Summary
Growth factors and mitogens use the Ras/Raf/MEK/ERK signaling cascade to transmit signals from their receptors to regulate gene expression and prevent apoptosis. Some components of these pathways are mutated or aberrantly expressed in human cancer (e.g., Ras, B-Raf). Mutations also occur at genes encoding upstream receptors (e.g., EGFR and Flt-3) and chimeric chromosomal translocations (e.g., BCR-ABL) which transmit their signals through these cascades. Even in the absence of obvious genetic mutations, this pathway has been reported to be activated in over 50% of acute myelogenous leukemia and acute lymphocytic leukemia and is also frequently activated in other cancer types (e.g., breast and prostate cancers). Importantly, this increased expression is associated with a poor prognosis. The Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt pathways interact with each other to regulate growth and in some cases tumorigenesis. For example, in some cells, PTEN mutation may contribute to suppression of the Raf/MEK/ERK cascade due to the ability of activated Akt to phosphorylate and inactivate different Rafs. Although both of these pathways are commonly thought to have anti-apoptotic and drug resistance effects on cells, they display different cell lineage specific effects. For example, Raf/MEK/ERK is usually associated with proliferation and drug resistance of hematopoietic cells, while activation of the Raf/MEK/ERK cascade is suppressed in some prostate cancer cell lines which have mutations at PTEN and express high levels of activated Akt. Furthermore the Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt pathways also interact with the p53 pathway. Some of these interactions can result in controlling the activity and subcellular localization of Bim, Bak, Bax, Puma and Noxa. Raf/MEK/ERK may promote cell cycle arrest in prostate cells and this may be regulated by p53 as restoration of wild-type p53 in p53 deficient prostate cancer cells results in their enhanced sensitivity to chemotherapeutic drugs and increased expression of Raf/MEK/ERK pathway. Thus in advanced prostate cancer, it may be advantageous to induce Raf/MEK/ERK expression to promote cell cycle arrest, while in hematopoietic cancers it may be beneficial to inhibit Raf/MEK/ERK induced proliferation and drug resistance. Thus the Raf/MEK/ERK pathway has different effects on growth, prevention of apoptosis, cell cycle arrest and induction of drug resistance in cells of various lineages which may be due to the presence of functional p53 and PTEN and the expression of lineage specific factors.
Keywords: Raf/MEK/ERK, Signaling, Apoptosis, Drug Resistance, PI3K/Akt, Cancer Therapy
Introduction
Overview of the Ras/Raf/MEK/ERK Signaling
The Ras/Raf/MEK/ERK cascade couples signals from cell surface receptors to transcription factors, which regulate gene expression. Futhermore, this cascade also regulates the activity of many proteins involved in apoptosis. A diagrammatic overview of the Ras/Raf/MEK/ERK pathway is presented in Figure 1. This pathway is often activated in certain tumors by chromosomal translocations such as BCR-ABL, mutations in cytokine receptors such as Flt-3, Kit, Fms or overexpression of wild type or mutated receptors, e.g., EGFR. The Raf/MEK/ERK pathway also has profound effects on the regulation of apoptosis by the post-translational phosphorylation of apoptotic regulatory molecules including Bad, Bim, Mcl-1, caspase 9 and more controversially Bcl-2. This pathway has diverse effects which can regulate cell cycle progression, apoptosis or differentiation [1]. A survey of the literature documents the daily increase in the complexity of this pathway, as there are multiple members of the kinase, transcription factor, apoptotic regulator and caspase executioner families, which can be activated or inactivated by protein phosphorylation. Furthermore, this pathway can induce the transcription of certain genes. Raf, either through downstream MEK and ERK, or independently of MEK and ERK, can induce the phosphorylation of proteins, which control apoptosis. Additional signal transduction pathways interact with the Raf/MEK/ERK pathway to positively or negatively regulate its activity. Abnormal activation of this pathway occurs in human cancer due to mutations at upstream membrane receptors and Ras and B-Raf as well as genes in other pathways (e.g., PI3K, PTEN, Akt), which serve to regulate Raf activity. The Raf/MEK/ERK pathway also influences chemotherapeutic drug resistance as ectopic activation of Raf induces resistance to doxorubicin and paclitaxel in breast cancer cells. Mutations at B-Raf have been frequently detected in some malignancies including melanoma and thyroid cancers [2]. For all the above reasons, the Raf/MEK/ERK pathway is an important pathway to target for therapeutic intervention. Inhibitors of Ras, Raf, and MEK and some downstream targets have been developed and many are currently in clinical trials. Naturally, some inhibitors are better than others and certain “specific” inhibitors may inhibit multiple kinases.
Figure 1. Overview of Raf/MEK/ERK Pathway.
The Raf/MEK/ERK pathway is regulated by Ras as well as various kinases, which serve to phosphorylate S/T and Y residues on Raf. Some of these phosphorylation events serve to enhance Raf activity (shown by a black P in a white circle) whereas others serve to inhibit Raf activity (shown by a white P in a black circle. Moreover there are phosphatases such as PP2A, which remove phosphates on certain regulatory residues. The downstream transcription factors regulated by this pathway are indicated in diamond shaped outlines.
Ras—An Upstream Activator of the Raf/MEK/ERK Kinase Cascade
Ras is a small GTP-binding protein, which is the common upstream molecule of several signaling pathways including Raf/MEK/ERK, PI3K/Akt and RalEGF/Ral [3]. Four Ras proteins have been identified, namely Ha-Ras, N-Ras, Ki-Ras 4A and Ki-Ras 4B. The 2 isoforms of Ki-Ras are produced from the same gene by alternative splicing. Ras proteins show varying abilities to activate the Raf/MEK/ERK and PI3K/Akt cascades. They may differ in terms of their potency to activate the Raf/MEK/ERK cascde with Ki-Ras being a stronger induce of Raf/MEK/ERK than Ha-Ras [4]. In contrast, Ha-Ras may be a stronger inducer of PI3K/Akt than Raf/MEK/ERK. Different mutation frequencies have been observed between Ras genes in human cancer and it is important to realize that Ki-Ras is the more frequently mutated Ras isoform in human cancer, although in some particular cancer subsets N-Ras may be more frequently mutated.
For Ras to be targeted to the cell membrane, it must be farnesylated by farnesyl transferase (Ha-, Ki-, and N-Ras) or geranylgeranylated by geranylgeranyl transferase (N-and Ki-Ras). Farnesylation and geranylgeranylation both occur on the same cysteine residue. Ras preferentially undergoes farnesylation, however, in the presence of farnesylation inhibitors, N-Ras and Ki-Ras can undergo gernylgernylation. Ha-Ras and N-Ras can also undergo palmitoylation with Ha-Ras having two palmitoylation sites and N-Ras having one palmitoylation site. Ki-Ras appears to lack a palmitoylation site. It is believed that palmitoylation has a role in plasma membrane microlocalization. These post-translational modifications are important as they represent sites for therapeutic intervention which will be discussed later.
Following binding of cytokines, growth factors or mitogens to their appropriate receptors, activation of the coupling complex Shc/Grb2/SOS occurs. Upon stimulation by Shc/Grb2/SOS, the inactive Ras exchanges GDP for GTP and undergoes a conformational change and becomes active. The GTP bound active Ras can then recruit Raf to cell membrane. Ras is frequently mutated in human cancer and these point mutations occur in condons 12, 13, 59, and 61 [1]. These mutations result in the constitutive activation of the protein which no longer requires ligand for activation.
Complex Activation and Inactivation of Raf by Phosphorylation
The mammalian Raf gene family consists of A-Raf, B-Raf and Raf-1 (C-Raf). Raf is a serine/threonine (S/T) kinase and is normally activated by a complex series of events including: (i) recruitment to the plasma membrane mediated by an interaction with Ras [4] (Yan et al., 1998); (ii) dimerization of Raf proteins [5]; (iii) phosphorylation/dephosphorylation on different domains [6]; (iv) disassociation from the Raf kinase inhibitory protein (RKIP) [7–8] and (v) association with scaffolding complexes (e.g., kinase suppressor of Ras, (KSR) [9–11]. Raf activity is further modulated by chaperonin proteins including Bag1, 14-3-3 [12] and heat shock protein 90 (Hsp90) [13].
There are at least thirteen regulatory phosphorylation sites on Raf-1 [1]. Some of these sites [e.g., S43, S259 and S621] are phosphorylated when Raf-1 is inactive. This allows 14-3-3 to bind Raf-1 and confer a configuration which is inactive. Upon cell stimulation, S621 becomes transiently dephosphorylated by an unidentified phosphatase. Phosphatases such as protein phosphatase 2A (PP2A) dephosphorylate S259 [8]. 14-3-3 then disassociates from Raf-1. This allows Raf-1 to be phosphorylated at S338, Y340, and Y341, rendering Raf-1 active. A Src family kinase is likely responsible for phosphorylation at Y340 and Y341 [14]. Thus a side effect of Src family kinase inhibitors such as Dasatinib, which is being used to treat certain Imatinib resistant chronic myeloid leukemia (CML) patients and other cancer patients who overexpresses mutant c-Kit [15–16] would be suppression of Raf-1/MEK/ERK signaling in certain cells. This may be desired or undesired side effect.
Y340 and Y341, the phosphorylation targets of Src family kinases, are conserved in A-Raf (Y299 and Y300), but are replaced with aspartic acid (D) at the corresponding positions in B-Raf (D492 and D493) [6,14]. The negatively charged aspartic acid residues mimic activated residues, which confers elevated basal activity to B-Raf. Maximal activation of Raf-1 and A-Raf requires both Ras and Src activity while B-Raf activation is Src-independent [14]. Interestingly, as will be discussed later, a greater number of mutations are detected at B-Raf than either Raf-1 or A-Raf, in human cancer. This may have resulted from a simpler mode of activation and selection of cells containing B-Raf mutations. Dasatinib would be predicted to not inhibit B-Raf signaling, however, the story may be more complex as in some scenarios B-Raf signals through B-Raf: Raf-1 heterodimers [2]. Whether this B-Raf:Raf-1 heterodimers require phosphorylation at Y340 and Y341 for activation is unclear.
The S338 residue present in Raf-1 is conserved among the three Raf isoforms, however, in B-Raf (S445), this corresponding site is constitutively phosphorylated [17] S338 phosphorylation on Raf-1 is stimulated by Ras and is dependent on p21-activated protein kinase (PAK) [18]. Other phosphorylation sites in Raf-1 that may modulate its activity include: S43, S339, T491, S494, S497, S499, S619 and S621. Protein kinase C (PKC) has been shown to activate Raf and induce cross-talk between PKC and Raf/MEK/ERK signaling pathways [19]. S497 and S499 were identified as the target residues on Raf-1 for PKC phosphorylation. However, other studies suggest that these sites are not necessary for Raf-1 activation [20].
Raf activity is negatively regulated by phosphorylation on the conserved region (CR)2 regulatory domain. Akt and protein kinase A (PKA) phosphorylate S259 on Raf-1 and inhibit its activity [21]. Furthermore, Akt or the related serum/glucocorticoid regulated kinase (SGK) phosphorylate B-Raf on S364 and S428 and inactivate its kinase activity [22–23]. These S-phosphorylated Rafs associate with 14-3-3 and become inactive.
The scaffolding protein RKIP has been shown do inhibit Raf-1 activation and downstream signaling [7,24]. RKIP is a member of the phosphatidylethanolamine-binding protein (PEBP) family. This multi-gene family is evolutionarily conserved and has related members in bacteria, plants and animals [24]. Interesting RKIP can bind either Raf or MEK/ERK but not to Raf, MEK and ERK all together. Various isoforms of PKC have been shown to phosphorylate RKIP on S153 which results in the disassociation of Raf and RKIP. The role of RKIP in metastasis will be discussed later.
Hsp90 may serve to stabilize activated Raf. Drugs such as geldanamycin inhibit Hsp90 and result in the rapid degradation of Raf [13]. Although Raf is a target of these drugs, they are relatively non-specific and also result in the degradation of Src, EGFR, HER2, CDK4 and Akt. Raf is also a target of caspases. In this case, Raf is degraded and the cycle of Raf activation and inactivation by kinases and phosphatases is irreversibly broken.
The importance of Raf-1 in the Raf/MEK/ERK signal transduction pathway has come into question due to the discovery that B-Raf was a much more potent activator of MEK compared to Raf-1 and A-Raf. Many of the “functions” of Raf-1 persist in Raf-1 knock-out mice, and are likely maintained by endogenous B-Raf [25]. Interestingly and controversially it was recently proposed that B-Raf is not only the major activator of MEK1, but B-Raf is also involved in Raf-1 activation. B-Raf may be temporally activated before Raf-1. However, there may be different subcellular localizations of B-Raf and Raf-1 within the cell that exert different roles in signaling and apoptotic pathways [26]. In some cases, B-Raf may transduce its signal through Raf-1 and B-Raf can form heterodimers with Raf-1. Dimerization is one important component involved in Raf activation [27–28]. The reasons for these added complexities in the Raf/MEK/ERK kinase cascade are not obvious but may represent another layer of fine-tuning. Alternatively, B-Raf:Raf-1 heterodimers may have different substrate specifities or affinities than B-Raf:B-Raf and Raf-1:Raf-1 homodimers. Furthermore, as will be discussed later, some B-Raf mutants which are kinase deficient may transmit their signals via forming heterodimers with Raf-1 [2]
Activation of MEK1 by Raf
Mitogen-activated protein kinase/ERK kinase (MEK1) is a tyrosine (Y-) and S/T-dual specificity protein kinase [29]. Its activity is positively regulated by Raf phosphorylation on S residues in the catalytic domain. All three Raf family members are able to phosphorylate and activate MEK but different biochemical potencies have been observed (B-Raf > Raf-1≫ A-Raf) [29]. Activated MEK1 mutants have been constructed which will abrogate the cytokine-dependence of hematopoietic cells and morphologically transform NIH-3T3 cells [30]. Another interesting aspect regarding MEK1 is that it’s predominate downstream target is ERK. In contrast, downstream ERK has multiple targets. Thus, therapeutic targeting of MEK1 is relatively specific.
Activation of ERK1,2 by MEK1 and Downstream Targets of ERKs
Extracellular-signal-regulated kinases 1,2 (ERK), are S/T kinases and their activities are positively regulated by phosphorylation mediated by MEK1 and MEK2. ERKs can directly phosphorylate many transcription factors including Ets-1, c-Jun and c-Myc. ERK can also phosphorylate and activate the 90 kDa ribosomal S6 kinase (p90Rsk), which then leads to the activation of the transcription factor CREB [1] (See Figure 1). Moreover, through an indirect mechanism, ERK can lead to activation of the NF-κB transcription factor (nuclear factor immunoglobulin κ chain enhancer-B cell) by phosphorylating and activating inhibitor κB kinase (IKK) [31–32]. ERK1 and ERK2 are differentially regulated. These are only a few examples of the downstream targets of ERK 1,2. ERK has many other targets (over 160). ERK can enter the nucleus to phosphorylate many transcription factors [1]. ERK can also phosphorylate many proteins involved in cell cycle regulation as will be discussed later. ERK2 has been positively associated with proliferation while ERK1 may inhibit the effects of ERK2 in certain cells [33].
