Abstract
Objective:
Little is known about the influence of race and ethnicity on mortality from acute lung injury. We sought to determine whether black race or Hispanic ethnicity are independently associated with mortality among patients with acute lung injury.
Design:
Retrospective cohort study of patients enrolled in the Acute Respiratory Distress Syndrome (ARDS) Network randomized controlled trials.
Setting:
Adult intensive care units participating in the ARDS Network trials.
Patients:
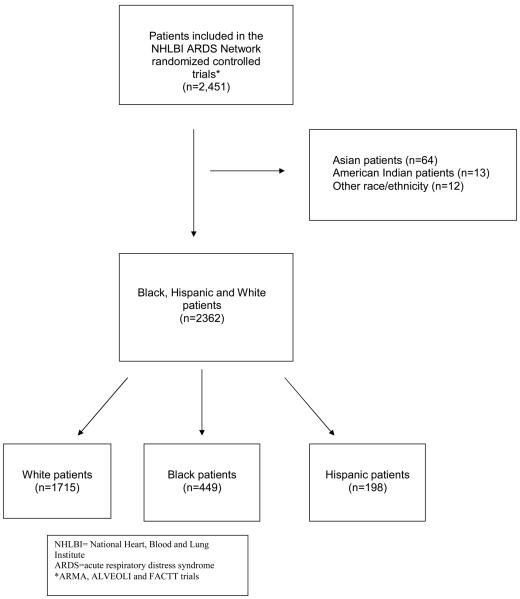
2362 mechanically ventilated patients (1,715 white, 449 black and 198 Hispanic) with acute lung injury.
Measurements and Main Results:
The primary outcome was 60-day mortality. A secondary outcome was number of ventilator-free days. Crude mortality was 33% for both blacks and Hispanics compared with 27% for whites (p=0.02). After adjusting for demographic and clinical covariates, the association between race/ethnicity and mortality persisted (OR = 1.42; 95% CI 1.10-1.84 for blacks; OR=1.94; 95% CI, 1.36-2.77 for Hispanics; OR=1 for whites, reference). After adjustment for severity of illness (Acute Physiology Score), black race was no longer significantly associated with mortality (OR =1.25; 95% CI, 0.95-1.66), whereas the association with Hispanic ethnicity persisted (OR=2.00; 95% CI, 1.37-2.90). Hispanics had significantly fewer ventilator-free days compared with whites after adjustment for demographic and clinical covariates (mean difference in days = -2.3; 95% CI -3.9 to -0.7).
Conclusions:
Black and Hispanic patients with acute lung injury have a significantly higher risk of death compared to white patients. This increased risk appeared to be mediated by increased severity of illness at presentation for blacks, but was unexplained among Hispanics.
Keywords: acute lung injury, acute respiratory distress syndrome, race, ethnicity, mortality, epidemiology
Introduction
Racial and ethnic disparities have been well described in many acute and chronic health conditions, but the influence of race and ethnicity on outcomes of acute lung injury has not been well investigated. One population-based epidemiologic study found that black men had the highest annual age-adjusted mortality rate from acute respiratory distress syndrome, the most severe form of acute lung injury, compared with other racial/ethnic and gender subgroups. (12.8 per 100,000 compared to 9.1 per 100,000 for white men).1 Whether this higher mortality rate reflected a higher overall incidence of acute lung injury among blacks or higher case fatality from acute lung injury remains unknown. In that study, the investigators were unable to adjust for differences in comorbid conditions or treatment measures that may have confounded or mediated the higher risk among blacks. Even less is known about outcomes for Hispanic patients with acute lung injury, as they have not been sufficiently represented in prior studies. To date, no study has specifically examined the association between race/ethnicity and mortality (case fatality) from acute lung injury.
The Acute Respiratory Distress Syndrome (ARDS) Network randomized controlled trials enrolled a racially and ethnically diverse sample of patients with acute lung injury to investigate potential treatments. The comprehensive collection of patient information in these trials allows the opportunity to compare outcomes across racial and ethnic subgroups. We evaluated whether race/ethnicity was independently associated with increased 60-day mortality among patients with acute lung injury enrolled in the ARDS Network randomized controlled trials.
