Abstract
Objective:
Individual differences in subjective response to alcohol and the occurrence of blackouts and hangover are associated with the development of alcohol-use disorders. As such, subjective responses to alcohol, blackouts, and hangover may share a biological vulnerability to excessive alcohol consumption. The purpose of the current study was to examine subjective responses to alcohol as predictors of estimated blood alcohol concentration (BAC), blackouts, and hangover for a single heavy drinking event.
Method:
Data were collected on 150 (50% female) college students at a large, public university who reported consuming alcohol during their 21st birthday celebration. Using semi-structured interviews and self-report measures, subjective responses to alcohol (at midpoint of a 21st birthday celebration) were examined as predictors of final estimated BAC, blackouts, and hangover.
Results:
Stimulant effects reported for the midpoint of the drinking event predicted final estimated BAC. Both stimulant and sedative alcohol effects directly predicted blackouts during the drinking event and the occurrence of a hangover. Neither stimulant nor sedative effects were mediated by final estimated BAC.
Conclusions:
Retrospective reports of subjective responses to alcohol were associated with the level of intoxication, blackouts, and hangover during a heavy drinking event. Findings therefore suggest the utility of incorporating subjective responses to alcohol into event-specific interventions that are designed to reduce or prevent heavy episodic drinking.
Excessive drinking among college students is a significant health concern that has received attention from the popular media and scientific community (National Institute on Alcohol Abuse and Alcoholism, 2002, 2003). Whereas the majority of attention focuses on typical and heavy drinking patterns, recent research indicates that specific drinking events may be associated with higher-than-usual alcohol consumption and alcohol-related problems (Del Boca et al., 2004; Neighbors et al., 2005; Smith et al., 2006). In particular, 21st birthday celebrations are typically a time to drink excessively as a rite of passage into the ability to legally purchase alcohol. More than 80% of college students drink alcohol to celebrate their 21st birthday, and they consume an average of nearly 13 drinks, with estimated blood alcohol concentrations (BACs) of .19% and higher, which indicates that 21st birthday drinking can be extremely dangerous (Neighbors et al., 2006; Rutledge et al., 2008). Despite the known prevalence and severity of 21st birthday drinking, researchers have just begun to characterize the individual factors that contribute to event-specific drinking patterns and associated negative consequences.
One individual factor that contributes to drinking patterns and development of alcohol-use disorders is “the quality and magnitude of acute subjective responses to alcohol” (King et al., 2002, p. 827; see also Holdstock and de Wit, 1999; Krueger et al., 2002; Schuckit et al., 2001). Early research on variations in subjective responses to alcohol focused on individuals with a family history of alcoholism. Schuckit and colleagues found that children of alcoholics have a lower subjective response to alcohol and are at an increased risk for the development of alcohol-use disorders compared with children of nonalcoholics (Schuckit and Smith, 1996, 2000; Schuckit et al., 1996, 2000). Yet, the association between family history of alcoholism and low subjective responses to alcohol has been inconsistent (Conrod et al., 1997, 2001; Newlin and Thomson, 1990; Peterson et al., 1996). Several studies report that sons of alcoholics actually experience enhanced responses to alcohol compared with sons of nonalcoholics (Conrod et al., 1997, 2001; de Wit and McCracken, 1990; Gianoulakis et al., 1996). For example, sons of alcoholics show a heightened heart rate response and greater behavioral activation to alcohol (Brunelle et al., 2004, 2007; Conrod et al., 1997; Peterson et al., 1996). In response to the conflicting findings, Newlin and Thomson (1990, 1999) proposed the “differentiator model” as a way to explain both decreased and enhanced subjective responses among high-risk individuals. According to this model, high-risk individuals are more sensitive to the stimulating effects of alcohol during the ascending limb of the BAC curve and less sensitive to the sedating effects of alcohol during the descending limb. Extending findings of subjective response beyond individuals with a family history of alcoholism, research also indicates that young heavy drinkers experience greater stimulant-like effects on the ascending limb of the BAC curve and less sedative-like effects on the descending limb than light drinkers (Holdstock et al., 2000; King et al., 2002).
Subjective responses to alcohol also contribute to excessive alcohol consumption. Specifically, individuals who were given a priming dose of alcohol showed greater stimulatory effects and consumed more alcohol than individuals who were not given a priming dose (Duka et al., 1998). Furthermore, in a controlled laboratory setting, stimulant effects from a dose of alcohol targeting a BAC of .06% primed further drinking during subsequent ad libitum access to alcohol (Corbin et al., 2008). Thus, individual differences in subjective responses to alcohol may contribute to alcohol-related problems via priming of excessive alcohol consumption.
