Abstract
Injection drug use (IDU) plays a critical role in the HIV epidemic in several countries throughout the world. In these countries, injection drug users are at significant risk for both HIV and tuberculosis, and active IDU negatively impacts treatment access, adherence and retention. Comprehensive strategies are therefore needed to effectively deliver preventive, diagnostic and curative services to these complex patient populations. We propose that developing co-located integrated care delivery systems should become the focus of national programmes as they continue to scale-up access to antiretroviral medications for drug users. Existing data suggest that such a programme will expand services for each of these diseases; increase detection of tuberculosis (TB) and HIV; improve medication adherence; increase entry into substance use treatment; decrease the likelihood of adverse drug events; and improve the effectiveness of prevention interventions. Key aspects of integration programmes include: co-location of services convenient to the patient; provision of effective substance use treatment, including pharmacotherapies; cross-training of generalist and specialist care providers; and provision of enhanced monitoring of drug-drug interactions and adverse side effects. Central to implementing this agenda will be fostering the political will to fund infrastructure and service delivery, expanding street-level outreach to IDUs, and training community health workers capable of cost effectively delivering these services.
Keywords: HIV, AIDS, Injection drug use, Substance use, Tuberculosis, Health care integration, Health services, Prevention
Introduction
In many communities throughout the world, the HIV/AIDS, tuberculosis (TB) and substance use epidemics are intertwined, each contributing to increased incidence, morbidity and mortality of the other. Injection drug use (IDU) is a leading mode of HIV transmission in parts of Eastern Europe, China (Ouellet et al., 1998), the Russian Federation (Klein, Roberts, & Trace, 2004), and South (Solomon, Chakraborty, & Yepthomi, 2004) and Southeast Asia (Aceijas, Stimson, Hickman, & Rhodes, 2004; World Health Organization, 2005). Among HIV-infected injection drug users, tuberculosis is a leading cause of mortality (Aliyu & Salihu, 2003; Waldrop-Valverde et al., 2006). Both all-cause and TB-associated mortality rates are several-fold higher among HIV-infected drug users than in the general HIV-infected population (Corbett et al., 2003; Kourbatova et al., 2006; McShane, 2005; Mosam et al., 2005; Nunn et al., 2005; Sharma, Mohan, & Kadhiravan, 2005). These poor outcomes are largely attributed to the fact that HIV-infected drug users are both less likely to be prescribed highly active antiretroviral therapy (HAART) (Celentano et al., 1998; Strathdee et al., 1998), and, when prescribed, less likely to achieve clinical benefit (Broadhead et al., 2002; Celentano et al., 2001; Loughlin et al., 2004; Lucas, Cheever, Chaisson, & Moore, 2001; Moore, Keruly, & Chaisson, 2004; Rompalo et al., 2001; World Health Organization, 2005). Patients with these diseases thus present serious challenges for adherence, drug dosing, drug interactions, and clinical and laboratory monitoring.
Despite the scope and complexity of the problem, little attention has been paid to developing holistic, comprehensive programmes for these patients. The current health policy paradigm for managing these diseases is fragmented, with services financing and provision separated at international, national and community levels. This paradigm is not fully equipped to address the complexity of care required to effectively treat and care for HIV-infected drug users. Here, we develop an alternative to this paradigm in areas heavily affected by these epidemics. Specifically, we present a rationale for integrating the following three key clinical services:
Screening, detection and treatment with anti-tuberculosis therapy (ATT) for patients with tuberculosis.
Voluntary Counselling and Testing (VCT) and comprehensive medical management, including highly active anti-retroviral therapy for HIV/AIDS.
Screening and treatment of substance use and dependence, including pharmacotherapies for chemical dependence.
Key aspects of integration programmes include: colocation of services convenient to the patient; provision of screening for each disease; training of outreach workers and case managers, mid-level practitioners and physicians in the management of co-morbid conditions; cross-training of specialist providers in the management of each disease; and provision of enhanced monitoring of drug-drug interactions and adverse side effects. Through these strategies, a more holistic approach to these complex patients can be achieved.
Defining integrated care
We favour the definition provided by Mur-Veeman, Hardy, Steenbergen, and Wistow (2003) for integrated care as “an organisational process of coordination which seeks to achieve seamless and continuous care, tailored to the patients’ needs and based on a holistic view of the patient”. This definition, while useful for thinking about complex disease entities like diabetes, is particularly relevant for patients with multiple co-morbidities for whom severely fragmented care may result if services are not integrated.