Methods
Subjects
The ARDS Network has conducted multiple randomized controlled trials to evaluate therapeutic interventions for the treatment of acute lung injury. These trials have been described previously.2-7 Details of the three trials included in this study are shown in Table 1. Briefly, patients were eligible for trial participation if they met diagnostic criteria for acute lung injury and required mechanical ventilation (Figure 1). Similar inclusion and exclusion criteria were used for all three trials. All studies were approved by the National Institutes of Health, the National Heart, Lung and Blood Institute, and the institutional review boards at each participating study site.
Table 1.
Description of ARDS Network Randomized Controlled Trials
| Trial | ARMA* | ALVEOLI† | FACTT‡ |
|---|---|---|---|
| Years | 1996-1999 | 1999-2002 | 2000-2005 |
| Intervention | Low tidal volume ventilation (6 ml/kg vs. 12 ml/kg) |
Higher PEEP§ vs. lower PEEP§ |
Pulmonary artery catheter vs. central venous catheter + Conservative vs. liberal fluid management strategy |
| Number of patients enrolled (N) |
902 | 549 | 1000 |
| Did intervention(s) reduce mortality? |
Yes | No | No |
ARMA = Respiratory Management of Acute Lung Injury
ALVEOLI = Assessment of Low Tidal Volume and Elevated End-Expiratory Lung Volume to Obviate Lung Injury
FACTT = Fluid and Catheter Treatment Trial
PEEP = positive end-expiratory pressure
Figure 1.
Inclusion and Exclusion Criteria
Measurements
Patients' race/ethnicity was assigned by the study investigators using several sources: 1) examination of the patient 2) review of the medical record and 3) discussion with family members. Race/ethnicity was classified into five mutually exclusive categories: White/non-Hispanic, Black/African American, Hispanic, Asian, American Indian or other. We limited our analysis to patients identified as White, Black/African American or Hispanic because of the small numbers of patients in the other racial and ethnic groups.
Demographic and clinical data were collected and included age, gender, body mass index, cause of lung injury, and comorbid conditions. Baseline measurements were performed immediately prior to randomization (“Day 0”) and included APACHE (acute physiologic and chronic health evaluation) III score, vasopressor administration, hemodynamic and respiratory measurements including ventilator parameters.
The primary outcome measure for this analysis was 60-day mortality after study enrollment. Patients who were discharged home and breathing without mechanical ventilation were presumed to be alive at day 60. We also evaluated the secondary outcome of ventilator-free days at day 28 as defined in previous studies.2, 5-7
Statistical Methods
Demographic and baseline clinical characteristics of the different race/ethnicity groups were compared using a chi-square or Fisher exact test for categorical variables and an analysis of variance (ANOVA) or Kruskal-Wallis test for continuous variables.
We used logistic regression to evaluate the potential independent effect of race/ethnicity on 60-day mortality. Linear regression was used to evaluate the effect of race on the continuous outcome of ventilator-free days. To create the regression models, we selected variables for inclusion if they were thought to be possible confounders in the relationship between race/ethnicity and mortality from acute lung injury. We included variables if they were associated (p<0.20) with race/ethnicity in the bivariate analyses or if they were considered clinically relevant on an a priori basis. The initial model included age, gender, body mass index, cause of lung injury (pneumonia, sepsis, aspiration, trauma, transfusion, other or unknown) PaO2:FiO2 ratio, comorbid conditions (diabetes, chronic dialysis, HIV/AIDS, cirrhosis, solid tumors, leukemia, lymphoma and immunosuppression) and receipt of low tidal volume ventilation. Patients were categorized as having received low tidal volume ventilation if they were assigned to the low tidal volume arm in the first trial. All patients in the second and third trials were categorized as having received low tidal volume ventilation as specified by the study protocols.5-7 Backwards selection was used and the likelihood ratio test compared nested models. We removed variables if their removal did not cause a statistically significant change in the overall model (p>0.05). We also considered possible confounding by the trial in which the patient was enrolled. Finally, we used generalized estimating equations to take patient clustering by hospital site into account.8
To investigate the relationship between race/ethnicity and mortality, we used sequential stages of modeling by adding groups of covariates (Table 3). First, we examined 60-day mortality and adjusted for age only. Next we included gender, the trial of enrollment, receipt of low tidal volume ventilation, comorbid conditions (HIV/AIDS, lymphoma, immunosuppression), cause of lung injury (trauma and sepsis), and PaO2:FiO2 ratio (model 2). Finally, to assess whether baseline severity of illness explained any of the effect of race/ethnicity on mortality, the APS (Acute Physiology Score), calculated from the APACHE (Acute Physiology and Chronic Health Evaluation) III score, was added to the model (model 3). We performed the same sequential modeling using linear regression to investigate the relationship between race/ethnicity and ventilator-free days. Because 172 patients (7.3% of cohort) had missing values for one or more variables, they were excluded from the regression analysis. The models had approximately equivalent specification and performance for each racial/ethnic group.
