Abstract
Background
Cognitive impairment has long been recognized as a manifestation of human immunodeficiency virus (HIV) infection. However, highly active antiretroviral therapy (HAART) has altered the neurologic manifestations of HIV.
Objectives
To develop a measure to quantify the motor abnormalities included in the original descriptions of HIV-associated dementia (HAD); to determine whether motor, affective, and behavioral dysfunction predict cognitive impairment; and to determine whether quantitative motor testing is a helpful adjunct in the diagnosis of HAD in a complex population from the HAART era.
Design
Neurologic and neuropsychological data were collected from the Manhattan HIV Brain Bank, a longitudinal cohort study of patients with advanced HIV. The HIV-Dementia Motor Scale (HDMS) was developed and validated and cognitive and affective or behavioral function was quantified using global neuropsychological T scores, the Beck Depression Inventory (BDI), and an independent assessment of apathy. Relationships among cognitive, motor, affective, and behavioral performance were examined using correlation, linear regression, and analyses of variance.
Setting
An urban AIDS research center.
Participants
A total of 260 HIV-positive, predominantly minority patients.
Main Outcome Measures
The HDMS scores and global neuropsychological T scores.
Results
The HDMS and BDI scores were independent predictors of cognitive impairment. Significant cognitive impairment was found in patients with motor dysfunction. Patients diagnosed as having HAD had a greater degree of motor impairment than those with other neurocognitive diagnoses.
Conclusions
Motor, affective, and behavioral abnormalities predict cognitive impairment in HIV-positive patients in this HAART-era cohort. The HDMS may be useful in the assignment of HIV-associated neurocognitive impairment in HIV populations in which normative data or neuropsychological test design is not optimal.
COGNITIVE IMPAIRMENT HAS long been recognized as a manifestation of human immunodeficiency virus (HIV) infection. The original description of the AIDS dementia complex was published by Navia and colleagues1 in 1986. The authors described the cardinal features of the disorder: progressive dementia accompanied by motor and behavioral dysfunction. In 1991, the American Academy of Neurology (AAN)2 published diagnostic criteria for HIV-associated cognitive impairment using the terms HIV-associated dementia (HAD) and minor cognitive motor disorder (MCMD). Consistent with the description of the AIDS dementia complex, these criteria included motor, affective, and behavioral abnormalities.
Since these early definitions, highly active antiretroviral therapy (HAART) has altered the neurologic manifestations of HIV; however, neuropsychological impairment persists.3 The HIV-associated neurocognitive disorders (HAND) no longer display the virologic associations seen in pre-HAART-era cohorts.4-6 Furthermore, the epidemic has steadily shifted into populations with comorbidities that complicate cognitive assessment (eg, low literacy, sociocultural alienation, and substance abuse).7
In light of these changes, the National Institute of Mental Health and the National Institute of Neurological Diseases and Stroke charged a working group with revising the AAN criteria. Three HAND diagnoses resulted: asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder, and HAD. Mild neurocognitive disorder and HAD were similar to the AAN definitions of MCMD and HAD. However, ANI was a new diagnostic category, defined as performance at least 1 SD below the mean in at least 2 cognitive areas, without abnormality in daily functioning. Motor, affective, and behavioral dysfunction were not included in the revised research criteria because “there is insufficient evidence that these symptoms are reliably associated with neurocognitive impairments.”8(p1797) The role of quantitative motor assessment in the diagnosis of HAND was also noted to need further exploration.8
In this study, we developed the HIV-Dementia Motor Scale (HDMS) to quantify the motor findings described in the early definitions of the AIDS dementia complex, HAD, and MCMD: slowed movements, gait abnormality, limb incoordination, hyperreflexia, hypertonia, and weakness.2 We then sought to determine whether motor, affective, and behavioral dysfunction were associated with cognitive impairment in a low literacy, predominantly minority cohort from the HAART era. We hypothesized that motor impairment would predict cognitive impairment and, thus, be a helpful adjunct in the diagnosis of HIV-associated cognitive syndromes in today's complex populations.
