Abstract
Purpose of review
Events occurring in acute HIV-1 infection are now recognized to be critical determinants of the subsequent disease course. Innate responses constitute the first line of defence against pathogens, and also play a key role in triggering the early adaptive response; as such, the innate responses activated in acute HIV-1 infection and their contribution to control of viral replication or disease pathogenesis are the focus of much current research. We review recent advances in this area.
Recent findings
Dendritic cell subsets can play pleiotropic roles in acute HIV-1 infection, with in-vitro studies illustrating that HIV–dendritic cell interactions may have outcomes as diverse as virion destruction, virus dissemination, T-cell triggering or subversion of dendritic cell functions. Natural killer cells can be activated in acute HIV-1 infection, and mounting evidence suggests that they contribute to determining the ensuing course of disease; however, much remains to be learned about how they mediate their effects.
Summary
The importance of innate responses as determinants of the outcome of HIV infection is increasingly evident, but more work is needed to understand how innate immunity can be harnessed to combat this infection.
Keywords: cytokine, dendritic cell, human immunodeficiency virus, innate immunity, natural killer cell
Introduction
Innate responses are rapidly activated in response to pathogen exposure or infection, and play dual roles in containment of early pathogen replication and induction of the adaptive response. The innate responses triggered on exposure to HIV type 1 (HIV-1) during acute HIV-1 infection (AHI) may thus be one of several factors involved in determining whether infection initially becomes established, and if so, how the subsequent disease course progresses. Many pathogens have evolved means of subverting innate responses to promote their replication, and increasing evidence suggests that HIV is no exception. Here, we discuss recent advances in understanding the nature and kinetics of innate responses activated in AHI and the roles they may play in protection or pathogenesis. We also review new insights into how HIV exploits components of the innate immune system during virus dissemination within the infected host.
Pleiotropic roles of dendritic cell subsets in acute HIV-1 infection
Dendritic cells, sentinels of the immune system, are strategically located in skin, mucosal tissues and blood to monitor acquisition of infection. Through expression of a diverse array of receptors and signaling molecules, dendritic cells capture pathogens and trigger innate and adaptive immune responses. They are professional antigen presenting cells, and produce cytokines that mediate direct effector and immunoregulatory functions.
Distribution and characteristics of human dendritic cell subsets
In humans, there are two major dendritic cell subsets, conventional CD11c+ myeloid dendritic cells (cDCs) and plasmacytoid dendritic cells (pDCs, which are CD11clo). Located in skin (as Langerhans cells and dermal dendritic cells), genital/gut mucosa and in blood, cDCs are poised to encounter HIV-1 in the initial stages of infection. pDCs are found in blood, thymus, inflamed skin and mucosa and lymph nodes, and may first encounter HIV during early local viral replication/spread.
cDCs and pDCs serve as critical links between innate and adaptive immune responses through expression of pattern-recognition receptors (PRRs) such as Toll-like receptors (TLRs) and C-type lectins, and intracellular pathogen sensors, which trigger specific signaling pathways that lead to host defense. cDCs express TLRs, 2–9, whereas pDCs express TLR7 and TLR9 [1•]. TLR ligation induces dendritic cell activation, illustrated by production of proinflammatory cytokines, upregulation of costimulatory molecules, acquisition of lymph node homing capacity, enhanced antigen presentation and efficient activation of T cells. Given their expression of a wider panel of TLRs, cDCs are equipped to respond to a more diverse range of pathogens, and are specialized to produce interleukin (IL)-12. By contrast, pDCs preferentially recognize viruses containing ssRNA (via TLR7) or unmethylated CpG motifs (via TLR9), and produce large amounts of type I interferons, a property unique to this dendritic cell subset.
Insights from in-vitro studies into possible outcomes of HIV-1–dendritic cell interactions in acute HIV-1 infection
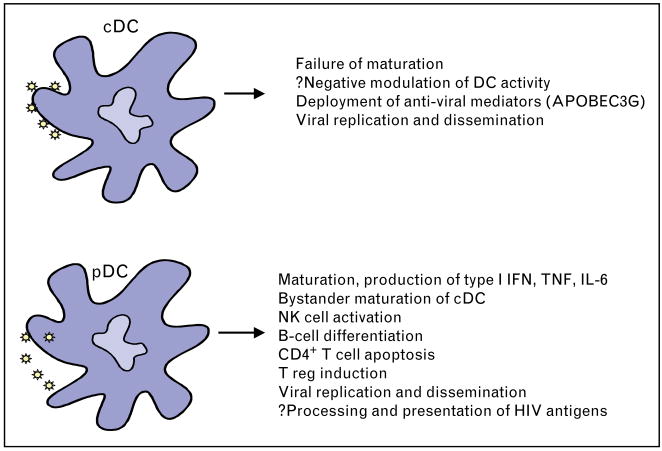
Dendritic cells at sites of pathogen exposure are thought to be among the first cells with which HIV interacts as infection occurs. HIV–dendritic cell interaction may involve contact of viral components with PRRs, capture of virions, or dendritic cell infection; and may have diverse consequences, including triggering of dendritic cell activation, impairment of dendritic cell maturation or modulation of dendritic cell behavior, and virion dissemination and amplification of infection [2] (Fig. 1). Although difficult to study in vivo, insight into the possible outcomes of HIV–dendritic cell interaction in AHI has been gained from in-vitro studies.
Figure 1. Pleiotropic functions of dendritic cell (DC) subsets in response to HIV encounter.