Table 3.
Association of race/ethnicity and 60-day mortality from acute lung injury among patients enrolled in the ARDSnet trials
| Outcome | White | Black | Hispanic |
|---|---|---|---|
| Odds Ratio (95% CI) |
Odds Ratio (95% CI) |
||
| 60-day mortality | |||
| Age-adjusted | Reference | 1.59 (1.26-2.01) | 1.85 (1.33-2.58) |
| Model 2* | Reference | 1.42 (1.10-1.84) | 1.94 (1.36-2.77) |
| Model 3† | Reference | 1.25 (0.95-1.66) | 2.00 (1.37-2.90) |
Adjusted for age, gender, receipt of low tidal volume ventilation, trial of enrollment, comorbid conditions (HIV/AIDS, lymphoma, immunosuppression) cause of lung injury (trauma, sepsis) and PaO2:FiO2 ratio.
Adjusted for variables in Model 2 + APS (Acute Physiology Score).
Rather than being a confounder, we hypothesized that severity of illness might be an important mechanism underlying any association between race/ethnicity and mortality from acute lung injury. Thus, we did not include APS as a covariate in the initial regression models. Instead, we used a bootstrap procedure to determine the extent to which severity of illness was mediating the effect of race/ethnicity on mortality. The bootstrap procedure compared the regression coefficients for race/ethnicity using two different models- one including and one excluding the APS. The bias-corrected bootstrap estimate provided the percentage decrease in the race/ethnicity coefficient due to mediation by severity of illness with 95% confidence intervals.
To investigate whether the effect of any trial interventions (lower tidal volume ventilation, higher PEEP, pulmonary artery catheter or a fluid conservative management strategy) varied by race/ethnicity, we evaluated possible statistical interaction (multiplicative or additive) between race/ethnicity and each intervention. To evaluate possible multiplicative interaction, we used logistic regression and the likelihood ratio test to compare a model including the race/ethnicity-treatment interaction terms with a nested model that included only the main effects and race/ethnicity and no interaction terms. A statistically significant interaction term would indicate that the efficacy of a treatment intervention differed by racial/ethnic subgroup. To evaluate possible additive interaction, we used generalized linear modeling (GLM) and the likelihood ratio test to compare a model including the race/ethnicity-treatment interaction terms with a nested model that included no interaction terms. We used the same techniques to evaluate whether the association of race/ethnicity and mortality varied by age, gender or BMI.
We used STATA, version 9.2 (College Station, Texas) for all analyses. Statistical significance was defined as a two-tailed p<0.05.
Results
There were a total of 2362 patients in the cohort including 449 blacks and 198 Hispanics. Baseline characteristics by race/ethnicity are shown in Table 2. Hispanic patients were the youngest on average. Black patients had greater severity of illness at baseline, as measured by the APS and were more likely to have pneumonia as the cause of acute lung injury. Blacks were also more likely to have HIV or AIDS, end-stage renal disease or cirrhosis. Hispanic and black patients had a greater degree of acidosis and lower partial pressure of carbon dioxide (PaCO2). Hispanic patients had the lowest PaO2:FiO2 ratio, while black patients had the highest. Prior to enrollment, blacks received lower weight-adjusted tidal volumes on average compared to whites and Hispanics.