We provide three broad levels of healthcare organisation as separate, partial and fully integrated models (see Fig. 1 and Table 1). Separate healthcare sites should work towards increased and more effective communication. Partial integration largely involves collaboration of different programmes in the forms of screening, testing and referral to other services. Full integration, on the other hand, provides “one-stop shopping” to the client, utilizing an integrated team to provide comprehensive screening, testing, treatment and management services for each co-morbidity. The integrated approach affords opportunities for disease screening, case finding and prevention of co-morbid conditions and directly observed therapy. Such treatment collaborations have demonstrated feasibility and cost effectiveness (Coetzee, Hilderbrand, Goemaere, Matthys, & Boelaert, 2004; Currie, Floyd, Williams, & Dye, 2005).
Fig. 1.
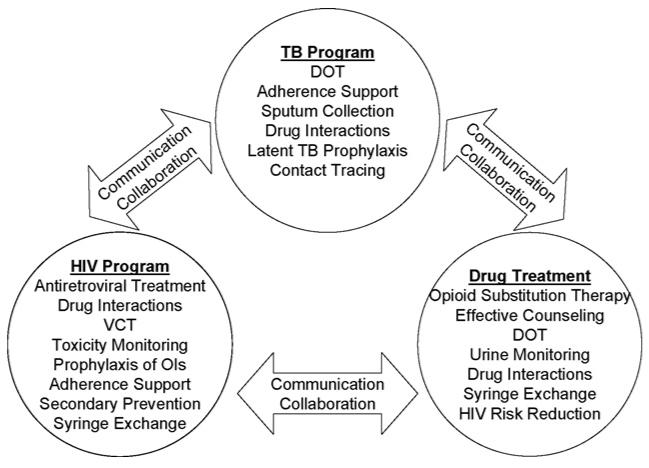
Initial approach towards successful integration.
Table 1.
Moving towards HIV/TB/drug dependency treatment integration
| Separated services | Site integration | Complete integration |
|---|---|---|
| Totally separated services | Where models are co-located physically, but not organically | Models are co-located physically as well as organically |
| Separate services with referral mechanisms in place | Screening on site for co-morbid conditions | Screening on site for co-morbid conditions |
| -Routine HIV/TB testing in drug treatment centres | -Routine HIV/TB testing in drug treatment centres | |
| -Routine HIV/drug dependency screening in TB clinics | -Routine HIV/drug dependency screening in TB clinics | |
| -Routine TB/drug dependency screening in HIV clinics | -Routine TB/drug dependency screening in HIV clinics | |
| Specialty clinics operate separately within same physical location OR share administrative systems/staff | Providers cross-trained in HIV/TB/drug dependency management | |
| Providers cross-trained in HIV/TB/drug dependency management but provide care in their primary specialty area and refer to other co-located programs for management of other conditions | Multiple specialists available on site, working as an integrated team; multi-disciplinary case discussions; shared patient charts and records OR cross-trained provider can manage HIV/TB/drug dependency in their patients with consultation as needed, including: Management of HIV with ARVs TB prophylaxis/treatment Opiate substitution therapy DOT for HIV/TB/opiate substitution provided by same DOT worker |
|
| Memorandums of understanding between programmes to facilitate prioritisation of shared patients |
While the fully integrated approach should be viewed as the ideal situation, it may not be attainable immediately due to funding shortfalls or organisational and political constraints. As such, it should be viewed as a goal to be evolved over time. Below, we provide some key aspects of a fully integrated programme. Note, however, that particular aspects may be implemented piecemeal depending upon local conditions, laws and policies (Fig. 2).
Fig. 2.
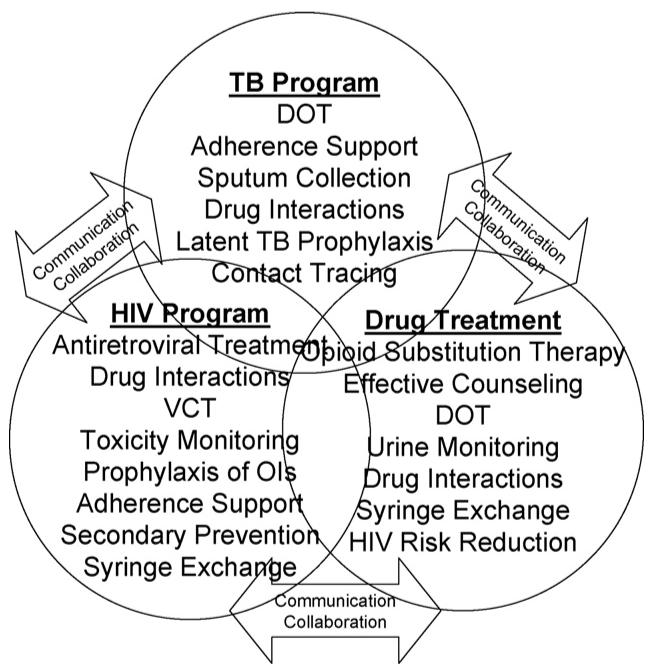
Later approach towards successful integration.