METHODS
STUDY PARTICIPANTS
Data pertaining to motor and neuropsychological function for 260 HIV-positive patients and 18 HIV-negative controls were collected from the Manhattan HIV Brain Bank (MHBB). The MHBB is a longitudinal observational and tissue donation study that includes semiannual neurologic, neuropsychological, medical, and psychiatric evaluations of adults with late-stage HIV and HIV-negative controls. The MHBB enrollment criteria and assessment protocol have been described elsewhere.9
ASSESSMENT OF NEUROPSYCHOLOGICAL FUNCTIONING
Detailed components and normative standards for the MHBB neuropsychological evaluation have been described previously and include tests validated in the assessment of HAND.10,11 For this study, a global neuropsychological T score was calculated from 6 domain-specific T scores: memory encoding, memory retrieval, speed of information processing, working memory, verbal fluency, and abstraction or executive functioning. The motor domain T score was excluded to reduce the effects of motor dysfunction on the global neuropsychological T score.
HIV-ASSOCIATED NEUROCOGNITIVE DIAGNOSTIC PROCEDURES
Neurocognitive diagnoses were assigned during multidisciplinary consensus meetings. The criteria used to assign the diagnoses of HAD and MCMD were those described by the AAN, with the exclusion of motor abnormalities from the diagnostic algorithm. Diagnoses of subsyndromic impairment and neuropsychological impairment attributable to other causes (NPI-O) were assigned according to the algorithm described by Woods et al.12 Subsyndromic impairment was assigned to patients whose impairments did not affect their daily functioning and is equivalent to ANI as described in the revised research nosology.8
ASSESSMENT OF PSYCHIATRIC, AFFECTIVE, AND BEHAVIORAL FACTORS
Patients were assessed for symptoms of depression using the Beck Depression Inventory (BDI).13 In addition, patients were independently queried about symptoms of apathy and social withdrawal and were assigned a score of 0 through 4 (0 indicates normal or usual, as good as it has always been; 1, less interest in family or friends than usual; 2,spends less time with family or friends; 3, lost contact with some family or friends; and 4, little or no contact with others). The Psychiatric Research Interview for Substance and Mental Disorders was used to assess the presence of substance use disorders.14
ASSESSMENT OF MOTOR FUNCTIONING
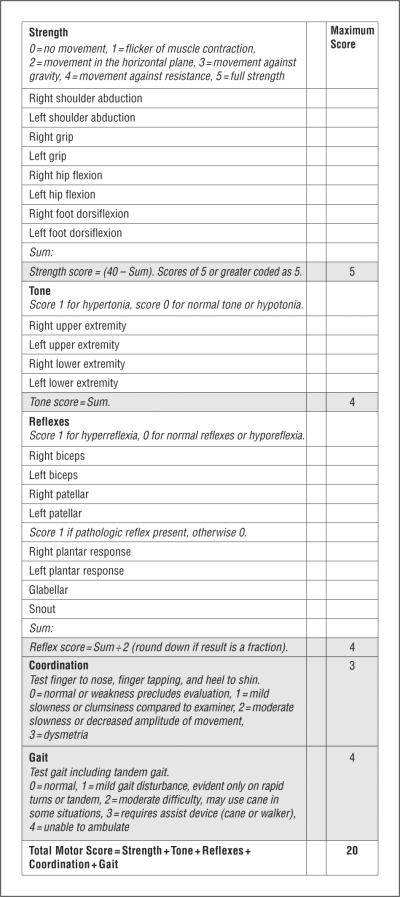
All patients underwent standardized, comprehensive neurologic examinations. The United Parkinson Disease Rating Scale (UPDRS) was included in all assessments after 2004.15 To quantify the motor abnormalities described in the original descriptions of HAND, the HDMS was developed. Using the AAN definition as a guide, 5 motor domains were defined (strength, tone, reflexes, coordination, and gait), and elements of a standard neurologic examination were selected to represent them (Figure 1).
Figure 1.
The Human Immunodeficiency Virus-Dementia Motor Scale.
STATISTICAL ANALYSES
The internal consistency reliability of the HDMS was assessed by performing reliability statistical analysis on the individual HDMS domain scores. Test-retest reliability was not assessed because data from 2 motor examinations close together in time were not available. However, as an approximation, a correlation analysis between the HDMS score at entry and at 6 months was performed for all study participants with both available data points. The validity of the HDMS was judged based on the strength of its correlation to validated measures of motor function: the UPDRS and the motor domain T score derived from performance on the grooved pegboard. A subset of 108 patients was selected for these analyses (all patients with a UPDRS and with neurologic and neuropsychological evaluations). This subset was also used for a linear multiple regression analysis in which HDMS score, BDI score, and apathy were the independent variables used to predict the global neuropsychological T score.