The figure illustrates the range of effects that HIV has on conventional CD11c+ myeloid dendritic cells (cDCs) and plasmacytoid dendritic cells (pDCs) and their potential consequences. APOBEC, apolipoprotein B mRNA editing enzyme catalytic polypeptide; IFN, interferon; IL, interleukin; NK, natural killer; TNF, tumor necrosis factor.
Dendritic cell infection
Both cDCs and pDCs express CD4 and CCR5/CXCR4, and can be productively infected with HIV-1 in vitro, although the frequency of cells producing virus is small (<5%). This fact may be because dendritic cells deploy viral restriction strategies to contain HIV replication. For example, cDCs can express apolipoprotein B mRNA editing enzyme catalytic polypeptides (APOBECs), proteins that deaminate cytidine to uridine in nascent minus-strand viral DNA, blocking HIV replication [3•]. Mature cDCs increase APOBECG expression, explaining their relative resistance to HIV-1 infection [4•]. APOBEC expression in the dendritic cells of HIV-infected patients has not been quantitated, but if substantial, may underlie the difficulty in consistently finding HIV+ dendritic cells in blood. Whether pDCs express APOBECG has yet to be determined, but type I interferons can promote its expression in myeloid cells [5].
HIV-1 transmission to T cells
Shortly after capture, cDCs transmit HIV-1 in trans to T cells [6] through virologic synapse formation or via exosomes and later, following direct viral replication. Transmission occurs preferentially to antigen-specific T cells [6] and is enhanced by dendritic cell maturation [7]. pDCs also transmit HIV to T cells, although production of type I interferons and other mechanisms limit replication in the latter [8,9].
Dermal and mucosal dendritic cells and monocyte-derived dendritic cells, but not circulating cDCs or pDCs, express dendritic cell-specific, intercellular adhesion molecule-grabbing nonintegrin (DC-SIGN), a C-type lectin thought to be prominent in capture of HIV-1 into specialized endosomes, from which virus can be routed to proteasomes or transmitted to CD4+ T cells in trans. DC-SIGN activation results in leukemia-associated Rho guanine nucleotide-exchange factor (LARG)-induced Rho-GTPase activation, required for virus T-cell synapse formation and increased virus replication [10]. Interestingly, ligation of DC-SIGN on monocyte-derived dendritic cells causes downregulation of major histocompatibility complex (MHC) class II molecules and interferon response genes [10]. By ligating and being sequestered via DC-SIGN, HIV-1 may simultaneously achieve dissemination and subversion of the host immune response.
Recent studies suggest that dendritic cells can capture, process or transfer virus through DC-SIGN-independent mechanisms [11–13]. One alternative HIV capture receptor is Langerin, a C-type lectin receptor expressed by Langerhans cells, a subset of cDCs that reside in the skin and in most mucosal epithelia. In contrast to HIV-1 captured by DC-SIGN, HIV-1 captured by Langerin is internalized into Birbeck granules and degraded [14••]. Unlike DC-SIGN+ cDCs in the subepithelium, Langerhans cells may thus effect clearance of captured virions rather than mediating HIV transmission to T cells.
Dendritic cell activation
In pDCs, endocytosis of HIV-1 after envelope–CD4 interactions is followed by ligation of TLR7 with viral RNA [15] and pDC activation. By contrast, HIV infection of cDCs does not lead to their activation, despite the fact that they also express TLR7 (although high doses of HIV-1 may induce phenotypic maturation of cDCs [16]). The paradox is that conventional TLR7 agonists such as resiquimod, or GU-rich sequences derived from genomic HIV RNA [17], activate both dendritic cell subsets. cDCs may route HIV-1 to endosomal compartments that do not intersect with those that contain TLR7. Notably, as discussed above, mucosal cDCs but not pDCs express DC-SIGN, virions captured by which are sequestered in specialized endosomes; and ligation of this C-type lectin also exerts immunomodulatory effects on cDCs [10].
Infection of cDCs with HIV not only fails to activate these cells, but may also interfere with their maturation in response to other activation stimuli. HIV-1-exposed cDCs elicit IL-10, are defective in IL-12 production and may adversely regulate T-cell function [18].
Immunoregulation by dendritic cells
cDCs capture and process HIV-1, and present associated antigens to T cells [13]. It is not yet known if pDCs display this capacity, although they present viral antigens to T cells following infection with influenza virus [19]. Nevertheless, pDC-derived interferon (IFN)-α participates in T-cell priming and cross presentation [1•]. HIV-1-activated pDCs also express indoleamine 2,3-dioxygenase (IDO) [20••]. This indirectly impairs CD4+ T-cell proliferation [20••] through tryptophan metabolism and has been implicated in the induction of T-regulatory cells.
In addition to its adjuvant effects, IFNα mediates direct antiviral activity against HIV [21]. Type 1 interferons may help to restrict viral replication by other means; for example, HIV-1-induced IFNα induces tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas/Fas ligand on CD4+ T cells, leading to T-cell apoptosis [22], which may limit virus spread from infected cells; however, pDC-derived IFNα may also induce apoptosis of uninfected CD4+ T cells, contributing to generalized T-cell loss [23].
In-vivo studies of the dendritic cell response in acute HIV-1 infection
Although HIV–dendritic cell interactions at the initial sites of virus infection and in lymphoid tissues are difficult to study in infected patients, some insight has been gained into the systemic dendritic cell response during AHI from analysis of plasma cytokine levels and peripheral blood dendritic cell subsets in recently infected individuals.