Activation of the Raf/MEK/ERK Pathway by Oxidative Stress
Reactive oxygen species (ROS) are well known to induce the activation of the Raf/MEK/ERK signaling pathways. Oxidative stress-induced ERK1/2 activation is reported in a variety of cell types [34–42]. In some cases reactive oxygen intermediates act directly on growth receptors, such as the EGFR, in a ligand-independent fashion and induce the activation of Ras and ERK1/2 signaling [43]. This type of responsiveness does not appear to be limited to the EGFR as ROS will also induce the ligand-independent activation of the platelet derived growth factor (PDGF) receptor and a subsequent increase in both Ras and ERK1/2 activity [43]. Ligand-independent receptor activation is not the only mechanism by which oxygen radicals activate the ERK1/2 signaling pathway. ROS mediate activation of Ras independently of reactive oxygen intermediate-induced receptor activation [44]. Furthermore, Ras expression is not an absolute requirement for ROS activation of the ERK1/2 signaling pathway either. ROS will induce the activation of the ERK1/2 signaling pathway in Ras negative cells [45]. This may occur in part via c-Src as c-Src can phosphorylate and activate phospholipase C (PLC)-gamma [46]. Activation of PLC-gamma results in the generation of diacyl-glycerol (DAG) and increases in intracellular calcium which in turn induce activation of several forms of PKC. Although PKC can lead to Ras activation it has also been shown to directly activate Raf [47]. Furthermore, ROS inhibit protein phosphatases [48–49] and inhibition of phosphatase activity results the activation of the ERK1/2 signaling pathways [50]. Thus, it would appear that the ERK1/2 kinase signaling cascade can be activated at multiple points by reactive oxygen species. It should be noted, however, that the MEK1 and 2 inhibitors U0126 and PD98059 both block oxidative stress-induced ERK1/2 activation [51–52] indicating that activating actions of oxidative stress do not occur directly on ERK1/2 but instead are localized at upstream targets.
Hydrogen peroxide was shown to stimulate the activation of the ERK family member ERK5/Big Map Kinase (BMK) in human skin fibroblasts, human vascular smooth muscle cells, and human umbilical vein endothelial cells [53]. In PC12 cells, hydrogen peroxide-induced ERK5/BMK1 activation requires the activation of a Src kinase [54]. Superoxide anion may have a role in ERK5/BMK1 activation as superoxide scavengers prevented Angiotensin II- and endothelin-1-induced ERK5/BMK1 phosphorylation.
Reactive oxygen species such as, singlet oxygen [55–57], hydrogen peroxide [58], nitric oxide [44,59], and peroxynitrite [60] induce the activation of the stress activated or JNK and p38 pathways. Activation of the JNK pathway by reactive oxygen intermediates likely occurs through the apoptosis signal related kinase (ASK1) and MEKK1 [61–64]. Reduced thioredoxin [62–63] binds to the N-terminal of ASK1 and inhibits ASK1 activity. Upon an oxidative stress, thioredoxin becomes oxidized and disassociates from ASK1; allowing ASK1 to oligomerize, autophosphorylate, and become activated [64]. Reactive nitrogen containing species can have multiple effects on ASK1 activation. First, reactive nitrogen species are able to nitrosylate thioredoxin and prevent thioredoxin binding to ASK1 [65]. This mechanism favors the activation of ASK1. The mechanism by which reactive oxygen intermediates induce the p38 pathway very likely occurs via mechanisms similar to the JNK pathway (i.e. Ask1). Many of the same signals activate both pathways concurrently and in many of the same cell types [62,64].
Non-MEK/ERK Mediated Functions of Raf-1
Raf-1 has also been proposed to regulate apoptosis at the mitochondrial membrane [67]. Expression of membrane targeted Raf was shown to complement a BCR-ABL mutant in abrogating the cytokine-dependence of hematopoietic cells [66–68] (see Figure 2). In these mutant BCR-ABL transfected cells, Bad was expressed in the hyperphosphorylated inactive form and released from the mitochondria into the cytosol. In contrast, in the cells containing the BCR-ABL mutant but lacking the membrane-targeted Raf-1, which were not cytokine-dependent, Bad was hypophosphorylated and present in the mitochondrial fraction. BCR-ABL may interact with mitochondrial targeted Raf-1 to alter the phosphorylation of Bad at the mitochondrial membrane and hence regulate (prevent) apoptosis in hematopoietic cells containing the BCR-ABL chromosomal translocation [69]. This survival mechanism was independent of MEK and ERK.
Figure 2. MEK1/ERK Independent Effects of Raf-1.
Many other non MEK1/ERK mediated functions of Raf-1 have been postulated. Some of the purported roles (e.g., cell cycle regulatory, anti-apoptotic effects on NF-κB activation) may be more likely than others (e.g., effects of Raf-1 on p53 transcription). BCR-ABL can activate Raf-1 activity and thus have anti-apoptotic and cell cycle effects. Raf can activate the Cdc25 phosphatase which results in CDK activation.
Effects of Ras/Raf/MEK/ERK Signaling on Cell Cycle Progression
Ras and its downstream effectors alter the expression of many molecules which regulate cell cycle including p16Ink4a, p15Ink4b and p21Cip1, and can lead to premature cell cycle arrest at the G1 phase. This p15Ink4b/p16Ink4a or p21Cip1-mediated premature G1 arrest and subsequent senescence is dependent on the Raf/MEK/ERK pathway [9,70,71].
Overexpression of activated Raf proteins is associated with such divergent responses as cell growth, cell cycle arrest or even apoptosis [70,72,73]. The fate of the cells depend on the level and isoform of Raf kinase expressed. Ectopic overexpression of Raf proteins is associated with cell proliferation in cells including hematopoietic cells [74]; erythroid progenitor cells [75]; and A10 smooth muscle cells [76]. However, overexpression of activated Raf proteins is associated with cell cycle arrest in rat Schwann cells, mouse PC12 cells, human promyelocytic leukemia HL-60 cells, small cell lung cancer cell lines, prostate cancer LNCaP cells, and some hematopoietic cells [77–79]. Depending on the Raf isoform, overexpression of Raf can lead to cell proliferation (A-Raf or Raf-1) or cell growth arrest (B-Raf) in NIH-3T3 fibroblast and FDC-P1 hematopoietic cells [70–73,80–81]. It is not clear why overexpression of the Raf gene can lead to such conflicting results, but it has been suggested that the opposite outcomes may be determined by the amount or activity of the particular Raf oncoprotein [70,81].
NIH-3T3 cells have been transfected with the three different Raf genes. The introduced A-Raf molecule was able to upregulate the expression of cyclin D1, cyclin E, Cdk2, and Cdk4 and down-regulate the expression of Cdk inhibitor p27Kip1 [80]. These changes induced the cells to pass through G1 phase and enter S phase. It should be remembered that A-Raf is the weakest Raf kinase and its role in cell proliferation is not clear. However, in B-Raf and Raf-1 transfected NIH-3T3 cells, there was also a significant induction of p21Cip1, which led to G1 arrest. Using cytokine-dependent FDC-P1 hematopoietic cells transfected with conditionally-active mutant Raf-1, A-Raf and B-Raf genes as a model, we have demonstrated that moderate Raf activation, such as A-Raf and Raf-1, led to cell proliferation, which was associated with the induction of cyclin expression and Cdk activity. However, ectopic expression of the much more potent B-Raf led to apoptosis [70,81].
An alternative explanation for the diverse proliferative results obtained with the three Raf genes is the different biological effects of A-Raf, B-Raf, and Raf-1. The individual functions of these three different Raf proteins are not fully understood. Even though it has been shown that all three Raf proteins are activated by oncogenic Ras, target the same downstream molecules, i.e. MEK1 and MEK2, and use the same adapter protein for conformational stabilization; different biological and biochemical properties among them have been reported and their functions are not always compensatable [82–85]. It is safe to say that even as we learn more about the intricacies of these Raf molecules, we discover that there are more questions regarding their specificities and mechanisms by which they exert their effects on cell proliferation.
Regulation of Apoptosis by the Raf/MEK/ERK Cascade
Clearly Raf has many roles in kinase cascades and downstream transcription factors which regulate apoptosis. The Raf/MEK/ERK cascade and Raf by itself also have diverse effects on key molecules involved in the prevention of apoptosis. For many years now, it has been known that the Raf/MEK/ERK pathway can phosphorylate Bad on S112 which contributes to its inactivation and subsequent sequestration by 14-3-3 proteins [86]. This allows Bcl-2 to form homodimers and an anti-apoptotic response is generated. Activation of the Raf/MEK/ERK cascade can also result in the phosphorylation of the anti-apoptotic Mcl-1 protein and the pro-apoptotic Bim protein. Phosphorylation of Bim results in its disassociation from Bcl-2, Bcl-XL, and Mcl-1 and Bim becomes ubiquitionated and targeted to the proteosome. This allows Bcl-2, Bcl-XL and Mcl-1 to bind Bax and prevent Bax activation and the formation of Bax:Bax homodimers. Thus apoptosis is inhibited [87–89]. ERK phosphorylation of Bim on S69 can result in ubiquitination of Bim and subsequent proteosomal degradation [90]. Bim can also be phosphorylated by Akt at S87 and this also attenuates Bim’s apoptotic potential and promotes binding to 14-3-3 proteins [91]. In contrast, phosphorylation of Bim at S65 by JNK can result in apoptosis due to stimulation of Bax:Bax interactions [92–93]. JNK also phosphorylates 14-3-3 family members which allow translocation of Bax from the cytosol to the mitochondria membrane where it can promote apoptosis [94]. Clearly depending upon the particular residues that Bim is phosphorylated can influence whether a cell undergoes apoptosis or survives.
Recently it has been shown that the Raf/MEK/ERK cascade can phosphorylate caspase 9 on residue T125 which contributes to the inactivation of this protein [95]. Interesting, both Bad and caspase 9 are also phosphorylated, by the Akt pathway indicating that the Raf/MEK/ERK and PI3K/Akt pathways can cross-talk and result in the prevention of apoptosis [96]. However, the significance of caspase 9 phosphorylation by Akt is controversial, as this phosphorylation site is not evolutionally conserved. More controversially, Bcl-2 is also phosphorylated by the Raf/MEK/ERK cascade on certain residues, in the loop region, which has been associated with enhanced anti-apoptotic activity [97–98]. As noted earlier, Raf-1 has MEK- and ERK-independent functions at the mitochondrial membrane by phosphorylating Bad, which results in its disassociation from the mitochondrial membrane.
Recently Raf-1 was shown to interact with mammalian sterile 20-like kinase (MST-2) and prevent its dimerization and activation [99]. MST-2 is a kinase, which is activated by pro-apoptotic agents such as staurosporine and Fas ligand. Raf-1 but not B-Raf binds MST-2. Depletion of MST-2 from Raf-1−/− cells abrogated sensitivity to apoptosis. Overexpression of MST-2 increased sensitivity to apoptosis. It was proposed that Raf-1 might control MST-2 by sequestering it into an inactive complex. This complex of Raf-1:MST-2 is independent of MEK and downstream ERK. Raf-1 can also interact with the ASK1 to inhibit apoptosis [100]. ASK1 is a general mediator of apoptosis and it is induced in response to a variety of cytotoxic stresses including TNF, Fas and ROS. ASK1 appears to be involved in the activation of the JNK and p38 MAP kinases. This is another interaction of Raf-1 which is independent of MEK and ERK.
Raf-Induced Autocrine and Paracrine Growth Factor Expression
A common feature of cells transformed by Raf is the expression of growth factors, which often have autocrine effects. NIH-3T3 cells transformed by activated Raf secrete heparin binding epidermal growth factor (hbEGF) [101]. Hematopoietic cells transformed by activated Raf genes often express granulocyte macrophage-colony stimulating factor (GM-CSF), which has autocrine growth factor effects [72,102–104]. Kaposi’s sarcoma transformed B cells, which express elevated levels of B-Raf, express high levels of vascular endothelial growth factor (VEGF) [105]. Recently it has been shown that B-Raf increases the infectivity of Kaposi’s Sarcoma Virus [106]. One mechanism responsible for this enhancement of viral infection by B-Raf is its ability to induce VEGF expression [107–109]. Many growth factor genes contain in their promoter regions binding sites for transcription factors phosphorylated by the Raf/MEK/ERK pathway [11]. Thus aberrant Raf expression may establish an autocrine loop, which results in the continuous stimulation of cell growth. Alternatively, the VEGF expression induced by Raf can promote angiogenesis. Raf-induced growth factor expression will contribute to both the prevention of apoptosis as well as chemotherapeutic drug resistance as growth factor expression has been associated with both the prevention of apoptosis and drug resistance [110].
The PI3K/PTEN/Akt Pathway and its Role in Regulating Raf/MEK/ERK
After ligand induced activation of specific receptors, PI3K can be activated by two mechanisms. First, a phosphorylated Y residue on the receptor serves as a docking site for the p85 regulatory subunit of PI3K [111]. This recruits the catalytic subunit of PI3K, p110, to this complex. Alternatively, upon activation of the cytokine receptor by the appropriate ligand, the Shc protein binds the receptor to enable the Grb-2 and Sos proteins to form a complex which results in the activation of Ras. Ras is then able to induce the membrane translocation and activation of the p110 subunit of PI3K.
Activated PI3K converts phosphatidylinositol 4,5 biphosphate (PIP2) into phosphatidylinositol 3,4,5 phosphate (PIP3) which results in the membrane localization of phosphoinositol-dependent kinase-1 (PDK1) via its pleckstrin homology (PH) domain. Akt is also recruited to the lipid-rich plasma membrane by its PH domain and is phosphorylated at residues by T308 and S473 by PDK1 and an unidentified kinase, respectively. An overview of the PI3K/Akt pathway is presented in Figure 3.
Figure 3. Overview of the PI3K/Akt Pathway.
The PI3K/Akt pathway is activated either by the p85 PI3K regulatory subunit binding to an activated tyrosine residue on the activated growth factor receptor or via interaction with Ras. Both result in the membrane localization of PI3K. Some of the downstream effects of activation of PI3K pathway are shown. PIP2 is phosphorylated by PI3K to create PIP3 which promotes membrane localization of PDK1 through its PH domain. PDK1 then phosphorylates and activates Akt. Proteins activated by S/T phosphorylation induced by the PI3K/Akt pathway are indicated by a clear circle with a black P. Proteins inactivated by S/T phosphorylation induced by the PI3K/Akt pathway are shown in black circles with white P. Note that depending on the phosphorylation residue, some proteins can either be activated or inactivated by phosphorylation. Transcription factors are indicated by diamonds are similarly marked. Phosphatases are indicated in squares and those which inhibit activity are indicated in black rectangles (PTEN & SHIP) while the PP2A phosphatase which activates Raf is shown in a white rectangle. Arrows from phosphatases to the regulatory phosphates are indicated in open arrows.