A larger subset of 237 individuals (all participants with a complete neurologic and neuropsychological evaluation at the entry visit) was chosen for further analysis of cognition and motor function. The linear regression analysis was repeated with the HDMS score as the sole independent variable. This analysis was also performed on the 18 HIV-negative controls.
Study participants were then divided into 3 groups (no motor impairment, mild motor impairment, and severe motor impairment) based on the HDMS score. Scores used to determine the 3 groups were derived from a quartile structure; approximately 25% of patients earned an HDMS score of 0, approximately 50% had scores between 1 and 4, and approximately 25% had scores of 5 or greater. Accordingly, a score of 0 was considered no motor impairment; a score of 1 through 4, mild motor impairment; and a score of 5 or greater, severe motor impairment. Global neuropsychological T scores and demographic and virologic variables were compared between groups using analysis of variance (ANOVA) and the χ2 test. Individuals were then divided into 5 groups based on neurocognitive diagnosis: neurocognitively normal, ANI (subsyndromic), MCMD, HAD, and NPI-O. The mean HDMS score was compared among groups using ANOVA.
Neurologic and neuropsychological data, as described herein, were also obtained at 6-, 12-, 18-, and 24-month visits when available to study the relation between the HDMS score and the global neuropsychological T score longitudinally. Each patient's HDMS score and global neuropsychological T score were regressed separately on time, and the correlation between the slopes was examined.
Statistical analyses were conducted with commercially available software programs (SPSS for Windows, version 14.1 [SPSS Inc, Chicago, Illinois]; and SAS for Windows, version 8 [SAS Institute Inc, Cary, North Carolina]).
RESULTS
COHORT CHARACTERISTICS
A total of 260 patients were selected for this study. The mean (SD) age of the group was 50.1 (7.7) years, 177 patients (68%) were male, and the group was predominantly of ethnic minority status (125 [48%] were African American and 70 [27%] were Hispanic). The mean (SD) CD4 cell count was 293/μL (319/μL), with a mean (SD) log-transformed plasma viral load (base 10) of 3.62 (1.31) copies per milliliter. The group displayed an average educational level of 12.3 years; however, the average reading level corresponded to a low-normal high school reading level.16
PSYCHOMETRIC PROPERTIES OF THE HDMS: RELIABILITY AND VALIDITY
The mean (SD) HDMS score for the sample was 3.24 (3.71) (range, 0-17). The internal consistency reliability of the HDMS met the criteria for acceptable reliability (Guttman split-half coefficient, 0.62; Spearman-Brown coefficient, 0.65; Cronbach α, 0.63).17 The correlation analysis between the HDMS score at entry and at 6 months demonstrated a significant positive correlation (r = 0.64, P<.001). The convergent validity analysis revealed significant associations between the HDMS and the UPDRS scores (r = 0.60, P<.001) and the HDMS score and the motor domain T score (r = -0.27, P = .007), both in the expected direction.
COGNITION, MOOD, AND THE HDMS
The multiple linear regression model with HDMS score, BDI score, and apathy as independent variables predicted the global neuropsychological T score (R2 = 0.31, F3,98 = 14.80, P < .001). The BDI (B = -0.39, β = -.41, t = -4.41, P < .001) and HDMS (B = -0.89, β = -.29, t = -3.44, P = .001) scores were significant predictors in the model. The apathy score was not (P = .41). The HDMS score was also a predictor of the global neuropsychological T score in the larger subset of 237 patients (R2 = 0.11, F1,235 = 30.00, P<.001).
COMPARISONS OF HDMS GROUPS
The ANOVA and post hoc pairwise comparisons revealed significant differences in the mean global neuropsychological T scores among the motor groups (F2,234 = 12.05, P<.001) (Figure 2). No significant difference was found between motor groups in age, ethnicity, educational level, substance abuse history, positive urine toxicologic test results, head trauma, loss of consciousness, learning disability, use of HAART, CD4 cell count, CD4 cell count nadir, plasma viral load, or cerebral spinal fluid viral load (Table). The groups with motor impairment contained more men and more patients with focal brain lesions and seizures. Of these factors, focal brain lesions were of particular concern because they may affect both motor and cognitive function. The comparison of mean global neuropsychological T scores between motor groups was repeated excluding the 18 study participants with focal brain lesions. All results remained significant at P≤.05.
Figure 2.

