Abstract
Neural induction is widely believed to be a direct consequence of inhibition of BMP pathways. Because of conflicting results and interpretations, we have reexamined this issue in Xenopus and chick embryos using the powerful and general TGFβ inhibitor, Smad7, which inhibits both Smad1- (BMP) and Smad2- (Nodal/Activin) mediated pathways. We confirm that Smad7 efficiently inhibits phosphorylation of Smad1 and Smad2. Surprisingly, however, over-expression of Smad7 in Xenopus ventral epidermis induces expression of the dorsal mesodermal markers Chordin and Brachyury. Neural markers are induced, but in a non-cell-autonomous manner and only when Chordin and Brachyury are also induced. Simultaneous inhibition of Smad1 and Smad2 by different approaches does not acount for Smad7 effects, indicating that Smad7 has activities other than inhibition of the TGFβ pathway. We provide evidence that these effects are independent of Wnt, FGF, Hedgehog and retinoid signalling. We also show that these effects are due to elements outside of the MH2 domain of Smad7. Together, these results indicate that BMP inhibition is not sufficient for neural induction even when Nodal/Activin is also blocked, and that Smad7 activity is considerably more complex than had previously been assumed. We suggest that experiments relying on Smad7 as an inhibitor of TGFβ-pathways should be interpreted with considerable caution.
Keywords: Xenopus, chick, neural induction, FGF, BMP, Wnt, hedgehog, retinoic acid, Smad6, Smad7, TGFβ
Introduction
BMPs are members of the transforming growth factor β (TGFβ) family of secreted proteins. The main mechanism of signal transduction for these proteins involves two serine/threonine kinase receptors: type-I and type-II. Upon ligand binding the receptor complex phosphorylates particular members of the Smad family of proteins, the receptor regulated Smads (R-Smads) (Hill, 2001; Massague et al., 2005; Park, 2005; Shi and Massague, 2003; ten Dijke and Hill, 2004; von Bubnoff and Cho, 2001). Phosphorylated R-Smads are released from the receptor complex and bind to Smad4, allowing the translocation of this complex to the nucleus, where it regulates transcription of target genes (von Bubnoff and Cho, 2001). Other than the R-Smads and Smad4, inhibitory Smads (I-Smads) have been shown to be important regulators of this pathway. Two major I-Smads have been characterized: Smad-6, which preferentially inhibits the BMP pathway and Smad-7, which blocks all TGFβ signalling. I-Smads bind to the intracellular domain of receptor type-I, recruit Smurf ubiquitin ligases and induce degradation of the receptor (Hill, 2001; ten Dijke and Hill, 2004). In addition, Smad6 inhibits BMP signalling by competing with Smad4 for binding to phosphorylated Smad1, yielding inactive Smad1-Smad6 complexes (Hata et al., 1998). Through these mechanisms I-Smads inhibit the TGFβ pathway in a cell-autonomous way.
BMP signalling plays numerous roles in development. One of the best studied processes involving regulation of BMP signalling is neural induction – an early embryonic event first demonstrated clearly almost a century ago when it was shown that signals emanating from the organizer (the dorsal lip of the blastopore in amphibians) can instruct ectoderm to acquire a neural fate (Spemann and Mangold, 1924). The first molecular explanation for this process (the “default model”) is that BMPs, which are broadly expressed in the early embryo, act as epidermal inducers and need to be inhibited in the prospective neural plate for this structure to form (Harland, 2000; Hemmati-Brivanlou and Melton, 1997a; Hemmati-Brivanlou and Melton, 1997b; Muñoz-Sanjuán and Brivanlou, 2002). Although there is some controversy concerning whether or not the default model provides a sufficient explanation for this process (see for example Bachiller et al., 2000; Belo et al., 2000; Bertrand et al., 2003; Delaune et al., 2005; Linker and Stern, 2004; McMahon et al., 1998; Mukhopadhyay et al., 2001; Stern, 2004; Streit et al., 2000; Streit et al., 1998; Streit and Stern, 1999a) it is clear that inhibition of BMP signalling is part of the neural induction process in vertebrates.
One of the ways in which the involvement of BMPs as epidermal inducers and neural inhibitors has been tested in Xenopus is by misexpression of the powerful inhibitory Smad, Smad7, a double-inhibitor of both the Smad1-,5-,8- (BMPs) and 2-,3-dependent (Nodal/Activin) TGFβ pathways (Casellas and Brivanlou, 1998; Chang and Harland, 2007; Nakao et al., 1997; Nakayama et al., 2001). Here we use this reagent to re-examine the role of BMP signalling in neural induction in Xenopus and chick. We confirm that Smad7 efficiently inhibits the phosphorylation of both Smad1 and Smad2. Overexpression of Smad7 does not induce neural markers in chick competent ectoderm. Surprisingly, in Xenopus, over-expression of Smad7 in ventral epidermis induces Chordin and Brachyury, markers of dorsal mesoderm, and as a secondary effect, neural markers are induced in a non-cell-autonomous manner. These effects cannot be explained entirely by inhibition of all (Nodal/Activin and BMP-related) TGFβ signalling, because inhibition of Nodal/Activin-related signalling by co-injection of either Cerberus Short (CerS) or a truncated form of a type I receptor (tAlk4), together with inhibition of BMP-related signals by either Smad6 or a truncated version of Smad7 (lacking the MH1 domain) does not produce the same effects. These results suggest that Smad7 has activities other than inhibition of TGFβ pathways and that these activities are due to functional elements outside of the MH2 domain. We also provide evidence that the effects of Smad7 unrelated to TGFβ signalling are independent of Wnt, FGF, hedgehog and retinoid signalling. Although these activities of Smad7 remain only partially understood, this study suggests that great caution should be exercised in interpreting the results of experiments using Smad7 as a BMP/TGFβ antagonist.
Results
Smad7 does not induce neural markers in the chick
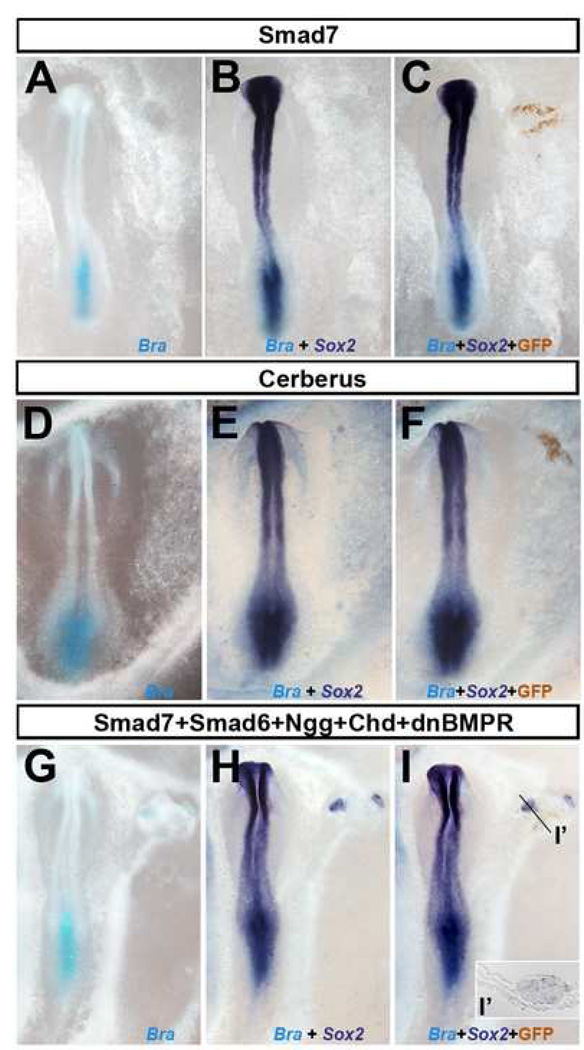
Previous work has shown that BMP inhibition, through over-expression of soluble or cell autonomous antagonists, does not induce neural markers in chick epiblast (Linker and Stern, 2004; Streit et al., 1998). It is possible that the absence of neural induction in these assays is due to an incomplete inhibition of the BMP pathway. Since it has been reported that Smad7 is one of the most effective BMP inhibitors (Casellas and Brivanlou, 1998; Ishisaki et al., 1999; Nakao et al., 1997; Nakayama et al., 2001), we used this to test whether Smad7 can induce neural markers in chick. When Smad7 was electroporated into the area opaca of stage 3+ embryos, either alone or in combination with other BMP inhibitors, no expression of mesodermal (brachyury, chordin) or neural (Sox2) markers was observed after 18–20 hours’ incubation (Smad7, 0/12; other BMP inhibitors 0/9; Fig. 1 and Supplementary Fig. 1). Thus, consistent with previous observations (Delaune et al., 2005; Linker and Stern, 2004), inhibition of BMP is not sufficient to induce Sox2 in competent chick epiblast.
