Summary
The transitional epithelium of the bladder normally turns over slowly but, upon injury, undergoes rapid regeneration, fueled by basal uroepithelial stem and/or early progenitor cells (USCs). Little is known about the mechanisms underlying the injury response. Here, we investigate the mechanism of bladder epithelial regeneration in response to infection with uropathogenic E. coli (UPEC). We show that infection resulted in rapid sloughing of superficial cells, a marked inflammatory response, and a substantial spike in basal cell proliferation. Epithelial renewal following infectious injury was mediated in part by Bmp signaling. In mice with Cre-recombinase-mediated ablation of the Bmp4 receptor, Bmpr1a, infection leads to aberrant urothelial renewal resulting in a block in USC differentiation into superficial cells. The response to chemical injury with protamine sulfate (PS) also caused sloughing but no inflammation or USC activation. Together, our study indicates that UPEC infection activates the USC niche, and Bmp signaling is required for regulation of the USC response to infection.
Keywords: bladder, Bmp4, bone morphogenetic protein, uropathogenic E. coli, injury, repair, stem cell, niche, urinary tract infections, interstitial cystitis, urothelium
Introduction
Self-renewing tissues like skin and intestine contain stem cells (SCs) that provide the tissue’s regenerative potential. Adult SCs typically reside in specialized niches where they receive microenvironmental cues that govern normal homeostasis and wound repair (Kobielak et al., 2007). The mammalian urinary bladder is lined by a self-renewing, 3–4 cell layer deep pseudo-stratified transitional epithelium known as the urothelium. The urothelium has a slow rate of turnover under physiological conditions (Hicks, 1975), yet, the bladder maintains a remarkable capacity for tissue regeneration. Adult urothelial cells, when challenged, are capable of completing the cell cycle as fast as stem cells in the embryonic bladder (Jost, 1986, 1989), suggesting the presence of a stem cell population. However, the slow rate of epithelial turnover has hindered the investigation of putative urothelial stem cells. In addition, urothelial stem cells have not been definitively identified at the ultrastructural level, nor their proliferative capacity defined in isolated cell populations, quantified by flow cytometry or correlated with patterns of marker expression (Huh et al., 2006). Nevertheless, 3H-thymidine labeling (Jost, 1986; Jost and Potten, 1986) and more recently with Bromodeoxy-uridine (BrdU) label retention assays (Kurzrock et al., 2008) have shown that the proliferative compartment localizes to the basal layer of the urothelium. These basal cells are characterized by small size (5–10 μM), low granularity, high β4 integrin expression, and demonstrate superior clonogenic and proliferative ability compared with unlabeled epithelial cells (Kurzrock et al., 2008); they also specifically express epithelial cell keratins 5, 6 (Mysorekar et al., 2002), which are basal cell specific markers in the prostate, lung and other epithelia (Reis-Filho et al., 2003; Riedel et al., 2001). These cells proliferate and terminally differentiate into large (80–120 μM) and typically binucleate superficial facet cells that line the luminal surface of the bladder (Hicks, 1975). Herein, we define these basally located cells which take up nucleotide analog upon activation, colocalize with Cytokeratin 5,6 as the putative urothelial stem and/or early progenitor cells (or USCs).
Unlike the undamaged urothelium, which may require up to 40 weeks to renew itself in the adult mouse (Jost, 1989), we and others have shown that acute injury caused by infectious or non-infectious agents leads to rapid renewal of the epithelium that can begin within hours of the molecular insult (Mulvey et al., 1998; Mysorekar et al., 2002). However, little is known about the regulatory mechanisms governing urothelial stem cells in response to infectious diseases that plague the bladder, namely, urinary tract infections (UTIs).
UTIs are amongst the most common infectious diseases in humans, resulting in an estimated 8 million outpatient visits yearly in the U.S. with an estimated cost of evaluating and treating this disease exceeding $1 billion (Foxman, 2003). Uropathogenic Escherichia coli (UPEC) are the major causative agents of UTIs. In a murine model of UTI, UPEC infection of the bladder elicits a host response which initiates superficial cell apoptosis concomitant with inflammation. Regeneration of the lost superficial cells proceeds rapidly over a 72 hour period post infection, with restoration of the intact urothelium by 7 days (Mulvey et al., 1998).
The rapid onset but limited duration of the epithelial renewal process in response to UPEC UTI suggests that regulation of stem cell proliferation and differentiation in the bladder is tightly controlled. In vitro studies have implicated a potential role for several pathways in urothelial renewal, e.g., TGF α/β, EGF and FGF, KGF families (de Boer et al., 1994) (Daher et al., 2003) (de Boer et al., 1996), but there are few in vivo studies that have attempted to elucidate the signaling governing urothelial regeneration following injury. A study investigating the host response to UPEC infection using microarrays with cDNA from infected C57BL/6J mouse bladders found prominent changes in the expression of molecular regulators and effectors of epithelial proliferation and differentiation (Mysorekar et al., 2002). One key regulator of urothelial proliferation and differentiation was determined to be a member of the TGFβ superfamily of secreted signaling molecules, bone morphogenetic protein 4 (Bmp4) (Mysorekar et al., 2002).
Bmp4 is a key developmentally regulated signaling molecule known to be important for cell survival, proliferation and differentiation in various embryonic tissues. Bmp4 is expressed in the developing urinary tract, and designates the sites of ureter formation (Miyazaki et al., 2000; Miyazaki et al., 2003). Bmp4 binds to and signals through two serine/threonine kinase receptors, Bmpr1a and Bmpr1b. The canonical Bmp signal is mediated by Smad transcriptional factors which upon activation translocate to the nucleus where they transactivate their target genes (Mishina, 2003). In the adult mouse bladder, Bmp4 mRNA is localized to the mesenchyme underlying the normal bladder urothelium, and UPEC infection promotes a decline in mRNA levels within this cellular compartment (Mysorekar et al., 2002). Bmpr1a is the only known receptor for Bmp4 that was found detectable by qRT-PCR of mRNAs in the bladders of uninfected mice but whose expression levels did not change upon infection (Mysorekar et al., 2002). Levels of Phospho-Smad1, the downstream mediator of Bmp4 pathway activity, were decreased in basal and suprabasal cells following infection, providing evidence for regulation of Bmp4 signaling during a UTI. These results suggested a hitherto unappreciated role for the bladder mesenchymal compartment in inducing or maintaining epithelial renewal.
Urothelial cells express pathogen pattern recognition receptors such as TLR4 to detect the presence of bacterial lipopolysaccharide (LPS) (Schilling et al., 2001). The secretion of cytokines and chemokines enhances recruitment of inflammatory cells such as neutrophils to the site of infection to remove extracellular bacteria (Mulvey et al., 2000 and references therein). There is little known about how inflammatory processes affect tissue stem cell niches. However, recent studies have shown that, in colonic epithelia, macrophages can coordinate inputs from luminal microbes and injured epithelium to transmit regenerative signals to neighboring progenitor cells of the colon (Pull et al., 2005).
Here, we show that inoculation of uropathogenic E. coli into adult female mouse bladders activates the presumptive USC niche. Next, we show that blocking the transduction of the mesenchymal Bmp4 signal by genetic ablation of Bmpr1a in the urothelium results in 1) reduced proliferation of the USC in response to infection and 2) aberrant proliferation of normally terminally differentiated superficial cells. Finally, we show that this inversion of normal patterns of renewal may be dependent on the concomitant induction of an inflammatory response, as non-infectious injury does not elicit activation of the progenitor niche.