Consequences of acute excessive alcohol consumption include, but are not limited to, alcohol-induced blackouts and hangover. Approximately 50% of college students who drink report having experienced a blackout at least once (Hartzler and Fromme, 2003; White et al., 2002), and 87% indicate having experienced a hangover (Slutske et al., 2003). Blackouts and hangover have been associated with heavy drinking behaviors, including higher amounts of alcohol consumed (Kruisselbrink et al., 2006; Zucker et al., 1985), frequency of drinking and intoxication (Anthenelli et al., 1994; McCaul et al., 1991), and heavy episodic (i.e., binge) drinking (Verster et al., 2003; Wechsler et al., 2000). Hartzler and Fromme (2003) found that the average BAC reached before blackouts occurred was .21%, with no blackouts reported at BACs lower than .06%. Also supporting a dose-dependent effect for hangover, the number of hangover symptoms after consuming six beers (.10% BAC) was two times larger than that after consuming four beers (.08% BAC; Kruisselbrink et al., 2006). Thus, it seems crucial to examine factors that contribute to excessive alcohol consumption as predictors of blackouts and hangover.
In sum, evidence indicates that subjective responses to alcohol lead to increased alcohol consumption and that increased alcohol consumption leads to blackouts and hangover. It remains to be determined, however, whether subjective responses to alcohol contribute to blackouts and hangover only through heavy drinking or if there might be a direct effect of subjective responses on blackouts and hangover that is independent of the amount of alcohol consumed.
In the current study, subjective responses to alcohol, blackouts, and hangover were examined within a single heavy drinking event (i.e., 21st birthday celebrations) to determine whether subjective responses to alcohol have direct effects on the occurrence of blackouts and hangover or if their effects are mediated by final BAC. Specifically, we sought to replicate and extend previous findings by evaluating (1) whether subjective responses to alcohol at the midpoint of the 21st birthday celebration predict acute excessive alcohol consumption (i.e., estimated BAC at the end of 21st birthday celebrations), (2) if subjective responses to alcohol are associated with the experience of blackouts and hangover, and (3) if final estimated BAC mediates the association between subjective responses to alcohol and the experience of blackouts and hangover. We hypothesized that the stimulant effects of alcohol would predict the BAC achieved at the end of the drinking event, thus supporting stimulant subjective effects as a reinforcing prime for further drinking. Second, based on the idea that subjective responses, blackouts, and hangover may share a common biological vulnerability, we hypothesized that subjective responses to alcohol would have direct effects on both blackouts and hangover that were not mediated by the final estimated BAC reached during the 21st birthday drinking celebration.
Method
Participants
Participants were recruited from a sample of 2,245 individuals at a large, public university in the southwest (for additional recruitment details, see Hatzenbuehler et al., 2008) who were participating in a 4-year longitudinal study on alcohol use and behavioral risks from high school through college (Fromme et al., 2008). Participants completed Web-based surveys during the summer before matriculation (Wave 1: 2004) and during the fall and spring semesters of the next 4 years (Waves 1-8: 2004-2007). During each spring of longitudinal data collection, subsets of the larger sample are recruited for in-person laboratory assessments. In the spring of 2007, individuals who would reach the legal U.S. drinking age of 21 during that semester (n = 575) were identified, and 212 were contacted by phone to determine study interest and eligibility. Of the participants contacted, 40 (19%) were ineligible because they did not plan to drink during their 21st birthday celebration, 20 (9%) refused, and 152 (72%) planned to consume alcohol during their 21st birthday celebration and completed a laboratory session within a week after their celebration. Two participants who planned to drink alcohol as part of their celebration but reported no alcohol consumption during the assessment were excluded from the current analyses. The final sample comprised 50% women and 38% white, 15% Asian, 12.5% Hispanic, and 3% black participants; 31.5% were mixed or other ethnicity.
Measures
Demographics.
Self-report measures assessed ethnicity and gender (male = 1, female = 0).
Alcohol consumption.
Using procedures similar to the Timeline Followback (TLFB; Sobell and Sobell, 1992), graduate-student interviewers assessed alcohol consumption at each drinking location during the 21st birthday celebration. Interviewers presented a 24-hour timeline (start time of 12 pm [noon] the day of the main celebration through 12 pm [noon] of the following day after the celebration) and asked participants to describe the time of their first drink and every subsequent drink during the entire celebration. Participants were also asked about their style of consumption (e.g., sip, gulp), type of drink consumed, and time interval over which drinking occurred at each location in which they celebrated. Information from the 21st birthday TLFB was used to identify the midpoint for each individual's drinking event by determining the midpoint between their first and last drink consumed.
Subjective responses to alcohol.