Fig. 1. Smad7 is not sufficient for neural induction in chick.
Electroporation of Smad7 (A–C; 0/12), Cerberus (D–F; 0/4) or a combination of Smad7+Smad6+Noggin+Chordin+dnBMPR (0/9) does not induce either Brachyury (light blue in A, D and G) or Sox2 (dark blue in B, E and H; the same embryo to the left). Electroporated cells were recognised by GFP expression (C, F, I and I’ in the same embryo to the left). The plane of section is indicated by a black line.
Neural induction by Smad7 in Xenopus is a secondary effect of dorsal mesoderm induction
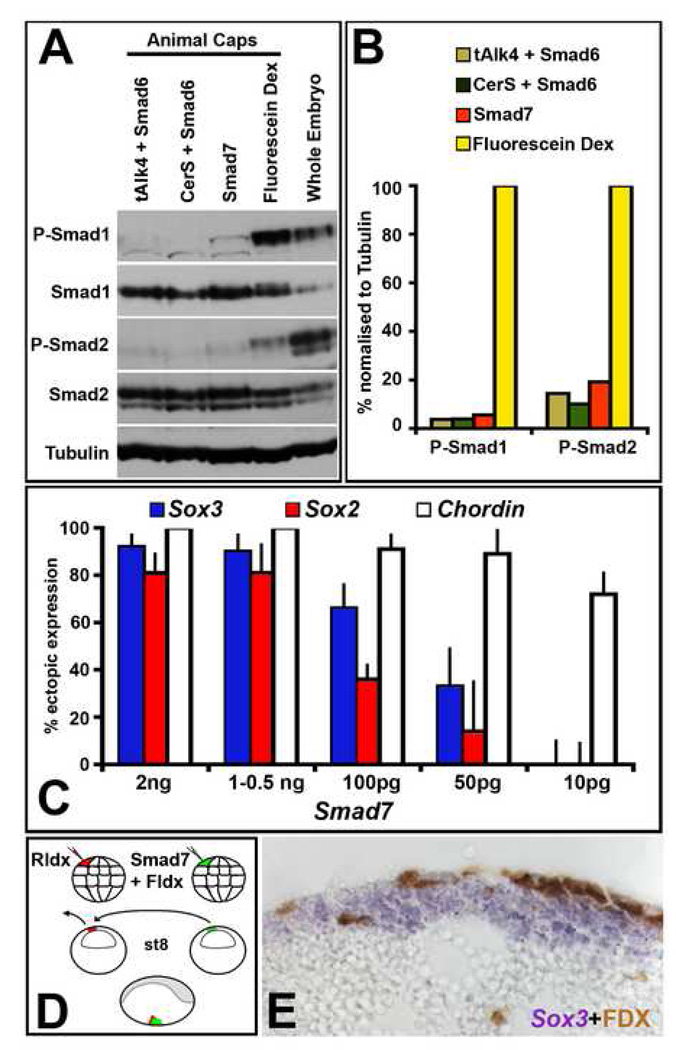
Some studies have reported that in Xenopus ventral epidermis, as in the chick, inhibition of BMP is not sufficient to induce neural markers (Chang and Harland, 2007; Delaune et al., 2005; Linker and Stern, 2004). However the possibility remains that BMP signalling was not completely inhibited in these studies. We therefore tested the activity of Smad7 in Xenopus embryos (Fig. 2). When injected into the ventral marginal zone of 4–8 cell stage embryos (where it should block mainly BMP signals), Smad7 (2ng) induces dorsal tissues and an ectopic axis (42/50; not shown), confirming that Smad7 does act as previously described (Casellas and Brivanlou, 1998). To test the efficiency of Smad7, we analyzed the levels of phosphorylation of Smad1 and Smad2 in Smad7-injected animal caps. Both are greatly reduced (Fig. 3 A, B), confirming the potent inhibitory effect of Smad7 on both Nodal/Activin (Smad2-dependent) and BMP-related (Smad1-dependent) TGFβ signalling (Casellas and Brivanlou, 1998).
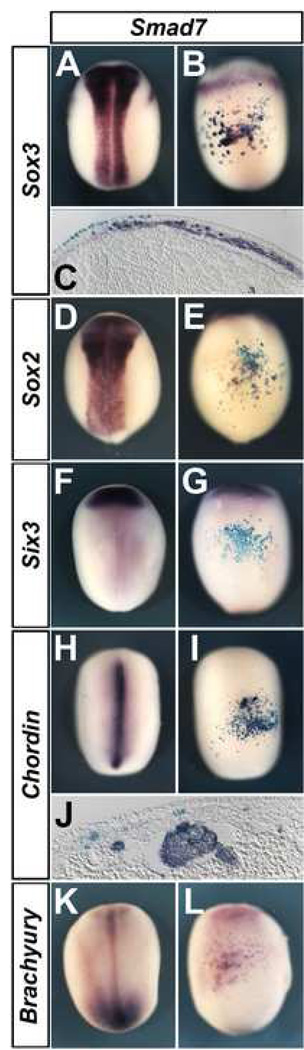
Fig. 2. Smad7 induces dorsal mesoderm in Xenopus ventral epidermis, and neural markers only indirectly.
Injection of Smad7 (2 ng) into the A4 blastomere induces Sox3 (A–C), Sox2 (D–E), Chordin (H–J) and Brachyury (K–L), but not Six3 (F–G). C, J. Histological sections through the levels indicated in B and I, respectively. A, D, F, H and K are dorsal views. B, E, G, I and L show ventral views of the embryo to their left.
Fig. 3. Smad7 blocks Smad1 and Smad2 phosphorylation but induces neural markers only indirectly.