Results
E. coli infection of the bladder results in increased epithelial proliferation and elicits rapid epithelial turnover fueled by basal stem/progenitor cells
UPEC invade into terminally differentiated superficial facet cells and rapidly replicate, forming intracellular bacterial communities (IBCs) that have biofilm-like properties allowing them to subvert innate defenses (Anderson et al., 2003) (Justice et al., 2004). The host responds to the pathogenic attack by exfoliation of superficial facet cells, thereby shedding infected cells, and new superficial facet cells begin to form within 72hrs. We characterized the regenerative response of the mouse bladder during acute UPEC infection. Adult (6–8 week old) female C57Bl/6 mouse bladders were infected with 107 colony forming units (CFU) of the UPEC strain UTI89, which was initially isolated from a patient with clinical cystitis (Mulvey et al., 2001). Mice were sacrificed at 3.5, 6, 12, 24, and 72 hours (h) post inoculation (hpi) for histopathologic analysis.
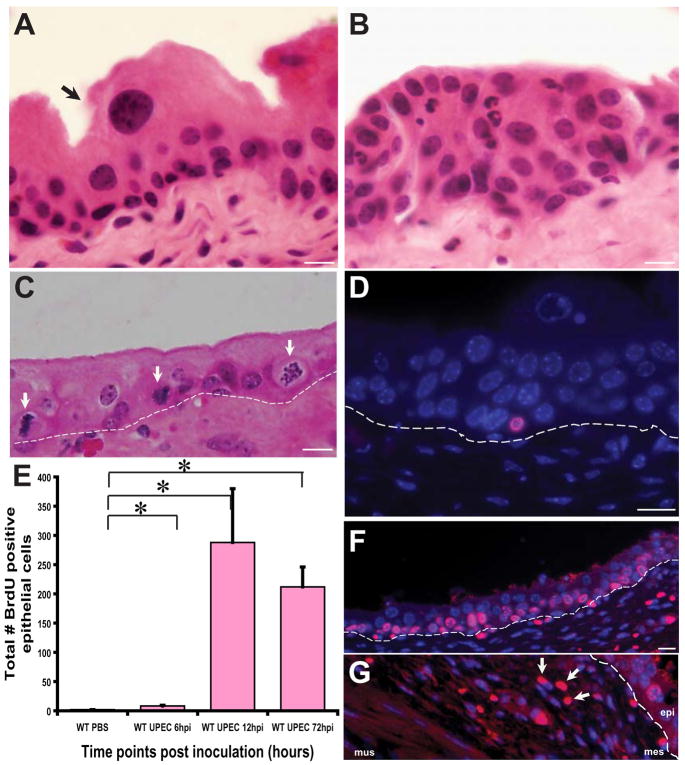
We found that, in contrast to untreated, intact urothelium with large mature superficial cells (Fig. 1A), UPEC infection of the bladder led to urothelial hyperplasia within 6 hpi, which was characterized by loss of superficial cells and additional strata of small, immature cells (Fig. 1B). At this timepoint, there was extensive infiltration of acute inflammatory cells, accompanied by hyperemia and edema (normal sequelae of an acute infection) of the lamina propria. The epithelium showed numerous mitotic figures (Fig. 1C). All mice were also inoculated intraperitoneally with a nucleotide analogue, Bromodeoxy-uridine (BrdU), 90 minutes prior to sacrifice in order to correlate mitotic activity with differentiation state in infected bladders at various time points. The short-pulse BrdU administration labels only cells in the basal layer and at 6hpi, a small number of BrDu+ cells can be found (Fig. 1D). These BrdU positive cells increased dramatically in number between 12–72 hpi (Fig. 1E–F, p<0.05). BrdU+ cells were also evident in the mesenchymal compartment at 12 hpi (Fig. 1G), suggesting activation of this compartment during acute infection. Short-term BrdU labeling of urothelial cells was confined throughout the course of acute infection to the basal layer where the USC cells reside. Together, these results suggest that UPEC infection leads to rapid induction of stem and early progenitor cell proliferation that then fuels regeneration of sloughed facet cells.
Figure 1. UPEC infection accelerates urothelial renewal.
(A) Hematoxylin and eosin (H&E) stained normal adult urothelium depicting an unperturbed USC niche. Arrows point to a mature superficial cell. (B) 12 hpi (hpi= hours post inoculation), there is exfoliation of mature superficial cells and hyperproliferation of the underlying immature basal cells concomitant with an inflammatory response. (C) Numerous mitotic figures (arrows) are evident localized to the basal layer housing presumptive stem cells. Dotted lines indicate the epithelial-mesenchymal boundary. (D) Immunofluorescence (IF) analysis reveals that BrdU (stained red with AlexaFluor 594-tagged anti-goat secondary antibodies) labels only basal cells at 6hpi. Nuclei are stained blue with biz-benzimide. (E) Total number of BrdU+ epithelial cells per tissue section (n= 1–2 sections/bladder; 4–7 mouse bladders/timepoint/condition) were counted. Depicted are data from 6h, 12h and 72h post UPEC inoculation relative to mock-infected bladders; p<0.05. P values were computed using a two-tailed Mann-Whitney test. (F) IF analysis of UPEC infected bladders at 72 hpi shows numerous BrdU+ cells in the USC niche. (G) BrdU+ mesenchymal cells are also evident (arrows) in the USC niche at 12 hpi and 72hpi. Bar= 10 μm.
Effect of UPEC infection on the Bmp4 signaling pathway
Our cellular and BrdU labeling studies indicated that the USC niche was activated following bacteria-induced injury to the superficial cells. Previously, we had demonstrated that concerted changes in several components of the Bmp4 signaling pathway occur within 6 hpi with UPEC in C57BL/6J mice (Mysorekar et al., 2002). For example, we demonstrated a decrease in expression of phospho-Smad1, one of the key effectors of Bmp4 signalling, upon bacterial infection. Thus, we hypothesized that Bmp4 signaling pathway is a key negative regulator of urothelial turnover. To address the possibility that other Bmp family members may play a role in signaling via Bmpr1a, we performed RT-PCR on other Bmp family members including Bmp 2, 5, 7 and a key Bmp4 antagonist, Noggin to determine baseline bladder expression of these family members as well as effects of infection on their expression in wild type mice. We found that Bmp2, 5 and 7 were expressed in the bladder but were not affected by infection at the 6 hour time point tested, when Bmp4 shows maximal change. Noggin does not appear to be expressed in the bladder at all (Supple. Fig. S1). We also examined the expression patterns of additional downstream targets of Bmp4 signaling, namely TGIF, p27Kip1 and p63. p63, a p53-related protein, has been demonstrated to be a direct transcriptional target of Bmp signaling (Bakkers et al., 2002) and TGF β-induced factor (TGIF) is a transcriptional co-repressor of Smad-mediated transcriptional activation and also a downstream target of Bmp4 (Wotton and Massague, 2001). p27Kip1 is a cyclin-dependent kinase (CDK)-inhibitor that suppresses the CDKs active at the G1/S-phase transition in the cell cycle (Glozak and Rogers, 2001; Koff and Polyak, 1995; Polyak et al., 1994a; Polyak et al., 1994b). Our results indicate that there is no change in TGIF levels upon infection. However, p27Kip1 and p63 were both reduced upon UPEC infection (see Figure 5 for details). These effects were specific to activation due to bacterial infection as they were not induced by PBS mock inoculation. Together, our results suggest that Bmp4 is the key family member playing a role in urothelial homeostasis and renewal.
