Abstract
Hispanic individuals in the U.S. have been disproportionately impacted by HIV/AIDS, yet little is known regarding the neuropsychological sequelae of HIV within the Hispanic population. This study characterized neuropsychological (NP) test performance of HIV+ English-speaking Hispanic participants (n= 51) and investigated the combined roles of sociocultural factors (e.g., ethnicity, socioeconomic status [SES] proxy, and reading level) on NP test performance among our HIV+ Hispanic and non-Hispanic White participants (n=49). Results revealed that the pattern of NP impairment in HIV+ Hispanic participants is consistent with the frontal-striatal pattern observed in HIV-associated CNS sequelae, and the overall prevalence of global NP impairment was high compared to previous reports with more ethnically homogeneous, non-Hispanic White cohorts. Multivariate prediction models that considered both sociocultural factors and CD4 count revealed that reading level was the only unique predictor of global NP functioning, learning, and attention/working memory. In contrast, ethnicity was the only unique predictor of abstraction/executive functioning. This study provides support for the use of neuropsychological evaluation in detecting HIV-associated NP impairment among HIV+ Hispanic participants, and adds to the growing literature regarding the importance of considering sociocultural factors in the interpretation of NP test performance.
Keywords: Hispanic, Human Immunodeficiency Virus, AIDS, Neuropsychological Functioning, Sociocultural Factors, Literacy
HIV has a unique affinity for the central nervous system (CNS), and neuropsychological impairment significantly increases mortality risk among HIV+ adults, regardless of physiological disease progression (Davis et al., 1992; Goudsmit et al., 1986; Mayeux et al., 1993; Sevigny et al., 2007; Wilkie et al., 1998). HIV-related neuropathology in fronto-striatal regions, along with elevated HIV viral load in CSF, are associated with neuropsychological sequelae in up to 50% of HIV+ persons (Cherner et al., 2002; Ellis et al., 2002; Heaton et al., 1995; Masliah, Ge, DeTeresa, Terry & Wiley, 1992; Masliah, Ge & Mucke, 1996; Zhao, Kim, Morgello & Lee, 2001). While HIV-associated CNS disease has been extensively studied over prior decades, little has been done to investigate how the epidemic's changing demographics might be associated with HIV's neuropsychological complications.
Initially, non-Hispanic White men accounted for the majority of HIV/AIDS cases in the U.S. (Centers for Disease Control; CDC, 2001, 2003). Hispanic individuals have since become a disproportionately affected at-risk group for HIV/AIDS. Hispanic individuals account for 20% of reported AIDS cases, but only represent 12.5% of the U.S. population (CDC, 2002). Between 1999 and 2002, the estimated number of Hispanic individuals living with HIV/AIDS rose at approximately twice the rate of non-Hispanic White individuals, and HIV/AIDS is now one of the leading causes of death for Hispanic men and women (ages 35 to 44; Anderson & Smith, 2003; CDC, 2002).
The U.S. Hispanic population is also disproportionately impacted by a myriad of sociocultural risk factors, each of which may play a role in the increased rates and possibly the virulence of HIV-1 infection (Cargill & Stone, 2005). Factors that have disproportionately impacted the U.S. Hispanic population include lower education/literacy, higher rates of poverty, and limited access to and/or use of health care (Cargill & Stone, 2005; Fiscella, Franks, Gold & Clancy, 2000; U.S. Census Bureau, 2003a). Thus, utilizing a biopsychosociocultural theoretical framework (briefly summarized here) for neurologic illness provides a rich tableau for conceptualizing neuroAIDS among the U.S. Hispanic population, by incorporating the following aspects of disease (Engel, 1980; Kiesler, 1999; Kuhn, 1970; Vatassery & Maletta, 1983):
the biomedical (the cellular to systemic changes produced by disease);
the psychosocial (the “person” level of analysis - how experience, behavior, emotion, cognition, etc. affect disease manifestations and outcomes); and
the sociocultural (the “community” and “cultural” levels of analysis - how social, socioeconomic, institutional, ethnic, and racial; as well as the shared attitudes, values, goals, and practices that characterize a group from one generation to the next modulate the group's health behaviors).
The field of neuropsychology has historically focused upon the biomedical and psychosocial aspects of neurologic illness, with relatively little focus upon the role of sociocultural factors in mediating the expression of neurologic disease. Consequently, with the increasing ethnic diversity of populations with HIV/AIDS, this approach provides an incomplete understanding of their neurologic illnesses. The expanded theoretical framework (above) for neuropsychology may have practical application in determining whether sociocultural factors associated with Hispanic ethnicity contribute to the overall cognitive profile of individuals with HIV/AIDS, and is essential if we are to disentangle how such factors (e.g., education, literacy, and socioeconomic status) impact the expression of neurologic illness with the U.S. Hispanic population - a group disproportionately impacted by the HIV/AIDS epidemic.
Hispanic individuals are the least educated and literate racial/ethnic group in the U.S. (Greenberg, Macias, Rhodes, & Chan, 2001; National Center for Education Statistics [NCES], 2006; The Pew Research Center, 2005). This is notable because lower education and/or literacy are associated with poorer neuropsychological test performance and decreased cognitive reserve (Byrd, Jacobs, Hilton, Stern, & Manly, 2005; de Ronchi et al., 2002; Manly, Jacobs, Touradji, Small & Stern, 2002; Ostrosky-Solis, Ardila, Rosselli, Lopez-Arango, & Uriel-Mendoza, 1998; Ryan et al., 2005; Satz et al., 1993; Stern et al., 2003). Lower premorbid functioning (e.g., lower education or literacy) thus appears associated with increased risk for neuropsychological abnormalities and more rapid decline secondary to neurological disease or insults (Le Carret et al., 2005; Stern, 2002; Stern, Silva, Chaisson, & Evans, 1996; Stern, Albert, Tang, & Tsai, 1999). However, these factors have yet to be examined among HIV+ Hispanic individuals.
Low socioeconomic status (SES) affects both access to and quality of healthcare (Shapiro et al., 1999). Hispanic individuals are approximately three times as likely as non-Hispanic White individuals to both live in poverty and be uninsured (Harrell & Carrasquillo, 2003; The Pew Hispanic Center, 2005). Moreover, Hispanic individuals who become infected with HIV are less likely to receive the pharmacological standard of care (highly active antiretroviral medications; HAART), have a higher mortality rate, and are more likely to die at significantly younger ages than non-Hispanic White individuals (Cargill & Stone, 2005; McGinnis, 2003; Morgello, Mahboob, Yakoushina, Khan, & Hague, 2002). Consequently, lower SES, and thus the lower standard of care Hispanic individuals often encounter, appears to have a salient impact on poorer health outcomes for HIV+ Hispanic individuals. Despite such findings, research has yet to examine the association between sociocultural risk factors and neuropsychological outcomes within this population.
The few available studies addressing U.S. Hispanic ethnicity and HIV-associated neuropsychological sequelae report contradictory findings. Compared to non-Hispanic White participants, one pre-HAART era study suggests worse performance for Hispanic participants on language and judgment/abstraction measures (Levin, Berger, Didona, & Duncan, 1992), another points to impairment in processing speed (Durvasula et al., 2001), while a third found no differences between ethnicities in global neuropsychological functioning (Richardson et al., 2002). A recent study also reports a higher prevalence of HIV-associated Dementia (HAD; 28.6%) among HIV+ Puerto Rican women residing in Puerto Rico compared to previous reports of prevalence among U.S. HIV+ populations (5.6% - 10.4%; see Wonjna et al., 2006). However, none of the above mentioned studies examined whether sociocultural risk factors might better account for these contradictory findings. Given the known neuropsychological complications of HIV, and the disproportionately higher rates of HIV-1 infection and poverty, as well as lower education and literacy rates among the U.S. Hispanic population, there is a need to characterize and understand HIV-associated neuropsychological complications within the U.S. Hispanic population.