Cytokines
The earliest perturbations in plasma cytokine levels detectable in AHI include increases in IFNα and IL-15, both of which are transiently elevated prior to the peak in acute plasma viraemia [24–26]. Elevations in levels of circulating tumor necrosis factor (TNF)α, IFNγ and other cytokines rapidly follow, temporally correlating with the increase in plasma viraemia [27,28]. The source of cytokine production is likely to be a combination of dendritic cells, monocytes, natural killer (NK) cells and possibly T cells, with IFNα probably being derived from pDCs, supporting the concept that this dendritic cell subset undergoes activation as plasma viraemia increases in AHI.
Dendritic cell subsets
There is a paucity of data on the activation or infection status of dendritic cell subsets in the blood during AHI, although in-vitro studies indicate that dendritic cells are initially preferentially infected compared with other blood leukocyte populations [29]. One study prospectively examined blood pDC and cDC frequencies in 88 therapy naïve patients undergoing AHI [30], and found that pDC, but not mDC, levels were reduced by almost half. Another study confirmed the decline in pDC counts [31], and showed that type I interferon production on in-vitro stimulation with herpes simplex virus type 1 was substantially impaired, albeit transiently. Interestingly, pDCs ligated with gp120 display impaired maturation, IFNα secretion and NK cell activation following stimulation via TLR9 [32]. This ‘refractory’ state of pDCs may mimic the natural unresponsiveness that occurs in vivo during AHI.
The mechanism behind the decline in pDC numbers in AHI is not clear, but this could be due to apoptosis as a direct result of infection [9] or mediated by TRAIL and Fas ligand–Fas interactions; may be a consequence of compromised production of pDC precursors due to bone marrow infection; or may reflect pDC migration to lymphoid tissues after HIV-induced activation. An accumulation of DC-SIGN+, CD40+ dendritic cells has been reported in lymph nodes of acutely infected patients [33].
The natural killer cell response in acute HIV-1 infection: a critical determinant of control of early viral replication?
NK cells are a subset of innate lymphocytes, capable, when mature, of responding rapidly to the presence of infection without the need for prior antigen exposure. They perform direct effector and immunoregulatory functions in the early response to many virus infections [34], acting via cytolysis and production of cytokines/chemokines. Triggering of NK cell effector functions on contact with target cells is controlled by the balance of signals received through cell-surface activating and inhibitory receptors [35,36•]. NK cell activity, however, is also modulated by accessory cells, via contact-dependent interactions and soluble factors including IFNα, IL-12, IL-15 and IL-18 [37•].
Historically, most studies of NK cells in HIV-1 infection have focused on analysis of NK cell numbers and function in chronic infection. The consensus from these is that NK cell activity is enhanced during viraemic HIV infection, but that in the context of chronic infection NK cell numbers are reduced, the NK cell subset composition is perturbed and there are alterations in NK cell functions [38,39•]. The contribution made by NK cells to control of infection has thus not been clear. Recent work, however, has begun to address the NK response in AHI, showing that NK cells are activated at this time and may play an important role in control of early virus replication.
Natural killer cell activation and functions in acute HIV-1 infection
As reviewed above, a transient elevation in plasma levels of IFNα and other innate cytokines occurs prior to the peak in plasma viraemia in AHI [24–26], likely reflecting systemic activation of dendritic cells at this time. Plasma levels of cytokines including TNFα and IFNγ increase shortly thereafter [25–28], providing suggestive evidence that systemic dendritic cell activation is rapidly (and most likely causally [40•]) followed by activation of other cell types including NK cells. Consistent with this, an increased percentage of NK cells has been observed in the blood in AHI in some [41], but not all [42], studies, with longitudinal analysis demonstrating that NK cell populations are expanded around the time of peak plasma viraemia, prior to HIV seroconversion and the development of HIV-specific CD8+ T-cell responses [43••].
During AHI, there is an increase in the proportion of CD3−CD56dim NK cells in the peripheral blood NK pool, while the proportion of CD3−CD56bright NK cells is reduced [41,42]. The former is likely due to expansion of the CD3−CD56dim NK cell population, which possesses cytolytic activity and is postulated to be the principal ‘direct effector’ NK subset [44]. Peripheral blood NK cells exhibit enhanced activity (degranulation and IFNγ production) during AHI [43••]. The reduced proportion of CD3−CD56bright NK cells in the blood during AHI may reflect recruitment of these cells, to which immunoregulatory functions are ascribed [44], into lymphoid tissues, where they could potentially play a role in activation of the adaptive response. The number/activity of NK cells present in lymph nodes during AHI, however, has not been addressed.
Evidence that natural killer cells impact on the control of viraemia in early HIV infection
The observation that NK cells are activated during AHI [41,42,43••] suggests that they may contribute directly or indirectly to control of primary viraemia. Likewise, reports of elevated NK cell activity in HIV-exposed, seronegative (ESN) individuals [45,46] imply that these cells may also play a role in preventing the establishment of infection. Further indirect evidence for a role for NK cells in combating HIV is that this virus possesses strategies for reducing NK cell activation on contact with infected cells – for example, although the HIV-1 Nef protein mediates downregulation of human leukocyte antigen (HLA)-A/B alleles from the surface of infected cells to impair recognition by virus-specific CD8+ T cells, expression of HLA-C/E, ligands for inhibitory NK cell receptors, is retained [47]; however, the strongest evidence for the importance of NK cells in HIV infection comes from genetic studies.