Mean global neuropsychological T scores in each of the 3 motor groups. Error bars indicate SD. P values shown are for pairwise comparisons with the normal motor group; P<.001 for the overall model. A global neuropsychological T score of 40 or greater is considered normal.
Table.
Motor Group Characteristics
| Characteristics | No Motor Impairment Group (n=72) | Mild Motor Impairment Group (n=106) | Severe Motor Impairment Group (n=59) | P Value |
|---|---|---|---|---|
| HDMS score range | 0 | 1-4 | 5-20 | NA |
| Age, mean (SD),y | 49 (8.3) | 51 (7.0) | 51 (7.1) | .17 |
| Male, No. (%) | 42 (58) | 74 (70) | 47 (80) | .03 |
| Ethnicity, No. (%) | ||||
| White | 12 (17) | 29 (27) | 19 (32) | .28 |
| Black | 37 (51) | 54 (51) | 23 (39) | |
| Hispanic | 22 (31) | 22 (21) | 17 (29) | |
| Other | 1 (1) | 1 (1) | 0 | |
| Educational level, mean (SD), y | 11.8 (2.8) | 12.4 (2.8) | 12.5 (2.8) | .23 |
| CNS lesion, No. (%) | 0 | 12 (11) | 6 (10) | .01 |
| Substance abuse by report, No. (%) | 56 (78) | 90 (85) | 46 (78) | .39 |
| Positive urine toxicologic test result, No. (%)a | 38 (57) | 58 (60) | 35 (67) | .49 |
| Receiving HAART, No. (%)a | 57 (80) | 80 (76) | 41 (70) | .44 |
| CNS trauma, No. (%) | 33 (46) | 37 (35) | 22 (37) | .33 |
| Loss of consciousness, No. (%) | 25 (35) | 37 (35) | 27 (46) | .32 |
| Seizures, No. (%) | 6 (8) | 28 (26) | 19 (32) | .002 |
| Learning disability, No. (%) | 10 (14) | 14 (13) | 8 (14) | .99 |
| CD4 lymphocyte count/μL, mean (SD) | 319 (379) | 271 (305) | 247 (268) | .59 |
| CD4 lymphocyte count nadir/μL, mean (SD)b | 187 (279) | 145 (221) | 151 (179) | .48 |
| Serum viral load, copies/mLc | 3.6 (1.2) | 3.8 (1.2) | 4.1 (1.2) | .27 |
| CSF viral load, mean (SD), copies/mLc | 2.3 (0.80) | 2.1 (0.72) | 2.6 (1.20) | .08 |
Abbreviations: CNS, central nervous system; CSF, cerebrospinal fluid; HAART, highly active antiretroviral therapy; HDMS, Human Immunodeficiency Virus-Dementia Motor Scale; NA, not applicable.
Percentages are not based on the totals given because of missing data.
Lowest recorded value for the study participant, as reported either at entry or on subsequent visits.
Log transformed (base 10).
HDMS COMPARISONS IN NEUROCOGNITIVE GROUPS
The study included 17 neurocognitively normal patients, 16 with ANI, 70 with MCMD, 46 with HAD, and 87 with NPI-O. The ANOVA and post hoc pairwise comparisons revealed a significant difference in the mean HDMS score among the neurocognitive groups (F4,232 = 5.30, P<.001). The mean HDMS score was significantly higher in the HAD group than in any other group (P <.007 for all groups), except NPI-O (P = .06). The HAD group also had the most patients with an abnormal HDMS score (36 patients [78%]), although differences were not statistically significant (neurocognitively normal group: 6 [35%] with an abnormal HDMS score, 9 [56%] with ANI, 52 [74%] with MCMD, and 61 [70%] with NPI-O; P = .02). Significant pairwise differences were found in the mean HDMS score between the neurocognitively normal group and all cognitively abnormal groups except the ANI group (Figure 3).
Figure 3.