Working from biopsychosociocultural theory, we hypothesized the following: 1) among HIV+ Hispanic participants, rates of NP-impairment would be consistent with a fronto-striatal pattern of HIV-associated NP impairment (i.e., higher rates of impairment in processing speed, learning, attention/working memory, and abstraction/executive function), 2) among the entire sample, reading level and SES would be significantly associated with NP functioning, and 3) among the entire sample, when the presence of a biological marker of disease progression (CD4 count) is also considered, reading level and SES would be better predictors of global and domain specific neuropsychological functioning than ethnicity.
Method
Participants
One hundred HIV+ adults participated in this study: 51 Hispanic participants and 49 non- Hispanic White participants, with 78% men, a mean age of 44.46 years (SD = 7.39), and a mean education of 12.87 years (SD = 2.43). Participants were recruited by the Manhattan HIV Brain Bank (MHBB, R24MH59724), an NIMH-funded longitudinal observational study. The sample's median CD4 lymphocyte was 66 cells μ/L (IQR = 28, 515). Participants had no reported history of learning disability or expressive or receptive language problems. Seventy-one percent of participants reported some form of prescribed HAART, and there was no difference between groups on rate of prescribed HAART therapies (p > .10). All participants provided written informed consent after the study's procedures were fully explained. The study was approved by the Mount Sinai School of Medicine Institutional Review Board (IRB). Additional demographic and medical characterization of the sample is provided in the results section.
Inclusion Criteria
All participants were at the advanced stage of the disease. MHBB participation eligibility criteria included: 1) presence of a condition indicative of advanced HIV without effective therapy; or 2) a CD4 count ≤ 50 cells/mm3 for at least a 3 month period of time or 3) substantive risk for imminent mortality in the judgment of the participant's primary physician. Indicator conditions for criteria 1 are progressive multifocal leukoencephalopathy, systemic or CNS lymphoma, disseminated mycobacterium avium-intracellular, wasting (>30% lean body mass), AIDS Dementia Complex, CMV end organ disease, visceral Kaposi's sarcoma, congestive heart failure, hemoglobin less than 10 mg/dl, or serum albumin <3.2 g/dl.
Procedure
All participants completed language assessments, as well as comprehensive neuromedical, psychiatric/substance use, and neuropsychological evaluations. Only baseline data were utilized in this study. All study protocols were administered in English, with the exception of one verbal fluency test given to Hispanic participants in Spanish for screening purposes.
Language Determination
All Hispanic study participants reported some degree of fluency in both English and Spanish, and were screened by a bilingual neuropsychologist (MRM) utilizing the following information to determine participants' language preference and proficiency: self-report on language use questionnaires, performance on the Controlled Oral Word Association Test (COWAT: F-A-S in English versus P-M-R in Spanish; Artiola, Fortuny, Hermosillo, Heaton & Pardee, 1999; Gladsjo et al., 1999), chart review and/or clinical interview. Our bilingual neuropsychologist (MRM) also assigned qualitative, clinical ratings of bilingual ability (i.e., Spanish-dominant, Balanced Bilinguals, English-dominant), based upon the information described above, to all Hispanic participants. Only participants with clear proficiency in English participated in this study.
Neuromedical Evaluation
All participants underwent a physical examination conducted by a neurologist specializing in HIV infection, including assessment of motor, sensory, and extrapyramidal systems; medical and medication use history obtained by trained nursing staff; and laboratory studies (CD4 lymphocyte counts, routine hematology, and chemistry).
Psychiatric/Substance Use Evaluation
Psychiatric symptomology and alcohol/substance use were assessed with the Psychiatric Research Interview for Substance Use and Mental Disorders (PRISM; Hasin et al., 1996), and administered by a trained examiner. The PRISM yields DSM-IV diagnoses for current and past psychiatric and alcohol/substance use disorders.
Neuropsychological Evaluation
All participants completed a detailed neuropsychological test battery that was administered and scored by trained psychometrists using standardized procedures. Table 1 summarizes the two-hour battery, which consists of measures assessing the following seven ability domains: abstraction/executive functioning, attention/working memory, learning, delayed recall, motor skills, processing speed and English-language verbal skills (Woods et al., 2004). Table 1 also provides the norms used to convert raw scores to age-, education-, and gender- corrected T-scores using published procedures based upon large normative data sets. To evaluate the rates of neuropsychological (NP) impairment across domains, Domain Average T-scores were derived from the mean T-scores of the individual tests in that particular domain, and the Global NP Average T-score is the mean of all individual NP test T-scores. NP Global and Domain Average T-scores below 40 were considered impaired.
Table 1.
Neuropsychological test battery and normative data arranged by the seven major ability areas for computation of average T-scores.
| Neuropsychological Domain and Tests | Normative Data Sources |
|---|---|
| Abstraction/Executive Functioning | |
| Wisconsin Card Sorting Task-64 Item Version | Kongs, Thompson, Iverson & Heaton (2000)1,2 |
| Trail Making Test (Part B) | Heaton, Grant & Matthews (1991)1,2,3 |
| Attention/Working Memory | |
| WAIS-III Letter Number Sequencing | Wechsler (1997)1 |
| PASAT Total Correct | Diehr et al. (2003)1,2,3 |
| Learning | |
| Hopkins Verbal Learning Test-Revised (Total Recall) | Benedict, Schretlen, Groninger & Brandt (1998)1 |
| Brief Visuospatial Memory Test-Revised (Total Recall) | Benedict (1997)1 |
| Delayed Recall | |
| Hopkins Verbal Learning Test (Delayed Recall Trial) | Benedict, Schretlen, Groninger & Brandt (1998)1 |
| Brief Visuospatial Memory Test-Revised (Delayed Recall Trial) | Benedict (1997)1 |
| Motor | |
| Grooved Pegboard Time (dominant hand) | Heaton, Grant & Matthews (1991)1,2,3 |
| Grooved Pegboard Time (non-dominant hand) | Heaton, Grant & Matthews (1991)1,2,3 |
| Speed of Information Processing | |
| WAIS-III Digit Symbol | Wechsler (1997)1 |
| WAIS-III Symbol Search | Wechsler (1997)1 |
| Trail Making Test (Part A) | Heaton, Grant & Matthews (1991)1,2,3 |
| Verbal Functioning | |
| Controlled Oral Word Association Test (F-A-S) | Gladsjo et al., 19991,2 |
Note: Wechsler Adult Intelligence Scale (WAIS); Paced Auditory Serial Arithmetic Test (PASAT). Normative data corrects for the following demographic characteristics indicated by superscript number:
Age
Education
Gender
Reading Level
As part of the NP evaluation, all participants' reading level was evaluated using the Reading Recognition subtest of the Wide Range Achievement Test - third edition, a test of single word reading (WRAT-3; Wilkinson, 1993). This measure has been used ubiquitously in clinical and research settings as an estimate of premorbid intelligence, as a “hold” test, due to its stability over time in a normal population (Blair & Spreen, 1989; Franzen, Burgess, & Smith-Seemiller, 1997; Griffin, Rivera Mindt, Rankin, Ritchie, & Scott, 2002). Moreover, there is strong empirical support showing that performance on single word reading tests (such as WRAT-3 Reading Recognition subtest) is among the most valid estimates of quality of educational experience among multiethnic samples (Constantino, Manly, & Mungas, 2007; Del Ser, Gonzalez-Montalvo, Martinez-Espinosa, Delgado-Villaplos, & Bermejo, 1997).