In 2002, a significant association was found between coexpression of killer inhibitory receptor (KIR)3DS1 (an allotype of the KIR3DL1 gene encoding a putative activating NK cell receptor), with HLA-Bw4 alleles having an isoleucine residue at position 80 (HLA-B Bw4-80Ile) (ligands for the inhibitory KIR3DL1 allotypes, which may also be involved in KIR3DS1 binding), and slower HIV disease progression [48]. Importantly, the strongest synergistic effect between these loci was on progression to depletion of CD4+ T cells, suggesting that a protective NK response involving KIR3DS1 and its class I ligands may begin soon after HIV infection. Likewise KIR3DS1/Bw4-80Ile was subsequently found to be associated with a low viral load in early HIV infection [49•]. Recent studies have given more insight into the expression, function and ligand specificity of the previously poorly characterized KIR3DS1 gene product, demonstrating that it is expressed on peripheral blood NK cells and when ligated, activates NK cell cytotoxicity and IFNγ production, plus suggesting that its interaction with HLA-Bw4 ligands may be peptide dependent [50•,51•].
Further to this, a large study addressing the effects of inhibitory KIR3DL1 subtypes in combination with HLA-B allelic groups on HIV disease progression and viral load provided evidence for an important role for epistatic effects of distinct KIR3DL1 and HLA-B Bw4 combinations in HIV infection [52••]. Notably, highly expressed, highly inhibitory KIR3DL1 alleles were found to strongly enhance the protection conferred by Bw4-80Ile alleles [52••]. This observation, which is seemingly inconsistent with the protective effect of KIR3DS1/Bw4-80Ile in HIV infection, may be explained by a model in which NK cells bearing the highly expressed KIR3DL1 allotypes are strongly inhibited in the presence of their Bw4 ligands, resulting in these cells being potent effectors against HIV-infected cells with reduced surface HLA-B expression. KIR3DS1-expressing NK cells may, by contrast, be stimulated by HIV-infected cells if the residual surface Bw4-80Ile presents viral or stress peptides, generating their cognate ligand.
Two recent studies have also documented patterns of NK receptor or ligand expression that could lower the threshold for NK cell activation in ESN individuals [53•,54•], supporting the hypothesis that NK cells may be involved in mediating resistance to HIV infection.
Natural killer cell recognition of HIV-infected cells
NK cells could combat HIV replication and spread both directly, via lysis of infected cells or production of antiviral cytokines (e.g. IFNγ) and coreceptor-blocking CC-chemokines; and indirectly, via activation of adaptive responses. The contribution of these different mechanisms to NK cell-mediated protection in AHI, however, is not known. In-vitro studies have demonstrated the capacity for NK cells to suppress HIV replication in infected cells via noncytolytic mechanisms [55,56]. By contrast, it is less clear how well NK cells are able to lyse HIV-infected cells [57].
The receptor–ligand interactions involved in determining whether target cell lysis is triggered following the interaction of an NK cell with an HIV-infected cell (Fig. 2) are currently under investigation. One set of interactions involved are those between class I molecules on the target cell and cognate inhibitory NK receptors: as mentioned above, surface levels of HLA-A/B are reduced on infected cells, whilst HLA-C/E expression is maintained [47] (or HLA-E may be upregulated [58]), resulting in inhibition of target cell lysis by NK cells expressing inhibitory receptors that interact with HLA-C/E (certain KIRs and NKG2A/CD94), but giving the potential for lysis by NK cells inhibited via receptors that interact with HLA-A/B [47,58]. Recent studies have also shown that ligands for activating receptors are expressed on HIV-infected cells, including a ligand for the natural cytotoxicity receptor NKp44 induced by a motif in the HIV envelope gp41 protein [59] and the NKG2D ligands UL-16 binding protein (ULBP)-1, 2 and 3 [60•] plus, as discussed above, a KIR3DS1 ligand.
Figure 2. Diagram to illustrate some of the putative interactions between ligands on (a) an uninfected cell and (b) an HIV-infected cell and activating/inhibitory natural killer (NK) cell receptors.
The signals NK cells receive following interaction with uninfected cells are likely to be predominantly inhibitory (−); but on HIV-infected cells expression of ligands for inhibitory NK cell receptors is postulated to be reduced, whilst ligands for activating NK cell receptors are thought to be induced, resulting in many NK cells receiving a greater proportion of activating (+) signals and hence being triggered into functional activity.
HIV possesses a variety of strategies for impairing NK cell activation by infected cells. In addition to the Nef protein modulating surface class I levels [47], Nef was also recently reported to reduce the upregulation of cell-surface expression of MHC-I related chain (MIC)A and ULBP-1 and 2, ligands for the activating NK receptor NKG2D [61•]. Expression of NK-T-B cell antigen (NTB-A) and CD48, ligands for the NK cell coreceptors NTB-A and 2B4, is also downmodulated on HIV-infected cells [60•]. Other mechanisms by which HIV may interfere with the NK cell response include inhibition of NK cell functions by HIV-1 gp120 [62] and an indirect effect of the HIV-1 Nef protein on NK cell activity mediated via impairment of NK/dendritic cell cross-talk [63].
Activation of other innate cell subsets in acute HIV-1 infection
In addition to dendritic cells and NK cells, other innate cell subsets also become activated in AHI, although relatively little is known about the kinetics with which this occurs or the contributions that these subsets may make to protection or pathogenesis.