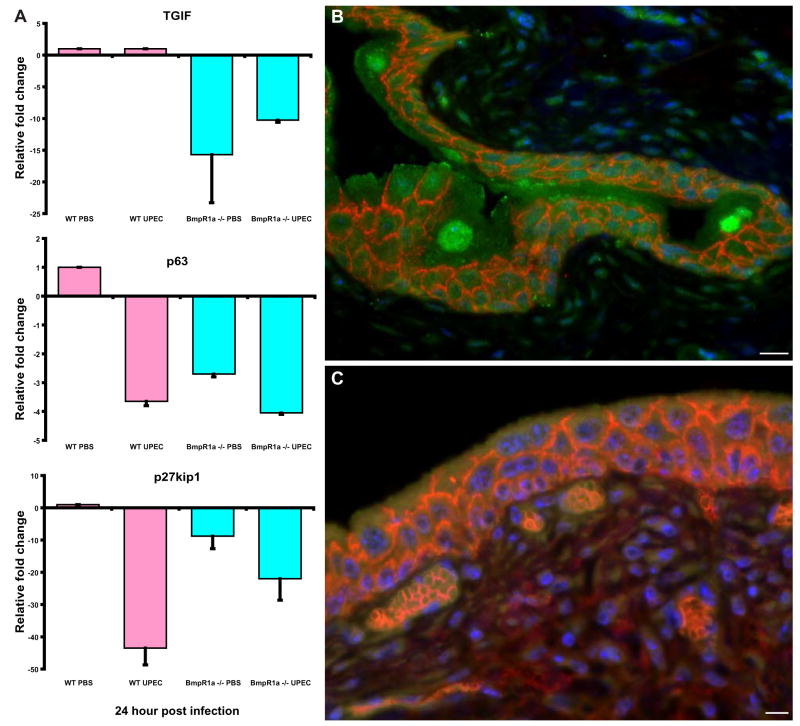
Figure 5. Bmp4 pathway activity is altered in Bmpr1a KO bladders.
(A) Real time quantitative RT-PCR analysis of fold changes in bladder expression of Bmp4 targets, p63, TGIF and p27kip1 at 24h following inoculation of UPEC. Mean values ± S.E. are plotted for 3 independent assays of RNAs, each pooled from 3–4 animals. mRNA levels were first normalized to 18S rRNA, which served as an internal reference control. The normalized values were then referenced to levels of the transcript in bladder RNA prepared from mock-inoculated mice. Levels in control bladder RNA are arbitrarily set at 1. (B) IF analysis shows high levels of p27kip1 expression (stained green with AlexaFluor 488-tagged secondary antibodies) in wild type bladders, with highest expression in mature superficial facet cells and minimally present in the basal and intermediate cells (E-cadherin–stained red/orange with AlexaFluor 594-tagged secondary antibodies). (C) Bmpr1a −/− bladders show markedly reduced levels of p27kip1 expression. Nuclei are stained blue with biz-benzimide. Bar= 10 μm.
Generation of mice with induced ablation of urothelial Bmpr1a eliminates Bmp4-mediated paracrine signaling from the mesenchymal compartment
To determine the role for Bmp signaling in activation of the USC niche, we first assayed the location of the key signaling pathway components. In untreated bladders, Bmp4 protein itself localized to the mesenchymal compartment (Fig. 2A; yellow staining), whereas Bmpr1a, the receptor for Bmp4, localized to the epithelium with highest expression, as might be expected, at the basolateral surfaces of the basal progenitor cells (Fig. 2B; green staining).
Figure 2. Bmp4 signaling pathway is active in the urothelium.
(A) IF analysis shows the ligand Bmp4 is localized to the mesenchymal compartment (green with AlexaFluor 488 tagged secondary antibodies; co-stained with a mesenchymal marker, Ezh2, red). (B) Rabbit polyclonal antibodies to Bmpr1a (stained green with AlexaFluor 488 tagged secondary antibodies) reveals that Bmpr1a is localized to the basolateral surface of the basal cells in the urothelial stem cell niche. Nuclei are stained blue with bis-benzimide. (C) qRT-PCR analysis of Bmpr1a expression in wild type and KO mice, levels decrease upon inducing the KO. Data depicted from 3–4 mouse bladders, analysis was performed in triplicate. (D) IF analysis indicates Bmpr1a protein is minimally expressed in KO mice. Bar= 10 μm.
Although roles for key signaling pathways are well known in development of tissues, the role of such pathways in adaptation/response to injury is relatively unstudied. To test the hypothesis that Bmp signaling may play a role in the bladder response to infection, we inactivated Bmpr1a, the only Bmp receptor known to be expressed in the bladder (Mysorekar et al., 2002). Since null Bmpr1a mutation leads to embryonic lethality, we used an inducible Bmpr1a conditional mutant mouse using a Cre-loxP system (Mishina et al., 2002). Mice with the Bmpr1a locus flanked by lox P sites were mated with inducible βActinCre-ERT mice, which harbor a fusion between Cre-recombinase and a mutated hormone-binding domain of the human estrogen receptor ERT expressed from the beta-actin promoter. We established matings between βActinCre+:Bmpr1afx/+ mice with Bmpr1afx/+ mice to generate litters with homozygous βActinCre+Bmpr1afx/fx (Bmpr1a mutant hereafter), heterozygous βActinCre+Bmpr1afx/+, plus wild type βActinCre-Bmpr1a+/f+ pups and their βActinCre- littermates. Offspring yielded litters of the expected numbers, genotype, and Mendelian ratios (Suppl. Fig. S2A–B). Cre-mediated elimination of Bmp4 pathway activity was induced by tamoxifen (TM) injection intraperitoneally into adult Bmpr1afx/fx: CreERT+ mice and control mice at specific times, once a day for a total of three injections. TM treatment activates the modified estrogen receptor which in turn relocates cre recombinase into the nucleus to eliminate the floxed Bmpr1a alleles.
Mouse bladders were examined histologically prior to TM treatment to verify that there were no effects of unintended (“leaky”) Cre expression. We found that untreated Bmpr1afx/fx/βActinCre+ mice were indistinguishable from βActinCre+Bmpr1afx/+, βActinCre+Bmpr1a+/+ pups and their βActinCre- littermates. Upon TM injection, the Bmpr1a gene was efficiently targeted as judged by genotyping tail DNA (see Suppl. Fig. S2B and Experimental Procedures); however, histological analysis of H&E sections showed, surprisingly, that even 2 weeks after TM induction, the knockout, heterozygous and wild type control mice showed no gross defects in urothelial architecture other than slight edema of the lamina propria. These changes were evident in all littermate controls and were likely non-specific results of TM injection (data not shown).
Genetic ablation of Bmpr1a should result in reduction in mRNA and protein expression of the receptor in the KO mouse bladders. Accordingly, we found that Bmpr1a expression levels were reduced relative to wild type bladders as determined by qRT-PCR (Fig. 2C) and the Bmpr1a protein was minimally expressed in the KO mice as ascertained by immunostaining (Fig. 2D).
Bmpr1a−/− epithelia show markedly reduced proliferation in the basal/stem cell compartment post infection at 12 hours post infection
We reasoned that since the urothelial USC niche is normally quiescent, ablation of Bmp4 signaling may not induce any changes in urothelial architecture in the absence of an activating stimulus. Thus, we induced injury in the bladders of TM-treated Bmpr1afx/fx:Cre-ERT+ and heterozygous and wildtype littermates by inoculation with 107 CFU of UTI89 and examined bladders histologically and by immunofluorescence at 6, 12, 24, and 72 hpi. All mice were also inoculated intraperitoneally with BrdU 90 minutes prior to sacrifice as described before.
