Abstract
Activity-dependent plasticity occurs throughout the CNS. However, investigations of skill acquisition usually focus on cortex. To expand the focus, we analyzed in humans the development of operantly conditioned H-reflex change, a simple motor skill that develops gradually and involves plasticity in both the brain and the spinal cord. Each person completed 6 baseline and 24 conditioning sessions over 10 weeks. In each conditioning session, the soleus H-reflex was measured while the subject was or was not asked to increase (HRup subjects) or decrease (HRdown subjects) it. When the subject was asked to change H-reflex size, immediate visual feedback indicated whether a size criterion had been satisfied. Over the 24 conditioning sessions, H-reflex size gradually increased in six of eight HRup subjects and decreased in eight of nine HRdown subjects, resulting in final sizes of 140 ± 12 and 69 ± 6% of baseline size, respectively. The final H-reflex change was the sum of within-session (i.e., task-dependent) adaptation and across-session (i.e., long-term) change. Task-dependent adaptation appeared within four to six sessions and persisted thereafter, averaging +13% in HRup subjects and −15% in HRdown subjects. In contrast, long-term change began after 10 sessions and increased gradually thereafter, reaching +27% in HRup subjects and −16% in HRdown subjects. Thus, the acquisition of H-reflex conditioning consists of two phenomena, task-dependent adaptation and long-term change, that together constitute the new motor skill. In combination with previous data, this new finding further elucidates the interaction of plasticity in brain and spinal cord that underlies the acquisition and maintenance of motor skills.
Introduction
The motor outputs of the adult CNS comprise a broad repertoire of adaptive behaviors acquired through practice, commonly referred to as skills. These range from simple behaviors (e.g., withdrawal reflexes) through complex behaviors (e.g., locomotion and speech) to the most demanding athletic, artistic, and intellectual performances. Skill acquisition remains a central problem of neuroscience. Most previous work focuses on cortical and subcortical plasticity (Pavlides et al., 1993; Jenkins et al., 1994; Nudo et al., 1996; Karni et al., 1998; Rioult-Pedotti et al., 1998, 2000; Kleim et al., 2002; Penhune and Doyon, 2002; Floyer-Lea et al., 2006). However, new skills entail plasticity from the cortex to the spinal cord (Wolpaw and Tennissen, 2001; Schneider and Capaday, 2003; Ung et al., 2005; Wolpaw, 2007). Thus, understanding their acquisition requires understanding how changes at different sites are created and coordinated to produce a new skill and ensure that other skills are maintained.
Models based on simple behaviors mediated by spinal cord pathways have unique advantages for addressing this difficult problem. Activity-dependent plasticity is abundant in the spinal cord (for review, see Wolpaw and Tennissen, 2001), and its study is facilitated by the relative simplicity and accessibility of the spinal cord. The anatomical separation of brain and spinal cord makes it possible to study interactions between supraspinal and spinal plasticity that underlie skill acquisition and maintenance. Although spinal reflexes usually function as components of complex skills such as locomotion (Yang and Stein, 1990; Stein, 1995; Sinkjaer et al., 1996; Brooke et al., 1997; Zehr and Stein, 1999b; Lamont and Zehr, 2006), they are themselves simple behaviors, and operantly conditioned changes in them are simple skills that can serve as models of skill acquisition (Wolpaw, 2007).
Operant conditioning of the largely monosynaptic spinal stretch reflex, or its electrical analog the H-reflex, changes both the brain and the spinal cord (Wolpaw, 2006). This simple skill requires the corticospinal tract (CST) and develops in two phases: phase 1 occurs in the first days; and phase 2 occurs gradually over weeks (Wolpaw and O'Keefe, 1984; Wolpaw et al., 1994; Chen et al., 2001). We hypothesize that these two phases reflect two components of skill acquisition: a rapid component in which the reward contingency modifies CST output to produce a small reflex change, and a slow component in which the CST gradually creates the spinal cord plasticity underlying most of the final reflex change. If this hypothesis is correct, it should be possible to track the development of each component separately and thereby confirm its distinct existence. This was the goal of the present study.
We designed a protocol that was intended to turn the rapid component on and off while leaving the slow component unaffected, and we repeated this protocol throughout the course of H-reflex conditioning. The results confirm that acquisition of a larger or smaller H-reflex consists of two components acquired at different times and different rates: rapid task-dependent reflex adaptation and gradual long-term reflex change.
Materials and Methods
Overview.
The protocol comprised 6 baseline sessions and 24 conditioning sessions spread over 10 weeks (i.e., 3 per week), and 4 follow-up sessions over the next 3 months. In each session, the soleus H-reflex was elicited while the subject maintained a natural standing posture and a stable level of soleus background EMG, and M-wave size was kept constant. In each baseline session, 225 control H-reflexes were elicited. In each conditioning or follow-up session, 20 control H-reflexes were elicited as in the baseline sessions and then 225 conditioned H-reflexes were elicited. In these conditioned H-reflex trials, the subject was asked to increase (HRup subjects) or decrease (HRdown subjects) the H-reflex and was given visual feedback after each stimulus to indicate whether the resulting H-reflex was larger (HRup subjects) or smaller (HRdown subjects) than a criterion value. Thus, in control H-reflex trials, the H-reflex was simply elicited, whereas in conditioned H-reflex trials the subject was encouraged to increase or decrease H-reflex size and the H-reflex was immediately followed by feedback indicating whether it satisfied the size criterion. As detailed in Materials and Methods, maximum voluntary contraction (MVC), maximum H-reflex (Hmax), maximum M-wave (Mmax), background EMG, and M-wave remained stable throughout data collection.
In sum, the conditioned H-reflexes tracked the overall development of H-reflex conditioning, the control H-reflexes tracked the development of the hypothesized slow component, and the within-session difference between the conditioned and control H-reflexes tracked the development of the hypothesized rapid component. Figure 1 summarizes the protocol, and Materials and Methods provides a detailed description.
Figure 1.
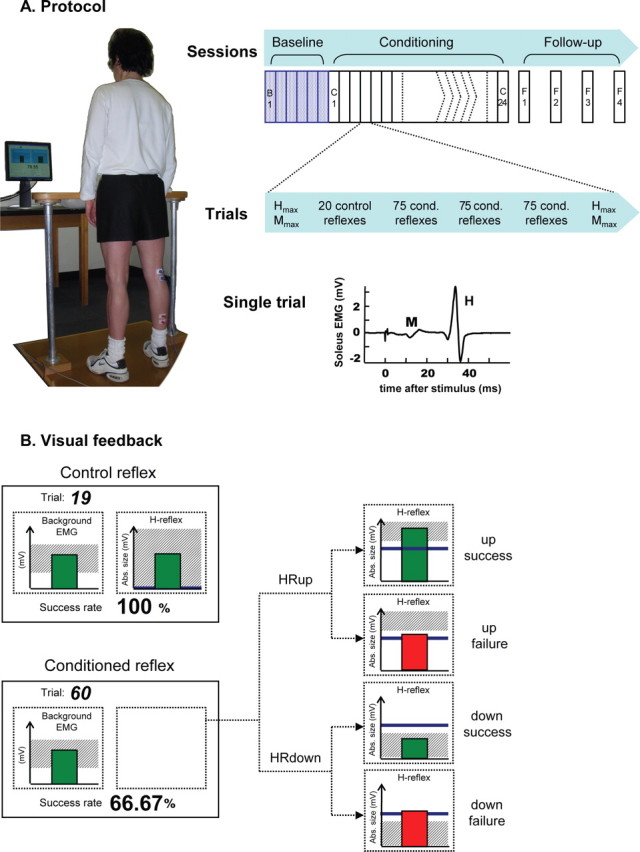
The study protocol. A, Left, Subject with electrodes on right leg faces monitor in standard study posture. Right top, Six baseline and 24 conditioning sessions occur at a rate of three per week and are followed by four follow-up sessions over the next 3 months. Right middle, At the beginning and end of each session, H–M recruitment curves (i.e., Hmax and Mmax) are obtained. In between, each baseline session has three 75 trial blocks of control H-reflexes, and each conditioning session has (as shown) a 20 trial block of control reflexes followed by three 75 trial blocks of conditioned H-reflexes. Right bottom, Soleus EMG recorded in a single representative trial. B, Visual feedback presented to the subject on the monitor. In all trials, the number of the current trial within its block and the running success rate for the current block are displayed, and the background EMG panel shows the correct range (shaded) and the current value (green vertical bar). If the EMG stays in the correct range for at least 2 s, an H-reflex is elicited. In control trials, the H-reflex panel is entirely shaded (indicating that every H-reflex is a success) and the green vertical bar is the H-reflex size for the most recent trial. In conditioned trials, the shading in the H-reflex panel indicates the rewarded H-reflex range for HRup (i.e., above a given value) or HRdown (i.e., below a given value) conditioning, the dark horizontal line is the average H-reflex size of the baseline sessions, and the vertical bar is the H-reflex size for the most recent trial. If that H-reflex size falls within the shaded area, the bar is green and the trial is a success. If it falls outside the shaded area, the bar is red and the trial is a failure. (For details, see Materials and Methods.)