Socioeconomic Status
Socioeconomic status (SES) was estimated using median income for each participant's respective zip code by household ethnicity utilizing the 2000 U.S. Census data (U.S. Census Bureau, 2000). U.S. Department of Housing and Urban Development (HUD, 2005) standards were used to categorize `Very Low' household income, which HUD defines as less than $22,000 per year.
Statistical Analyses
The sample size (N =100) provided adequate statistical power (≥ .80) to detect medium univariate effect sizes and medium multivariate effect sizes for planned multiple regression analyses using four predictors. All statistical analyses using NP data utilized demographically corrected average T-scores using the procedures described above. Effect sizes are presented with 95% confidence intervals.
For the final set of analyses, simultaneous multiple regressions were used to determine which sociocultural predictors (ethnicity, reading level, and SES) were most predictive of NP test performance (Global NP and Domain Average T-scores) in the presence of an indicator of disease progression (CD4 lymphocyte count). Multicollinearity diagnostics were also calculated with the use of variance inflation factor (VIF) values and tolerance levels (VIF values > 5.0 and tolerance values < .20 suggest high multicollinearity and unstable regression models). Ethnicity was dummy coded (0 = non-Hispanic White group and 1= Hispanic group).
Results
Within Group Analyses
Country of Origin
Country of origin data were available for 20 of the 51 Hispanic participants. Twelve of those participants (60%) were born in the U.S. and were of Caribbean ancestry and the remaining 40% were originally from the Caribbean (7 from Puerto Rico and 1 from Dominican Republic).
HIV+ Hispanic Participants and Neuropsychological Test Performance
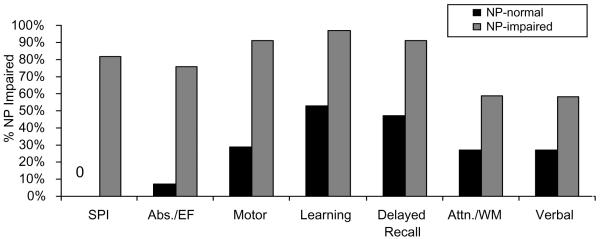
Among the 51 Hispanic participants, 70% were classified as NP-impaired. Of this subgroup, 27.8% had mild impairment, 36.1% had mild-to-moderate impairment, 30.6% had moderate impairment and 5.5% had moderate-to-severe impairment. The most prevalent deficits among the Hispanic NP-impaired participants were in the NP domains of Learning (97%), Motor Skills (91%), Delayed Recall (91%), and Processing Speed (82%).
Table 2 summarizes the results of t-test and chi-square analyses, which revealed no significant differences between the Hispanic NP-normal and NP-impaired groups on relevant demographic or medical characteristics. As Figure 1 illustrates, chi-square analyses revealed that the Hispanic NP-impaired group demonstrated a significantly higher rate of NP impairment on all NP domains compared to Hispanic NP-normal group (all p's< .05). However, the largest differences were observed on Processing Speed (χ2(1)=33.52, p< .0001), Abstraction/ Executive Functioning (χ2(1)=21.49,p< .0001), Motor Skills (χ2(1)=18.58, p< .0001), and Learning (χ2(1)=14.2, p= .0002). Of note, additional posthoc chi-square analyses revealed that the non- Hispanic White NP impaired group also demonstrated a significantly higher rate of NP impairment on all NP domains compared to their NP normal counterparts (all p's< .01), with the largest differences observed on: Processing Speed (χ2(1)=42.64, p<.0001), Attention/Working Memory (χ2(1)=23.79, p< .0001), and Learning (χ2(1)=22.26, p< .0001).
Table 2.
Comparison of demographic and disease characteristics of Hispanic participants by global neuropsychological (NP) status.
| NP-Normal | NP-Impaired | |||||
|---|---|---|---|---|---|---|
| M or P | SD | M or P | SD | t/χ2 | p | |
| Age (year) | 43.6 | 7.92 | 43.0 | 5.15 | 0.32 | .75 |
| Education (year) | 12.7 | 2.1 | 12.4 | 2.1 | 0.40 | .69 |
| CD4 Count* | 268 | 245 | 288 | 314 | 0.01 | .99 |
| Percent Male | 80 | -- | 78 | -- | 0.01 | .93 |
| Percent Immunosuppresseda | 53 | -- | 56 | -- | 0.02 | .88 |
Median test: NP-Normal CD4 Median = 154 (51, 510); NP-Impaired CD4 Median = 169 (27, 420); Note. NP = Neuropsychological. n = 15 for NP-normal, n = 36 for NP-impaired.
Immunosuppression as defined as CD4 lymphocyte count < 200 cells μ/L.
Figure 1.
Rate of neuropsychological impairment for global neuropsychological functioning and neuropsychological domains by neuropsychological status among Hispanic participants. Note: NP = Neuropsychological, Abs./EF = Abstraction/Executive Functioning, SPI = Speed of Information Processing, Attn./WM = Attention/Working Memory. All p's < .05
Post hoc analyses were also computed to examine the role of language fluency on NP test performance. As all of the Hispanic participants reported some degree of fluency in both English and Spanish, comparisons between purely English-only and bilingual Hispanic participants were not possible. Chi-square analyses to compare English-dominant Hispanic participants to balanced bilingual Hispanic participants revealed no significant differences between groups on Global NP function or any of the NP domains described above (all p's > .10; please see Methods for information on assignment of language groups). Thus, language fluency measures were not included in the following a priori analyses.
Between Group Analyses
Comparison of Ethnic Groups on Demographic, Medical, and Psychiatric Characteristics
Table 3 summarizes demographic and medical characteristics of the Hispanic participants who were comparable with their non-Hispanic White counterparts on a number of important demographic and medical variables. Of note, there was no significant difference between ethnic groups on time (years) since HIV diagnosis (p = .78). A series of posthoc Pearson correlations were also revealed that there was no significant association between time (years) since HIV diagnosis with Global NP or specific NP domains (all p's > .10).
Table 3.
Comparison of demographic and disease characteristics by ethnic minority status.
| Non-Hispanic White Group | Hispanic Group | |||||
|---|---|---|---|---|---|---|
| M or P | SD | M or P | SD | t/χ2 | p | |
| Age (year) | 45.8 | 8.5 | 43.2 | 6.0 | -1.74 | .08 |
| Education (year) | 13.2 | 2.7 | 12.5 | 2.1 | -1.35 | .18 |
| CD4 Count* | 309 | 368 | 282 | 294 | 0.50 | .48 |
| Percent Male | 78 | -- | 78 | -- | 0.01 | .92 |
| Percent Immunosuppresseda | 59 | -- | 55 | -- | 0.19 | .67 |
| Years Since Diagnosis | 10.1 | 5.4 | 9.9 | 3.9 | 0.28 | .78 |
Median test: non-Hispanic White group CD4 Median = 148 (25, 554); Hispanic group CD4 Median =169 (32, 491); Note: n = 49 for non-Hispanic White group, n = 51 for Hispanic group.
Immunosuppression as defined as CD4 lymphocyte count < 200 cells μ/L.