A., B. Inhibition of phospho-Smad1 and phospho-Smad2 by Smad7, tAlk4+Smad6 and CerS+Smad6, revealed by Western blot analysis of animal caps from injected embryos. Fldx-injection and whole embryos are included as controls. B. Quantification of the level of phosphorylated Smad1 and Smad2 in the above experiment. C. Effects of decreasing concentration of Smad7 on Sox3, Sox2 and Chordin induction after injection into the A4 blastomere. 2 ng: Sox3 92%, Sox2 81%, Chordin 100%; 1–0.5 ng: Sox3 89%, Sox2 80%, Chordin 100%; 100 pg: Sox3 66%, Sox2 36%, Chordin 91%; 50 pg: Sox3 33%, Sox2 14%, Chordin 88%; 10 pg: Sox3 0%, Sox2 0%, Chordin 72%. D. The induction of Sox3 is not cell autonomous to the progeny of the Smad7-injected A4 blastomere. Embryos were injected with either Rldx or Smad7+Fldx in the A4 blastomere at the 32-cell stage, and isotopic transplants performed at stage 8, replacing Rldx-labelled cells with the progeny of Smad7(2ng)+Fldx-injected cells. Embryos were grown to stage 18 and stained for Sox3 and Fldx. E. Section through the transplanted area showing expression of Sox3 in uninjected cells of the host (8/8).
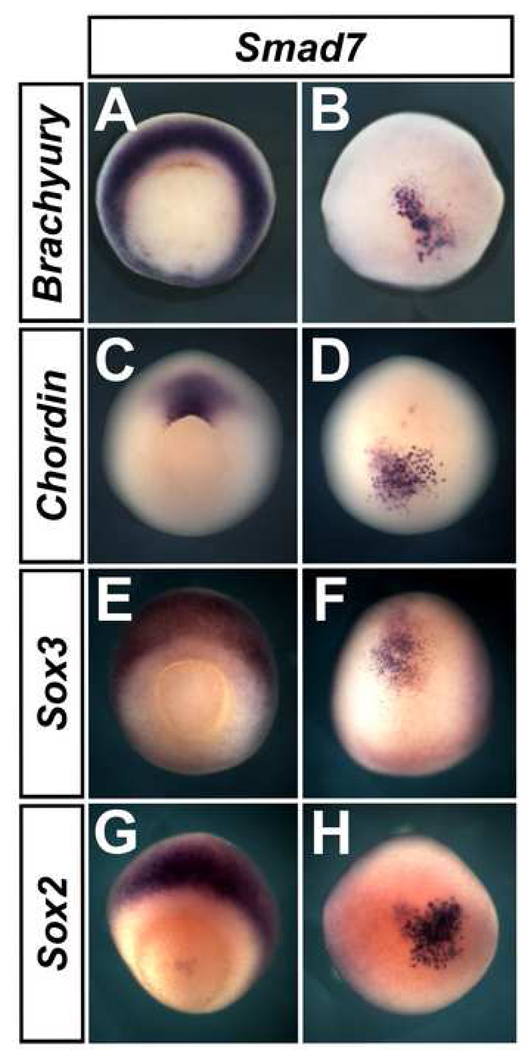
Next, we analysed whether Smad7 can induce neural markers in ventral ectoderm. 32-cell-stage embryos were injected into the A4 blastomere and cultured to stage 18. In these experiments both Sox3 (66/72) and Sox2 (47/58) are induced in ventral epidermis (Fig. 2 A–E). To test whether this induction is direct, we analysed the expression of mesodermal markers. We were surprised to find that Smad7 strongly induces the organizer/notochord makers chordin (52/52; Fig. 2 H–J) and brachyury (25/32; Fig. 2 K–L), although other dorsal markers are not induced [goosecoid 0/36; pintallavis 0/24; Loxl3 (Geach and Dale, 2005) 0/39; not shown]. In normal embryos, chordin is expressed at low levels in the anterior neural plate (in addition to stronger expression in the notochord). To test the possibility that the Smad7-induced chordin expression represents anterior neural plate rather than dorsal mesoderm, we analysed the expression of Six3, a specific marker for anterior neural tissue. Six3 was never expressed in Smad7-injected embryos (0/41; Fig. 2 F–G), suggesting that the brachyury- and chordin-expressing cells are not anterior neural plate but rather dorsal mesoderm, also consistent with the observation that chordin-expressing cells are located internally and have a notochord-like morphology (Fig. 2J). We then tested if the ectopic induction of dorsal mesoderm and neural markers by Smad7 mirrors the timing of its endogenous induction. We find that brachyury (42/45; Fig. 4 A–B), chordin (22/24; Fig. 4 C–D), Sox3 (25/34; Fig. 4 E–F) and Sox2 (24/31; Fig. 4 G–H) are expressed in the embryo at the gastrula stage, indicating that Smad7 may modulate the endogenous signals that regulate expression of these markers.
Fig. 4. Smad7 induces dorsal mesodermal and neural markers in Xenopus ventral epidermis at gastrula stages.
Injection of Smad7 (2 ng) into the A4 blastomere induces Brachyury (A–B), Chordin (C–D), Sox3 (E–F) and Sox2 (G–H). A, C, E and G are shown in vegetal view, B, D, F and H in animal view.
One concern raised by this experiment is the possibility of non-specific effects due to the high dose of Smad7 used (2ng mRNA). We therefore analyzed the response of neural and mesodermal markers to different doses of Smad7. As the concentration decreases, neural markers are lost first, until at 10pg only dorsal mesodermal markers are induced (Fig. 3C). These results strongly suggest that the induction of neural markers in ventral epidermis by Smad7 is indirect, a secondary consequence of mesoderm induction. Consistent with this, histological sections reveal Chordin expression in LacZ-positive cells (derived from the injected blastomere; Fig. 2J), while Sox3 expression is observed in both LacZ-positive and -negative cells, some of which are located more than 7 cell-diameters away from the nearest descendants of the injected cell (Fig. 2C). This suggests that the induction of neural markers is not cell-autonomous to the Smad7-injected cells. To confirm this, we used transplantation to test whether Smad7-injected cells are able to induce neural markers in neighbouring cells. Smad7-injected cells were grafted isotopically into control host embryos (Fig. 3D). Sections reveal Sox3 expression in host cells (Fig. 3E), confirming that the neural inducing effect of Smad7 is not cell-autonomous. In conclusion, Smad7 only induces neural markers in a non-cell-autonomous manner, through a prior induction of dorsal mesendoderm.
Smad7 has activities distinct from inhibition of TGFβ signalling
The unexpected induction of dorsal mesoderm by Smad7 described above could indicate that a different signalling pathway is involved in its ability to induce neural and/or mesodermal markers. Since Smad7 antagonises both BMP and Nodal signalling we tested whether this effect could be mimicked by separate inhibition of both pathways using other antagonists. We injected a combination of the BMP inhibitor Smad6 (1–4 ng) together with either the secreted Nodal inhibitor Cerberus-Short (CerS, 1.5–2ng; Piccolo et al., 1999) or with the Activin inhibitor, truncated TGFβ receptor type I (tAlk4, which acts cell-autonomously (Chang et al., 1997). These combinations only partially mimic the effects of Smad7. Chordin is induced in both cases (Smad6+CerS:110/112, Fig. 5 E–F; Smad6+tAlk4 45/57 not shown), but neural markers are not (Sox2 in Smad6+CerbS: 0/44, Fig. 5 A–B, Sox2 in Smad6+tAlk4: 1/56, not shown; Sox3 in Smad6+CerbS: 0/53, Fig. 5 C–D, Sox3 in Smad6+tAlk4: 2/45, not shown). It is possible that these results are due to differences in the level of Smad1 and/or Smad2 inhibition resulting from each combination of factors. However, the levels of phosphorylated Smad1 and Smad2 are similar in all three combinations (Fig. 3 A–B), indicating that inhibition of the Smad1 and Smad2 pathways alone cannot account for all of the effects of Smad7 injection.