Akt is the primary mediator of PI3K-initiated signaling; it has a number of downstream substrates. Among these targets are: Bad, Bim, procaspase-9, IκKalpha, the forkhead family of transcription factors (FKHR/AFX now called FOXO3a), GSK-3β, MDM-2, p21CIP1, p27KIP1 and others [91,111–112]. It is worth noting that Akt can cause the activation of specific substrates (e.g., IκKalpha and CREB) or may mediate the inactivation of other proteins (e.g., Raf, B-Raf [by the Akt related kinase SGK], p21Cip-1, Bim, Bad, procaspase-9, FOXO3a, and GSK-3β).
In addition, this pathway also includes phosphatases that serve to negatively regulate the growth-promoting effects of PI3K activity. The phosphatases PTEN and SHIP-1/2 can remove the phosphates from PIP3 [113]. Mutations in these phosphatases, which eliminate their activity, can lead to tumor progression. Consequently, the genes encoding these phosphatases are referred to as anti-oncogenes or tumor suppressor genes.
Ras/Raf/MEK/ERK and Human Cancer
Amplification of ras proto-oncogenes and activating mutations that lead to the expression of constitutively-active Ras proteins are observed in approximately 30% of human cancers [114–115]. B-Raf has been reported to be mutated in approximately 7% of all cancers [2]. However, this frequency may change as more and diverse tumors are examined for B-Raf mutation. Recent studies indicated that mutated alleles of Raf-1 are present in therapy induced acute myelogenous leukemia (t-AML) [116]. These leukemias arose after chemotherapeutic treatment of breast cancer patients. The mutated Raf-1 genes detected were transmitted in the germ line, thus they are not a spontaneous mutation in the leukemia but may be associated with the susceptibility to induction of t-AML in these Austrian breast cancer patients.
For many years, the Raf oncogenes were not thought to be frequently mutated in human cancer and more attention to abnormal activation of this pathway was dedicated to Ras mutations which can regulate both the Raf/MEK/ERK and PI3K/Akt pathways. However, recently it was shown that B-Raf is frequently mutated in certain types of cancer, especially melanoma (27 to 70%), papillary thyroid cancer (36 to 53%), colorectal cancer (5 to 22%) and ovarian cancer (30%) [2,117–119]. The reasons for mutation at B-Raf and not Raf-1 or A-Raf in melanoma patients are not entirely clear. Based on the mechanism of activation of B-Raf, it may be easier to select for B-Raf than either Raf-1 or A-Raf mutations. As stated previously, activation of B-Raf would require one genetic mutation whereas activation of either Raf-1 or A-Raf needs two genetic events. It has been proposed recently that the structure of B-Raf, Raf-1 and A-Raf may dictate the ability of activating mutations to occur at these molecules, which can permit the selection of oncogenic forms [2,119–120]. These predictions have arisen from determining the crystal structure of B-Raf [120]. Like many enzymes, B-Raf is proposed to have small and large lobes, which are separated, by a catalytic cleft. The structural and catalytic domains of B-Raf and the importance of the size and positioning of the small lobe may be critical in its ability to be stabilized by certain activating mutations. In contrast, the precise substitutions in A-Raf and Raf-1 are not predicted to result in small lobe stabilization thus preventing the selection of mutations at A-Raf and Raf-1, which would result in activated oncogenes [120]. Raf-1 has been known for years to interact with Hsp90. Hsp90 may stabilize activated Raf-1, B-Raf and A-Raf. The role that Hsp90 plays in selection of activated Raf mutations is highly speculative yet very intriguing.
The most common B-Raf mutation is a change at nucleotide 600 which converts a valine to a glutamic acid (V600E) [2]. This B-Raf mutation accounts for over 90% of the B-Raf mutations found in melanoma and thyroid cancer. It has been proposed that B-Raf mutations may occur in certain cells, which express high levels of B-Raf due to hormonal stimulation. Certain hormonal signaling events will elevate intracellular cAMP levels, which result in B-Raf activation, leading to proliferation. Melanocytes and thyrocytes are two such cell types, which have elevated B-Raf expression as they are often stimulated by the appropriate hormones [121]. Moreover, it is now thought that B-Raf is the most important kinase in the Raf/MEK/ERK cascade [2], thus mutation at B-Raf activates downstream MEK and ERK. In some models wild-type and mutant B-Raf activates Raf-1, which in turn activates MEK and ERK [2,27–28].
In some cells, B-Raf mutations are believed to be initiating events but not sufficient for full-blown neoplastic transformation [122–123]. Moreover, there appears to be cases where certain B-Raf mutations (V600E) and Ras mutations are not permitted in the transformation process as they might result in hyperactivation of Raf/MEK/ERK signaling and expression, which leads to cell cycle arrest [117]. In contrast, there are other situations, which depend on the particular B-Raf mutation and require both B-Raf and Ras mutations for transformation. The B-Raf mutations in these cases result in weaker levels of B-Raf activity [117,123].
Different B-Raf mutations have been mapped to various regions of the B-Raf protein. However, the mutations at the aforementioned 600 residue in B-Raf appear to be the most common. This mutation (V600E) results in activation of B-Raf and downstream MEK and ERK. Some of the other B-Raf mutations are believed to result in B-Raf molecules with impaired B-Raf activity, which must signal through Raf-1 [2,28]. Heterodimerzation between B-Raf and Raf-1 may allow the defective B-Raf to activate Raf-1. Other mutations, such as D593V, may activate alternative signal transduction pathways [2].
It has been reported that a high frequency of acute myeloid leukemias (AML) and acute lymphocytic leukemias (ALL) (>50%) display constitutive activation of the Raf/MEK/ERK pathway in absence of any obvious genetic mutation [124–125]. While there may be some unidentified mutation at one component of the pathway or a phosphatase which regulates the activity of the pathway, the genetic nature of constitutive activation of the Raf/MEK/ERK pathway is unknown. Elevated expression of ERK in AMLs and ALLs is associated with a poor prognosis [125]. Raf and potentially more effective MEK inhibitors may prove useful in the treatment of a large percentage of AML and ALLs.
Aberrant PI3K Activity, Oncogenic Transformation and Drug Resistance
The relationship between dysregulated PI3K activity and the onset of cancer is well-documented. For example, a mutated version of the p85 subunit of PI3K was detected in a Hodgkin’s Lymphoma-derived cell line (CO) [126]. The PI3K p110 subunit gene PI3KCA is a frequently mutated gene in human cancer [127]. The PI3K is the predominant growth factor-activated pathway in LNCaP human prostate carcinoma cells [128]. Other reports directly implicate PI3K activity in a variety of human tumors including breast cancer [129], lung cancer [130], melanomas [131], and leukemia [132] among others. Further evidence supports the notion that Akt (a.k.a., protein kinase B, PKB), a downstream kinase of PI3K, is also heavily-involved in the malignant transformation of cells as well as the induction of hormonal resistance [112]. Activated Akt can affect the expression and regulation of the responses of hormone receptors and hence lead to ineffectiveness of hormone ablation therapies [133–135].
Activated Akt has been reported to be detected in over 50% of primary AML samples and detection of activated Akt is associated with a poor prognosis [125]. Furthermore, the Akt pathway has been shown to be involved in regulation of multidrug resistance protein-1 (MRP-1) and drug resistance in AML [136–139]. Taken together, these data endorse the substantial role that PI3K signaling plays in oncogenesis and drug resistance. Moreover, targeted inhibition of the central components of this pathway appears to be an excellent choice for future therapeutic approaches. It has been observed that overexpression of both the Raf/MEK/ERK and PI3K/Akt pathways in AML is associated with a worse prognosis than overexpression of a single pathway [125]. Thus the development of inhibitors which target both pathways or the formulation of combinations of inhibitors may prove effective in the treatment of certain cancers.
Raf and Chemotherapeutic Drug Resistance
In certain cancer types, expression of the Raf/MEK/ERK pathway will modulate the expression of drug pumps and anti-apoptotic molecules such as Bcl-2 [140–145]. We have observed that ectopic expression of Raf will increase the levels of both the Mdr-1 drug pump and the anti-apoptotic Bcl-2 protein in breast cancer cells [144–145]. This increased expression of Mdr-1 and Bcl-2 most likely occurs by a transcriptional mechanism by downstream target kinases of the Raf/MEK/ERK pathway inducing the phosphorylation of transcription factors which bind the promoter regions of Mdr-1 and Bcl-2 and stimulate transcription. The increased expression of Mdr-1 and Bcl-2 is associated with the drug resistance of these breast cancer cells.
Roles of the Raf/MEK/ERK and PI3K/Akt Pathways in Growth and Drug Resistance of Hematopoietic Cells
In certain hematopoietic cell lines (FDC-P1 and TF-1), their cytokine-dependence can be relieved by expression of activated Raf and MEK genes [30,72,102]. Their growth is dependent upon the presence of the activated oncogene as we have used conditional Raf (ΔRaf:ER) and MEK (ΔMEK1:ER) oncogenes to transform the cells to grow in the absence of the previously required cytokine which is normally essential for their growth and survival. This Raf/MEK dependency can be easily determined, as removal of β-estradiol, which activates the ΔRaf:ER or ΔMEK1:ER results in the induction of apoptosis. Using these models (ΔB-Raf:ER, ΔRaf-1:ER, ΔA-Raf:ER and ΔMEK1:ER), we have also determined that B-Raf, which is the strongest inducer of downstream MEK and ERK activation, is a potent inducer of cell cycle arrest and apoptosis, while Raf-1, A-Raf and MEK1 are less prone to induce cell cycle arrest [70,73]. These studies indicate the Raf genes can effect cell cycle progression and the prevention of apoptosis in hematopoietic cells. In contrast, activated and conditional Akt and PI3K constructs did not readily relieve the cytokine dependency of either FDC-P1 or TF-1 cells, however, introduction of a conditional Raf and an activated/conditional Akt or PI3K construct would increase the frequency of cytokine-independent cells 10- to 1000- fold depending upon the particular Raf/MEK and PI3K/Akt combination.
The effects of activated Raf/MEK and PI3K/Akt genes on the cytokine-dependence of FL5.12 cells was also examined [81,146]. The cytokine-dependence of FL5.12 cells is not readily relieved by Raf, MEK, PI3K or Akt activated proteins. However the cytokine dependence of FL5.12 cells is relieved when both pathways are activated in the cells, for example introduction of an activated Raf gene (ΔRaf-1:ER) and introduction of an activated Akt gene (ΔAkt:ER). These results indicate that these two pathways can interact to increase the proliferation of certain hematopoietic cells in the absence of exogenous cytokines.
We were also interested in determining the roles of the Raf/MEK/ERK and PI3K/Akt pathways on the induction of drug resistance in hematopoietic cells. To address this question we isolated doxorubicin and paclitaxel resistant hematopoietic cells by culturing cytokine-dependent FL5.12 cells in the presence of different concentrations of doxorubicin or paclitaxel in different cell/well concentrations (0.1 cell/well to 100,000 cells/well). Doxorubicin and paclitaxel resistant hematopoietic cells were determined to express elevated levels of activated ERK and were hypersensitive to Raf/MEK inhibitors. The drug resistant cells were resistant to the apoptosis inducing effects of doxorubicin and displayed a reduced caspase 3 activation. The role of MEK in the process was further examined by introduction of activated or dominant negative (DN) MEK genes into the cells. Activated MEK increased the resistance of the cells to doxorubicin by approximately 10-fold as determined by growth and apoptosis assays as well as the detection of cleaved caspase 3. In contrast, introduction of DN-MEK increased the sensitivity of the cells to doxorubicin.
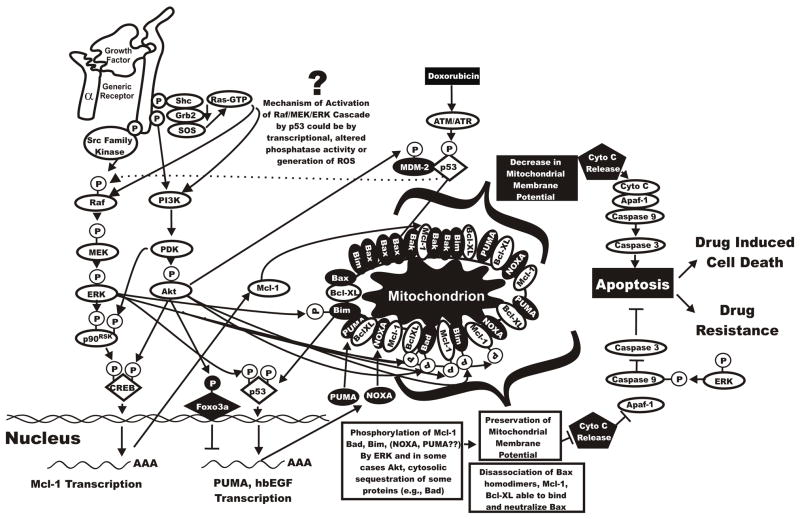
The drug resistant FL5.12 cells still activate p53 after doxorubicin treatment, suggesting that they retained a functional p53 circuit. Furthermore, the drug resistance of the cells could be increased by introduction of DN-p53 suggesting that inhibition of the normal functions of p53, increases drug resistance. A model for the roles of Raf/MEK/ERK, PI3K/Akt, and p53 in the induction of drug resistance is presented in Figure 4.
Figure 4. Interactions between Raf/MEK/ERK and Apoptotic Pathways Resulting in Drug Resistance.
Some of the potential downstream molecules activated by the Raf/MEK/ERK, PI3K/Akt and p53 and their effects on the induction of apoptosis are illustrated. The effects of Raf/MEK/ERK and PI3K/Akt on phosphorylation of apoptotic regulatory molecules which exert their effects on mitochondrial membrane potential and the prevention of apoptosis are indicated. Phosphorylation of these molecules by Raf/MEK/ERK and PI3K/Akt is associated with prevention of the caspase cascade and the induction of apoptosis. Activation of p53 by genotoxic stresses can result in the activation of PUMA and NOXA which can have pro-apoptotic effects. Activation of p53 can also result in transcription of hbEGF which would be predicted to have growth stimulatory effects. Drug resistance can result from Raf/MEK/ERK and PI3K/Akt pathways disrupting the balance between induction and prevention of apoptosis.