No significant differences between ethnic groups were observed on any measures of current (within the past year) or past diagnoses of depression or substance abuse or dependence (based on PRISM DSM-IV diagnoses; all p's>.10). In addition, a posthoc independent samples t-test comparing Global NP functioning (Global NP Average T-score) of participants with a current diagnosis of any substance dependence or abuse (including opiates) versus those without any such a diagnosis revealed that there was no significant difference between the two groups (t=-0.74(98), p= .46). An independent samples t-test specifically comparing Global NP functioning of participants with a current diagnosis of opiate dependence versus those without such diagnosis also revealed no significant difference (t=-0.90(93), p= .37). A similar analysis with a diagnosis of opiate abuse was not possible as none of the participants met criteria for this diagnosis. Consequently, substance dependence or abuse does not appear to contribute to the current NP test results.
Reading Level and Neuropsychological Test Performance
The results of an independent samples t-test revealed that the Hispanic group had significantly lower WRAT-3 Reading subtest T-scores compared to the non-Hispanic White group (M=40.73 (9.62) vs. M=48.57 (10.09), respectively; t(93)=-3.88, p< .001). A large effect size was also observed (.80; CI=.37,1.21). A Pearson correlation revealed that performance on the WRAT-3 Reading subtest was significantly associated with Global NP functioning (r=.43, p< .0001). Posthoc Pearson correlations revealed that the WRAT-3 Reading subtest was also significantly associated with Learning (r=.51, p< .0001), Attention/Working Memory (r=.44, p< .0001), Delayed Recall (r=.42, p< .0001), and Verbal Fluency (r=.39, p=.0001).
Socioeconomic Status (SES) and Neuropsychological Test Performance
In order to assess whether SES was associated with NP performance, we evaluated estimated income level. Hispanic participants lived in neighborhoods where the median income level for their ethnicity was significantly less than the non-Hispanic White participants (M= $28,734/ year ($15,173) vs. M= $50,936/year ($25,936), respectively; t(98)=5.25, p< .0001), and was approximately four times as likely to meet criteria for Very Low household income according to HUD (2005) standards (10% vs. 43%; χ2(1)=14.17, p< .001). However, there was no association between income and Global NP functioning or any of the NP domains (all p's>.10).
Ethnicity, Reading Level, and SES
Lastly, a series of linear multiple regression analyses were computed to evaluate the combined contribution of ethnicity (non-Hispanic White group vs. Hispanic group), SES (median income level based on participants' zip code per household ethnicity), reading level (WRAT-3 Reading T-score) and CD4 count to predict Global NP Function, Attention/Working Memory, Abstraction/Executive Functioning, Processing Speed & Learning.
Table 4 summarizes the results of Spearman's rho correlations among the predictor variables. As three sociocultural predictors in the following regressions were mild-to-moderately intercorrelated, multicollinearity diagnostics were computed for all of the regression analyses to evaluate the stability of the models. The results revealed that all VIF values (.688 - .975) and all tolerance values (1.026 - 1.453) were within acceptable range for all five regression models.
Table 4.
Spearman's rho correlation matrix for the four regression model predictor variables: Ethnicity (non-Hispanic white vs. Hispanic), Socioeconomic Status (SES)+ WRAT-3 Reading subtest T-score and CD4 absolute T-cell count; and linear multiple regression models predicting participants' Global Neuropsychological (NP) Average T-score, Learning Domain Average T-score, Attention/Working Memory (WM) Average T-score, and Abstraction/Executive Functioning (EF) Average T-score by predictor variables.
| Spearman's rho | Global NP Average T-score (N =93) | Learning Average T-score (N=93) | Attention/WM Average T-score (N=91) | Abstraction/EF Average T-score (N=90) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Ethnicity | SES+ | WRAT-3 Reading | CD4 Count | β | p | β | p | B | p | β | p |
| Ethnicity | 1.00 | -.12 | .30 | -.06 | .59 | -.11 | .34 | -.33 | .01 | |||
| SES+ | -.44** | 1.00 | -.18 | .11 | -.06 | .54 | -.08 | .47 | -.21 | .08 | ||
| WRAT-3 Reading | -.33* | .37* | 1.00 | .43 | <.0001 | .49 | <.0001 | .43 | COOOl | .15 | .18 | |
| CD4 Count | .01 | .06 | .10 | 1.00 | .08 | .41 | .08 | .38 | .05 | .58 | .03 | .78 |
SES = median income level based on participants' zip code
p<.01
p<.0001
Table 4 also illustrates the results of each of the multiple regressions, all of which were significant (even after correcting for alpha inflation), with the exception of the model predicting Processing Speed (R2=.09; F(4,84)=2.11, p= .09). The model predicting Global NP Functioning accounted for 22% of the variance (R2=.22; F(4,88)=6.29, p< .0001). For the respective NP domains, the models accounted for 13% to 27% of the variance (respectively) in predicting: Learning (R2=.27; F(4,88)=8.12, p<.0001); Attention/Working Memory (R2=.22; F(4,86)= 6.00, p< .0001), and Abstraction/Executive Functioning (R2=.13; F(4,85)=3.20, p= .02). The WRAT-3 Reading score was the only significant predictor of Global NP function, Learning, and Attention/Working Memory (all p's < .0001). In contrast, ethnicity (p=.009) was the only significant predictor of Abstraction/Executive Functioning.
In order to better understand the role of ethnicity in Abstraction/Executive Function, two posthoc analyses were undertaken. First, a simultaneous linear multiple regression analysis was computed exclusively within the Hispanic group to examine whether with-in group variance on English language verbal skills (based on the Verbal Skills Domain) provided a unique contribution to the prediction of Abstraction/Executive Functioning when the other 6 NP domains (see Methods for complete list) were also considered as predictors. The results revealed that the model was significant (R2=.71; F(6,36)=14.95, p<.0001), but only Processing Speed (p<.0001) and Motor Skills (p=.01) provided a unique contribution to the model while English-language Verbal Skills did not (p=.20). Second, a posthoc Pearson correlation was computed to examine whether bilingual ability (based upon difference scores between the English- and Spanish-language verbal fluency measures: COWAT F-A-S vs. P-M-R) was associated with Abstraction/Executive Functioning, but the association was not significant (r =-.13, p=.67).
Discussion
The Hispanic population represents the largest and fastest growing racial/ethnic minority group in the U.S., and has also been disproportionately affected by the HIV/AIDS epidemic. This study is the first to begin to systematically characterize the neuropsychological sequelae of HIV-1 infection within an advanced HIV+ Hispanic cohort utilizing a biopsychosociocultural framework to inform measurement of sociocultural factors that may be involved in the expression of neurologic illness among HIV+ Hispanic individuals. This study is also the first of which we are aware to begin to disentangle the role of sociocultural factors (reading level and SES) from ethnicity in the prediction of neuropsychological test performance among HIV+ Hispanic participants. The current results suggest that the pattern of neuropsychological impairment demonstrated by our Hispanic cohort is consistent with other reports of HIV/AIDS-related neuropsychological impairment and higher prevalence of global neuropsychological impairment compared to previous reports with more non-Hispanic White cohorts. Further, the present findings highlight the prominent and separable roles of reading level and ethnicity in neuropsychological test performance.
