Abstract
Background
Radiosurgery is a special treatment method that employs highly focused radiation to destroy tumors with high precision in a single session. A broad base of scientific evidence already exists for the radiosurgical treatment of brain metastases. Recent advances in medical technology now allow radiosurgery to be extended to the spine as well.
Methods
Selective literature review based on a PubMed search using the search terms stereotaxis, radiosurgery, stereotactic radiotherapy, accuracy, quality assurance, spine, spine metastasis, pain, Novalis, CyberKnife, Synergy, and robotics. We also present and analyze our own data as an illustration of the application of spinal radiosurgery.
Results
The literature search identified 20 scientific original publications and one recent review. The data indicate that, within the specific constraints of the method, radiosurgery can arrest the growth of up to 96% of spinal metastases. Durable pain relief can be achieved in patients with tumor-associated pain syndromes. The morbidity of spinal radiosurgery is low, with a less than 1% risk of myelopathy.
Conclusions
Spinal radiosurgery is an independent, essentially noninvasive method of treatment. Different types of radiosurgical treatment apparatus are available. For properly selected patients, radiosurgery offers a good chance of therapeutic success with relatively rare complications.
Keywords: radiation therapy, cancer treatment, medical technology, surgery, quality of life
The term "radiosurgery" refers to a therapeutic concept originally described in 1951 by Lars Leksell, a Swedish neurosurgeon (1). Although radiosurgery was first proposed for the treatment of functional disorders of the central nervous system, its major area of use shifted to neuro-oncology in the 1980s. The shift became definitive with the initial description of radiosurgery for brain metastases by Volker Sturm and his colleagues in Germany in 1987 (2).
In radiosurgery, as it is classically defined, the growth of malignant tumors is arrested with a single, high dose of focused ionizing radiation. In this article, we will use the term "radiosurgery" in accordance with this definition, even though its use has been extended recently to cover treatments in up to five divided doses (fractions) of radiation (3, 4). Fractionated stereotactic radiotherapy is another related technique. Fractionation is defined as the division of the overall dose into multiple individual doses, which are usually given once per day. In so-called hypofractionation, the single daily fractional dose is greater than 2 Gray (Gy) (e1). Hypofractionation shortens the treatment time compared to conventional fractionated radiotherapy with the same overall radiation dose. Thus, with respect to dose fractionation, radiosurgery represents the limiting case of a treatment in which the overall dose and the fractional dose are the same, and the time needed for treatment is the shortest. Radiosurgery began as a specialized method within neurosurgery; it now belongs to radiation oncology as well. In the German-speaking countries, it is also known by the synonymous term "stereotaktische Einzeitkonvergenzbestrahlung" (stereotactic single-session convergent beam irradiation) (e2).
Until just a few years ago, radiosurgery could only be used to treat pathological lesions in the brain and skull. In this region of the body, the target structures bear a fixed topographical relationship to the cranial bone, and this fact is exploited when a so-called stereotactic ring or frame is rigidly applied to the patient’s head. Stereotaxy enables target localization in a coordinate system. Only radiotherapeutic techniques that employ stereotactic target localization can be called radiosurgery or stereotactic radiotherapy. Ever since the introduction of sectional digital imaging (computerized tomography, CT; magnetic resonance imaging, MRI), these techniques have been used to define the target volumes for radiosurgery.