We have also developed a model for understanding the effects of Raf and Akt on the cytokine dependence and drug resistance of hematopoietic cells by infecting a hematopoietic cell line, FL5.12, with retroviruses encoding conditional Raf (ΔRaf-1:AR) and Akt (ΔAkt:ER*). The resulting cells (FL/ΔAkt:ER* +ΔRaf-1:AR) proliferate in the absence of exogenous cytokines when Raf is activated by testosterone and Akt is activated by tamoxifen. Testosterone binds the androgen receptor (AR) domain present in ΔRaf-1:AR and results in Raf activation. Tamoxifen binds the modified estrogen receptor (ER*, mutated to bind tamoxifen 100-fold more efficiently than β-estradiol) domain in ΔAkt:ER* which results in Akt activation. The IC50 for doxorubicin was increased 10-fold when Raf was activated as compared to when Akt was activated. Furthermore, in the drug resistant clones, Raf activation increased the IC50 for doxorubicin 80-fold compared to Akt activation. Thus Raf induced resistance to doxorubicin. Akt activation was not necessary for growth in small culture volumes (100 to 200 microliter cultures) over a 5 day incubation period, however, Akt activation was necessary in larger culture volumes (5 to 50 ml) as well as for prolonged time periods (>5 days). Growth of the cells in the presence of chemotherapeutic drugs was increased when both Raf and Akt were activated, implicating a role for Akt in the drug resistant phenotype.
The drug resistant phenotype in the FL/ΔAkt:ER* +ΔRaf-1:AR cells was not due to the lack of a p53 response as these cells displayed a prominent stabilization of p53 and downstream p21Cip-1 was induced after doxorubicin treatment. These clones displayed increased p27Kip-1 (a cyclin dependent kinase [cdk] inhibitor) expression compared to the non-drug resistant cells. The mechanism by which Raf-1 induces the drug resistance of these cells is being further determined. Raf-1 may induce the phosphorylation of downstream targets which allow the cells to grow in the presence of chemotherapeutic drugs. These targets may be molecules involved in apoptotic pathways or may be transcription factors which control gene expression. Alternatively Raf-1 may induce the phosphorylation of proteins involved in centrosome amplification which may occur following genotoxic stress.
The Raf/MEK/ERK pathway may also induce the phosphorylation of Bcl-2 and other key molecules involved in the regulation of apoptosis. Certain phosphorylation events on Bcl-2 have been associated with prolonged activation [97], whereas other phosphorylation events on Bcl-2 induced by chemotherapeutic drugs such as paclitaxel have been associated with inactivation of Bcl-2 [142,147–148]. Likewise, phosphorylation of ERK is normally associated with a proliferative response. However, certain chemotherapeutic drugs such as paclitaxel can induce the phosphorylation of ERK at the same residues, which is associated with the prevention of apoptosis. There is an ever growing list of apoptotic regulatory molecules which are phosphorylated by the Raf/MEK/ERK and PI3K/Akt pathways. This list includes Bad, Bim, Mcl-1, Caspase 9 and Foxo3A. Foxo3A is mentioned as it is a transcription factor which can control the expression of genes such as Puma which regulates apoptosis through interactions with Mcl-1 and Bax/Bak. Phosphorylation of Foxo3A by Akt suppresses the ability of Foxo3A to induce the transcription of Puma and other genes.
Clearly phosphorylation of these molecules involved in apoptosis and transcription is complicated, as some phosphorylation events may stimulate activity while other phosphorylation events may inhibit activity. We know very little about the phosphatases which are responsible for removal of the phosphate groups added by Raf/MEK/ERK and PI3K/Akt and even less is known concerning the mechanisms which regulate the activity of these phosphatases. Clearly such information is very important, as many of the phosphatases which have been identified so far, also play important roles as tumor suppressor genes.
Expression of the Raf/MEK/ERK Pathway in Prostate Cancer
Increased expression of the Ras/Raf/MEK/ERK pathway has been associated with advanced prostate cancer, hormonal independence and a poor prognosis [149–153]. The mechanisms of activation of this cascade in prostate cancer have not been well established as mutation at Ras, Raf, MEK or ERK have not been frequently reported in prostate cancer, however, amplification of Ras has been detected in some prostate cancers. Alternatively, the Ras/Raf/MEK/ERK pathway could be induced by autocrine and paracrine acting growth factors in prostate cancer cells.
Interestingly, we observed that introduction of activated Raf genes (Raf-1 and B-Raf) did not increase the chemoresistance of prostate cancer cells, while introduction of activated Akt genes did increase their chemoresistance [154–155]. Some prostate cancer cell lines such as LNCaP and PC3 cells have PTEN mutations and express high levels of active Akt, and express low levels of active Raf/MEK/ERK pathway members. Hormonal independent advanced prostate cancer cell lines such as DU145 and PC3 tend to express low levels of activated Raf, MEK and ERK. Thus while it is logical to believe that activation of the Ras/Raf/MEK/ERK cascade could contribute to prostate cancer progression and hormonal independence, it may not be as simple as one thinks as some of these established prostate cancer lines express low levels of Raf/MEK/ERK.
Recently, a role for RKIP in prostate cancer was hypothesized. Certain advanced prostate cancers express lower amounts of RKIP than less malignant prostate cancer specimens [156–157]. Inhibition of RKIP expression makes certain prostate cancer cells more metastatic [156]. The mechanism responsible for this increase in metastasis is believed to be due to the enhanced activity of the Raf/MEK/ERK signaling pathways. RKIP is not thought to alter the tumorigenic properties of prostate cancer cells; rather it is thought to be a suppressor of metastasis and may function by decreasing vascular invasion [156].
The role of the Raf/MEK/ERK pathway in prostate cancer remains controversial. The studies with RKIP suggest that increasing Raf activity, by inhibition of RKIP after phosphorylation by PKC, is somehow linked with metastasis in prostate cancer [157]. However, the Raf/MEK/ERK pathway may be shut off in advanced prostate cancer due to the deletion of the PTEN gene, which normally regulates the activity of Akt by counterbalancing PI3K activity [158]. In some but not all cell lineages, Akt may inhibit Raf-1 activity by phosphorylation of Raf-1 on S259.
The expression of the Raf/MEK/ERK pathway may be decreased in some prostate cancer cell lines isolated from advanced prostate cancer patients by the deletion or inactivation of p53. p53 could influence the expression and activation of the Raf/MEK/ERK pathway by multiple mechanisms. For example, p53 could alter the expression of phosphatases which regulate the activity of Raf/MEK/ERK or stimulate the transcription of the Raf/MEK/ERK genes [159]. After DNA damage, p53 may activate the PAC1, DUSP5 or other phosphatases which serve to fine tune the Raf/MEK/ERK cascade. In contrast, after growth factor stimulation, p53 may induce map kinase phosphatase (MKP1) or other phosphatases which alter activity of the Raf/MEK/ERK cascade. Some events associated with phosphatases involve the removal of a phosphate group which results in inactivation of the protein, in contrast, some dephosphorylation events (e.g., removal of the phosphate from S259) of Raf-1 by PP2A contributed to activation of Raf-1.
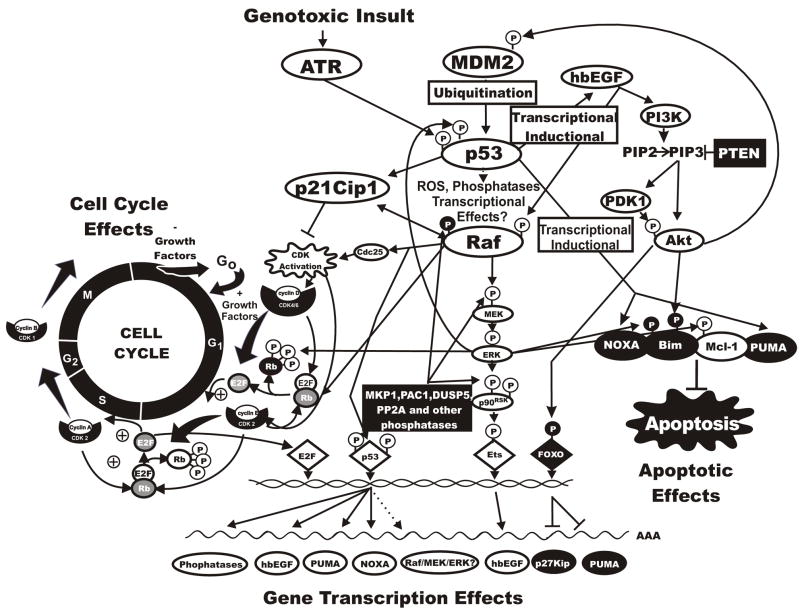
hbEGF has been observed to be a transcriptional target of p53. p53 can transactivate hbEGF which in turn activates both the Raf/MEK/ERK and PI3K/Akt pathways suggesting that p53 can activate survival pathways which affect the balance between life and death in response to genotoxic stresses [159–161]. As stated previously, Raf activates hbEGF expression in some cell types and the effects of this induction on p53 activity are poorly characterized. Furthermore, Akt can phosphorylate MDM-2 which in turn affects p53 activity. Clearly, the interactions between p53, and the Raf/MEK/ERK and PI3K/Akt are very complicated and likely feed-back upon one another. Some of the interactions between p53 and the Raf/MEK/ERK and PI3K/Akt pathways are illustrated in Figure 5.
Figure 5. Interactions Between p53 and the Raf/MEK/ERK and PI3K/Akt Pathways.
This figure summarizes some of the complex interactions between p53 and the Raf/MEK/ERK and PI3K/Akt pathways. These pathways are linked by complex interactions both directly and indirectly. These interactions can result in regulation of cell cycle progression as well as apoptosis. Dotted lines indicate indirect pathways.
We have observed low expression of the Raf/MEK/ERK pathway in certain prostate cancer cells (LNCaP and PC3), which often have overexpression of activated Akt due to deletion or mutation of the PTEN phosphatase [154–155]. Although the Raf/MEK/ERK genes are present in these cells and Raf/MEK/ERK can be induced upon treatment with certain chemotherapeutic drugs, their expression under normal growth conditions are very low. This may indicate that elevated Akt expression shuts off the Raf/MEK/ERK pathway, which may normally induce the cell cycle arrest or senescence of prostate cells (see Figure 6). The situation becomes even more complicated in advanced prostate cancer cells which have lost functional p53 activity. The expression of the Raf/MEK/ERK cascade is detected at lower levels in these cell lines (PC3 and DU145) than in prostate cancer lines that have both functional p53 and PTEN (22Rv1). Introduction of wild-type p53 into PC3 and DU145 cells increases both the sensitivity of the cells to chemotherapeutic drugs and expression and activation of the Raf/MEK/ERK cascade. Thus therapies aimed at increasing Raf/MEK/ERK activation might be more effective in the treatment of certain prostate cancers as they may induce terminal differentiation, senescence or cell cycle arrest of the cells [162–164]. In contrast, therapies aimed at decreasing Raf/MEK/ERK activity are likely to be more appropriate for the treatment of hematopoietic and breast malignancies where overexpression of this cascade is associated with proliferation and drug resistance.
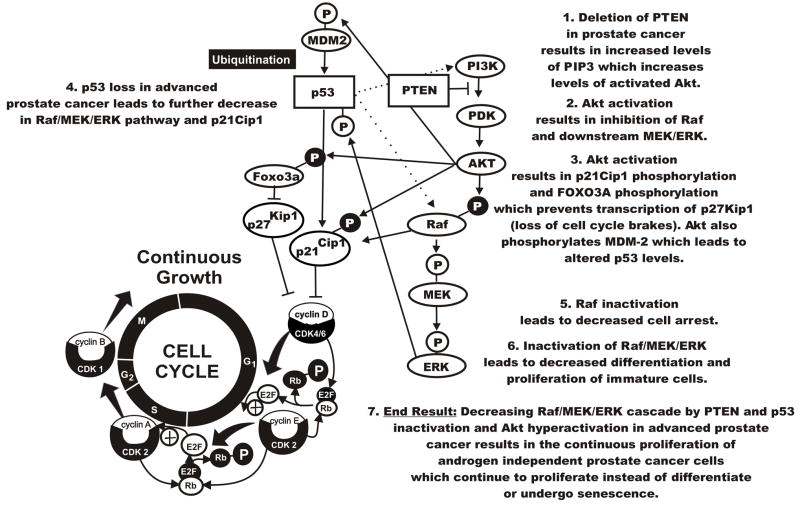
Figure 6. Effects of PTEN Deletion on PI3K/Akt and Raf/MEK/ERK Activation in Prostate Cancer.
PTEN deletion results in Akt activation. Akt activation can result in the phosphorylation and inactivation of Raf. This decrease in downstream MEK and ERK activation may lead to the loss of differentiation or senescence. Lack of Raf activation blocks p21Cip1 activation which may result in cell cycle progression. Akt activation can also result in the phosphorylation and inactivation of p21Cip1 and Foxo3a. Fox3a normally leads to the transcription of p27zlip1 and Puma which can act to inhibit cell cycle progression and promote apoptosis. Phosphorylated Foxo3a results in the suppression of p27kip1 and Puma transcription. Dotted lines indicate indirect pathways.
In some AML patients, MDM2 is overexpressed which enhances the tumorgencity and the loss of apoptotic processes resulting in a poor prognosis. When MDM2 ubiquitionates WT p53, it becomes targeted for degradation in the proteosome. p53 controls the transcription of many genes and is critical for the fate of the cells. Many of these targets are involved in p53-dependent apoptotic processes (e.g., Puma and Noxo). Recently, Andreeff and his group have observed that the small molecular weight, membrane permeable, Nutlins bind MDM2 and activate WT p53 and induce apoptosis. Therefore, by using this treatment, the role of p53 is enhanced and tumor growth is supressed. More studies need to be performed, but according to this group, Nutlins are a novel therapeutic approach in the treatment of AML and other chemorefractory conditions [165]. Thus targeting MDM2 with Nutlins may be a therapeutic approach in cancers which have WT p53.