Hispanic participants were primarily of Caribbean descent, and this is consistent with the demographic characteristics of the area in which this study was conducted (East Harlem, New York City; NYC Department of Health and Mental Hygiene, 2006). To characterize the neuropsychological sequelae of HIV+ Hispanic participants, we conducted a series of analyses exclusively within the Hispanic group. We found that 71% of the Hispanic group was globally neuropsychologically impaired, with the great majority (95%) of those demonstrating a mild to moderate level of impairment. When the Hispanic NP-impaired group was compared with the Hispanic NP-normal group, the former demonstrated the greatest deficits in the areas of processing speed, abstraction/executive functioning, fine motor skills, and learning. This pattern of deficits is consistent with previous reports of HIV-associated neuropsychological sequelae and with frontal-striatal involvement, suggesting that these deficits truly represent HIV disease- induced CNS abnormality in this group (Becker et al., 1997; Grant & Heaton, 1990; Heaton et al., 1995; Heaton et al., 2004; Martin, Sorensen, Edelstein, & Robertson, 1992; White et al., 1997). Parallel analyses conducted with the non-Hispanic White group revealed a similar pattern of results thereby bolstering support for the detection of HIV-related neuropsychological sequelae within both ethnic groups.
The present study observed a higher prevalence of global neuropsychological impairment (70%) among the HIV+ Hispanic group than has been previously reported with samples with greater proportions of HIV+ non-Hispanic white participants (Heaton et al., 1995; Heaton et al., 2004). The results also demonstrated that time since HIV diagnosis and substance abuse/dependence were not associated with ethnicity or neuropsychological function. Thus, this finding provides preliminary support for Wojna et al.'s (2006) report that HIV+ Puerto Rican women residing in Puerto Rico had a higher prevalence of HIV-Associated Dementia (HAD), and those findings by examining global and specific aspects of NP functioning (rather than just an HAD diagnosis) with U.S. Hispanic individuals of Caribbean origin that include a majority of men (78%) in the sample. Still, these findings must be interpreted with caution as several factors may be influencing these results.
The greater pervasiveness of observed neuropsychological impairment among the HIV+ Hispanic participants in this study compared to previous studies is likely to be associated with several factors, including: 1) cultural bias of the neuropsychological measures used in this study (e.g., inadequate normative data, cultural variability in response set, participant/examiner interactions, test-taking attitudes, etc.; Manly, 2005), 2) methodological and/or sampling differences between the current and previous studies on HIV+ Hispanic & non-Hispanic white participants, and/or 3) bio-behavioral factors associated with differing Hispanic populations (e.g., genetics, health disparities, medication adherence, etc.). While certainly a more speculative hypothesis, it is also possible that there is a lowered threshold for the onset of HIV-associated neuropsychological sequelae secondary to reduced cognitive reserve within this HIV+ Hispanic population given their myriad sociocultural risk factors (educational, socioeconomic, and health disparities; Durvasula et al., 2001; Manly et al., 2003; Satz et al., 1993; Stern et al., 1996). The current findings, in concert with the recent findings of Wojna et al. (2006) and a report of earlier onset of Alzheimer Disease symptoms among Hispanic participants compared to non-Hispanic White participants (Clark et al., 2005), make this latter hypothesis worth future examination.
The significant association between reading level and neuropsychological functioning observed in this study goes far to contextualize the current findings. Hispanic individuals are more likely than non-Hispanic White individuals to live in poverty and reside within geographically concentrated urban areas (Llorente, Ponton, Taussig, & Satz, 1999; The Pew Hispanic Center, 2005; U.S. Census Bureau, 2003b), both of which are risk factors for lower per pupil resource allocation (Carey, 2004). Moreover, while national literacy scores have risen for African- and Asian- Americans, and have remained essentially unchanged for non-Hispanic White individuals between 1992 and 2003, they have decreased significantly for Hispanic individuals (NCES, 2006). Therefore, although our Hispanic sample was relatively well educated in terms of duration of schooling (M=12.5 years), this appears to be strongly mitigated by their lower reading level, which serves as a proxy for poorer quality of education. Ultimately, such educational disparities appear to put HIV+ Hispanic individuals at greater risk for poorer neuropsychological test performance.
SES was not associated with neuropsychological functioning in this study. However, due to the fact that this study's SES measure was actually a proxy (median income based on zip code), which used household ethnicity as part of the determination of SES, caution must be taken when interpreting this finding. This study's measure of SES may lack the specificity necessary to draw more definitive conclusions regarding the association between SES and neuropsychological functioning in this population.
Our most important hypotheses, regarding the combined roles of sociocultural variables (ethnicity, reading level, SES) and disease progression (CD4 count) in the prediction of neuropsychological functioning, were partially supported. As predicted, we found that only reading level significantly predicted global neuropsychological functioning, learning, and attention/working memory. In contrast to our hypotheses, only ethnicity significantly predicted abstraction/executive functioning. Posthoc analyses revealed that neither English-language verbal skills nor bilingual ability uniquely predicted abstraction/executive functioning. However, our current assessment of English and Spanish language fluency was circumscribed to brief verbal fluency measures, and more comprehensive assessment may have yielded different results. Further research is needed to examine the association between English-language fluency, bilingual ability, and abstraction/executive functioning.
Beyond potential language considerations, the unique role of ethnicity in abstraction/executive functioning may also be associated with acculturation, a construct not examined in the current study. Previous research suggests that lower acculturation (to majority, mainstream culture) is associated with poorer performance on measures of abstraction/executive functioning (Arnold, Montgomery, Castaneda, & Longoria, 1994). It is possible that the process of nonverbal, abstract reasoning is more culturally mediated than other neuropsychological processes within the Hispanic population. However, additional research specifically examining these associations is needed in order to test this hypothesis.
The findings on the association between ethnicity and abstraction/executive function highlight a limitation of this study, e.g., that it lacked the resources to do more comprehensive cultural evaluations to examine within group variation among Hispanic participants. Additional research is needed to replicate and expand the current findings with larger, more thoroughly characterized Hispanic samples of English- and Spanish-speakers to better evaluate the prevalence of HIV-associated neuropsychological sequelae within this population and to examine the role of with-in group variation on several sociocultural factors (acculturation, language, etc.) that may associated with neuropsychological test performance.
Given the relative homogeneity of our Hispanic sample, one could make the argument that the generalizability of the current findings is limited to primarily English-speaking Hispanic individuals of Caribbean origin. While this may indeed be the case, it is alternately possible that the current findings are applicable to other Hispanic subpopulations, and potentially other racial/ethnic groups who experience similar sociocultural disadvantages. Thus, more research is needed to replicate this study with other Hispanic subpopulations as Hispanic individuals tend to exhibit nonrandom, preferential geographical affinity that could interact with other demographic/cultural factors to create distinct patterns of neuropsychological test performance (Llorente et al., 1999).
The lack of demographically corrected norms, which include ethnicity, for English- speaking Hispanic individuals represents a major limitation, and within the field more broadly, to all neuropsychological studies of U.S. Hispanic individuals. While we utilized the best available norms, they did not address current educational disparities and the current findings provide strong support for the fact that even relatively well educated Hispanic individuals are likely to experience poorer quality of education compared to their non-Hispanic White counterparts. Additional research using improved norms for English-speaking Hispanic individuals is needed.
An additional limitation of this study is that HIV-seronegative controls were not included. While inclusion of such a control group would certainly enhance the current study, the goal of this study was not to compare HIV-seropositives to seronegative controls. Rather, this study sought to characterize the NP function of HIV-seropositive Hispanic participants, and to examine the combined roles of sociocultural variables in the prediction of their NP test performance. As a first step in a new area of investigation, this study met its goals using the current sample.
