Abstract
SOX transcription factors have the capacity to modulate stem/progenitor cell proliferation and differentiation in a dose-dependent manner. SOX9 is expressed in the small intestine epithelial stem cell zone. Therefore, we hypothesized that differential levels of SOX9 may exist, influencing proliferation and/or differentiation of the small intestine epithelium. Sox9 expression levels in the small intestine were investigated using a Sox9 enhanced green fluorescent protein (Sox9EGFP) transgenic mouse. Sox9EGFP levels correlate with endogenous SOX9 levels, which are expressed at two steady-state levels, termed Sox9EGFPLO and Sox9EGFPHI. Crypt-based columnar cells are Sox9EGFPLO and demonstrate enriched expression of the stem cell marker, Lgr5. Sox9EGFPHI cells express chromogranin A and substance P but do not express Ki67 and neurogenin3, indicating that Sox9EGFPHI cells are postmitotic enteroendocrine cells. Overexpression of SOX9 in a crypt cell line stopped proliferation and induced morphological changes. These data support a bimodal role for SOX9 in the intestinal epithelium, where low SOX9 expression supports proliferative capacity, and high SOX9 expression suppresses proliferation.
Keywords: intestinal epithelial development, stem cell, progenitor cell
complete renewal of the adult mammalian small intestinal epithelium occurs every 3 to 14 days and is driven by multipotential stem cells located in the base of the crypts (4, 11). This impressive rate of tissue turnover requires well-coordinated maintenance of the stem cell pool and also the appropriate differentiation of the transit-amplifying progenitors into the four mature postmitotic lineages of the small intestine (7–11). Although most progenitors differentiate into absorptive cells, a small minority of progenitors differentiates into the remaining three secretory cell types, goblet cells, enteroendocrine cells, and Paneth cells.
The multipotent stem cells and committed progenitor cells that give rise to the postmitotic intestinal epithelium have been difficult to study because of the lack of specific biomarkers, animal models, and methods to isolate live stem/progenitor cell populations for gene expression analysis. The specific location of the small intestine epithelial stem cell has been thought for many years to be located at cell position +4 (as numbered from the bottom of the crypt up the crypt-villus axis) (4). This putative stem cell location was based on a slow cell division criterion of the cells at an average position of +4 using 3H-thymidine and BrdU label retention assays, which do not assess the multipotency and self-renewal characteristics of a stem cell.
Recently, an elegant in vivo lineage-tracing experiment, using Lgr5 as a biomarker, demonstrated that a population of multipotential intestinal epithelial stem cells localize to the very base of the crypt (cell positions 0, 1′, 2′, 4; as defined by Barker et al., Ref. 2) in cells that have been previously characterized as crypt-based columnar cells (CBCs) (4, 9, 11). More recently, lineage tracing has demonstrated that the polycomb group gene, Bmi1, which is expressed primarily in a pool of cells around the +4 region, also has multipotent capacity but only in the proximal small intestine (36). Although it is not clear whether the Lgr5+ and Bmi1+ cells are functionally equivalent, it has been hypothesized that one population may be a slower-dividing “quiescent” stem cell (cells at position +4), which gives rise to more rapidly dividing multipotent stem cells (the CBCs) (24, 34).
Nonetheless, both cell types are under the influence of intrinsic and extrinsic signals directing the decisions to self renew and differentiate into all postmitotic cell types of the small intestine epithelium. The intrinsic and extrinsic genetic pathways controlling the defining properties of stem cells— self-renewal capacity and multipotency—are not fully understood in the intestine. Perhaps the most well-studied pathways critical to the development and maintenance of the intestinal epithelium include the Wnt/β-catenin and Notch pathways. When Wnt signaling is engaged, a cascade of events leads to nuclear localization of β-catenin and transcriptional enhancement of target genes involved in supporting proliferation (i.e., c-myc, cyclin-D1). Genetic ablation of Wnt signaling components results in crypt loss and the eventual disruption of intestinal epithelium homeostasis (23, 31).
Studies describing perturbations in normal Notch1 receptor signaling (by gain or loss of function) have likewise demonstrated the critical role of the Notch pathway in intestinal epithelial development, specifically in controlling the cell fate decisions of intestinal stem/progenitor cells (39, 46). When the Notch1 pathway is activated, its downstream target gene, Hes1, represses Math1 expression and promotes an absorptive cell fate over a secretory lineage fate. Negative disruption of the Notch1 pathway, either pharmacologically (41) or genetically, results in an aberrant increase in the numbers of secretory cell lineages. Collectively, these studies indicate that precise control of the Wnt and Notch1 pathways by modulating factors is critical for normal proliferation and differentiation of intestinal stem/progenitor cells.
A common feature of both the Wnt and Notch pathways is that they appear to be modulated by Sox (Sry-Box) genes (1, 3, 40), indicating a critical role for SOX transcription factors in normal intestinal epithelium homeostasis. Sox genes are a family of at least 20 closely related transcription factors, which are defined by the presence of a high-mobility group domain (28). All Sox factors bind a relatively loose consensus sequence, (A/T)(A/T)CAA(A/T)G (28), resulting in dramatic DNA bending (37, 44) that has been hypothesized to be critical for bringing distal control elements to proximal transcriptional start sites (44). SOX factor competition for a single cis-control element combined with the physical interaction of SOX factors with other transcription factors (45) influences the specificity of target gene transcription (21). The ability of SOX factors to bind the same consensus sequence has been linked to highly redundant function in some cell types (14, 19, 27), obscuring the specific role of some Sox genes using gene-targeting technologies. The notion that multiple SOX factors with differential capacities to modulate transcription are able to compete for transcriptional control of the same target gene points to the concept of how differential levels of SOX factors in a single cell might influence proliferation or the outcome of lineage specification.
An interesting feature of SOX factors is that they can function as dose-dependent regulators of stem/progenitor cell potency and competence (33, 40). Disruption in the proper balance of SOX factor levels can result in severe congenital defects. Several conditions in humans and mice are caused by subtle changes in regulation or haploinsufficiency of some Sox genes. For example, distinct hypomorphic levels of Sox2 result in esophageal atresia and tracheoesophageal fistula (33) and a range of eye phenotypes from anophthalmia to microphthalmia (15, 40). Likewise, the loss of a single Sox9 allele presents clinically as sex reversal with Campomelic Dysplasia (22, 42). In both cases of SOX factor deficiency, aberrant maintenance and differentiation of the stem/progenitor cell pool is causative to the clinical sequelae. Taken together, these reports indicate that discrete levels of SOX factors play a critical role in normal differentiation of stem/progenitor cell populations.
RT-PCR analysis demonstrates that Sox3, 4, 5, 7, 9, 10, 17, and 18 mRNAs are expressed in whole intestine preparations (5). Of these Sox factors, Sox9 has been the most widely studied in the intestine. Expression of SOX9 protein has been localized to the stem cell and transit-amplifying zones of the crypt (5). Interestingly, sporadic SOX9-expressing cells have also been reported to be located throughout the nonproliferating villus epithelium (3, 5, 26).
