SUMMARY
Changes in cytosolic calcium are crucial for numerous processes including neuronal plasticity. This study investigates the regulation of cytosolic calcium by corticotropin releasing factor (CRF) in midbrain dopamine neurons. The results demonstrate that CRF stimulates the release of intracellular calcium from stores through activation of adenylyl cyclase and PKA. Imaging and photolysis experiments showed that the calcium originated from dendrites and required both functional IP3 and ryanodine receptor channels. The elevation in cytosolic calcium potentiated inhibition mediated by calcium sensitive potassium channels (sK) activated by action potentials and metabotropic Gq-coupled receptors for glutamate and acetylcholine. This increase in cytosolic calcium activated by postsynaptic Gs-coupled CRF receptors may represent a fundamental mechanism by which stress peptides and hormones can shape Gq-coupled receptor mediated regulation of neuronal excitability and synaptic plasticity in dopamine neurons.
INTRODUCTION
In both in neurons and non-neuronal cells, changes in the concentrations of cytosolic (free) calcium regulate diverse processes including hormone secretion (Verkhratsky, 2005), cytoskeletal rearrangement (Spira et al., 2001), gene expression (West et al., 2001), receptor trafficking (Ziff, 2007) and neuronal plasticity (Ghosh and Greenberg, 1995). Calcium enters the cytosol from two spatially distinct sources: intracellular stores and the extracellular space (Bootman et al., 2000;Bootman and Berridge, 1995). Intracellular stores release calcium through channels located on the endoplasmic reticulum membrane such as the inositol triphosphate (IP3) receptor and the ryanodine receptor (Berridge et al., 2000;Choi et al., 2006;Foskett et al., 2007). IP3 receptors are recruited following activation of Gq-protein coupled receptors and phospholipase C (PLC) (Augustine et al., 2003). Calcium release from IP3 receptors activates ryanodine receptor channels through calcium-induced calcium-release (Augustine et al., 2003). Calcium entry from the extracellular space occurs through voltage gated calcium channels that are activated upon depolarization. Increases in cytosolic calcium originating from both sources can activate intrinsic conductances, including small conductance calcium-activated potassium (sK) channels that are involved in synaptic integration and spike frequency adaptation (Bean, 2007;Bond et al., 1999;Madison and Nicoll, 1984;Womack et al., 2004;Womack and Khodakhah, 2003).
Mesocorticolimbic dopamine neurons, which originate from the ventral tegmental area and substantia nigra, encode signals important for movement and reinforcing behaviors such as drug abuse (Bjorklund and Dunnett, 2007;Grace et al., 2007;Koob and Le Moal, 2001;Robinson and Berridge, 2000;Wise, 2004). Recent studies implicate corticotropin-releasing factor (CRF) in many central actions of drugs of abuse (Sarnyai et al. 2001). CRF is a 41 amino acid peptide that mediates behavioral responses to stress through coordinated actions at two Gs-coupled protein receptors (CRF-1 and CRF-2) (Chen et al., 1993;Stenzel et al., 1995;Vale et al., 1981). CRF functions as a hormone within the hypothalamic pituitary axis (Hauger et al., 2006), but is also synthesized and released in areas outside the hypothalamus (Orozco-Cabal et al., 2006;Swanson et al., 1983). In non-neuronal cells, CRF and many other hormones increase cytosolic calcium through protein kinase A (PKA)- and PKC-dependent actions at internal stores and voltage gated calcium channels on the plasma membrane (Dermitzaki et al., 2004;Petersen et al., 1994).
CRF-1 and CRF-2 receptors are present on midbrain dopamine neurons, but their impact on neuronal excitability remains poorly understood (Foote and Cha, 1988;Sauvage and Steckler, 2001). For instance, both activation and blockade of CRF receptors stimulate dopamine neuron firing and inconsistently alter dopamine release from terminals (Korotkova et al., 2006;Lavicky and Dunn, 1993;Lodge and Grace, 2005;Lu et al., 2003). Known actions of CRF on dopamine neurons involve the CRF-binding protein, PKC and voltage gated calcium channels (Kim et al., 2007;Ungless et al., 2003). A CRF-mediated increase in concentrations of cytosolic calcium in dopamine neurons has not been reported. However, the release of calcium from intracellular stores in dopamine cells activates an sK conductance following synaptic activation of metabotropic glutamate receptors (mGluRs) (Fiorillo and Williams, 1998;Morikawa et al., 1998;Morikawa et al., 2003a), alpha-1-adrnoceptors (Paladini et al., 2001;Paladini and Williams, 2004) and muscarinic receptors (Fiorillo and Williams, 2000). Moreover, calcium entry during action potentials results in an increase in an sK-dependent conductance (Cui et al., 2007;Wolfart et al., 2001a;Wolfart and Roeper, 2002). Such observations raise the possibility that in dopamine neurons, CRF could regulate sK-dependent currents by influencing the calcium associated with intracellular stores, calcium entry through voltage dependent channels, or both.
This study investigates the regulation of cytosolic calcium concentrations by CRF receptors in dopamine neurons. The results demonstrate that CRF stimulates the release of intracellular calcium from stores through activation of adenylyl cyclase and PKA. This release of calcium is sufficient to potentiate the sK channel inhibition mediated by Gq-coupled receptors. The increase in calcium release from intracellular stores mediated by Gs-activation may represent a fundamental mechanism by which stress peptides in particular and various hormones in general can shape the neuronal excitability and synaptic plasticity of dopamine neurons.
RESULTS
CRF increases the current induced by mGluRs
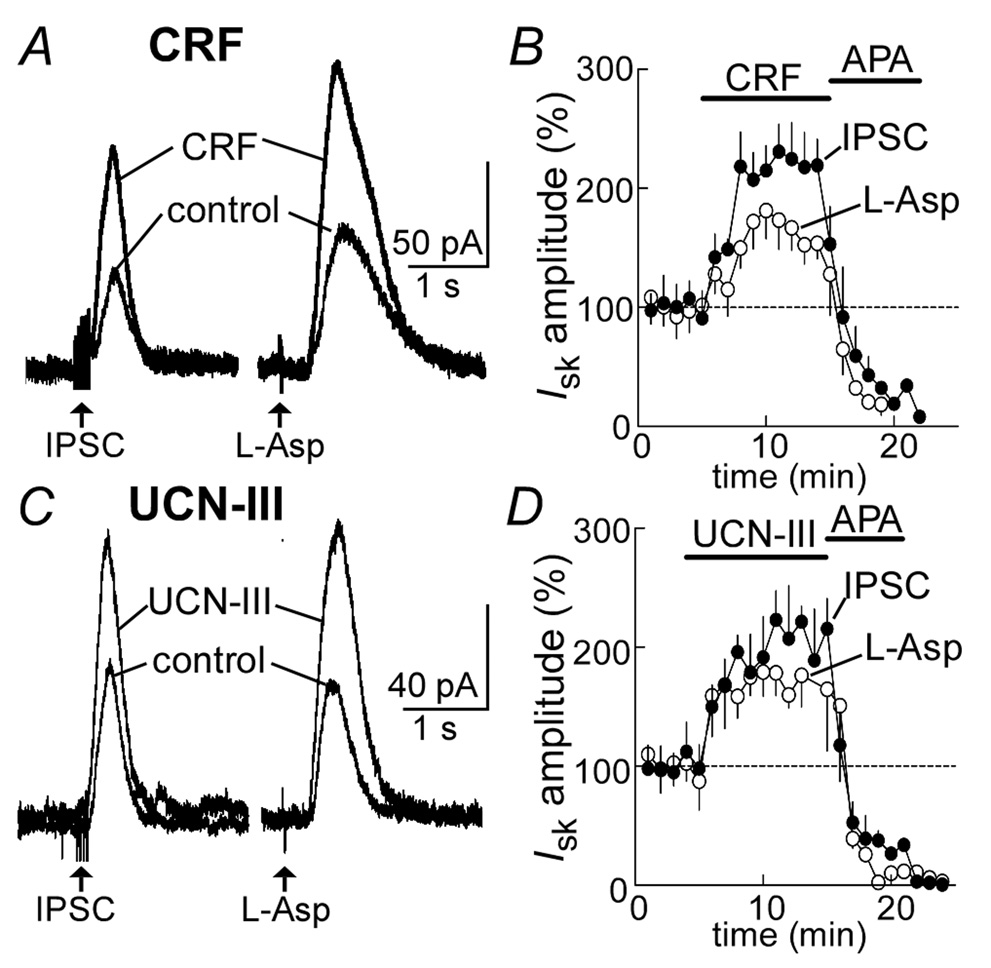
Superfusion of CRF (300 nM) caused a significant increase in the amplitude of the inhibitory postsynaptic current (IPSC) mediated by the mGluR (230±21% of control, n=8, Figure 1A, B). All data are expressed as a percent of control. Likewise CRF increased the amplitude of the current induced by iontophoretic application of aspartate (182±22%, n=6, Figure 1B). The increase in both the IPSC and the current induced by aspartate peaked within 6–8 min following the onset of the CRF treatment and occurred without alterations in the holding current (control: −31± 3.8 pA; CRF: −34±4 pA, n=12, p>0.05). At the end of the experiment apamin (100 nM) was used to block both currents. A second agonist specific for CRF-2 receptors, urocortin-III (UCN-III, 300 nM), produced a similar augmentation of both the IPSC (223±18%, n=6) and aspartate induced current (189±15%, n=5, Figures 1C, D). Application of the CRF-2 receptor subtype antagonist antisauvagine-30 (1 µM) blocked CRF-evoked increases in the current induced by iontophoretic application of aspartate (106±15%, n=6, p>0.05) (Jahn et al., 2004). Neither the agonist (control: −63±10 pA; UCN-III: −70±11 pA, n=6, p>0.05) nor antagonist (control: −58±14 pA; antisauvagine-30: −50±11 pA, n=6, p>0.5) altered the holding current. The results suggest that the CRF-induced augmentation of the sK current was a consequence of activation of the CRF-2 receptor subtype.