A final study limitation relates to the limited assessment of literacy. Consistent with previous research, this study's assessment of literacy was limited to evaluation of reading level based upon a single word reading test (WRAT-3 Reading subtest) as a proxy for quality of education (Byrd et al., 2005; Constantino et al., 2007; Manly et al., 2002, 2003; Ryan et al., 2005). Consequently, the current results cannot be used to make broad generalizations regarding the association between literacy and neuropsychological test performance, but rather provide initial insight into the role of quality of education on neuropsychological test performance among those with advanced HIV/AIDS.
Despite these limitations, this study has three important strengths. This study is the first to suggest that HIV+ Hispanic participants with advanced disease demonstrate a pattern of neuropsychological sequelae that is consistent with the frontal-striatal pattern observed in HIV-induced CNS disease. Second, this study is the first to systematically evaluate HIV-related neuropsychological complications within a sample of advanced HIV+ Hispanic participants in the U.S. In using a larger and better characterized Hispanic group with a demographically and medically equivalent non-Hispanic White comparison group, this study was able to more confidently make inferences based on the current findings than previous studies with smaller or less thoroughly characterized Hispanic groups. Third and finally, in utilizing biopsychosociocultural theory to inform hypotheses, this investigation will hopefully help to move NeuroAIDS research beyond categorical racial/ethnic variables as terminal explanatory constructs of neuropsychological functioning in favor of more conceptually and theoretically driven constructs, such as quality of education and SES (Helms, Jernigan, & Mascher, 2005).
The current findings have implications for treatment interventions and public policy. Given that HIV has a strong affinity for the CNS and that neuropsychological impairment is a significant risk factor for non-adherent medical behaviors and mortality in HIV (Hinkin et al., 2002; Hinkin et al., 2004; Heaton et al., 2004; Mayeux et al., 1993; Rivera Mindt et al., 2003; Sevigny et al., 2007; Wilkie et al., 1998), accurate neuropsychological evaluation of HIV+ Hispanic individuals is critical for optimal care and public planning for disease burden.
With regard to disease, this study's results affirm the utility of commonly used neuropsychological test measures in the detection of HIV-related neuropsychological impairment among HIV+ Hispanic participants. However, the results also demonstrate the need for providing culturally competent neuropsychological evaluations as an integral part of the standard of care. Specifically, it is recommended that a comprehensive sociocultural evaluation (e.g., literacy, quality of education, acculturation, linguistic information, etc.) and improved normative data be utilized. Integration of such information could potentially improve the interpretation of neuropsychological test data, aid in making more precise cognitive-diagnoses, and assist in developing culturally tailored treatment recommendations.
Research suggests HIV-related neuropsychological impairment is related to significant functional impairment across a spectrum of areas, including medication adherence (Hinkin et al., 2002; Hinkin et al., 2004; Heaton et al., 2004; Rivera Mindt et al., 2003). If it is indeed the case that a high rate of neuropsychological impairment is present among HIV+ Hispanic individuals as this and previous research suggests (Wojna et al., 2006), then research is needed to develop culturally tailored interventions to address likely associated functional impairments (particularly in the area of medication adherence - a critical factor for positive health outcomes) - with public policy support to implement such interventions within Hispanic communities. Public policy would also go far to address the dearth of linguistically/culturally competent neuropsychologists currently in practice and research in the U.S. in order to better provide evaluation and treatment services to HIV+ Hispanic individuals. In sum, the current results suggest that HIV+ Hispanic individuals with global neuropsychological impairment demonstrate a pattern of impairments consistent with other reports of HIV-related CNS complications. Moreover, this study highlights the need to consider sociocultural factors in the interpretation of neuropsychological test performance with this population. Given the importance of sociocultural factors in the neuropsychological evaluation of HIV+ Hispanic individuals, it is likely that these same factors play important roles for consideration at every stage of the disease, from prevention to palliative care, and more research is needed to better understand these factors at every stage of care.
Acknowledgements
The authors wish to thank the patients and staff of the Manhattan HIV Brain Bank, and Dr. Ramani Durvasula and Mr. Napoleon Wells for their assistance with this manuscript. Supported by grant R24MH59724 (to SM) and the Clinical Research Center of the Mount Sinai School of Medicine (M01-RR-00071) from the National Institutes of Health.
References
- Anderson RN, Smith BL. Deaths: Leading Causes for 2001. National Vital Statistics Report. 2003;52:1–85. [PubMed] [Google Scholar]
- Arnold BR, Montgomery GT, Castaneda I, Longoria R. Acculturation and performance of Hispanics on selected Halstead-Reitan neuropsychological tests. Assessment. 1994;1:239–248. [Google Scholar]
- Artiola I, Fortuny L, Hermosillo RD, Heaton RK, Pardee RE., III . Manual de normas y procedimientos para la bateria neuropsicologica en Espanol. Tucson, AZ: 1999. [Google Scholar]
- Becker JT, Caldararo R, Lopez OL, Dew MA, Dorst SK, Banks G. Qualitative features of the memory deficit associated with HIV infection and AIDS: Cross-validation of a discriminant function classification scheme. Journal of Clinical and Experimental Neuropsychology. 1997;17:134–142. doi: 10.1080/13803399508406588. [DOI] [PubMed] [Google Scholar]
- Benedict RH. Brief Visuospatial Memory Test-Revised. Psychological Assessment Resources; Odessa, Florida: 1997. [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test- revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Byrd DA, Jacobs DM, Hilton HJ, Stern Y, Manly J. Sources of errors on visuoperceptual tasks: Role of education, literacy, and search strategy. Brain and Cognition. 2005;58:251–257. doi: 10.1016/j.bandc.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Byrd DA, Sanchez D, Manly JJ. Neuropsychological test performance among Caribbean-born and U.S.-born African-American elderly: The role of age, education, and reading level. Journal of Clinical and Experimental Neuropsychology. 2005;27:1056–1069. doi: 10.1080/13803390490919353. [DOI] [PubMed] [Google Scholar]
- Carey K. The Funding Gap 2004: Many states still shortchange low-income and minority students. The Education Trust. 2004;2004:1–17. [Google Scholar]
- Cargill VA, Stone VE. HIV/AIDS: A minority health issue. The Medical Clinics of North America. 2005;89:895–912. doi: 10.1016/j.mcna.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control HIV and AIDS- United States, 1981-2000. MMWR Weekly. 2001 Retrieved August 21, 2005, from [HYPERLINK “ http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5021a2.htm”]
- Centers for Disease Control . HIV/AIDS among Hispanics in the United States. Surveillance fact sheet. 2002. issued March 11, 2002. [Google Scholar]
- Centers for Disease Control HIV/AIDS Surveillance by Race/Ethnicity. 2003 Retrieved June 12, 2005, from www.cdc.gov/hiv/graphics/minority.htm.