Table 2.
| Characteristic | White (n=1715) |
Black (n=449) |
Hispanic (n=198) |
P value |
|---|---|---|---|---|
| Age | 52 ± 17 | 48 ± 16 | 44 ± 16 | <0.0005 |
| Male | 936 (55) | 251 (56) | 128 (65) | 0.16 |
| BMI, kg/m2 | 28.0 ± 7.3 | 27.0 ± 7.5 | 28.0 ± 6.6 | 0.049 |
| Acute Physiology Score (calculated from APACHE III) |
78 ± 30 | 86 ± 30 | 83 ± 30 | <0.0005 |
| Vasopressor use | 506 (33) | 131 (32) | 70 (38) | 0.36 |
| Cause of lung injury | 0.001 | |||
| pneumonia | 667 (39) | 210 (47) | 77 (39) | |
| sepsis | 397 (23) | 107 (24) | 61 (31) | |
| aspiration | 260 (15) | 71 (16) | 17 (9) | |
| trauma | 161 (9) | 25 (6) | 20 (10) | |
| transfusion | 46 (3) | 5 (1) | 7 (4) | |
| other or unknown | 184 (11) | 31 (7) | 16 (8) | |
| Comorbid conditions‡ | ||||
| Diabetes | 241 (14) | 89(20) | 41(22) | 0.001 |
| Chronic Dialysis | 21 (1) | 23 (3) | 1(0.5) | 0.03 |
| HIV or AIDS | 56 (3) | 76 (17) | 20 (11) | <0.001 |
| Cirrhosis | 45 (3) | 21 (5) | 9 (5) | 0.04 |
| Solid tumors | 31 (2) | 4 (1) | 3 (2) | 0.40 |
| Leukemia | 40(2) | 5 (1) | 3(2) | 0.26 |
| Lymphoma | 26 (2) | 0 (0) | 2(1) | 0.009 |
| Immunosuppression | 188 (11) | 54 (12) | 13(7) | 0.14 |
| Baseline respiratory variables | ||||
| Tidal volume (ml)/kg PBW§ | 8.6 ± 2.2 | 7.9 ± 2.0 | 8.6 ± 2.4 | <0.001 |
| Plateau pressure, cm H20 | 28 ± 8 | 28 ± 8 | 28 ± 7 | 0.31 |
| Peak airway pressure, cm H20 | 34 ± 10 | 34 ± 9 | 34 ± 9 | 0.90 |
| Mean airway pressure, cm H20 | 16 ± 9 | 16 ± 8 | 16 ± 5 | 0.97 |
| PEEP, cm H20 | 9.1± 4.1 | 8.8 ± 4.0 | 9.3 ± 3.7 | 0.16 |
| Arterial pH | 7.38 ± 0.09 | 7.37 ± 0.09 | 7.37 ± 0.09 | 0.003 |
| PaO2, mm Hg | 86 ± 34 | 93 ± 46 | 87 ± 38 | 0.08 |
| PaCO2, mm Hg | 39 ±9 | 38 ± 10 | 37 ± 9 | 0.008 |
| PaO2:FiO2 ratio | 149 ± 66 | 158 ± 81 | 144 ± 61 | 0.03 |
Values are mean ± SD or n (%).
All clinical variables were measured at the time of trial enrollment, prior to randomization.
Comorbid conditions available for n=1686 whites, n=443 blacks and n=187 Hispanics.
Predicted body weight
There was 29% overall mortality at day 60 among the study participants. Blacks and Hispanics had higher mortality, 33% in each group, compared to 27% for whites (p-value=0.02). Both blacks and Hispanics had a significantly increased risk for mortality after age adjustment (Table 3). Further adjustments for gender, receipt of low tidal volume ventilation, trial of enrollment, comorbid conditions (HIV/AIDS, lymphoma, immunosuppression) cause of lung injury (sepsis, trauma) and PaO2:FiO2 minimally affected the association between black race and mortality (OR=1.42; 95% CI, 1.10-1.84) and Hispanic ethnicity and mortality (OR=1.94; 95% CI 1.36-2.77). After adjustment for severity of illness, the association between black race and mortality was no longer statistically significant, whereas the increased mortality risk associated with Hispanic ethnicity persisted (Table 3).
Using the bootstrap procedure, we estimated that severity of illness, measured by APS, accounted for 30% (95% CI 7-95%) of the association between black race and mortality. The addition of APS did not substantively alter the association of Hispanic ethnicity with mortality risk.
White patients had the greatest number of ventilator-free days in the unadjusted analysis with a median of 16 days (IQR 0-27) compared to 15 days (IQR 0-27) for blacks and 12 days (IQR 0-27) for Hispanics (p= 0.06). After age adjustment, black and Hispanic patients had significantly fewer ventilator-free days compared to white patients (Table 4). Further adjustments for gender, receipt of low tidal volume ventilation, trial of enrollment, comorbid conditions (HIV/AIDS, lymphoma, immunosuppression), cause of lung injury (trauma, sepsis) and PaO2:FiO2 ratio attenuated the difference between blacks and whites and the confidence interval included no effect. Hispanic ethnicity remained associated with fewer ventilator-free days after controlling for covariates including APS.