Figure 1.
Activation of CRF receptors potentiates an outward current evoked by the activation of metabotropic glutamate receptors. A, raw traces of an IPSC and outward current induced by the iontophoretic application of aspartate (L-Asp) in control and in the presence of CRF (300 nM). B, summarized results showing the time course of the facilitation of the IPSC and outward current. At the end of each experiment the sK channel antagonist apamin (APA, 100 nM) was applied to block the current. C, raw traces of an IPSC and outward aspartate-evoked current before and after treatment with the CRF-2 agonist urocortin-III (UCN-III, 300 nM). D, summarized results showing the time course of facilitation induced by urocortin-III and subsequent block by apamin.
CRF acts through adenylyl cyclase and protein kinase A (PKA)
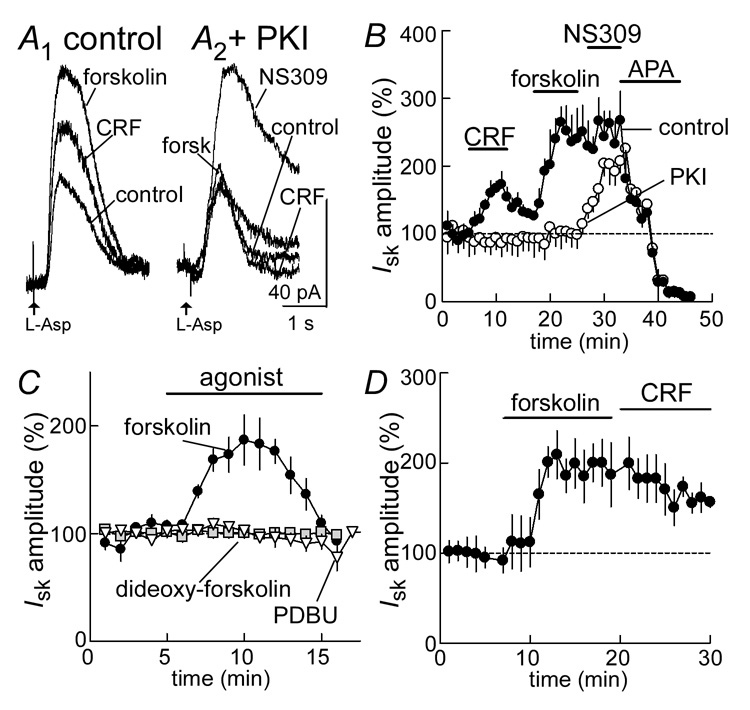
CRF receptors are known to be Gs-coupled receptors (Hsu and Hsueh, 2001;Jahn et al., 2004;Lewis et al., 2001). The actions of CRF were compared with the effect of forskolin, a potent activator of adenylate cyclase. In this experiment the current induced by iontophoretic application of aspartate was used because forskolin facilitates the presynaptic release of glutamate (Manzoni and Williams, 1999). In the initial experiments the actions of CRF (300 nM) on the mGluR-induced current were tested prior to the application of forskolin (Figure 2A, B). CRF increased the mGluR current (177±17%, n=14; Figure 2A, B) and forskolin (3 µM) caused an additional potentiation of the current (265±23%, n=9, Figure 2A, B). Application of forskolin following washout of CRF resulted in a larger forskolin-induced increase in the mGluR current than forskolin application without prior CRF application (187±16%, n=8, Figure 2B compared with 265±23%, Figure 2D). When forskolin (3 µM) was applied for a longer period (15 min) followed by additional application of CRF (300 nM), CRF had no additive effect (212±21%, n=6, compared with 181±22%, n=6, Figure 2D). In many cells, the forskolin-evoked increase in IsK peaked, and declined in continued presence of the agonist (Figure 2B, 2C, 2D, Figure 5B). This decline was reminiscent of the reported heterologous inhibition of the sK-dependent conductance following tonic activation of various Gq-coupled receptors (Paladini and Williams, 2004). In some cells, application of forskolin alone also generated a small standing inward current (control: −23±13 pA; forskolin: −38±15 pA, n=9, p>0.05; data not shown).
Figure 2.
The CRF-potentiation of metabotropic glutamate receptor (mGluR)-induced current is mediated by adenylate cyclase and PKA. A1 (left side), raw traces of the current induced by iontophoretic application of aspartate (L-Asp) in control, in the presence of CRF (300 nM) and in the presence of forskolin (3 µM). A2 (right side), raw traces taken from an experiment where the pseudo-substrate blocker PKI (1 mM) was included in the internal solution. The aspartate induced current was not changed by either CRF or forskolin, but was increased by NS308 (10 µM), a stabilizer of sK channels. B, summarized results showing in control the time course of the potentiation of the aspartate-evoked current by CRF (n=14) and forskolin (n=9). Inclusion of PKI in the internal solution blocked this potentiation (CRF: n=8; forskolin: n=8). All currents were inhibited by the sK channel antagonist, apamin (APA, 100 nM). C, summarized results showing the facilitation of the aspartate-induced current by forskolin (3 µM, n=8), but not the inactive analog dideoxy-forskolin (3 µM, n=5) or the PKC activating phorbol ester, PDBU (300 nM, n=6). D, Summarized result showing the increase in aspartate induced current caused by forskolin (3 µM) and the occlusion of an increase induced by CRF (300 nM, n=6).
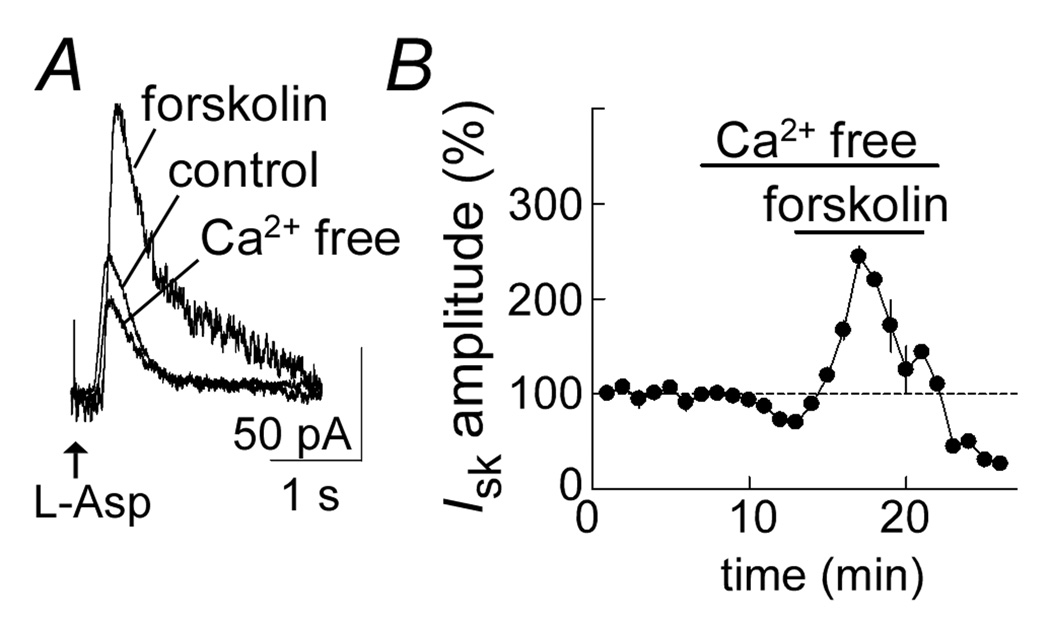
Figure 5.
The forskolin induced increase in aspartate current is not dependent on extracellular calcium. A, raw current traces showing the outward current induced by aspartate in control, in calcium-free solution and in the presence of forskolin in the calcium-free solution. B, summarized results showing the time course of the experiment. Forskolin caused a significant increase in current after treatment of the slice with calcium-free solution for 5 min (n=5). The calcium free solution contained EGTA (500 µM).
The role of protein kinase A (PKA) in the action of CRF and forskolin was tested using the pseudo-substrate blocker protein kinase inhibitor (PKI) (Scott et al., 1985;Scott et al., 1986). The PKI was included in the intracellular solution, and cells were equilibrated before CRF and forskolin were tested. Under these conditions neither CRF (4±17% change, n=8) nor forskolin (10±15% change, n=8) had any significant effect on the current induced by aspartate (Figure 2A, B). As an indication that the potassium channels could still be modulated, a stabilizer of sK-channels, NS309 was tested at the end of these experiments (Pedarzani et al., 2005;Strobaek et al., 2004). Application of NS309 (10 µM) increased the current (204±18%, n=5) even in the presence of PKI (Figure 2A, B). The inactive analog of forskolin 1,9-dideoxyforskolin (3 µM) had no effect on the aspartate-induced current (4 % ± 4 %, n = 5, Figure 2C). Likewise when phorbol-ester-dybutyrate (PDBu) was used to activate protein kinase C (PKC), the current induced by asparate was not changed (Figure 2C). Thus, CRF and forskolin enhanced the mGluR-mediated outward current by a PKA dependent mechanism.
