Abstract
We report a transgenic line with highly penetrant cre recombinase activity in the somatotrope cells of the anterior pituitary gland. Expression of the cre transgene is under the control of the locus control region of the human growth hormone gene cluster and the rat growth hormone promoter. Cre recombinase activity was assessed with two different lacZ reporter genes that require excision of a floxed stop sequence for expression: a chick β-actin promoter with the CMV enhancer transgene and a ROSA26 knock-in. Cre activity is detectable in the developing pituitary after initiation of Gh transcription and persists through adulthood with high penetrance in Gh expressing cells and lower penetrance in lactotropes, a cell type that shares a common origin with somatotropes. This Gh-cre transgenic line is suitable for efficient, cell-specific deletion of floxed regions of genomic DNA in differentiated somatotropes and a subset of lactotrope cells of the anterior pituitary gland.
Keywords: anterior pituitary gland, cell specific expression, transgene, growth hormone, prolactin
Introduction
The pituitary gland is the central endocrine organ in vertebrates. Through communication with the hypothalamus, the pituitary regulates a wide range of functions including growth, fertility, lactation, stress response, homeostasis and metabolism. Disruption in normal pituitary gland function may result in failed expansion of pituitary cell types, hormone deficiency, pituitary dwarfism, or pituitary tumors (Cushman and Camper, 2001). Somatotropes are cells responsible for growth hormone (GH) production in the pituitary gland. GH is a key component of the somatotropic axis, stimulating the production of insulin-like growth factor-I (IGF-I) in the liver, which in turn acts on numerous target organs to regulate growth and a variety of other physiological processes including hearing, vision, and glucose homeostasis (Chandrashekar et al., 2007; Schneider et al., 2003; Walenkamp and Wit, 2007).
Generation of pituitary cell-specific cre transgenic lines has allowed for the study of particular pituitary cell functions and cell lineage relationships (Bingham et al., 2006; Charles et al., In press; Charles et al., 2006; Cushman et al., 2000; Jorgez et al., 2006; Luque et al., 2007; Naik et al., 2006; Yin et al., 2008). We sought to generate a tool that would be useful for studying the development and function of somatotropes, which represent approximately 40% of the cell population of the adult anterior lobe. Cell specific gene targeting is invaluable for studying the function of genes that have pleiotropic effects on development, function, or carcinogenesis of vital organs causing embryonic lethality or complications due to multi-organ effects. For example, a systemic knockout of the growth hormone receptor (Ghr) produces cardiac and pulmonary phenotypes, precluding assessment of functions in other organs (Beyea et al., 2006; Egecioglu et al., 2007). Here we present a transgenic cre line, Tg(Gh-cre)SAC1, which expresses cre recombinase in pituitary somatotropes, with limited expression in lactotropes, and no detectable expression in other pituitary cell types. We predict this to be a useful tool for targeted gene ablation in committed somatotropes, and therefore a valuable resource for the scientific community.
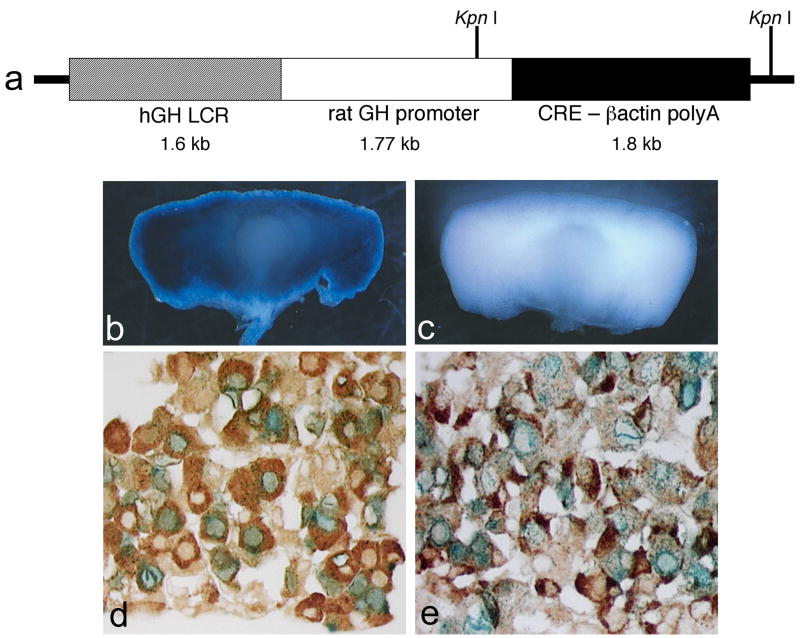