Persuasive scientific evidence (level 2 evidence) has now accumulated to demonstrate the benefit of radiosurgery for brain metastases (5, 6). The resulting clinical attractiveness of the method has led to the application of the radiosurgery concept extracranially as well, particularly in the spine. As early as 1969, E. Hitchcock described a prototype device for spinal radiosurgery (7), yet this, like other early innovations, did not become established in clinical practice. It was nonetheless possible to prove the principle of spinal radiosurgery in initial, small series of spinal malignant tumors (e3, e4). In this early phase of development of spinal radiosurgical technology, the spinal segment to be treated had to be invasively immobilized so that the necessary spatial precision could be attained. Other means of fixation, such as body frames or casts of plaster or plastic, generally cannot achieve the system precision of 1 mm that is required in radiosurgery; they can, however, be used for fractionated stereotactic radiotherapy (e5). Later, so-called fiducials were developed, i.e., small metal markers or screws that are usually implanted with a minimally invasive technique (8, 9). These markers can be localized on orthogonal plain x-ray images and then used for target localization. The most recent development for precise target localization in spinal radiosurgery is a software program that recognizes skeletal structures and uses them for targeting (10, 11). With this latest improvement, it is often no longer necessary to affix markers to the patient’s body, and thus spinal radiosurgery has become, in principle, a fully noninvasive therapeutic procedure.
Various different commercially available systems (4, 8, 12) and individually designed systems in large institutes and clinics (4) are now available for spinal radiosurgery. For a detailed discussion of these technologies, the reader is referred to the current review by Sahgal and colleagues (4), and to the primary literature cited in this article. All of these systems consist of medical high technology in the midst of continuous, rapid development. Thus, the authors will here simply state some of the basic principles of spinal radiosurgery and describe the current state of the art of radiosurgery for malignant spinal tumors, on the basis of the major publications in the literature as well as our own experience.
Basic principles of spinal radiosurgery
In spinal radiosurgery, a radiation dose with relatively high biological effectiveness (4) is delivered from outside the body to a vertebral metastasis, either in a single fraction or in a small number of fractions (3, 4). The convergence of the radiation beams concentrates the therapeutic radiation dose in the tumor; it is achieved in different ways, depending on the particular device used (figure 1). The main quality criteria for a radiosurgical treatment are the precision with which the radiation dose is delivered into the tumor and the quality of the dose concentration. Less important parameters are the homogeneity of the dose distribution and the degree of "coverage" of the target. In all cases, the dose distribution must be conformal, i.e., it must follow the three-dimensional shape of the tumor to be treated, as defined on the CT and MR images (figure 2). The precision of targeting in spinal radiosurgery should not be much higher than 1 millimeter. The figures that have been determined by various methods for the individual radiosurgical systems currently in use vary from 0.5 to 3 millimeters (4, 10, 11, 12). Good dose concentration results in a steep fall-off of the dose at the interface between the tumor and the surrounding healthy tissue (dose gradient). The most important organ at risk in spinal radiosurgery is the spinal cord (20). The dose gradient may be asymmetrical if necessary, being particularly steep in the region of the spinal cord, for example. The fact that the size of the tumor—in addition to the applied dose and the quality of the treatment plan—is a major determinant of the steepness of the dose gradient explains why radiosurgery cannot be used to treat tumors that are too large (figure 3). Only tumors measuring about 50 cm3 or less can be treated radiosurgically.
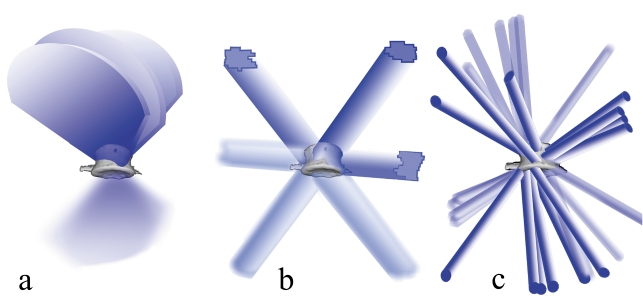
Figure 1.
(a) Radiation over segments of a circle in radiotherapy with a moving source. Three arc segments are schematically depicted; in practice, 9 to 15 arc segments are usually used.
(b) Radiation with stationary fields. The diagram shows three stationary fields shaped by a multileaf collimator. In practice, 7 to 13 stationary fields with multileaf collimation are usually used. Figures 1(a) and 1(b) correspond to treatment with gantry-based systems such as Novalis and Synergy.