- Cherner M, Masliah E, Ellis RJ, Marcotte TD, Moore DJ, Grant I, et al. Neurocognitive dysfunction predicts postmortem findings of HIV encephalitis. Neurology. 2002;59:1563–1567. doi: 10.1212/01.wnl.0000034175.11956.79. [DOI] [PubMed] [Google Scholar]
- Clark C, DeCarli C, Mungas D, Chui HI, Higdon R, Nunez J, Fernandez H, Negron H, Manly J, Ferris S, Perez A, Torres M, Ewbank D, Glosser G, van Belle G. Earlier onset of Alzheimer's Disease symptoms in Latino individuals compared with Anglo individuals. Archives of Neurology. 2005;642:774–778. doi: 10.1001/archneur.62.5.774. [DOI] [PubMed] [Google Scholar]
- Constantino S, Manly JJ, Mungas D. Do reading tests measure the same construct in multiethnic and multilingual persons? Journal of the International Neuropsychological Society. 2007;13:228–236. doi: 10.1017/S1355617707070257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, et al. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- de Ronchi D, Faranca I, Berardi D, Scudellari P, Borderi M, Manfredi R, et al. Risk factors for cognitive impairment in HIV-1 infected persons with different risk behaviors. Archives of Neurology. 2002;59:812–818. doi: 10.1001/archneur.59.5.812. [DOI] [PubMed] [Google Scholar]
- Del Ser T, Gonzalez-Montalvo J, Martinez-Espinosa S, Delgado-Villaplos C, Bermejo F. Estimation of premorbid intelligence in Spanish people with the Word Accentuation Test and its application to the diagnosis of dementia. Brain and Cognition. 1997;33:343–356. doi: 10.1006/brcg.1997.0877. [DOI] [PubMed] [Google Scholar]
- Diehr MC, Cherner M, Wolfson TJ, Miller SW, Grant I, Heaton RK. The 50 and 100-item short forms of the Paced Auditory Serial Addition Task (PASAT): demographically corrected norms and comparisons with the full PASAT in normal and clinical samples. Journal of Clinical and Experimental Neuropsychology. 2003;25:571–585. doi: 10.1076/jcen.25.4.571.13876. [DOI] [PubMed] [Google Scholar]
- Durvasula RS, Miller EN, Myers HF, Wyatt GE. Predictors of neuropsychological performance in HIV positive women. Journal of Clinical and Experimental Neuropsychology. 2001;23:149–163. doi: 10.1076/jcen.23.2.149.1211. [DOI] [PubMed] [Google Scholar]
- Durvasula RS, Myers HF, Satz P, Miller E, Morgenstern H, Richardson MA, et al. HIV-1, cocaine, and neuropsychological performance in African American men. Journal of the International Neuropsychological Society. 2000;6:335. doi: 10.1017/s1355617700633076. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Moore DJ, Childers ME, Letendre S, McCutchan JA, Wolfson T, et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Archives of Neurology. 2002;59:923–928. doi: 10.1001/archneur.59.6.923. [DOI] [PubMed] [Google Scholar]
- Engel GL. The clinical application of the biopsychosocial model. American Journal of Psychiatry. 1980;137:535–544. doi: 10.1176/ajp.137.5.535. [DOI] [PubMed] [Google Scholar]
- Fiscella K, Franks P, Gold MR, Clancy Inequality in quality: Addressing socioeconomic, racial, and ethnic disparities in health care. Journal of the American Medical Association. 2000;283:2579–2584. doi: 10.1001/jama.283.19.2579. [DOI] [PubMed] [Google Scholar]
- Franzen MD, Burgess EJ, Smith-Seemiller L. Methods of estimating premorbid functioning. Archives of Clinical Neuropsychology. 1997;12:711–738. [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peavey GM, Miller SW, Heaton RK. Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- Goudsmit J, de Wolf F, Paul DA, Epstein LG, Lange JM, Krone WJ, et al. Expression of human immunodeficiency virus antigen (HIV-Ag) in serum and cerebrospinal fluid during acute and chronic infection. Lancet. 1986;2:177–180. doi: 10.1016/s0140-6736(86)92485-2. [DOI] [PubMed] [Google Scholar]
- Grant I, Heaton RK. Human immunodeficiency virus-type 1 (HIV-1) and the brain. Journal of Consulting and Clinical Psychology. 1990;58:22–30. doi: 10.1037//0022-006x.58.1.22. [DOI] [PubMed] [Google Scholar]
- Greenberg E, Macias EG, Rhodes D, Chan T. English Literacy and Language Minorities in the United States (NCES Publication No. 2001-464) U.S. Department of Education and National Center for Education Statistics; Washington, DC: 2001. [Google Scholar]
- Griffin SL, Rivera Mindt M, Rankin EJ, Ritchie AJ, Scott JG. Estimating premorbid intelligence: Comparison of traditional and contemporary methods across the intelligence continuum. Archives of Clinical Neuropsychology. 2002;17:495–505. [PubMed] [Google Scholar]
- Harrell J, Carrasquillo O. The Latino disparity in health coverage. The Journal of the American Medical Association. 2003;289:1167. doi: 10.1001/jama.289.9.1167. [DOI] [PubMed] [Google Scholar]
- Hasin D, Trautman K, Miele G, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): Reliability for substance abusers. American Journal of Psychiatry. 1996;153:1195. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, et al. HIV Neurobehavioral Research Center The HNRC 500--neuropsychology of HIV infection at different disease stages. J.Int.Neuropsychol.Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- Heaton RK, Marcotte TD, Rivera Mindt M, Sadek J, Moore DJ, Bentley H, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J.Int.Neuropsychol.Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Helms JE, Jernigan M, Mascher J. The meaning of race in psychology and how to change it: A methodological perspective. American Psychologist. 2005;60:27–36. doi: 10.1037/0003-066X.60.1.27. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon S, Durvasula RS, Hardy DJ, Lam MN, Mason KI. Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy D, Mason KI, Castellon S, Durvasula RS, Lam MN. Medication adherence in HIV-infected adults: Effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18:S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesler DJ. Beyond the Disease Model of Mental Disorders. Praeger; New York: 1999. [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test-64 Card Computerized Version. Psychological Assessment Resources; Odessa, Florida: 2000. [Google Scholar]
- Kuhn TS. The Structure of Scientific Revolution. University of Chicago Press; Chicago: 1970. [Google Scholar]
- Le Carret N, Auriacombe S, Letenneur l., Bergua V, Dartigues J, Fabrigoule C. Influence of education on the patter of cognitive deterioration in AD patients: The cognitive reserve hypothesis. Brain and Cognition. 2005;57:120–126. doi: 10.1016/j.bandc.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Levin BE, Berger JR, Didona T, Duncan R. Cognitive function in asymptomatic HIV-1 infection: The effects of age, education, ethnicity, and depression. Neuropsychology. 1992;6:303–313. [Google Scholar]
- Llorente AM, Ponton MO, Taussig IM, Satz P. Patterns of American immigration and their influence on acquisition of neuropsychological norms for Hispanics. Archives of Clinical Neuropsychology. 1999;14:603–614. [PubMed] [Google Scholar]
- Manly JJ. Advantages and disadvantages of separate norms for African Americans. The Clinical Neuropsychologist. 2005;2:270–275. doi: 10.1080/13854040590945346. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropsychological test performance between African American and White elders. Journal of the International Neuropsychological Society. 2002;8:341–348. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Touradji P, Tang M-X, Stern Y. Literacy and memory decline among ethnically diverse elders. Journal of Clinical and Experimental Neuropsychology. 2003;25:680–690. doi: 10.1076/jcen.25.5.680.14579. [DOI] [PubMed] [Google Scholar]
- Martin EM, Sorensen DJ, Edelstein HE, Robertson LC. Decision-making speed in HIV-1 infection: A preliminary report. AIDS. 1992;6:109–113. doi: 10.1097/00002030-199201000-00015. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, DeTeresa R, Terry RD, Wiley CA. Cortical dendritic pathology in human immunodeficiency virus encephalitis. Laboratory Investigation: A Journal of Technical Methods and Pathology. 1992;66:291. [PubMed] [Google Scholar]
- Masliah E, Ge N, Mucke L. Pathogenesis of HIV-1 associated neurodegeneration. Critical Reviews in Neurobiology. 1996;10:57–67. doi: 10.1615/critrevneurobiol.v10.i1.30. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Stern Y, Tang M, Todak G, Marder K, Sano M, Richards M, Stein Z, Ehrhadt A, Gorman J. Mortality risks in gay men with human immunodeficiency virus infection and cognitive impairment. Neurology. 1993;43:176–182. doi: 10.1212/wnl.43.1_part_1.176. [DOI] [PubMed] [Google Scholar]
- McGinnis K. Understanding racial disparities in HIV using data from the Veterans Aging Cohort - 3 site study and VA administrative data. American Journal of Public Health. 2003;93:1728–1733. doi: 10.2105/ajph.93.10.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgello S, Mahboob R, Yakoushina T, Khan S, Hague K. Autopsy findings in a human immunodeficiency virus-infected population over 2 decades: Influences of gender, ethnicity, risk factors, and time. Archives of Pathology Lab Med. 2002;126:182–190. doi: 10.5858/2002-126-0182-AFIAHI. [DOI] [PubMed] [Google Scholar]
- National Center for Education Statistics . National assessment of adult literacy (NAAL): A first look at the literacy of America's adults in the 21st century (Report No. NCES 206-470) U.S. Department of Education; Jessup, MD: 2006. [Google Scholar]
- New York Department of Health and Mental Hygiene East Harlem Neighborhood Health Profile. 2006 http://www.nyc.gov/html/doh/downloads/pdf/data/2006chp-303.pdf, Retrieved August 13, 2006.