The Gh-cre transgenic construct contains the coding sequences for a nuclear localized cre, 1.6 kb of the human GH locus control region (LCR), 1.77 kb of rat GH promoter, and β-actin polyadenylation sequences (Fig. 1a). The rat GH promoter has produced strong expression in transgenic mice (Akita et al., 1997; Behringer et al., 1988; Burton et al., 1991; Lira et al., 1988; Lira et al., 1993). The hGH LCR fragment (−14.6 kb to −16.2 kb relative to the hGH promoter) contains two pituitary-specific DNase I-hypersensitive sites and is sufficient for restricting expression to somatotropes and somatolactotropes, for appropriately timed induction of hGH transgene expression in mice starting at embryonic day e15.5–e16.5, and for selective extinction of hGH in mature lactotropes (Bennani-Baiti et al., 1998). The human GH LCR improves the penetrance of rat GH promoter-driven transgene expression (Behringer et al., 1988; Bennani-Baiti et al., 1998; Jin et al., 1999; Jones et al., 1995; Nasonkin, 2002).
Figure 1. Structure and function of Tg(Gh-cre)SAC1 transgene.
A total of 1.6 kb of the human growth hormone locus control region was cloned upstream of a 1.77 kb fragment containing the rat growth hormone promoter, the cre recombinase coding sequences, and the beta-globin poly adenylation and terminator sequences (a). Pituitaries of adult mice doubly transgenic for Tg (Gh-cre)SAC1 and the Rosa26 floxed stop lacZ reporter mouse strain stained blue with X-gal indicating cre-mediated recombination (b), while cre transgene negative mice carrying the reporter have no X-gal staining (c). Tg(Gh-cre)SAC1 mice were mated to the cβ-actin-CMV-floxed stop NLS-LacZ reporter and tissues from doubly transgenic progeny stained with X-gal (Cushman et al., 2000). Nuclear localized blue staining, evidence of cre activity, is detected in cells immunostained for GH (d) and PRL (e). Black arrows identify hormone-positive cells with cre activity, and white arrows identify hormone-positive cells without cre activity.
Twelve transgenic founder mice were initially surveyed for transgene expression by mating with the chick β-actin-LacZ cre reporter strain, known as Tg(flox-lacZ)J7Sac (Cushman et al., 2000), and analysis of X-gal staining in doubly transgenic progeny (Nasonkin, 2002). Five of those lines exhibited robust staining in the adult pituitary gland, and the best line, Tg(Gh-cre)SAC1, was chosen for detailed analysis with two cre reporter strains: chick β-actin-LacZ and Rosa26-LacZ, officially known as B6;129S4-Gt(ROSA)26Sortm1Sor/J (Soriano, 1999).
Strong X-gal staining is evident in the anterior lobe of pituitaries from mice carrying both the transgene Tg(Gh-cre)SAC1 and the Rosa26-LacZ reporter allele, and there is no significant background activity in mice with the Rosa-LacZ allele but no cre transgene (Figure 1b). The cell-specificity of cre-excision was assessed in mice carrying Tg(Gh-cre)SAC1 and the chick β-actin-LacZ cre reporter by immunohistochemical staining for pituitary hormones and X-gal staining. Nearly all X-gal stained cells also immunostained for GH, and the majority of GH cells were X-gal stained (Figure 1d). Despite this 1:1 correspondence between X-gal staining and GH immunostaining, a subset of the prolactin (PRL) expressing cells stain for X-gal (Figure 1e). These PRL, X-gal double positive cells are likely to represent somatomammotropes, which express both PRL and GH (Frawley and Boockfor, 1991).