Targeting the Raf/MEK/ERK Pathways for Therapeutic Intervention
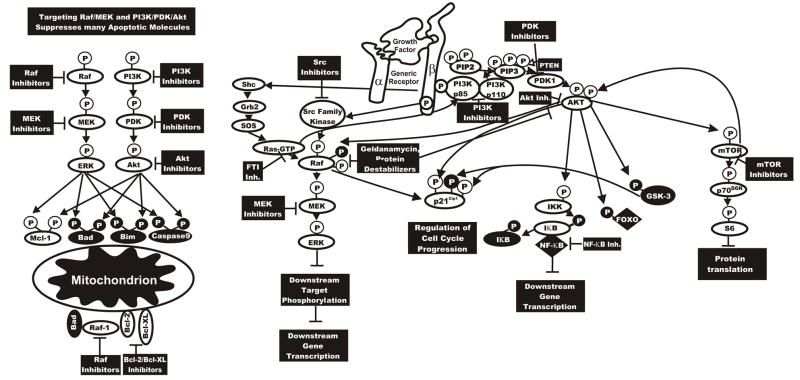
Raf inhibitors have been developed and some are being evaluated in clinical trials [104,166–168]. Certain Raf inhibitors have been developed which are small molecule competitive inhibitors of the ATP-binding site of Raf protein. These inhibitors (e.g., L-779,450, ZM 336372, Bay 43-9006) bind the Raf kinase domain and therefore prevent its activity. Some Raf inhibitors may affect a single Raf isoform (e.g., Raf-1), others may affect Raf proteins, which are more similar (Raf-1 and A-Raf), and while other pan Raf inhibitors may affect all three Raf proteins (Raf-1, A-Raf and B-Raf). We have observed that the L-779,450 inhibitor suppresses the effects of A-Raf and Raf-1 more than the effects of B-Raf [104]. Like many Raf inhibitors, L-779,450 is not specific for Raf; it also inhibits the closely related p38MAPK. Likewise, Bay 49-9006 inhibits other kinases besides Raf (e.g., VEGF-II Receptor, PDGF-R, Kit, Flt-3). Knowledge of the particular Raf gene mutated or overexpressed in certain tumors may provide critical information regarding how to treat the patient as some cancers which overexpress a particular Raf gene may be more sensitive to inhibition by agents which target that particular Raf protein. Inhibition of certain Raf genes might prove beneficial while suppression of other Raf genes under certain circumstances might prove detrimental. Thus the development of unique and broad-spectrum Raf inhibitors may prove useful in human cancer therapy. Some of the targets which are affected by signal transduction pathway inhibitors are illustrated in Figure 7.
Figure 7. Sites of Action of Small Molecular Weight Signal Transduction Pathway Inhibitors.
Potential sites of action of small molecular weight inhibitors are indicated. In some cases inhibitors will suppress growth, apoptotic and cell cycle regulatory pathways. This diagram serves to illustrate the concept that targeting Raf/MEK/ERK and PI3K/Akt can have dramatic effects on many growth regulatory molecules. Proteins inactivated by S/T phosphorylation induced by the PI3K/Akt pathway are shown in black circles with white P in a black circle.
Chaperonin proteins such as 14-3-3 and Hsp90 regulate Raf activity [13]. Raf activity is regulated by dimerization. These biochemical properties result in Raf activity being sensitive to drugs which block protein: protein interactions such as geldanamycin [169]. Geldanamycin and its 17-allylamino-17-demethoxy analog (17-AAG) are non-specific Raf inhibitors as they also affect the activity of many proteins which are stabilized by interaction with Hsp90. Geldanamycin and 17-AAG are currently in clinical trials [170]. We often think of a single Raf protein carrying out its biochemical activity. However, Raf isoforms dimerize with themselves and other Raf isoforms to become active. Drugs such as coumermycin, which inhibit Raf dimerization and others such as geldanmycin, which prevent interaction of Raf with Hsp90 and 14-3-3 proteins suppress Raf activity [13].
An alternative approach to targeting Raf is to prevent Raf activation by targeting kinases (e.g., Src, PKC, PKA, PAK or Akt) and phosphatases (e.g., PP2A) involved in Raf activation. As mentioned earlier, some Src kinase inhibitors such as Dastinib would be predicted to inhibit Raf activation as it should suppress Raf-1 activation by Src. It is worth noting that some of these kinases normally inhibit Raf activation (Akt and PKA). A major limitation of this approach would be that these kinases and phosphatases could result in activation or inactivation of other proteins and would have other effects on cell physiology.
Currently it is believed that MEK1 is not frequently mutated in human cancer. However, aberrant expression of MEK1 is observed in many different cancers due to the activation of the Raf/MEK/ERK pathway by upstream kinases (e.g., BCR-ABL) and growth factor receptors (e.g., EGFR) as well as other unknown mechanisms. Specific inhibitors to MEK have been developed (PD98059, U0126, PD184352 (a.k.a., CI1040), PD-0325901, Array MEK inhibitors [ARRY-142886 and others]). The successful development of MEK inhibitors may be due to the relatively few phosphorylation sites on MEK involved in activation/inactivation.
An advantage of targeting the Raf/MEK/ERK cascade is that it can be targeted without knowledge of the precise genetic mutation, which results in its aberrant activation. This is important as the nature of the critical mutation(s), which leads to the malignant growth of at least 50% of AMLs and other cancers, is not currently known. An advantage of targeting MEK is that Raf/MEK/ERK pathway is a convergence point where a number of upstream signaling pathways can be blocked with the inhibition of a single kinase (MEK).
To our knowledge, no small molecular weight ERK inhibitors have been developed yet, however, inhibitors to ERK could prove very useful as ERK can phosphorylate many targets (Rsk, c-Myc, Elk, and at least 150 more). There are at least 2 ERK molecules regulated by the Raf/MEK/ERK cascade, ERK1 and ERK2. Little is known about the different in vivo targets of ERK1 and ERK2. However ERK2 has been postulated to have pro-proliferative effects while ERK1 has anti-proliferative effects [171]. Development of specific inhibitors to ERK1 and ERK2 might eventually prove useful in the treatment of certain diseases.
Combination Therapies to Enhance Toxicity
An approach, which we have been investigating recently, is to determine whether inhibition of two signal transduction pathways is a more effective means to induce apoptosis than inhibiting a single signal transduction pathway. We have observed that inhibition of the Raf/MEK/ERK and PI3K/Akt pathways is usually a more effective means to induce apoptosis and synergy between the two inhibitors is often observed. Many transformed cells have elevated Raf/MEK/ERK and/or PI3K/Akt signaling. These two pathways play prominent roles in the promotion of growth and the prevention of apoptosis. The PI3K/Akt pathway may be inhibited with PI3K (LY294002, PX-866), PDK1 (OSU-03012, Celecoxib), Akt (A-443654) inhibitors or downstream mTOR inhibitors such as rapamycin and modified rapamycin (CCI-779). Initially mTOR inhibitors showed much promise as PTEN is often deleted in various tumors. However, it has been recently determined that the mTOR pathway has a complicated feed-back loop which actually involves suppression of Akt, hence mTOR inhibitors would be predicted to activate Akt in some cells. Recent evidence has highlighted that mTOR can also be activated by Raf/MEK/ERK [139,172–173]. This may well be another relevant cross-talk between the Ras/Raf/MEK/ERK and the PI3K/Akt pathways and might offer a further rationale for treatments combining drugs which inhibit both signaling networks.
In some cases, the precise gene responsible for driving proliferation of the malignant cell is known (e.g., BCR-ABL in CML, EGFR in some cases of non small cell lung cancer, FLT-3 in some AMLs and B-Raf in melanoma). Treatment of some of these diseases with specific kinase inhibitors is often effective; however, resistance to the inhibitors may develop due to further mutations in aberrant kinases which often prevent the signal transduction inhibitor from inhibiting the altered kinase. In these novel “drug resistant” cases, additional therapeutic approaches are necessary. In some of these cases, it may be possible to inhibit the drug resistant cells with combinations of MEK and PI3K/Akt inhibitors. We have observed that Imatinib resistant hematopoietic cells (which have mutated BCR-ABL kinase) are sensitive to MEK inhibitors. This result is not surprising as a Src inhibitor (Dasatinib) is being used to inhibit Imatinib resistant cells as they often have overexpression of an activated Src family kinase, such as Lyn, which likely acts by inducing the Raf/MEK/ERK cascade.
Classical chemotherapy often remains the most used anti-cancer therapy for many different types of cancer treatment. Drugs such as doxorubicin and taxol are effective in the treatment of many cancers, even though in some cases drug resistance does develop after prolonged treatment. Doxorubicin and taxol target cellular events such as DNA replication and cell division which are downstream of the targets of signal transduction pathway inhibitors. Thus by combining classical chemotherapy with targeted therapy, it may be possible to enhance toxicity while lowering the effective concentrations of classical chemotherapeutics necessary for effective elimination of the particular tumor.
We have investigated the effects of combining classical chemotherapy with signal transduction inhibitors in suppressing the growth of hematopoietic cells which grow in response to activated Raf and Akt or ErbB. Treatment of transformed cells with MEK or PI3K/Akt inhibitors with either doxorubicin or taxol resulted in a synergistic response documenting the effectiveness of classical chemotherapy with targeted therapy.
Role of the Raf/MEK/ERK Pathways in Drug Resistance to Reactive Oxygen Intermediate Inducing Cancer Treatments
Many cancer therapies induce the generation of oxygen radicals within cells. These therapies include treatments such as, chemotherapeutic drugs, irradiation, and newer treatments such as photodynamic therapy (PDT). Doxorubicin, one of the most effective chemotherapeutic drugs against a wide variety of cancers, works via two main mechanisms to exert anti-tumor effects and toxicity. Doxorubicin intercalates in the DNA and interferes with DNA polymerase with helicase activity [174]. It also induces the production of free radicals and oxidative stress which are involved in its anti-tumor effects [175–176]. The generation of oxygen radicals is important for the therapeutic effectiveness of doxorubicin because scavenging reactive oxygen intermediates results in decreased cell killing to this drug [177].
The initial reactive oxygen species generated as a consequence of ionization radiation is OH−, which is short-lived and only diffuses about 4 nm before reacting. Secondary reactive oxygen species, produced in response to ionizing radiation include O2− and H202. Studies with fluorescent dyes demonstrated generation of reactive oxygen species within cells within 15 minutes after irradiation [178]. Similar to doxorubicin the generation of oxygen radicals is important for the therapeutic effectiveness of radiation therapy because scavenging reactive oxygen intermediates results in decreased cell killing in response to radiation [179].
PDT is a three-component treatment that is used for the treatment of cancer [180]. PDT requires a photosensitizer, molecular oxygen and a laser of a wavelength matching the absorption spectrum of the photosensitizer (porphyrins and porphyrin-related compounds). When a porphyrin molecule absorbs light, it can transform an oxygen molecule to an activated state. Similar to doxorubicin and irradiation, PDT also requires the production of oxygen radicals to mediate some of its anti-tumor effects [181]. Thus, three well known cancer treatments result in the generation of reactive oxygen intermediates. These same three treatments have also been shown to lead to the activation of both ERK1,2 and ERK5 [182–185].
The Raf/MEK/ERK signaling pathway can play an adaptive role in protecting cells from oxidative stress [186]. In a non-malignant murine alveolar epithelial cell line, blocking MEK activation using the MEK inhibitor U0126 prevents hypoxia-induced Nrf2 up-regulation [186]. Deletion of ASK1 protects cells from oxidant-induced cell death but not death receptor-induced apoptosis [187]. Conversely, hydrogen peroxide is capable of inducing apoptosis in cardiomyocytes which can be increased in MEKK1 negative cells [188]. Deletion of ASK1 protects against hydrogen peroxide-induced apoptosis in fibroblasts and also prevents prolonged p38 activation, suggesting an apoptotic role for p38 in response to oxidative stress [189]. Ras activation and subsequent signaling via Rho can also activate this pathway as does ligation of the TNF receptor [190–193]. Redox activation of ERK5/BMK1 exhibits an anti-apoptotic effect [54]. U0126 and PD98059 are also reported to inhibit the activity of MEK5, the MAPKK involved in ERK5/BMK1 activation [194–196]. Suzuki et al. found that these inhibitors decreased PC12 cell viability in response to hydrogen peroxide treatment. This decrease in cell viability occurred when the ERK5/BMK1 protein was completely down-regulated using siRNA, suggesting that the effects of U0126 and PD98059 were mediated in part via ERK5/BMK1 pathway [54]. These data indicate the potential for both the ERK1/2 and ERK5/BMK pathways to promote treatment resistance to currently used reactive oxygen intermediate inducing cancer treatments.
Conclusions
Over the past 25 years, there has been much progress in elucidating the involvement of the Ras/Raf/MEK/ERK cascade in promoting normal cell growth as well as the etiology of human neoplasia and the induction of chemotherapeutic drug resistance. From initial seminal studies which elucidated the oncogenes present in avian and murine oncogenes, we learned that ErbB, Ras, Src, Abl, Raf, PI3K, Akt, Jun, Fos, Ets and NF-κB (Rel) were originally cellular genes which were captured by retroviruses. Biochemical studies defined and continue to elucidate the roles that these cellular and viral oncogenes had in cellular transformation. We have learned that many of these oncogenes are connected to the Ras/Raf/MEK/ERK and PI3K/PTEN/PDK/Akt pathways and either feed into this pathway (e.g., BCR-ABL, ErbB) or are downstream targets, which regulate gene expression (e.g., Jun, Fos, Ets, and NF-κB).
The Ras/Raf/MEK/ERK pathway has what often appears to be conflicting roles in cellular proliferation, differentiation, and the prevention of apoptosis. Classical studies have indicated that Ras/Raf/MEK/ERK can promote proliferation and malignant transformation in part due to the stimulation of cell growth and at the same time the prevention of apoptosis. Furthermore, an often overlooked aspect of Raf/MEK/ERK is the production of growth factors which can stimulate growth. The latest “hot” area of the Ras/Raf/MEK/ERK pathway is the discovery of mutation of the B-Raf gene in human cancer, which can promote proliferation and transformation [2]. However, it should be remembered that only a few years ago, hyperactivtion of B-Raf and Raf-1 was proposed to promote cell cycle arrest [70,79,81]. Thus it is probably fine-tuning of these mutations, which dictates whether there is cell cycle arrest or malignant transformation.
Initially it was thought that Raf-1 was the most important Raf isoform. Raf-1 was the earliest studied Raf isoform and homologous genes are present in both murine and avian transforming retroviruses. Originally it was shown that Raf-1 was ubiquitously expressed, indicating a more general and important role while B-Raf and A-Raf had more limited patterns of expression. However, it is now believed that not only B-Raf is the more important activator of the Raf/MEK/ERK cascade and in some cases, activation of Raf-1 may require B-Raf. However, Raf-1 rears its head again in the cancer field by the recent discovery that there are mutant Raf-1 alleles in certain therapy induced t-AMLs which are transmitted in a Mendelian fashion [116]. The role of A-Raf remains poorly defined yet it is an interesting isoform. It is the weakest Raf kinase, yet it can stimulate cell cycle progression and proliferation without having the negative effects on cell proliferation that B-Raf and Raf-1 can exert [70,81].
Activation of the Raf proteins is very complex as there are many phosphorylation sites on Raf. Phosphorylation at different sites can lead to either activation or inactivation. Clearly there are many kinases and phosphatases which regulate Raf activity and the state of phosphorylation will determine whether Raf is active or inactive. While the kinases involved in regulation in Raf/MEK/ERK have been extensively studied, we have only a very limited knowledge of the specific phosphatases involved in these regulatory events.
Raf-1 has many roles, which are apparently independent of downstream MEK/ERK. Some of these functions occur at the mitochondria and are intimately associated with the prevention of apoptosis. Raf-1 may function as a scaffolding molecule to inhibit the activity of kinases which promote apoptosis.
