as hepatocytes. We are currently investigating the expression and activities of alcohol dehydrogenase and aldehyde dehydrogenase 2 in RKC1 or macrophages. Our study provides new insights into understanding the molecular mechanisms of alcoholic steatohepatitis. Finally, we suggest that resveratrol or adiponectin might be useful therapeutic agents against elevated TNF-α through their ability to stimulate SIRT1.
GRANTS
This study was supported by National Institute on Alcoholism and Alcohol Abuse Grants AA-015951 and AA-013623 (to M. You).
Acknowledgments
We thank Dr. Qi Cao for outstanding technical contributions.
REFERENCES
- 1.Abdelmohsen K, Pullmann R Jr, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, Furneaux H, Gorospe M. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell 25: 543–557, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol 295: G833–G842, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Q, Mak KM, Lieber CS. Dilinoleoylphosphatidylcholine decreases acetaldehyde-induced TNF-alpha generation in Kupffer cells of ethanol-fed rats. Biochem Biophys Res Commun 299: 459–464, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Cao Q, Mak KM, Lieber CS. Dilinoleoylphosphatidylcholine decreases LPS-induced TNF-alpha generation in Kupffer cells of ethanol-fed rats: respective roles of MAPKs and NF-kappaB. Biochem Biophys Res Commun 294: 849–853, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med 4: e76, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol 294: H2721–H2735, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Minicis S, Brenner DA. Oxidative stress in alcoholic liver disease: role of NADPH oxidase complex. J Gastroenterol Hepatol 23: S98–S103, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Feige JN, Auwerx J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr Opin Cell Biol 20: 303–309, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng YH, Zou JP, Li XY. Effects of resveratrol and ethanol on production of pro-inflammatory factors from endotoxin activated murine macrophages. Acta Pharmacol Sin 23: 1002–1006, 2002. [PubMed] [Google Scholar]
- 10.Fukui H Relation of endotoxin, endotoxin binding proteins and macrophages to severe alcoholic liver injury and multiple organ failure. Alcohol Clin Exp Res 29: 172S–179S, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Gao Z, Ye J. Inhibition of transcriptional activity of c-JUN by SIRT1. Biochem Biophys Res Commun 376: 793–796, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gobejishvili L, Barve S, Joshi-Barve S, McClain C. Enhanced PDE4B expression augments LPS-inducible TNF expression in ethanol-primed monocytes: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 295: G718–G724, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, Louis H, Deviere J, Le Moine O. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology 43: 989–1000, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 283: 20015–20026, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, El May M, Gharbi N, Kamoun A, El-Fazaâ S. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci 80: 1033–1039, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, Gharbi N, Kamoun A, El-Fazaa S. Protective effect of resveratrol on ethanol-induced lipid peroxidation in rats. Alcohol Alcohol 41: 236–239, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr-1 contribute to increased TNF-α production in rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol 282: G6–G15, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Kowalski J, Samojedny A, Paul M, Pietsz G, Wilczok T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor-alpha genes in J774.2 macrophages. Pharmacol Rep 57: 390–394, 2005. [PubMed] [Google Scholar]
- 19.Lieber CS, Leo MA, Wang X, Decarli LM. Alcohol alters hepatic FoxO1, p53, and mitochondrial SIRT5 deacetylation function. Biochem Biophys Res Commun 373: 246–252, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Lieber CS, Leo MA, Wang X, Decarli LM. Effect of chronic alcohol consumption on hepatic SIRT1 and PGC-1alpha in rats. Biochem Biophys Res Commun 370: 44–48, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Ma ZH, Ma QY, Wang LC, Sha HC, Wu SL, Zhang M. Effect of resveratrol on NF-kappaB activity in rat peritoneal macrophages. Am J Chin Med 34: 623–630, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Meier P, Seitz HK. Age, alcohol metabolism and liver disease. Curr Opin Clin Nutr Metab Care 11: 21–26, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Mello T, Ceni E, Surrenti C, Galli A. Alcohol induced hepatic fibrosis: role of acetaldehyde. Mol Aspects Med 29: 17–21, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Park PH, Huang H, McMullen MR, Mandal P, Sun L, Nagy LE. Suppression of lipopolysaccharide-stimulated tumor necrosis factor-alpha production by adiponectin is mediated by transcriptional and post-transcriptional mechanisms. J Biol Chem 283: 26850–26858, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park PH, Thakur V, Pritchard MT, McMullen MR, Nagy LE. Regulation of Kupffer cell activity during chronic ethanol exposure: role of adiponectin. J Gastroenterol Hepatol 21: S30–S3, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Peng Y, Murr MM. Establishment of immortalized rat Kupffer cell lines. Cytokine 37: 185–191, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA 105: 9793–9798, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life 60: 790–797, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Salminen A, Kauppinen A, Suuronen T, Kaarniranta K. SIRT1 longevity factor suppresses NF-kappaB-driven immune responses: regulation of aging via NF-kappaB acetylation? Bioessays 30: 939–942, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Shi L, Kishore R, McMullen MR, Nagy LE. Chronic ethanol increases lipopolysaccharide-stimulated Egr-1 expression in RAW 264.7 macrophages: contribution to enhanced tumor necrosis factor alpha production. J Biol Chem 277: 14777–14785, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Thakur V, McMullen MR, Pritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J Gastroenterol Hepatol 22: S53–S56, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol 79: 1348–1356, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchikura K, Wada T, Hoshino S, Nagakawa Y, Aiko T, Bulkley GB, Klein AS, Sun Z. Lipopolysaccharides induced increases in Fas ligand expression by Kupffer cells via mechanisms dependent on reactive oxygen species. Am J Physiol Gastrointest Liver Physiol 287: G620–G626, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology 34: 101–108, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Wu A, Ying Z, Gomez-Pinilla F. Oxidative stress modulates Sir2alpha in rat hippocampus and cerebral cortex. Eur J Neurosci 23: 2573–2580, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-κB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol 292: L567–L576, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J Biol Chem 272: 17795–17801, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369–2380, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol 29: 1363–1374, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 127: 1798–1808, 2004. [DOI] [PubMed] [Google Scholar]
- 41.You M, Cao Q, Liang X, Ajmo JM, Ness GC. Mammalian sirtuin 1 is involved in the protective action of dietary saturated fat against alcoholic fatty liver in mice. J Nutr 138: 497–501, 2008. [DOI] [PubMed] [Google Scholar]
- 42.You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol 294: G892–G898, 2008. [DOI] [PubMed] [Google Scholar]