For example, studies carried out in macaques have shown that peripheral blood monocyte counts increase prior to the peak in plasma viraemia in acute simian immunodeficiency virus (SIV) infection, and that circulating monocytes exhibit an increased functional capacity at this time [64,65•]. Monocytes may contribute directly or indirectly to control of infection via their ability to produce cytokines, chemokines and other soluble factors; but conversely may also act as a substrate for viral replication and may contribute to spread of virus within the infected host, including transport of virus into the brain [65•].
CD3+CD56+ natural T cells and CD1d-restricted natural killer T (NKT) cells (most of which express a restricted T-cell receptor repertoire consisting of a Vα24 chain preferentially paired to Vβ11) are also thought to be activated in AHI. These are lymphocyte subsets that possess features of both NK cells and T cells, and are capable of mediating immunoregulatory and effector functions via production of T helper type 1 (Th1) and Th2 cytokines and via cytolysis. Peripheral blood NKT cell numbers are known to be reduced from early HIV infection onwards [66], which may be due in part to the susceptibility of CD4+ NKT cells to infection and destruction by HIV. Recent studies have shown, however, that administration of antiretroviral therapy results in recovery of (predominantly CD4−) NKT cells with kinetics similar to those of the increase in peripheral blood T cells [67•,68]. The latter is thought to reflect the return of cells previously sequestered in lymphoid tissues back into the circulation. The initial decrease in peripheral blood NKT cell numbers during AHI may thus be due to a combination of cell death and sequestration of NKT cells into tissues. NKT cell numbers in lymphoid tissues, however, remain to be addressed, as do the role(s) that these cells may be playing in control of viral replication or regulation of other innate or adaptive responses.
Conclusion
Although understanding of the innate responses activated in AHI is rapidly increasing, there is still much to be learned. Dendritic cell subsets exhibit a pleiotropic response to encounter with HIV, mediating effects that may be beneficial (e.g. capture and destruction of HIV by Langerhans cells; activation of HIV-specific T-cell responses by cDCs), detrimental (e.g. dissemination of infection to HIV-specific T cells by subepithelial cDCs), or perhaps a combination of the two (e.g. pDC production of IFNα, which may mediate antiviral effects but also drive CD4 T-cell apoptosis). Recent evidence suggests that NK cells may play an important role in mediating both resistance to HIV infection and containment of viral replication in infected individuals – but the mechanisms by which they mediate these effects and viral strategies for subverting NK control remain poorly understood. More work is thus required to dissect beneficial and detrimental aspects of innate responses in AHI, so that prophylactic and therapeutic strategies can be designed to exploit the former and block the latter.
Acknowledgments
P.B. is a Jenner Institute investigator, and is supported by a Jenner Institute senior fellowship and funding from the Bill and Melinda Gates Foundation Grand Challenges in Global Health Initiative (37874) and the Division of AIDS, NIAID, NIH (AI67854). N.B. is a Doris Duke Distinguished Scientist, and is supported by grants from the NIH (AI44628, AI55274 and AI67854), the Emerald Foundation, the Cancer Research Institute, the Burroughs Wellcome Foundation and the Elizabeth Glaser Pediatric AIDS Foundation.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 82–85).
- 1•.Lakshmanan V, Alter G et al. Biology of plasmacytoid dendritic cells and natural killer cells in HIV-1 infection. Curr Opin HIV AIDS. 2007;2:189–200. doi: 10.1097/COH.0b013e32810996db. A recent detailed review of pDC–HIV-1 interactions. [DOI] [PubMed] [Google Scholar]
- 2.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Takaori-Kondo A. APOBEC family proteins: novel antiviral innate immunity. Int J Hematol. 2006;83:213–216. doi: 10.1532/IJH97.05187. A comprehensive review summarizing the APOBEC family of proteins. [DOI] [PubMed] [Google Scholar]
- 4•.Pion M, Granelli-Piperno A, Mangeat B et al. APOBEC3G/3F mediates intrinsic resistance of monocyte-derived dendritic cells to HIV-1 infection. J Exp Med. 2006;203:2887–2893. doi: 10.1084/jem.20061519. A study describing the antiviral role of ABOBEC3G in dendritic cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng G, Greenwell-Wild T, Nares S, et al. Myeloid differentiation and susceptibility to HIV-1 are linked to APOBEC3 expression. Blood. 2007;110:393–400. doi: 10.1182/blood-2006-10-051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lore K, Smed-Sorensen A, Vasudevan J, et al. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J Exp Med. 2005;201:2023–2033. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izquierdo-Useros N, Blanco J, Erkizia I, et al. Maturation of blood-derived dendritic cells enhances human immunodeficiency virus type 1 capture and transmission. J Virol. 2007;81:7559–7570. doi: 10.1128/JVI.02572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groot F, van Capel TMM, Kapsenberg ML, et al. Opposing roles of blood myeloid and plasmacytoid dendritic cells in HIV-1 infection of T cells: transmission facilitation versus replication inhibition. Blood. 2006;108:1957–1964. doi: 10.1182/blood-2006-03-010918. [DOI] [PubMed] [Google Scholar]