Subjects.
The participants were seven women and eight men aged 21–55, with no history of neurological disease or injury. Thirteen people participated in either up- or down-conditioning, and two participated in both. (In these two people, the effect of the first study on H-reflex size had disappeared by its last follow-up session; moreover, the first baseline session of the second study did not begin until 3 and 6 additional months, respectively, had passed since the end of the first study.) The study was approved by the New York State Department of Health Institutional Review Board, and all subjects gave informed consent before participation.
Eight subjects (four women and four men; age, 34.8 ± 11.3 years; age range, 25–54) were randomly assigned to the HRup conditioning group, and nine subjects (four women and five men; age, 34.4 ± 13.0 years; age range, 21–55) were randomly assigned to the HRdown group. In each subject, the leg to be conditioned was chosen randomly, without regard to handedness or weight-bearing tendency.
Session schedule.
In one to three preliminary sessions, the subject was familiarized with the protocol and appropriate background EMG and M-wave criteria were defined. The background EMG level was set to be similar to the EMG range during natural standing. The M-wave size criterion was set to be the size of the M-wave elicited by a stimulus just above M-wave threshold. For all subjects, the stimulus that elicited an M-wave of the required size elicited an H-reflex on the rising phase of the H-reflex recruitment curve. After these preliminary sessions, each subject completed the 6 baseline sessions, 24 conditioning sessions, and up to 4 follow-up sessions (Fig. 1A). Baseline and conditioning sessions usually occurred three times per week. Follow-up sessions occurred 10–14 d, 1 month, 2 months, and 3 months after the final conditioning session. To prevent the normal diurnal variation in H-reflex size (Wolpaw and Seegal, 1982; Chen and Wolpaw, 1994; Carp et al., 2006a; Lagerquist et al., 2006) from affecting the results, a subject's sessions always occurred at the same time of day (i.e., within the same 3 h time window). Each session lasted 60–90 min. This relatively brief session length avoided the subject fatigue and time-dependent changes in EMG recording and reflex responses found in other studies (Crone et al., 1999; Zehr, 2002).
Session protocol.
Each session began with placement of EMG recording and stimulating electrodes (see below, Electrical stimulation and EMG recording) and measurement of rectified soleus EMG amplitude during MVC while the subject sat in a chair with hip, knee, and ankle joints kept at ∼90° by a custom-made apparatus. Next, an H-reflex/M-wave recruitment curve was obtained while the subject stood and maintained a defined level of rectified soleus EMG activity (see below, Electrical stimulation and EMG recording; Visual feedback). Stimulus intensity was varied in increments of 1.2–2.5 mA from soleus H-reflex threshold to an intensity just above that needed to elicit the maximum M-wave (Zehr and Stein, 1999a; Kido et al., 2004). Approximately 10 different intensities were used to obtain each recruitment curve, and four EMG responses were averaged to measure the H-reflex and M-wave at each intensity. Analyses of the H–M recruitment data yielded Hmax and Mmax. After the Hmax and Mmax were determined, the session continued by following the protocol of either a baseline session or a conditioning session (see below). For all trials in all sessions, H-reflexes were obtained while the subject stood and provided the same defined level of soleus EMG activity as for the H–M recruitment curve, and stimulus intensity was selected so that the stimulus produced the predetermined M-wave size (i.e., just above M-wave threshold).
In each of the six baseline sessions, the H–M recruitment curve was followed by 225 control H-reflex trials separated into three blocks of 75 trials each. In these control H-reflex trials, the subject was not asked to increase or decrease the H-reflex and was not given visual feedback as to H-reflex size.
In each of the 24 conditioning sessions, the H–M recruitment curve was followed by a 20 trial block of “within-session” control H-reflex trials identical with those of the baseline sessions. This was followed by 225 conditioned H-reflex trials separated into three blocks of 75 each. In these conditioned H-reflex trials, the subject was asked to increase (HRup subject) or decrease (HRdown subject) H-reflex size and was provided with immediate visual feedback that indicated his or her success in doing so (see below, Visual feedback) (Fig. 1B). Throughout these 20 within-session control trials and 225 conditioned trials, the size of the M-wave was monitored. Small adjustments in stimulus strength were occasionally needed to maintain the predetermined M-wave size.
Each of the four follow-up sessions followed the same protocol used in the conditioning sessions. At the end of every session (baseline, conditioning, or follow-up), another H–M curve was obtained to determine Hmax and Mmax again.
Electrical stimulation and EMG recording.
To avoid session-to-session variability in the location of stimulating and recording electrodes, the positions of all electrodes were mapped in relation to landmarks on the skin (e.g., scars or moles) in the first preliminary session. These measures were used to place the electrodes in all subsequent sessions.
To elicit the H-reflex, the tibial nerve was stimulated in the popliteal fossa, using surface self-adhesive Ag–AgCl electrodes (2.2 × 2.2 cm for the cathode and 2.2 × 3.5 cm for the anode; Vermed) and a Grass S48 stimulator (with CCU1 constant current unit and SIU5 stimulus isolation unit; Astro-Med). The stimulating electrode pair was placed so as to minimize the H-reflex threshold and to avoid stimulation of other nerves. Soleus EMG was recorded with another pair of the 2.2 × 3.5 cm surface Ag–AgCl electrodes placed longitudinally on the soleus muscle just below the gastrocnemii with their centers 3 cm apart and their long dimension perpendicular to a line between their centers. For each subject, the medial-lateral EMG electrode position was chosen so as to minimize H-reflex and M-wave thresholds and to maximize their sizes. For evaluation of concurrent antagonist activity, additional EMG electrodes were placed over the belly of the tibialis anterior muscle. EMG activity was amplified, bandpass filtered (3–3000 Hz), sampled at 5000 Hz, and stored.
EMG activity was always rectified before measurement. Every 100 ms, the rectified EMG activity was averaged and the result was immediately provided as visual feedback to help the subject maintain soleus background EMG activity within the specified range (usually 10–20% of maximum voluntary contraction) (see below, Visual feedback). When the subject had maintained soleus EMG activity within the specified range for at least 2 s, a square stimulus pulse (1 ms in duration) was delivered to elicit the H-reflex and M-wave. The minimum interstimulus interval was 5 s. In general, the interstimulus interval varied within and across sessions and subjects, because the subject was permitted to move or rest between trials (see below, Instructions to and interaction with subjects) and a trial occurred only after the subject had maintained the required level of soleus background EMG activity for 2 s (see above).
Visual feedback.