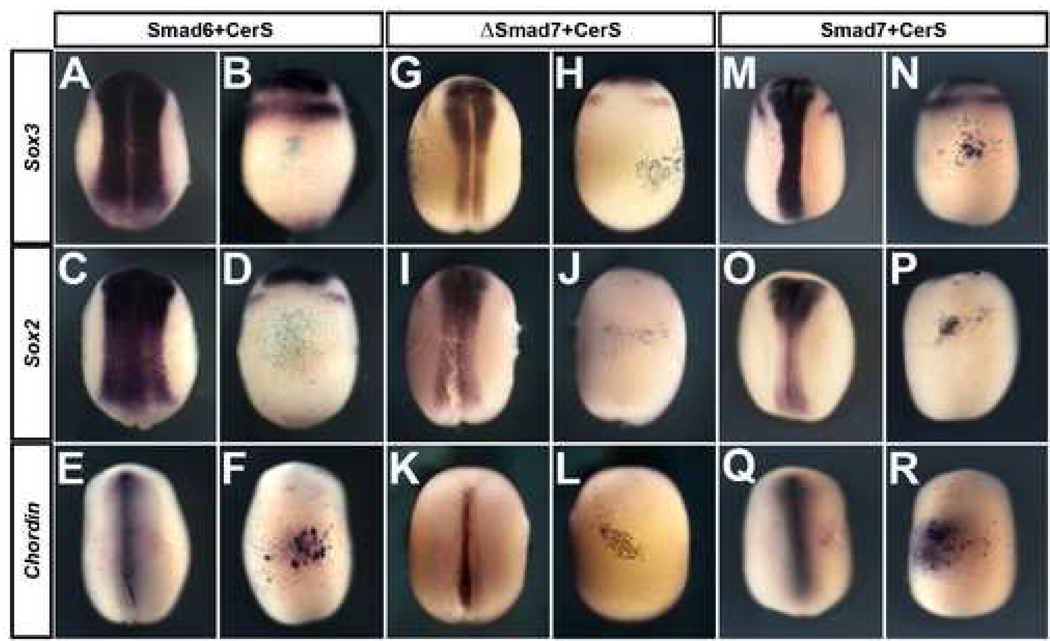
Fig. 5. Inhibition of TGFβ signalling by Smad7 has different consequences from its inhibition by Smad6 (or ΔSmad7) with CerS.
Inhibition of the BMP and the Nodal/Activin pathway does not recapitulate the effect of Smad7 injection. Injection of Smad6+CerS into A4 does not induce Sox3 (A–B) or Sox2 (C–D), but does induce Chordin (E–F). Injection of ΔSmad7+CerS into A4 does not induce Sox3 (G–H) or Sox2 (I–J), but does induce Chordin (K–L). Smad7+CerS injection induces Sox3 (M–N), Sox2 (O–P) and Chordin (Q–R).
The C-terminal (MH2) domain of Smad7 is responsible for the BMP inhibitory functions of the protein (Nakayama et al., 2001). We tested whether expression of this domain together with inhibition of the Nodal/Activin signalling by CerS could recapitulate the effects of Smad7. An inducible form of the MH2 domain (Wawersik et al., 2005) (ΔSmad7 10pg; Supplementary Fig. 2) was injected together CerS (1ng) into the A4 blastmere. This combination (similar to Smad6+CerS or Smad6+Alk4, see above), only partially mimicked the effect of Smad7 injections. Chordin is induced (Fig. 5 K–L; 36/39), but neural markers are not (Fig. 5 G–J; Sox2: 0/42, Sox3: 1/28). This experiment confirms that simultaneous inhibition of BMP- and Nodal/Activin-pathways does not recapitulate all of the activities of Smad7. It also shows that the additional activities reside outside of the MH2 domain.
The loss of neural marker induction in these experiments raises the possibility that a Nodal activity, emitted by Smad7-injected cells, is responsible for the non-cell-autonomous induction of neural markers in adjacent cells (Fig. 2 A–C, Fig. 3 D–E). To test this directly, we co-injected the secreted Nodal antagonist CerS together with Smad7 into A4. CerS does not inhibit either neural (Sox3 8/13; Sox2 11/17; Fig. 5 M–P) or dorsal mesoderm (chordin 23/23; Fig. 5 Q–R) induction by Smad7. Taken together, these experiments strongly suggest that Smad7 has activities other than inhibition of TGFβ signalling.
Smad7 activities are independent of FGF, Wnt, retinoid and hedgehog signalling
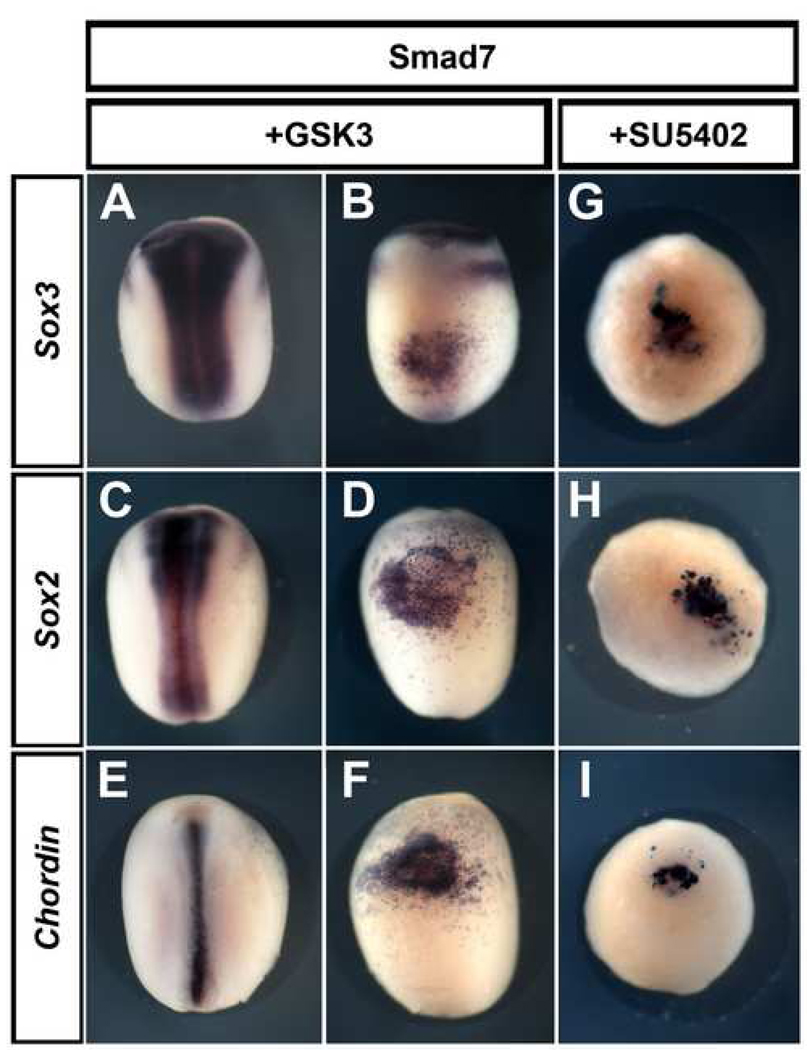
Together with Nodal, FGF and Wnt/β–catenin signalling are the major pathways implicated in mesoderm induction (Kimelman and Bjornson, 2004; Kimelman et al., 1992; Kimelman and Kirschner, 1987), raising the possibility that the induction of dorsal mesoderm by Smad7 might be due to activation of one of these pathways. Furthermore, the induction of neural markers (Sox2 and Sox3) by misexpression of Smad7 in A4 was shown to be non-cell-autonomous, suggesting that a factor secreted by the induced dorsal mesoderm is responsible for the neural induction. To examine which factors might underlie both of these activities, we inhibited Wnt/β–catenin or FGF signalling in Smad7-injected embryos. GSK3 is an intracellular inhibitor of Wnt/β–catenin signalling. Over-expression of GSK3 (2ng) in one half of the embryo efficiently inhibits Slug expression (31/54, not shown; Bastidas et al., 2004) and causes ectopic cement gland formation (93/105, data not shown; Itoh et al., 1995) when injected into one of the dorsal-animal blastomeres at the 8-cell stage, confirming that GSK3 effectively inhibits Wnt/βcatenin signalling. However, cells co-injected with Smad7 and GSK3 still express neural (Sox3: 28/28, Fig. 6 A–B; Sox2: 25/26, Fig. 6 C–D) and mesodermal markers (Chordin: 35/35, Fig. 6 E–F). We then tested the possible involvement of FGF signalling in the effects of Smad7, using the FGF-receptor inhibitor SU5402 (Mohammadi et al., 1997). Embryos cultured in SU5402 (120µM; (Delaune et al., 2005) from the 32-cell-stage fail to gastrulate and do not produce neural or mesodermal tissue (Fig. 6 G–I), confirming that the inhibitor is effective. Nevertheless, injection of Smad7 into A4 still induces Sox3 (30/30, Fig. 6G), Sox2 (27/29, Fig. 6H) and Chordin (26/27, Fig. 6I) when these embryos are grown in SU5402, while the normal expression of these genes in the same embryos is abolished.