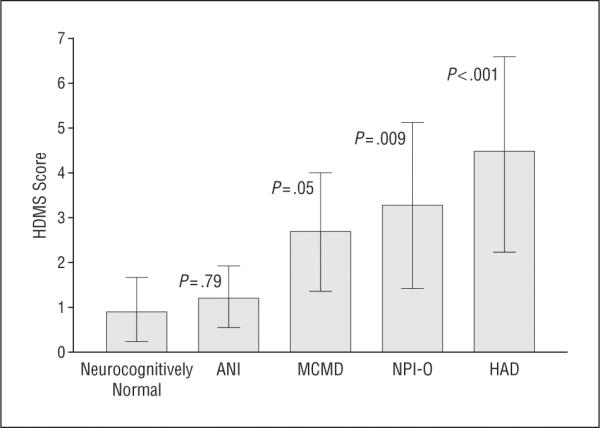
Mean Human Immunodeficiency Virus (HIV)-Dementia Motor Scale (HDMS) scores by neurocognitive diagnosis. ANI indicates asymptomatic neurocognitive impairment; HAD, HIV-associated dementia; MCMD,minor cognitive motor disorder; and NPI-O,neuropsychological impairment attributable to other causes. Error bars indicate SD. P values shown are for pairwise comparisons with the neurocognitively normal group; P=.001 for the overall model.
LONGITUDINAL ANALYSIS
The Spearman rank correlation coefficient between the slope representing the change in the HDMS score and the slope representing the change in the global neuropsychological T score was -0.20 (P = .01). This finding suggests that over time worse motor performance correlated with worse neuropsychological test performance. Sample sizes at subsequent visits were substantially smaller than at entry. If this phenomenon represented a survivor effect, a correlation might be observed even if the HDMS score and global neuropsychological T score were truly independent. However, the agreement of the longitudinal results with those obtained from the cross-sectional analysis lends them credibility.
HIV-NEGATIVE CONTROLS
A linear regression analysis revealed a trend for worsening of the global neuropsychological T score with worsening of the HDMS score (R2 = 0.16, F1,16 = 3.00, P = .10).
COMMENT
We undertook this study in the context of the recent publication8 of an updated research nosology that removes motor abnormalities from the definition of HAND and with the need for culturally less biased assessments of cognitive functioning in mind. We developed and validated the HDMS, the first quantitative measure of the motor abnormalities classically attributed to HAND: slowed movements, gait abnormality, limb incoordination, hyperreflexia, hypertonia, and weakness. We then used the HDMS, in conjunction with the BDI and an apathy score, in a multiple linear regression model to show that the combination of motor, affective, and behavioral abnormalities is predictive of cognitive impairment in HIV-positive patients in the HAART era. Although the triad of motor, affective, and behavioral, and cognitive abnormalities has been considered the hallmark of HAND since it was first described, the association has never before been rigorously demonstrated. We showed that motor impairment is worse in HAD than in other neurocognitive diagnoses. Finally, in a longitudinal analysis, we showed that the correlation between the HDMS score and the global neuropsychological T score tended to be preserved over time.
The significance of our findings is best understood in the context of the current HIV epidemic. In the United States, HIV infection is steadily shifting into populations with greater levels of socioeconomic deprivation, lower literacy, and nontraditional cultural backgrounds.7 In both nonmainstream US and developing world populations, the neuropsychological tests used to arrive at diagnoses of HAND continue to require adjustments and validation. For example, in our cohort, the impact of low literacy in biasing neuropsychological characterization toward diagnoses of impairment has previously been described.18 In addition, virologic markers no longer correlate with HAND in HAART-era cohorts, further complicating diagnosis.
In populations similar to ours, the HDMS may help establish the presence of HAND and distinguish it from pseudoimpairment, reflecting bias from a test unsuited to the individual or population under analysis. The relative insensitivity of motor testing to population bias has been noted in a prior study19 of HIV-associated dementia, in which premorbid and actual intelligence did not correlate with motor test results, such as rapid alternating movements and contraction times, but did correlate with more cognitively influenced HIV Dementia Scale scores. This prior study and the results we describe herein suggest that current recommendations to eliminate affective, behavioral, and motoric adjuncts to neurocognitive diagnosis should be carefully assessed when they are applied to nonmainstream populations. In our cohort, the only group that did not have a significantly different HDMS score from neurocognitively normal individuals was the group of functionally normal individuals with ANI. This finding may suggest that caution should be used in the assignment of ANI because it is currently unclear whether ANI represents a manifestation of brain dysfunction or a lack of suitability of testing modalities for nonmainstream populations.
The ability to distinguish the cognitive effects of HIV from other comorbidities has become one of the most pressing problems in the development of treatment strategies, particularly pertaining to the demonstration of efficacy in clinical treatment trials. Although, in our cohort, motor function was most severely abnormal in individuals with HAD, high levels of impairment were also seen in those with cognitive abnormalities attributable to other causes. The lack of specificity for motor phenomena to HIV underscores the need for continued analysis of culturally less biased, ancillary modalities in the diagnosis of HAND.