Figure 1B shows the visual feedback provided to the subject during H-reflex trials. The screen presented two graphs, one for soleus background EMG activity (left) and one for H-reflex size (right). The background EMG graph was the same for both control and conditioned H-reflex trials. As Figure 1B illustrates, the shaded area showed the specified range of background activity while the bar, which was updated every 100 ms, showed the current level of background activity. If the subject kept the bar in the specified range for 2 s and if at least 5 s had passed since the last stimulus, a stimulus pulse elicited the H-reflex and M-wave (see below, Electrical stimulation and EMG recording).
As Figure 1B also shows, the H-reflex feedback graph differed for control and conditioned H-reflex trials. For the control trials, the graph showed only a green vertical bar that reflected H-reflex size [i.e., represented here by the average rectified EMG in the H-reflex interval (typically 30–45 ms after the stimulus)] and appeared 200 ms after the stimulus. For the conditioned trials, a vertical bar reflecting H-reflex size also appeared 200 ms after the stimulus, but it was green only if the H-reflex size satisfied the criterion (i.e., was more than the criterion for HRup subjects or less than the criterion for HRdown subjects), and it thereby indicated that the trial was a success. If the H-reflex size did not satisfy the criterion, the bar was red and the trial was a failure. In addition, for the conditioned trials, the screen constantly showed a heavy horizontal line that indicated the subject's average H-reflex size for the six baseline sessions and a shaded area that indicated the H-reflex size range that satisfied the criterion. Finally, for conditioned trials, the current success rate (i.e., the percentage of the trials of the current 75 trial block that were successful) was shown at the bottom of the screen and was updated after each trial.
In sum, for control H-reflex trials, the visual feedback simply helped the subject maintain the required prestimulus background EMG. In contrast, for conditioned H-reflex trials, the visual feedback also informed the subject as to whether he or she had succeeded in producing an H-reflex that satisfied the size criterion, and it showed the success rate for the current block of trials.
The reward criterion.
As described above, during conditioned H-reflex trials the subject received visual feedback that indicated whether the H-reflex size satisfied the criterion value. The criterion was based on the average H-reflex size for the previous block of trials. Thus, in each conditioning session, the criterion value for the first block of 75 conditioned H-reflex trials was based on the immediately preceding block of 20 control trials, and the criterion values for the second and third conditioned blocks were based on the immediately preceding block of 75 conditioned trials. The criterion was selected so that if H-reflex values for the new block were similar to those for the previous block, 50–60% of the trials would be successful (Chen and Wolpaw, 1995). For each block, the subject earned a modest extra monetary reward if the success rate exceeded 50%. It is important to note that, as described above, each subject's background EMG level and M-wave size were kept the same for all the control and conditioned trials of all the sessions (see below).
Instructions to and interaction with subjects.
The same investigator (A. K. Thompson) conducted or directly supervised every session for every subject. For the ≥2 s prestimulus period during which the subject maintained correct soleus background EMG, he or she was asked to maintain a natural, relaxed standing posture (Fig. 1A) and to avoid tensing any part of the body [e.g., a Jendrassick maneuver (Zehr and Stein, 1999a)] or flexing or rotating the hip, knee, or ankle joints. It was occasionally necessary for the investigator to correct the subject's posture between trials. After each H-reflex trial, and before again providing the required level of soleus background EMG for the next trial, the subject was free to rest in a chair or move within a limited area (radius, ≤1.5 m). Although some subjects tended to stand still throughout a conditioned block, others sometimes moved (e.g., stretching and taking a few steps) between trials. If the subject preferred, music was played throughout data collection. In this case, the same category of music (e.g., soft rock, classical) was played for all the subject's sessions. The average interstimulus intervals were not significantly different for HRup and HRdown subjects (mean ± SE, 5.4 ± 0.2 s for HRup subjects; 6.3 ± 0.3 s for HRdown subjects; p = 0.08, unpaired t test, two-tailed). When the 6 baseline sessions and 24 conditioning sessions were analyzed as successive six session groups, there was no significant change in the interstimulus interval over the course of study (p = 0.24 for HRup subjects and 0.38 for HRdown subjects, by repeated-measures ANOVA).
In the first conditioning session, the subject was notified of his/her conditioning direction (i.e., either HRup or HRdown) and asked to try to change the H-reflex in the assigned direction. Over the course of conditioning, the subject was urged (1) to try to maximize success rate, (2) to try to change H-reflex size in the correct direction as much as possible, and (3) to try for success on every conditioned H-reflex trial. To maximize the subject's motivation for and attention to improving success rate, the investigator sought to increase the subject's conscious involvement in each conditioned trial (beyond that produced by the green-bar/red-bar feedback) by giving verbal encouragement between conditioned blocks.
Data analysis.
For each session of each subject, we calculated average H-reflex sizes for the 20 within-session control trials, for each of the three 75 trial blocks, and for all three 75 trial blocks together. For these calculations, H-reflex size was defined as average rectified value in the H-reflex interval minus average soleus background EMG. Changes in these H-reflex sizes across sessions were quantified in percentage of their average values for the six baseline sessions.
To determine for each subject whether HRup or HRdown conditioning was successful, the average conditioned H-reflexes of the final six conditioning sessions were compared with the average H-reflexes of the six baseline sessions by unpaired t test (two-tailed). In addition, we also determined for each subject the final effects of HRup or HRdown conditioning on the conditioned H-reflex and on the control H-reflex. The final effect on the conditioned H-reflex was calculated by averaging the H-reflexes for the 75 trial conditioned blocks of conditioning sessions 22–24, and expressing the result in percentage of the average H-reflex for the 75 trial blocks of the six baseline sessions. (Thus, a value of 100% indicated no change in the H-reflex.) The final effect on the control H-reflex was calculated by averaging the H-reflexes for the 20 within-session control trials of conditioning sessions 22–24, and expressing the result in percentage of the average H-reflex for the first 20 trials of the six baseline sessions. To determine for the HRup and HRdown subject groups the effect of HRup or HRdown conditioning, a repeated-measures ANOVA was used to evaluate conditioned and control H-reflex sizes across successive six session blocks (i.e., baseline sessions 1–6 and conditioning sessions 1–6, 7–12, 13–18, and 19–24).
We also assessed over all sessions the stability of MVC, Hmax, and Mmax, and the stability for control and conditioned H-reflex trials of soleus M-wave size and of soleus and tibialis anterior background EMG levels in the 50 ms before the stimulus.
Stability of MVC, Hmax, Mmax, background EMG, and M-wave size.
Soleus MVC, measured at the beginning of each session, did not change significantly in the HRup or HRdown group (p = 0.06 for the HRup group and p = 0.91 for the HRdown group; one-way repeated-measures ANOVA). MVC did increase slightly in four HRup subjects and one HRdown subject over the course of study; this occurred mainly in the baseline and early conditioning sessions and may have reflected practice in achieving MVC. Because the background EMG level and M-wave size criteria were in millivolts (rather than in percentage of MVC) and were set in the preliminary sessions and never changed after that, this slight MVC change had no direct effects on H-reflex size and could not account for increase in the H-reflex.
In neither HRup nor HRdown subjects did Mmax and Hmax change significantly over the course of study (p = 0.19 and p = 0.07, respectively, for the HRup subjects; p = 0.41 and p = 0.74, respectively, for the HRdown subjects; one-way repeated-measures ANOVA). Neither group showed any consistent within-session change in Mmax or Hmax. This finding contrasted with the significant decreases over 1–3 h found by Crone et al. (1999). It should be noted that the sessions of the present study never exceeded 1.5 h and all the measurements were made while the subject maintained a constant level of soleus background EMG.