(c) Radiation with multiple microstationary fields according to the CyberKnife concept. The diagram shows 14 such fields, shaped by a cylindric collimator. In practice, 150 to 350 fields of this type are usually used.
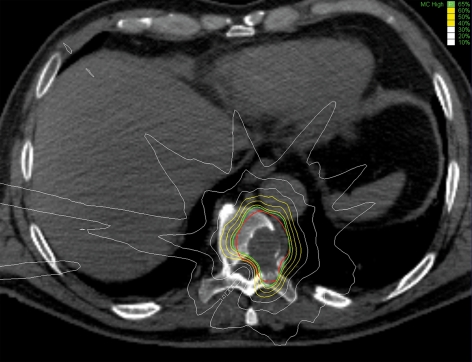
Figure 2.
Axial CT section through a 10th thoracic vertebra containing a metastasis of undifferentiated sarcoma. CyberKnife radiosurgery was performed; the target was defined as the involved area of the skeletal structures. The red line indicates the target volume. The radiosurgical dose was 35 Gy in the center of the tumor and 22.8 Gy at the 65% isodose line (green line). The 60%, 50%, and 40% isodose lines are shown in yellow, while the 30%, 20%, and 10% isodose lines are shown in white. The dose distribution was calculated with a special planning algorithm (inverse planning principle, Monte Carlo simulation). This method yields not only the dose within the tumor, but also a protective value for the spinal cord. The low water content of pulmonary tissue, through which part of the applied radiation passes, was also taken into account.
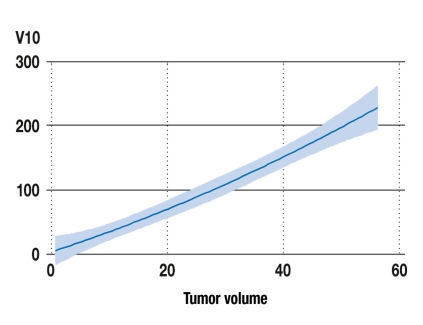
Figure 3.
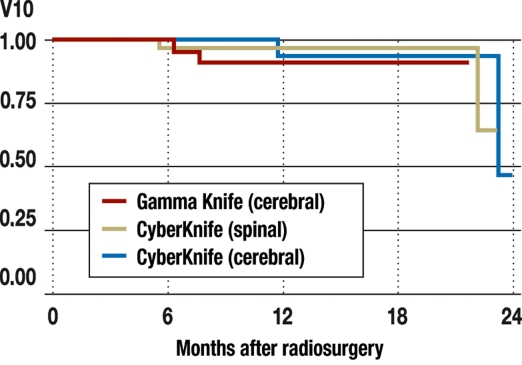
The radiation exposure of normal tissue as a function of tumor volume. The parameter V10 designates the volume of tissue (in cm3) that is exposed to a dose of 10 Gy or higher. The diagram is based on data from 70 patients with solitary spinal metastases who were treated radiosurgically with the CyberKnife. The tumor volume is the volume (in cm3) of the spinal metastases treated. The blue line represents a polynomial fitting function of these two parameters; the shaded area corresponds to the 95% confidence interval.
In addition to these basic principles and quality criteria, the various technologies now available for spinal radiosurgery have some other features in common:
They can all be considered types of "image-guided (high-precision) radiotherapy," or IGRT
They use specially designed linear accelerators for dose application
The target structures and the structures at risk are localized stereotactically in a coordinate system.