Table 4.
Association of race/ethnicity and ventilator-free days among patients enrolled in the ARDSnet trials
| Outcome | White | Black | Hispanic |
|---|---|---|---|
| Mean Difference in Days (95%CI) | Mean Difference in Days (95% CI) | ||
| Ventilator-free days | |||
| Age-adjusted | Reference | −1.1 (−2.2 to −0.3) | −2.2 (−3.7 to −0.7) |
| Model 2* | Reference | −1.0 (−2.2 to 0.1) | −2.2 (−3.9 to −0.7) |
| Model 3† | Reference | −0.8 (−2.0 to 0.3) | −2.3 (−3.9 to −0.7) |
Adjusted for age, gender, receipt of low tidal volume ventilation, trial of enrollment, comorbid conditions (HIV/AIDS, lymphoma, immunosuppression) cause of lung injury (trauma, sepsis) and PaO2:FiO2 ratio.
Adjusted for variables in Model 2 + APS (Acute Physiology Score).
There were no significant multiplicative or additive interactions between race/ethnicity and any of the treatment interventions (p>0.10 for all interaction terms). Nor were there significant multiplicative or additive interactions between race/ethnicity and age, gender or BMI (p>0.10 for all interaction terms). The hierarchical analyses that accounted for patient clustering within hospitals did not substantively change the point estimates or confidence intervals for either 60-day mortality or ventilator-free days.
Discussion
In this study of patients with acute lung injury who were enrolled in the ARDS Network randomized controlled trials, both black and Hispanic patients had a significantly higher risk of 60-day mortality compared to white patients and Hispanics had significantly fewer ventilator-free days. The increased mortality risk among blacks was largely explained by their greater severity of illness at presentation. In contrast, the increased mortality risk among Hispanics was not fully explained by differences in patient characteristics or disease severity.
The primary implication of these findings for black patients with acute lung injury is that their greater severity of illness at presentation may be primarily responsible for their elevated mortality risk. Prior studies have also reported that black patients present later in their disease course with greater severity of illness at hospital admission compared with white patients, yet the reasons underlying this finding are not clear.9, 10 Delay in the diagnosis of acute lung injury may have occurred, which could be related to either patient-related or physician practice factors. Because severity of illness is a major determinant of mortality risk in patients with acute lung injury, it is not surprising to find that it contributes to worse outcomes among blacks.11, 12
Our finding of worse outcomes among critically ill Hispanic patients highlights a potentially vulnerable subgroup of acute lung injury patients. For Spanish-speaking patients, language barriers may be significant and lead to delay both in seeking medical care and in being appropriately diagnosed with acute lung injury. This may be a potential explanation for our findings among Hispanic patients, though we did not collect data about language preference or fluency. Nor did we have information about language or cultural discordance between patients and their providers. However, unlike black patients, severity of illness was not a mediator of the association between Hispanic ethnicity and increased mortality risk. Language discordance between patient and provider has been shown to be a predictor of worse health outcomes for outpatients with chronic diseases, but this is of less certain significance in the intensive care unit setting.13 Further research is needed to determine whether our finding of worse outcomes for Hispanics is related to language discordance, medical treatment, cultural beliefs or practices, socioeconomic status or biological differences.
Although we had information about many comorbid conditions, we were not able to make comprehensive adjustments for all comorbid conditions. Also, we did not have information about the severity of patients' comorbid conditions like HIV/AIDS or diabetes. Blacks and Hispanics have a higher burden of comorbid conditions and our inability to fully adjust for all of these conditions and their severity may have accounted for some of the increased mortality risk among these groups.14-16
An additional explanation for our findings is that black and Hispanic patients had higher rates of chronic alcohol abuse, which has been identified as a risk factor for the development of acute lung injury and is also associated with increased frequency and severity of non-pulmonary organ dysfunction in patients with sepsis.17, 18 The prevalence of cirrhosis was higher among black and Hispanic patients in our study and may be indicative of more chronic alcohol abuse among these groups in our study sample. In a large sample of hospitalized patients who had diagnoses that require intensive care unit admission, alcohol use disorders were more common among blacks and Hispanics and Native Americans.19 Because the trials included in our study did not systematically collect information on alcohol use, we cannot fully evaluate its importance as a contributor to poor acute lung injury outcomes.