In both HRup and HRdown subjects, soleus background EMG level was maintained within the preset range (typically 10–20% of MVC) throughout the study. At the same time, in HRup subjects, its average value rose 10% during conditioning sessions 1–12, from 20 ± 3 μV (mean ± SE) to 22 ± 3 μV (p = 0.02, one-way repeated-measures ANOVA). It then fell 5% for conditioning sessions 13–24 (to 21 ± 3 μV) and was no longer significantly different from the baseline sessions. In HRdown subjects, soleus background EMG averaged 23 ± 3 μV in the baseline sessions and did not change significantly over the subsequent 24 conditioning sessions (p = 0.07, ANOVA). Soleus background EMG also remained stable over the follow-up sessions, averaging 21 ± 2 μV for HRup subjects and 22 ± 3 μV for HRdown subjects.
The background EMG of the antagonist tibialis anterior muscle and the soleus M-wave size [calculated as average absolute value of amplitude in the M-wave interval (typically 6–23 ms after the stimulus) minus average level of soleus background EMG] were also stable throughout study in both groups (tibialis anterior EMG, p = 0.68 for HRup and p = 0.32 for HRdown; M-wave, p = 0.86 for HRup and p = 0.14 for HRdown; one-way repeated-measures ANOVA).
Results
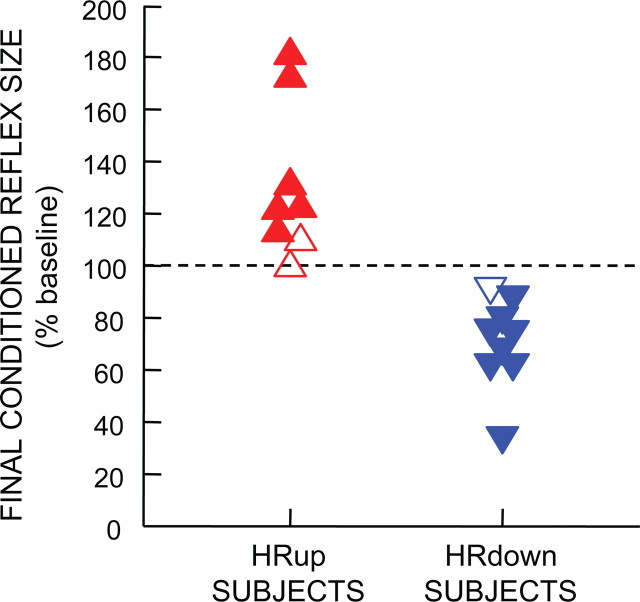
Figure 2 shows the final conditioned H-reflex sizes of the eight HRup and nine HRdown subjects. The filled symbols represent the subjects in whom conditioning was successful. For each of these subjects, the average conditioned H-reflexes for conditioning sessions 19–24 were significantly greater (for an HRup subject) or significantly less (for an HRdown subject) than the average H-reflexes of the six baseline sessions (p < 0.05, two-tailed t test). H-reflex conditioning was successful in six of the eight HRup subjects and in eight of the nine HRdown subjects, and was unsuccessful in two HRup subjects and one HRdown subject (open symbols). The overall success rate for HRup and HRdown subjects, 14 of 17 or 82%, is similar to that in monkeys and rodents (Wolpaw et al., 1993; Chen et al., 2001, 2002; Chen and Wolpaw, 2002; Carp et al., 2006b). Because the purpose of this study was to analyze the development of H-reflex conditioning, this presentation focuses on the data from the six successful HRup subjects and eight successful HRdown subjects.
Figure 2.
Final conditioned H-reflex sizes for individual subjects. The filled symbols represent the 14 successful subjects [i.e., subjects whose average conditioned H-reflexes for conditioning sessions 19–24 were significantly greater (for an HRup subject) or significantly less (for an HRdown subject) than the average H-reflexes of the six baseline sessions (p < 0.05, two-tailed t test)]. The open symbols represent the three subjects in whom conditioning was unsuccessful.
H-reflex stability in the baseline sessions
In the baseline session, 225 control H-reflex trials were separated into three blocks of 75 trials. Block order (first, second, or third) or session order (first to sixth) did not affect H-reflex size (block by session interaction, p = 0.99 for HRup group and p = 0.95 for HRdown group; sessions by blocks two-way repeated-measures ANOVA). Furthermore, there was no significant difference in either group between the first 20 control reflexes and all 225 control reflexes (p = 0.66 for HRup group and p = 0.07 for HRdown group; paired t test, two-tailed). These results indicated that any within-session changes in the conditioning sessions (i.e., between the 20 control reflexes and the three blocks of conditioned reflexes, or over the three blocks of conditioned reflexes) were probably not nonspecific effects. Rather, they were likely to be related to HRup or HRdown conditioning.
The effect of conditioning on the size of the conditioned H-reflex
In the conditioned trials, the H-reflex was elicited and the subject was immediately informed as to whether the trial was a success, that is, whether H-reflex size was greater than the criterion in an HRup subject or less than the criterion in an HRdown subject. In neither HRup nor HRdown subjects did block order (i.e., first, second, or third) affect H-reflex size (p = 0.94 for HRup group, p = 0.40 for HRdown group; sessions by blocks two-way repeated ANOVA). Thus, the data from the three blocks were combined to calculate the average conditioned H-reflex size for each of the conditioning and follow-up sessions. This value is referred to as “the conditioned H-reflex” size and is calculated as percentage of the subject's average H-reflex for the six baseline sessions.
Figure 3A summarizes the course of conditioned H-reflex size for the successful HRup and HRdown subjects. It shows the group averages (±SE) for each session. Over the 24 conditioning sessions, the conditioned H-reflex gradually increases in the HRup subjects and decreases in the HRdown subjects [r2 for linear regression, 0.53 (p < 0.0001) for HRup; 0.81 (p < 0.0001) for HRdown]. The final size of the conditioned H-reflex (i.e., its average size for conditioning sessions 22–24) averaged 140 ± 12% of baseline in the HRup subjects and 69 ± 6% in the HRdown subjects.
Figure 3.
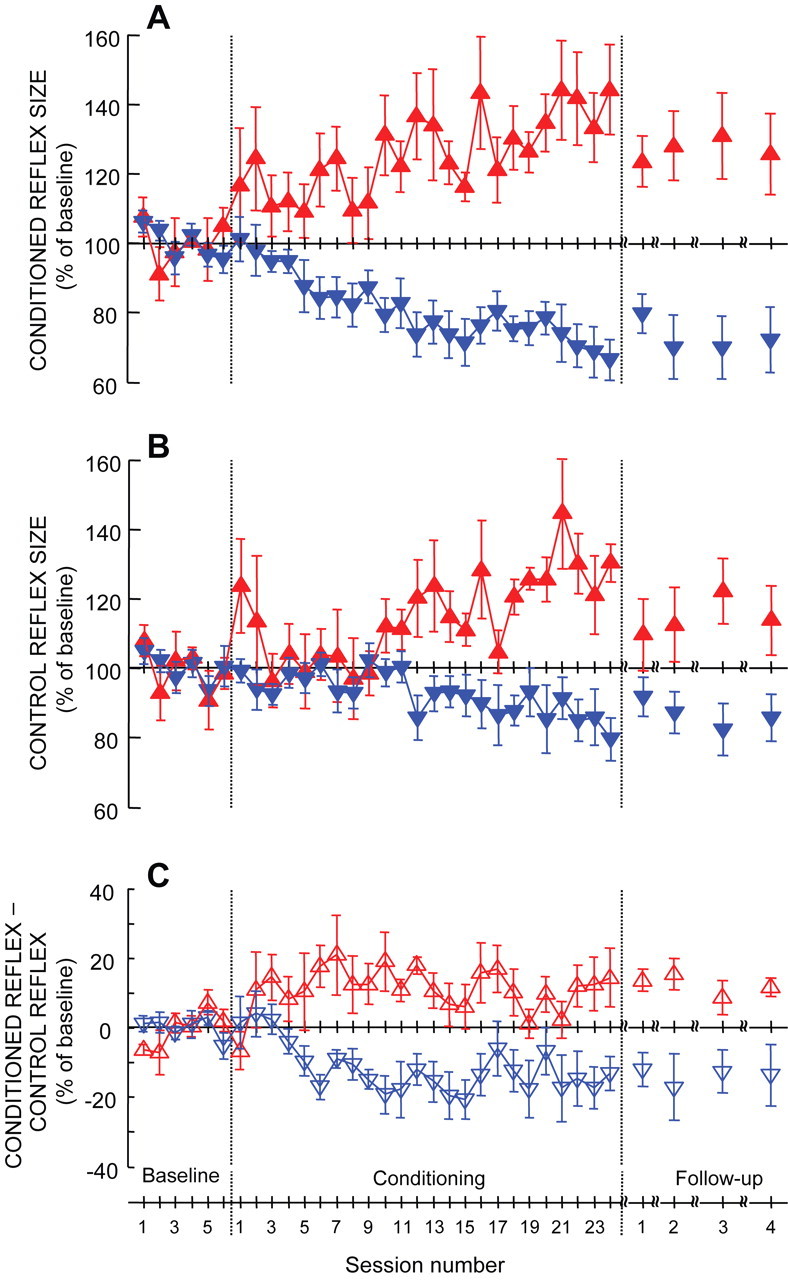
Average (±SE) H-reflex measures for all successful HRup (upward triangle) and HRdown (downward triangle) subjects over the baseline, conditioning, and follow-up sessions. A, Average conditioned H-reflex size. B, Average control H-reflex size. C, Average of conditioned H-reflex size minus control H-reflex size. (For details, see Results.)