Key aspects of integration programmes
Screening and testing for comorbid conditions
A first step towards integration is to develop screening and testing services for each condition. The most studied programmes in this realm are from providing HIV voluntary counselling and testing (VCT) at TB clinics, VCT and tuberculin skin testing at drug treatment programmes (Altarac & Dansky, 1995), and screening for substance use and tuberculosis (Alaei, Alaei, & Mansouri, 2002; Brassard, Bruneau, Schwartzman, Senecal, & Menzies, 2004) at HIV care sites (Table 2).
Table 2.
Key characteristics of an integration programme
| Characteristic | Rationale | Challenges |
|---|---|---|
| Co-located services | Convenience for client | Coordinating multiple providers |
| Logistical simplification | Space limitations | |
| Monitoring for adverse drug effects (ADEs) | High incidence of ADEs in co-morbid patients treated for multiple diseases | Limited resources |
| Detrimental impact of ADEs on adherence | Lower access to technology | |
| Staff less accustomed to technology | ||
| Cross-training of specialists | Effectively engage specialists in the comprehensive care of patients | Rigidity on the part of staff to new, foreign areas of medicine |
| Each specialist can help in educating each other, building team synergy | Defining roles, setting safe limits, and avoiding “turf wars” | |
| Overcoming stigma providers may hold towards those with “other” condition | ||
| Generalist staff | Likely cost effectiveness benefits | Difficulty mastering multiple complex domains of knowledge |
| Coherence in care | Training and recruitment | |
| Improved patient-provider relations | Establishing expertise limits and maintaining specialist referral link ages |
Both patient- and provider-initiated VCT can be provided at substance use treatment sites (Grusky et al., 2006; Gunn et al., 2005; Lally et al., 2005). There is increasing evidence, however, that opt-out, provider-initiated HIV testing may be more effective at identifying cases of HIV and has been demonstrated to be cost effective (Paltiel et al., 2005; Walensky et al., 2005). In settings where testing facilities are limited, providing HIV screening as well as screening for other infectious diseases (e.g., hepatitis B and C) at health clinics and rehabilitation programmes for drug users is one way to target individuals at greatest risk. To reduce costs and improve acceptability, appropriate use of technologies such as urine or saliva-based HIV testing may be considered (Lee, Lee, Wan, & Wong, 2006).
A major issue with screening drug users for tuberculosis is the high rate of failure to follow-up for readings of tuberculin skin testing. To increase return for tuberculin skin screening among drug users, financial incentives have been used (FitzGerald et al., 1999). Additionally, linkage to other daily treatment programmes can increase uptake. For example, integrating tuberculin skin testing with methadone treatment services can increase uptake because methadone is positively reinforcing. Indeed, integration of screening and chemoprophylaxis for latent tuberculosis at methadone maintenance clinics is likely highly cost effective (Snyder et al., 1999).
Co-location of services
Co-located services are being utilised more extensively as a means towards providing comprehensive care to complex, marginalised patient populations. These are especially useful for IDUs, who have complex preventive, psychosocial and medical needs. Co-location is not simply about physical proximity of distinct services, but rather represents an attempt to integrate the care of patients with multiple co-morbid conditions under the supervision of a single clinical team.
Co-location is substantially complicated by substance use. On the one hand, drug users are less likely to utilise government or other conventional health services. Making these services more favourable towards drug users is crucial if integration is going to be centred on existing conventional HIV or TB services. On the other hand, non-drug-using HIV+ or tuberculosis-infected clients may view drug users unfavourably, making it less likely for communities and providers to accommodate drug services. In some cases, the only way to solve this may be to develop integrated services that cater particularly to drug-using clientele. For example, services could build off of existing drug rehabilitation (Gunn et al., 2005) or syringe exchange programmes (Altice, Springer, Buitrago, Hunt, & Friedland, 2003; Altice, Bruce, Walton, & Buitrago, 2005).
Provision of effective substance use treatment
Provision of pharmacotherapy for substance dependence is an expanding and crucial aspect of providing comprehensive care to patients with multiple co-morbid conditions. Currently, the paradigm for this comes from opioid agonist therapies, notably methadone and buprenorphine. Pharmacotherapies for alcohol exist and are in development for stimulants (e.g., cocaine and methamphetamine). The lessons learned through opioid dependence therapy should be directly transferable. In the US and France, programmes integrating buprenorphine and HIV treatment have been piloted and achieved improved clinical outcomes (Altice et al., 2006; Basu, Smith-Rohrberg, Bruce, & Altice, 2006; Carrieri et al., 2003; Lucas, 2004; Sullivan & Fiellin, 2005; Sullivan, Metzger, Fudala, & Fiellin, 2005). In this issue, there are emerging data from the Ukraine demonstrating successful integration of HIV and buprenorphine treatment services as buprenorphine became publicly available (Bruce, Dvoryak, Sylla, & Altice, 2007).