In summary, our study shows that, in the HAART era, motor and behavioral abnormalities are associated with and predict cognitive impairment in HIV-positive patients and may be important ancillary information in the assignment of cognitive impairment in marginalized populations. More work is needed to reliably distinguish HIV-associated cognitive impairments from those attributable to other causes.
Announcement.
Visit www.archneurol.com. As an individual subscriber to Archives of Neurology, you have full-text online access to the journal from 1998 forward. In addition, you can find abstracts to the journal as far back as 1975.
Acknowledgments
Funding/Support: This study was supported by grant R24MH59724 (Dr Morgello) and grant M01-RR-00071 (Clinical Research Center of Mount Sinai School of Medicine) from the National Institutes of Health.
Additional Contributions: We thank the participants and staff of the MHBB and the staff of the National Coordination Office of the National NeuroAIDS Tissue Consortium. Geoffrey Cohen, MS, contributed to the statistical analysis.
Footnotes
Financial Disclosure: None reported.
Group Information: The Manhattan HIV Brain Bank investigators and staff are listed at the end of this article.
REFERENCES
- 1.Navia BA, Jordan B, Price R. The AIDS dementia complex, I: clinical features. Ann Neurol. 1986;19(6):517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 2.Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection: report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology. 1991;41(6):778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- 3.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment. J Acquir Immune Defic Syndr. 2007;45(2):174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 4.Brew BJ, Pemberton L, Cunningham P, Law MG. Levels of human immunodeficiency virus type 1 RNA in cerebrospinal fluid correlate with AIDS dementia stage. J Infect Dis. 1997;175(4):963–966. doi: 10.1086/514001. [DOI] [PubMed] [Google Scholar]
- 5.McArthur JC, McClernon DR, Cronin MF, et al. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42(5):689–698. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- 6.McArthur JC, McDermott MP, McClernon D, et al. Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch Neurol. 2004;61(11):1687–1696. doi: 10.1001/archneur.61.11.1687. [DOI] [PubMed] [Google Scholar]
- 7.Dean HD, Steele CB, Satcher AJ, Nakashima AK. HIV/AIDS among minority races and ethnicities in the United States, 1999. J Natl Med Assoc. 2003;2005;97(7suppl):5S–12S. [PMC free article] [PubMed] [Google Scholar]
- 8.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgello S, Estanislao L, Simpson D, et al. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank. Arch Neurol. 2004;61(4):546–551. doi: 10.1001/archneur.61.4.546. [DOI] [PubMed] [Google Scholar]
- 10.Ryan EL, Morgello S, Isaacs K, Naseer M, Gerits P. The Manhattan HIV Brain Bank. Neuropsychiatric impact of hepatitis C on advanced HIV. Neurology. 2004;62(6):957–962. doi: 10.1212/01.wnl.0000115177.74976.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey CL, Woods SP, Rippeth JD, et al. Initial validation of a screening battery for the detection of HIV-associated cognitive impairment. Clin Neuropsychol. 2004;18(2):234–248. doi: 10.1080/13854040490501448. [DOI] [PubMed] [Google Scholar]
- 12.Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26(6):759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 13.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7(0):151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 14.Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. Am J Psychiatry. 1996;153(9):1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- 15.Fahn S, Elton R, Members of the UPDRS Development Committee . Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. Vol 2. Macmillan Health Care Information; Florham Park, NJ: 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- 16.Wilkinson G. Wide Range Achievement Test, 3rd Edition: Administration Manual. Wide Range Inc; Wilmington, DE: 1993. [Google Scholar]
- 17.Nunnally JC, Bernstein IH. Psychometric Theory. McGraw-Hill Inc; New York, NY: 1994. [Google Scholar]
- 18.Ryan EL, Baird R, Mindt MR, et al. Neuropsychological impairment in racial/ethnic minorities with HIV infection and low literacy levels: effects of education and reading level in participant characterization. J Int Neuropsychol Soc. 2005;11(7):889–898. doi: 10.1017/S1355617705051040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Giesen HJ, Haslinger BA, Rohe S, Köller H, Arendt G. HIV Dementia Scale and psychomotor slowing: the best methods in screening for neuro-AIDS. J Neuropsychiatry Clin Neurosci. 2005;17(2):185–191. doi: 10.1176/jnp.17.2.185. [DOI] [PubMed] [Google Scholar]