Because distinct levels of SOX factors appear to be critical for proper proliferation and differentiation in stem cell biology, we hypothesized that differential SOX9 expression may be playing a similar role in stem/progenitor cell populations in the intestinal epithelium. To explore this possibility we utilized a bacterial artificial chromosome (BAC) transgenic Sox9EGFP animal model where EGFP is expressed by the regulatory regions of Sox9 and stands for enhanced green fluorescent protein. In this study, we examine both the Sox9 transcriptional activity (via EGFP expression) and endogenous levels of SOX9 protein throughout the crypt/villus axis of the small intestine. Additionally, we investigate the molecular characteristics and cellular identities of Sox9-expressing stem/progenitor cells and postmitotic populations and test whether increasing the levels of SOX9 is able to confer phenotypic changes in a nontransformed intestinal epithelial crypt cell line. The results of this study provide further insight into a function for Sox9 in both the stem/progenitor cell populations and in a subset of postmitotic cells in the crypts and villi.
MATERIALS AND METHODS
Mice/genotyping.
The Sox9EGFP mouse line was originally generated as part of the GENSAT Brain Atlas Project (17) and contains genomic integration of a modified BAC (RP32–140D18) with ∼75.5 kb upstream and ∼151 kb downstream sequence to Sox9. Frozen Sox9EGFP mouse embryos were obtained from the Mutant Mouse Regional Resource Center (University of California-Davis) and reconstituted by transfer into foster mice. All mice are on the outbred CD-1 strain and were maintained as heterozygotes on the CD-1 genetic background. Mice breed normally and live to adulthood with no overt phenotypes because of the transgene. At ∼12 days postnatal, tail snips were viewed under an epifluorescent microscope fitted with filters for EGFP visualization. A high level of EGFP fluorescence compared with transgene negative control mice was scored as a positive for the Sox9EGFP transgene. All protocols for animal use were reviewed and approved by the University of North Carolina Institutional Animal Care and Use Committee (IACUC).
Immunostaining/microscopy.
For tissue preparation, small intestines were dissected from adult Sox9EGFP mice (>8 wk of age), and luminal contents were flushed out with PBS followed immediately by a single flush with freshly made 4% paraformaldehyde. The intestine was opened along the duodenal-ileal axis, placed on filter paper, and fixed for an additional 14–18 h at 4°C. The tissues were then prepared for cryosectioning by immersion in 30% sucrose solution for at least 24 h at 4°C. Tissues were then embedded in optimal cutting temperature (OCT) medium and frozen on dry ice. The OCT blocks were stored at −80°C until cryosectioning. Thin sections (8–10 μm) were cut on a cryostat and placed on positively charged microscope slides for staining and microscopy. This tissue preparation technique is critical for preserving the EGFP fluorescence.
For immunostaining, the sections were washed twice in PBS to remove OCT, followed by incubation in blocking medium [5% normal goat serum (NGS), in PBS-0.3% Triton-X 100] for at least 30 min at room temperature (21–25°C). Primary antibodies were applied to the tissue sections in antibody staining solution (1% NGS, in PBS-0.3% Triton-X 100). Dilutions were as follows: α-EGFP (chicken, 1:500, no. GFP-1020; Aves Laboratories, Tigard, OR), α-SOX9 (rabbit, 1:1,000, no. AB5535; Chemicon, Temecula, CA), α-NGN3 (rabbit, 1:250, no. AB5684; Chemicon), α-substance P (rat; 1:500, no. MAB356; Chemicon), α-chromogranin A (rabbit, 1:500, no. 20086; ImmunoStar, Hudson, WI), α-Ki67 (mouse, 1:100, no. M7249; Dako, Carpinteria, CA), α-lysozyme (rabbit, 1:1,000, no. RP 028; Diagnostics Biosystems, Pleasanton, CA). All secondary antibodies [α-Rabbit-Cy3, no. C2306 (Sigma, Saint Louis, MO); α-Rabbit- Alexafluor 488, no. Z-25302 (Molecular Probes, Eugene, OR); α-Rat-Alexafluor 555, no. Z-25305 (Molecular Probes)] were used at a 1:500 dilution in staining buffer. For Ki67 it was necessary to amplify the signal using α-Mouse IgG-Biotinylated (1:200; Jackson Immunoresearch Laboratories, West Grove, PA) followed by Alexafluor-555-labeled streptavidin (1:250, no. S32355, Molecular Probes). Antigen retrieval using Reveal Buffer (no. RV1000L2J; Biocare Medical, Concord, CA) was required for NGN3, Ki67, and chromogranin A. Nuclei were stained with Draq5 (1:10,000, no. BOS-889-001; Biostatus, San Diego, CA). Background staining was negligible as determined by nonspecific IgG staining. Unless otherwise noted, costaining/positive staining statistics were generated from cells from at least 50 crypts/villi.
For whole mount staining of crypts, whole crypts were isolated as described in Epithelium dissociation without the dispase treatment. Approximately 500 crypts were fixed in 4% paraformaldehyde at 4°C for ∼18 h, then washed five times with PBS. For all wash procedures, crypts were gently mixed and allowed to settle to the bottom of tube (and not centrifuged). Washes and staining buffers were removed from crypts by gentle suction. Crypts were blocked in 500 μl of 5% NGS/PBS-T for 1 h at room temperature. Primary antibodies were added to 500 μl of staining buffer and allowed to incubate for ∼18 h at 4°C. The crypts were then washed five times with 1.5 ml PBS-T/wash. Secondary antibodies were added 1:500 in 1% NGS/PBS-T and incubated at room temperature for at least 2 h. The secondary antibodies were removed, and the crypts were washed five times with PBS-T for 30 min each. For costaining with α-lysozyme and α-SOX9, it was necessary to label the lysozyme antibody with Alexafluor 488 using the Zenon primary antibody labeling kit (Invitrogen, Carlsbad, CA). Staining for SOX9 was conducted first followed by staining for lysozyme. Nuclei were stained with Draq5.
Epifluorescent images were captured on an Olympus IX70 fitted with an Olympus digital camera. Objective lenses used were ×20 and ×40 with numerical apertures of 0.55 and 1.40, respectively. All confocal images represent 1.5 μm optical sections unless otherwise noted, and crypts were captured using confocal optical Z-sectioning (1.0-μm optical sections) and compiled using Zeiss LSM Image Software (Zeiss, Thornwood, NY). Objective lenses for the confocal images were ×40 with a numerical aperture of 0.1.
Immunoblotting.
Whole cell lysates were made from virus-infected intestinal epithelial cells (IEC)-18 cells by adding RIPA buffer (0.15 M NaCl, 50 mM Tris·Cl, pH-7.2, 1% deoxycholic acid, 1% Triton-X 100, 0.1% SDS) and 1× protease inhibitors (Sigma, P8340) to two 15-cm plates. Before cell lysis, virus-infected cells had been selected for stable transgene integration using puromycin selection for 10 days. Whole cell lysates were separated on a 10% SDS-PAGE gel, and proteins were transferred to PVDF membrane (Hybond LFP; GE Healthcare, Piscataway, NJ). The membranes were probed with anti-c-MYC (SC-40; Santa Cruz Biotechnology), anti-proliferating cell nuclear antigen (PCNA) (MAB424R; Chemicon), and anti-β-actin (A5316; Sigma), blocked in Blotto (5% dried milk in Tris-buffered Saline-Tween, 0.75%) for 30 min, followed by incubation with primary antibodies diluted in Blotto. Primary antibodies were detected with anti-IgG-horseradish peroxidase-conjugated secondary antibodies diluted in Blotto. The membranes were then incubated in the horseradish peroxidase detection agent (ECL, GE Healthcare) for 5 min and then exposed to film. Films were scanned on a flatbed film scanner to generate digital images.