Figure 3A also shows the average conditioned H-reflex sizes for the follow-up sessions. In these follow-up sessions, the conditioned H-reflex increase present in HRup subjects at the end of the conditioning sessions declined by ∼30%, whereas the conditioned H-reflex decrease present in HRdown subjects at the end of the conditioning sessions persisted unchanged.
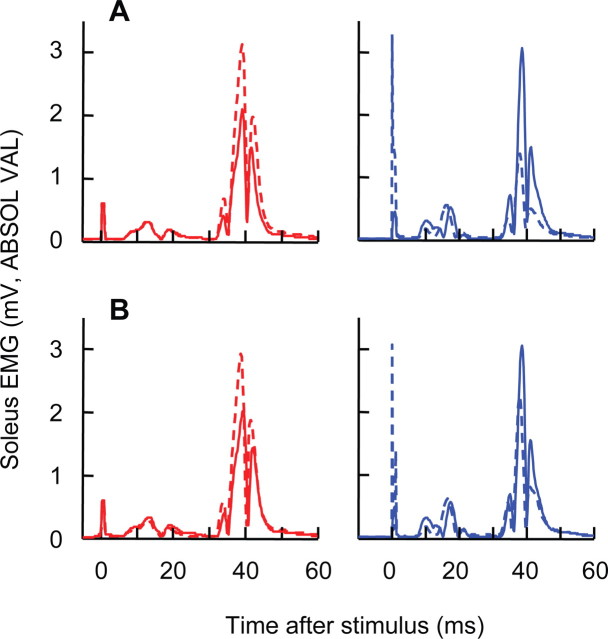
Figure 4A shows baseline H-reflexes and final conditioned H-reflexes from two representative subjects. At the end of the conditioning sessions, the H-reflex is increased in the HRup subject and decreased in the HRdown subject. Background soleus EMG level and M-wave size do not change.
Figure 4.
Average conditioned (A) and control (B) H-reflexes from two representative subjects for a baseline session (solid) and the last conditioning session (dashed). Both conditioned and control H-reflexes are larger after 24 conditioning sessions in the HRup subject (left) and smaller in the HRdown subject (right). Background EMG and M-wave size do not change.
In neither HRup nor HRdown subjects did the change in the conditioned H-reflex correlate with age (r2 < 0.1 for each group). Older (i.e., >50 years) and younger (<30 years) subjects were similarly successful.
The effect of conditioning on the size of the control H-reflex
In every conditioning or follow-up session, the subject completed 20 control H-reflex trials before the three blocks of conditioned H-reflex trials. In these within-session control H-reflex trials, the H-reflex was simply measured: the subject was asked not to try to increase or decrease H-reflex size and no feedback was provided as to whether H-reflex size satisfied a criterion. The average H-reflex size for these 20 within-session control trials is referred to as the “control H-reflex” size of the session, and is expressed in percentage of the average H-reflex size for the first 20 trials of the six baseline sessions.
Figure 3B summarizes the courses of the control H-reflex size of each session for the successful HRup and HRdown subjects. It shows the group averages (±SE) for each session. Over the conditioning sessions, the control H-reflex size increases in the HRup subjects and decreases in the HRdown subjects. The final control H-reflex size (i.e., the average size of the control H-reflexes in conditioning sessions 22–24) averaged 127 ± 7% of baseline in HRup subjects and 84 ± 6% in HRdown subjects. Most importantly, the timing of the changes in the control H-reflex differs from the timing of the changes in the conditioned reflex. Whereas the changes in the conditioned reflexes begin in the first few sessions, the changes in the control reflex are not apparent until conditioning session 10 in HRup subjects and conditioning session 12 in HRdown subjects. Figure 3B also shows the average control H-reflex sizes for the follow-up sessions. In these sessions, the control H-reflex increase in HRup subjects evident at the end of the conditioning sessions has declined by ∼45%, whereas the control H-reflex decrease evident in HRdown subjects at the end of the conditioning sessions has declined by only ∼20%.
Figure 4B shows average baseline H-reflexes and final control H-reflexes from two representative subjects. At the end of the conditioning sessions, control H-reflex size is increased in the HRup subject and decreased in the HRdown subject. Background soleus EMG level and M-wave size do not change.
Difference between the control and conditioned H-reflexes
As Figure 3, A and B, shows, over the 24 conditioning sessions both the conditioning and control H-reflexes change. However, they differ in both the course and final magnitude of change. The conditioned H-reflexes begin to change in the first few conditioning sessions, whereas the control H-reflexes do not show consistent mode-appropriate change until conditioning sessions 10–12. By the end of the 24 conditioning sessions, the conditioned H-reflex has changed more than the control H-reflex. As noted above, no detectable within-session changes occurred during the baseline sessions. This implies that any difference observed between the control and conditioned H-reflexes in the conditioning sessions results from asking the subject to change H-reflex size, and represents the subject's adaptation to the task.
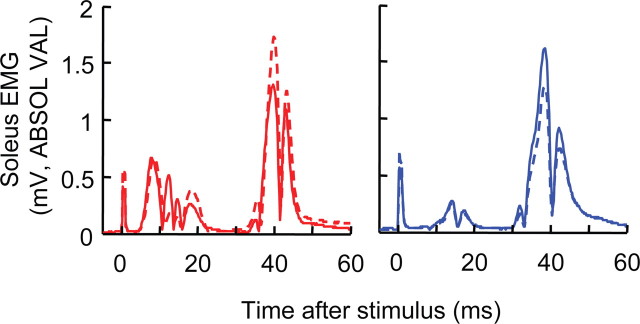
To see this difference, we subtracted the within-session control H-reflex (Fig. 3B) from the conditioned H-reflex (Fig. 3A) for each session. Figure 3C shows the results. For HRup subjects, a within-session task-dependent H-reflex increase is apparent by conditioning sessions 2–3. For HRdown subjects, a within-session task-dependent H-reflex decrease is apparent by conditioning sessions 5–6. The subjects retain this task-dependent difference through the remaining conditioning sessions and the follow-up sessions. For the final three conditioning sessions, it averages +13% in HRup subjects and −15% in HRdown subjects. Figure 5 illustrates this task-dependent effect by showing conditioning and control H-reflexes for single sessions from an HRup and an HRdown subject.
Figure 5.
Average control (solid) and conditioned (dashed) H-reflexes for a representative conditioning session of an HRup (left) and an HRdown subject (right). In each subject, task-dependent reflex change is evident: the conditioned H-reflex is larger than the control H-reflex in the HRup subject and smaller in the HRdown subject. Background EMG and M-wave size do not change.