Providing substance use services in the clinical setting produces additional complexities. Substance misusers can be frustrating to treat, and some medical providers may not wish to engage in substance use management. Many providers who are comfortable managing HIV or TB are not comfortable working with drug users, and may themselves have “moral issues” with those who use drugs.
Drug users tend to be amongst society’s most marginalised individuals, and often are the targets of harassment by police. This problem is particularly severe in Central Asia, and likely contributes to the high prevalence of syringe sharing, which reaches up to 70% in some areas (Hunt, Trace, & Bewley-Taylor, 2005; Klein et al., 2004).
Given the often hostile political climate towards substance use in general and substitution therapy in particular (Dworkin et al., 2005), these academic efforts need to be combined with grassroots and international activism. Lessons learned from the international movements to expand access to primary care and to anti-retroviral medications should be applied in developing the political will to translate research findings into legislative and policy changes.
Enhanced monitoring for adverse events
A major benefit of effective, integrated services providing multiple interventions to individual patients is avoiding adverse clinical events and unfavourable drug-drug interactions. Hepatic side effects are prominent among these. For example, in one study of 372 episodes of culture-confirmed tuberculosis among HIV-infected patients (of whom 44% reported IDU as the mode of HIV transmission), 25% of patients had evidence of hepatic disease, and 31% developed elevations of liver transaminases greater than two times the upper limit of normal during anti-tuberculosis therapy (Bruce, Altice, Gourevitch, & Friedland, 2006; Foisy & Akai, 2004). These topics have been explored elsewhere (Narita, Ashkin, Hollender, & Pitchenik, 1998; Price et al., 2001).
Compounding the complexities of drug-drug interactions is the phenomenon of immune reconstitution syndrome (IRS), which occurs in over 30% of patients who receive both HAART and antituberculosis therapy (Bruce & Altice, 2003). Managing IRS effectively requires clinical astuteness to differentiate from clinical failure or other AIDS-associated conditions.
Laboratory monitoring, particularly of hepatitis, providing dosage adjustments for dealing with drug-drug interactions, and dialoguing with patients about adherence to all regimens, are critical for managing co-morbid conditions and avoiding adverse effects. Integrating services can help to reduce duplication of lab tests and to provide more effective dosing, which may decrease costs. Clinical staff and pharmacists must receive adequate training about drug-drug interactions and managing adverse side effects. Some possible strategies include the use of electronic medication reading devices, point-of-care laboratory diagnostics and standardised clinical screening tools such as the Clinical Opioid Withdrawal Scale.
Cross-training of generalists and specialists
The optimal management of complex patients requires comprehensive cross-training and integration amongst specialists in the fields of HIV, TB and substance use (Abdool-Karim, Abdool-Karim, Friedland, Lalloo, & El-Sadr, 2004). There also remains a need for generalist outreach workers or case managers and clinicians who can manage complex patients. Unless there is one clinician who is responsible for coordinating the overall care of the patient, it is likely that certain conditions will be attributed to the treatment of one of the other co-morbid conditions and not get resolved properly because it is the expectation that someone else must deal with the issue. In an HIV-infected patient with elevated hepatic transaminases, for example, such an adverse effect could easily be attributable to one or more of the agents treating HIV or tuberculosis, to various drug-drug interactions, or to acute or chronic viral hepatitis. Without someone coordinating care, the HIV specialist might wait for the TB specialist to alter the regimen and vice versa and the patient may ultimately go untreated.
There are several important challenges associated with cross-training specialist and generalist providers. Clinicians already overburdened with managing one disease may be reluctant to invest the time to develop clinical proficiency in the other disease or to take-on additional clinical management responsibility. The work culture and philosophy of different disease-based programmes may be vastly different. For example, TB has a well-established standardised system of care, focused on identification and cure of smear-positive using the Directly Observed Therapy-Short Course strategy and public health enforcement measures. In contrast, HIV care tends to be more patient centred.
Evaluation of integration
Effective methodologies for the evaluation of these programmes must be developed. Evaluation can be divided into both programmatic and patient-oriented outcomes, the former dealing with the number and quality of integrated services provided, and the latter assessing the rates of morbidity, mortality and adverse events amongst the different patient populations. Table 3 provides an overview of these key outcomes. It will be critical for integration programmes, as they expand, to prospectively collect these types of outcomes data, both for internal monitoring purposes and for publication and dissemination amongst providers and programme managers worldwide. Without such documentation, it is unlikely that replication efforts will persist.