The 14-item Biphasic Alcohol Effects Scale (Martin et al., 1993) was used to assess the participant's retrospective report of their subjective stimulation and sedation at the midpoint of their drinking event (as determined by the TLFB). The stimulant subscale consists of the following descriptors: elated, energized, excited, stimulated, talkative, up, and vigorous. The sedative subscale consists of the following descriptors: down, heavy head, inactive, sedated, slow thoughts, sluggish, and difficulty concentrating. Response options ranged from 0 (not at all) to 10 (extremely), and the stimulation (seven items) and sedation (seven items) scores were summed to form stimulation (α = .89) and sedation (α = .80) subscales.
Blackouts and hangover.
A 38-item yes/no behavioral experience checklist assessed whether the participant engaged in a variety of behaviors (e.g., acted provocatively, sang karaoke) and, most importantly for the current study, whether they experienced alcohol-related consequences (e.g., blackout, hangover). For the current analyses, experiencing a blackout or hangover was coded as 1, and not experiencing these consequences was coded as 0.
In response to positive endorsement of blackout or hangover on the behavioral experience checklist, interviewers questioned participants about the time of the blackout or hangover, how many drinks were consumed before experiencing the blackout or hangover, and how experiencing the blackout or hangover might influence their future behavior.
Procedures
After completing a telephone screen, eligible participants attended an individual laboratory session within 1 week after their 21st birthday (mean [SD] = 4 [2] days after celebration). On arrival at the laboratory, participants provided informed consent and completed a paper-and-pencil behavioral experience checklist, which included the occurrence of hangovers and blackouts. Postdoctoral and upper-level graduate students then conducted the 21st birthday TLFB and a semi-structured interview. Self-reported subjective responses to alcohol were collected by paper-and-pencil assessment but relied on information from the interview to query the midpoint of the drinking event. Participants were paid $40.
Statistical analyses
Direct and mediated effects of subjective responses to alcohol on blackouts and hangover were tested in separate analyses using Baron and Kenny's (1986) procedures. For tests of mediation, three significant direct effects must first be found: (1) there is a significant association between the predictor (stimulant and sedative effects of alcohol) and outcome (blackouts, hangover), (2) there is a significant association between the predictor and mediator (final estimated BAC), and (3) there is a significant association between the mediator and the outcome. Mediation is demonstrated when the effect of the predictor on criterion is no longer significant or substantially reduced when controlling for the mediator.
All analyses were conducted using SPSS Version 15.0 (SPSS Inc., Chicago, IL) and STATA Version 9.2 (StataCorp LP, College Station, TX), and all variables were examined for outliers and normality. Evaluations of BAC indicated one extreme outlier (BAC > +3 SD) that was dropped from the analyses. Next, subjective response variables were mean centered to reduce multicollinearity (Aiken and West, 1991). For the first criterion and hypothesis, interaction terms were created by multiplying gender and subjective effects variables (e.g., Gender × Stimulant Response).
Results
Descriptive data
Analyses were first conducted to provide descriptive information about participants' typical drinking patterns (Table 1) so that average alcohol use could be compared with event-specific drinking on 21st birthday celebrations. Overall, participants typically consumed a mean of 3.01 (2.63) drinks on a mean of 2.04 (1.44) days a week. Analyses then characterized alcohol consumption, subjective responses to alcohol, blackouts, and hangover during the 21st birthday celebrations, as well as individual midpoints of their drinking events. The midpoint of participants' 21st birthday celebrations varied by the overall duration of their event, with the median duration being 4.0 hours (range: 0.5-12 hours). Because pace of drinking may vary during the 21st birthday celebration, participants' BAC curves were computed and examined. Only one participant had a peak BAC (.19%) greater than their final BAC (.18%), which supports the use of final BAC in the current analyses. The pace of consumption (number of drinks per hour) was computed for each location and showed the fastest pace at the first location, 3.66 (3.31) drinks per hour, then decreasing speed at subsequent locations, 2.97 (1.87) and 2.16 (0.98) drinks per hour, respectively. Mean values for final BAC and midpoint subjective responses by gender were computed (see Table 1). The mean BAC reached during 21st birthday celebrations was .22% (.15), with 60 individuals reporting a blackout, 68 reporting a hangover, and 43 reporting both a blackout and hangover. Men and women did not differ with respect to final estimated BAC (t = 1.16, 148 df, p = .27), stimulant effects (t = 1.63, 147 df, p = .11), or sedative effects (t = −0.37, 147 df, p = .72), nor did they differ with respect to the occurrence of blackouts (χ2 = 2.35, 1 df, p = .13) or hangover (χ2 = 0.02, 1df, p = .88).