We found that complete ablation of Bmp4 signaling led to substantially reduced basal cell proliferation in response to infection at 12 hpi compared to wild type bladders (Fig. 3A, p<0.05). While the total numbers of cycling urothelial cells was reduced, they remained localized to the basal layer as determined by the co-localization of BrdU and cytokeratins 5 and 6 (Fig. 3B). Thus, ablation of Bmp4 signaling leads to an apparent deficiency in basal progenitor cell proliferation immediately following UPEC infection. We also determined that Bmpr1a ablation had no detectable effect on the ability of UPEC to undergo IBC formation (Fig. 3C). Thus, during the acute stages of infection, UPEC pathogenesis was not dramatically affected in the KO mice.
Figure 3. Bmp4 signaling regulates basal cell proliferation.
(A) Loss of Bmp4 signaling leads to reduced basal cell proliferation. Depicted are counts of BrdU positive cells in 12h post UPEC infection of wild type and KO bladders plus mock infected bladders. The number of BrdU+ cells in infected Bmpr1a KO bladders are markedly reduced relative to infected wild type bladders (p<0.05).(B) Immunofluorescence studies of the KO bladders reveal that BrdU+ (pink) cells are localized to the basal layer (Cytokeratins 5, 6 positive, green). (C) Immunofluorescence studies of Bmpr1a KO bladders reveal that IBCs (orange, costained with rabbit antibodies to E. coli and BrDu) form normally in superficial cells. BrDu+ epithelial cells (red nuclei) are evident in the basal layer (Cytokeratins 5, 6 positive, green). Nuclei are stained blue with bis-benzimide. Bar= 10 μm.
Inducible inactivation of Bmpr1a results in a block in terminal differentiation of superficial epithelial cells at 72h post infection
To further explore the role of Bmp signaling in urothelial renewal, we examined the bladders at 72 hpi, a time of maximal regeneration of superficial facet cells following bacterial-induced damage of the epithelial layers. Overall, the total number of cycling cells at this timepoint was still reduced relative to infected littermate control bladders (Fig. 4A, p<0.05). However, a striking feature emerged in the Bmpr1a -deficient bladders at this later time point. There existed an aberrant presence of proliferating cells in the superficial layer: immunofluorescence analysis demonstrated that many superficial cells were still synthesizing DNA (i.e., were BrdU+) (Fig. 4B) while concomitantly expressing the terminal differentiation marker Uroplakin III (Fig. 4C). Basal cells labeling with BrdU were no longer identifiable at this timepoint, despite their abundant presence in wildtype controls (Fig. 4F). Thus, at 72 hours after infection with UPEC, in the Bmpr1a mutant mice, the BrdU+ cells of the bladder (red) were found in the superficial layer of cells, separated from the cytokeratin-positive basal layer (green), (Fig. 4D). Normally, in wild type mice, these superficial cells are post-mitotic, but in the Bmpr1a mutant mice, they were often in various stages of mitoses (Fig. 4E). In wild type mice at 72 hpi, only cells in the basal layer are positive for BrdU (Fig. 4F) and mitotic activity is evident only in the basal cell layer (Fig. 4G, arrow points to a basal layer mitotic figure). Thus, not only was basal cell proliferation reduced overall in the Bmpr1a−/− epithelia, but the location of proliferation at 72 hpi was also altered suggesting that the entire differentiation program was disrupted.
Figure 4. Bmp4 signaling is necessary for terminal differentiation of superficial facet cells.
(A) Bmpr1a −/− bladders 72 hpi show reduced proliferation relative to infected wild type control bladders (p<0.05). Depicted are total number of BrdU+ epithelial cells/section (n=2 sections/bladder, 4–7 mouse bladders/timepoint/condition) from 72h post UPEC infection of wild type and KO bladders and from mice mock-inoculated with PBS. (B) All BrdU+ (pink) nuclei are in the luminal/superficial layer at 72 hpi. (C) Immunofluorescence studies of the KO bladders at 72 hpi reveal that the BrdU+(pink) cell is also Uroplakin+ (green). (D) IF of 72h UPEC infected Bmpr1a −/− bladder co-stained with BrdU and Cytokeratin 5,6 and 14 (basal cell markers). No colocalization is evident. (E) H&E stained section of a bladder from 72h UPEC infected Bmpr1a KO mouse shows a cell undergoing mitosis in the superficial layer while exhibiting differentiated cell characteristics. (F) BrdU staining of 72h UPEC infected wild type bladders reveals BrdU+ cells localizing to the basal cell layer only. Nuclei are stained blue with bis-benzimide. (G) H&E stained 72h UPEC infected wild type bladder shows mitotic cell localized to basal layer. Bar= 10 μm.
Bmpr1a ablation elicits alterations in targets of Bmp4 signaling pathway
To determine whether targets of Bmp4 signaling were affected by Bmpr1a ablation, we examined the expression of TGIF, p63 and p27Kip1. Quantitative real-time PCR detection demonstrated a reduction in these three downstream targets of Bmp signaling in Bmpr1a−/− mouse bladders. TGIF, p63 and p27Kip1 were reduced 15 fold, 3-fold and 7-fold, respectively, in Bmpr1a−/− mouse bladders relative to wild type mice (Fig. 5A). We examined immunolocalization of the p27kip1 protein and found that p27kip1 exhibits high levels of expression in wild type bladders with highest expression in mature superficial cells (green, Fig. 5B) and shows markedly lowered levels in Bmpr1a KO bladders (Fig. 5C). This reduction in p27Kip1 expression may be one underlying mechanism driving the block in terminal differentiation of facet cells. High levels of p27Kip1 are known to be critical for a proliferating cell to exit the cell cycle and terminally differentiate. Thus, reduced levels of this protein may contribute to the block in terminal differentiation seen in the Bmpr1a knockout mice.
Together, our results reveal that ablation of Bmpr1a leads to a decrease in Bmp4 pathway activity indicated by reduced expression of various Bmp4 downstream targets. This altered expression of Bmp4 downstream targets may be responsible, at least in part, for the aberrant renewal in the Bmpr1a −/− epithelia.
Requirement for Bmp4 signaling in urothelial renewal is specific to uropathogenic bacteria and is not induced by an isogenic avirulent mutant
To determine whether USC proliferation and differentiation required bacterial colonization, invasion and establishment of infection, 21 adult female mice wild type, heterozygous and null for the Bmpr1a alleles were infected with an isogenic but avirulent strain of UTI89 (UTI89 Delta FimH, ΔfimH). FimH is the bacterial adhesin of the type 1 pilus required for bladder colonization, invasion and IBC formation (Mulvey et al., 1998). UTI89 ΔfimH does not produce adhesive type 1 pili (Wright et al., 2007). Mice were treated as described with TM once daily for 3 days and then infected with UTI89 ΔfimH for 12, 24, 48, 72h and 7days. Bladders were isolated from the mice at various time points after infection and analyzed histologically. We found that there was no significant difference in appearance of the UTI89 ΔfimH infected bladders relative to the uninfected bladders. In addition, there were no discernible differences between the Bmpr1a wild type and null mice. All mice were injected with BrdU prior to sacrifice and BrdU positive cells were counted at each time point following inoculation. No significant differences above mock infected bladders were seen (data not shown). Thus, the activation of the USC niche was dependent not only on colonization, but upon adherence to or invasion of the urothelium and subsequent activation of the pathogenesis cycle described previously (Justice et al., 2004).
Protamine Sulfate induced injury to the bladder results in regeneration that does not activate stem/progenitor cells
Although ΔfimH bacteria could not induce USC activation, these mutant bacteria also did not cause exfoliation of superficial facet cells. To determine whether superficial cell exfoliation alone, in the absence of an invasive pathogenic stimulus, was sufficient to induce USC activation, we transurethrally administered protamine sulfate (PS) to adult C57Bl/6J mice. We had previously shown that PS induces exfoliation of the superficial facet cells in a dose-dependent manner within the first 12 hrs (Mysorekar and Hultgren, 2006). Although an effective superficial cell exfoliant, PS did not induce host inflammation. Superficial cells began regenerating by 48hrs, similar to the timecourse following infection with UPEC.