Another possibility is that the quality of care differed across racial/ethnic groups despite all patients being enrolled in randomized controlled trials with explicit protocols for the interventions under investigation. Aspects of care that did not involve the trial intervention were left to the treating physicians' discretion and may potentially have been influenced by race/ethnicity. Although lower tidal volume ventilation is the only therapy proven to reduce mortality for patients with acute lung injury, other therapies directed at the underlying cause of lung injury may have been preferentially given to white patients. Also, it is possible that more time elapsed between the onset of acute lung injury and trial enrollment for black and Hispanic patients. During this time, these patients could have been exposed to factors associated with poorer outcome (e.g., higher tidal volume ventilation). In these trials, we did not have information about the number of hours that elapsed prior to trial enrollment. However, all patients needed to be enrolled within 48 hours of meeting criteria for acute lung injury. Given this short time frame required for trial enrollment, any differences in exposures that occurred during this period were likely small and were therefore unlikely to have accounted for the differences in outcomes for black and Hispanic patients. Additionally, recent studies have shown that hospitals that treat larger proportions of black patients may provide worse quality of care. 20, 21 Importantly, we found no change in the association between race/ethnicity and mortality after adjusting for patient clustering within hospitals, which was intended to capture hospital-specific differences in the quality of care.
Our study has some limitations. Because this was a secondary analysis of data from randomized controlled trials, there may have been confounding by unmeasured variables. For instance, we did not have information about socioeconomic status and lower socioeconomic status has been associated with worse outcomes from acute illnesses.22, 23 In addition, all of the patients in our study were enrolled in one of three randomized controlled trials, and it is possible that enrollment targeted black and Hispanic patients with greater severity of illness compared with white participants. While there could have been barriers to enrolling minority patients in the ARDS Network trials, this seems less likely, as greater proportions of screened black and Hispanic patients were enrolled in the trials compared to whites (12.5% blacks enrolled, 18.1% Hispanics enrolled and 10.4% whites enrolled). In addition, we may have misclassified the race of certain participants, but that would likely have biased the results toward the null. Also, data about orders for limitations of care were not collected for the first two ARDS Network studies, therefore we were unable to determine whether there were differences in do-not-resuscitate orders by race/ethnicity. Our results may have been more marked if we had been able to account for this variable, given that many studies have shown that whites more frequently prefer limiting care when compared to blacks.24, 25
While we did not have detailed information about the racial and ethnic composition of the populations served by ARDS Network Hospitals, it is likely that these populations did not have the same racial and ethnic makeup as the U.S. Census. The centers participating in the ARDS Network trials were located throughout the country, but were more often located in larger cities with higher proportions of black Americans (Baltimore, New Orleans, Philadelphia, Chicago, Raleigh). Comparatively few centers were located in areas with higher concentrations of Hispanic Americans (California, Texas, Arizona, New Mexico, New York, Florida). Thus, our study population had a greater proportion of blacks (19%) and fewer Hispanics (8%) compared to the 2000 U.S. Census (12% black and 13% Hispanic).26 Because we sought to measure the case fatality of ALI among black and Hispanic patients and not the incidence of ALI among black and Hispanic populations, we do not believe these differences introduced systematic bias to our results.
These results have important implications. Future study should evaluate the relative importance of socioeconomic status, health-related behaviors and comorbid conditions as confounding factors, and should more closely examine provider challenges in recognizing acute lung injury among racial and ethnic minority populations as well as provider preferences and beliefs about caring for critically ill minority patients.
Acknowledgements
Supported in part by NHLBI P50 HL74005 (MAM, ME), NHLBI R01 HL51856 (MAM) and NHLBI HR046059 (ARDS Network).