Activation of adenylyl cyclase increases calcium in dendrites
In dopamine neurons, the outward current induced by activation of metabotropic glutamate receptors is dependent on the release of calcium from intracellular stores (Morikawa et al., 2000;Morikawa et al., 2003b). The increase in the current that is induced by CRF or forskolin could result from a number of different mechanisms including an increase in the release of calcium from intracellular stores or an increase in the sensitivity of the calcium sensitive potassium channel for calcium.
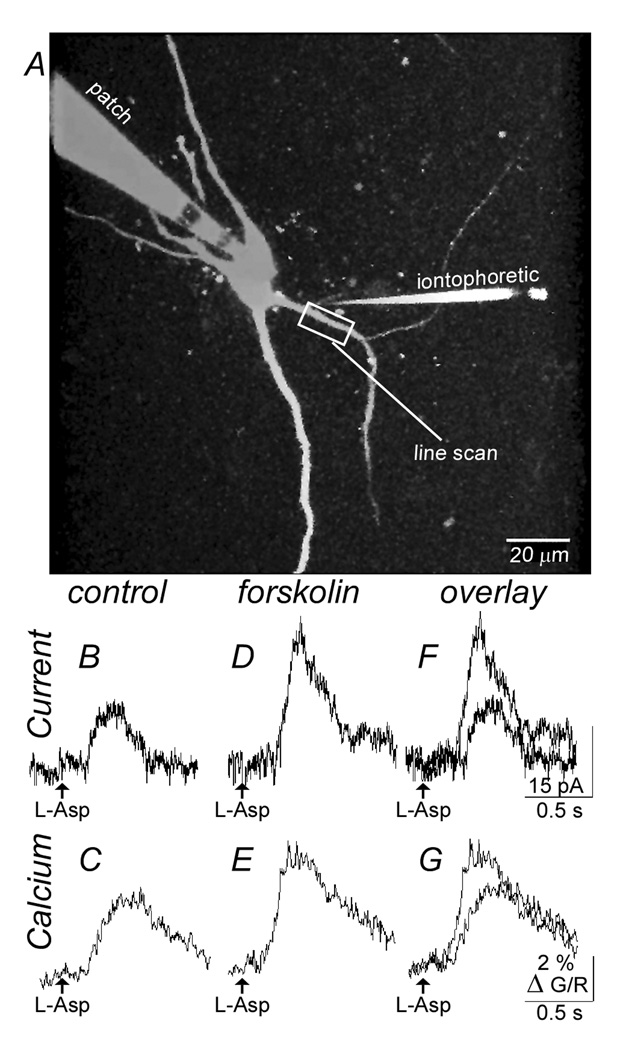
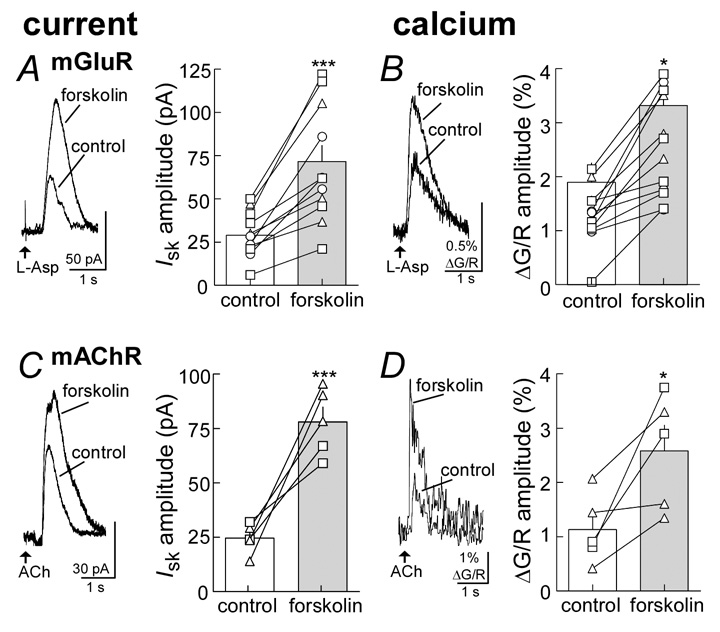
These potential mechanisms were tested first by measuring the rise and fall in intracellular calcium induced by aspartate before and during application of forskolin (3 µM) (Figure 3, Figure 4). Forskolin increased both the outward current induced by aspartate (control: 28.9±3.6 pA, n=12; forskolin: 71.6±9.3 pA, n=12; p<0.001, Figure 4A) and simultaneously increased the dendritic calcium transients (ΔG/R; control: 1.90±0.36%, n=12; forskolin: 3.32±0.54%, n=12; p<0.05, Figure 4B). This increase was observed with the use of each of the calcium indicators (Fluo5F: n=6 cells; Oregon Green BAPTA-5N: n=3; Fluo4: n=3). The facilitation of calcium was dendritic in origin at or near the point where the initial rise in calcium was evoked.
Figure 3.
Both the aspartate induced current and the rise in dendritic calcium are increased by forskolin. A, image of a midbrain dopamine neuron taken with a two-photon microscope using the red channel (Alexaflor594, 5 µM). Indicated are the recording electrode (patch), the iontophoretic electrode (iontophoretic) and the dendrite where the line scan was taken. B, is a current trace showing the outward current induced by aspartate (L-Asp). C, is the rise in fluorescence (Δ G/R) induced by the application of aspartate. D and E, shows the current (D) and fluorescence (E) from the same cell in the presence of forskolin (3 µM). F and G are an overlay of the traces shown in B and D, and C and E.
Figure 4.
Forskolin potentiates both the current and rise in dendritic calcium induced by the activation of metabotropic glutamate and muscarinic receptors. A (left) raw current traces showing the aspartate (L-Asp)-induced current potentiated by forskolin (3 µM). Right side, summarized results showing individual experiments (lines) and the averaged results (bars) in control and during forskolin. B left side, raw trace of the aspartate-induced increase in fluorescence (Δ G/R) and the facilitation caused by forskolin. Right side, summarized results showing individual (lines) and averaged data (bars) indicated the facilitation in calcium release induced by forskolin. The fluorescencent indicators tested included OGB488-5N (-Δ-), Fluo-5 (-□-) and Fluo-4 (-○-). The summarized data from A and B are from the same cells. C and D show results using the iontophoretic application of acetylcholine (ACh) in the presence of hexamethonium to activate muscarinic acetylcholine receptors, using the same protocol as described in A and B. Statistical significance is signified by * p < 0.05 and *** p < 0.001.
These results suggest that these increases in sK-channel induced current by forskolin result from an increased release of calcium. One possibility is that there is a PKA dependent effect directly on the metabotropic glutamate receptor. The effect of forskolin was therefore tested on a second phosphatidylinositol (PI)-coupled receptor, i.e., the M1-muscarinic receptor. Activation of M1-receptors results in the release of calcium from intracellular stores and the activation of a calcium dependent outward current (Fiorillo and Williams, 2000). Iontophoresis of acetylcholine (ACh), in the presence of the nicotinic acetylcholine receptor antagonist, hexamethonium (200 µM), caused an outward current and a transient rise in calcium (Figure 4C,D). Forskolin increased the ACh-induced current (control: 24.6±3.1 pA, n=5; forskolin: 78.0±6.9 pA, n=5; p<0.001) and the rise in intracellular calcium (ΔG/R control: 1.13±0.29, n=5; forskolin: 2.58±0.47, n=5; p<0.05). This increase in calcium was observed with the use of both calcium indicators (Fluo5F: n=2 cells; Oregon Green BAPTA488-5N: n=3).
Next, the activation of PI-coupled receptors was bypassed and an increase in intracellular calcium was evoked by depolarizing the cell to 0 mV for 50–200 ms. The resulting apamin-sensitive outward current was measured upon repolarization to −60 mV (Cui et al., 2007;Wolfart et al., 2001a). Forskolin increased this outward current (control: 154±28 pA, n=4; forskolin: 306±51 pA, n=4, p<0.05; data not shown) and the calcium transients measured with Fluo5F (40 µM) (ΔG/R: control: 0.47±0.22 %, n=4; forskolin: 1.8±0.12%, n=4; p<0.05; data not shown).
Thus, forskolin had the same action to increase the sK current and intracellular calcium transient following stimulation of metabotropic glutamate and M1-muscarinic receptors as well as the outward current induced by calcium entry through voltage dependent calcium channels. It is therefore likely that the target of PKA-phosphorylation is not Gq-coupled receptors.
The potentiation does not require extracellular calcium
The results suggest that activation of adenylyl cyclase increases the calcium transient to facilitate the activation of the apamin sensitive potassium current. The role of extracellular calcium was tested by examining the effect of forskolin on the aspartate-induced current in calcium-free solution (with EGTA, 500µM, Figure 5). The aspartate-induced current was only slightly reduced during a 10 min treatment with calcium free solution and forskolin caused a large increase in the current under those conditions (244±1%, n=5, Figure 5A). This result suggests that the PKA-mediated facilitation of IsK is not dependent on extracellular calcium.