The Raf/MEK/ERK pathway is both positively (Hsp90, KSR, MP-1) and negatively (RKIP, 14-3-3) regulated by association with scaffolding proteins. The expression of some of the scaffolding proteins is altered in human cancer (e.g., RKIP) in some cases. Some of these scaffolding proteins (e.g., Hsp90) are being evaluated as potential therapeutic targets (geldanamycin). Potential roles of Hsp90 in stabilizing activated forms of Raf are intriguing and may allow the evolution of activated mutant forms of Raf.
The Raf/MEK/ERK pathway is intimately linked with the PI3K/PTEN/Akt pathway. Ras can regulate both pathways. Furthermore, in some cell types, Raf activity is negatively regulated by Akt indicating a cross-talk between the two pathways. Both pathways may result in the phosphorylation of many downstream targets and impose a role in the regulation of cell survival and proliferation. Interestingly, Ras and Raf mutations may not always result in similar outcomes. For example a Ras mutation would be predicted to activate both the Raf/MEK/ERK and PI3K pathways. Activation of PI3K/Akt could result in the suppression of Raf/MEK/ERK. However, mutation at either B-Raf or Raf-1 would result in only activation of Raf/MEK/ERK.
Although we often think of phosphorylation of these molecules as being associated with the prevention of apoptosis and the induction of gene transcription, this view is oversimplified. For example, in certain situations such as in advanced prostate cancer, the Raf/MEK/ERK pathway may be inhibited; hence the phosphorylation of Bad and CREB normally mediated by the Raf/MEK/ERK cascade, which is associated with the prevention of apoptosis, will be inhibited. Likewise it is important to remember that phosphorylation at certain protein residues will result in enhanced activity whereas phosphorylation at different residues will result in decreased activity. For example, phosphorylation of Bim by JNK is associated with the promotion of apoptosis while phosphorylation of Bim by Raf/MEK/ERK or PI3K/Akt pathways is associated with the prevention of apoptosis.
A consequence of diverse cancer therapies (chemotherapy, radiation therapy, photodynamic therapy) is the induction of the Raf/MEK/ERK pathway which may in some cases provide a survival function. The mechanism of induction of these pathways may be in part in response to the ROS generated by the different therapies. Thus in some cases it may be appropriate to combine these conventional therapies with small molecular weight inhibitors which target the Raf/MEK/ERK pathway.
Although it has been known for many years that the Raf/MEK/ERK pathway can effect cell cycle arrest, differentiation and senescence, these are probably some of the least studied research areas in the field due to the often cell lineage specific effects which must be evaluated in each cell type. An intriguing aspect of human cancer therapy is that in some cases stimulation of the Raf/MEK/ERK pathway may be desired to promote terminal differentiation, while in other types of malignant cancer cells which proliferate in response to Raf/MEK/ERK activity, inhibition of the Raf/MEK/ERK pathway may be desired to suppress proliferation. Thus we must be flexible in dealing with the Raf/MEK/ERK pathway. As we learn more, our conceptions continue to change.
Acknowledgments
JAM, LSS and RAF have been supported in part by a grant from the NIH (R01098195). AMM and CE were supported in part from a grant from Associazione Italiana Ricerca sul Cancro (AIRC Regional grants).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004a;18:189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- 2.Garnett MJ, Marais R. Guilty as charged: B-Raf is a human oncogene. Cancer Cell. 2004;6:313–319. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Peyssonnaux C, Provot S, Felder-Schmittbuhl MP, Calothy G, Eychéne A. Induction of postmitotic neuroretina cell proliferation by distinct Ras downstream signaling pathways. Mol Cell Biol. 2000;20:7068–7079. doi: 10.1128/mcb.20.19.7068-7079.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan J, Roy S, Apolloni A, Lane A, Hancock JF. Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. J Biol Chem. 1998;273:24052–24056. doi: 10.1074/jbc.273.37.24052. [DOI] [PubMed] [Google Scholar]
- 5.Luo Z, Tzivion G, Belshaw PJ, Vavvas D, Marshall M, Avruch J. Oligomerization activates c-Raf-1 through a Ras-dependent mechanism. Nature. 1996;383:181–185. doi: 10.1038/383181a0. [DOI] [PubMed] [Google Scholar]
- 6.Fabian DR, Daar IO, Morrison DK. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol Cell Biol. 1993;13:7170–7179. doi: 10.1128/mcb.13.11.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, Sedivy JM, Kolch W. Suppression of Raf-1 kinase activity and MAP kinase signaling by RKIP. Nature. 1999;401:173–177. doi: 10.1038/43686. [DOI] [PubMed] [Google Scholar]
- 8.Dhillon AS, Meikle S, Yazici Z, Eulitz M, Kolch W. Regulation of Raf-1 activation and signaling by dephosphosphorylation. EMBO J. 2002;21:64–71. doi: 10.1093/emboj/21.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blalock WL, Weinstein-Oppenheimer C, Chang F, Hoyle PE, Wang X-Y, Algate PA, Franklin RA, Oberhaus SM, Steelman LS, McCubrey JA. Signal transduction, cell cycle regulatory, and anti-apoptotic pathways regulated by IL-3 in hematopoietic cells: possible sites for intervention with anti-neoplastic drugs. Leukemia. 1999;13:1109–1166. doi: 10.1038/sj.leu.2401493. [DOI] [PubMed] [Google Scholar]
- 10.Lee JT, Jr, McCubrey JA. The Raf/MEK/ERK Signal Transduction Cascade as a Target for Chemotherapeutic Intervention. Leukemia. 2002;16:486–507. doi: 10.1038/sj.leu.2402460. [DOI] [PubMed] [Google Scholar]
- 11.Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003a;17:1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- 12.Fantl WJ, Muslin AJ, Kikuchi A, Martin JA, MacNicol AM, Gross RW, Williams LT. Activation of Raf-1 by 14-3-3 proteins. Nature. 1994;371:612–614. doi: 10.1038/371612a0. [DOI] [PubMed] [Google Scholar]
- 13.Blagosklonny MV. Hsp-90-associated oncoproteins: multiple targets of geldanamycin and its analogs. Leukemia. 2002;16:455–462. doi: 10.1038/sj.leu.2402415. [DOI] [PubMed] [Google Scholar]
- 14.Marais R, Light Y, Paterson HF, Mason CS, Marshall CJ. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272:4378–4783. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- 15.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O’Brien S, Nicalse C, Bleickardt E, Blackwood-Chirchir MA, Iver V, Chen TT, Huang F, Decillis AP, Sawyers CL. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 16.Schittenhelm MM, Shiraga S, Schroeder A, Corbin AS, Griffith D, Lee FY, Bokemeyer C, Deininger MW, Druker BJ, Heinrich MC. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66:473–481. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- 17.Mason CS, Springer CJ, Cooper RG, Superti-Furga G, Marshall CJ, Marais R. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 1999;18:2137–2148. doi: 10.1093/emboj/18.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz B, Barnard D, Filson A, MacDonald S, King A, Marshall M. Phosphorylation of Raf-1 serine 338-serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling. Mol Cell Biol. 1997;17:4509–4516. doi: 10.1128/mcb.17.8.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp UR. Protein kinase Cα activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 20.Barnard D, Diaz B, Clawson D, Marshall M. Oncogenes, growth factors and phorbol esters regulate Raf-1 through common mechanisms. Oncogene. 1998;17:1539–1547. doi: 10.1038/sj.onc.1202061. [DOI] [PubMed] [Google Scholar]
- 21.Rommel C, Clarke BA, Zimmermann S, Nuñez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 22.Guan KL, Figueroa C, Brtva TR, Zhu T, Taylor J, Barber TD, Vojtek AB. Negative regulation of the serine/threonine kinase B-Raf by Akt. J Biol Chem. 2000;275:27354–27359. doi: 10.1074/jbc.M004371200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang BH, Tang ED, Zhu T, Greenberg ME, Vojtek AB, Guan KL. Serum- and glucocorticoid-inducible kinase SGK phosphorylates and negatively regulates B-Raf. J Biol Chem. 2001;276:31620–31826. doi: 10.1074/jbc.M102808200. [DOI] [PubMed] [Google Scholar]
- 24.Corbit KC, Trakul N, Eves EM, Diaz B, Marshall MMR. Rosner. Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J Biol Chem. 2003;278:13061–13068. doi: 10.1074/jbc.M210015200. [DOI] [PubMed] [Google Scholar]
- 25.Mercer KE, Pritchard CA. Raf proteins and cancer: B-Raf is identified as a mutational target. Biochimica et Biophysica Acta. 2003;1653:25–40. doi: 10.1016/s0304-419x(03)00016-7. [DOI] [PubMed] [Google Scholar]
- 26.Brummer T, Shaw PE, Reth M, Misawa Y. Inducible gene deletion reveals different roles for B-Raf and Raf-1 in B-cell antigen receptor signalling. EMBO J. 2002;21:5611–5622. doi: 10.1093/emboj/cdf588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rushworth LK, Hindley AD, O’Neil E, Kolch W. Regulation and role of Raf-1.B-Raf heterodimerization. Mol Cell Biol. 2006;26:2262–2272. doi: 10.1128/MCB.26.6.2262-2272.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garnett MJ, Rana S, Paterson H, Barford D, Marais R. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol Cell. 2005;20:963–969. doi: 10.1016/j.molcel.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Alessi DR, Saito Y, Campbell DG, Cohen P, Sithanandam G, Rapp U, Ashworth A, Marshall CJ, Cowley S. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J. 1994;13:1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blalock WL, Pearce M, Steelman LS, Franklin RA, McCarthy SA, Cherwinski H, McMahon M, McCubrey JA. A conditionally-active form of MEK1 results in autocrine transformation of human and mouse hematopoietic cells. Oncogene. 2000;19:526–536. doi: 10.1038/sj.onc.1203337. [DOI] [PubMed] [Google Scholar]
- 31.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K. Differential regulation of IkappaB kinase alpha and beta by two upstream kinases, NF-kappaB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc Natl Acad Sci USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao O, Lee FS. Mitogen-activated protein kinase/ERK kinase kinases 2 and 3 activated nuclear factor-kappaB through IkappaB kinase-alpha and IkappaB kinase-beta. J Biol Chem. 1999;274:8355–8358. doi: 10.1074/jbc.274.13.8355. [DOI] [PubMed] [Google Scholar]
- 33.Pouyssegur J, Volmat V, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signaling. Biochem Pharm. 2002;64:755–763. doi: 10.1016/s0006-2952(02)01135-8. [DOI] [PubMed] [Google Scholar]
- 34.Jimenez LA, Zanella C, Fung H, Janssen YM, Vacek P, Charland C, Goldberg J, Mossman BT. Role of extracellular signal-regulated protein kinases in apoptosis by asbestos and H2O2. Am J Physiol. 1997;273:1029–1035. doi: 10.1152/ajplung.1997.273.5.L1029. [DOI] [PubMed] [Google Scholar]
- 35.Tournier C, Thomas G, Pierre J, Jacquemin C, Pierre M, Saunier B. Mediation by arachidonic acid metabolites of the H2O2-induced stimulation of mitogen-activated protein kinases (extracellular-signal-regulated kinase and c-Jun NH2-terminal kinase) Eur J Biochem. 1997;244:587–595. doi: 10.1111/j.1432-1033.1997.00587.x. [DOI] [PubMed] [Google Scholar]
- 36.Griffith CE, Zhang W, Wange RL. ZAP-70-dependent and -independent activation of Erk in Jurkat T cells. Differences in signaling induced by H2o2 and Cd3 cross-linking. J Biol Chem. 1998;273:10771–10776. doi: 10.1074/jbc.273.17.10771. [DOI] [PubMed] [Google Scholar]
- 37.Buder-Hoffmann S, Palmer C, Vacek P, Taatjes D, Mossman B. Different accumulation of activated extracellular signal-regulated kinases (ERK 1/2) and role in cell-cycle alterations by epidermal growth factor, hydrogen peroxide, or asbestos in pulmonary epithelial cells. Am J Respir Cell Mol Biol. 2001;24:405–413. doi: 10.1165/ajrcmb.24.4.4290. [DOI] [PubMed] [Google Scholar]
- 38.Kim BY, Han MJ, Chung AS. Effects of reactive oxygen species on proliferation of Chinese hamster lung fibroblast (V79) cells. Free Radic Biol Med. 2001;30:686–698. doi: 10.1016/s0891-5849(00)00514-1. [DOI] [PubMed] [Google Scholar]
- 39.Xiao L, Pimentel DR, Wang J, Singh K, Colucci WS, Sawyer DB. Role of reactive oxygen species and NAD(P)H oxidase in alpha(1)-adrenoceptor signaling in adult rat cardiac myocytes. Am J Physiol Cell Physiol. 2002;282:C926–934. doi: 10.1152/ajpcell.00254.2001. [DOI] [PubMed] [Google Scholar]
- 40.Blanc A, Pandey NR, Srivastava AK. Synchronous activation of ERK 1/2, p38mapk and PKB/Akt signaling by H2O2 in vascular smooth muscle cells: potential involvement in vascular disease (review) Int J Mol Med. 2003;11:229–234. [PubMed] [Google Scholar]
- 41.Usatyuk PV, Vepa S, Watkins T, He D, Parinandi NL, Natarajan V. Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid Redox Signal. 2003;5:723–730. doi: 10.1089/152308603770380025. [DOI] [PubMed] [Google Scholar]
- 42.Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, Moshage H. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol. 2005;12:12. doi: 10.1016/j.jhep.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 43.Knebel A, Rahmsdorf HJ, Ullrich A, Herrlich P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. Embo J. 1996;15:5314–5325. [PMC free article] [PubMed] [Google Scholar]