- 9.Meyers JH, Justement JS, Hallahan CW, et al. Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells. PLoS ONE. 2007;2:e458. doi: 10.1371/journal.pone.0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodges A, Sharrocks K, Edelmann M, et al. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol. 2007;8:569–577. doi: 10.1038/ni1470. [DOI] [PubMed] [Google Scholar]
- 11.Magerus-Chatinet A, Yu H, Garcia S, et al. Galactosyl ceramide expressed on dendritic cells can mediate HIV-1 transfer from monocyte-derived dendritic cells to autologous T cells. Virology. 2007;362:67–74. doi: 10.1016/j.virol.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Boggiano C, Manel N, Littman DR. Dendritic cell-mediated trans-enhancement of human immunodeficiency virus type 1 infectivity is independent of DC-SIGN. J Virol. 2007;81:2519–2523. doi: 10.1128/JVI.01661-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabado RL, Babcock E, Kavanagh DG, et al. Pathways utilized by dendritic cells for binding, uptake, processing and presentation of antigens derived from HIV-1. Eur J Immunol. 2007;37:1752–1763. doi: 10.1002/eji.200636981. [DOI] [PubMed] [Google Scholar]
- 14••.de Witte L, Nabatov A, Pion M et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13:367–371. doi: 10.1038/nm1541. A study demonstrating a novel function of Langerin in anti-HIV immunity. [DOI] [PubMed] [Google Scholar]
- 15.Beignon AS, McKenna K, Skoberne M, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor–viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harman AN, Wilkinson J, Bye CR, et al. HIV induces maturation of monocyte-derived dendritic cells and Langerhans cells. J Immunol. 2006;177:7103–7113. doi: 10.4049/jimmunol.177.10.7103. [DOI] [PubMed] [Google Scholar]
- 17.Meier A, Alter G, Frahm N, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded toll-like receptor ligands. J Virol. 2007;81:8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granelli-Piperno A, Golebiowska A, Trumpfheller C, et al. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc Natl Acad Sci U S A. 2004;101:7669–7674. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonteneau JF, Larsson M, Beignon AS, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Boasso A, Herbeuval JP, Hardy AW et al. HIV inhibits CD4+ T-cell prolifera- tion by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–3359. doi: 10.1182/blood-2006-07-034785. This study shows that HIV can induce indoleamine 2,3-dioxygenase in pDCs and thereby indirectly compromises CD4+ T cell function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto JK, Barre-Sinoussi F, Bolton V, et al. Human alpha- and beta-interferon but not gamma- suppress the in vitro replication of LAV, HTLV-III, and ARV-2. J Interferon Res. 1986;6:143–152. doi: 10.1089/jir.1986.6.143. [DOI] [PubMed] [Google Scholar]
- 22.Herbeuval JP, Grivel JC, Boasso A, et al. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood. 2005;106:3524–3531. doi: 10.1182/blood-2005-03-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbeuval JP, Shearer GM. HIV-1 immunopathogenesis: how good interferon turns bad. Clin Immunol. 2007;123:121–128. doi: 10.1016/j.clim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaines H, von Sydow M, von Stedingk LV, et al. Immunological changes in primary HIV-1 infection. AIDS. 1990;4:995–999. doi: 10.1097/00002030-199010000-00008. [DOI] [PubMed] [Google Scholar]
- 25.von Sydow M, Sonnerborg A, Gaines H, Strannegard O. Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res Hum Retroviruses. 1991;7:375–380. doi: 10.1089/aid.1991.7.375. [DOI] [PubMed] [Google Scholar]
- 26.Stacey A, Haygreen E, Taylor E, et al. Program and Abstracts of the Keystone Symposium on ‘HIV vaccines: from basic research to clinical trials’. Whistler, Canada: Mar 25–30, 2007. Elevations in plasma levels of innate cytokines prior to the peak in plasma viremia in acute HIV-1 infection. Keystone Symposia. [Google Scholar]
- 27.Graziosi C, Gantt KR, Vaccarezza M, et al. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Sci U S A. 1996;93:4386–4391. doi: 10.1073/pnas.93.9.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norris PJ, Pappalardo BL, Custer B, et al. Elevations in IL-10, TNF-alpha, and IFN-gamma from the earliest point of HIV type 1 infection. AIDS Res Hum Retroviruses. 2006;22:757–762. doi: 10.1089/aid.2006.22.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cameron PU, Handley AJ, Baylis DC, et al. Preferential infection of dendritic cells during human immunodeficiency virus type 1 infection of blood leukocytes. J Virol. 2007;81:2297–2306. doi: 10.1128/JVI.01795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Killian MS, Fujimura SH, Hecht FM, Levy JA. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV infection and treatment. AIDS. 2006;20:1247–1252. doi: 10.1097/01.aids.0000232231.34253.bd. [DOI] [PubMed] [Google Scholar]
- 31.Kamga I, Kahi S, Develioglu L, et al. Type 1 interferon production is profoundly and transiently impaired in primary HIV-1 infection. J Infect Dis. 2005;192:303–310. doi: 10.1086/430931. [DOI] [PubMed] [Google Scholar]
- 32.Martinelli E, Cicala C, Van Ryk D, et al. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2007;104:3396–3401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lore K, Sonnerborg A, Brostrom C, et al. Accumulation of DC-SIGN+ CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS. 2002;16:683–692. doi: 10.1097/00002030-200203290-00003. [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama WM. Natural killer cell immune responses. Immunol Res. 2005;32:317–325. doi: 10.1385/IR:32:1-3:317. [DOI] [PubMed] [Google Scholar]