Epithelium dissociation/FACS.
To isolate intestinal crypt cells for fluorescence-activated cell sorting (FACS), small intestine epithelium was dissociated into single cells essentially as previously described (13) with slight modifications. For FACS experiments, mouse intestine were flushed with cold PBS, cut open lengthwise in ∼10-cm-long pieces, and immersed in PBS/30 mM EDTA/1.5 mM DTT over ice for 20 min. The solution was disposed of, and the tissue was shaken vigorously in fresh PBS/30 mM EDTA for ∼30 s before being incubated at 37°C for 10 min. Intact tissue was discarded, and dissociated crypts and villi were pelleted at 2,500 revolution/min for 5 min. The cells were washed twice with cold PBS, resuspended in HBSS/0.3 U/ml dispase at 37°C, and shaken approximately every 2 min for 10 min. Then, (5%) and 100 μg DNaseI was added before the cells were passed through a 70-μm filter. Cells were pelleted at 2,500 revolution/min for 5 min and resuspended in 4 ml HBSS with 5% FBS, then passed through a 100-μm filter and combined with an additional 100 μg DNaseI. Equivalent numbers of cells from three animals were combined before FACSorting. Sox9EGFPNEGATIVE, Sox9EGFPHI, and Sox9EGFPLO cells were isolated using a MoFlo FACS machine (Dako/Cytomation). Cells were collected in ice-cold HBSS and kept on ice throughout the sort. Time between death of the mouse to RNA extraction was kept to 3.5 to 4 h to ensure the highest quality of RNA.
cDNA preparation/real-time PCR analysis.
cDNA from ∼5 × 104 cells from each FACSorted population (Sox9EGFPNEGATIVE, Sox9EGFPHI, and Sox9EGFPLO) was made using RNAqueous Micro Kit (Ambion, Austin, TX) according to the manufacturer's protocols. Real-time PCR was conducted for each sample in triplicate on ∼1/20,000 of the total amount of cDNA generated. TaqMan probes (Hes1, Mm00468601_m1; Notch1, Mm00435245_m1; Lgr5, Mm00438890_m1; Msi1, Mm00485224_m1; Sox9, Mm00448840_m1; 18S, HS99999901) for each gene were obtained from Applied Biosystems and used in reactions according to the manufacturer's protocol. 18S ribosomal RNA was amplified and used as the internal control gene for sample comparison. ΔCt values were calculated to obtain fold changes for sample comparison (30).
Lentivirus production/cell culture.
The lentiviral vectors were constructed using the plasmid backbone, pLVX-Puro, from Clontech (Madison, WI). To generate the pLVX-SOX9-EGFP vector, the coding sequence for murine SOX9 was cloned into the polylinker of a shuttle vector just 5′ of an internal ribosomal entry site (IRES) linked to EGFP coding sequence. The SOX9-IRES-EGFP DNA fragment was then cloned into the pLVX-Puro vector. The control viral vector, pLVX-EGFP, contains all the elements of pLVX-SOX9-EGFP minus the SOX9 coding sequence. High titer lentivirus was generated according the Lenti-X system specifications (Clontech).
The IEC-18 cell line was cultured in medium according to previously established optimal conditions (18): high-glucose DMEM-H, 10% FBS, 0.1% zinc/insulin (Gibco, Grand Island, NY), 10 U/ml penicillin/streptomycin, and 2 mM l-glutamine. At ∼80% cell confluence, the medium was replaced with the normal medium containing the lentivirus and polybrene at 4.0 μg/ml for overnight transduction. At 48 h, ∼75–80% of the cells were infected as assessed by EGFP fluorescence. The transduction medium was replaced after 24 h with the standard IEC-18 medium. After 48 h, the cells were puromycin selected (at 2.0 μg/ml) in the standard IEC-18 culture medium. Selection continued for 10 days with cells analyzed daily for EGFP levels and morphology by microscopy.
RESULTS
Sox9EGFP is expressed primarily in the base of the crypt in non-Paneth cell populations.
The Sox9EGFP mouse was originally generated as part of the GENSAT brain-mapping project and constructed by insertion of a BAC into the mouse genome (17). The BAC containing the Sox9 locus was genetically modified to introduce the EGFP coding sequence just 5′ of the Sox9 transcriptional start site, thus destroying any transgenic expression of Sox9 from the BAC. The Sox9EGFP mice breed normally and do not have any deleterious phenotype resulting from EGFP transgene expression.
Upon initial examination of the small intestine, we observed a restricted expression pattern of EGFP that localized exclusively to epithelial cells primarily at the base of the crypt in the Paneth cell zone (Fig. 1A). As has been previously reported (5), we also observed sporadic Sox9EGFP-positive cells in the villus (Fig. 1B). This expression pattern of the Sox9EGFP transgene was observed throughout the duodenal-ileal axis. No Sox9EGFP expression was observed in the lamina propria, muscularis, bone marrow, or circulating lymphocytes (as assessed by flow cytometry).
Fig. 1.