The devices differ from one another mainly in the mode of use of the linear accelerator, the beam-shaping (collimation) system, the patient-positioning system, and the method of checking position during the treatment (4). In gantry-based linear accelerators, the radiation head of the linear accelerator rotates along a fixed, circular path, whose central axis intersects with the axis around which the patient can be turned. The point of intersection is called the isocenter, and procedures carried out in this way are called isocentric (figure 1b). Nonisocentric treatments can be performed with arc radiation (figure 1a), or with free movement of the linear accelerator through space at the end of a robotized arm (8, 11) (figure 1c). All systems use round-aperture collimators with a fixed opening for beam-shaping. Iris collimators (8) and multileaf collimators (12) have variable openings; the latter permit irregular shaping of the field that is to be radiated (figure 1b). The various patient-positioning and fixation systems range from vacuum mattresses to special stereotactic body frames. Patients treated with the CyberKnife can, in principle, be freely positioned and treated without any fixation at all, because this technology includes rapid positional control and correction in real time, while the treatment fraction is being delivered. The Novalis system, too, includes a stereoscopic x-ray device for positional control before delivery of the treatment fraction (4); during delivery of the fraction, the position is controlled with an optical system that recognizes infrared markers attached to the patient’s skin (4). In other radiosurgery systems, the x-ray or CT devices that are installed as a fixed component of the system can be used to check the position of the tumor before each treatment fraction, but not during the fraction (4). The CyberKnife automatically corrects for the patient’s frequent, small movements with corresponding movements of the robotized arm that bears the linear accelerator. Large movements are compensated for by repositioning of the patient table. In gantry-based systems, positioning is performed by movement of the patient table.
Clinical application
Radiosurgery can be used to supplement or even replace conventional treatment methods such as surgery and radiotherapy, not only in the brain and skull, but also in the spine. In principle, an ambulatory and noninvasive radiosurgical treatment can always be assumed to be advantageous when the imaging studies reveal a small, well-demarcated tumor posing a significant threat to the patient’s health. This aspect limits the number of tumors that can be reasonably treated radiosurgically in a single patient. Generalized metastatic involvement of the axial skeleton is certainly not an indication for radiosurgery, but radiosurgical treatment may well be reasonable for one or two metastatic tumors in the spine. In such situations, single-dose radiation with a steep dose fall-off outside the tumor is more effective, and less fraught with complications, than fractionated radiotherapy (4). Furthermore, radiosurgery requires only a few hours, much less time than fractionated radiotherapy, and can, in principle, always be performed on an outpatient basis. This may be an advantage for patients with advanced malignant disease, who often require multimodal treatment. Concomitantly administered treatments need not be deferred till later, and the patients’ time out of treatment becomes larger. With respect to open surgical procedures, radiosurgery cannot be considered an alternative in emergency situations, or when debulking or stabilization is necessary. Both of the latter techniques, however, can be advantageously supplemented by radiosurgery (18, 19). Radiosurgery can be used to sterilize a vertebral body before vertebroplasty is performed to stabilize the affected segment (23). A combined treatment of this type is also possible with debulking and stabilizing procedures that involve metal osteosynthetic implants, but, in this situation, particular attention must be paid to the temporal sequence of the treatment steps. Experience shows that metal implants do not interfere with the positional control and targeting of skeletal structures during radiosurgical treatment (11), but the artifacts that they produce in CT and MRI scans may render the target volume impossible to define. In such situations, therefore, it is generally better to perform radiosurgery before the stabilizing operation.
Spinal radiosurgery is most often used to treat the following types of malignant tumor: metastases of renal cell carcinoma, carcinomas of the breast, lung, colon, and prostate gland, spinal plasmacytoma, and primary and secondary sarcomatous tumors of various types. The CyberKnife can be used for the radiosurgical treatment of tumors of these types in the entire spine and below it down to the pelvic ring.