- 35.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 36•.Stewart CA, Vivier E, Colonna M. Strategies of natural killer cell recognition and signaling. Curr Top Microbiol Immunol. 2006;298:1–21. doi: 10.1007/3-540-27743-9_1. This informative review describes the receptor–ligand interactions and intracellular signaling pathways controlling NK effector responses. [DOI] [PubMed] [Google Scholar]
- 37•.Newman KC, Riley EM. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol. 2007;7:279–291. doi: 10.1038/nri2057. An excellent review of the interactions involved in accessory-cell-dependent activation of NK cells in response to infection with viral, bacterial and protozoan pathogens. [DOI] [PubMed] [Google Scholar]
- 38.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 39•.Alter G, Altfeld M. NK cell function in HIV-1 infection. Curr Mol Med. 2006;6:621–629. doi: 10.2174/156652406778195035. A well written review covering the effects of HIV infection on NK cell activity and also evidence suggesting that NK cells may impact on the course of HIV infection. [DOI] [PubMed] [Google Scholar]
- 40•.Alter G, Suscovich TJ, Teigen N et al. Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells. J Immunol. 2007;178:7658–7666. doi: 10.4049/jimmunol.178.12.7658. This study demonstrates the ability of single-stranded RNA derived from HIV to promote NK cell activation via an accessory cell-dependent pathway, illustrating a mechanism that may help to drive NK cell activation in acute infection and contribute to persistent immune activation in chronic infection. [DOI] [PubMed] [Google Scholar]
- 41.Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–3369. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 42.Titanji K, Sammicheli S, De Milito A et al. Altered distribution of natural killer cell subsets identified by CD56, CD27 and CD70 in primary and chronic human immunodeficiency virus-1 infection. Immunology. 2007 doi: 10.1111/j.1365-2567.2007.02657.x. 11 July [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Alter G, Teigen N, Ahern R et al. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J Infect Dis. 2007;195:1452–1460. doi: 10.1086/513878. An important paper that addresses the kinetics of functional activation of NK cells and CD8+ T cells in AHI and shows that the NK cell response is activated prior to the detection of adaptive (T cell or antibody) responses, dominating the initial effector lymphocyte response to HIV. [DOI] [PubMed] [Google Scholar]
- 44.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 45.Scott-Algara D, Truong LX, Versmisse P, et al. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J Immunol. 2003;171:5663–5667. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 46.Montoya CJ, Velilla PA, Chougnet C, et al. Increased IFN-gamma production by NK and CD3+/CD56+ cells in sexually HIV-1-exposed but uninfected individuals. Clin Immunol. 2006;120:138–146. doi: 10.1016/j.clim.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Bonaparte MI, Barker E. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility class I molecules. Blood. 2004;104:2087–2094. doi: 10.1182/blood-2004-02-0696. [DOI] [PubMed] [Google Scholar]
- 48.Martin MP, Gao X, Lee JH, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 49•.Qi Y, Martin MP, Gao X et al. KIR/HLA pleiotropism: protection against both HIV and opportunistic infections. PLoS Pathogens. 2006;2:741–745. doi: 10.1371/journal.ppat.0020079. This group had previously reported an association between the compound genotype KIR3DS1/HLA-B Bw4Ile and delayed disease progression in HIV infection. In this study they dissect the protective effect(s) of this genotype further, demonstrating that it confers dual protection, being associated with early containment of HIV viraemia and late defence against opportunistic infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Carr WH, Rosen DB, Arase H et al. Cutting edge: KIR3DS1, a gene implicated in resistance to progression to AIDS, encodes a DAP12-associated receptor expressed on NK cells that triggers NK cell activation. J Immunol. 2007;178:647–651. doi: 10.4049/jimmunol.178.2.647. When genetic studies first suggested a role for interactions between KIR3DS1 and HLA-Bw4 in determining the course of HIV infection, little was known about the expression and functions of this KIR. Here, and in Ref. [51•], KIR3DS1 expression on a subset of peripheral blood NK cells is demonstrated, and KIR3DS1 ligation is shown to trigger NK cell functional activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Pascal V, Yamada E, Martin MP et al. Detection of KIR3DS1 on the cell surface of peripheral blood NK cells facilitates identification of a novel null allele and assessment of KIR3DS1 expression during HIV-1 infection. J Immunol. 2007;179:1625–1633. doi: 10.4049/jimmunol.179.3.1625. As in Ref. [50•], KIR3DS1 expression on a subset of peripheral blood NK cells is demonstrated, and KIR3DS1 ligation is shown to trigger NK cell functional activation. [DOI] [PubMed] [Google Scholar]