Fig. 6. The non-cell-autonomous effects of Smad7 are not mediated by the Wnt/β-catenin or the FGF pathways.
When co-injected with the Wnt/β-catenin inhibitor GSK3, Smad7 still induces Sox3 (A–B), Sox2 (C–D) and Chordin (E–F). The same result is obtained when Smad7-injected embryos are grown in the presence of the FGF inhibitor SU5402: Sox3 (G), Sox2 (H) and Chordin (I) are still induced. Note that the concentration of SU5402 used is sufficient to block gastrulation and endogenous expression of these markers.
Another barely studied pathway that has been implicated in neural induction involves retinoids (Chen et al., 1992; Chen and Solursh, 1992; Sharpe, 1991; Sharpe and Gurdon, 1990). To test whether these may underlie the non-cell-autonomous effects of Smad7 we incubated Smad7- injected embryos in the retinoid antagonist citral (Schuh et al., 1993). At a concentration sufficient to affect axial patterning (at 60–90 µM 21/21 embryos displayed defects in hindbrain patterning; not shown), incubation in citral did not inhibit the induction of Sox3 (18/19), Sox2 (14/15) or Chordin (4/6) in Smad7-injected embryos (not shown). Finally, since Sonic Hedgehog is expressed in the late organizer and in its midline derivatives (Charrier et al., 2002; Charrier et al., 1999; Krauss et al., 1993; Roelink et al., 1994), we used cyclopamine to test whether Hedgehog signalling might be responsible for the non-cell-autonomous effects. At concentrations sufficient to cause holoprosencephaly (100 µM, 32/32 with defects; not shown) (Dunn et al., 1995; Martin et al., 2007; Perron et al., 2003), cyclopamine did not inhibit the induction of Sox3 (7/7) or Sox2 (8/8) in Smad7-injected embryos (not shown). Taken together, these results suggest that the unexplained effects of Smad7 are mediated by a pathway other than Wnt/β–catenin, FGF, retinoids or Hedgehog.
DISCUSSION
Smad7 induces neural tissue only indirectly
Previous studies showed that inhibition of BMP is not sufficient to trigger neural induction either in Xenopus or in chick (Delaune et al., 2005; Launay et al., 1996; Linker and Stern, 2004; Sasai et al., 1996; Streit et al., 1998). To confirm this conclusion we used the more potent TGFβ inhibitor Smad7. We find that neither in chick nor in Xenopus is Smad7 able to induce neural tissue directly. While in chick the expression of Smad7 in competent epiblast does not induce any of the markers tested, dorsal mesodermal markers are induced in Xenopus along with neural markers. This indicates that the neural induction by Smad7 in Xenopus is indirect, a secondary consequence of dorsal mesoderm induction. In agreement with this conclusion, as the concentrations of Smad7 are reduced, neural markers disappear first until, at 10pg, only dorsal mesodermal markers are induced. Moreover, grafting experiments show that induction of mesodermal markers is cell-autonomous, whereas induction of neural markers is not. These results strongly argue that the “neural inducing” activity of Smad7 is indirect and cannot be explained solely by its inhibition of the BMP pathway in the injected cells.
It is possible that Smad7 acts by dorsalising some prospective ventral mesoderm descendants of the injected cell. We used two lineage markers: fluorescein and LacZ. The former allowed us to see cells entering the blastopore at gastrula stage in a few embryos (where the wrong cell may have been targeted), and these were discarded. The latter was used at the end of the experiment, and again, embryos with labelled cells in regions outside the ventral epidermis were discarded. In all control embryos, labelled cells end up exclusively in ventral epidermis, consistent with the fate maps of Dale and Slack (1987). This makes it unlikely that the primary effect of Smad7 is dorsalisation of mesoderm in this assay - neural marker expression is only seen when Chordin/Brachyury are also expressed, again strongly suggesting that this neural induction is indirect.
These results conflict directly with a recent report (Chang and Harland, 2007) claiming that simultaneous inhibition of Smad1-mediated BMP signals and of Nodal/Activin signalling is sufficient for neural induction. However the figures in their study (Fig. 2, 3 and 5 of Chang and Harland, 2007) reveal clearly that this induction is also non-cell-autonomous to the injected cells, similar to what we have found here. Therefore both studies support the notion that inhibition of TGFβ-related pathways is not sufficient for neural induction.
Unexpected effects of Smad7
It is particularly surprising that Smad7, which inhibits both the Smad1/5/8-mediated pathway (used by BMPs) and the Smad2/3-pathway (used by Nodal/Activin), paradoxically induces dorsal mesoderm, especially since it is well established that mesoderm induction is mediated by Nodal/Activin (Kessler, 2004; Kimelman and Bjornson, 2004; Schier, 2004). We had expected no mesoderm to be induced by Smad7 since the Smad2-dependent signalling pathway downstream of Nodal/Activin is blocked. Several recent reports suggest that Smad7 may have other functions in addition to being an inhibitory Smad. One study (Liu et al., 2006) reported a direct association between Smad7, Arkadia (a Nodal effector) and the Wnt effector Axin as part of a larger complex, opening the possibility that this complex somehow provides a link between the Nodal and Wnt pathways. Another possible mechanism arises from the discovery (Edlund et al., 2005; Han et al., 2006) of connections that include induction of β-catenin by Smad7 as well as a direct physical association between these two proteins and with Lef1. In mouse skin, Smad7 promotes the degradation of β-catenin, perhaps via its association with the ubiquitin ligase Smurf1 (Han et al., 2006). These findings prompted us to test whether canonical Wnt signalling might be implicated in the effects of Smad7. Inhibition of the Wnt/βcatenin pathway by GSK3 does not alter the mesodermal inducing activity of Smad7, indicating that this effect is not due to activation of the Wnt pathway. In addition, it has been reported that Smurf1 can inhibit the JNK MAPK cascade (involved in FGF signalling) by targeting MEKK2 for degradation (Yamashita et al., 2005), raising the possibility that the FGF pathway could be affected by misexpression of Smad7 in our experiments. However, we find that the mesodermal inducing activity of Smad7 is unaffected by the FGF receptor inhibitor, SU5402. Although the possibility remains open that the effects of Smad7 on FGF signalling may occur downstream of this receptor, our results suggest that the mesoderm inducing activity of Smad7 is independent of the activation of the Wnt/β–catenin or FGF signals, and that the non-cell-autonomous effects of Smad7 on induction of neural markers is also unlikely to involve either of these pathways. Likewise, we show that neither retinoid nor hedgehog signalling accounts for the non-cell-autonomous neural inducing effects of Smad7 injection.