Footnotes
The following persons and institutions participated in the trials:
Steering Committee Chair — G.R. Bernard; Clinical Coordinating Center — D.A. Schoenfeld, B.T. Thompson, N. Ringwood, C. Oldmixon, F. Molay, A. Korpak, R. Morse, D. Hayden, M. Ancukiewicz, A. Minihan; Protocol-Review Committee — J.G.N. Garcia, R. Balk, S. Emerson, M. Shasby, W. Sibbald; Data Safety and Monitoring Board — R. Spragg, G. Corbie-Smith, J. Kelley, K. Leeper, A.S. Slutsky, B. Turnbull, C. Vreim; National Heart, Lung, and Blood Institute — A.L. Harabin, D. Gail, P. Lew, M. Waclawiw; ARDS Clinical Trials Network Consultant — P. Parsons; Clinical Centers — University of Washington, Harborview — L. Hudson, K. Steinberg, M. Neff, R. Maier, K. Sims, C. Cooper, T. Berry-Bell, G. Carter, L. Andersson; University of Michigan — G.B. Toews, R.H. Bartlett, C. Watts, R. Hyzy, D. Arnoldi, R. Dechert, M. Purple; University of Maryland — H. Silverman, C. Shanholtz, A. Moore, L. Heinrich, W. Corral; Johns Hopkins University — R. Brower, D. Thompson, H. Fessler, S. Murray, A. Sculley; Cleveland Clinic Foundation — H.P. Wiedemann, A.C. Arroliga, J. Komara, T. Isabella, M. Ferrari; University Hospitals of Cleveland — J. Kern, R. Hejal, D. Haney; MetroHealth Medical Center — A.F. Connors; University of Colorado Health Sciences Center — E. Abraham, R. McIntyre, F. Piedalue; Denver Veterans Affairs Medical Center — C. Welsh; Denver Health Medical Center — I. Douglas, R. Wolkin; St. Anthony Hospital — T. Bost, B. Sagel, A. Hawkes; Duke University — N. MacIntyre, J. Govert, W. Fulkerson, L. Mallatrat, L. Brown, S. Everett, E. VanDyne, N. Knudsen, M. Gentile; University of North Carolina — P. Rock, S. Carson, C. Schuler, L. Baker, V. Salo; Vanderbilt University — A.P. Wheeler, G. Bernard, T. Rice, B. Christman, S. Bozeman, T. Welch; University of Pennsylvania — P. Lanken, J. Christie, B. Fuchs, B Finkel, S. Kaplan, V. Gracias, C.W. Hanson, P. Reilly, M.B. Shapiro, R. Burke, E. O'Connor, D. Wolfe; Jefferson Medical College — J. Gottlieb, P. Park, D.M. Dillon, A. Girod, J. Furlong; LDS Hospital — A. Morris, C. Grissom, L. Weaver, J. Orme, T. Clemmer, R. Davis, J. Gleed, S. Pies, T. Graydon, S. Anderson, K. Bennion, P. Skinner; McKay-Dee Hospital — C. Lawton, J. d'Hulst, D. Hanselman; Utah Valley Regional Medical Center — K. Sundar, T. Hill, K. Ludwig, D. Nielson; University of California, San Francisco — M.A. Matthay, M. Eisner, B. Daniel, O. Garcia; San Francisco General — J. Luce, R. Kallet; University of California, San Francisco, Fresno — M. Peterson, J. Lanford; Baylor College of Medicine — K. Guntupalli, V. Bandi, C. Pope; Baystate Medical Center — J. Steingrub, M. Tidswell, L. Kozikowski; Louisiana State University Health Sciences Center — B. deBoisblanc, J. Hunt, C. Glynn, P. Lauto, G. Meyaski, C. Romaine; Louisiana State University Earl K. Long Center — S. Brierre, C. LeBlanc, K. Reed; Alton-Ochsner Clinic Foundation — D. Taylor, C. Thompson; Tulane University Medical Center — F. Simeone, M. Johnston, M. Wright; University of Chicago — G. Schmidt, J. Hall, S. Hemmann, B. Gehlbach, A. Vinayak, W. Schweickert; Northwestern University — J. Dematte D'Amico, H. Donnelly; University of Texas Health Sciences Center — A. Anzueto, J. McCarthy, S. Kucera, J. Peters, T. Houlihan, R. Steward, D. Vines; University of Virginia — J. Truwit, A.F. Connors, M. Marshall, W. Matsumura, R. Brett; University of Pittsburgh — M. Donahoe, P. Linden, J. Puyana, L. Lucht, A. Verno; Wake Forest University — R.D. Hite, P. Morris, A. Howard, A. Nesser, S. Perez; Moses Cone Memorial Hospital — P. Wright, C. Carter-Cole, J. McLean; St. Paul's Hospital, Vancouver — J. Russell, L. Lazowski, K. Foley; Vancouver General Hospital — D. Chittock, L. Grandolfo; Mayo Foundation — M. Murray.