- 44.Lander HM, Milbank AJ, Tauras JM, Hajjar DP, Hempstead BL, Schwartz GD, Kraemer RT, Mirza UA, Chait BT, Burk SC, Quilliam LA. Redox regulation of cell signalling. Nature. 1996b;381:380–381. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 45.Zou Y, Komuro I, Yamazaki T, Aikawa R, Kudoh S, Shiojima I, Hiroi Y, Mizuno T, Yazaki Y. Protein kinase C, but not tyrosine kinases or Ras, plays a critical role in angiotensin II-induced activation of Raf-1 kinase and extracellular signal-regulated protein kinases in cardiac myocytes. J Biol Chem. 1996;271:33592–33597. doi: 10.1074/jbc.271.52.33592. [DOI] [PubMed] [Google Scholar]
- 46.Wang XT, McCullough KD, Wang XJ, Carpenter G, Holbrook NJ. Oxidative stress-induced phospholipase C-gamma 1 activation enhances cell survival. J Biol Chem. 2001;276:28364–28371. doi: 10.1074/jbc.M102693200. [DOI] [PubMed] [Google Scholar]
- 47.Buhl AM, Osawa S, Johnson GL. Mitogen-activated protein kinase activation requires two signal inputs from the human anaphylatoxin C5a receptor. J Biol Chem. 1995;270:19828–19832. doi: 10.1074/jbc.270.34.19828. [DOI] [PubMed] [Google Scholar]
- 48.Whisler RL, Goyette MA, Grants IS, Newhouse YG. Sublethal levels of oxidant stress stimulate multiple serine/threonine kinases and suppress protein phosphatases in Jurkat T cells. Arch Biochem Biophys. 1995;319:23–35. doi: 10.1006/abbi.1995.1263. [DOI] [PubMed] [Google Scholar]
- 49.Rao RK, Clayton LW. Regulation of protein phosphatase 2A by hydrogen peroxide and glutathionylation. Biochem Biophys Res Commun. 293:610–616. doi: 10.1016/S0006-291X(02)00268-1. [DOI] [PubMed] [Google Scholar]
- 50.Lee K, Esselman WJ. Inhibition of PTPs by H(2)O(2) regulates the activation of distinct MAPK pathways. Free Radic Biol Med. 2002;33:1121–1132. doi: 10.1016/s0891-5849(02)01000-6. [DOI] [PubMed] [Google Scholar]
- 51.Lee JS, Kim SY, Kwon CH, Kim YK. EGFR-dependent ERK activation triggers hydrogen peroxide-induced apoptosis in OK renal epithelial cells. Arch Toxicol. 2006;80:337–346. doi: 10.1007/s00204-005-0052-2. [DOI] [PubMed] [Google Scholar]
- 52.Lee WC, Choi CH, Cha SH, Oh HL, Kim YK. Role of ERK in hydrogen peroxide-induced cell death of human glioma cells. Neurochem Res. 2005b;30:263–270. doi: 10.1007/s11064-005-2449-y. [DOI] [PubMed] [Google Scholar]
- 53.Abe J, Kusuhara M, Ulevitch RJ, Berk BC, Lee JD. Big mitogen-activated protein kinase 1 BMK1 is a redox-sensitive kinase. J Biol Chem. 1996;271:16586–16590. doi: 10.1074/jbc.271.28.16586. [DOI] [PubMed] [Google Scholar]
- 54.Suzaki Y, Yoshizumi M, Kagami S, Koyama AH, Taketani Y, Houchi H, Tsuchiya K, Takeda E, Tamaki T. Hydrogen peroxide stimulates c-Src-mediated big mitogen-activated protein kinase 1 (BMK1) and the MEF2C signaling pathway in PC12 cells: potential role in cell survival following oxidative insults. J Biol Chem. 2002;277:9614–9621. doi: 10.1074/jbc.M111790200. [DOI] [PubMed] [Google Scholar]
- 55.Klotz LO, Pellieux C, Briviba K, Pierlot C, Aubry JM, Sies H. Mitogen-activated protein kinase (p38-, JNK-, ERK-) activation pattern induced by extracellular and intracellular singlet oxygen and UVA. Eur J Biochem. 1999;260:917–922. doi: 10.1046/j.1432-1327.1999.00255.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhuang S, Demirs JT, Kochevar IE. p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J Biol Chem. 2000;275:25939–25948. doi: 10.1074/jbc.M001185200. [DOI] [PubMed] [Google Scholar]
- 57.Cheng A, Chan SL, Milhavet O, Wang S, Mattson MP. p38 MAP kinase mediates nitric oxide-induced apoptosis of neural progenitor cells. J Biol Chem. 2001;276:43320–43327. doi: 10.1074/jbc.M107698200. [DOI] [PubMed] [Google Scholar]
- 58.Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 59.Lander HM, Jacovina AT, Davis RJ, Tauras JM. Differential activation of mitogen-activated protein kinases by nitric oxide-related species. J Biol Chem. 1996a;271:19705–19709. doi: 10.1074/jbc.271.33.19705. [DOI] [PubMed] [Google Scholar]
- 60.Schieke SM, Briviba K, Klotz LO, Sies H. Activation pattern of mitogen-activated protein kinases elicited by peroxynitrite: attenuation by selenite supplementation. FEBS Lett. 1999;448:301–303. doi: 10.1016/s0014-5793(99)00372-5. [DOI] [PubMed] [Google Scholar]
- 61.Janssen-Heininger YM, Macara I, Mossman BT. Cooperativity between oxidants and tumor necrosis factor in the activation of nuclear factor (NF)-kappaB: requirement of Ras/mitogen-activated protein kinases in the activation of NF-kappaB by oxidants. Am J Respir Cell Mol Biol. 1999;20:942–952. doi: 10.1165/ajrcmb.20.5.3452. [DOI] [PubMed] [Google Scholar]
- 62.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. Embo J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheikh MS, Antinore MJ, Huang Y, Fornace AJ., Jr Ultraviolet-irradiation-induced apoptosis is mediated via ligand independent activation of tumor necrosis factor receptor 1. Oncogene. 1998;17:2555–2563. doi: 10.1038/sj.onc.1202292. [DOI] [PubMed] [Google Scholar]
- 64.Matsukawa J, Matsuzawa A, Takeda K, Ichijo H. The ASK1-MAP kinase cascades in mammalian stress response. J Biochem (Tokyo) 2004;136:261–265. doi: 10.1093/jb/mvh134. [DOI] [PubMed] [Google Scholar]
- 65.Yasinska IM, Kozhukhar AV, Sumbayev VV. S-nitrosation of thioredoxin in the nitrogen monoxide/superoxide system activates apoptosis signal-regulating kinase 1. Arch Biochem Biophys. 2004;428:198–203. doi: 10.1016/j.abb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 66.Hindley A, Kolch W. Extracellular signal regulated kinase (ERK)/mitogen activated protein kinase (MAPK)-independent functions of Raf kinases. J Cell Sci. 2002;115:1575–1581. doi: 10.1242/jcs.115.8.1575. [DOI] [PubMed] [Google Scholar]
- 67.Wang HG, Rapp UR, Reed JC. Bcl-2 targets the protein kinase Raf-1 to mitochondrin. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 68.Salomoni P, Wasik MA, Riedel RF, Reiss K, Choi JK, Skorski T, Calabretta B. Expression of constitutively active Raf-1 in the mitochondria restores antiapoptotic and leukemogenic potential of a transformation-deficient BCR/ABL mutant. J Exp Med. 1998;187:1995–2007. doi: 10.1084/jem.187.12.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neshat MS, Raitano AB, Wang HG, Reed JC, Sawyers CL. The survival function of the Bcr-Abl oncogene is mediated by Bad-dependent and -independent pathways: roles for phosphatidylinositol 3-kinase and Raf. Mol Cell Biol. 2000;20:1179–1186. doi: 10.1128/mcb.20.4.1179-1186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang F, McCubrey JA. P21Cip1 induced by Raf is associated with increased Cdk4 activity in hematopoietic cells. Oncogene. 2001;20:4354–4364. doi: 10.1038/sj.onc.1204564. [DOI] [PubMed] [Google Scholar]
- 71.Malumbres M, Castro IPD, Hernández MI, Jiménez M, Corral T, Pellicer A. Cellular response to oncogenic ras involves induction of the Cdk4 and Cdk6 inhibitor p15INK4b. Mol Cell Biol. 2000;20:2915–2925. doi: 10.1128/mcb.20.8.2915-2925.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoyle PE, Moye PW, Steelman LS, Blalock WL, Franklin RA, Pearce M, Cherwinski H, Bosch E, McMahon M, McCubrey JA. Differential abilities of the Raf family of protein kinases to abrogate cytokine dependency and prevent apoptosis in murine hematopoietic cells by a MEK1-dependent mechanism. Leukemia. 2000;14:642–656. doi: 10.1038/sj.leu.2401720. [DOI] [PubMed] [Google Scholar]
- 73.Chang F, Steelman LS, McCubrey JA. Raf-induced cell cycle progression in human TF-1 hematopoietic cells. Cell Cycle. 2002;1:220–226. [PubMed] [Google Scholar]
- 74.McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D’Assoro AB, Salisbury JL, Mazzarino MC, Stivala F, Libra M. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006 doi: 10.1016/j.advenzreg.2006.01.004. July Online. In Press. [DOI] [PubMed] [Google Scholar]
- 75.Sanders MR, Lu H, Walker F, Sorbad S, Dainiak N. The Raf-1 protein mediates insulin-like growth factor-induced proliferation of erythroid progenitor cells. Stem Cells. 1998;16:200–207. doi: 10.1002/stem.160200. [DOI] [PubMed] [Google Scholar]
- 76.Cioffi CL, Garay M, Johnston JF, McGraw K, Boggs RT, Hreniuk BD, Monia BP. Selective inhibition of A-Raf and C-Raf mRNA expression by antisense oligodeoxynucleotides in Rat vascular smooth muscle cells: role of A-Raf and C-Raf in serum-induced proliferation. Mol Pharmacol. 1997;51:383–389. [PubMed] [Google Scholar]
- 77.Yen A, Williams M, Platko JD, Der C, Hisaka M. Expression of activated Raf accelerates cell differentiation and RB protein down-regulation but not hypophosphorylation. Eur J Cell Biol. 1994;65:103–113. [PubMed] [Google Scholar]
- 78.Ravi RK, Weber E, McMahon M, Williams JR, Baylin S, Mal A, Harter ML, Dillehay LE, Claudio PP, Giordano A, Nelkin BD, Mabry M. Activated Raf-1 causes cell cycle arrest in small cell lung cancer cells. J Clin Invest. 1998;101:153–159. doi: 10.1172/JCI831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lloyd AC, Obermuller F, Staddon S, Barth CF, McMahon M, Land H. Cooperating oncogenes converge to regulate cyclin/Cdk complexes. Genes Devel. 1997;11:663–677. doi: 10.1101/gad.11.5.663. [DOI] [PubMed] [Google Scholar]
- 80.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip. Mol Cell Biol. 1997;19:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shelton JG, Chang F, Lee JT, Franklin RA, Steelman LS, McCubrey JA. B-Raf and insulin synergistically prevent apoptosis and induce cell cycle progression in hematopoietic cells. Cell Cycle. 2004;3:189–196. [PubMed] [Google Scholar]
- 82.Wadewitz AG, Winer MA, Wolgemuth DJ. Developmental and cell lineage specificity of raf family gene expression in mouse. Oncogene. 1993;8:1055–1062. [PubMed] [Google Scholar]
- 83.Pritchard CA, Bolin L, Slattery R, Murray R, McMahon M. Post-natal lethality and neurological and gastrointestinal defects in mice with targeted disruption of the A-Raf protein kinase gene. Curr Biol. 1996;6:614–617. doi: 10.1016/s0960-9822(02)00548-1. [DOI] [PubMed] [Google Scholar]
- 84.Wojnowski L, Zimmer AM, Bec TW, Hahn H, Bernal R, Rapp UR, Zimmer A. Endothelial apoptosis in Braf-deficient mice. Nat Genet. 1997;16:293–297. doi: 10.1038/ng0797-293. [DOI] [PubMed] [Google Scholar]
- 85.Kolch W. To be or not to be: a question of B-Raf. Trends Neurosci. 2001;21:498–500. doi: 10.1016/s0166-2236(00)01889-0. [DOI] [PubMed] [Google Scholar]
- 86.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not Bcl-XL. Cell. 1996;87:589–592. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 87.Weston CR, Balmanno K, Chalmers C, Hadfield K, Molton SA, Ley R, Wagner EF, Cook SJ. Activation of ERK1/2 by deltaRaf-1:ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene. 2003;22:128–1293. doi: 10.1038/sj.onc.1206261. [DOI] [PubMed] [Google Scholar]
- 88.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein. Bim, J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 89.Harada H, Quearry B, Ruiz-Vela A, Korsmeyer SJ. Survival factor-induced extracellar signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc Natl Acad Sci USA. 2004;101:15313–15317. doi: 10.1073/pnas.0406837101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, Auberger P. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 91.Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL, apoptotic function. J Biol Chem. 2005;281:813–823. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- 92.Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, Alix S, Youle RJ, LaMarche A, Maroney AC, Johnson EM., Jr JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 93.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Okuno S, Saito A, Hayashi T, Chan PH. The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J Neurosci. 2004;24:7879–7887. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nature Cell Biol. 2003;7:647–654. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- 96.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase 9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 97.Deng X, Ruvolo P, Carr B, May WS., Jr Survival function of ERK1/2 as an IL-3 activated, staurosporine-resistant Bcl2 kinases. Proc Nat Acad USA. 2000;97:1578–1583. doi: 10.1073/pnas.97.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deng X, Kornblau SM, Ruvulo PP, May WS., Jr Regulation of Bcl2 phosphorylation and potential significance for leukemic cell chemoresistance. JNCI Monographs. 2001;28:30–37. doi: 10.1093/oxfordjournals.jncimonographs.a024254. [DOI] [PubMed] [Google Scholar]
- 99.O’Neill E, Rushworth L, Baccarini M, Kolch W. Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. 2004;306:2267–2270. doi: 10.1126/science.1103233. [DOI] [PubMed] [Google Scholar]
- 100.Du J, Cai SH, Shi Z, Nagase F. Binding activity of H-Ras is necessary for in vivo inhibition of ASK1 activity. Cell Research. 2004;14:148–154. doi: 10.1038/sj.cr.7290214. [DOI] [PubMed] [Google Scholar]
- 101.McCarthy SA, Samuels ML, Pritchard CA, Abraham JA, McMahon M. Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Genes Dev. 1995;9:1953–1964. doi: 10.1101/gad.9.16.1953. [DOI] [PubMed] [Google Scholar]
- 102.McCubrey JA, Steelman LS, Hoyle PE, Blalock WL, Weinstein-Oppenheimer C, Franklin RA, Cherwinski H, Bosch E, McMahon M. Differential abilities of activated Raf oncoproteins to abrogate cytokine-dependency, prevent apoptosis and induce autocrine growth factor synthesis in human hematopoietic cells. Leukemia. 1998;12:1903–1929. doi: 10.1038/sj.leu.2401215. [DOI] [PubMed] [Google Scholar]