Table 3.
Key outcomes in assessing the impact of an integration programme
| Programmatic outcomes: screening and testing |
| Number of TB patients offered, HIV VCT, receiving an HIV test, and detected as HIV+ |
| Number of HIV patients screened for TB |
| Number of HIV and TB clients screened for substance abuse |
| Number of patients receiving drug treatment screened for TB |
| Number of patients receiving drug treatment offered, HIV VCT, receiving an HIV test, and detected as HIV+ |
| Programmatic outcomes: treatment |
| Number of HIV-positive clients with LTBI receiving chemotherapy |
| Number of active TB clients with HIV receiving HAART |
| Number of HIV-positive drug users receiving drug treatment counselling |
| Number of HIV-positive drug users receiving substitution pharmacotherapy |
| Number of drug users receiving TB treatment |
| Programmatic outcomes: staff issues |
| Number and percent of providers routinely screening/increasing screening for co-morbid conditions |
| Number and percent of providers participating in cross-training opportunities |
| Providers’ self-reported comfort and satisfaction levels in managing co-morbid conditions |
| Changes in clinic organisational and communication structures |
| Patient-oriented outcomes |
| Urine toxicology positivity rates amongst TB and HIV drug users |
| Drug treatment entry rates amongst TB and HIV drug users |
| Morbidity from TB amongst HIV-positive clients |
| Incidence of ARV-related, TB-related, and substitution pharmacotherapy-related adverse drug events |
| Incidence of adverse drug events specifically related to drug interactions |
| Ancillary medication utilisation for the treatment of side effects |
Model programme: our experience in inner-city America
Integration may be catalysed by generalist providers who realise that, in order to meet the complex needs of their patient population, they need to provide comprehensive services. This has certainly been our experience developing a mobile health care programme in New Haven, CT, USA, where primary health care was made available to IDUs at sites proximate to the local syringe exchange programme.
Initially, VCT, case management and acute care were provided. Upon recognizing the multiple needs of these complex patients, services were expanded to include screening and treatment for HIV/AIDS, sexually transmitted diseases, viral hepatitis, tuberculosis and opioid dependence (Liebman, Pat Lamberti, & Altice, 2002). Treatment for HIV and TB are now supervised as directly administered therapy. Buprenorphine treatment has been integrated into onsite HIV services as well. We have shown in this context that the use of co-located medical and case management services resulted in improved clinical outcomes (Smith-Rohrberg, Mezger, Walton, Bruce, & Altice, 2006). As individual components are added to existing programmes, the expertise of the generalist staff has also grown, and they have become more adept at managing multiple conditions and at dialoguing with specialists.
Conclusions
The case for integration of HIV/AIDS, tuberculosis and substance use treatment services is strong, potentially allowing clinicians and policymakers alike to treat single patients and populations as whole rather than as fragmented parts. Large resources and considerable political will by capable planners will be needed for infrastructure development of treatment integration-friendly national policies. As more data become available about these programmes, refinements and improvements can be made, hopefully producing rigorous and flexible guidelines for how countries and communities can provide comprehensive, integrated care to their most complex and vulnerable patient populations.
Acknowledgements
The authors would like to thank the National Institute on Drug Abuse [K24-DA 017072 (Altice) and K23-DA 022143 (Bruce)] and the Substance Abuse and Mental Health Services Agency [H79 TI 15767 (Altice)] for their funding and continued support.