Dependent on intracellular calcium
Although the forskolin induced increase in outward current was accompanied by an increase in intracellular calcium, it is also possible that the sensitivity of the potassium channel to calcium was also increased. This possibility was tested with the use of photolytic release of caged-calcium, which transiently increases intracellular calcium. For these experiments the intracellular solution contained caged-calcium NP-EGTA (1.5 mM). Photolysis experiments began 10 min after the onset of recording and calcium-induced currents were evoked once every 2 min.
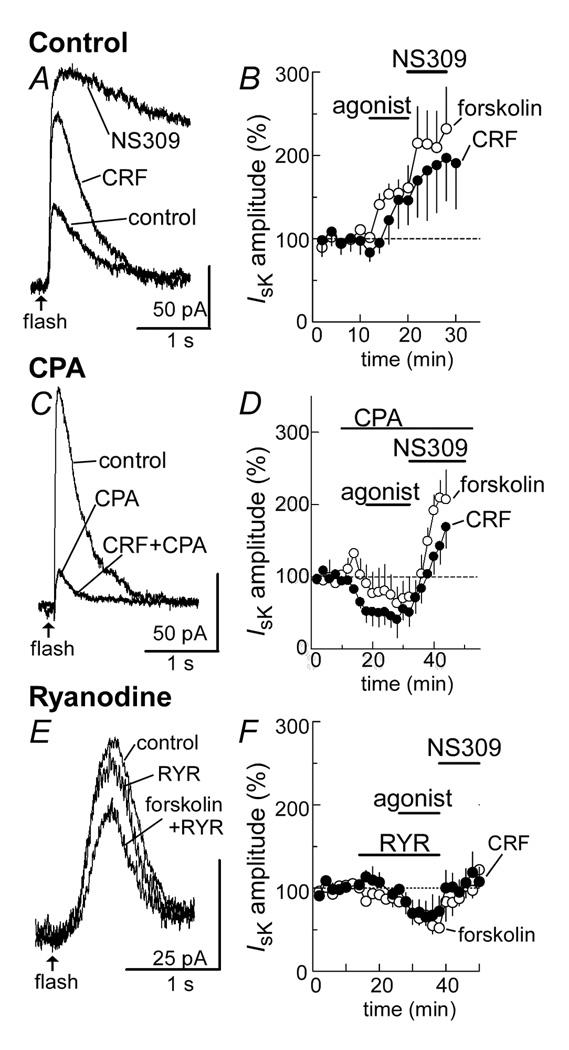
The outward current induced by the uncaging of calcium was stable over time and in all instances could be eliminated by apamin (100 nM, data not shown). Both CRF and forskolin (Figure 6A, B) increased the outward current induced by calcium photolysis (CRF: 146±29%, n=5; forskolin: 155±16%, n=6, Figure 6 A, B). Application of NS309 (10 µM) to stabilize the sK channel produced a further increase in the outward calcium induced current (CRF + NS309: 197±51%, n=5; Forskolin + NS309: 215±44%, n=5, Figure 6 A, B). These results do not rule out a direct action of PKA on the potassium channel.
Figure 6.
Photolytic release of caged-calcium induces an outward current that is facilitated by the CRF- and forskolin-evoked release of calcium from intracellular stores. A, raw traces of the outward current induced by photolysis of caged-calcium (flash) in: control, CRF (300 nM), and the sK channel stabilizer NS309 (10 µM). B, summarized results showing the increase in the current evoked by both forskolin (3 µM, n=6) and CRF (300 nM, n=5). C, raw current traces showing that the current resulting from photolysis of caged calcium is dramatically decreased after depletion of intracellular calcium stores with cyclopiazonic acid (CPA 10 µM). After CPA, CRF (300 nM) does not increase the outward current. D, summarized data showing the decline in current induced by CPA and the blockade of the forskolin- (3 µM, n=5) and CRF- (300 nM, n=5) potentiations. The current was still augmented by NS309 (10 µM). E, raw current trace showing that treatment of the slice with a high concentration of ryanodine (10 µM) to block ryanodine receptors decreased the current resulting from photolysis and blocked the facilitation caused by forskolin. F, summarized results showing the time course of the inhibition of calcium-induced currents and facilitations induced by forskolin (3 µM, n=5) and CRF (300 nM, n=5). The current was restored after treatment of the slice with NS309.
The involvement of intracellular calcium stores in calcium-dependent calcium release was examined by depleting intracellular stores with cyclopiazonic acid (CPA, 10 µM). Treatment with CPA alone produced a decrease in the outward current induced by the photolysis of caged calcium (Figure 6C,D, CRF: −48±15%, n=5; forskolin: −13±26%, n=5). This observation indicated that the photolytic release of calcium resulted in a further release of calcium from intracellular stores. Once CPA had depleted internal stores, neither CRF nor forskolin had an effect (Figure 6C, D). The increase induced by NS309 was however maintained, indicating that CPA did not change the function of sK-channels (Figure 6C, D). Thus, functional intracellular calcium stores are essential for PKA-dependent augmentation of the calcium sensitive potassium channels.
Dependent on ryanodine receptors
Blockade of ryanodine receptors with a high concentration of ryanodine (10 µM) decreased the amplitude of the current induced by photolysis of caged calcium (Figure 6E,F, ryanodine alone in CRF experiments: −24±11%, n=5; ryanodine alone in forskolin experiment: −21±14%, n=5). Likewise, ryanodine blocked the CRF- and forskolin-mediated increase in the calcium-induced outward current (Figure 6F). Thus the activation of adenylate cyclase causes a PKA dependent increase in calcium-dependent calcium-release from intracellular stores and requires active ryanodine receptors.
Two pathways
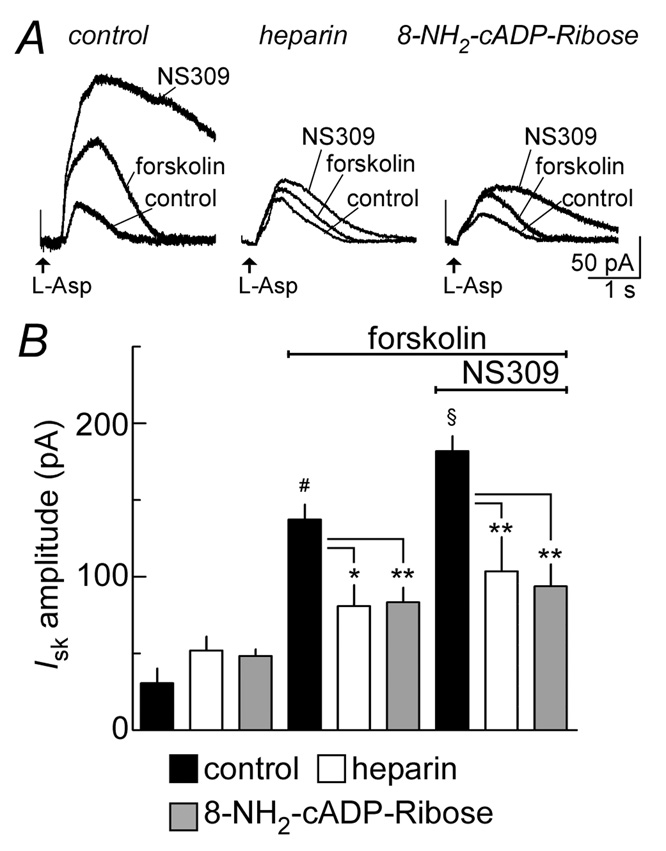
The outward current induced by the activation of metabotropic glutamate receptors is mediated by two pathways, one that is sensitive to heparin (IP3 receptor dependent) and a second that is sensitive to 8-amino-cADP-ribose (cADP/ryanodine receptor dependent, Morikawa et al 2003b). To determine if both pathways are sensitive to forskolin, two experiments were preformed. The first experiment was to block IP3 receptors with intracellular heparin (1 mg/ml), and test the effect of forskolin on the outward current induced by aspartate. The second experiment was to block cADP-ribose receptors with intracellular 8-amino-cADP-ribose (50 µM).
Under control conditions, forskolin potentiated the aspartate-induced current (control: 31±10 pA; forskolin: 137±10 pA, n=5 cells/group, p<0.001, Figure 7A, B). Inclusion of heparin or 8-NH2-cADP-ribose reduced the effectiveness of forskolin by about half (heparin + forskolin: 81±14 pA, n=6, p<0.05; 8-NH2-cADP-ribose + forskolin: 83±10 pA, n=5, p<0.01, Figure 7A, B), but forskolin still caused a significant augmentation. Subsequent addition of NS309 to forskolin further increased the IsK (forskolin + NS309: 182±10 pA, n=5 cells, p<0.01 vs. forskolin alone, Figure 7A, B). In the presence of heparin or 8-NH2-cADP-ribose the effectiveness of NS309 at the end of the experiment was reduced (heparin + forskolin +NS309: 104±22 pA, n=6, p<0.01; 8-NH2-cADP-ribose + forskolin + NS309: 94±14 pA, n=5, p<0.001, Figure 7A, B). Simultaneous infusion of both heparin and 8-NH2-cADP-ribose is know to eliminate the IsK and was not repeated in this study (Morikawa et al., 2003a). Thus, forskolin enhances both ryanodine and IP3 receptor mediated release of calcium from intracellular stores, and thereby increases the activation of sK. Together these results imply that the CRF Gs-linked pathway amplifies perhaps all Gq-receptor dependent signaling via PKA phosphorylation, leading to increased release of calcium from intracellular stores. A schematic summarizing these results is illustrated in Figure 8.
Figure 7.