References
- 1.Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979- 1996) Crit Care Med. 2002;30:1679–85. doi: 10.1097/00003246-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. The ARDS Network. JAMA. 2000;283:1995–2002. doi: 10.1001/jama.283.15.1995. [DOI] [PubMed] [Google Scholar]
- 4.Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30:1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–36. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–24. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 7.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 8.Zeger SLLK. Longitudinal analysis using generalized linear models. Biometrika. 1986;42:121–130. [Google Scholar]
- 9.Johnson PA, Lee TH, Cook EF, Rouan GW, Goldman L. Effect of race on the presentation and management of patients with acute chest pain. Ann Intern Med. 1993;118:593–601. doi: 10.7326/0003-4819-118-8-199304150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Weissman JS, Stern R, Fielding SL, Epstein AM. Delayed access to health care: risk factors, reasons, and consequences. Ann Intern Med. 1991;114:325–31. doi: 10.7326/0003-4819-114-4-325. [DOI] [PubMed] [Google Scholar]
- 11.Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 12.Ware LB. Prognostic determinants of acute respiratory distress syndrome in adults: impact on clinical trial design. Crit Care Med. 2005;33:S217–22. doi: 10.1097/01.ccm.0000155788.39101.7e. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Stable EJ, Napoles-Springer A, Miramontes JM. The effects of ethnicity and language on medical outcomes of patients with hypertension or diabetes. Med Care. 1997;35:1212–9. doi: 10.1097/00005650-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Racial/ethnic disparities in prevalence, treatment, and control of hypertension--United States, 1999-2002. MMWR Morb Mortal Wkly Rep. 2005;54:7–9. [PubMed] [Google Scholar]
- 15.Racial/ethnic disparities in diagnoses of HIV/AIDS--33 states, 2001-2005. MMWR Morb Mortal Wkly Rep. 2007;56:189–93. [PubMed] [Google Scholar]
- 16.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit Care Med. 2006;34:2576–82. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50–4. [PubMed] [Google Scholar]
- 18.Moss M, Parsons PE, Steinberg KP, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31:869–77. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 19.de Wit M, Best AM, Gennings C, Burnham EL, Moss M. Alcohol use disorders increase the risk for mechanical ventilation in medical patients. Alcohol Clin Exp Res. 2007;31:1224–30. doi: 10.1111/j.1530-0277.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- 20.Bradley EH, Herrin J, Wang Y, et al. Racial and ethnic differences in time to acute reperfusion therapy for patients hospitalized with myocardial infarction. Jama. 2004;292:1563–72. doi: 10.1001/jama.292.13.1563. [DOI] [PubMed] [Google Scholar]
- 21.Jha AK, Orav EJ, Li Z, Epstein AM. Concentration and quality of hospitals that care for elderly black patients. Arch Intern Med. 2007;167:1177–82. doi: 10.1001/archinte.167.11.1177. [DOI] [PubMed] [Google Scholar]
- 22.Kim C, Diez Roux AV, Hofer TP, Nallamothu BK, Bernstein SJ, Rogers MA. Area socioeconomic status and mortality after coronary artery bypass graft surgery: the role of hospital volume. Am Heart J. 2007;154:385–90. doi: 10.1016/j.ahj.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 23.Rao SV, Schulman KA, Curtis LH, Gersh BJ, Jollis JG. Socioeconomic status and outcome following acute myocardial infarction in elderly patients. Arch Intern Med. 2004;164:1128–33. doi: 10.1001/archinte.164.10.1128. [DOI] [PubMed] [Google Scholar]
- 24.Degenholtz HB, Arnold RA, Meisel A, Lave JR. Persistence of racial disparities in advance care plan documents among nursing home residents. J Am Geriatr Soc. 2002;50:378–81. doi: 10.1046/j.1532-5415.2002.50073.x. [DOI] [PubMed] [Google Scholar]
- 25.Garcia JA, Romano PS, Chan BK, Kass PH, Robbins JA. Sociodemographic factors and the assignment of do-not-resuscitate orders in patients with acute myocardial infarctions. Med Care. 2000;38:670–8. doi: 10.1097/00005650-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Census http://www.census.gov/prod/2001pubs/c2kbr01-1.pdf.