References
- Abdool-Karim SS, Abdool-Karim Q, Friedland G, Lalloo U, El-Sadr WM. Implementing anti-retroviral therapy in resource-constrained settings: Opportunities and challenges in integrating HIV and tuberculosis care. AIDS. 2004;18(7):975–979. doi: 10.1097/00002030-200404300-00004. [DOI] [PubMed] [Google Scholar]
- Aceijas C, Stimson GV, Hickman M, Rhodes T. Global overview of injecting drug use and HIV infection among injecting drug users. AIDS. 2004;18(17):2295–2303. doi: 10.1097/00002030-200411190-00010. [DOI] [PubMed] [Google Scholar]
- Alaei K, Alaei A, Mansouri D. Reduction of clinical tuberculosis in HIV-infected males with isoniazid prophylaxis. Eastern Mediterranean Health Journal. 2002;8(6):754–757. [PubMed] [Google Scholar]
- Aliyu MH, Salihu HM. Tuberculosis and HIV disease: Two decades of a dual epidemic. Wien Klin Wochenschr. 2003;115(1920):685–697. doi: 10.1007/BF03040884. [DOI] [PubMed] [Google Scholar]
- Altarac D, Dansky SF. Tuberculosis treatment through directly observed therapy in a large multisite methadone maintenance treatment programme: Addressing the public health needs of a high-risk population. Journal of Public Health Management & Practice. 1995;1(4):40–47. [PubMed] [Google Scholar]
- Altice FL, Bruce RD, Walton MR, Buitrago MI. Adherence to hepatitis B virus vaccination at syringe exchange sites. Journal of Urban Health. 2005;82(1):151–161. doi: 10.1093/jurban/jti016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Springer S, Buitrago M, Hunt DP, Friedland GH. Pilot study to enhance HIV care using needle exchange-based health services for out-of-treatment injecting drug users. Journal of Urban Health. 2003;80(3):416–427. doi: 10.1093/jurban/jtg053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Sullivan LE, Smith-Rohrberg D, Basu S, Stancliff S, Eldred L. The potential role of buprenorphine in the treatment of opioid dependence in HIV-infected individuals and in HIV infection prevention. Clinical Infectious Diseases. 2006;43(Suppl 4):S178–S183. doi: 10.1086/508181. [DOI] [PubMed] [Google Scholar]
- Basu S, Smith-Rohrberg D, Bruce RD, Altice FL. Models for integrating buprenorphine therapy into the primary HIV care setting. Clinical Infectious Diseases. 2006;42(5):716–721. doi: 10.1086/500200. [DOI] [PubMed] [Google Scholar]
- Brassard P, Bruneau J, Schwartzman K, Senecal M, Menzies D. Yield of tuberculin screening among injection drug users. International Journal of Tuberculosis and Lung Disease. 2004;8(8):988–993. [PubMed] [Google Scholar]
- Broadhead RS, Heckathorn DD, Altice FL, van Hulst Y, Carbone M, Friedland GH, et al. Increasing drug users’ adherence to HIV treatment: Results of a peer-driven intervention feasibility study. Social Science & Medicine. 2002;55(2):235–246. doi: 10.1016/s0277-9536(01)00167-8. [DOI] [PubMed] [Google Scholar]
- Bruce RD, Altice FL. Editorial comment: Why treat three conditions when it is one patient? AIDS Reader. 2003;13(8):378–379. [PubMed] [Google Scholar]
- Bruce RD, Altice FL, Gourevitch M, Friedland GH. A review of pharmacokinetic drug interactions between opioid agonist therapy and anti-retroviral medications: Implications and management for clinical practice. Journal of Acquired Immune Deficiency Syndromes. 2006;41(5) doi: 10.1097/01.qai.0000219769.89679.ec. [DOI] [PubMed] [Google Scholar]
- Bruce RD, Dvoryak S, Sylla L, Altice FL. HIV treatment access and scale-up for delivery for IDU in Ukraine with opiate substitution therapy with buprenorphine—Programme description and policy implications. International Journal of Drug Policy. 2007;18:326–328. doi: 10.1016/j.drugpo.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri MP, Rey D, Loundou A, Lepeu G, Sobel A, Obadia Y, et al. Evaluation of buprenorphine maintenance treatment in a French cohort of HIV-infected injecting drug users. Drug and Alcohol Dependence. 2003;72(1):13–21. doi: 10.1016/s0376-8716(03)00189-3. [DOI] [PubMed] [Google Scholar]
- Celentano DD, Galai N, Sethi AK, Shah NG, Strathdee SA, Vlahov D, et al. Time to initiating highly active anti-retroviral therapy among HIV-infected injection drug users. AIDS. 2001;15(13):1707–1715. doi: 10.1097/00002030-200109070-00015. [DOI] [PubMed] [Google Scholar]
- Celentano DD, Vlahov D, Cohn S, Shadle VM, Obasanjo O, Moore RD. Self-reported anti-retroviral therapy in injection drug users. JAMA. 1998;280(6):544–546. doi: 10.1001/jama.280.6.544. [DOI] [PubMed] [Google Scholar]