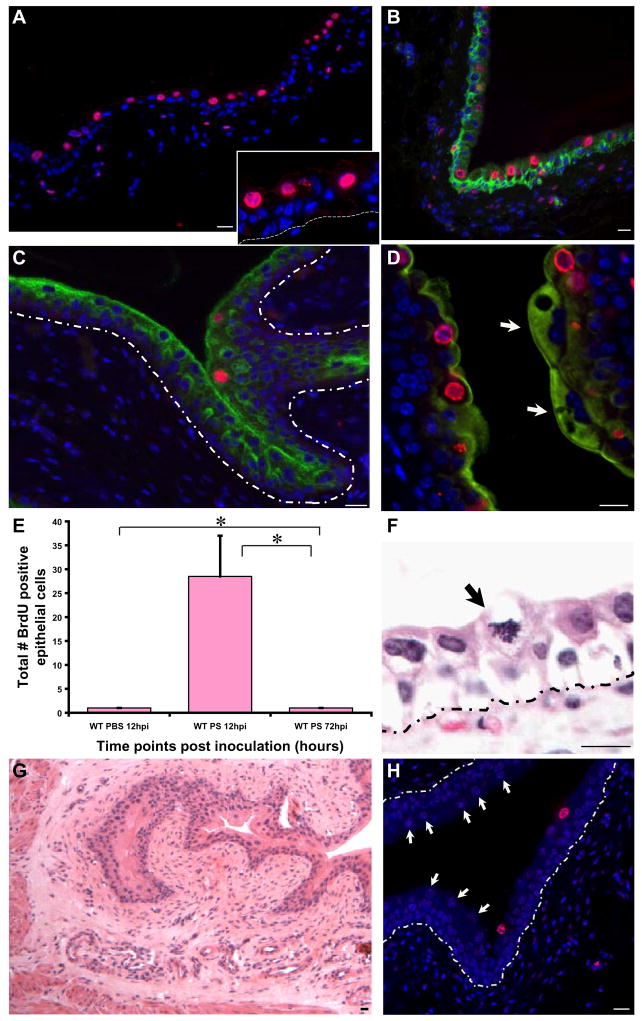
To determine the source of the new superficial cells, we performed 90 minute BrdU pulse labels, as for UPEC, prior to sacrifice at 1.5, 3.5, 6, 12, 48 and 72 hrs post PS treatment. Surprisingly, BrdU was incorporated only by cells above the basal layer at 12hpi (i.e., supra-basal cells; Fig. 6A, inset shows a higher magnification). To confirm absence of proliferation in presumptive USCs, we co-stained cells expressing BrdU with a cytokeratin marker that exclusively labels basal cells. Our results showed that the BrdU+ cells (red) localized to an intermediate layer, and almost never in the basal layer (green) (Fig. 6B), suggesting that renewal following PS administration is catalyzed by transiently amplifying (TA) cells excluded from the basal layer. In addition, we examined the colocalization between BrdU and uroplakin (a marker of terminal differentiation). Our results show BrdU+ cells are faintly positive for uroplakin III expression (Fig. 6C). These levels are consistent with the cells being of intermediate character (i.e., neither basal nor superficial cells). We also observed BrdU-negative, mature multinucleated superficial facet cells (arrow, Fig. 6D) with high and dense expression of uroplakin, which were nearby BrdU+ intermediate cells. Quantification of the BrdU+ cells revealed that at 12 hpi, there were numerous dividing cells and these numbers decrease dramatically by 72 h (Fig. 6E, p<0.05). Interestingly, despite the frequent mitotic activity of the supra-basal cells, the epithelium is not hyperplastic (Fig. 6F), and, as expected, there was no evidence of accumulation or infiltration by inflammatory cells (Fig. 6G). Even at 72hpi, a few presumptive TA cells remain positive for BrdU although they continue to be localized to the suprabasal layer adjacent to newly regenerated mature superficial cells (arrows)(Fig. 6H).
Figure 6. PS treatment activates transiently amplifying cell populations.
(A) IF analysis reveals that BrdU (stained red with AlexaFluor 594-tagged anti-goat secondary antibodies) labels non-basally located (intermediate) cells (shown in higher magnification in A_Inset). Nuclei are stained blue with biz-benzimide. Dotted lines indicate the epithelial-mesenchymal boundary. (B) BrdU+ cells (red) do not co-label with the cytokeratins 5, 6 (green)-expressing basal urothelial cell layer. (C) IF analysis of a 12h PS treated bladder, showing slight colocalization between BrdU (red) and uroplakin III, a terminal differentiation marker (stained green with AlexaFluor 488-tagged anti-mouse secondary antibodies). (D) Higher magnification, with a mature, multinucleated superficial facet cell densely expressing uroplakin III (arrow) opposite several BrdU+ intermediate cells. (E) Total number of BrdU+ epithelial cells were counted in 12h and 72h post PS treated bladders (n= 1–2 sections/bladder; 4–7 mouse bladders/timepoint/condition). (F) H&E stained PS treated bladders showing a mitotic figure in the suprabasal layer of the urothelium (arrow). (G) H & E stained 12h PS treated wild type bladder, showing no evidence of an inflammatory infiltrate. (H) IF analysis of BrdU+ cells in PS treated wild type bladders, PS treated for 72h, with few proliferating cells in suprabasal layer with presence of numerous regenerated mature facet cells present (arrows). Bar= 10 μm.
The above results argue that renewal following PS administration is mediated by cells of intermediate differentiation or TA cells– and not the basal USCs. To further illustrate the marked difference in the bladder response to inflammation-inducing injury (e.g., infection with UPEC) compared to chemical, non-inflammatory injury, we used Affymetrix GeneChips to assay global expression profiles. We compared global gene expression profiles in the bladders of PS-treated vs UPEC-infected C57BL/6J mice. We found that the pattern of gene expression changes induced by PS at timepoints (<6 h) were dramatically different from those induced by UPEC infection (Supple. Fig. 3). Thus, at time points in which infection is known to induce marked changes in the expression of cell cycle and inflammation-related genes, a completely different pattern of gene expression was induced by PS treatment. Thus, the molecular regulation of urothelial response to chemical injury is distinct from the response to infectious injury.
Chemical injury to the Bmpr1a−/− bladders did not induce aberrant renewal
We investigated whether inactivation of Bmp4 signaling affected response to chemical injury since UPEC infection seemingly led to Bmp4-dependent activation of the USC niche. If the PS induced bladder response is independent of Bmp4, then we hypothesized that inoculation of Bmpr1a −/− mouse bladders with PS should not have a response different from wild type or heterozygous littermates. Mice were treated with TM once daily for 3 days and inoculated with 10 mg/ml PS for 12h, 48h, 72, and 2 weeks. Bladders were isolated from the mice at 12, 48, and 72 h, and 14 days after inoculation and analyzed histopathologically by hematoxylin and eosin staining of bladder sections, presence or absence of epithelial hyperplasia, mitotic activity, and quantitation of BrdU positive cells at each timepoint. We did not detect any discernible differences between the knockout and heterozygous mice at 12 or 72 hours (data not shown). Our results further provide evidence that PS treatment does not activate the USC niche and that urothelial regeneration from PS-mediated injury does not require the Bmp4 pathway.