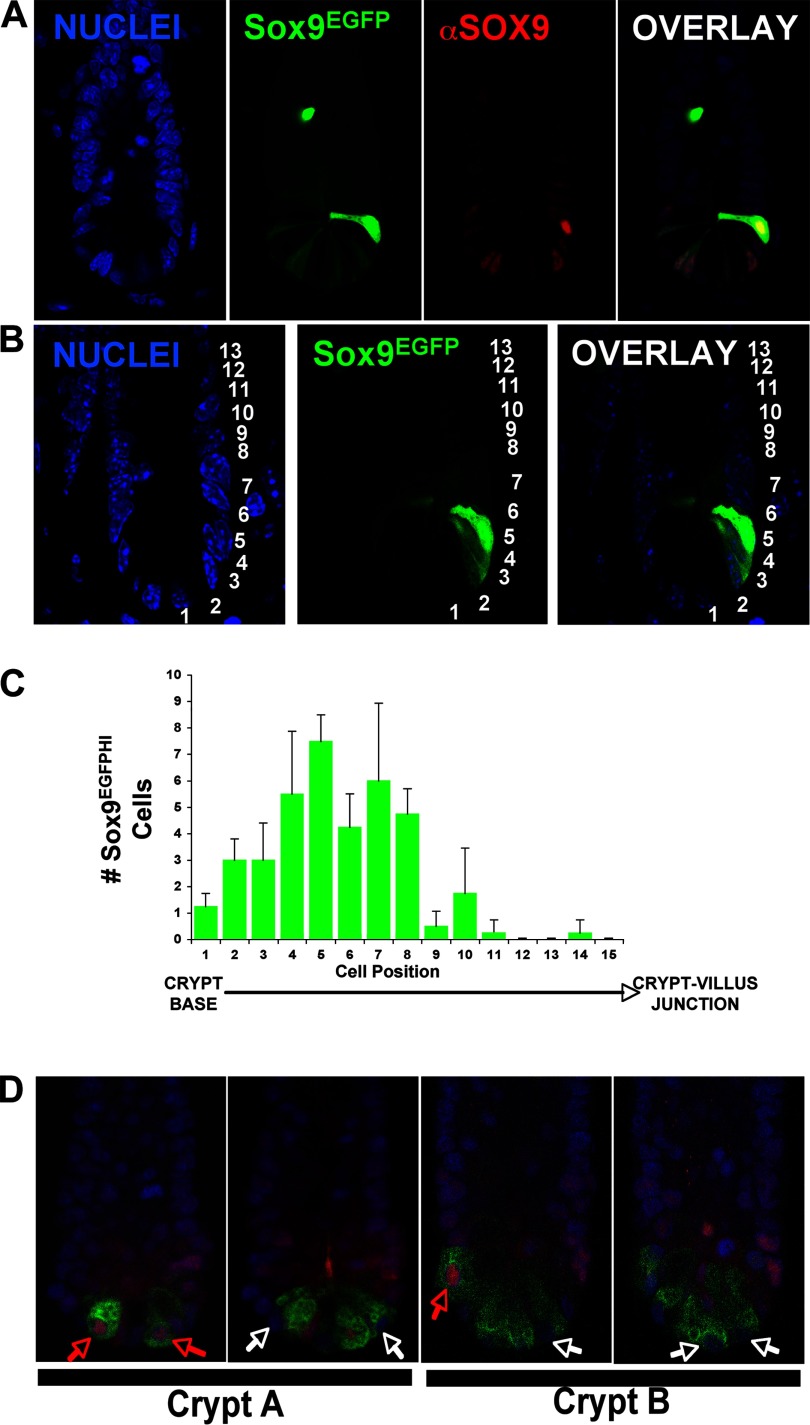
Expression analysis of Sox9EGFP in the small intestine. A: Sox9EGFP expression in jejunal crypts is localized to the base in intercalating crypt-based columnar cells (CBCs) and supra-Paneth cell locations. There are two distinct levels termed “HI” and “LO”. EGFP, enhanced green fluorescent protein. B: sporadic Sox9EGFPHI-expressing cells are found throughout the villus epithelium. White arrows mark representative Sox9EGFPHI cells. Image at far right is a high magnification to demonstrate morphology. C: Sox9EGFP expression does not colocalize with granulated Paneth cells. D: molecular confirmation that Sox9EGFP is not expressed in Paneth cells as marked by lysozyme.
Upon further examination of the crypts with the use of brightfield microscopy, Sox9EGFP appeared to be mostly expressed in a cell population that was intercalated between the granulated Paneth cells and supra-Paneth cell location but not in Paneth cells themselves (Fig. 1C). To rule out the possibility that a small subset of Sox9EGFP-expressing Paneth cells might have been overlooked in a thin cryostat section, with the use of Paneth cell morphology as an identifying criterion, we costained whole crypts for the Paneth cell marker, lysozyme (6), and conducted three-dimensional (3-D) confocal microscopy. Three-dimensional reconstruction of the entire crypt demonstrates that Sox9EGFP is expressed in all cells at the base of the crypt except Paneth cells. (Fig. 1D, Supplemental Fig. S1; supplemental material for this article is available at the American Journal of Physiology Gastrointestinal and Liver Physiology website). Thus a population of Sox9EGFP-positive cells in the crypt base appears to be consistent with intercalating CBCs.
Sox9EGFP is expressed at two discrete levels.
Consistent with our hypothesis, there are two distinct levels of Sox9EGFP expression, termed Sox9EGFPHI and Sox9EGFPLO, in the base of the crypt (Fig. 2A). To establish whether endogenous SOX9 levels correlated with Sox9EGFP transgene levels, we stained Sox9EGFP small intestines for SOX9 protein. One-micron confocal microscopy slices demonstrate a direct correlation between the graded levels of Sox9EGFP and endogenous SOX9. Empirical evidence suggested that the Sox9EGFP high cells were concentrated in a supra-Paneth cell region, a region traditionally accepted as part of the stem cell niche. To quantify this observation we counted the position of Sox9EGFPHI cells using the standard cell numbering scheme where cell position 1 is at the base of the crypt (Fig. 2B). Additionally, we assessed the total number of Sox9EGFPHI cells from the crypt base to the villus junction in intact crypt preparations. The results indicate that there are between 1 and 6 Sox9EGFPHI cells per crypt (mean = 3.1; SE = 1.4) and that most Sox9EGFPHI cells localize to position +4–6 (average +5) (Fig. 2C). On the villus, all Sox9EGFP-positive cells had a Sox9EGFPHI status and did not appear to be clustered in any particular region or pattern.
Fig. 2.
Validation of the Sox9EGFP transgene and whole crypt analysis of SOX9 expression in Paneth cells. A: SOX9 protein levels directly correlate with Sox9EGFP transgene expression levels. The nucleus of the Sox9EGFPHI cell located higher up the crypt is not in the focal plane of the confocal image, and thus endogenous SOX9 is not observable. B: numbering scheme used to quantify the location of Sox9EGFPHI cells. Cell position 1 is located at the very base of the crypt. C: distribution of Sox9EGFPHI cells in the small intestine crypt. Variation is depicted as the standard error of the mean of 3 individuals counting one cell position difference either up or down the average cell position along crypt-villus axis. D: whole crypts were immunostained for lysozyme and SOX9 and digitally reconstructed from 1-μm confocal slices. By assessing every Paneth cell in an intact jejunal crypt, we can define two populations of Paneth cells by either the presence or absence of SOX9. The ratio of SOX9+ to SOX9− Paneth cells was on average 1:1 (n = 5 intact crypts, SOX9+ mean = 2.6/crypt, SE = 1.5; SOX9− mean = 2.6/crypt, SE = 1.1). The images represent 2 different representative crypts (designated A and B). Each image represents a different optical slice on the z-plane. Blue = nuclei, red = SOX9, green = lysozyme. Red arrows point to SOX9-positive Paneth cells. White arrows point to SOX9-negative Paneth cells.
Distinct SOX9 expression signatures define two populations of Paneth cells.
Several investigations have associated SOX9 immunostaining with granulated Paneth cells; however, Sox9 mRNA expression in Paneth cells has never been established (3, 5, 26). Although it appears from these previous studies that SOX9 protein is expressed in some Paneth cells, it is unclear in the absence of a lineage-specific biomarker whether the nuclear staining is in the Paneth cells or the intercalating CBCs. In light of the absence of any Sox9EGFP expression in lysozyme-positive cells (Fig. 1D), we indeed questioned whether there was SOX9 protein expression in the entire lysozyme-positive Paneth cell compartment. Using 3-D confocal imaging of intact crypts, we demonstrate that there are two populations of Paneth cells (defined as Lysozyme+SOX9+ or Lysozyme+SOX9−) existing in a 1:1 ratio per crypt (Fig. 2D, Supplemental Fig. S2). Neither of these two populations appears to localize to a particular region of the base of the crypt (i.e., at the very base of the crypt or higher up the crypt axis). One possible interpretation for the lack of Sox9EGFP expression in the SOX9+ Paneth cells is that the cis-regulatory elements controlling Sox9 expression in Paneth cells are not present in the Sox9 BAC clone used to generate the mice.