- Coetzee D, Hilderbrand K, Goemaere E, Matthys F, Boelaert M. Integrating tuberculosis and HIV care in the primary care setting in South Africa. Tropical Medicine and International Health. 2004;9(6):A11–A15. doi: 10.1111/j.1365-3156.2004.01259.x. [DOI] [PubMed] [Google Scholar]
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: Global trends and interactions with the HIV epidemic. Archives of Internal Medicine. 2003;163(9):1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- Currie CS, Floyd K, Williams BG, Dye C. Cost, affordability and cost-effectiveness of strategies to control tuberculosis in countries with high HIV prevalence. BMC Public Health. 2005;5:130. doi: 10.1186/1471-2458-5-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin MS, Adams MR, Cohn DL, Davidson AJ, Buskin S, Horwitch C, et al. Factors that complicate the treatment of tuberculosis in HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes. 2005;39(4):464–470. doi: 10.1097/01.qai.0000152400.36723.85. [DOI] [PubMed] [Google Scholar]
- FitzGerald JM, Patrick DM, Strathdee S, Rekart M, Elwood RK, Schecter MT, et al. Vancouver Injection Drug Use Study Group Use of incentives to increase compliance for TB screening in a population of intravenous drug users. International Journal of Tuberculosis and Lung Disease. 1999;3(2):153–155. [PubMed] [Google Scholar]
- Foisy MM, Akai PS. Pharmaceutical care for HIV patients on directly observed therapy. Annals of Pharmacotherapy. 2004;38(4):550–556. doi: 10.1345/aph.1D444. [DOI] [PubMed] [Google Scholar]
- Grusky O, Liu H, Li X, Swanson AN, Duan N, Zhu Y, et al. Is voluntary counselling and testing of drug users in China feasible? International Journal of STD & AIDS. 2006;17(5):354–355. doi: 10.1258/095646206776790196. [DOI] [PubMed] [Google Scholar]
- Gunn RA, Lee MA, Callahan DB, Gonzales P, Murray PJ, Margolis HS. Integrating hepatitis, STD, and HIV services into a drug rehabilitation programme. American Journal of Preventive Medicine. 2005;29(1):27–33. doi: 10.1016/j.amepre.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Hunt N, Trace M, Bewley-Taylor D. Reducing drug related harms to health: Overview of the global evidence. No Four. The Beckley Foundation Drug Policy Programme; 2005. [Google Scholar]
- Klein A, Roberts M, Trace M. Drug policy and the HIV pandemic in Russia and the Ukraine. No Two. The Beckley Foundation Drug Policy Programme; 2004. [Google Scholar]
- Kourbatova EV, Borodulin BE, Borodulina EA, del Rio C, Blumberg HM, Leonard MK., Jr. Risk factors for mortality among adult patients with newly diagnosed tuberculosis in Samara, Russia. International Journal of Tuberculosis and Lung Disease. 2006;10(11):1224–1230. [PubMed] [Google Scholar]
- Lally MA, MacNevin R, Sergie Z, Hitt R, DiSpigno M, Cenedella C, et al. A model to provide comprehensive testing for HIV, viral hepatitis, and sexually transmitted infections at a short-term drug treatment center. AIDS Patient Care STDS. 2005;19(5):298–305. doi: 10.1089/apc.2005.19.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Lee KC, Wan WY, Wong KH. A urine-based approach to scale up HIV testing in drug users. AIDS. 2006;20(9):1349–1351. doi: 10.1097/01.aids.0000232254.12275.fc. [DOI] [PubMed] [Google Scholar]
- Liebman J, Pat Lamberti M, Altice F. Effectiveness of a mobile medical van in providing screening services for STDs and HIV. Public Health Nursing. 2002;19(5):345–353. doi: 10.1046/j.1525-1446.2002.19504.x. [DOI] [PubMed] [Google Scholar]
- Loughlin A, Metsch L, Gardner L, Anderson-Mahoney P, Barrigan M, Strathdee S. Provider barriers to prescribing HAART to medically-eligible HIV-infected drug users. AIDS Care. 2004;16(4):485–500. doi: 10.1080/09540120410001683411. [DOI] [PubMed] [Google Scholar]
- Lucas GM. Buprenorphine in primary HIV care clinics: A big pill to swallow. Hopkins HIV Report. 2004;16(4):5–7. [PubMed] [Google Scholar]
- Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. Journal of Acquired Immune Deficiency Syndromes. 2001;27(3):251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- McShane H. Co-infection with HIV and TB: Double trouble. International Journal of STD & AIDS. 2005;16(2):95–100. doi: 10.1258/0956462053057576. 101 quiz. [DOI] [PubMed] [Google Scholar]
- Moore RD, Keruly JC, Chaisson RE. Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. Journal of Acquired Immune Deficiency Syndromes. 2004;35(1):46–51. doi: 10.1097/00126334-200401010-00006. [DOI] [PubMed] [Google Scholar]
- Mosam A, Cassol E, Page T, Bodasing U, Cassol S, Dawood H, et al. Generic anti-retroviral efficacy in AIDS-associated Kaposi’s sarcoma in sub-Saharan Africa. AIDS. 2005;19(4):441–443. doi: 10.1097/01.aids.0000161775.36652.85. [DOI] [PubMed] [Google Scholar]