For these types of malignant tumor in the spine, the results of radiosurgery are relatively uniform, varying only to a small degree from one type of primary tumor to another. Tumor growth can be effectively arrested by radiosurgery in up to 96% of cases (81% to 96%) (4, 13– 19, 21) (figures 4a and b, table 1). Radiosurgery can also be used to treat tumors that have recurred after fractionated radiotherapy (4) and surgery (4, 18, 23). With regard to recurrent spinal metastases after radiosurgery, the following observations have been made: recurrent tumors only rarely arise in neighboring vertebrae (4, 24); they most commonly arise in the epidural space and the posterior elements of the vertebrae (pedicles, laminae) (4, 13); after the treatment of large metastases, recurrences tend to arise at the edge of the treated area (4). These facts have led to the proposals, which the authors also support, that radiosurgery for spinal metastases should be limited to those no larger than about 50 cm3, and that the vertebral pedicles should be included in the radiation field whenever appropriate. The radiosurgical treatment of spinal metastases with epidural spread is problematic because of the high risk of recurrence and the danger of spinal cord injury (20). The authors consider radiosurgery to be contraindicated if such metastases compress the spinal cord or are disseminated in the epidural space (epidural carcinomatosis). The risk of side effects from spinal CyberKnife radiosurgery is low. Only a few patients react with nausea or vomiting. Rare cases of mucositis, dysphagia, diarrhea, and similar symptoms have been reported (4). The risk of spinal cord toxicity is less than 1% according to the published literature, as well as in the authors’ own experience (4, 20). No cases of osteonecrosis have been observed to date.
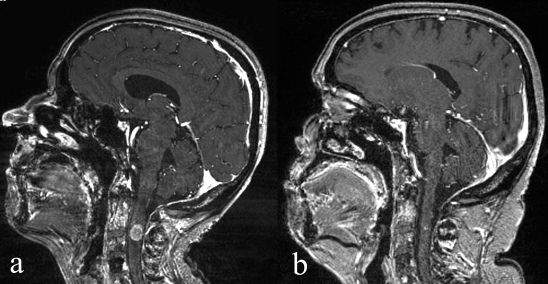
Figure 4.
(a) Intramedullary metastasis of breast carcinoma at the C2 vertebral level, causing incipient quadriparesis.
(b) Complete remission of the clinical manifestations and subtotal radiological remission of the metastasis upon follow-up 4 weeks after treatment. (Contrast-enhanced sagittal MR images.)
Table 1. Summary of treatment results with CyberKnife radiosurgery for spinal metastases in the largest clinical series published to date (15).
| Long-term improvement of pain | |
| Overall group (n=294) | 86% |
| Renal cell carcinoma | 94% |
| Breast carcinoma | 96% |
| Bronchial carcinoma | 93% |
| Malignant melanoma | 96% |
| Long-term radiological tumor control | |
| Overall group (n=294) | 88% |
| Renal cell carcinoma | 87% |
| Breast carcinoma | 100% |
| Bronchial carcinoma | 100% |
| Malignant melanoma | 75% |
Moreover, the analgesic effect of spinal radiosurgery merits special mention. It has long been recognized that a single dose of radiation can relieve the pain caused by a malignant tumor in the spine (25). The magnitude of the single dose that can be delivered in conventional radiotherapy, however, is limited by the radiosensitivity of the spinal cord to such a degree that a single dose cannot achieve lasting tumor control. Spinal radiosurgery is a better therapeutic option in this situation, in that it not only provides very effective analgesia, but also continues to exert this effect for a markedly longer time, as it also devitalizes the tumor (4, 15, 18, 19) (table 1). The affected spinal nerve roots show a surprisingly good tolerance to the radiosurgical dose. It must, nonetheless, be said that the individual published studies of spinal radiosurgery vary widely in their methodological details, and it is therefore difficult to draw any generally applicable conclusions from them. The scientific level of evidence of the currently available studies on spinal radiosurgery is no higher than level 3 (demonstration of efficacy in noncontrolled, but methodologically well-designed studies). Yet it is clear that the efficacy of radiosurgery for spinal metastases is comparable to its efficacy for brain metastases (5, 6) (figures 4–6, table 2). The indication for spinal radiosurgery should be determined by an interdisciplinary consensus, and randomized, controlled studies would certainly be desirable, so that the value of this particular method in the repertoire of treatments for spinal metastases can be conclusively demonstrated.
Figure 6.