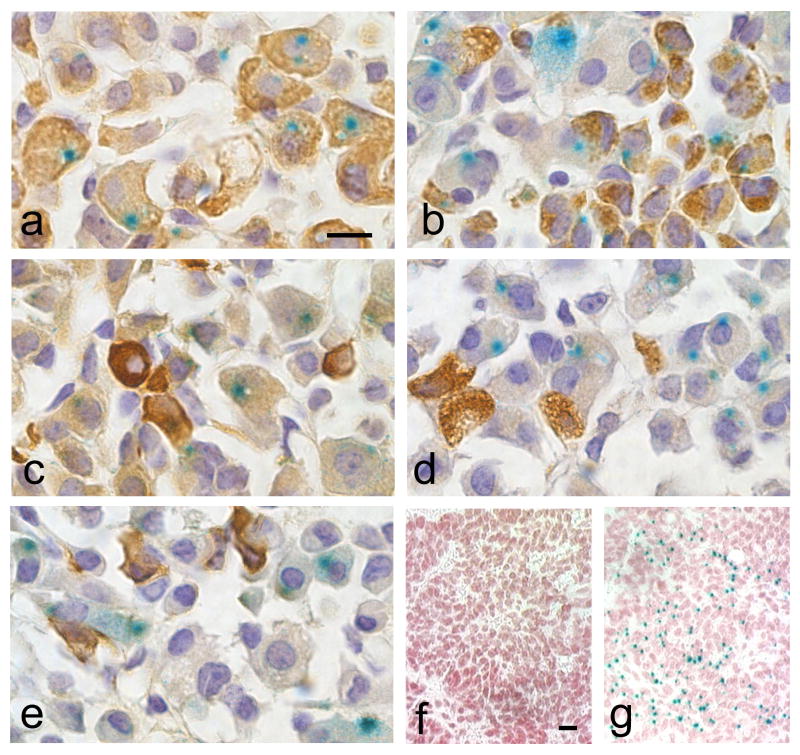
To facilitate comparison of the Tg(Gh-cre)SAC1 transgene with other cre strains, an analysis of cell specificity and developmental activation was performed using the popular Rosa26-LacZ allele. X-gal staining was observed in the majority of GH immunopositive cells and a subset of the PRL immunopositive cells (Figure 2a, b). Quantitation of cre-mediated excision revealed 61 ± 11% of the somatotropes are X-gal positive and 9 ± 5% of the lactotropes. These results are similar to those obtained with the chick β-actin-LacZ reporter. X-gal stained cells expressing the Rosa26-LacZ reporter have a very small blue precipitate in a restricted cytosolic region, while the entire nucleus turns blue using the chick β-actin-LacZ reporter. This could result in underestimated penetrance with Rosa26-LacZ. No cre-mediated excision is observed in adult thyrotropes, gonadotropes, or corticotropes (Figure 2c–e, respectively). Although it is not uncommon for transgenes to exhibit variable activity, even within the same line, the majority of Tg(Gh-cre)SAC1;Rosa-LacZ double transgenic mice exhibited similar X-gal staining in the pituitary gland (17/18), suggesting that Tg(Gh-cre)SAC1 is a reliable resource for deletion in somatotropes. Gh expression is normally detected in the mouse pituitary by embryonic day 15.5 (e15.5) (Japon et al., 1994). Tg(Gh-cre)SAC1 transgene activity is not detectable at e14.5 (Fig. 2f), but it becomes readily apparent between e15.5 and e18.5 (Fig. 2g).
Figure 2. Tg(Gh-cre)SAC1 is specific for the somatotrope, lactotrope lineages.
Adult pituitaries from mice doubly transgenic for Tg(Gh-cre)SAC1 and the Rosa26 lacZ reporter strain were stained with X-gal and antibodies specific for GH (a), prolactin (b), TSHβ (c), LHβ (d), or POMC (e), developed with DAB to produce a brown color, and photographed at 100x oil immersion magnification. Although doubly transgenic embryos stained for X-gal show no blue staining at e14.5 (f), it is detected at e15.5 (g) when endogenous growth hormone expression begins. Magnification bars (a, f) represent 10μm (a–e, f–g, respectively).