- Mur-Veeman I, Hardy B, Steenbergen M, Wistow G. Development of integrated care in England and the Netherlands: Managing across public-private boundaries. Health Policy. 2003;65(3):227–241. doi: 10.1016/s0168-8510(02)00215-4. [DOI] [PubMed] [Google Scholar]
- Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following anti-retroviral therapy in patients with AIDS. American Journal of Respiratory and Critical Care Medicine. 1998;158(1):157–161. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- Nunn P, Williams B, Floyd K, Dye C, Elzinga G, Raviglione M. Tuberculosis control in the era of HIV. Nature Reviews Immunology. 2005;5(10):819–826. doi: 10.1038/nri1704. [DOI] [PubMed] [Google Scholar]
- Ouellet D, Hsu A, Qian J, Lamm JE, Cavanaugh JH, Leonard JM, et al. Effect of fluoxetine on pharmacokinetics of ritonavir. Antimicrobial Agents and Chemotherapy. 1998;42(12):3107–3112. doi: 10.1128/aac.42.12.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltiel AD, Weinstein MC, Kimmel AD, Seage GR, 3rd, Losina E, Zhang H, et al. Expanded screening for HIV in the United States—An analysis of cost-effectiveness. The New England Journal of Medicine. 2005;352(6):586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- Price P, Mathiot N, Krueger R, Stone S, Keane NM, French MA. Immune dysfunction and immune restoration disease in HIV patients given highly active anti-retroviral therapy. Journal of Clinical Virology. 2001;22(3):279–287. doi: 10.1016/s1386-6532(01)00200-1. [DOI] [PubMed] [Google Scholar]
- Rompalo AM, Shah N, Mayer K, Schuman P, Klein RS, Smith DK, et al. Influence of injection drug use behavior on reported anti-retroviral therapy use among women in the HIV epidemiology research study: On-site versus referral care. Journal of Acquired Immune Deficiency Syndromes. 2001;28(1):28–34. doi: 10.1097/00042560-200109010-00005. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Mohan A, Kadhiravan T. HIV-TB co-infection: Epidemiology, diagnosis & management. The Indian Journal of Medical Research. 2005;121(4):550–567. [PubMed] [Google Scholar]
- Smith-Rohrberg D, Mezger J, Walton M, Bruce RD, Altice FL. Impact of enhanced services on virologic outcomes in a directly administered anti-retroviral therapy trial for HIV-infected drug users. Journal of Acquired Immune Deficiency Syndromes. 2006;43(1 Suppl):S48–S53. doi: 10.1097/01.qai.0000248338.74943.85. [DOI] [PubMed] [Google Scholar]
- Snyder DC, Paz EA, Mohle-Boetani JC, Fallstad R, Black RL, Chin DP. Tuberculosis prevention in methadone maintenance clinics. Effectiveness and cost-effectiveness. American Journal of Respiratory and Critical Care Medicine. 1999;160(1):178–185. doi: 10.1164/ajrccm.160.1.9810082. [DOI] [PubMed] [Google Scholar]
- Solomon S, Chakraborty A, Yepthomi RD. A review of the HIV epidemic in India. AIDS Education and Prevention: Official Publication of the International Society for AIDS Education. 2004;16(3 Suppl A):155–169. doi: 10.1521/aeap.16.3.5.155.35534. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Palepu A, Cornelisse PG, Yip B, O’Shaughnessy MV, Montaner JS, et al. Barriers to use of free anti-retroviral therapy in injection drug users. JAMA. 1998;280(6):547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Fiellin DA. Buprenorphine: Its role in preventing HIV transmission and improving the care of HIV-infected patients with opioid dependence. Clinical Infectious Diseases. 2005;41(6):891–896. doi: 10.1086/432888. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Metzger DS, Fudala PJ, Fiellin DA. Decreasing international HIV transmission: The role of expanding access to opioid agonist therapies for injection drug users. Addiction. 2005;100(2):150–158. doi: 10.1111/j.1360-0443.2004.00963.x. [DOI] [PubMed] [Google Scholar]
- Waldrop-Valverde D, Ownby RL, Wilkie FL, Mack A, Kumar M, Metsch L. Neurocognitive aspects of medication adherence in HIV-positive injecting drug users. AIDS Behavior. 2006 doi: 10.1007/s10461-005-9062-6. [DOI] [PubMed] [Google Scholar]
- Walensky RP, Weinstein MC, Kimmel AD, Seage GR, 3rd, Losina E, Sax PE, et al. Routine human immunodeficiency virus testing: An economic evaluation of current guidelines. The American Journal of Medicine. 2005;118(3):292–300. doi: 10.1016/j.amjmed.2004.07.055. [DOI] [PubMed] [Google Scholar]
- World Health Organization, United Nations Office on Drugs and Crime . Policy brief: Anti-retroviral therapy and injecting drug users. Report No. WHO/HIV/2005.06. World Health Organization; Geneva: 2005. [Google Scholar]