Table 1.
Descriptive data
| Variable | Males |
Females |
Total |
||||
| Mean (SD) | Samplea % | Mean (SD) | Sample % | Mean (SD) | Sample % | ||
| Typical alcohol use | |||||||
| no. drinks | |||||||
| Frequency | 2.24 (1.44) | — | 1.91 (1.42) | — | 2.04 (1.44) | — | |
| Quantity | 3.73 (2.89) | — | 2.37 (2.14) | — | 3.01 (2.63) | — | |
| Event-specific alcohol use | |||||||
| Total no. of drinks | 12.40 (6.63) | 8.77 (5.25) | — | 10.70 (6.25) | — | ||
| Final BAC, % | .20 (.13) | .23 (.16) | — | .22 (.15) | — | ||
| Stimulant alcohol effectsb | 37.73 (15.63) | — | 42.11 (16.88) | — | 39.92 (16.35) | — | |
| Sedative alcohol effectsb | 9.82 (8.76) | — | 9.29 (8.96) | — | 9.56 (8.83) | — | |
| Blackouts | — | 46.7 | — | 33.3 | — | 40.0 | |
| Hangover | — | 45.3 | — | 45.3 | — | 45.3 | |
| Blackout and hangover | — | 33.3 | — | 24.0 | — | 28.7 | |
Notes: BAC = blood alcohol concentration.
Percentage of sample reporting blackouts, hangover, or both;
subjective response scores at the midpoint of the 21st birthday celebration.
Subjective responses to alcohol as a prime for 21st birthday alcohol consumption
Subjective responses to the initial drink(s) were examined as a prime for further alcohol consumption during 21st birthday celebrations through a regression model using stimulant and sedative effects reported for the midpoint of the drinking event as predictors of the final estimated BAC achieved. Gender was entered in Step 1, followed by subjective effects (stimulation and sedation) in Step 2. Interaction terms were entered in Step 3. In Step 1, gender was not significant. In Step 2, the stimulant alcohol effects were significantly and positively associated with final BAC (β = .48, p < .001), but sedative effects were not (β = .14, p < .21). Interactive terms did not account for significant variance. Thus, stimulant alcohol effects predicted BAC during a natural heavy drinking event.
Subjective responses to alcohol and 21st birthday alcohol consumption as predictors of blackout and hangover
Separate logistic regression analyses assessed self-reported subjective responses to alcohol (i.e., stimulation and sedation) for the midpoint of the drinking event and final estimated BAC as predictors of blackouts and hangover (see Figures 1–4). Because there were no differences between genders for final BAC or the occurrence of blackouts and hangover, gender was not included in the logistic regression models.
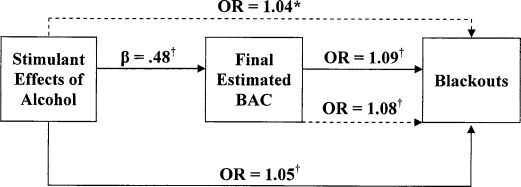
Figure 1.
Direct versus mediated effects of stimulant alcohol effects on blackouts. Solid lines represent first and second steps; dashed lines represent full model analysis in mediator analyses. OR = odds ratio; BAC = blood alcohol concentration. *p < .05; † p < .01.
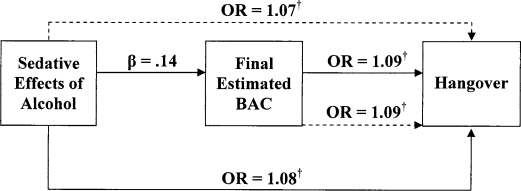
Figure 4.
Direct versus mediated effects of sedative alcohol effects on hangover. Solid lines represent first and second steps; dashed lines represent full model analysis in mediator analyses. OR = odds ratio; BAC = blood alcohol concentration. † p < .01.
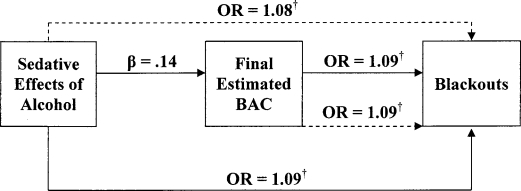
Figure 2.
Direct versus mediated effects of sedative alcohol effects on blackouts. Solid lines represent first and second steps; dashed lines represent full model analysis in mediator analyses. OR = odds ratio; BAC = blood alcohol concentration. † p < .01.
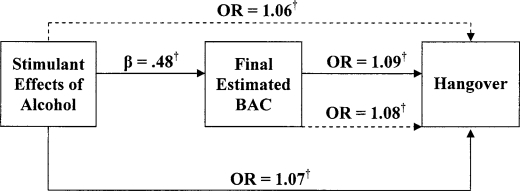
Figure 3.