Sox9EGFPLO cells demonstrate enhanced signaling for maintenance of “stemness”.
To better characterize the stemness and genetic and cellular differences between Sox9EGFPLO and Sox9EGFPHI cells, we investigated the expression of Notch1 pathway genes. Studies in stem/progenitor cells indicate that decreases in Notch1 receptor signaling lead to decreases in Hes1 repression of Math1, thus pushing stem/progenitor cell fate toward a secretory lineage (16, 38, 39, 46). To determine whether there was differential Notch1 expression and downstream signaling between Sox9EGFPHI and Sox9EGFPLO cells, we assessed the mRNA levels for Notch1 and its downstream target gene, Hes1, in these two cell populations. Small intestine epithelium was dissociated and the single cells were FACSorted on the basis of either Sox9EGFPLO or Sox9EGFPHI expression status. Real-time semi-quantitative PCR was used to assess relative mRNA levels between the two samples (Fig. 3A). The data show that there is a fivefold enrichment of Sox9 mRNA in Sox9EGFPHI cells compared with Sox9EGFPLO cells, validating the EGFP intensity-based cell sorting. The data also demonstrate that there is a 10-fold enrichment of Notch1 mRNA in Sox9EGFPLO cells with a concomitant 11-fold increase in Hes1, the downstream target of Notch signaling (Fig. 3B). These data indicate that the Notch1 pathway is activated to higher degree in Sox9EGFPLO cells, suggesting that secretory lineage fate is being suppressed and an uncommitted state and/or absorptive fate is being maintained in Sox9EGFPLO cells.
Fig. 3.
Gene expression analysis in isolated populations of Sox9EGFP-expressing cells. A: FACS histogram demonstrates the 3 distinct populations of Sox9EGFP-expressing cells. Sox9EGFP cells were sorted on the basis of EGFP intensities [either negative (NEG), HI, or LO]. B: FACS on the basis of EGFP levels (and thus Sox9 mRNA levels) was validated because Sox9EGFPHI cells express 5-fold higher (SE = 1.2-fold) Sox9 mRNA than Sox9EGFPLO cells. Sox9EGFPLO cells have 10.2-fold more Notch1 (SE = 0.65-fold) and 11.4-fold more Hes1 (SE = 1.3-fold) mRNA compared with Sox9EGFPHI cells. Images to the right of the graph represent the cells postsort. C: Sox9EGFPLO cells are enriched in stem/progenitor cell marker genes. Sox9 (mean = 6.7-fold higher, SE = 2.3-fold), Lgr5 (mean = 8.9-fold, SE = 3.8-fold), Msi1 (mean = 13.3, SE = 3.6-fold); all P values <0.001.
Sox9EGFPLO cells demonstrate enriched levels of stemness gene expression.
Two characteristics of Sox9EGFPLO cells suggest that they in part comprise the multipotent stem cell population. These characteristics include 1) the localization of Sox9EGFPLO to the intercalating CBC population and 2) an active Notch1 pathway, which generally indicates suppression of secretory lineage differentiation (16). To further characterize the genetic signatures of Sox9EGFPLO cells, we assessed gene expression patterns for other putative intestinal stem cell biomarkers in Sox9EGFPLO FACSorted cells (Fig. 3C). The data show enriched expression of the multipotent stem cell marker, Lrg5 (2), in Sox9EGFPLO cells compared with the Sox9EGFPNEGATIVE population. Additionally, the mRNA for another putative intestinal stem cell marker, Musashi1 (32), was enriched in Sox9EGFPLO cells (Fig. 3C).
Sox9EGFPHI cells are mature/postmitotic enteroendocrine cells.
The decrease in Notch1 signaling in Sox9EGFPHI cells compared with Sox9EGFPLO cells (Fig. 3B) led us to hypothesize that Sox9EGFPHI cells would be fate restricted to a secretory lineage. Because the Notch1 pathway activation appeared to be suppressed in the Sox9EGFPHI cells, we predicted that the lineage identity of this population would be either goblet or enteroendocrine cells. The Paneth cell lineage was ruled out because of the location of Sox9EGFPHI cells along the crypt-villus axis, a non-Paneth cell morphology, and the lack of colocalization with the Paneth cell marker, lysozyme (Fig. 1D). To identify the lineage of Sox9EGFPHI cells, we stained the small intestine of the Sox9EGFP mice with the goblet marker, MUC2, and the enteroendocrine markers, substance P and chromogranin A. No colocalization was observed with MUC2, indicating that Sox9EGFPHI cells are not of the goblet lineage (data not shown). However, the results clearly demonstrate colocalization of Sox9EGFPHI cells with the enteroendocrine cell markers, substance P (61% colocalized) and chromogranin A (100% colocalized) (Fig. 4, A and B).
Fig. 4.
Sox9EGFPHI cells are postmitotic enteroendocrine cells. Sox9EGFPHI cell lineage was identified by colocalization of EGFP with enteroendocrine-specific markers, substance P (A) and chromogranin A (B). Postmitotic status of Sox9EGFPHI cells was assessed by colocalization of EGFP with Ki67 (C) and Neurogenin3 (Ngn3) (D). White arrows depict representative cells. All images are 1-μm confocal optical slices. Note: A is EGFP fluorescence, and is immunofluorescence staining for EGFP (B–C). Immunodetection of EGFP is required because of destruction of EGFP fluorescence by the antigen retrieval methods. SUB P, substance P; CHG A, chromogranin A.
To assess whether the Sox9EGFPHI cells in the crypts were immature cycling enteroendocrine precursors, we stained small intestine from Sox9EGFP mice with Neurogenin3 (Ngn3) (a biomarker of secretory/enteroendocrine progenitors) and the general proliferation marker, Ki67. We never detected colocalization of Ngn3 or Ki67 with Sox9EGFPHI in the adult small intestine (Fig. 4, C and D). These data, combined with the observation of substance P/chromogranin A expression in Sox9EGFPHI cells, indicate that Sox9EGFPHI cells are mature enteroendocrine cells.
Increasing SOX9 levels in an intestinal progenitor cell line halts proliferation and elicits morphological changes.