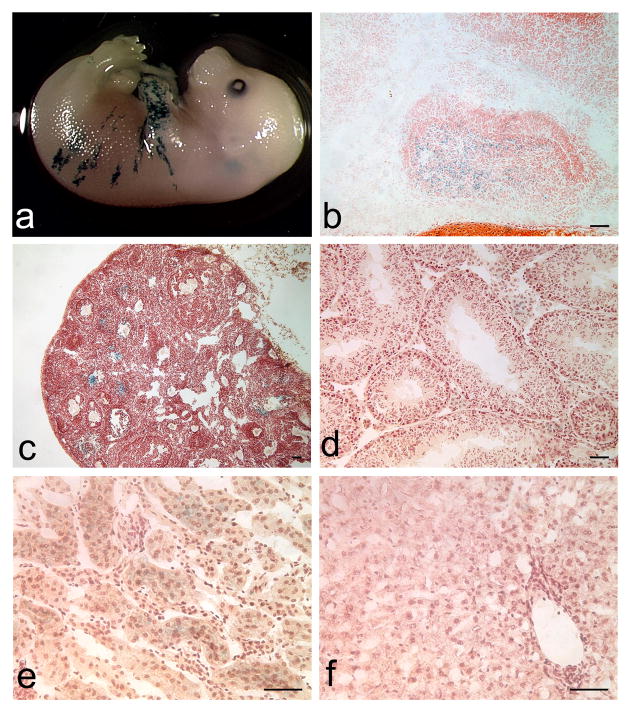
Recombination in non-pituitary tissues was assessed in Tg(Gh-cre)SAC1, Rosa-LacZ reporter mice. X-gal staining was observed in the skin surrounding the forearm of e16.5-e17.5 embryos. Low level X-gal staining is detected in the kidney, ovary and testis of Tg(Gh-cre)SAC1;Rosa-LacZ mice (Figure 3). The recombination in nonpituitary tissues may not represent ectopic expression because GH is expressed human skin, and Gh transcripts are detectable in the brain and testis of wild type mice (Slominski et al., 2000), (http://www.informatics.jax.org/searches/reference.cgi?46734). No X-gal staining was observed in the adult liver, skeletal muscle, adipose tissue, lung, spleen, or heart, and in one occurrence minimal staining was observed in pancreas (data not shown). No X-gal staining was seen in the placentas of double transgenic embryos at e16.5. In addition, we detected no evidence of leaky cre activity in the germline (data not shown).
Figure 3. Minimal ectopic Tg(Gh-cre)SAC1 transgene expression.
Very limited X-gal staining is detected in non-pituitary tissues such as the skin surrounding the limbs and trunk of developing embryos (a). At e18.5, X-gal staining is appropriately restricted to the developing anterior lobe (A) and is absent from the intermediate (I) and posterior (P) lobes (b). In adult tissues, X-gal staining is seen in the developing follicles of some ovaries (c). Sporadic but very faint X-gal staining appears in the Leydig cells of the testes (d) and in the renal cortex (e). No staining is detected in the adult liver (f). All magnification bars represent 50μm.
Mouse strains that confer gene deletion in somatotropes have been developed using the promoter from the rat GH releasing hormone receptor (Ghrhr) (Yin et al., 2008) and the rat Gh promoter, similar to our strategy, except without the human growth hormone locus control region (Luque et al., 2007). In contrast to the Ghrhr-cre strain, we find no evidence of cre-activity in thyrotropes. The Tg(Gh-cre)SAC1 strain is the first to be fully characterized for ectopic excision activity during development and in adult tissues. Thus, the thorough histological information we provide using a well-characterized cre-reporter gene is an advantage of the Tg(Gh-cre)SAC1 strain.
We expect that these transgenic mice will have many uses. Permanently marked somatotropes could be dispersed and sorted for gene expression profiling (Muzumdar et al., 2007) or used in intact tissue to study the role of the interconnected cellular network in producing sex-specific pulsatile patterns of GH secretion (Bonnefont et al., 2005). Another potentially intriguing application would be somatotrope-specific deletion of Ghr to discover the role of GH feedback on the anterior pituitary, given the many metabolic effects of systemic Ghr deletion on skeletal and cardiac muscle function, adiposity, liver function, carcinogenesis and aging (reviewed in (Clark et al., 2006).