It remains possible that, rather than affecting a different pathway, Smad7 alters the balance between levels of activation of the Smad1/5/8 and Smad2/3 pathways, and it is this balance rather than the absolute levels of any one phosphorylated Smad, that determines whether ventralization (BMP-dependent) or mesoderm induction and dorsalization (Nodal/Activin-dependent) occurs. However, our experiments confirm the finding that Smad7 inhibits both Smad1 and Smad2 phosphorylation (Casellas and Brivanlou, 1998), therefore this still does not provide a satisfactory explanation for the results obtained. Moreover, inhibition of the Smad1 and the Smad2 pathways by co-injection of Smad6 (or truncated Smad7) and CerS (or Alk4) does not completely mimic the effects of Smad7, which further supports the idea that the mesoderm inducing activity of Smad7 cannot be explained solely by an inhibition of Smad-dependent pathways.
For the moment therefore we can only conclude that there is considerably more complexity to the activity of Smad7 than has hitherto been assumed. We suggest that experiments relying on Smad7 as an inhibitor of Smad pathways should be interpreted with considerable caution.
Experimental Procedures
Xenopus
Xenopus oocytes were fertilised in vitro and embryos staged according to (Nieuwkoop and Faber, 1967). Injections were performed as described (Marchant et al., 1998) into the A4 blastomere at the 32 cell stage or into one of its descendants at the 64 cell stage. Capped mRNAs were made with mMessage mMachine (Ambion) as described (Linker and Stern, 2004). mRNA was transcribed from Smad7-pBSK (kind gift of Ali H. Brivanlou (Casellas and Brivanlou, 1998), ΔSmad7-pCS2+, TEV2GR-pCS2+ (kind gifts of M. Whitman (Wawersik et al., 2005), Smad6-pCS2+ (Linker and Stern, 2004; Yamada et al., 1999), CerberusShort-pCS2+ (kind gift of E M de Robertis (Piccolo et al., 1999), and GSK3-pCS2 (kind gift of S. Wilson (Shimizu et al., 2000), tAlk4 (kind gift of C. Chang (Chang et al., 1997), the mRNA was injected together with 200–600pg of nuclear LacZ mRNA (kind gift of Ali H. Brivanlou) as a lineage tracer. The accuracy of injection was assessed by the fate of the progeny of the injected cell: embryos with labelled cells in regions other than the ventral epidermis were discarded.
To investigate the cell autonomy of Smad7 effects, a host embryo was injected with rhodamine lysine dextran (Rdlx) in A4 and a donor with fluorescein lysine dextran (Fdlx) together with Smad7 in the same blastomere. After culture at 14°C for 3–3.5 hours to early stage 8, isotopic grafts were performed to replace RDX cells with Smad7/FDX cells. Embryos were allowed to heal in ¾ NAM for 1 hour and grown overnight (to stage 17) in 1/10 NAM at 14°C. Paraffin sections were performed as described (Linker and Stern, 2004).
The FGF receptor inhibitor SU5402 (Calbiochem) was dissolved in DMSO at 30 mM and was used at 160 µM (Delaune et al., 2005; Mohammadi et al., 1997). The retinoid inhibitor citral (Sigma) was made up as a 60 mM stock in ethanol and was used at 60–90 µM (Schuh et al., 1993). The hedgehog antagonist cyclopamine (Sigma) was made up as a 10 mM stock in ethanol and was used at 100 µM (Dunn et al., 1995; Martin et al., 2007; Perron et al., 2003).
Whole cell extracts from Xenopus animal caps explants and Western blotting procedures were performed as described (Dorey and Hill, 2006). The following commercial antibodies were used: anti-phosphorylated Smad2 (465/467, Cell Signaling Technology), anti-phosphorylated Smad1 (463/465, Cell Signaling Technology), anti-Smad2/3 (BD Biosciences Pharmingen), anti-Smad1 (Upstate) and anti-tubulin (YL1/2, AbCam).
Chick experiments
Fertilised hens’ eggs (Brown Bovan Gold; Henry Stewart) were incubated at 38°C and staged according to (Hamburger and Hamilton, 1951). Factors were delivered to the inner third of the lateral/anterior area opaca at stage 3+/4 by electroporation or by grafting transfected COS cells. Electroporation was performed as previously described (Sheng et al., 2003) using the following cloned into pCAβ: xSmad7 (Casellas and Brivanlou, 1998), cSmad6 (Linker and Stern, 2004; Yamada et al., 1999), cChordin (Streit et al., 1998), Xenopus truncated BMP receptor (tBR (Linker and Stern, 2004; Suzuki et al., 1994) and cCerberus (Bertocchini et al., 2004; Zhu et al., 1999). An expression plasmid (pCDNAII) encoding Noggin (Streit and Stern, 1999b), was used to transfect COS cells as previously described (Streit et al., 1998).
In situ hybridisation and whole mount immunocytochemistry were performed as previously described (Stern, 1998). In situ hybridisation with Sox2 always produces background staining in grafted cell pellets; expression of the markers in the host was therefore assessed in histological sections.
Supplementary Material
A. Diagram showing the experimental approach. An expression plasmid is electroporated into the inner third of the area opaca at stage 3+ (for Noggin, a stable cell line secreting Noggin is implanted in this location); embryos are then incubated for 18–20hs. None of the following molecules induces either Brachyury (light blue in B, D, G and J) or Sox2 (dark blue in C, E, H, K): Noggin (0/6, B–C); dnBMPR (0/5, D–F); Smad6 (0/12, G–I); Chordin (0/10, J–L). Electroporated cells were recognized by GFP expression (F,I and L in the same embryo to the left). The inset in C shows a section of the embryo at the level (shown by a black line in the main panel) of the implanted cells.
Injection of ΔSmad7 (together with 10 pg of TEV) and immediate activation by Dexamethasone in one blastomere at the 2-cell stage expands the neural plate, as seen by Sox3 (A, B) and Sox2 (C, D) expression. E. Effects of decreasing concentrations of ΔSmad7 mRNA on the expansion of Sox3 expression, in a similar experiment. +DEX, injected embryos activated by Dexamethasone; −DEX, non-activated controls. This experiment shows that at 10 pg ΔSmad7 the effect is maximal.