To analyze the trends in Figure 3, we combined the 24 conditioning sessions into four groupings of six sessions each (i.e., conditioning sessions 1–6, 7–12, etc.) and calculated average results for the conditioned H-reflex, the control H-reflex, and the difference between them. Table 1 shows the results. It also indicates whether the values are significantly different from the values of the six baseline sessions. The contrasts among the three measures are clear. The conditioned H-reflex begins to change in the correct direction in sessions 1–6; this change becomes significant in sessions 7–12; and it continues to grow thereafter. In contrast, the control H-reflex shows little change during sessions 1–12, changes more in sessions 13–18, and finally shows significant change in sessions 19–24. Finally, the within-session reflex change appears in sessions 1–6, becomes significant in sessions 7–12, and then remains with little apparent change during sessions 13–24.
Table 1.
H-reflex values for all successful HRup and HRdown subjects for each group of six conditioning sessions
| C1–C6 (%) | C7–C12 (%) | C13–C18 (%) | C19–C24 (%) | ||
|---|---|---|---|---|---|
| Conditioned reflex | HRup | 115.6 ± 6.2 | 122.4 ± 5.5* | 127.6 ± 7.5* | 137.3 ± 8.6* |
| HRdown | 93.4 ± 4.0 | 81.7 ± 4.4* | 75.1 ± 4.9* | 72.3 ± 5.3* | |
| Control reflex | HRup | 106.4 ± 6.0 | 106.7 ± 3.7 | 116.5 ± 4.9 | 128.2 ± 5.4* |
| HRdown | 97.1 ± 1.8 | 95.5 ± 2.8 | 90.5 ± 4.5 | 86.7 ± 5.9* | |
| Within-session change | HRup | 9.2 ± 5.4 | 15.6 ± 4.1* | 11.6 ± 4.2* | 12.4 ± 6.0* |
| HRdown | −3.8 ± 3.2 | −13.9 ± 3.2* | −14.4 ± 5.1* | −14.4 ± 6.3* |
Values represent average (±SE) and are expressed as percentage of baseline values.
*Significant differences from the six baseline sessions (p < 0.05, Dunnett's post hoc).
Subject reports of their conditioning techniques
In the conditioned trials, subjects were encouraged to change H-reflex size in the correct direction as much as possible and to try for success on every conditioned trial. Most successful subjects reported that, during the initial three to nine conditioning sessions, they explored different techniques for changing H-reflex size in the correct direction and identified an effective technique. They then used that technique through the conditioned trials of the subsequent conditioning and follow-up sessions. Each subject's report that he or she had identified a successful technique coincided with the subject's achieving a task-dependent change in H-reflex size like that evident in the average data of Figure 3C.
Table 2 lists the techniques used by different subjects. Although these reports are interesting, they do not necessarily reflect in any meaningful way the CNS processes responsible for the task-dependent changes in H-reflex size. The uncertainty of the connection between these subjective reports and the mechanisms of reflex change is emphasized by the fact that the techniques of “anticipating stimulus occurrence” and “meditation” were each used by both an HRup subject and an HRdown subject.
Table 2.
The strategies for changing H-reflex size reported by the subjects
| HRup subjects | HRdown subjects |
|---|---|
| Focusing on the H-reflex feedback bar | Trying to relax |
| Focusing on the background EMG feedback bar | Ignoring the stimulus |
| Praying | Thinking about anything but the reflex |
| Imagining a toe flick right after the stimulus | Turning off the reflex at the time of the stimulus |
| Controlling breathing (rhythm and ventilation) | Moving in between trials |
| Anticipating stimulus occurrence | Anticipating stimulus occurrence |
| Meditation | Meditation |
Discussion
This study shows for the first time that the human soleus H-reflex can be changed with an operant conditioning protocol like those used to change the H-reflex in monkeys, rats, and mice or the spinal stretch reflex (SSR) in monkeys and humans (Wolpaw et al., 1983; Wolpaw, 1987; Evatt et al., 1989; Chen and Wolpaw, 1995; Carp et al., 2006b). H-reflex increase (HRup subjects) or decrease (HRdown subjects) occurs while background EMG, M-wave, and subject posture remain stable. The prolonged course of H-reflex change and its persistence over subsequent months are also consistent with previous data (Wolpaw et al., 1986; Segal and Wolf, 1994; Wolf and Segal, 1996). The H-reflex is elicited by direct nerve stimulation. Thus, unlike human SSR conditioning (Segal and Wolf, 1994; Wolf and Segal, 1996), human H-reflex conditioning could not be explained by a change in fusimotor drive. It can only be explained by a change that affects the spinal pathway of the H-reflex.
The primary significance of this study is that it dissects the course of acquisition of a simple skill (i.e., a larger or smaller H-reflex) and thereby distinguishes two phenomena, task-dependent adaptation and long-term change, that together constitute the skill. Thus, it confirms the two-phase hypothesis that was previously based simply on analyses of the overall courses of reflex conditioning in animals (Table 3). Furthermore, combined with previous studies, this new result illuminates the complementary roles of brain and spinal cord in skill acquisition.
Table 3.
Comparison of the task-dependent adaptations (TDAs) and long-term changes (LTCs) found in the present human study with the phase 1 and phase 2 changes found in monkeys and rats (Wolpaw and O'Keefe, 1984; Wolpaw et al., 1994; Chen et al., 2001)
| Conditioningmode | Monkey biceps SSR | Monkey TS H-reflex | Rat soleus H-reflex | Human soleus H-reflex | |
|---|---|---|---|---|---|
| Up | Phase 1 or TDA | 8.7% | 24.0% | 17.0% | 13.0% |
| Phase 2 or LTC | 1.2%/d | 1.4%/d | 1.7%/d | 1.2%/session | |
| Down | Phase 1 or TDA | −7.7% | −7.0% | −4.8% | −15.0% |
| Phase 2 or LTC | −0.8%/d | −0.8%/d | −0.9%/d | −0.7%/session |
To determine the LTC values, the average change in the control H-reflex for conditioning sessions 22–24 was divided by 22.5 (the midpoint of the trials of these three sessions). TS, Triceps surae.
Task-dependent adaptation
The protocol measured soleus H-reflex size in two different situations. In the control H-reflex situation, the H-reflex was simply measured. In the conditioned H-reflex situation, the H-reflex was measured while the subject was encouraged to produce H-reflexes that were larger (HRup subjects) or smaller (HRdown subjects) than a criterion, was immediately informed as to whether he or she had succeeded, and was rewarded for success. Thus, the conditioned reflex situation imposed a task: to change H-reflex size as requested.
The impact of this task is reflected in the within-session difference between the control and conditioned H-reflexes (Fig. 3C). The within-session difference appeared in the early sessions (session 2 for HRup subjects and sessions 5–6 for HRdown subjects), and then remained about the same through the follow-up sessions. Since these within-session task-dependent differences increased the number of successful trials, they were adaptive and are called task-dependent adaptations. In both magnitude and rate (i.e., the number of trials over which they develop), these HRup and HRdown task-dependent adaptations resemble the phase 1 changes in animals exposed to the up- or down-conditioning mode (Wolpaw and O'Keefe, 1984; Wolpaw et al., 1994; Chen et al., 2001). Table 3 compares these task-dependent adaptations to the phase 1 changes in monkeys and rats (Wolpaw and O'Keefe, 1984; Wolpaw et al., 1994; Chen et al., 2001). Unlike the animal protocols, which simply imposed the conditioning task and left it in effect, the present human protocol repeatedly turned the task from “off” (i.e., within-session control trials) to “on” (i.e., conditioned trials). It thereby indicated that the rapid phase 1 change is not simply the first part of a long conditioning process, but is rather a discrete component of the skill of a larger or smaller H-reflex.