METHODS
Generation of Transgene Construct
The Gh-cre transgene construct was generated by PCR amplification of 1.77 kb of sequence from the rat GH (rGH) promoter (accession #X12967) using primers F 5′-GGGTACCTCTAGAAGCTTAGTTTCTAGTAGG and R, 5′-CCTGAGCAGTTTGGAATCTGG. The PCR product was amplified using the Expand High Fidelity polymerase mix (Roche, Indianapolis, IN) under the following conditions: 95°C for 2 minutes, followed by 5 cycles of 94°C for 45 seconds, 58°C for 1 minute and 72°C for 2 minutes, then 25 cycles of 94°C for 45 seconds, 61°C for 1 minute and 72°C for 2 minutes, and a final 10 minute extension at 72°C. The rGH promoter was cloned upstream of the cassette containing the nuclear localized Cre and βactin-polyadenylation sequences, in the pML78 plasmid at the XhoI site (Meyers et al., 1998). To minimize the possibility of position effect variegation (PEV) of the transgene insertion site, a 1.6 kb BglII fragment of sequence from the human growth hormone (hGH) locus control region (LCR) was cloned upstream of the 1.77 kb rGH promoter (Bennani-Baiti et al., 1998; Jin et al., 1999; Jones et al., 1995). hGH LCR sequences (accession # AF039413) were obtained by PCR amplification of human DNA using hGH LCR primers F, 5 ′-CGGGGTACCTCTAGAGATCTTGTCTCAGAAAAACCC, (KpnI and XbaI cloning sites underlined) and R, 5′-GGGGTACCTCTAGAGATCTTGGCCTAGGCCTCG with Expand High Fidelity polymerase under the following conditions: 95°C for 3 minutes, followed by 5 cycles of 94°C for 45 seconds, 60°C for 1 minute and 72°C for 2 minutes, then 25 cycles of 94°C for 40 seconds, 65°C for 1 minute and 72°C for 1 minute 45 seconds, and a final extension at 72°C for 10 minutes.
Generation, Characterization and Maintenance of Mice
The GH-cre transgene was injected into (C57BL/6J × SJL/J) F1 × F1 zygotes, and the fertilized eggs were then transferred into pseudopregnant foster mothers. Transgene positive progeny were genotyped using PCR amplification of genomic DNA for the presence of cre using the following primers: 5′-GCATAACCAGTGAAACAGCATTGCTG-3′ and 5′-GGACATGTTCAGGGATCGCCAGGCG-3′, under the following conditions: 94°C for 3 minutes, followed by 32 cycles of 94°C for 30 seconds, 60°C for 60 seconds and 72°C for 90 seconds, and a final 10 minute extension at 72°C.
To determine the number of copies of the transgene inserted into the genome, a Southern analysis was performed using 10μg of KpnI digested genomic tail DNA. The copy number of Tg(GH-cre)SAC1 was estimated at 30–50 copies per haploid genome (data not shown).
Dissected pituitaries from adult progeny were assayed for X-gal activity, as previously described (Brinkmeier et al., 1998). The pituitaries of the doubly transgenic progeny (n=8) were stained for X-gal activity as previously described (Cushman et al., 2000). Tissues were embedded in paraffin and sectioned at 5μm thickness. Immunohistochemistry was performed for each of the pituitary hormones as previously described (Kendall et al., 1991).
Quantitation of cre-mediated excision in somatotropes and lactotropes was accomplished using three doubly transgenic mice positive for Tg(GH-cre)SAC1 and the Rosa26-LacZ reporter. Three separate regions of the pituitary were photographed after histochemical staining for PRL or GH and X-gal. The average percentage of cells positive for GH and X-gal was calculated by dividing the total number of double positive cells by the total number of GH positive cells. The same calculations were done for PRL.
Acknowledgments
We thank Jill Karolyi, the University of Michigan Transgenic Animal Model Core, and NIH R01-HD34283. ION generated the transgenic mice and characterized them with the chick β-actin-LacZ reporter and identified the best line. MAP characterized the best line with the Rosa26-LacZ reporter and wrote the manuscript.