Forskolin recruits release of intracellular calcium via IP3 and ryanodine receptors. A, raw traces of experiments done under three conditions: control internal (left), blockade of IP3 receptors with heparin (1 mg/ml) containing internal (middle) and blockade of ryanodine receptors with 8-amino-cADP-ribose (50 µM) containing internal (right). Each experiment shows the increase in the aspartate (L-Asp)-induced outward current caused by forskolin (3 µM) and NS309 (10 µM), a stabilizer of sK channels. B, summarized results showing the average outward current induced by aspartate in control under all three experimental conditions (left three bars, n=5 for each group), after treatment with forskolin (middle three bars) and with the addition of NS309 (right three bars). The facilitation induced by forskolin was present but reduced in experiments where IP3 receptors were blocked by heparin or cADP-ribose receptors were blocked by 8-amino-cADP-ribose. Statistical significance is signified by: # forskolin vs. control, p<0.01; § forskolin vs. forskolin + NS309, p<0.01, * p< 0.05 and **p<0.001.
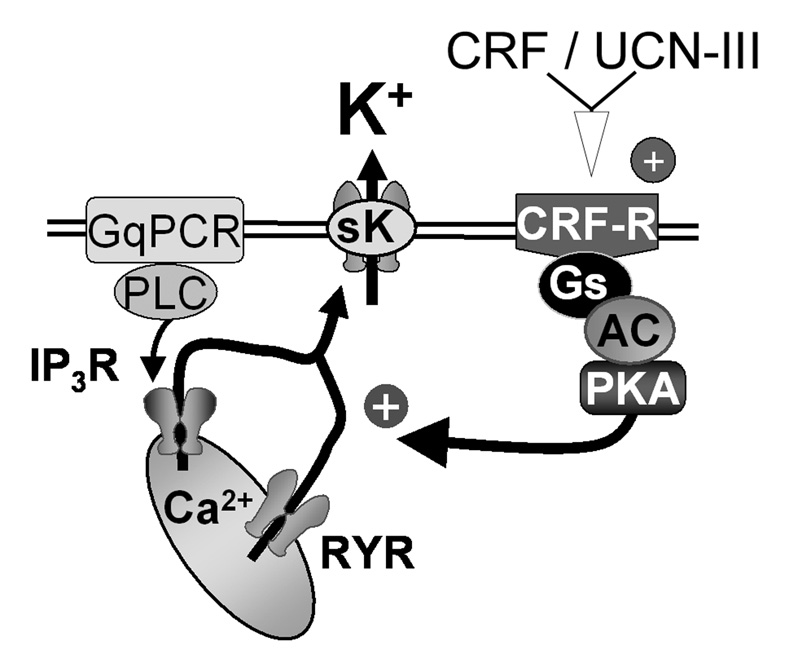
Figure 8.
A schematic summarizing the synergy between Gs- and Gq-coupled receptors in the regulation of calcium release from intracellular stores and subsequent activation of sK. Stimulation of a Gq-coupled receptors (metabotropic glutamate or the M1-muscarinic receptor) activates PLC to activate IP3- and cADP-ribose/ryanodine receptors to release calcium from intracellular stores. This release of calcium activates sK channels. The CRF receptor results in an activation of adenylate cyclase to increase cAMP production and stimulate PKA. PKA potentiates the release of calcium from intracellular stores gated by both IP3 and ryanodine receptors and thereby increases the outward calcium sensitive potassium current, sK.
DISCUSSION
This study demonstrates that in dopamine neurons, the stress hormone CRF facilitates the release of calcium from intracellular stores by a PKA-dependent mechanism. Thus the excitability of dopamine neurons would be affected by any mechanism that caused calcium release from intracellular stores. The local increase in calcium was sufficient to augment the inhibition induced by both synaptic (mGluR) and intrinsic (after-hyperpolarization) mechanisms. Photolysis experiments defined the involvement of both calcium permeable (IP3 and ryanodine) receptor channels. Given that dopamine neuron excitability is regulated by calcium-dependent (both intrinsic and synaptic) mechanisms, this synergy between Gs- and Gq-coupled systems may represent a fundamental strategy for shaping neuronal excitability.
Comparatively little is known about the cellular actions of CRF in dopamine neurons. To date, the only other reported action in these cells involves NMDA receptors, the CRF-2 receptor subtype, PKC and the CRF-binding protein (Ungless et al., 2003). Although our results with selective agonist (urocortin-III) and antagonist (antisauvagine-30) implicate the CRF-2 subtype, urocortin-III has no measurable affinity for the CRF-binding protein (Jahn et al., 2004). Moreover, activation of PKC with the phorbol ester (PDBU) did not potentiate the sK current. Thus, the PKA-dependent facilitation of cytosolic calcium in dopamine cells is distinct from other reports.
Activation of adenylyl cyclase potentiates cytosolic calcium
Four different experiments indicated that activation of adenylyl cyclase potentiates sK currents through increases in cytosolic calcium, rather than enhancing influx of extracellular calcium. First, in extracellular solution containing no added calcium (and inclusion of EGTA), CRF facilitated the sK current evoked by activation of metabotropic glutamate receptors. Second, calcium imaging experiments measured local increases in dendritic calcium, time-locked to facilitation of the sK current. Third, depletion of intracellular calcium stores with CPA or blockade of ryanodine receptors with ryanodine decreased photolysis-evoked currents and eliminated the CRF- and forskolin-evoked facilitation of sK current. Fourth, blockade of IP3-receptors with heparin or cADP-ribose receptors with 8-amino-cADP-ribose attenuated the forskolin-evoked increases in the sK current.
CRF-evoked increases in cytosolic calcium may represent an elementary action of hormones in general, with the underlying mechanism dictated by the specific neuron or cell studied. For instance, in other neuronal or non-neuronal systems, CRF also increases intracellular calcium (Dermitzaki et al., 2004;Kageyama et al., 2006;Tao et al., 2006;Yamamori et al., 2004). The increase in intracellular calcium can result from the release from internal stores and/or the activation of voltage dependent calcium channels (Duridanova et al., 1999;Haug and Storm, 2000;Huang et al., 2003;Kanno et al., 1999;Petkova-Kirova et al., 2000). Moreover, the actual release of calcium is regulated by multiple kinases (Fazal et al., 1998;Guerineau et al., 1991;Kim et al., 2007;Kuryshev et al., 1996;Lee and Tse, 1997;Luini et al., 1985;Spada et al., 1990;Takuma et al., 1994). Similar results are reported with CCK, serotonin, vasoactive intestinal peptide, neurotensin, vasopressin and norepinephrine (Belmeguenai et al., 2003;Gaisano et al., 1994;Kudoh et al., 2003;Petersen et al., 1994;Tse and Lee, 1998). The spatial and temporal properties of the increases in intracellular calcium, however, vary considerably with each hormone. The same hormone can generate global, long lasting changes in one region of the cell and yet discrete, short lasting calcium spikes in another region (Petersen et al., 1994). Thus, although our results highlight the actions of CRF and PKA on internal stores at or near dendrites, we do not exclude other possible actions of CRF in dopamine neurons.
PKA regulates increases in cytosolic calcium
Experiments with PKI revealed a PKA-dependence of CRF. These results in brain slices are consistent with other reports using expression systems to investigate CRF-R2, urocortin-III and cAMP (Hsu and Hsueh, 2001;Jahn et al., 2004;Lewis et al., 2001). The activation of PKA could augment calcium release through several actions. First, PKA phosphorylation is known to increases both the size and the rate of calcium uptake into the calcium pool (Bruce et al., 2003;Bugrim, 1999;Furuichi et al., 1994). Increasing concentrations of IP3 increases the sensitivity of IP3 receptors, and can thereby further potentiate the release of calcium (Bezprozvanny et al., 1991;Bootman and Berridge, 1995;Finch et al., 1991;Iino, 1990). Second, PKA can directly increase the open probability of ryanodine receptors, which like the IP3 contains a consensus sequences for PKA (Marx et al., 2000;Mignery et al., 1990;Sudhof et al., 1991). Third, PKA can phosphorylate certain signaling scaffolds and thereby reposition Gs-signaling components (i.e., cAMP) in close proximity to clusters of IP3 and ryanodine receptors (Augustine et al., 2003;Berridge et al., 2003;Tu et al., 1998). One relevant example is the interaction between signaling scaffold A-kinase anchoring proteins (AKAPs) and the hormone vasoactive intestinal peptide (VIP) (Beene and Scott, 2007;Smith et al., 2006). Like CRF, VIP activates Gs, adenylyl cyclase, cAMP and PKA (Hagen et al., 2006). The application of either VIP or a cAMP analog increases the release of calcium from IP3 receptors. Blockade of ryanodine receptors does not attenuate release from IP3 receptors. However, either disruption of AKAPs or the blockade of ryanodine receptors eliminates the potentiation by both VIP and the cAMP analog (Hagen et al., 2006). It is conceivable that similar actions could be relevant to CRF.
Implications for dopamine neuron excitability
In many brain regions, recruitment of sK channels regulates synaptic integration and spike frequency adaptation (Bond et al., 1999;Madison and Nicoll, 1984;Womack et al., 2004;Womack and Khodakhah, 2003). The importance of calcium in this process can be inferred from studies in the cerebellum where deletion of IP3 and ryanodine receptors dramatically disrupts LTD and impairs synaptic plasticity (Balschun et al., 1999;Inoue et al., 1998;Taufiq et al., 2005). Such changes predominantly alter weaker forms of plasticity without affecting neuronal morphology, basal synaptic transmission or presynaptic function (Balschun et al., 1999).