Discussion
Modulating Bmp signaling to balance quiescence and activation of USC niche in response to UPEC infection
Our previous data (Mysorekar et al., 2002) showed that urothelial basal cells normally display signs of activated Bmp signaling in vivo; however, unlike the hair follicle, where ablation of Bmp4 pathway activity induced the follicular stem cells to precociously enter the proliferative phases associated with the new hair cycle (Kobielak et al., 2007), our current results show that ablation of Bmp signaling alone was not sufficient to disrupt the quiescent state of the USC niche in the bladder. Instead, our loss-of-function studies highlighted an essential role for Bmp signaling in maintaining the responsiveness of the USC niche, because, in its absence, USC proliferation was reduced 100-fold in response to infection. Further, our studies demonstrate that Bmp signaling is required for proper terminal differentiation following UPEC infection. The UPEC pathogenic cycle includes exfoliation of the superficial facet cells and subsequent basal cell proliferation and upon resolution of infection, the terminally differentiated facet cell layer is replaced, thus restoring the impermeable uroplakin barrier that protects the bladder epithelium from the contents of the bladder lumen. However, in the absence of Bmpr1a, induction of basal cell proliferation by infection and the regenerative response was severely hampered. Intriguingly, superficial cells continued to divide and failed to undergo terminal differentiation in the KO mice. Thus, we propose a model whereby the USC niche maybe defined as comprising the proliferative basal epithelial cell compartment and the underlying mesenchyme. In normal unperturbed bladders, Bmp signaling from the mesenchyme may be required to maintain both USC quiescence and terminal differentiation of superficial cells. UPEC infection triggers epithelial cell damage and inflammation, resulting in the modulation of this key pathway in order to trigger exit from quiescence (i.e., to fuel basal cell proliferation) and to induce their terminal differentiation into superficial cells and to restore normal physiological barriers. Ongoing studies in the laboratory suggest that the absence of normal patterns of renewal due to ablation of Bmp signaling predisposes to a chronic inflammatory condition characterized by lack of superficial cells, changes in tissue architecture, and luminal bacterial colonization (data not shown). Investigations aimed at defining the molecular and cellular nature of the observed abnormalities in long term pathogenesis of UPEC-infected Bmpr1a −/− mice are in progress.
Bmp4 signaling is essential for urothelial regeneration and repair in response to infectious, but not chemical, injury
In the current study, we have elucidated a novel urothelial regenerative response to infection, and use the currently available USC markers (e.g., BrdU labeling, position within the epithelium, Cytokeratins 5, 6 and BrdU colocalization) to characterize the cellular bases of the regenerative response. We have shown for the first time that the pattern of cellular and molecular response of the urothelium to chemical injury differs markedly from its response to infectious injury. These differences are seen despite similarities in the kinetics and magnitude of facet cell exfoliation and urothelial regeneration in both types of injury. Injury induced by the basic cationic protein protamine sulfate did not induce basal cell proliferation or change molecular aspects of the underlying USC niche despite facet cell exfoliation and epithelial regeneration of similar timecourse and magnitude as followed UPEC inoculation. Rather, PS treatment led to activation and proliferation of cells of intermediate differentiation residing above the basal level and led to no change in Bmp4 pathway activation. Thus, chemical agents may cause exfoliation but not inflammation and may induce transient amplifying (TA) cells, rather than USCs, to mediate repair of superficial cell damage. These studies have the caveat that stem cells likely constitute a subset of these basally located Cyt5,6+BrdU+ cells; molecular markers entirely specific for the stem cell remain to be defined, and as yet there are no clonogenic assays that functionally prove stemness in the urothelium (Huh et al., 2006).
Ablation of Bmp signaling did not alter the regenerative response to PS-mediated injury. Bmp4 signaling is apparently dispensable for urothelial renewal that does not involve proliferation of basally located, presumptive urothelial stem cells because exfoliation/regeneration-inducing injury by chemical treatment and by non-adherent or non-pathogenic bacteria does not require Bmp4. The different requirements for Bmp4 may reflect that mesenchymal Bmp4 elaboration may depend on inflammation mediated signals. GeneChip and histological studies demonstrated that PS-treated bladders have minimal inflammation compared with UPEC-infected bladders. Thus, chemical agents that cause exfoliation, but not inflammation, may induce transient amplifying (TA) cells rather than USCs to mediate repair of superficial cell damage. Our results suggest that exfoliation inducing injury in the absence of inflammation leads to bladder regeneration based almost exclusively on TA cell amplification. Recent work has stressed the difference between lineage-specific stem cells and transit amplifying (TA) cells, which carry the major proliferative duties of epidermis (Kobielak et al., 2003; Kobielak et al., 2007). Further studies aimed at dissecting specific roles for various immune cell populations in modulating the urothelial stem cell niche will shed light on the cells that might mediate the changes in Bmp signaling that are required for stem cell activation.
Significance of Bmp4 signaling in human disease
Bmp signaling has emerged as a common pathway for controlling stem cell self-renewal and lineage fate from Drosophila to mammals. However, this is the first time that Bmp signaling has been implicated in progression of an acute infection process in any tissue. Differences in regulation of urothelial renewal in humans may influence progression of UTIs (whether they are acute and self-limited or can become chronically recurring). Previously, we showed that UPEC can persist indefinitely within the immature basal cells enclosed within lysosomal compartments and can re-emerge from these quiescent reservoirs to seed recurrent UTIs (Mysorekar and Hultgren, 2006). Our study had suggested that UTI recurrence may depend on UPEC’s ability to manipulate urothelial differentiation/proliferation in the bladder and that stem and early progenitor cells serve as a protective niche for UPEC to escape immune detection and evade exfoliation. Dysregulation of urothelial renewal by inactivation of Bmp signaling could affect both formation of UPEC intracellular reservoirs and their re-emergence from this state concomitant with proliferation/differentiation cascades. Our findings may also have clinical significance for another major chronic disorder that is of low incidence and unknown etiology affecting bladders of women: interstitial cystitis (IC), characterized by damaged urothelial barriers including loss of a superficial facet cell layer and abnormally permeable membranes (Parsons, 2007). Indeed, aberrations in Bmp4 pathway activity could be one genetic etiological factor leading to certain women being more susceptible to IC. Thus, recombinant activators of Bmp4 signaling could be used to modulate activation of the USC niche and to induce terminal differentiation and restoration of normal physiologic barriers. Understanding how the urothelium renews itself following infection may be critical not only for understanding of acute and recurrent UTIs, but also for understanding another major malignancy: bladder cancer, which is characterized by aberrant urothelial turnover and chronic inflammation. Future studies may demonstrate how aberrations in important bladder signaling pathways regulating urothelial proliferation like the Bmp4 pathway, may predispose patients to recurrent UTIs, IC or to the aberrant proliferative and differentiation patterns that result in bladder cancers.
Prospectus
Together, our study distinguishes two pathways of epithelial injury response in the same tissue. One, the response to infection, involves activation of basal stem cells and is regulated at the molecular level by Bmp4 signaling. The other, response to non-inflammatory, exfoliative chemical injury, appears to involve increased proliferation of transient amplifying cells. The molecular regulation of this latter pathway remains to be elucidated but clearly does not seem to depend on Bmp4. Future studies will elucidate the potential role of inflammatory mediators in regulating Bmp4 signaling, as well as the principal mediators of non-inflammatory injury response, and might eventually shed light on how the bladder responds to chronic inflammation (e.g. interstitial cystitis) and how it might progress to development of bladder cancer.
Experimental Procedures
Mice
All experiments were performed using protocols approved by the animal studies committee of the Washington University School of Medicine (Animal Welfare Assurance # A-3381–01). Adult female mice were used in all experiments. All mice were maintained under specified pathogen free conditions in a barrier facility and under a strict 12h light cycle.