LITERATURE CITED
- Akita S, Readhead C, Stefaneanu L, Fine J, Tampanaru-Sarmesiu A, Kovacs K, Melmed S. Pituitary-directed leukemia inhibitory factor transgene forms Rathke’s cleft cysts and impairs adult pituitary function. A model for human pituitary Rathke’s cysts. J Clin Invest. 1997;99:2462–2469. doi: 10.1172/JCI119430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer RR, Mathews LS, Palmiter RD, Brinster RL. Dwarf mice produced by genetic ablation of growth hormone-expressing cells. Genes Dev. 1988;2:453–461. doi: 10.1101/gad.2.4.453. [DOI] [PubMed] [Google Scholar]
- Bennani-Baiti IM, Asa SL, Song D, Iratni R, Liebhaber SA, Cooke NE. DNase I-hypersensitive sites I and II of the human growth hormone locus control region are a major developmental activator of somatotrope gene expression. Proc Natl Acad Sci U S A. 1998;95:10655–10660. doi: 10.1073/pnas.95.18.10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyea JA, Sawicki G, Olson DM, List E, Kopchick JJ, Harvey S. Growth hormone (GH) receptor knockout mice reveal actions of GH in lung development. Proteomics. 2006;6:341–348. doi: 10.1002/pmic.200500168. [DOI] [PubMed] [Google Scholar]
- Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- Bonnefont X, Lacampagne A, Sanchez-Hormigo A, Fino E, Creff A, Mathieu MN, Smallwood S, Carmignac D, Fontanaud P, Travo P, Alonso G, Courtois-Coutry N, Pincus SM, Robinson IC, Mollard P. Revealing the large-scale network organization of growth hormone-secreting cells. Proc Natl Acad Sci U S A. 2005;102:16880–16885. doi: 10.1073/pnas.0508202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmeier ML, Gordon DF, Dowding JM, Saunders TL, Kendall SK, Sarapura VD, Wood WM, Ridgway EC, Camper SA. Cell-specific expression of the mouse glycoprotein hormone alpha-subunit gene requires multiple interacting DNA elements in transgenic mice and cultured cells. Mol Endocrinol. 1998;12:622–633. doi: 10.1210/mend.12.5.0103. [DOI] [PubMed] [Google Scholar]
- Burton FH, Hasel KW, Bloom FE, Sutcliffe JG. Pituitary hyperplasia and gigantism in mice caused by a cholera toxin transgene. Nature. 1991;350:74–77. doi: 10.1038/350074a0. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V, Dawson CR, Martin ER, Rocha JS, Bartke A, Kopchick JJ. Age-related Alterations in Pituitary and Testicular Functions in Long-lived Growth Hormone Receptor Gene-disrupted Mice. Endocrinology. 2007 doi: 10.1210/en.2007-0837. [DOI] [PubMed] [Google Scholar]
- Charles MA, Mortensen AH, Potok MA, Camper SA. Pitx2 deletion in pituitary gonadotropes is compatible with gonadal development, puberty and fertility. Genesis. doi: 10.1002/dvg.20398. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles MA, Saunders TL, Wood WM, Owens K, Parlow AF, Camper SA, Ridgway EC, Gordon DF. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Mol Endocrinol. 2006;20:1366–1377. doi: 10.1210/me.2005-0378. [DOI] [PubMed] [Google Scholar]
- Clark RP, Schuenke M, Keeton SM, Staron RS, Kopchick JJ. Effects of growth hormone and insulin-like growth factor I on muscle in mouse models of human growth disorders. Horm Res. 2006;66(Suppl 1):26–34. doi: 10.1159/000096620. [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Burrows HL, Seasholtz AF, Lewandoski M, Muzyczka N, Camper SA. Cre-mediated recombination in the pituitary gland. Genesis. 2000;28:167–174. doi: 10.1002/1526-968x(200011/12)28:3/4<167::aid-gene120>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Camper SA. Molecular basis of pituitary dysfunction in mouse and human. Mamm Genome. 2001;12:485–494. doi: 10.1007/s003350040002. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Andersson IJ, Bollano E, Palsdottir V, Gabrielsson BG, Kopchick JJ, Skott O, Bie P, Isgaard J, Bohlooly YM, Bergstrom G, Wickman A. Growth hormone receptor deficiency in mice results in reduced systolic blood pressure and plasma renin, increased aortic eNOS expression, and altered cardiovascular structure and function. Am J Physiol Endocrinol Metab. 2007;292:E1418–1425. doi: 10.1152/ajpendo.00335.2006. [DOI] [PubMed] [Google Scholar]