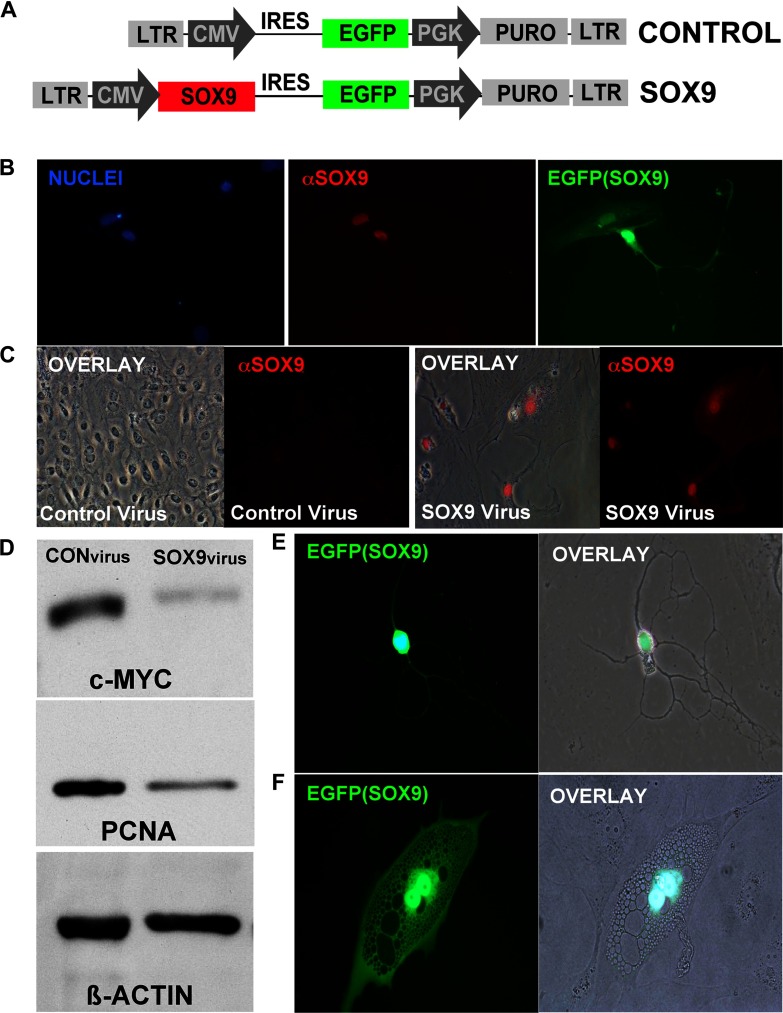
To define a functional role for increased levels of SOX9 enteroendocrine differentiation, we developed a gain-of-function assay in a nontransformed small intestine epithelial crypt cell line, IEC-18. Spontaneous differentiation of the IEC-18 cells into enteroendocrine-like cells has been demonstrated, indicating the multipotent capacity of these cells in culture (18). On the basis of the exclusive expression of high SOX9 levels in noncycling enteroendocrine cells in vivo, we predicted that increasing the levels of SOX9 beyond a particular threshold might fate specify these cells to an enteroendocrine-like fate and/or halt proliferation. To test this hypothesis we generated a lentiviral vector in which the constitutive cytomegalovirus (CMV) promoter drives Sox9 cDNA expression (Fig. 5A). The recombinant lentiviral transduction results in stable integration of the Sox9 cDNA transgene into the IEC-18 genome and high levels of SOX9 expression (Fig. 5B). After positive selection with puromycin, equal numbers of control- or SOX9 virus-infected IEC-18 cells were plated and allowed to grow over a 10-day period. Control virus-infected cells proliferated normally, growing to a confluent monolayer, whereas SOX9 virus-infected cells remained viable as single cells with no clonal populations appearing on the plate (Fig. 5C). Additionally, Western blot analysis demonstrated a decrease in PCNA and also a decrease in c-MYC, a downstream target of β-catenin signaling that supports proliferation (Fig. 5D).
Fig. 5.
Phenotypic analysis of lentiviral-mediated increases in SOX9 levels. A: schematic of lentiviral construct generated. The control lentiviral vector contains all the elements as the SOX9 lentiviral vector except the Sox9 cDNA. EGFP is translated from an internal ribosomal entry site (IRES) as a reporter gene to assess infection. The puromycin resistance (PURO) gene is included to allow positive selection for viral integration into the genome. cDNA expression is driven by strong constitutive promoters [either cytomegalovirus (CMV) or phosphoglycerate kinase (PGK)]. LTR, long terminal repeats. B: validation of the SOX9 lentivirus. 48 h postinfection, intestinal epithelial cell (IEC)-18 cells were assessed for SOX9 by immunostaining (red), and EGFP autofluorescence (green). C: IEC-18 cells were infected with equivalent titers of either control lentivirus or SOX9 lentivirus and selected with puromycin to deplete the cultures of untransduced cells. Equivalent numbers of cells were plated and allowed to grow for an additional 5 days. Images (left and middle left) depict monolayers of IEC-18 cells that emerged in the control infected cells that express low endogenous levels of SOX9 (red). Other images (right and middle right) depict cells infected with the SOX9 lentivirus. No proliferation (as detected by clonal populations) was observed in SOX9 lentivirus-infected cells. Immunostaining for SOX9 (red) validates high expression of SOX9 in these cells. D: immunoblotting analysis of whole cell extracts made from control-virus (left lane) or SOX9-virus infected (right lane) IEC-18 cells. Blots were probed for c-MYC and proliferating cell nucleus antigen (PCNA) using β-actin expression as an internal control. E: morphological phenotypes of SOX9 lentivirus-infected IEC-18 cells. Note neuroendocrine-like morphologies (axonal/dendritic processes and dense vesicle formations) were evident 6–8 days postinfection.
A second striking observation was a distinct morphological change in SOX9 virus-infected cells (Fig. 5, D and E). Between 8–10 days postinfection, IEC-18 cells expressing high levels of SOX9 transitioned from a typical IEC-18 epithelial cell morphology (small, uniform, flat cell body) to a neuroendocrine morphology with a condensed cell body, dendritic/axonal-like projections (Fig. 5E), and/or possessing multiple vesicles (Fig. 5F). These cells remained adherent and survived in culture until completion of the 10-day experiment. To determine whether SOX9-infected IEC-18 cells had differentiated into enteroendocrine cells, we immunostained these cells for mature enteroendocrine markers chromogranin A and substance P. Neither chromogranin A nor substance P antibodies demonstrated cross reactivity with SOX9 virus-infected cells (data not shown), indicating that SOX9 virus-infected cells had not fully differentiated into mature enteroendocrine cells.
DISCUSSION
The phenotypic effects of differential transcription factor levels have recently been the focus of several studies, which illustrate the importance of this biological property during the development of several tissues (15, 22, 33, 40, 42). In this study, we utilized a transgenic reporter gene mouse to determine whether Sox9 was expressed at different levels in cells of the small intestinal epithelium. Our results indicate that there are two discrete steady-state levels of Sox9 mRNA and protein in the adult intestinal epithelium (now termed Sox9LOW and Sox9HIGH to reflect the levels of the endogenous Sox9 gene). Sox9LOW-expressing cells exist in the lower crypt, whereas Sox9HIGH-expressing cells are distributed primarily in the cell +4–6 position of the crypt and also sporadically throughout the villus epithelium. Our study shows that Sox9LOW-expressing cells are in part intercalated between the Paneth cells in the CBC population. Three-dimensional confocal crypt reconstruction showing Sox9LOW expression in CBCs, combined with gene expression analysis showing enriched Lgr5 expression in Sox9LOW cells, demonstrates that Sox9LOW cells include the Lgr5+ multipotent stem cell population. Presently, an Lgr5 antibody that is able to detect mouse LGR5 does not exist, thus precluding formal immunostaining analysis of the Sox9LOW population. Sox9HIGH cells in the crypt and villus are chromogranin A+ Ki67−, postmitotic enteroendocrine cells. Gain-of-function studies in the IEC-18 intestinal crypt cell line indicate that high levels of SOX9 are involved in repressive modulation of proliferation in intestinal epithelial cells and are perhaps influencing enteroendocrine cell fate specification.