Bacterial Strains
UTI89 (Mulvey et al., 2001), a pathogenic UPEC strain recovered from a patient with UTI (Langermann et al., 2000) was transformed with a pCOM-GFP plasmid (Valdivia et al., 1996) and grown for 16h in LB as a static culture at 37 °C. Bacterial strains were grown using standard techniques. UTI89 ΔfimH (Wright et al., 2007) contains a deletion of the fimH gene and produces fim encoded rods lacking an adhesin (type1+/FimH−).
Inoculations of mice
Mice were anesthetized and inoculated via transurethral catheterization with 50 μl of bacterial suspension (107 CFU/ml) in phosphate buffered saline (PBS) as previously described (Hannan et al., 2008; Mysorekar and Hultgren, 2006). At the indicated times, mice were sacrificed and their bladders were aseptically removed and processed for microscopy, histology and CFU titration. All analysis was performed in the urothelia of adult female mice (n=4–7 mice per experimental group for all experiments, n=1–2 experiments).
Protamine sulfate (PS) treatment
PS (Sigma) was delivered transurethrally at a concentration of 10 mg/ml in water (Mysorekar and Hultgren, 2006). All analysis was performed in the urothelia of adult female C57BL6 mice (n=4–7 mice per experimental group for all experiments, n=1–2 experiments).
Histochemical and immunofluorescence analysis
For histological and immunofluorescence studies, bladders were dissected into two halves and incubated in 10% formalin overnight at 4°C. The bladder tissues were embedded in 2% agar for paraffin processing. For immunohistochemical analysis, 4–5μm serial sections were cut longitudinally, de-paraffinized in fresh xylene (2× 10min, RT), rehydrated in isopropanol (3× 5min, RT), antigen retrieved by boiling for 30min in 10mM NaCitrate buffer, blocked in 1% BSA/0.3% Triton X-100 for 1hr at RT, and subsequently incubated overnight at 4°C with the following primary antibodies:(i) rabbit polyclonal antibodies to E. coli (1:500; US Biological), (ii) rat anti-mouse E-cadherin (1:500, Zymed), (iii) mouse monoclonal antibody (mAb) to uroplakin III (Research Diagnostics); (iii) mouse mAb to cytokeratins 5,6 (clone D5/16B4; Chemicon; 1:500); (iv) goat polyclonal antibody to Bmpr1a (R&D Systems) and (v) mouse monoclonal antibody to Bmp4 (Novocastra and R&D Systems). After 3 PBS washes (3× 5 min at RT), antigen-antibody complexes were detected with AlexaFluor-488, 594, 647-conjugated secondary antibodies (1:500, Molecular Probes).
Profiling Gene Expression Using DNA Microarrays
UTI89 was grown as described before. 8–10-week-old female C57Bl/6J mice (The Jackson Laboratories) were inoculated, via transurethral catheterization, with 50 μl of a suspension of 107 colony-forming units of UTI89 in PBS. Total cellular RNA was isolated (RNeasy kit, Qiagen) from bladders of mice sacrificed prior to and 3, 6 h after inoculation (n = 10 mice/time point/bacterial strain/experiment; n = 2 independent experiments). Equivalent amounts of RNA from each mouse in each group/experiment were pooled and biotinylated cRNA targets prepared. Each cRNA target was hybridized to U74 GeneChips (Affymetrix). GeneChip software was then used to compute an average fluorescence intensity across all probe sets on the GeneChips.
Comparison of profiles using functional GO-term annotated databases
Profiles of each timepoint were generated by standard thresholds for significance (90% confidence of fold change >1.3 and intensity level >100), comparing each timepoint to the reference untreated/uninfected GeneChips using dChip software. Additional, higher level analysis of changes in genes across the timecourse following PS or UPEC were corroborated by the commercial visualization tool Spotfire. Further, analysis of each profile for over- and under-represented Gene Ontology categories was carried out using the GOurmet software (Doherty et al., 2006) to quantitatively analyze differences between the profiles based on overall changes in distribution of the GO terms inherent to each profile (Doherty et al., 2008; Lugus et al., 2007).
SYBR Green-based Real Time Quantitative PCR (qRT-PCR)
Age-matched female wild type C57Bl/6 mice or Bmpr1a −/− mice were infected with UTI89 strain for multiple; timepoints (n = 4 animals/time point/strain). Bladder RNAs were pooled as above and used for qRT-PCR studies. qRT-PCR assays were performed using the same RNAs employed for the DNA microarray analysis. cDNAs were added to 25-μl qRT-PCRs containing 12.5 μl of 2× SYBR Green master mix (Applied Biosystems), and 900 nM gene-specific primers. A melting curve was used to identify a temperature where only the amplicon, and not primer dimers, accounted for the SYBR Green-bound fluorescence. Assays were performed in triplicate with a BioRad MyiQ instrument. All data were normalized to an internal standard (18S rRNA; ΔΔCT method). Primer Sequences used: p63_5′: GTG TTG GCA AGG ATT CTG AGA CC; 3′: GGA AGA CAA TAG CAG GCA TGC TG TGIF 5′: GAT GGC AAA GAT CCA AAT CAG TTC; 3′: GCA TGA AGT TTT TGA TAC CCA TT p27kip1 5′: CGG CGG CAA GGT TTG GAG AGG; 3′: GGA GGA GGC AGG AGG AGG TGG.
Generation of Bmpr1a knockout mice
In the Bmpr1afx/fx mouse line, the second exon of the Bmpr1a gene is flanked by two loxP sites (Mishina et al., 2002). Using the Bmpr1afx/fx line and the tamoxifen inducible Cre line βActinCre (Jackson Laboratory). BActinCre+Bmpr1afx/fx homozygous, βActinCre +Bmpr1afx/+ heterozygous, and βActinCre -Bmpr1afx/fx or fx/+ wild type control mice were generated. To introduce Bmpr1a inactivation, adult female mice were intraperitoneally (IP) injected with 25 mg/ml tamoxifen (TM, Sigma, P-0913) at specific time points once every day for a total of 3 injections. TM induces Cre expression. Cre recombinase in turn mediates loxP dependent DNA recombination. Tails were collected on postnatal day 21 (P21) and at the time of termination to extract genomic DNA. Genotyping of loss of Bmpr1a alleles were carried out as described previously by Mishina and coworkers (Mishina et al., 2002). Genotyping was done by PCR assay with different combinations of primers (Pr). Pr1:GCAGCTGCTGCTGCAGCCTCC, Pr2:TGGCTACAATTTGTCTCATGC, Pr3:GGTTTGGATCTT AACCTTAGG. Pr1 and Pr2 amplify 230bp and 150bp products specific for the floxed and wild-type alleles, respectively. The combination of Pr2 and Pr3 amplifies a 180bp product specific for the targeted allele after TM-induced Cre mediated recombination.
BrdU labeling
To determine mitotic activity, all mice were injected intraperitoneally with an aqueous solution of 5-bromo-2′-deoxyuridine (BrdU, 120 mg/kg) and 5′-fluoro-2′-deoxyuridine (12 mg/kg) (Sigma), 90 min prior to sacrifice. All BrdU count analyses were performed on two separate sections, each containing bisected bladders from 4 to 7 adult female mouse bladders/timepoint and per condition.
Statistical Analysis
Statistical analysis was performed using Mann-Whitney U-test (two-tailed). A value of p<0.05 was considered to be significant.
Supplementary Material
Acknowledgments
We thank Drs. Thomas Hannan, Swaine Chen and Jennifer Elam for thoughtful review of the manuscript. The Bmpr1a fx/fx mice were a kind gift of Dr. Yuji Mishina (NIEHS). We thank the Multiplexed Gene Analysis Core of the Siteman Cancer Center (supported in part by National Cancer Institute Grant P30-CA91842). This work was supported by National Institutes of Health Grant DK51406 and Office of Research on Women’s Health Specialized Center of Research Grant DK64540 (to S.J.H.), K08-DK066062 and R01-DK079798 (to J.C.M.); postdoctoral support by Individual National Research Service Award F32-CA108328 and a Pathway to independence Award K99- DK080643 (to I.U.M).