- Frawley LS, Boockfor FR. Mammosomatotropes: presence and functions in normal and neoplastic pituitary tissue. Endocr Rev. 1991;12:337–355. doi: 10.1210/edrv-12-4-337. [DOI] [PubMed] [Google Scholar]
- Japon MA, Rubinstein M, Low MJ. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem. 1994;42:1117–1125. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- Jin Y, Surabhi RM, Fresnoza A, Lytras A, Cattini PA. A role for A/T-rich sequences and Pit-1/GHF-1 in a distal enhancer located in the human growth hormone locus control region with preferential pituitary activity in culture and transgenic mice. Mol Endocrinol. 1999;13:1249–1266. doi: 10.1210/mend.13.8.0332. [DOI] [PubMed] [Google Scholar]
- Jones BK, Monks BR, Liebhaber SA, Cooke NE. The human growth hormone gene is regulated by a multicomponent locus control region. Mol Cell Biol. 1995;15:7010–7021. doi: 10.1128/mcb.15.12.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgez CJ, De Mayo FJ, Matzuk MM. Inhibin alpha-iCre mice: Cre deleter lines for the gonads, pituitary, and adrenal. Genesis. 2006;44:183–188. doi: 10.1002/dvg.20198. [DOI] [PubMed] [Google Scholar]
- Kendall SK, Saunders TL, Jin L, Lloyd RV, Glode LM, Nett TM, Keri RA, Nilson JH, Camper SA. Targeted ablation of pituitary gonadotropes in transgenic mice. Mol Endocrinol. 1991;5:2025–2036. doi: 10.1210/mend-5-12-2025. [DOI] [PubMed] [Google Scholar]
- Lira SA, Crenshaw EB, 3rd, Glass CK, Swanson LW, Rosenfeld MG. Identification of rat growth hormone genomic sequences targeting pituitary expression in transgenic mice. Proc Natl Acad Sci U S A. 1988;85:4755–4759. doi: 10.1073/pnas.85.13.4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira SA, Kalla KA, Glass CK, Drolet DW, Rosenfeld MG. Synergistic interactions between Pit-1 and other elements are required for effective somatotroph rat growth hormone gene expression in transgenic mice. Mol Endocrinol. 1993;7:694–701. doi: 10.1210/mend.7.5.8316253. [DOI] [PubMed] [Google Scholar]
- Luque RM, Amargo G, Ishii S, Lobe C, Franks R, Kiyokawa H, Kineman RD. Reporter expression, induced by a growth hormone promoter-driven Cre recombinase (rGHp-Cre) transgene, questions the developmental relationship between somatotropes and lactotropes in the adult mouse pituitary gland. Endocrinology. 2007;148:1946–1953. doi: 10.1210/en.2006-1542. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre-and Flp-mediated recombination. Nat Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Naik K, Pittman It, Wolfe A, Miller RS, Radovick S, Wondisford FE. A novel technique for temporally regulated cell type-specific Cre expression and recombination in the pituitary gonadotroph. J Mol Endocrinol. 2006;37:63–69. doi: 10.1677/jme.1.02053. [DOI] [PubMed] [Google Scholar]
- Nasonkin IO. Human Genetics. Ann Arbor: University of Michigan; 2002. Targeting Prophet of Pit1 gene and designing mouse lines for cre-mediated gene targeting in somatotrophs; p. 145. [Google Scholar]
- Schneider HJ, Pagotto U, Stalla GK. Central effects of the somatotropic system. Eur J Endocrinol. 2003;149:377–392. doi: 10.1530/eje.0.1490377. [DOI] [PubMed] [Google Scholar]
- Slominski A, Malarkey WB, Wortsman J, Asa SL, Carlson A. Human skin expresses growth hormone but not the prolactin gene. J Lab Clin Med. 2000;136:476–481. doi: 10.1067/mlc.2000.110605. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Walenkamp MJ, Wit JM. Genetic disorders in the GH IGF-I axis in mouse and man. Eur J Endocrinol. 2007;157(Suppl 1):S15–26. doi: 10.1530/EJE-07-0148. [DOI] [PubMed] [Google Scholar]
- Yin Z, Williams-Simons L, Parlow AF, Asa S, Kirschner LS. Pituitary-specific knockout of the Carney complex gene Prkar1a leads to pituitary tumorigenesis. Mol Endocrinol. 2008;22:380–387. doi: 10.1210/me.2006-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]