In dopamine neurons, opening of the sK channel is precisely controlled by increases in intracellular calcium arising from two sources (Bean, 2007;Stocker, 2004). First, synaptic activation of metabotropic glutamate receptors activates PLC to release calcium from internal stores, and thus slow pacemaker firing (Fiorillo and Williams, 1998;Morikawa et al., 2000;Morikawa et al., 2003b). Second, action potentials activate voltage gated calcium channels to initiate calcium influx across the plasma membrane, and thus stabilize spiking precision during increased action potential firing (Wolfart et al., 2001a;Wolfart and Roeper, 2002). Coordination between these systems exists, as the calcium released by a burst of action potentials is potentiated by the increases in IP3 receptor tone initiated by metabotropic glutamate receptors (Cui et al., 2007). Unlike most neurons in the brain, the axon of dopamine neurons originates from a dendrite (Hausser et al., 1995). Consequently, the generation of action potentials in dopamine cells is dendritic in origin followed by back-propagation into soma (Gentet and Williams, 2007). This arrangement renders the dendrite that posses the axon disproportionably important for generation of action potentials (Gentet and Williams, 2007). Potentiation of the sK current by CRF in that dendrite would be predicted to have a strong influence on excitability (Gentet and Williams, 2007). Thus, CRF could serve as an important coincidence detector to correlate activity inputs by integrating the calcium originating from action potentials with the release of calcium from intracellular stores induced by Gq-couple receptors (Cui et al., 2007;Nakamura et al., 1999).
Experimental Procedures
Slices and solutions
Slices containing midbrain DA neurons from adult male rats (P30–75) were prepared as described previously (Williams et al., 1984). Briefly, horizontal midbrain slices (220 µm) were cut in ice-cold saline containing (in mM): 126 NaCl, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.4 NaH2PO4, 25 NaHCO3, 11 D-glucose, 0.4 ascorbate and 0.01 MK801 using a vibratome (Leica, Nussloch, Germany). Slices were stored in 35°C oxygenated (95% O2–5% CO2) saline containing MK-801 (10 µM) for 30 min before recording. Electrophysiological recordings were performed at 35°C. The perfusion rate of oxygenated saline (95% O2–5% CO2) was 2 ml/min. Neurons were visualized with either an Olympus BX51WI (Olympus America, San Diego, CA) or Zeiss Axioskop (Germany) microscope. Microscopes were equipped with gradient contrast infrared optics (Dodt et al., 2002).
Whole cell recordings
Whole cell recordings were made using Axopatch 1D or Axopatch 200A amplifiers (Axon Instruments, Foster City, CA). Neurons were voltage clamped at −60 mV using 1.5–2.0 MΩ pipettes. Pipette internal solution contained (in mM) 115 K-methylsulfate, 20 NaCl, 1.5 MgCl2, 5 HEPES, 2 ATP and 0.3 GTP (pH 7.3, 265–270 mOsm). Unless noted otherwise, internal solution contained 0.1 mM EGTA. Series resistance (3–15 MΩ) was compensated at 80%. DA neurons near (<30 µm) the terminal nucleus of the accessory optic tract (MT) displayed pacemaker firing (1–5 Hz), broad action potentials (≥1.2 ms) and large H-currents (>300 pA at −120 mV) (Fiorillo and Williams, 1998;Ford et al., 2006;Wolfart et al., 2001b). The hyperpolarizing mGluR-mediated current carried by the sK channel is observed only in dopaminergic neurons in midbrain slices (Marino et al., 2001). Recordings were collected using AxoGraph X (AxoGraph Scientific, Sydney, Australia) and filtered at 1–2 kHz, digitized at 2–5 kHz.
A monopolar saline-filled glass electrode (3–6 MΩ) was used to evoke synaptic release. A train of stimuli (2–5 stimuli, 0.2 ms duration, 40 Hz, once per minute) was used to evoke IPSCs mediated by the activation of mGluRs (Fiorillo and Williams, 1998). The mGluR IPSC was isolated pharmacologically with picrotoxin (100 µM), 6,7-dinitroquinoxaline-2,3-dione (DNQX, 10 µM), CGP55845 (200 nM), sulpiride (1 µM) and MK-801 (10 µM). Iontophoretic application of aspartate (200 nA, 5–75 ms) was used to activate postsynaptic mGluRs. The amplitude of all sK currents were adjusted to approximately 20–30 % of maximum.
Imaging experiments
Simultaneous calcium imaging and whole cell voltage-clamp recording was preformed using an electrophysiological setup equipped with a purpose-built two-photon microscope controlled via Scan Image software (Pologruto et al., 2003). Neurons were filled for 20–25 min with an internal (K-methylsulfate) solution containing Alexa Fluor-594 (5 µM) and either Fluo-4, Fluo-5F or OregonGreenBAPTA488-5N (40 µM). The EGTA in the internal solution was omitted in these experiments. Alexa Fluor 594 (5 µM), which was not sensitive to calcium, was used to normalize the changes in fluorescence of the calcium sensitive dye (change in green fluorescence/the red fluorescence, ΔG/R, (Sabatini et al., 2002). The red fluorescence was also used to visualize the cellular morphology and to identify dendrites ~10–35 µm distal to soma where line scans were taken to measure aspartate induced change in fluorescence. By limiting the amplitude of the aspartate induced current to 10–20% of maximum, the problem of saturating the calcium indicator was reduced. The fluorescence of dendritic process (10–45 µm from soma) was measured over a period of 4 s using a line scan. Offline data analysis was performed using Image-J (NIH) and AxoGraph X (AxoGraph Scientific, Sydney, Australia). The values of ΔG/R represent averages of 3–5 line scans surrounding the maximum changes induced by each drug. Summary data are reported as mean ± SEM. Significance was defined as p < 0.05, using the two-tailed Student's t test.
Photolysis experiments
Photolysis experiments were conducted using UV pulses supplied by a shuttered mercury lamp (100 W, Olympus America, San Diego, CA) and NP-EGTA (Ellis-Davies et al., 1996;Ellis-Davies and Kaplan, 1994;Zimmermann et al., 1995). Immediately prior to use, NP-EGTA (1.5 mM) was mixed with CaCl2 (1.2 mM) (Neher and Zucker, 1993) in a K-methylsulfate internal solution lacking EGTA. Solutions were stored at −70°C and discarded after 3 days. Neurons were loaded for ~5–10 min prior to photolysis. The amplitude of the flash-evoked current increased proportionally with increasing shutter duration (12–200 ms). Baseline amplitudes were adjusted to ~10–20% of the maximum resulting from a long shutter opening duration (200–400 ms). The interval between two pulses was 2 min to allow re-equilibration of caged compound.
Drugs
DNQX, MK-801, CGP55845, CRF, cyclopiazonic acid (CPA), PKI (6–22) were obtained from Tocris Bioscience (Ellisville, MO). Heparin and forskolin were purchased from Calbiochem (La Jolla, CA). Alexa 594, Fluo-5F, fluo-4FF, OGB488-5N and NP-EGTA were purchased from Invitrogen (San Diego, CA). All other chemicals were obtained from Sigma-RBI (St. Louis, MO).
Statistical analysis
Data are expressed as means ± SEM. Statistical significance was determined by Student's t test or ANOVA followed by Bonferroni's post hoc test. The difference was considered significant at p < 0.05.
Acknowledgements
We would like to thank Drs. J. Christie, C. Jahr, G. Cui, and H. Morikawa for helpful advice regarding imaging and photolysis. We would also like to thank members of the Williams lab for their constructive criticism. This work was supported by NIH grants KO1DA020751 (A.C.R) and DA4523 (J.T.W).