Acknowledgements
We are indebted to Roberto Mayor, Despina Stamataki and Andrea Streit for useful comments on the manuscript and advice, to Chenbei Chang and Richard Harland for sharing data prior to publication, to Sharon Boast for technical support, to Octavian Voiculescu for help with data analysis and to Federica Bertocchini for useful discussions. We also thank Tim Geach, Leslie Dale, Lorena Marchant, Sei Kuriyama, Helen Mathews, Carlos Carmona-Fontaine and Ben Steventon for help with Xenopus manipulations. This study was funded by grants from the Medical Research Council, NIH (GM60156) and the European Union FP6 Network of Excellence “Cells into Organs”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Bastidas F, De Calisto J, Mayor R. Identification of neural crest competence territory: role of Wnt signaling. Dev Dyn. 2004;229:109–117. doi: 10.1002/dvdy.10486. [DOI] [PubMed] [Google Scholar]
- Belo JA, Bachiller D, Agius E, Kemp C, Borges AC, Marques S, Piccolo S, De Robertis EM. Cerberus-like is a secreted BMP and nodal antagonist not essential for mouse development. Genesis. 2000;26:265–270. [PubMed] [Google Scholar]
- Bertocchini F, Skromne I, Wolpert L, Stern CD. Determination of embryonic polarity in a regulative system: evidence for endogenous inhibitors acting sequentially during primitive streak formation in the chick embryo. Development. 2004;131:3381–3390. doi: 10.1242/dev.01178. [DOI] [PubMed] [Google Scholar]
- Bertrand V, Hudson C, Caillol D, Popovici C, Lemaire P. Neural tissue in ascidian embryos is induced by FGF9/16/20, acting via a combination of maternal GATA and Ets transcription factors. Cell. 2003;115:615–627. doi: 10.1016/s0092-8674(03)00928-0. [DOI] [PubMed] [Google Scholar]
- Casellas R, Brivanlou AH. Xenopus Smad7 inhibits both the activin and BMP pathways and acts as a neural inducer. Dev Biol. 1998;198:1–12. doi: 10.1006/dbio.1998.8893. [DOI] [PubMed] [Google Scholar]
- Chang C, Harland RM. Neural induction requires continued suppression of both Smad1 and Smad2 signals during gastrulation. Development. 2007;134:3861–3872. doi: 10.1242/dev.007179. [DOI] [PubMed] [Google Scholar]
- Chang C, Wilson PA, Mathews LS, Hemmati-Brivanlou A. A Xenopus type I activin receptor mediates mesodermal but not neural specification during embryogenesis. Development. 1997;124:827–837. doi: 10.1242/dev.124.4.827. [DOI] [PubMed] [Google Scholar]
- Charrier JB, Lapointe F, Le Douarin NM, Teillet MA. Dual origin of the floor plate in the avian embryo. Development. 2002;129:4785–4796. doi: 10.1242/dev.129.20.4785. [DOI] [PubMed] [Google Scholar]
- Charrier JB, Teillet MA, Lapointe F, Le Douarin NM. Defining subregions of Hensen's node essential for caudalward movement, midline development and cell survival. Development. 1999;126:4771–4783. doi: 10.1242/dev.126.21.4771. [DOI] [PubMed] [Google Scholar]
- Chen Y, Huang L, Russo AF, Solursh M. Retinoic acid is enriched in Hensen's node and is developmentally regulated in the early chicken embryo. Proc Natl Acad Sci U S A. 1992;89:10056–10059. doi: 10.1073/pnas.89.21.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Solursh M. Comparison of Hensen's node and retinoic acid in secondary axis induction in the early chick embryo. Dev Dyn. 1992;195:142–151. doi: 10.1002/aja.1001950209. [DOI] [PubMed] [Google Scholar]
- Dale L, Slack JMW. Regional specification within the mesoderm of early embryos of Xenopus laevis. Development. 1987;100:279–295. doi: 10.1242/dev.100.2.279. [DOI] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]
- Dorey K, Hill CS. A novel Cripto-related protein reveals an essential role for EGF-CFCs in Nodal signalling in Xenopus embryos. Dev Biol. 2006;292:303–316. doi: 10.1016/j.ydbio.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Dunn MK, Mercola M, Moore DD. Cyclopamine, a steroidal alkaloid, disrupts development of cranial neural crest cells in Xenopus. Dev Dyn. 1995;202:255–270. doi: 10.1002/aja.1002020305. [DOI] [PubMed] [Google Scholar]
- Edlund S, Lee SY, Grimsby S, Zhang S, Aspenstrom P, Heldin CH, Landstrom M. Interaction between Smad7 and beta-catenin: importance for transforming growth factor beta-induced apoptosis. Mol Cell Biol. 2005;25:1475–1488. doi: 10.1128/MCB.25.4.1475-1488.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geach TJ, Dale L. Members of the lysyl oxidase family are expressed during the development of the frog Xenopus laevis. Differentiation. 2005;73:414–424. doi: 10.1111/j.1432-0436.2005.00041.x. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Han G, Li AG, Liang YY, Owens P, He W, Lu S, Yoshimatsu Y, Wang D, Ten Dijke P, Lin X, Wang XJ. Smad7-induced beta-catenin degradation alters epidermal appendage development. Dev Cell. 2006;11:301–312. doi: 10.1016/j.devcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Harland R. Neural induction. Curr Opin Genet Dev. 2000;10:357–362. doi: 10.1016/s0959-437x(00)00096-4. [DOI] [PubMed] [Google Scholar]
- Hata A, Lagna G, Massagué J, Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998;12:186–197. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton D. Vertebrate embryonic cells will become nerve cells unless told otherwise. Cell. 1997a;88:13–17. doi: 10.1016/s0092-8674(00)81853-x. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton D. Vertebrate neural induction. Annu Rev Neurosci. 1997b;20:43–60. doi: 10.1146/annurev.neuro.20.1.43. [DOI] [PubMed] [Google Scholar]
- Hill CS. TGF-beta signalling pathways in early Xenopus development. Curr Opin Genet Dev. 2001;11:533–540. doi: 10.1016/s0959-437x(00)00229-x. [DOI] [PubMed] [Google Scholar]
- Ishisaki A, Yamato K, Hashimoto S, Nakao A, Tamaki K, Nonaka K, ten Dijke P, Sugino H, Nishihara T. Differential inhibition of Smad6 and Smad7 on bone morphogenetic protein- and activin-mediated growth arrest and apoptosis in B cells. J Biol Chem. 1999;274:13637–13642. doi: 10.1074/jbc.274.19.13637. [DOI] [PubMed] [Google Scholar]
- Itoh K, Tang TL, Neel BG, Sokol SY. Specific modulation of ectodermal cell fates in Xenopus embryos by glycogen synthase kinase. Development. 1995;121:3979–3988. doi: 10.1242/dev.121.12.3979. [DOI] [PubMed] [Google Scholar]
- Kessler DS. Activin and Vg1 and the search for embryonic inducers. In: Stern CD, editor. Gastrulation: from cells to embryo. New York: Cold Spring Harbor Press; 2004. pp. 505–520. [Google Scholar]
- Kimelman D, Bjornson C. Vertebrate mesoderm induction. In: Stern CD, editor. Gastrulation: from cells to embryo. New York: Cold Spring Harbor Press; 2004. pp. 363–372. [Google Scholar]
- Kimelman D, Christian JL, Moon RT. Synergistic principles of development: overlapping patterning systems in Xenopus mesoderm induction. Development. 1992;116:1–9. doi: 10.1242/dev.116.Supplement.1. [DOI] [PubMed] [Google Scholar]
- Kimelman D, Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987;51:869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Launay C, Fromentoux V, Shi DL, Boucaut JC. A truncated FGF receptor blocks neural induction by endogenous Xenopus inducers. Development. 1996;122:869–880. doi: 10.1242/dev.122.3.869. [DOI] [PubMed] [Google Scholar]
- Linker C, Stern CD. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development. 2004;131:5671–5681. doi: 10.1242/dev.01445. [DOI] [PubMed] [Google Scholar]