Conditional deletion of Sox9 at E10.5 has been shown to ablate the Paneth cell population in the adult mouse (3, 26). An interesting observation from our study is that the Sox9EGFP transgene is never expressed in the Paneth cell population, yet 50% of Paneth cells express SOX9 protein. Because of the rapid generation of the intestinal epithelium, a reasonable interpretation is that transcription from the Sox9 gene is terminated before Paneth cell differentiation and that SOX9 protein remains stabilized in “younger” Paneth cells. In “older” Paneth cells, SOX9 has degraded and results in a lysozyme+/SOX9− population. The expression of SOX9 protein in only half of the Paneth cells may point to a unique role for the two populations in normal gut homeostasis or implicate the requirement for SOX9 protein during the final stages of Paneth cell fate specification just before terminal differentiation and/or maturation at which time SOX9 is degraded.
Our results clearly indicate that high levels of SOX9 are expressed in the enteroendocrine compartment of the small intestine epithelium. However, unlike the Paneth cell lineage, SOX9 expression does not seem to be required for differentiation of enteroendocrine cells since there are no observable differences in the number of chromogranin A-positive cells in an intestinal epithelium-specific knockout for SOX9 (26). It might be predicted, because of the high levels of SOX9 specifically in enteroendocrine cells, that SOX9 normally influences the generation of the enteroendocrine cell lineage, but its conditional loss at E10.5 is being compensated for by other SOX factors. By contrast, no such compensation exists for Paneth cell differentiation as demonstrated by both Sox9 conditional knockout studies (3, 26).
The literature is replete with examples of Sox factor redundancy. For example, Sox1, Sox2, and Sox3 appear to functionally compensate for each other in neural stem/progenitor populations during development (12, 29, 35); Sox17 and Sox18 show redundant functions in postnatal angiogenesis (25); and Sox4 and Sox11 can compensate for Sox12 during early embryonic development (20). The mRNAs for Sox3, 4, 5, 7, 10, 17, and 18 have been detected in whole intestine preparations (5); therefore, it is reasonable that another Sox factor(s) may be functionally compensating for the loss of Sox9 during enteroendocrine cell fate decisions. An intriguing observation from our study that may support a role of SOX9 in differentiation is that, when SOX9 was increased to high levels in IEC-18 cells, there was a cellular phenotype that was characterized by a dramatic morphological change in which cells acquired characteristics resembling a neuroendocrine morphology. We are cautious not to overinterpret these morphological changes because of the absence of extrinsic influences on differentiation of IEC-18 cells in the in vitro context. In vivo studies are being conducted to address the role of SOX9 levels on differentiation of the intestinal epithelium.
An alternative hypothesis for the role of Sox9 in the intestinal epithelium that was not tested in the conditional Sox9 knockout studies is that SOX9 may be required for proper expression of enteroendocrine specific genes. To date, no direct target genes for SOX9 have been identified in cells of the small intestine epithelium. Although there were no other gross phenotypic abnormalities observed in the Sox9 conditional knockout mice besides the loss of Paneth cells and crypt hyperproliferation, variations in enteroendocrine-specific SOX9 target genes might exist with a less overt phenotype.
A generalized role for Sox9 in modulating proliferation through the Wnt/β-catenin pathway is becoming clear (1, 3, 5, 26). Sox9 has been reported to be a downstream target of Wnt signaling by acting in a feedback loop to repress Wnt signaling, thus keeping proliferation under tight regulatory control (1, 3). The molecular mechanism responsible for this proliferation control has been established by overexpressing SOX9 in human colon cancer lines (3). In these studies, high levels of SOX9 were able to decrease transcription of β-catenin target genes that positively regulate proliferation (i.e., c-myc, cyclin-D1) and increase inhibitors of β-catenin-Tcf proliferative activity [i.e., inhibitor of β-catenin and T cell factor (ICAT), transducin-like enhancer of splits 2–4 (TLE2–4)]. Both of these events would presumably result in decreased proliferative capacity, which was not formally tested. The data presented in our study are consistent with the findings in human colon cancer cell lines by showing that high levels of SOX9 expression in the nontransformed IEC-18 cell line decreases the expression of the β-catenin target gene, c-MYC. Our study further demonstrates a functional decrease in proliferation and expression of PCNA. Together, these data suggest that increasing SOX9 from low levels to high levels suppresses proliferation by decreasing β-catenin signaling.
Since Sox9 appears to be a Wnt target gene, one question our data poses is whether Wnt signaling is responsible for the high Sox9 levels observed in noncycling enteroendocrine cells. A recent study, however, demonstrates that, even at the enteroendocrine precursor stage, Ngn3+ cells differentiate into enteroendocrine cells independently of Wnt signaling (43), indicating that Sox9HIGH enteroendocrine cells would develop independently of Wnt signaling. To reconcile these studies with our data, we propose a bimodal role for Sox9 where low levels of Sox9 modulate proliferative capacity in the stem/progenitor cell compartment in a Wnt-dependent manner, whereas high levels of Sox9 in enteroendocrine precursors ablate proliferative capacity and influence terminal differentiation/maturation in a Wnt-independent manner (Fig. 6). On the basis of our in vivo data demonstrating that all Sox9HIGH cells are mature and postmitotic, combined with the in vitro data showing that high levels of SOX9 abrogate the proliferative capacity of IEC-18 cells, we support a model where Sox9 expression becomes uncoupled from Wnt signaling at some point during enteroendocrine fate specification and acts to repress the proliferative capacity of cycling enteroendocrine precursors at the final stages of terminal differentiation. Our results indicate that the uncoupling event would occur downstream of Ngn3 since colocalization of Ngn3 and Sox9HIGH is never observed in the Ngn3-fated progenitors. The crypt position for this “uncoupling event” appears to occur on average at cell position +5 because there is a greater proportion of Sox9HIGH cells in this region of the crypts (Fig. 2C). It has been previously reported that cell position +5 defines a niche for the differentiation of secretory lineages (4); thus the observation that Sox9HIGH cells in the crypt appear to primarily localize to cell position +4–6 (average +5) may point to an important source of extrinsic factors at this crypt position that may be influencing high levels of Sox9 expression and the development/maturation of enteroendocrine cells.
Fig. 6.
Model describing the relationship between Wnt and Sox9 in the adult intestinal epithelium. Multipotent CBCs universally express low levels of Sox9 in a Wnt-dependent manner. Post-Ngn3 lineage specification, instructive intrinsic and/or extrinsic signaling increases Sox9 expression to high levels in a Wnt-independent manner decreasing proliferative capacity and promoting terminal differentiation/maturation of enteroendocrine cells.