Local tumor control rate of radiosurgery of solitary cerebral and spinal metastases. The brain metastases were treated radiosurgically with either the Leksell Gamma Knife or the CyberKnife, while the spinal metastases were treated with the CyberKnife. Kaplan-Meier curves for recurrence-free survival are shown. The recurrence-free survival 18 months after treatment was 94% to 96% in all three groups. (No significant differences between groups were found with the Cox proportional hazard model.)
Table 2. Comparison of tumor size (cm3), minimal tumor dose (Gy), and tissue exposure to radiation (V10; cm3) for singular cerebral and spinal metastases treated with the Gamma Knife or the CyberKnife.
| Technique | Organ | n | Volume | Dose | V10 |
| Units | cm3 | Gy | cm3 | ||
| Gamma Knife | Brain | 57 | 3.6 ± 3.7 | 19.3 ± 2.2 | 9.5 ± 8.6 |
| CyberKnife | Brain | 57 | 3.6 ± 3.5 | 18.4 ± 1.2 | 7.3 ± 7.4 |
| CyberKnife | Spine | 70 | 21.3 ± 16.0 | 19.0 ± 2.0 | 81.1 ± 74.3 |
n, number of patients with solitary metastases; volume, volume of the radiosurgically treated metastases (mean ± standard deviation, cm3); dose, minimal tumor dose (mean ± standard deviation, Gy); V10, volume of tissue outside the tumor exposed to a radiation dose of 10 Gy or more, a measure of the dose concentration in the tumor and the exposure of normal tissue. The selection of brain metastases was representative for the Munich overall patient population. The patients with brain metastases who were treated with either the Gamma Knife or the CyberKnife were matched pairwise with respect to age, tumor size, functional status, and type of primary tumor. The spinal metastases on which data are presented here were all of the singular spinal metastases treated in Munich in the first two years of operation of our CyberKnife radiosurgery center. The brain metastases were considerably smaller than the spinal metastases. The radiosurgical dose level was comparable for all three groups. V10 depends on the size of the tumor and thus tends to be larger for spinal tumors.
Conclusion
Spinal radiosurgery is now available as a noninvasive, ambulatory method with few side effects for the treatment of selected malignant tumors of the spine, spinal cord, spinal nerve roots, and pelvic skeleton. It has a wide therapeutic range. It can permanently eliminate tumor-associated pain, thereby ameliorating the quality of life of patients suffering from advanced malignant disease (14). The fiducial-free CyberKnife technology occupies a highly competitive position among current systems for spinal radiosurgery. The scientific evidence for spinal radiosurgery is now compelling so that it can be expected, in future, to be as well established in clinical practice as classical radiosurgery for brain metastases has already become.
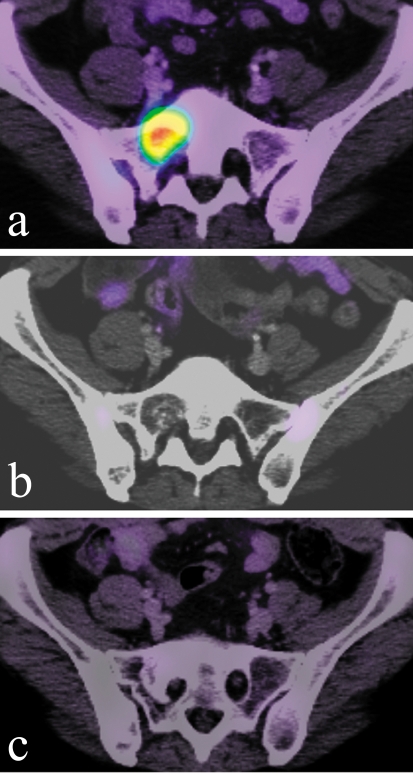
Figure 5.
(a) Metastasis of breast carcinoma in the right side of the sacrum causing radicular pain.
(b) Complete remission of clinical manifestations and radiological remission of the metastasis as seen in a follow-up study 3 months after treatment.