- 52••.Martin MP, Qi Y, Gao X et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV. Nat Genet. 2007;39:733–740. doi: 10.1038/ng2035. This is a key large-scale genetic study, in which it is shown that various distinct allelic combinations of the KIR3DL1 and HLA-B loci strongly influence both the viral load and disease course in HIV-infected individuals. The results provide strong evidence to support the hypothesis that NK cells play a critical role in the response to HIV infection; and also illustrate and help to cast light on the complexity of the KIR3DL1 locus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Jennes W, Verheyden S, Demanet C et al. Cutting edge: resistance to HIV-1 infection among African female sex workers is associated with inhibitory KIR in the absence of their HLA ligands. J Immunol. 2006;177:6588–6592. doi: 10.4049/jimmunol.177.10.6588. Both this study and Ref. [54•] document patterns of NK receptor and ligand expression that could lower the threshold for NK cell activation in HIV-exposed, seronegative individuals, providing suggestive evidence that NK cells may be involved in mediating resistance to HIV infection. [DOI] [PubMed] [Google Scholar]
- 54•.Ravet S, Scott-Algara D, Bonnet E et al. Distinctive NK-cell receptor repertoires sustain high-level constitutive NK-cell activation in HIV-exposed uninfected individuals. Blood. 2007;109:4296–4305. doi: 10.1182/blood-2006-08-040238. Both this study and Ref. [53•] document patterns of NK receptor or ligand expression that could lower the threshold for NK cell activation in HIV-exposed, seronegative individuals, providing suggestive evidence that NK cells may be involved in mediating resistance to HIV infection. [DOI] [PubMed] [Google Scholar]
- 55.Kottilil S. Natural killer cells in HIV-1 infection: role of NK cell-mediated noncytolytic mechanisms in pathogenesis of HIV-1 infection. Indian J Exp Biol. 2003;41:1219–1225. [PubMed] [Google Scholar]
- 56.Zhang T, Li Y, Wang Y-J, et al. Natural killer cell inhibits human immunodeficiency virus replication in chronically-infected immune cells. Antiviral Res. 2007;73:132–139. doi: 10.1016/j.antiviral.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 57.Bonaparte M, Barker E. Inability of natural killer cells to destroy autologous HIV-infected T lymphocytes. AIDS. 2003;17:487–494. doi: 10.1097/00002030-200303070-00003. [DOI] [PubMed] [Google Scholar]
- 58.Martini F, Agrati C, D’Offizi G, Poccia F. HLA-E up-regulation induced by HIV infection may directly contribute to CD94-mediated impairment of NK cells. Int J Immunopathol Pharmacol. 2005;18:269–276. doi: 10.1177/039463200501800209. [DOI] [PubMed] [Google Scholar]
- 59.Vieillard V, Strominger JL, Debre P. NK cytotoxicity against CD4+ T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc Natl Acad Sci U S A. 2005;102:10981–10986. doi: 10.1073/pnas.0504315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60•.Ward J, Bonaparte M, Sacks J et al. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood. 2007;110:1207–1214. doi: 10.1182/blood-2006-06-028175. Much less is known about ligands involved in triggering activating NK receptors than those involved in NK cell inhibition. In this study, the expression of ligands for several activating NK cell receptors on HIV-infected target cells is analysed, and their role in triggering NK cell lysis of the target cells addressed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Cerboni C, Neri F, Casartelli N et al. Human immunodeficiency virus Nef protein downmodulates the ligands of the activating NK receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol. 2007;88:242–250. doi: 10.1099/vir.0.82125-0. In this study, a novel mechanism by which the Nef protein may reduce NK cell lysis of HIV-infected cells is described. [DOI] [PubMed] [Google Scholar]
- 62.Kottilil S, Shin K, Jackson JO, et al. Innate immune dysfunction in HIV infection: effect of HIV envelope–NK cell interactions. J Immunol. 2006;176:1107–1114. doi: 10.4049/jimmunol.176.2.1107. [DOI] [PubMed] [Google Scholar]
- 63.Quaranta MG, Napolitano A, Sanchez M, et al. HIV-1 Nef impairs the dynamic of DC/NK crosstalk: different outcome of CD56dim and CD56bright NK cell subsets. FASEB J. 2007;21:2323–2334. doi: 10.1096/fj.06-7883com. [DOI] [PubMed] [Google Scholar]
- 64.Kuwata T, Kodama M, Sato A, et al. Contribution of monocytes to viral replication in macaques during acute infection with simian immunodeficiency virus. AIDS Res Hum Retroviruses. 2007;23:372–380. doi: 10.1089/aid.2006.0208. [DOI] [PubMed] [Google Scholar]
- 65•.Clay CC, Rodrigues DS, Ho YS et al. Neuroinvasion of fluorescein+ mono- cytes in acute simian immunodeficiency virus infection. J Virol. 2007 doi: 10.1128/JVI.00133-07. 22 August [Epub ahead of print]. Using the SIV macaque model, this study shows that there is an increase in peripheral blood monocyte numbers in acute infection and documents monocyte trafficking into the central nervous system coincident with the first detection of virus in the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Vliet HJ, von Blomberg BM, Hazenberg MD, et al. Selective decrease in circulating V alpha 24+ V beta 11+ NKT cells during HIV type 1 infection. J Immunol. 2002;168:1490–1495. doi: 10.4049/jimmunol.168.3.1490. [DOI] [PubMed] [Google Scholar]
- 67•.van der Vliet HJJ, van Vonderen MGA, Molling JW et al. Cutting edge: rapid recovery of NKT cells upon institution of highly active antiretroviral therapy for HIV-1 infection. J Immunol. 2006;177:5775–5778. doi: 10.4049/jimmunol.177.9.5775. This study gives insight into mechanisms that may contribute to the decrease in peripheral blood NKT numbers in AHI, suggesting that in addition to CD4+ NKT cell infection and loss, NKT cell relocation into tissues may also be involved. [DOI] [PubMed] [Google Scholar]
- 68.Vasan S, Poles MA, Horowitz A, et al. Function of NKT cells, potential anti-HIV effector cells, are improved by beginning HAART during acute HIV-1 infection. Int Immunol. 2007;19:943–951. doi: 10.1093/intimm/dxm055. [DOI] [PubMed] [Google Scholar]