Subtle yet significant changes in the levels of steady-state gene expression have traditionally been difficult to assess at the cellular level because of the inability of existing technologies to accurately detect slight transcriptional variations. Transgenic reporter gene mice now enable high-resolution and real-time in vivo monitoring of gene transcription in normal and diseased states. Using this technology, we have demonstrated that distinct levels of SOX9 in the intestinal epithelium are associated with both proliferative and postmitotic cell types and, furthermore, that these variable SOX9 levels likely play an important role in both the control of proliferative capacity of stem/progenitor populations and also the maturation of enteroendocrine cells. The Sox9EGFP mouse will also be a useful tool to investigate stem/progenitor populations during disease/injury and subsequent regeneration of the small intestine epithelium. Moreover, the ability to specifically FACS isolate Sox9-expressing stem/progenitor populations for array-formatted gene expression studies will greatly enhance the ability to dissect important genetic pathways influencing normal gut homeostasis and diseased states involving the stem/progenitor cell compartment.
GRANTS
Funding was provided by the American Gastroenterological Association Research Scholar Award, the National Institutes of Health Grant 1-K01-DK080181-01, and the UNC-Chapel Hill Center for Gastrointestinal Biology and Disease, 5P30DK034987 (to S. T. Magness).
Supplementary Material
Acknowledgments
We acknowledge the UNC Neuroscience Confocal Imaging Facility (P30 NS045892-04), the Center for GI Biology and Disease Imaging and Histology COREs (5P30DK034987), the UNC Chapel Hill Mutant Mouse Regional Resource Center, and the UNC Flow Cytometry CORE. We also thank Priya Soni for technical support, and Drs. Kay Lund, Susan Henning, Michael Helmrath, Christopher Dekaney, Ajay Gulati, and Aaron Garrison for useful discussions.
REFERENCES
- 1.Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev 18: 1072–1087, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, Blache P, Jay P. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol 178: 635–648, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. III. Evidence from columnar, enteroendocrine, and mucous cells in the adult mouse. Am J Anat 160: 77–91, 1981. [DOI] [PubMed] [Google Scholar]
- 5.Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, Clevers H, Jay P. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol 166: 37–47, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bry L, Falk P, Huttner K, Ouellette A, Midtvedt T, Gordon JI. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc Natl Acad Sci USA 91: 10335–10339, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng H Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. II. Mucous cells. Am J Anat 141: 481–501, 1974. [DOI] [PubMed] [Google Scholar]
- 8.Cheng H Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. IV. Paneth cells. Am J Anat 141: 521–535, 1974. [DOI] [PubMed] [Google Scholar]
- 9.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat 141: 461–479, 1974. [DOI] [PubMed] [Google Scholar]
- 10.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am J Anat 141: 503–519, 1974. [DOI] [PubMed] [Google Scholar]
- 11.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat 141: 537–561, 1974. [DOI] [PubMed] [Google Scholar]
- 12.Collignon J, Sockanathan S, Hacker A, Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, Goodfellow PN, Lovell-Badge R. A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development 122: 509–520, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Dekaney CM, Rodriguez JM, Graul MC, Henning SJ. Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology 129: 1567–1580, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Denny P, Swift S, Brand N, Dabhade N, Barton P, Ashworth A. A conserved family of genes related to the testis determining gene, SRY. Nucleic Acids Res 20: 2887, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fantes J, Ragge NK, Lynch SA, McGill NI, Collin JR, Howard-Peebles PN, Hayward C, Vivian AJ, Williamson K, van Heyningen V, FitzPatrick DR. Mutations in SOX2 cause anophthalmia. Nat Genet 33: 461–463, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature 435: 964–968, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425: 917–925, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Gordon PV, Paxton JB, Fox NS. A methodology for distinguishing divergent cell fates within a common progenitor population: adenoma- and neuroendocrine-like cells are confounders of rat ileal epithelial cell (IEC-18) culture. BMC Cell Biol 6: 2, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron 39: 749–765, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Hoser M, Potzner MR, Koch JM, Bosl MR, Wegner M, Sock E. Sox12 deletion in the mouse reveals non-reciprocal redundancy with the related Sox4 and Sox11 transcription factors. Mol Cell Biol 28: 4675–4687, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev 15: 1272–1286, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kist R, Schrewe H, Balling R, Scherer G. Conditional inactivation of Sox9: a mouse model for campomelic dysplasia. Genesis 32: 121–123, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19: 379–383, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Lobachevsky PN, Radford IR. Intestinal crypt properties fit a model that incorporates replicative ageing and deep and proximate stem cells. Cell Prolif 39: 379–402, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui T, Kanai-Azuma M, Hara K, Matoba S, Hiramatsu R, Kawakami H, Kurohmaru M, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J Cell Sci 119: 3513–3526, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Mori-Akiyama Y, van den Born M, van Es JH, Hamilton SR, Adams HP, Zhang J, Clevers H, de Crombrugghe B. SOX9 is required for the differentiation of Paneth cells in the intestinal epithelium. Gastroenterology 133: 539–546, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Overton PM, Meadows LA, Urban J, Russell S. Evidence for differential and redundant function of the Sox genes Dichaete and SoxN during CNS development in Drosophila. Development 129: 4219–4228, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Pevny LH, Lovell-Badge R. Sox genes find their feet. Curr Opin Genet Dev 7: 338–344, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development 125: 1967–1978, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Pfaffl MW A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17: 1709–1713, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potten CS, Booth C, Tudor GL, Booth D, Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S, Okano H. Identification of a putative intestinal stem cell and early lineage marker: musashi-1. Differentiation 71: 28–41, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134: 2521–2531, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radford IR, Lobachevsky PN. An enteroendocrine cell-based model for a quiescent intestinal stem cell niche. Cell Prolif 39: 403–414, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R. SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet 36: 247–255, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40: 915–920, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scaffidi P, Bianchi ME. Spatially precise DNA bending is an essential activity of the sox2 transcription factor. J Biol Chem 276: 47296–47302, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: Development and differentiation of gut endocrine cells. Endocrinology 145: 2639–2644, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci USA 102: 12443–12448, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, Pevny LH. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev 20: 1187–1202, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435: 959–963, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Huster E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and Campomelic Dysplasia are caused by mutations in and around the Sry-related gene SOX9. Cell 9: 1111–1120, 1994. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Giel-Moloney M, Rindi G, Leiter AB. Enteroendocrine precursors differentiate independently of Wnt and form serotonin expressing adenomas in response to active beta-catenin. Proc Natl Acad Sci USA 104: 11328–11333, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss MA Floppy SOX: mutual induced fit in hmg (high-mobility group) box-DNA recognition. Mol Endocrinol 15: 353–362, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Wissmuller S, Kosian T, Wolf M, Finzsch M, Wegner M. The high-mobility-group domain of Sox proteins interacts with DNA-binding domains of many transcription factors. Nucleic Acids Res 34: 1735–1744, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294: 2155–2158, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.