Reference List
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wolfer DP, Bertocchini F, Barone V, Conti A, Zuschratter W, Missiaen L, Lipp HP, Frey JU, Sorrentino V. Deletion of the ryanodine receptor type 3 (RyR3) impairs forms of synaptic plasticity and spatial learning. EMBO J. 1999;18:5264–5273. doi: 10.1093/emboj/18.19.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Beene DL, Scott JD. A-kinase anchoring proteins take shape. Current opinion in cell biology. 2007;19:192–198. doi: 10.1016/j.ceb.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmeguenai A, Desrues L, Leprince J, Vaudry H, Tonon MC, Louiset E. Neurotensin stimulates both calcium mobilization from inositol trisphosphate-sensitive intracellular stores and calcium influx through membrane channels in frog pituitary melanotrophs. Endocrinology. 2003;144:5556–5567. doi: 10.1210/en.2003-0176. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bond CT, Maylie JA, Adelman JP. Small-Conductance Calcium-Activated Potassium Channels. Ann NY Acad Sci. 1999;868:370. doi: 10.1111/j.1749-6632.1999.tb11298.x. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Berridge MJ. The elemental principles of calcium signaling. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Thomas D, Tovey SC, Berridge MJ, Lipp P. Nuclear calcium signalling. Cell Mol. Life Sci. 2000;57:371–378. doi: 10.1007/PL00000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce JI, Straub SV, Yule DI. Crosstalk between cAMP and Ca2+ signaling in non-excitable cells. Cell Calcium. 2003;34:431–444. doi: 10.1016/s0143-4160(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Bugrim AE. Regulation of Ca2+ release by cAMP-dependent protein kinase. A mechanism for agonist-specific calcium signaling? Cell Calcium. 1999;25:219–226. doi: 10.1054/ceca.1999.0027. [DOI] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YM, Kim SH, Chung S, Uhm DY, Park MK. Regional Interaction of Endoplasmic Reticulum Ca2+ Signals between Soma and Dendrites through Rapid Luminal Ca2+ Diffusion. J. Neurosci. 2006;26:12127–12136. doi: 10.1523/JNEUROSCI.3158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Bernier BE, Harnett MT, Morikawa H. Differential regulation of action potential- and metabotropic glutamate receptor-induced Ca2+ signals by inositol 1,4,5-trisphosphate in dopaminergic neurons. J. Neurosci. 2007;27:4776–4785. doi: 10.1523/JNEUROSCI.0139-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermitzaki I, Tsatsanis C, Alexaki VI, Castanas E, Margioris AN. Roles of protein kinase A (PKA) and PKC on corticotropin-releasing hormone (CRH)-induced elevation of cytosolic calcium from extra-and intra-cellular sources. Hormones. (Athens. ) 2004;3:252–258. doi: 10.14310/horm.2002.11134. [DOI] [PubMed] [Google Scholar]
- Dodt HU, Eder M, Schierloh A, Zieglgansberger W. Infrared-guided laser stimulation of neurons in brain slices. Sci. STKE. 2002;2002:L2. doi: 10.1126/stke.2002.120.pl2. [DOI] [PubMed] [Google Scholar]
- Duridanova DB, Petkova-Kirova PS, Lubomirov LT, Gagov H, Boev K. Corticotropin-releasing hormone acts on guinea pig ileal smooth muscle via protein kinase A. Pflugers Arch. 1999;438:205–212. doi: 10.1007/s004240050899. [DOI] [PubMed] [Google Scholar]
- Ellis-Davies GC, Kaplan JH. Nitrophenyl-EGTA, a photolabile chelator that selectively binds Ca2+ with high affinity and releases it rapidly upon photolysis. Proc. Natl. Acad. Sci. U. S. A. 1994;91:187–191. doi: 10.1073/pnas.91.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Davies GC, Kaplan JH, Barsotti RJ. Laser photolysis of caged calcium: rates of calcium release by nitrophenyl-EGTA and DM-nitrophen. Biophys. J. 1996;70:1006–1016. doi: 10.1016/S0006-3495(96)79644-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazal N, Slominski A, Choudhry MA, Wei ET, Sayeed MM. Effect of CRF and related peptides on calcium signaling in human and rodent melanoma cells. FEBS Lett. 1998;435:187–190. doi: 10.1016/s0014-5793(98)01067-9. [DOI] [PubMed] [Google Scholar]
- Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature. 1998;394:78–82. doi: 10.1038/27919. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. Cholinergic inhibition of ventral midbrain dopamine neurons. J. Neurosci. 2000;20:7855–7860. doi: 10.1523/JNEUROSCI.20-20-07855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Cha CI. Distribution of corticotropin-releasing-factor-like immunoreactivity in brainstem of two monkey species (Saimiri sciureus and Macaca fascicularis): an immunohistochemical study. J. Comp Neurol. 1988;276:239–264. doi: 10.1002/cne.902760208. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J. Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak D-OD. Inositol Trisphosphate Receptor Ca2+ Release Channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi T, Kohda K, Miyawaki A, Mikoshiba K. Intracellular channels. Curr. Opin. Neurobiol. 1994;4:294–303. doi: 10.1016/0959-4388(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Gaisano HY, Wong D, Sheu L, Foskett JK. Calcium release by cholecystokinin analogue OPE is IP3 dependent in single rat pancreatic acinar cells. Am. J. Physiol. 1994;267:C220–C228. doi: 10.1152/ajpcell.1994.267.1.C220. [DOI] [PubMed] [Google Scholar]
- Gentet LJ, Williams SR. Dopamine gates action potential backpropagation in midbrain dopaminergic neurons. J. Neurosci. 2007;27:1892–1901. doi: 10.1523/JNEUROSCI.5234-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Guerineau N, Corcuff JB, Tabarin A, Mollard P. Spontaneous and corticotropin-releasing factor-induced cytosolic calcium transients in corticotrophs. Endocrinology. 1991;129:409–420. doi: 10.1210/endo-129-1-409. [DOI] [PubMed] [Google Scholar]
- Hagen BM, Bayguinov O, Sanders KM. VIP and PACAP regulate localized Ca2+ transients via cAMP-dependent mechanism. Am J Physiol Cell Physiol. 2006;291:C375–C385. doi: 10.1152/ajpcell.00495.2005. [DOI] [PubMed] [Google Scholar]
- Haug T, Storm JF. Protein kinase A mediates the modulation of the slow Ca(2+)-dependent K(+) current, I(sAHP), by the neuropeptides CRF, VIP, and CGRP in hippocampal pyramidal neurons. J Neurophysiol. 2000;83:2071–2079. doi: 10.1152/jn.2000.83.4.2071. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS. Neurol. Disord. Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser M, Stuart G, Racca C, Sakmann B. Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron. 1995;15:637–647. doi: 10.1016/0896-6273(95)90152-3. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat. Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- Huang Y, Chan FL, Lau CW, Tsang SY, Chen ZY, He GW, Yao X. Roles of cyclic AMP and Ca2+-activated K+ channels in endothelium-independent relaxation by urocortin in the rat coronary artery. Cardiovasc. Res. 2003;57:824–833. doi: 10.1016/s0008-6363(02)00773-3. [DOI] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J. Gen. Physiol. 1990;95:1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Kato K, Kohda K, Mikoshiba K. Type 1Inositol 1,4,5-Trisphosphate Receptor Is Required for Induction of Long-Term Depression in Cerebellar Purkinje Neurons. J. Neurosci. 1998;18:5366–5373. doi: 10.1523/JNEUROSCI.18-14-05366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn O, Tezval H, van WL, Eckart K, Spiess J. Three-amino acid motifs of urocortin II and III determine their CRF receptor subtype selectivity. Neuropharmacology. 2004;47:233–242. doi: 10.1016/j.neuropharm.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Kimura R, Suga S, Ogawa Y, Suda T, Wakui M. Modulation of Ca2+ influx by corticotropin-releasing factor (CRF) family of peptides via CRF receptors in rat pancreatic beta-cells. Peptides. 2006;27:1814–1819. doi: 10.1016/j.peptides.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Kanno T, Suga S, Nakano K, Kamimura N, Wakui M. Corticotropin-releasing factor modulation of Ca2+ influx in rat pancreatic beta-cells. Diabetes. 1999;48:1741–1746. doi: 10.2337/diabetes.48.9.1741. [DOI] [PubMed] [Google Scholar]
- Kim Y, Park MK, Uhm DY, Chung S. Modulation of T-type Ca(2+) channels by corticotropin-releasing factor through protein kinase C pathway in MN9D dopaminergic cells. Biochem. Biophys. Res. Commun. 2007 doi: 10.1016/j.bbrc.2007.04.198. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacol. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur. J. Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Kudoh A, Kudoh E, Katagai H, Takazawa T. Norepinephrine-induced inositol 1,4,5-trisphosphate formation in atrial myocytes is regulated by extracellular calcium, protein kinase C, and calmodulin. Jpn. Heart J. 2003;44:547–556. doi: 10.1536/jhj.44.547. [DOI] [PubMed] [Google Scholar]
- Kuryshev YA, Childs GV, Ritchie AK. Corticotropin-releasing hormone stimulates Ca2+ entry through L- and P-type Ca2+ channels in rat corticotropes. Endocrinology. 1996;137:2269–2277. doi: 10.1210/endo.137.6.8641175. [DOI] [PubMed] [Google Scholar]
- Lavicky J, Dunn AJ. Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis. J. Neurochem. 1993;60:602–612. doi: 10.1111/j.1471-4159.1993.tb03191.x. [DOI] [PubMed] [Google Scholar]
- Lee AK, Tse A. Mechanism underlying corticotropin-releasing hormone (CRH) triggered cytosolic Ca2+ rise in identified rat corticotrophs. J Physiol. 1997;504(Pt 2):367–378. doi: 10.1111/j.1469-7793.1997.367be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Acute and Chronic CRF1 Receptor Blockade Inhibits Cocaine-induced Dopamine Release: Correlation with Dopamine Neuron Activity. J Pharmacol Exp Ther. 2005 doi: 10.1124/jpet.105.084913. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu Z, Huang M, Zhang Z. Dopamine-dependent responses to cocaine depend on corticotropin-releasing factor receptor subtypes. J. Neurochem. 2003;84:1378–1386. doi: 10.1046/j.1471-4159.2003.01635.x. [DOI] [PubMed] [Google Scholar]