Footnotes
Conflict of Interest Disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science (New York, NY. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- Bakkers J, Hild M, Kramer C, Furutani-Seiki M, Hammerschmidt M. Zebrafish DeltaNp63 is a direct target of Bmp signaling and encodes a transcriptional repressor blocking neural specification in the ventral ectoderm. Developmental cell. 2002;2:617–627. doi: 10.1016/s1534-5807(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Daher A, de Boer WI, El-Marjou A, van der Kwast T, Abbou CC, Thiery JP, Radvanyi F, Chopin DK. Epidermal growth factor receptor regulates normal urothelial regeneration. Laboratory investigation; a journal of technical methods and pathology. 2003;83:1333–1341. doi: 10.1097/01.lab.0000086380.23263.52. [DOI] [PubMed] [Google Scholar]
- de Boer WI, Rebel JM, Vermey M, de Jong AA, van der Kwast TH. Characterization of distinct functions for growth factors in murine transitional epithelial cells in primary organotypic culture. Experimental cell research. 1994;214:510–518. doi: 10.1006/excr.1994.1288. [DOI] [PubMed] [Google Scholar]
- de Boer WI, Vermeij M, Diez de Medina SG, Bindels E, Radvanyi F, van der Kwast T, Chopin D. Functions of fibroblast and transforming growth factors in primary organoid-like cultures of normal human urothelium. Laboratory investigation; a journal of technical methods and pathology. 1996;75:147–156. [PubMed] [Google Scholar]
- Doherty JM, Carmichael LK, Mills JC. GOurmet: a tool for quantitative comparison and visualization of gene expression profiles based on gene ontology (GO) distributions. BMC bioinformatics. 2006;7:151. doi: 10.1186/1471-2105-7-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JM, Geske MJ, Stappenbeck TS, Mills JC. Diverse adult stem cells share specific higher-order patterns of gene expression. Stem cells (Dayton, Ohio) 2008;26:2124–2130. doi: 10.1634/stemcells.2008-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49:53–70. doi: 10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- Glozak MA, Rogers MB. Retinoic acid- and bone morphogenetic protein 4-induced apoptosis in P19 embryonal carcinoma cells requires p27. Experimental cell research. 2001;268:128–138. doi: 10.1006/excr.2001.5281. [DOI] [PubMed] [Google Scholar]
- Hannan TJ, Mysorekar IU, Chen SL, Walker JN, Jones JM, Pinkner JS, Hultgren SJ, Seed PC. LeuX tRNA-dependent and -independent mechanisms of Escherichia coli pathogenesis in acute cystitis. Molecular microbiology. 2008;67:116–128. doi: 10.1111/j.1365-2958.2007.06025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks RM. The mammalian urinary bladder: an accommodating organ. Biological reviews of the Cambridge Philosophical Society. 1975;50:215–246. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Huh WJ, Pan XO, Mysorekar IU, Mills JC. Location, allocation, relocation: isolating adult tissue stem cells in three dimensions. Current opinion in biotechnology. 2006;17:511–517. doi: 10.1016/j.copbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Jost SP. Renewal of normal urothelium in adult mice. Virchows Archiv. 1986;51:65–70. doi: 10.1007/BF02899016. [DOI] [PubMed] [Google Scholar]
- Jost SP. Cell cycle of normal bladder urothelium in developing and adult mice. Virchows Archiv. 1989;57:27–36. doi: 10.1007/BF02899062. [DOI] [PubMed] [Google Scholar]
- Jost SP, Potten CS. Urothelial proliferation in growing mice. Cell and tissue kinetics. 1986;19:155–160. doi: 10.1111/j.1365-2184.1986.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. The Journal of cell biology. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff A, Polyak K. p27KIP1, an inhibitor of cyclin-dependent kinases. Progress in cell cycle research. 1995;1:141–147. doi: 10.1007/978-1-4615-1809-9_11. [DOI] [PubMed] [Google Scholar]
- Kurzrock EA, Lieu DK, Degraffenried LA, Chan CW, Isseroff RR. Label-retaining cells of the bladder: candidate urothelial stem cells. American journal of physiology. 2008;294:F1415–1421. doi: 10.1152/ajprenal.00533.2007. [DOI] [PubMed] [Google Scholar]
- Langermann S, Mollby R, Burlein JE, Palaszynski SR, Auguste CG, DeFusco A, Strouse R, Schenerman MA, Hultgren SJ, Pinkner JS, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. The Journal of infectious diseases. 2000;181:774–778. doi: 10.1086/315258. [DOI] [PubMed] [Google Scholar]
- Lugus JJ, Chung YS, Mills JC, Kim SI, Grass J, Kyba M, Doherty JM, Bresnick EH, Choi K. GATA2 functions at multiple steps in hemangioblast development and differentiation. Development (Cambridge, England) 2007;134:393–405. doi: 10.1242/dev.02731. [DOI] [PubMed] [Google Scholar]
- Mishina Y. Function of bone morphogenetic protein signaling during mouse development. Front Biosci. 2003;8:d855–869. doi: 10.2741/1097. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32:69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. The Journal of clinical investigation. 2000;105:863–873. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Oshima K, Fogo A, Ichikawa I. Evidence that bone morphogenetic protein 4 has multiple biological functions during kidney and urinary tract development. Kidney international. 2003;63:835–844. doi: 10.1046/j.1523-1755.2003.00834.x. [DOI] [PubMed] [Google Scholar]
- Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science (New York, NY. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a Persistent Escherichia coli Reservoir during the Acute Phase of a Bladder Infection. Infection and immunity. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. The Journal of biological chemistry. 2002;277:7412–7419. doi: 10.1074/jbc.M110560200. [DOI] [PubMed] [Google Scholar]
- Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. 2007;69:9–16. doi: 10.1016/j.urology.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes & development. 1994a;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994b;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis-Filho JS, Simpson PT, Martins A, Preto A, Gartner F, Schmitt FC. Distribution of p63, cytokeratins 5/6 and cytokeratin 14 in 51 normal and 400 neoplastic human tissue samples using TARP-4 multi-tumor tissue microarray. Virchows Arch. 2003;443:122–132. doi: 10.1007/s00428-003-0859-2. [DOI] [PubMed] [Google Scholar]
- Riedel I, Czernobilsky B, Lifschitz-Mercer B, Roth LM, Wu XR, Sun TT, Moll R. Brenner tumors but not transitional cell carcinomas of the ovary show urothelial differentiation: immunohistochemical staining of urothelial markers, including cytokeratins and uroplakins. Virchows Arch. 2001;438:181–191. doi: 10.1007/s004280000315. [DOI] [PubMed] [Google Scholar]
- Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol. 2001;166:1148–1155. doi: 10.4049/jimmunol.166.2.1148. [DOI] [PubMed] [Google Scholar]
- Valdivia RH, Hromockyj AE, Monack D, Ramakrishnan L, Falkow S. Applications for green fluorescent protein (GFP) in the study of host-pathogen interactions. Gene. 1996;173:47–52. doi: 10.1016/0378-1119(95)00706-7. [DOI] [PubMed] [Google Scholar]
- Wotton D, Massague J. Smad transcriptional corepressors in TGF beta family signaling. Curr Top Microbiol Immunol. 2001;254:145–164. [PubMed] [Google Scholar]
- Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cellular microbiology. 2007;9:2230–2241. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.