(c) Another follow-up study 11 months after treatment shows recalcification of the area that originally harbored the metastasis. (PET-CT images in (a), (b), and (c).)
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Berndt Wowra is a member of the Clinical Advisory Board of Accuray Inc., Sunnyvale, CA, USA, and receives reimbursement of travel expenses in this capacity. Alexander Muacevic is a member of the Board of Directors of the CyberKnife Society and receives reimbursement of travel expenses in this capacity. Stefan Zausinger and Jörg-Christian Tonn declare that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316–319. [PubMed] [Google Scholar]
- 2.Sturm V, Kober B, Hover KH, et al. Stereotactic percutaneous single dose irradiation of brain metastases with a linear accelerator. Int J Radiat Oncol Biol Phys. 1987;13:279–282. doi: 10.1016/0360-3016(87)90140-4. [DOI] [PubMed] [Google Scholar]
- 3.Barnett GH, Linskey ME, Adler JR, et al. Stereotactic radiosurgery—an organized neurosurgery-sanctioned definition. J Neurosurg. 2007;106:1–5. doi: 10.3171/jns.2007.106.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: a critical review. Int J Radiat Oncol Biol Phys. 2008;71:652–665. doi: 10.1016/j.ijrobp.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 5.Muacevic A, Wowra B, Siefert A, Tonn JC, Steiger HJ, Kreth FW. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. 2008;87:299–307. doi: 10.1007/s11060-007-9510-4. [DOI] [PubMed] [Google Scholar]
- 6.Smith ML, Lee JY. Stereotactic radiosurgery in the management of brain metastasis. Neurosurg Focus. 2007;22 E5:1–8. doi: 10.3171/foc.2007.22.3.6. [DOI] [PubMed] [Google Scholar]
- 7.Hitchcock E. An apparatus for stereotactic spinal surgery. Lancet. 1969;1:705–706. doi: 10.1016/s0140-6736(69)92653-1. [DOI] [PubMed] [Google Scholar]
- 8.Adler JR, Jr, Chang SD, Murphy MJ, Doty J, Geis P, Hancock SL. The Cyberknife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg. 1997;69:124–128. doi: 10.1159/000099863. [DOI] [PubMed] [Google Scholar]
- 9.Yu C, Main W, Taylor D, Kuduvalli G, Apuzzo ML, Adler JR., Jr An anthropomorphic phantom study of the accuracy of Cyberknife spinal radiosurgery. Neurosurgery. 2004;55:1138–1149. doi: 10.1227/01.neu.0000141080.54647.11. [DOI] [PubMed] [Google Scholar]
- 10.Ho AK, Fu D, Cotrutz C, et al. A study of the accuracy of Cyberknife spinal radiosurgery using skeletal structure tracking. Neurosurgery. 2007;60:147–156. doi: 10.1227/01.NEU.0000249248.55923.EC. [DOI] [PubMed] [Google Scholar]
- 11.Muacevic A, Staehler M, Drexler C, Wowra B, Reiser M, Tonn JC. Technical description, phantom accuracy, and clinical feasibility for fiducial-free frameless real-time image-guided spinal radiosurgery. J Neurosurg Spine. 2006;5:303–312. doi: 10.3171/spi.2006.5.4.303. [DOI] [PubMed] [Google Scholar]
- 12.Teh BS, Paulino AC, Lu HH, et al. Versatility of the Novalis system to deliver image-guided stereotactic body radiation therapy (SBRT) for various anatomical sites. Technol Cancer Res Treat. 2007;6:347–354. doi: 10.1177/153303460700600412. [DOI] [PubMed] [Google Scholar]
- 13.Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007:151–160. doi: 10.3171/SPI-07/08/151. [DOI] [PubMed] [Google Scholar]