This task-dependent adaptation took practice to acquire: two sessions (i.e., 450 conditioned trials) for HRup subjects and five to six sessions (i.e., ∼1200 trials) for HRdown subjects. The difference in the amount of practice required is further confirmation that up- and down-conditioning are not mirror images of each other, but instead have different mechanisms (Wolpaw, 2006, 2007). By clearly isolating this task-dependent component, the present study supports the two-phase hypothesis that was previously based only on the overall course of conditioning (Wolpaw and O'Keefe, 1984; Wolpaw et al., 1994; Chen et al., 2001).
In its rapid development and flexibility, this within-session task-dependent adaptation is similar to other motor skills associated with plasticity in cortex and subcortical areas (Jenkins et al., 1994; Penhune and Doyon, 2002; Floyer-Lea and Matthews, 2005; Lehéricy et al., 2005; Floyer-Lea et al., 2006). Animal data indicate that H-reflex conditioning depends on sensorimotor cortex and the CST and does not depend on other major descending pathways (Chen and Wolpaw, 2002; X. Y. Chen et al., 2002, 2006), and also indicate that H-reflex conditioning involves plasticity in cortex or closely related areas (Wolpaw and Chen, 2006). Furthermore, patient data suggest that sensorimotor cortex is essential for reflex conditioning in humans (Segal, 1997). Together, these findings suggest that cortical activity produces CST output that causes the observed task-dependent adaptation and that this cortical activity reflects supraspinal plasticity that is acquired in the first few conditioning sessions.
Long-term change
As Figure 3 and Table 1 indicate, task-dependent adaptation did not entirely account for the final effect of the conditioning protocol on the conditioned H-reflex. Moreover, since task-dependent adaptation developed in the first few sessions, it did not explain the additional change in the conditioned H-reflex in subsequent sessions. This additional change reflected gradual change in the control reflex (Fig. 3B).
In its gradual development and persistence, this long-term change in the control H-reflex resembles the phase 2 change seen in animals (Wolpaw and O'Keefe, 1984; Wolpaw et al., 1994; Chen et al., 2001). Table 3 compares the control H-reflex changes described here to the phase 2 changes found in animals. Unlike the animal protocols, which could not measure the control H-reflex once conditioning started, the present human protocol allowed the long-term change in the control H-reflex to be separated from task-dependent adaptation. This revealed that the two components occurred at different points in the conditioning process and developed at different rates; they are essentially two distinct phenomena that together account for the final change in the conditioned H-reflex.
The fact that long-term change in the control H-reflex was not accompanied by change in Hmax implies that it reflected a change that was focused in the ascending limb of the H-reflex recruitment curve. This change could be a shift in H-reflex threshold and/or an alteration of recruitment slope.
Animal studies of H-reflex conditioning link the long-term reflex changes to spinal cord plasticity (Wolpaw, 1997, 2007; Wolpaw and Tennissen, 2001). A positive shift in motoneuron firing threshold [probably caused by a change in sodium channel activation voltage (Halter et al., 1995)] accounts for most of the H-reflex decrease (Carp and Wolpaw, 1994). Plasticity in spinal interneurons is the most likely mechanism for H-reflex increase (Wolpaw and Chen, 2001). The gradual changes in the control H-reflex (Fig. 3B) almost certainly reflect such spinal cord plasticity. Since the CST is the only major descending tract essential for H-reflex conditioning (Chen and Wolpaw, 2002; X. Y. Chen et al., 2002, 2006), it is presumably CST activity that changes the spinal cord. Given the likelihood that this CST activity reflects supraspinal plasticity (see above), H-reflex conditioning appears to depend on a hierarchy in which plasticity in the brain induces plasticity in the spinal cord.
In its rate and persistence, the long-term change in the control H-reflex resembles the changes in spinal reflexes associated with acquisition of more complex motor skills. Reflexes change gradually early in life as skills (e.g., walking) are acquired, and they continue to change throughout life as new skills (e.g., ballet, backward walking) are mastered (Nielsen et al., 1993; Wolpaw and Tennissen, 2001; Earles et al., 2002; Ozmerdivenli et al., 2002; Schneider and Capaday, 2003; Ung et al., 2005). Like H-reflex conditioning, these other long-term reflex changes are likely to reflect plasticity in both the brain and the spinal cord.
Therapeutic applications
Because it affects a pathway important in locomotion, H-reflex conditioning can help to restore locomotion in rats with partial spinal cord injuries (Y. Chen et al., 2006). Reflex conditioning might help to improve motor function in people with spinal cord injuries or other disorders. Protocols could be designed for the particular deficits of each person. They might be especially useful when significant regeneration becomes possible and precise methods for reeducating the regenerated spinal cord neurons and synapses are needed to restore function (Wolpaw, 2006).
This study addresses two issues affecting the potential therapeutic value of reflex conditioning: practicality and persistence. The results show that human soleus H-reflex conditioning, like human biceps brachii SSR conditioning (Segal and Wolf, 1994; Wolf and Segal, 1996), requires only a small fraction (<3%) of the trials used in animals and persists for several months at least. Thus, reflex conditioning protocols may prove clinically practical and may have lasting functional benefit. Initial efforts to test this possibility in humans with spinal cord injuries have begun (Thompson et al., 2008).
Conclusions
This study is, to our knowledge, the first demonstration of H-reflex operant conditioning in humans. Its major finding is that the simple skill of a larger or smaller soleus H-reflex consists of two separable components, task-dependent adaptation and long-term change, that differ in time of onset, rate of development, and flexibility. Combined with previous results, this new finding suggests that the conditioning protocol induces plasticity in the brain that in turn induces plasticity in the spinal cord.
Footnotes
This work was supported in part by National Institutes of Health Grants NS22189 and HD36020, and the New York State Spinal Cord Injury Research Trust. We thank Dr. Gerwin Schalk for technical support, Thomas N. Nielsen for assisting in data collection, and Drs. Jonathan S. Carp, Dennis J. McFarland, and Elizabeth Winter Wolpaw for comments on this manuscript.