- Liu W, Rui H, Wang J, Lin S, He Y, Chen M, Li Q, Ye Z, Zhang S, Chan SC, Chen YG, Han J, Lin SC. Axin is a scaffold protein in TGF-beta signaling that promotes degradation of Smad7 by Arkadia. Embo J. 2006;25:1646–1658. doi: 10.1038/sj.emboj.7601057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant L, Linker C, Ruiz P, Guerrero N, Mayor R. The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev Biol. 1998;198:319–329. [PubMed] [Google Scholar]
- Martin BL, Peyrot SM, Harland RM. Hedgehog signaling regulates the amount of hypaxial muscle development during Xenopus myogenesis. Dev Biol. 2007;304:722–734. doi: 10.1016/j.ydbio.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Belmonte JC, Westphal H. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- Muñoz-Sanjuán I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci. 2002;3:271–280. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P. Identification of Smad7, a TGFbeta-inducible antagonist of TGFbeta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Berg LK, Christian JL. Dissection of inhibitory Smad proteins: both N- and C-terminal domains are necessary for full activities of Xenopus Smad6 and Smad7. Mech Dev. 2001;100:251–262. doi: 10.1016/s0925-4773(00)00533-5. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Amsterdam: North-Holland Publ. Co.; 1967. [Google Scholar]
- Park SH. Fine tuning and cross-talking of TGF-beta signal by inhibitory Smads. J Biochem Mol Biol. 2005;38:9–16. doi: 10.5483/bmbrep.2005.38.1.009. [DOI] [PubMed] [Google Scholar]
- Perron M, Boy S, Amato MA, Viczian A, Koebernick K, Pieler T, Harris WA. A novel function for Hedgehog signalling in retinal pigment epithelium differentiation. Development. 2003;130:1565–1577. doi: 10.1242/dev.00391. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba A, Tanabe Y, Placzek M, Edlund T, Jessell TM, et al. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Piccolo S, De Robertis EM. Endoderm induction by the organizer-secreted factors chordin and noggin in Xenopus animal caps. Embo J. 1996;15:4547–4555. [PMC free article] [PubMed] [Google Scholar]
- Schier AF. Nodal signaling during gastrulation. In: Stern CD, editor. Gastrulation: from cells to embryo. New York: Cold Spring Harbor Press; 2004. pp. 491–504. [Google Scholar]
- Schuh TJ, Hall BL, Kraft JC, Privalsky ML, Kimelman D. verbA and citral reduce the teratogenic effects of all-trans retinoic acid and retinol, respectively, in Xenopus embryogenesis. Development. 1993;119:785–798. doi: 10.1242/dev.119.3.785. [DOI] [PubMed] [Google Scholar]
- Sharpe CR. Retinoic acid can mimic endogenous signals involved in transformation of the Xenopus nervous system. Neuron. 1991;7:239–247. doi: 10.1016/0896-6273(91)90262-x. [DOI] [PubMed] [Google Scholar]
- Sharpe CR, Gurdon JB. The induction of anterior and posterior neural genes in Xenopus laevis. Development. 1990;109:765–774. doi: 10.1242/dev.109.4.765. [DOI] [PubMed] [Google Scholar]
- Sheng G, dos Reis M, Stern CD. Churchill, a zinc finger transcriptional activator, regulates the transition between gastrulation and neurulation. Cell. 2003;115:603–613. doi: 10.1016/s0092-8674(03)00927-9. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yamanaka Y, Ryu SL, Hashimoto H, Yabe T, Hirata T, Bae YK, Hibi M, Hirano T. Cooperative roles of Bozozok/Dharma and Nodal-related proteins in the formation of the dorsal organizer in zebrafish. Mech Dev. 2000;91:293–303. doi: 10.1016/s0925-4773(99)00319-6. [DOI] [PubMed] [Google Scholar]
- Spemann H, Mangold H. Über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Roux' Arch EntwMech Org. 1924;100:599–638. [Google Scholar]
- Stern CD. Detection of multiple gene products simultaneously by in situ hybridization and immunohistochemistry in whole mounts of avian embryos. Curr Top Dev Biol. 1998;36:223–243. doi: 10.1016/s0070-2153(08)60505-0. [DOI] [PubMed] [Google Scholar]
- Stern CD. Neural induction. In: Stern CD, editor. Gastrulation: from cells to embryo. New York: Cold Spring Harbor Press; 2004. pp. 419–432. [Google Scholar]
- Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–78. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- Streit A, Lee KJ, Woo I, Roberts C, Jessell TM, Stern CD. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development. 1998;125:507–519. doi: 10.1242/dev.125.3.507. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech Dev. 1999a;82:51–66. doi: 10.1016/s0925-4773(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Mesoderm patterning and somite formation during node regression: differential effects of chordin and noggin. Mech Dev. 1999b;85:85–96. doi: 10.1016/s0925-4773(99)00085-4. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Thies RS, Yamaji N, Song JJ, Wozney JM, Murakami K, Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci U S A. 1994;91:10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- von Bubnoff A, Cho KW. Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev Biol. 2001;239:1–14. doi: 10.1006/dbio.2001.0388. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Evola C, Whitman M. Conditional BMP inhibition in Xenopus reveals stage-specific roles for BMPs in neural and neural crest induction. Dev Biol. 2005;277:425–442. doi: 10.1016/j.ydbio.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Yamada M, Szendro PI, Prokscha A, Schwartz RJ, Eichele G. Evidence for a role of Smad6 in chick cardiac development. Dev Biol. 1999;215:48–61. doi: 10.1006/dbio.1999.9419. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Ying SX, Zhang GM, Li C, Cheng SY, Deng CX, Zhang YE. Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell. 2005;121:101–113. doi: 10.1016/j.cell.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Marvin MJ, Gardiner A, Lassar AB, Mercola M, Stern CD, Levin M. Cerberus regulates left-right asymmetry of the embryonic head and heart. Current Biology. 1999;9:931–938. doi: 10.1016/s0960-9822(99)80419-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Diagram showing the experimental approach. An expression plasmid is electroporated into the inner third of the area opaca at stage 3+ (for Noggin, a stable cell line secreting Noggin is implanted in this location); embryos are then incubated for 18–20hs. None of the following molecules induces either Brachyury (light blue in B, D, G and J) or Sox2 (dark blue in C, E, H, K): Noggin (0/6, B–C); dnBMPR (0/5, D–F); Smad6 (0/12, G–I); Chordin (0/10, J–L). Electroporated cells were recognized by GFP expression (F,I and L in the same embryo to the left). The inset in C shows a section of the embryo at the level (shown by a black line in the main panel) of the implanted cells.
Injection of ΔSmad7 (together with 10 pg of TEV) and immediate activation by Dexamethasone in one blastomere at the 2-cell stage expands the neural plate, as seen by Sox3 (A, B) and Sox2 (C, D) expression. E. Effects of decreasing concentrations of ΔSmad7 mRNA on the expansion of Sox3 expression, in a similar experiment. +DEX, injected embryos activated by Dexamethasone; −DEX, non-activated controls. This experiment shows that at 10 pg ΔSmad7 the effect is maximal.