- 14.Degen JW, Gagnon GJ, Voyadzis JM, et al. CyberKnife stereotactic radiosurgical treatment of spinal tumors for pain control and quality of life. J Neurosurg Spine. 2005;2:540–549. doi: 10.3171/spi.2005.2.5.0540. [DOI] [PubMed] [Google Scholar]
- 15.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32:193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs IC, Kamnerdsupaphon P, Ryu MR, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82:185–190. doi: 10.1016/j.radonc.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Jin JY, Ryu S, Rock J, et al. Evaluation of residual patient position variation for spinal radiosurgery using the Novalis image guided system. Med Phys. 2008;35:1087–1093. doi: 10.1118/1.2839097. [DOI] [PubMed] [Google Scholar]
- 18.Rock JP, Ryu S, Shukairy MS, et al. Postoperative radiosurgery for malignant spinal tumors. Neurosurgery. 2006;58:891–898. doi: 10.1227/01.NEU.0000209913.72761.4F. [DOI] [PubMed] [Google Scholar]
- 19.Ryu S, Fang Yin F, Rock J, et al. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97:2013–2018. doi: 10.1002/cncr.11296. [DOI] [PubMed] [Google Scholar]
- 20.Ryu SI, Jin JY, Jin R, et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109:628–636. doi: 10.1002/cncr.22442. [DOI] [PubMed] [Google Scholar]
- 21.Yamada Y, Lovelock DM, Yenice KM, et al. Multifractionated image-guided and stereotactic intensity-modulated radiotherapy of paraspinal tumors: a preliminary report. Int J Radiat Oncol Biol Phys. 2005;62:53–61. doi: 10.1016/j.ijrobp.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Yan H, Yin FF, Kim JH. A phantom study on the positioning accuracy of the Novalis Body system. Med Phys. 2003;30:3052–3060. doi: 10.1118/1.1626122. [DOI] [PubMed] [Google Scholar]
- 23.Gerszten PC, Germanwala A, Burton SA, Welch WC, Ozhasoglu C, Vogel WJ. Combination kyphoplasty and spinal radiosurgery: a new treatment paradigm for pathological fractures. Neurosurg Focus. 2005;18 doi: 10.3171/foc.2005.18.3.9. [DOI] [PubMed] [Google Scholar]
- 24.Ryu S, Rock J, Rosenblum M, Kim JH. Patterns of failure after single-dose radiosurgery for spinal metastasis. J Neurosurg. 2004;101(Suppl 3):402–405. [PubMed] [Google Scholar]
- 25.Wu JS, Wong R, Johnston M, Bezjak A, Whelan T. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys. 2003;55:594–605. doi: 10.1016/s0360-3016(02)04147-0. [DOI] [PubMed] [Google Scholar]
- e1.Baumann M, Molls M. Die „4Rs“ der Strahlentherapie und prädiktive Tests. In: Bamberg M, Molls M, Sack H, editors. Radioonkologie. Grundlagen. Germering: W. Zuckschwerdt Verlag; 2003. pp. 220–228. [Google Scholar]
- e2.Engenhart R, Wowra B, Wannenmacher M. Stellenwert der stereotaktischen Einzeitbestrahlung bei der Therapie benigner und maligner Hirnprozesse. Jahrbuch der Radiologie. 1993:141–157. [Google Scholar]
- e3.Hamilton AJ, Lulu BA, Fosmire H, Gossett L. LINAC-based spinal stereotactic radiosurgery. Stereotact Funct Neurosurg. 1996;66:1–9. doi: 10.1159/000099658. [DOI] [PubMed] [Google Scholar]
- e4.Takacs II, Hamilton AJ, Lulu B, et al. Frame based stereotactic spinal radiosurgery: experience from the first 19 patients treated. Stereotact Funct Neurosurg. 1999;73 doi: 10.1159/000029755. [DOI] [PubMed] [Google Scholar]
- e5.Lohr F, Debus J, Frank C, et al. Noninvasive patient fixation for extracranial stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 1999;45:521–527. doi: 10.1016/s0360-3016(99)00190-x. [DOI] [PubMed] [Google Scholar]