Direct versus mediated effects of stimulant alcohol effects on hangover. Solid lines represent first and second steps; dashed lines represent full model analysis in mediator analyses. OR = odds ratio; BAC = blood alcohol concentration. † p < .01.
For the blackout models, stimulant and sedative effects of alcohol predicted the occurrence of blackouts (stimulant effects: odds ratio [OR] = 1.05, p < .00; sedative effects: OR = 1.09, p < .00). Final BAC was also predictive of blackouts (OR = 1.09, p < .00). In the logistic regression models for hangover, a similar pattern of results emerged, with both stimulant and sedative effects of alcohol predicting the occurrence of a hangover (OR = 1.07, p < .00; OR = 1.08, p < .01, respectively). Similarly, final BAC was predictive of hangover occurrence (OR = 1.09, p < .00).
Twenty-first birthday final BAC as a mediator of the effects of subjective responses on blackouts and hangover
Mediation was examined using separate logistic regression models. For blackouts, final BAC (OR = 1.08, p < .00), stimulant effects (OR = 1.04, p < .02), and sedative effects (OR = 1.08, p < .00) were significantly associated with the occurrence of blackouts. Similarly, final BAC (OR = 1.08, p < .00), stimulant effects (OR = 1.06, p < .00), and sedative effects (OR = 1.07, p < .00) were positively associated with hangover. As shown in Figures 1–4, when final BAC was included in the models, the associations between subjective effects of alcohol and blackouts and hangover were not significantly reduced. Thus, final BAC did not mediate the effects of subjective responses to alcohol on blackouts or hangovers.
Discussion
Current findings indicate that subjective responses to alcohol have direct effects on both the final BAC achieved and on the experience of blackouts and hangover that are not explained by the level of intoxication. Whereas a variety of social factors, such as peer pressure and 21st birthday traditions (e.g., 21 shots), may influence the amount of alcohol people consume, their subjective experiences of alcohol have clear influences on both consumption and the physiological consequences of drinking. These physiological responses to alcohol may have a biological vulnerability that extends beyond the dose-dependent effects of alcohol.
Replicating previous laboratory findings (Corbin et al., 2008; Duka et al., 1998) in a social, naturalistic drinking situation, stimulant alcohol effects predicted the final BAC achieved during 21st birthday celebrations. Because laboratory studies lack several real-world situations, such as crowds, dancing, and loud music, which may affect subjective responses, current findings provide ecological validity for the priming effects of subjective response on subsequent alcohol consumption. Current findings also extended previous research by showing that stimulant effects primed heavier alcohol consumption (median BAC = .21%) than could be ethically studied in the laboratory. Thus self-reported responses to alcohol during a natural drinking event provide a richer understanding of the factors that lead people to drink excessively, especially during high-risk situations.
Whereas only stimulant alcohol effects predicted final BAC, subjective responses of stimulation and sedation predicted blackouts and hangover. Specifically, both stimulant and sedative effects predicted blackouts during the event and hangovers the next morning. Consistent with the opponent process model, whereby initial drug effects are followed by effects that are opposite in nature and valence (Solomon and Corbit, 1974), the initial stimulant (e.g., feelings of increased energy, sociability, and euphoria) and sedative (e.g., sleepy and slow thoughts) effects of acute alcohol consumption were associated with subsequent hangover symptoms during withdrawal from alcohol. Thus, acute stimulatory effects may be associated with sedative hangover symptoms (e.g., tired), whereas acute sedative effects may be followed by stimulatory hangover symptoms (e.g., agitation, sweating).
Blackouts were predicted by subjective responses of stimulation and sedation. This finding was not surprising because blackouts are associated with a rapid rate of alcohol consumption (White et al., 2002), and according to the opponent process model, positive drug effects follow administration (e.g., rate) of the drug closely in time. As such, we expected stimulant effects of alcohol to predict blackouts. Furthermore, sedative effects were also expected to predict blackouts because substance-induced amnesia is caused by the sedative effects of depressants. This prediction of negative consequences by subjective alcohol responses demonstrates the prognostic utility and importance of subjective responses to alcohol in understanding alcohol-related consequences. According to neurobiological research, substance dependence involves a shift from a preponderance of positive (e.g., stimulant-like effects) to more dominant negative (e.g., sedative-like effects) reinforcement (Koob, 2006; Koob et al., 1997). Based on our findings and previous research, blackouts and hangover may be a sign of early alcohol-use problems, associated with both the stimulating and sedating effects of alcohol, before shifting to more severe alcohol-use problems or dependence (Jennison and Johnson, 1994; Piasecki et al., 2005). Future studies should explore whether subjective responses to alcohol predict hangover and blackouts as heavy drinking evolves into alcohol dependence.