- Luini A, Lewis D, Guild S, Corda D, Axelrod J. Hormone secretagogues increase cytosolic calcium by increasing cAMP in corticotrophin-secreting cells. Proc. Natl. Acad. Sci. U. S. A. 1985;82:8034–8038. doi: 10.1073/pnas.82.23.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J. Physiol. 1984;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni OJ, Williams JT. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J. Neurosci. 1999;19:6629–6636. doi: 10.1523/JNEUROSCI.19-15-06629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MJ, Wittmann M, Bradley SR, Hubert GW, Smith Y, Conn PJ. Activation of Group I Metabotropic Glutamate Receptors Produces a Direct Excitation and Disinhibition of GABAergic Projection Neurons in the Substantia Nigra Pars Reticulata. J. Neurosci. 2001;21:7001–7012. doi: 10.1523/JNEUROSCI.21-18-07001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Mignery GA, Newton CL, Archer BT, III, Sudhof TC. Structure and expression of the rat inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1990;265:12679–12685. [PubMed] [Google Scholar]
- Morikawa H, Fukuda K, Mima H, Shoda T, Kato S, Mori K. Desensitization and resensitization of delta-opioid receptor-mediated Ca2+ channel inhibition in NG108-15 cells. Br. J. Pharmacol. 1998;123:1111–1118. doi: 10.1038/sj.bjp.0701733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Khodakhah K, Williams JT. Two intracellular pathways mediate metabotropic glutamate receptor-induced Ca2+ mobilization in dopamine neurons. J Neurosci. 2003a;23:149–157. doi: 10.1523/JNEUROSCI.23-01-00149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Imani F, Khodakhah K, Williams JT. Inositol 1,4,5-Triphosphate-Evoked Responses in Midbrain Dopamine Neurons. J. Neurosci. 2000;20:103RC. doi: 10.1523/JNEUROSCI.20-20-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Khodakhah K, Williams JT. Two Intracellular Pathways Mediate Metabotropic Glutamate Receptor-Induced Ca2+ Mobilization in Dopamine Neurons. J. Neurosci. 2003b;23:149–157. doi: 10.1523/JNEUROSCI.23-01-00149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Barbara JG, Nakamura K, Ross WN. Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials. Neuron. 1999;24:727–737. doi: 10.1016/s0896-6273(00)81125-3. [DOI] [PubMed] [Google Scholar]
- Neher E, Zucker RS. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993;10:21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Orozco-Cabal L, Pollandt S, Liu J, Shinnick-Gallagher P, Gallagher JP. Regulation of synaptic transmission by CRF receptors. Rev. Neurosci. 2006;17:279–307. doi: 10.1515/revneuro.2006.17.3.279. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Fiorillo CD, Morikawa H, Williams JT. Amphetamine selectively blocks inhibitory glutamate transmission in dopamine neurons. Nat. Neurosci. 2001;4:275–281. doi: 10.1038/85124. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Williams JT. Noradrenergic Inhibition of Midbrain Dopamine Neurons. J. Neurosci. 2004;24:4568–4575. doi: 10.1523/JNEUROSCI.5735-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, McCutcheon JE, Rogge G, Jensen BS, Christophersen P, Hougaard C, Strobaek D, Stocker M. Specific enhancement of SK channel activity selectively potentiates the afterhyperpolarizing current I(AHP) and modulates the firing properties of hippocampal pyramidal neurons. J. Biol. Chem. 2005;280:41404–41411. doi: 10.1074/jbc.M509610200. [DOI] [PubMed] [Google Scholar]
- Petersen OH, Petersen CC, Kasai H. Calcium and hormone action. Annu. Rev. Physiol. 1994;56:297–319. doi: 10.1146/annurev.ph.56.030194.001501. [DOI] [PubMed] [Google Scholar]
- Petkova-Kirova PS, Gagov HS, Duridanova DB. Urocortin hyperpolarizes stomach smooth muscle via activation of Ca2+-sensitive K+ currents. J Muscle Res. Cell Motil. 2000;21:639–645. doi: 10.1023/a:1005653218639. [DOI] [PubMed] [Google Scholar]
- Pologruto TA, Sabatini BL, Svoboda K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng Online. 2003;2:13. doi: 10.1186/1475-925X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 Suppl 2:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Oertner TG, Svoboda K. The Life Cycle of Ca2+ Ions in Dendritic Spines. Neuron. 2002;33:439–452. doi: 10.1016/s0896-6273(02)00573-1. [DOI] [PubMed] [Google Scholar]
- Sauvage M, Steckler T. Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei--potential implication for arousal and attention. Neuroscience. 2001;104:643–652. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Scott JD, Fischer EH, Demaille JG, Krebs EG. Identification of an inhibitory region of the heat-stable protein inhibitor of the cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. U. S. A. 1985;82:4379–4383. doi: 10.1073/pnas.82.13.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JD, Glaccum MB, Fischer EH, Krebs EG. Primary-structure requirements for inhibition by the heat-stable inhibitor of the cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. U. S. A. 1986;83:1613–1616. doi: 10.1073/pnas.83.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FD, Langeberg LK, Scott JD. The where's and when's of kinase anchoring. Trends Biochem. Sci. 2006;31:316–323. doi: 10.1016/j.tibs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Spada A, Reza-Elahi F, Lania A, Bassetti M, Atti E. Inhibition of basal and corticotropin-releasing hormone-stimulated adenylate cyclase activity and cytosolic Ca2+ levels by somatostatin in human corticotropin-secreting pituitary adenomas. J Clin. Endocrinol. Metab. 1990;70:1262–1268. doi: 10.1210/jcem-70-5-1262. [DOI] [PubMed] [Google Scholar]
- Spira ME, Oren R, Dormann A, Ilouz N, Lev S. Calcium, protease activation, and cytoskeleton remodeling underlie growth cone formation and neuronal regeneration. Cell Mol. Neurobiol. 2001;21:591–604. doi: 10.1023/A:1015135617557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel P, Kesterson R, Yeung W, Cone RD, Rittenberg MB, Stenzel-Poore MP. Identification of a novel murine receptor for corticotropin-releasing hormone expressed in the heart. Mol. Endocrinol. 1995;9:637–645. doi: 10.1210/mend.9.5.7565810. [DOI] [PubMed] [Google Scholar]
- Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- Strobaek D, Teuber L, Jorgensen TD, Ahring PK, Kjaer K, Hansen RS, Olesen SP, Christophersen P, Skaaning-Jensen B. Activation of human IK and SK Ca2+ -activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) Biochim. Biophys. Acta. 2004;1665:1–5. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Sudhof TC, Newton CL, Archer BT, III, Ushkaryov YA, Mignery GA. Structure of a novel InsP3 receptor. EMBO J. 1991;10:3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Takuma K, Matsuda T, Yoshikawa T, Kitanaka J, Gotoh M, Hayata K, Baba A. Corticotropin-releasing factor stimulates Ca2+ influx in cultured rat astrocytes. Biochem. Biophys. Res. Commun. 1994;199:1103–1107. doi: 10.1006/bbrc.1994.1344. [DOI] [PubMed] [Google Scholar]
- Tao J, Zhang Y, Soong TW, Li S. Expression of urocortin 2 and its inhibitory effects on intracellular ca2+ via L-type voltage-gated calcium channels in rat pheochromocytoma (PC12) cells. Neuropsychopharmacol. 2006;31:2600–2609. doi: 10.1038/sj.npp.1301123. [DOI] [PubMed] [Google Scholar]
- Taufiq AM, Fujii S, Yamazaki Y, Sasaki H, Kaneko K, Li J, Kato H, Mikoshiba K. Involvement of IP3 receptors in LTP and LTD induction in guinea pig hippocampal CA1 neurons. Learn. Mem. 2005;12:594–600. doi: 10.1101/lm.17405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse A, Lee AK. Arginine vasopressin triggers intracellular calcium release, a calcium-activated potassium current and exocytosis in identified rat corticotropes. Endocrinology. 1998;139:2246–2252. doi: 10.1210/endo.139.5.5999. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-Releasing Factor Requires CRF Binding Protein to Potentiate NMDA Receptors via CRF Receptor 2 in Dopamine Neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons. J. Neurosci. 2001a;21:3443–3456. doi: 10.1523/JNEUROSCI.21-10-03443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart J, Neuhoff H, Franz O, Roeper J. Differential Expression of the Small-Conductance, Calcium-Activated Potassium Channel SK3 Is Critical for Pacemaker Control in Dopaminergic Midbrain Neurons. J. Neurosci. 2001b;21:3443. doi: 10.1523/JNEUROSCI.21-10-03443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart J, Roeper J. Selective Coupling of T-Type Calcium Channels to SK Potassium Channels Prevents Intrinsic Bursting in Dopaminergic Midbrain Neurons. J. Neurosci. 2002;22:3404–3413. doi: 10.1523/JNEUROSCI.22-09-03404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J. Neurosci. 2004;24:8818–8822. doi: 10.1523/JNEUROSCI.2915-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar purkinje neurons. J. Neurosci. 2003;23:2600–2607. doi: 10.1523/JNEUROSCI.23-07-02600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori E, Iwasaki Y, Oki Y, Yoshida M, Asai M, Kambayashii M, Oiso Y, Nakashima N. Possible involvement of ryanodine receptor-mediated intracellular calcium release in the effect of corticotropin-releasing factor on adrenocorticotropin secretion. Endocrinology. 2004;145:36–38. doi: 10.1210/en.2003-1222. [DOI] [PubMed] [Google Scholar]
- Ziff EB. TARPs and the AMPA receptor trafficking paradox. Neuron. 2007;53:627–633. doi: 10.1016/j.neuron.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Zimmermann B, Somlyo AV, Ellis-Davies GC, Kaplan JH, Somlyo AP. Kinetics of prephosphorylation reactions and myosin light chain phosphorylation in smooth muscle. Flash photolysis studies with caged calcium and caged ATP. J. Biol. Chem. 1995;270:23966–23974. doi: 10.1074/jbc.270.41.23966. [DOI] [PubMed] [Google Scholar]