References
- Brooke JD, Cheng J, Collins DF, McIlroy WE, Misiaszek JE, Staines WR. Sensori-sensory afferent conditioning with leg movement: gain control in spinal reflex and ascending paths. Prog Neurobiol. 1997;51:393–421. doi: 10.1016/s0301-0082(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Carp JS, Wolpaw JR. Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. J Neurophysiol. 1994;72:431–442. doi: 10.1152/jn.1994.72.1.431. [DOI] [PubMed] [Google Scholar]
- Carp JS, Tennissen AM, Chen XY, Wolpaw JR. Diurnal H-reflex variation in mice. Exp Brain Res. 2006a;168:517–528. doi: 10.1007/s00221-005-0106-y. [DOI] [PubMed] [Google Scholar]
- Carp JS, Tennissen AM, Chen XY, Wolpaw JR. H-reflex operant conditioning in mice. J Neurophysiol. 2006b;96:1718–1727. doi: 10.1152/jn.00470.2006. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Circadian rhythm in rat H-reflex. Brain Res. 1994;648:167–170. [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Operant conditioning of H-reflex in freely moving rats. J Neurophysiol. 1995;73:411–415. doi: 10.1152/jn.1995.73.1.411. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Probable corticospinal tract control of spinal cord plasticity in the rat. J Neurophysiol. 2002;87:645–652. doi: 10.1152/jn.00391.2001. [DOI] [PubMed] [Google Scholar]
- Chen XY, Chen L, Wolpaw JR. Time course of H-reflex conditioning in the rat. Neurosci Lett. 2001;302:85–88. doi: 10.1016/s0304-3940(01)01658-5. [DOI] [PubMed] [Google Scholar]
- Chen XY, Carp JS, Chen L, Wolpaw JR. Corticospinal tract transection prevents operantly conditioned H-reflex increase in rats. Exp Brain Res. 2002;144:88–94. doi: 10.1007/s00221-002-1026-8. [DOI] [PubMed] [Google Scholar]
- Chen XY, Carp JS, Chen L, Wolpaw JR. Sensorimotor cortex ablation prevents H-reflex up-conditioning and causes a paradoxical response to down-conditioning in rats. J Neurophysiol. 2006;96:119–127. doi: 10.1152/jn.01271.2005. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT, Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci. 2006;26:12537–12543. doi: 10.1523/JNEUROSCI.2198-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Hultborn H, Orsnes GB. Amplitude of the maximum motor response (Mmax) in human muscles typically decreases during the course of an experiment. Exp Brain Res. 1999;124:265–270. doi: 10.1007/s002210050621. [DOI] [PubMed] [Google Scholar]
- Earles DR, Dierking JT, Robertson CT, Koceja DM. Pre- and post-synaptic control of motoneuron excitability in athletes. Med Sci Sports Exerc. 2002;34:1766–1772. doi: 10.1097/00005768-200211000-00012. [DOI] [PubMed] [Google Scholar]
- Evatt ML, Wolf SL, Segal RL. Modification of human spinal stretch reflexes: preliminary studies. Neurosci Lett. 1989;105:350–355. doi: 10.1016/0304-3940(89)90646-0. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Matthews PM. Distinguishable brain activation networks for short- and long-term motor skill learning. J Neurophysiol. 2005;94:512–518. doi: 10.1152/jn.00717.2004. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006;95:1639–1644. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- Halter JA, Carp JS, Wolpaw JR. Operantly conditioned motoneuron plasticity: possible role of sodium channels. J Neurophysiol. 1995;73:867–871. doi: 10.1152/jn.1995.73.2.867. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE. Motor sequence learning: a study with positron emission tomography. J Neurosci. 1994;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol. 2004;82:238–248. doi: 10.1139/y04-017. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- Lagerquist O, Zehr EP, Baldwin ER, Klakowicz PM, Collins DF. Diurnal changes in the amplitude of the Hoffmann reflex in the human soleus but not in the flexor carpi radialis muscle. Exp Brain Res. 2006;170:1–6. doi: 10.1007/s00221-005-0172-1. [DOI] [PubMed] [Google Scholar]
- Lamont EV, Zehr EP. Task-specific modulation of cutaneous reflexes expressed at functionally relevant gait cycle phases during level and incline walking and stair climbing. Exp Brain Res. 2006;173:185–192. doi: 10.1007/s00221-006-0586-4. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Benali H, Van de Moortele PF, Pélégrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Crone C, Hultborn H. H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur J Appl Physiol Occup Physiol. 1993;66:116–121. doi: 10.1007/BF01427051. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozmerdivenli R, Bulut S, Urat T, Ayar A. The H- and T-reflex response parameters of long- and short-distance athletes. Physiol Res. 2002;51:395–400. [PubMed] [Google Scholar]
- Pavlides C, Miyashita E, Asanuma H. Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. J Neurophysiol. 1993;70:733–741. doi: 10.1152/jn.1993.70.2.733. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Doyon J. Dynamic cortical and subcortical networks in learning and delayed recall of timed motor sequences. J Neurosci. 2002;22:1397–1406. doi: 10.1523/JNEUROSCI.22-04-01397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Schneider C, Capaday C. Progressive adaptation of the soleus H-reflex with daily training at walking backward. J Neurophysiol. 2003;89:648–656. doi: 10.1152/jn.00403.2002. [DOI] [PubMed] [Google Scholar]
- Segal RL. Plasticity in the central nervous system: operant conditioning of the spinal stretch reflex. Top Stroke Rehabil. 1997;3:76–87. doi: 10.1080/10749357.1997.11754130. [DOI] [PubMed] [Google Scholar]
- Segal RL, Wolf SL. Operant conditioning of spinal stretch reflexes in patients with spinal cord injuries. Exp Neurol. 1994;130:202–213. doi: 10.1006/exnr.1994.1199. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Larsen B. Soleus stretch reflex modulation during gait in humans. J Neurophysiol. 1996;76:1112–1120. doi: 10.1152/jn.1996.76.2.1112. [DOI] [PubMed] [Google Scholar]
- Stein RB. Presynaptic inhibition in humans. Prog Neurobiol. 1995;47:533–544. doi: 10.1016/0301-0082(95)00036-4. [DOI] [PubMed] [Google Scholar]
- Thompson AK, Wolpaw JR, Lichtman SW, DeFrancesco E, Pomerantz F. Operant down-conditioning of soleus H-reflex in incomplete spinal cord injury. Soc Neurosci Abstr. 2008;34:73–4. [Google Scholar]
- Ung RV, Imbeault MA, Ethier C, Brizzi L, Capaday C. On the potential role of the corticospinal tract in the control and progressive adaptation of the soleus h-reflex during backward walking. J Neurophysiol. 2005;94:1133–1142. doi: 10.1152/jn.00181.2005. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Segal RL. Reducing human biceps brachii spinal stretch reflex magnitude. J Neurophysiol. 1996;75:1637–1646. doi: 10.1152/jn.1996.75.4.1637. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Operant conditioning of primate spinal reflexes: the H-reflex. J Neurophysiol. 1987;57:443–459. doi: 10.1152/jn.1987.57.2.443. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. The complex structure of a simple memory. Trends Neurosci. 1997;20:588–594. doi: 10.1016/s0166-2236(97)01133-8. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. The education and re-education of the spinal cord. Prog Brain Res. 2006;157:261–280. doi: 10.1016/s0079-6123(06)57017-7. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol (Oxf) 2007;189:155–169. doi: 10.1111/j.1748-1716.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Chen XY. Operant conditioning of rat H-reflex: effects on mean latency and duration. Exp Brain Res. 2001;136:274–279. doi: 10.1007/s002210000609. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Chen XY. The cerebellum in maintenance of a motor skill: a hierarchy of brain and spinal cord plasticity underlies H-reflex conditioning. Learn Mem. 2006;13:208–215. doi: 10.1101/lm.92706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, O'Keefe JA. Adaptive plasticity in the primate spinal stretch reflex: evidence for a two-phase process. J Neurosci. 1984;4:2718–2724. doi: 10.1523/JNEUROSCI.04-11-02718.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, Seegal RF. Diurnal rhythm in the spinal stretch reflex. Brain Res. 1982;244:365–369. doi: 10.1016/0006-8993(82)90099-3. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci. 2001;24:807–843. doi: 10.1146/annurev.neuro.24.1.807. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Braitman DJ, Seegal RF. Adaptive plasticity in primate spinal stretch reflex: initial development. J Neurophysiol. 1983;50:1296–1311. doi: 10.1152/jn.1983.50.6.1296. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, O'Keefe JA, Noonan PA, Sanders MG. Adaptive plasticity in primate spinal stretch reflex: persistence. J Neurophysiol. 1986;55:272–279. doi: 10.1152/jn.1986.55.2.272. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Herchenroder PA, Carp JS. Operant conditioning of the primate H-reflex: factors affecting the magnitude of change. Exp Brain Res. 1993;97:31–39. doi: 10.1007/BF00228815. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Maniccia DM, Elia T. Operant conditioning of primate H-reflex: phases of development. Neurosci Lett. 1994;170:203–207. doi: 10.1016/0304-3940(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB. Phase-dependent reflex reversal in human leg muscles during walking. J Neurophysiol. 1990;63:1109–1117. doi: 10.1152/jn.1990.63.5.1109. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. Interaction of the Jendrassik maneuver with segmental presynaptic inhibition. Exp Brain Res. 1999a;124:474–480. doi: 10.1007/s002210050643. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol. 1999b;58:185–205. doi: 10.1016/s0301-0082(98)00081-1. [DOI] [PubMed] [Google Scholar]
- Zehr PE. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol. 2002;86:455–468. doi: 10.1007/s00421-002-0577-5. [DOI] [PubMed] [Google Scholar]