Several limitations to the current research should be acknowledged. First, the assessment of subjective responses was based on retrospective self-report. Biased recall of subjective responses to alcohol, based on the overall valence and experiences during the drinking event, is a possibility. In other words, the degree of enjoyment of the celebration and any consequences experienced could have influenced participants' retrospective accounts of their stimulation and sedation at the midpoint of the drinking event. To further understand the predictive value of both stimulant and sedative alcohol effects, future studies should include ratings of subjective effects throughout the drinking event to provide greater specificity and generalizability of findings. For example, ecological momentary assessment (i.e., palm pilots) would allow for real-time assessment of subjective response to alcohol throughout a drinking episode and outside of the laboratory.
Lastly, findings were based on estimated, rather than actual, BAC. Although estimated BAC is not ideal, research indicates that the TLFB is accurate in assessing alcohol consumption (Carney et al., 1998; Sobell and Sobell, 2003) and that the Matthews and Miller (1979) formula for computing BAC in the current study provides the highest level of agreement between estimated BACs and breath alcohol tests when number of drinks, time spent drinking, gender, and body weight are used (Hustad and Carey, 2005). We also ran the same analyses using the number of standard drinks consumed rather than estimated BAC and the findings remained the same. Field tests using handheld breath alcohol analyzers would provide actual BAC ratings but could introduce bias and ethical issues. Repeated assessments with breathalyzer units could lead to reactivity that might alter the participant's natural pace or amount of consumption. Moreover, if realtime BACs were assessed, researchers could be ethically responsible for (1) notifying participants of dangerous BACs and taking appropriate action if necessary and (2) making sure participants did not drive if they had a BAC greater than .0%.
Despite limitations, the current study contributes to the literature on individual differences in subjective responses to alcohol and has important implications for future research. Findings support previous experimental laboratory studies that subjective responses to alcohol prime further alcohol consumption (Corbin et al., 2008) and extend these findings to a heavy drinking event in the natural environment. Because this was the first study to examine subjective responses, blackouts, and hangover in a single sample and for a single event, we were also able to demonstrate the utility of subjective alcohol effects in priming further drinking and predicting the experience of blackouts and hangover. Findings suggest that individual differences in subjective responses to alcohol may help identify individuals who are more susceptible to drinking excessively, experiencing negative consequences, and potentially developing alcohol-use disorders. Although current findings are promising, they should be replicated in individuals with a family history of alcoholism to fully parse out the effects of family history of alcoholism from those of subjective responses to alcohol. Additional genotyping studies are also needed to explore the neurobiological correlates (e.g., variation in the ALDH2 gene) of subjective responses to alcohol, blackouts, and hangover. Future investigations examining subjective responses to alcohol with functional magnetic resonance imaging techniques may also help elucidate the neurobiological systems associated with these effects of alcohol intoxication. Finally, continued examination of subjective responses to alcohol and negative consequences might also be extended to other heavy drinking events (e.g., New Year's Eve, graduation) to further examine the influence of contextual factors on subjective responses and the experience of blackouts and hangover. In sum, subjective responses to alcohol are important determinants of excessive alcohol consumption, blackouts, and hangover, and it is possible that these three factors confer risk for the development of alcohol-use disorders.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism grant AA013967 and a grant from the Waggoner Center for Alcohol and Addiction Research.