- Ostrosky-Solis F, Ardila A, Rosselli M, Lopez-Arango G, Uriel-Mendoza V. Neuropsychological test performance in illiterate subjects. Arch.Clin.Neuropsychol. 1998;13:645–660. doi: 10.1093/arclin/13.7.645. [DOI] [PubMed] [Google Scholar]
- Richardson JL, Martin EM, Jimenez N, Danley K, Cohen M, Carson VL, et al. Neuropsychological functioning in a cohort of HIV infected women: Importance of antiretroviral therapy. Journal of the International Neuropsychological Society. 2002;8:781–793. doi: 10.1017/s1355617702860064. [DOI] [PubMed] [Google Scholar]
- Rivera Mindt M, Cherner M, Marcotte TD, Moore D, Bentley H, Esquivel MM, Lopez Y, Grant I, Heaton RK, the HNRC Group The functional impact of HIV- associated neuropsychological impairment in Spanish-speaking adults: A pilot study. The Journal of Clinical and Experimental Neuropsychology. 2003;25:122–132. doi: 10.1076/jcen.25.1.122.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EL, Baird R, Rivera Mindt M, Byrd D, Monzones J, Morgello S, the Manhattan HIV Brain Bank Neuropsychological impairment in racial/ethnic minorities with HIV infection and low literacy levels: Effects of education and reading level in participant characterization. Journal of the International Neuropsychological Society. 2005;11:889–898. doi: 10.1017/S1355617705051040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satz P, Morgenstern H, Miller EN, Selnes OA, McArthur J, Cohen B, et al. Low education as a possible risk factor for cognitive abnormalities in HIV-1: Findings from the Multicenter AIDS Cohort Study (MACS) Journal of Acquired Immune Deficiency Syndrome: JAIDS. 1993;6:503–511. [PubMed] [Google Scholar]
- Sevigny JJ, Albert SM, McDermott MP, Schifitto G, McArthur JC, Sacktor N, Conant K, Selnes OA, Stern Y, McClernon DR, Palumbo D, Kieburtz K, Riggs G, Cohen B, Marder K, Epstein LG. An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. Archives of Neurology. 2007;64(1):97–102. doi: 10.1001/archneur.64.1.97. [DOI] [PubMed] [Google Scholar]
- Shapiro MF. Variations in the care of HIV-infected adults in the United States: results from the HIV Cost and Services Utilization Study. JAMA: Journal of the American Medical Association. 1999;281:2305–2315. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8:448–460. [PubMed] [Google Scholar]
- Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: Cognitive reserve? Neurology. 1999;53:1942–1947. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- Stern RA, Silva SG, Chaisson N, Evans DL. Influence of cognitive reserve on neuropsychological functioning in asymptomatic human immunodeficiency virus-1 infection. Archives of Neurology. 1996;53:148–153. doi: 10.1001/archneur.1996.00550020052015. [DOI] [PubMed] [Google Scholar]
- Stern Y, Zarahn E, Hilton HJ, Flynn J, De La Paz R, Rakitin B. Exploring the neural basis of cognitive reserve. Journal of Clinical and Experimental Neuropsychology. 2003;25:691–701. doi: 10.1076/jcen.25.5.691.14573. [DOI] [PubMed] [Google Scholar]
- The Pew Hispanic Center . Hispanics: A people in motion. Pew Research Center; Washington, D.C.: 2005. [Google Scholar]
- U.S. Census Bureau 2000 Summary File 3 (SF 3) 2000 Available at: http://factfinder.census.gov. Accessed June 16, 2005.
- U.S. Census Bureau . Census facts for Hispanic Heritage Month. 2003a. Press release issued CB03-FF.14 - issued September 18, 2003. [Google Scholar]
- U.S. Census Bureau . The Hispanic Population in the United States: March 2002. 2003b. Press release P20-545, issued June, 2003. [Google Scholar]
- U.S. Department of Housing and Urban Development FY 2005 Income Limits. 2005 Available at: http://www.huduser.org/datasets/il/IL05/index.html. Accessed June 18, 2005.
- Vatassery GT, Maletta GJ. Relationship between nutrition and dementia in the elderly. Journal of Psychiatric Medicine. 1983;1:429–443. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Third Edition: Administration and Scoring Manual. Harcourt Brace; San Antonio, TX: 1997. [Google Scholar]
- White DA, Taylor MJ, Butters N, Mack C, Salmon DP, Peavy G, et al. Memory for verbal information in individuals with HIV-associated dementia complex. Journal of Clinical and Experimental Neuropsychology. 1997;19:357–366. doi: 10.1080/01688639708403864. [DOI] [PubMed] [Google Scholar]
- Wilkie FL, Goodkin K, Eisdorfer C, Feaster D, Morgan R, Fletcher MA, Blaney N, Baum M, Szapocznik J. Mild cognitive impairment and risk of mortality in HIV-1 infection. The Journal of Neuropsychiatry and Clinical Neurosciences. 1998;10:125–132. doi: 10.1176/jnp.10.2.125. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. Wide Range Achievement Test (3rd ed.) Administration Manual. Wide Range Inc; Delaware: 1993. [Google Scholar]
- Wonja V, Skolasky RL, Hechavarria R, Mayo R, Selnes O, McArthur JC, et al. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. Journal of NeuroVirology. 2006;12:356–364. doi: 10.1080/13550280600964576. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Hinkin CH, Lazzaretto D, Cherner M, Marcotte TD, Gelman BB, Morgello S, Singer EJ, Grant I, Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Journal of Clinical and Experimental Neuropsychology. 2004;26:759–78. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- Zhao ML, Kim MO, Morgello S, Lee SC. Expression of inducible nitric oxide synthase, interleukin-1 and caspase-1 in HIV-1 encephalitis. Journal of Neuroimmunology. 2001;115:182–191. doi: 10.1016/s0165-5728(00)00463-x. [DOI] [PubMed] [Google Scholar]