References
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- Anthenelli RM, Klein JL, Tsuang JW, Smith TL, Schuckit MA. The prognostic importance of blackouts in young men. J. Stud. Alcohol. 1994;55:290–295. doi: 10.15288/jsa.1994.55.290. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Social Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Brunelle C, Assaad J-M, Barrett SP, Avila C, Conrod PJ, Tremblay RE, Pihl RO. Heightened heart rate response to alcohol intoxication is associated with a reward-seeking personality profile. Alcsm Clin. Exp. Res. 2004;28:394–401. doi: 10.1097/01.alc.0000117859.23567.2e. [DOI] [PubMed] [Google Scholar]
- Brunelle C, Barrett SP, Pihl RO. Relationship between the cardiac response to acute intoxication and alcohol-induced subjective effects throughout the blood alcohol concentration curve. Human Psychopharmacol. Clin. Exp. 2007;22:437–443. doi: 10.1002/hup.866. [DOI] [PubMed] [Google Scholar]
- Carney MA, Tennen H, Affleck G, Del Boca FK, Kranzler HR. Levels and patterns of alcohol consumption using timeline follow-back, daily diaries and real-time “electronic interviews.”. J. Stud. Alcohol. 1998;59:447–454. doi: 10.15288/jsa.1998.59.447. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology. 2001;157:20–30. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO, Mankowski S. Biphasic effects of alcohol on heart rate are influenced by alcoholic family history and rate of alcohol ingestion. Alcsm Clin. Exp. Res. 1997;21:140–149. [PubMed] [Google Scholar]
- Corbin WR, Gearhardt A, Fromme K. Stimulant alcohol effects prime within session drinking behavior. Psychopharmacology. 2008;197:327–337. doi: 10.1007/s00213-007-1039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J, Greenbaum PE, Goldman MS. Up close and personal: Temporal variability in the drinking of individual college students during their first year. J. Cons. Clin. Psychol. 2004;72:155–164. doi: 10.1037/0022-006X.72.2.155. [DOI] [PubMed] [Google Scholar]
- de Wit H, McCracken SG. Ethanol self-administration in males with and without an alcoholic first-degree relative. Alcsm Clin. Exp. Res. 1990;14:63–70. doi: 10.1111/j.1530-0277.1990.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Duka T, Tasker R, Stephens DN. Alcohol choice and outcome expectancies in social drinkers. Behav. Pharmacol. 1998;9:643–653. doi: 10.1097/00008877-199811000-00019. [DOI] [PubMed] [Google Scholar]
- Fromme K, Corbin WR, Kruse MI. Behavioral risks during the transition from high school to college. Devel. Psychol. 2008;44:1497–1504. doi: 10.1037/a0012614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C, Krishnan B, Thavundayil J. Enhanced sensitivity of pituitary βendorphin to ethanol in subjects at high risk of alcoholism. Arch. Gen. Psychiat. 1996;53:250–257. doi: 10.1001/archpsyc.1996.01830030072011. [DOI] [PubMed] [Google Scholar]
- Hartzler B, Fromme K. Fragmentary and en bloc blackouts: Similarity and distinction among episodes of alcohol-induced memory loss. J. Stud. Alcohol. 2003;64:547–550. doi: 10.15288/jsa.2003.64.547. [DOI] [PubMed] [Google Scholar]
- Hatzenbuehler ML, Corbin WR, Fromme K. Trajectories and determinants of alcohol use among LGB young adults and their heterosexual peers: Results from a prospective study. Devel. Psychol. 2008;44:81–90. doi: 10.1037/0012-1649.44.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Individual differences in subjective responses to ethanol and triazolam. Behav. Pharmacol. 1999;10:283–295. doi: 10.1097/00008877-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcsm Clin. Exp. Res. 2000;24:789–794. [PubMed] [Google Scholar]
- Hustad JTP, Carey KB. Using calculations to estimate blood alcohol concentrations for naturally occurring drinking episodes: A validity study. J. Stud. Alcohol. 2005;66:130–138. doi: 10.15288/jsa.2005.66.130. [DOI] [PubMed] [Google Scholar]
- Jennison KM, Johnson KA. Drinking-induced blackouts among young adults: Results from a national longitudinal study. Int. J. Addict. 1994;29:23–51. doi: 10.3109/10826089409047367. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcsm Clin. Exp. Res. 2002;26:827–835. [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: A Hedonic Calvinist view. In: Miller WR, Carroll KM, editors. Rethinking Substance Abuse: What the Science Shows, and What We Should Do About It. New York: Guilford Press; 2006. pp. 25–45. [Google Scholar]
- Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacol. Biochem. Behav. 1997;57:513–521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior and personality: Modeling the externalizing spectrum. J. Abnorm. Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Kruisselbrink LD, Martin KL, Megeney M, Fowles JR, Murphy RJL. Physical and psychomotor functioning of females the morning after consuming low to moderate quantities of beer. J. Stud. Alcohol. 2006;67:416–420. doi: 10.15288/jsa.2006.67.416. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Turkkan JS, Svikis DS, Bigelow GE. Alcohol and secobarbital effects as a function of familial alcoholism: Extended intoxication and increased withdrawal effects. Alcsm Clin. Exp. Res. 1991;15:94–101. doi: 10.1111/j.1530-0277.1991.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcsm Clin. Exp. Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Miller WR. Estimating blood alcohol concentration: Two computer programs and their applications in therapy and research. Addict. Behav. 1979;4:55–60. doi: 10.1016/0306-4603(79)90021-2. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; Changing the Culture of Campus Drinking. Alcohol Alert, No. 58. 2002
- National Institute on Alcohol Abuse and Alcoholism. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; Underage Drinking: A Major Public Health Challenge. Alcohol Alert, No. 59. 2003
- Neighbors C, Oster-Aaland L, Bergstrom RL, Lewis MA. Event- and context-specific normative misperceptions and high-risk drinking: 21st birthday celebrations and football tailgating. J. Stud. Alcohol. 2006;67:282–289. doi: 10.15288/jsa.2006.67.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors C, Spieker CJ, Oster-Aaland L, Lewis MA, Bergstrom RL. Celebration intoxication: An evaluation of 21st birthday alcohol consumption. J. Amer. Coll. Hlth. 2005;54:76–80. doi: 10.3200/JACH.54.2.76-80. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychol. Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Chronic tolerance and sensitization to alcohol in sons of alcoholics: II. Replication and reanalysis. Exp. Clin. Psychopharmacol. 1999;7:234–243. doi: 10.1037//1064-1297.7.3.234. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Pihl RO, Gianoulakis C, Conrod P, Finn PR, Stewart SH, LeMarquand DG, Bruce KR. Ethanol-induced change in cardiac and endogenous opiate function and risk for alcoholism. Alcsm Clin. Exp. Res. 1996;20:1542–1552. doi: 10.1111/j.1530-0277.1996.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Sher KJ, Slutske WS, Jackson KM. Hangover frequency and risk for alcohol use disorders: Evidence from a longitudinal high-risk study. J. Abnorm. Psychol. 2005;114:223–234. doi: 10.1037/0021-843X.114.2.223. [DOI] [PubMed] [Google Scholar]
- Rutledge PC, Park A, Sher KJ. 21st birthday drinking: Extremely extreme. J. Cons. Clin. Psychol. 2008;76:511–516. doi: 10.1037/0022-006X.76.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, Bierut L, Goate A, Foroud T. A genome-wide search for genes that relate to a low level of response to alcohol. Alcsm Clin. Exp. Res. 2001;25:323–329. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch. Gen. Psychiat. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. J. Stud. Alcohol. 2000;61:827–835. doi: 10.15288/jsa.2000.61.827. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Tsuang J, Hesselbrock V, Bucholz K. Response to alcohol in daughters of alcoholics: A pilot study and a comparison with sons of alcoholics. Alcohol Alcsm. 2000;35:242–248. doi: 10.1093/alcalc/35.3.242. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tsuang JW, Anthenelli RM, Tipp JE, Nurnberger JI., Jr Alcohol challenges in young men from alcoholic pedigrees and control families: A report from the COGA report. J. Stud. Alcohol. 1996;57:368–377. doi: 10.15288/jsa.1996.57.368. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Piasecki TM, Hunt-Carter EE. Development and initial validation of the Hangover Symptoms Scale: Prevalence and correlates of hangover symptoms in college students. Alcsm Clin. Exp. Res. 2003;27:1442–1450. doi: 10.1097/01.ALC.0000085585.81711.AE. [DOI] [PubMed] [Google Scholar]
- Smith BH, Bogle KE, Talbott L, Gant R, Castillo H. A randomized study of four cards designed to prevent problems during college students' 21st birthday celebrations. J. Stud. Alcohol. 2006;67:607–615. doi: 10.15288/jsa.2006.67.607. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totawa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sobell LC, Sobell MB. Allen JP, Wilson VB, editors. Alcohol consumption measures. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; Assessing Alcohol Problems: A Guide for Clinicians and Researchers, 2nd Edition, NIH Publication No. 03-3745. 2003:75–99.
- Solomon RL, Corbit JD. An opponent-process theory of acquired motivation: I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Verster JC, van Duin D, Volkerts ER, Schreuder AHCML, Verbaten MN. Alcohol hangover effects on memory functioning and vigilance performance after an evening of binge drinking. Neuropsychopharmacology. 2003;28:740–746. doi: 10.1038/sj.npp.1300090. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Lee JE, Kuo M, Lee H. College binge drinking in the 1990s: A continuing problem. Results of the Harvard School of Public Health 1999 College Alcohol Study. J. Amer. Coll. Hlth. 2000;48:199–210. doi: 10.1080/07448480009599305. [DOI] [PubMed] [Google Scholar]
- White AM, Jamieson-Drake DW, Swartzwelder HS. Prevalence and correlates of alcohol-induced blackouts among college students: Results of an e-mail survey. J. Amer. Coll. Hlth. 2002;51:117–119. doi: 10.1080/07448480209596339. 122–131, [DOI] [PubMed] [Google Scholar]
- Zucker DK, Austin FM, Branchey L. Variables associated with alcoholic blackouts in men. Amer. J. Drug Alcohol Abuse. 1985;11:295–302. doi: 10.3109/00952998509016867. [DOI] [PubMed] [Google Scholar]