- 103.McCubrey JA, Lee JT, Steelman LS, Blalock WL, Moye PW, Chang F, Pearce M, Shelton JG, White MK, Franklin RA, Pohnert SC. Interactions between the PI3K and Raf signaling pathways can result in the transformation of hematopoietic cells. Cancer Detection and Prevention. 2001;25:375–393. [PubMed] [Google Scholar]
- 104.Shelton JG, Moye PW, Steelman LS, Blalock WL, Lee JT, Franklin RA, McMahon M, McCubrey JA. Differential effects of kinase cascade inhibitors on neoplastic and cytokine-mediated cell proliferation. Leukemia. 2003;17:1765–1782. doi: 10.1038/sj.leu.2403052. [DOI] [PubMed] [Google Scholar]
- 105.Akula SM, Ford PW, Whitman AG, Hamden KE, Bryan BA, Cook PP, McCubrey JA. B-Raf dependent expression of vascular endothelial growth factor-A in Kaposi’s sarcoma-associated herpesvirus infected human B cells. Blood. 2005;105:4516–22. doi: 10.1182/blood-2004-09-3683. [DOI] [PubMed] [Google Scholar]
- 106.Akula SM, Ford PW, Whitman AG, Hamden KE, Shelton JG, McCubrey JA. Raf promotes human herpesvirus-8 (HHV-8/KSHV) infection. Oncogene. 2004;23:5227–5241. doi: 10.1038/sj.onc.1207643. [DOI] [PubMed] [Google Scholar]
- 107.Ford PW, Hamden KE, Whitman AG, McCubrey JA, Akula SM. Vascular endothelial growth factor augments human Herpesvirus-8 (HHV-8/KSHV) infection. Cancer Biol Ther. 2004;3:876–881. doi: 10.4161/cbt.3.9.1054. [DOI] [PubMed] [Google Scholar]
- 108.Hamden KE, Ford PW, Whitman AG, McCubrey JA, Akula SM. Raf induced Vascular Endothelial Growth Factor Augments Kaposi’s Sarcoma-Associated Herpesvirus (KSHV/HHV-8) Infection. J Virol. 2004;78:13381–13390. doi: 10.1128/JVI.78.23.13381-13390.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hamden KE, Ford PW, Whitman AG, Shelton JG, McCubrey JA, Akula SM. Raf and VEGF: Emerging Therapeutic targets in Kaposi’s Sarcoma-Associated Herpesviurs Infection and Angiogenesis in Hematopoietic and Non-Hematopoietic Tumors. Leukemia. 2005;19:18–26. doi: 10.1038/sj.leu.2403532. [DOI] [PubMed] [Google Scholar]
- 110.Alexia C, Fallot G, Lasfer M, Schweizer-Groyer G, Groyer A. An evaluation of the role of insulin-like growth factors (IGF) and of type-I IGF receptor in signaling in hepatocarcinogenesis and in the resistance of hepatocarcinoma cells against drug-induced apoptosis. Biochemical Pharmacology. 2004;68:1003–1015. doi: 10.1016/j.bcp.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 111.Chang F, Lee JT, Navolanic PM, Steelman LS, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003b;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 112.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 113.Steelman LS, Bertrand FE, McCubrey JA. The complexity of PTEN mutation, marker and potential target for therapeutic intervention. Expert Opin Ther Targets. 2004;8:537–550. doi: 10.1517/14728222.8.6.537. [DOI] [PubMed] [Google Scholar]
- 114.Flotho C, Valcamonica S, Mach-Pascual S, Schmahl G, Corral L, Ritterbach J, Hasle H, Arico M, Biondi A, Niemeyer CM. RAS mutations and clonality analysis in children with juvenile myelomonocytic leukemia (JMML) Leukemia. 1999;13:32–37. doi: 10.1038/sj.leu.2401240. [DOI] [PubMed] [Google Scholar]
- 115.Stirewalt DL, Kopecky KJ, Meshinchi S, Appelbaum FR, Slovak ML, Willman CL, Radich JP. FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood. 2001;97:3589–3595. doi: 10.1182/blood.v97.11.3589. [DOI] [PubMed] [Google Scholar]
- 116.Zebisch A, Staber PB, Delavar A, Bodner C, Hiden K, Fischereder K, Janakiraman M, Linkesch W, Auner HW, Embergber W, Windpassinger C, Schimek MG, Hoefler G, Troppmair J, Sill H. Two transforming C-RAF germ-line mutations identified in patients with therapy-related acute myeloiid leukemia. Cancer Res. 2006;166:3401–3408. doi: 10.1158/0008-5472.CAN-05-0115. [DOI] [PubMed] [Google Scholar]
- 117.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin J, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Prichard-Jones K, Maitland N, Chenevix-Trench G, Cossu A, Flanagan A, Nicolson A, Ho JWC, Leung ST, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 118.Libra M, Malaponte G, Navolanic PM, Gangemi P, Bevelacqua V, Proietti L, Bruni B, Stivala F, Mazzarino MC, Travali S, McCubrey JA. Analysis of BRAF Mutation in Primary and Metastatic Melanoma. Cell Cycle. 2006;4:968–970. doi: 10.4161/cc.4.10.2026. [DOI] [PubMed] [Google Scholar]
- 119.Fransen K, Klinntenas M, Osterstrom A, Dimberg J, Monsteis HJ, Soderkvist P. Mutation analysis of the B-Raf, A-Raf and Raf-1 genes in human colorectal adenocarcinomas. Carcinogenesis. 2004;25:527–533. doi: 10.1093/carcin/bgh049. [DOI] [PubMed] [Google Scholar]
- 120.Wan PT, Garnett MJ, Ros SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R. Mechanism of activation of the Raf-MEK signaling pathway by oncogenic mutations of B-Raf. Cell. 2004;116:856–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 121.Busca R, Abbe P, Mantous F, Aberdam E, Peyssonnaux C, Eychene A, Ortonne JP, Ballot R. Ras mediates the cAMP-dependent activation of extracellular signal-regulated in melanocytes. EMBO J. 2000;19:2900–2910. doi: 10.1093/emboj/19.12.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rajagopalan H, Bordelli A, Lengauer C, Kinzler KN, Vogelstein B, Velculescu VE. Tumorigenesis: Raf/Ras oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 123.Yuen ST, Davies H, Chan TL, Ho JW, Bignell GR, Cox C, Stephens P, Edkins S, Tsui WW, Chan AS, Futreal PA, Stratton MR, Wooster R, Leung SY. Similarity of the phenotypic patterns associated with B-Raf and KRAS mutations in colorectal neoplasia. Cancer Research. 2002;62:6451–6455. [PubMed] [Google Scholar]
- 124.Ricciardi MR, McQueen T, Chism D, Millella M, Estey E, Kaldjian E, Leopold J, Konopleva M, Andreeff M. Quantitative single cell determination of ERK phosphorylation and regulation in relapsed and refractory primary acute myeloid leukemia. Leukemia. 2005;29:1543–1549. doi: 10.1038/sj.leu.2403859. [DOI] [PubMed] [Google Scholar]
- 125.Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M, Estey EH, Andreeff M. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006 doi: 10.1182/blood-2006-02-003475. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jucker M, Sudel K, Horn S, Sickel M, Wegner W, Fiedler W, Feldman RA. Expression of a mutated form of the p85alpha regulatory subunit of phosphatidylinositol 3-kinase in a Hodgkin’s lymphoma-derived cell line (CO) Leukemia. 2002;16:894–901. doi: 10.1038/sj.leu.2402484. [DOI] [PubMed] [Google Scholar]
- 127.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 128.Lin J, Adam RM, Santiestevan E, Freeman MR. The phosphatidylinositol 3′-kinase pathway is a dominant growth factor-activated cell survival pathway in LNCaP human prostate carcinoma cells. Cancer Res. 1999;59:2891–2897. [PubMed] [Google Scholar]
- 129.Fry MJ. Phosphoinositide 3-kinase signalling in breast cancer: how big a role might it play? Breast Cancer Res. 2001;3:304–312. doi: 10.1186/bcr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin X, Bohle AS, Dohrmann P, Leuschner I, Schulz A, Kremer B, Fandrich F. Overexpression of phosphatidylinositol 3-kinase in human lung cancer. Langenbecks Arch Surg. 2001;386:293–301. doi: 10.1007/s004230100203. [DOI] [PubMed] [Google Scholar]
- 131.Krasilnikov M, Adler V, Fuchs SY, Dong Z, Haimovitz-Friedman A, Herlyn M, Ronai Z. Contribution of phosphatidylinositol 3-kinase to radiation resistance in human melanoma cells. Mol Carcinog. 1999;24:64–69. doi: 10.1002/(sici)1098-2744(199901)24:1<64::aid-mc9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 132.Martinez-Lorenzo MJ, Anel A, Monleon I, Sierra JJ, Pineiro A, Naval J, Alava MA. Tyrosine phosphorylation of the p85 subunit of phosphatidylinositol 3-kinase correlates with high proliferation rates in sublines derived from the Jurkat leukemia. Int J Biochem Cell Biol. 2000;32:435–445. doi: 10.1016/s1357-2725(99)00142-9. [DOI] [PubMed] [Google Scholar]
- 133.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 134.Frogne T, Jepsen JS, Larsen SS, Fog CK, Brockdorff BL, Lykkesfeldt AE. Antiestrogen-resistant human breast cancer cells require activated protein kinase B/Akt for growth. Endocr Relat Cancer. 2005;12:599–614. doi: 10.1677/erc.1.00946. [DOI] [PubMed] [Google Scholar]
- 135.Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, Bartlett JM. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol. 2005;207:139–146. doi: 10.1002/path.1829. [DOI] [PubMed] [Google Scholar]
- 136.Nyakern M, Tazzari PL, Finelli C, Bosi C, Follo MY, Grafone T, Piccaluga PP, Martinelli G, Cocco L, Martelli AM. Frequent elevation of Akt kinase phosphorylation in blood marrow and peripheral blood mononuclear cells from high-risk myelodysplastic syndrome patients. Leukemia. 2006;20:230–238. doi: 10.1038/sj.leu.2404057. [DOI] [PubMed] [Google Scholar]
- 137.Mantovani I, Cappellini A, Tazzari PL, Papa V, Cocco L, Martelli AM. Caspase-dependent cleavage of 170-kDa P-glycoprotein during apoptosis of human T-lymphoblastoid CEM cells. J Cell Physiol. 2006;207:836–844. doi: 10.1002/jcp.20628. [DOI] [PubMed] [Google Scholar]
- 138.Nyakern M, Cappellini A, Mantovani J, Martelli AM. Synergistic induction of apoptosis in yhuman leukemia T cells by the Akt inhibitor perifosine and etoposide through activation of intrinsic and Fas-mediated extrinsic cell death pathways. Mol Cancer Ther. 2006;5:1559–1570. doi: 10.1158/1535-7163.MCT-06-0076. [DOI] [PubMed] [Google Scholar]
- 139.Martelli AM, Nyakern M, Tabellini G, Bortul R, Tazzsari PL, Evangelisti C, Cocco L. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myelid leukemia. Leukemia. 2006;20:911–928. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- 140.Mitenberger RJ, Farham PJ, Smith DE, Stommell JM, Cornwell MM. v-Raf activates transcription of growth-responsive promoters via GC-rich sequences that bind the transcription factor Sp1. Cell Growth & Differentiation. 1995;6:549–556. [PubMed] [Google Scholar]
- 141.Kim SH, Lee SH, Kwak NH, Kang CD, Chung BS. Effect of the activated Raf protein kinase on the human multidrug resistance 1 (MDR1) gene promoter. Cancer Letters. 1996;98:199–205. [PubMed] [Google Scholar]
- 142.Blagosklonny MV, Schulte T, Nguyen P, Trepel J, Neckers LM. Taxol-induced apoptosis and phosphorylation of Bcl-2 protein involves c-Raf-1 and represents a novel c-Raf-1 signal transduction pathway. Cancer Res. 1996;56:1851–1854. [PubMed] [Google Scholar]
- 143.Blagosklonny MV. Drug-resistance enables selective killing of resistant leukemia cells: exploiting of drug resistance instead of reversal. Leukemia. 1999;13:2031–2035. doi: 10.1038/sj.leu.2401623. [DOI] [PubMed] [Google Scholar]
- 144.Weinstein-Oppenheimer CR, Henríquez-Roldán CF, Davis J, Navolanic PM, Saleh OA, Steelman LS, Franklin RA, Robinson PJ, McMahon M, McCubrey JA. Role of the Raf signal transduction cascade in the in vitro resistance to the anticancer drug doxorubicin. Clinical Cancer Res. 2001;7:2892–2907. [PubMed] [Google Scholar]
- 145.Davis JM, Weinstein-Oppenheimer CR, Steelman LS, Navolanic PM, Hu W, Konopleva M, Blagosklonny MV, McCubrey JA. Raf-1 and Bcl-2 induce distinct and common pathways which contribute to breast cancer drug resistance. Clinical Cancer Res. 2003;9:1161–1170. [PubMed] [Google Scholar]
- 146.Shelton JG, Steelman LS, Lee JT, Knapp SL, Blalock WL, Moye PM, Franklin RA, Pohnert SC, Mizra AM, McMahon M, McCubrey JA. Effects of the Raf/MEK/ERK and PI3K signal transduction pathways on the abrogation of cytokine dependence and prevention of apoptosis in hematopoietic cells. Oncogene. 2003;24:2478–2492. doi: 10.1038/sj.onc.1206321. [DOI] [PubMed] [Google Scholar]
- 147.Haldar S, Jena N, Croce CM. Antiapoptosis potential of bcl-2 oncogene by dephosphorylation. Biochemistry & Cell Biology. 1994;72:455–462. doi: 10.1139/o94-061. [DOI] [PubMed] [Google Scholar]
- 148.Basu A, Haldar S. Microtubule-damaging drugs triggered bcl2 phosphorylation-requirement of phosphorylation on both serine-70 and serine-87 residues of bcl2 protein. Int J Oncol. 1998;13:659–664. doi: 10.3892/ijo.13.4.659. [DOI] [PubMed] [Google Scholar]
- 149.Gioeli D, Mandell JW, Petroni GR, Frierson HF, Jr, Weber MJ. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59:279–284. [PubMed] [Google Scholar]
- 150.Abreu-Martin MY, Chari A, Palladino AA, Craft NA, Sawyers CL. Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcripition and apoptosis in prostate cancer. Mol Cell Biol. 1999;19:5143–5154. doi: 10.1128/mcb.19.7.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weber MJ, Gioeli D. Ras signaling in prostate cancer progression. J Cell Biochem. 2004;91:13–25. doi: 10.1002/jcb.10683. [DOI] [PubMed] [Google Scholar]
- 152.Bakin RE, Gioeli D, Sikes RA, Bissonette EA, Weber MJ. Constitutive activation of the Ras/Mitogen-activated protein kinase signaling pathway promotes androgen hypersensitivity in LNCaP prostate cancer cells. Cancer Res. 2003;63:1981–1989. [PubMed] [Google Scholar]
- 153.Mukherjee R, Bartlett JMS, Krishna NS, Underwood MA, Edwards J. Raf-1 expression may influence progression to androgen insensitive prostate cancer. The Prostate. 2005;64:101–107. doi: 10.1002/pros.20211. [DOI] [PubMed] [Google Scholar]
- 154.Lee JT, Steelman LS, McCubrey JA. Modulation of Raf/MEK/ERK pathway in prostate cancer drug resistance. Int J Oncol. 2005;26:1637–1645. [PubMed] [Google Scholar]
- 155.Lee JW, Soung YH, Park WS, Kim SY, Nam SW, Min WS, Lee JY, Yoo NJ, Lee SH. BRaf mutations in acute leukemias. Leukemia. 2004;1818:170–172. doi: 10.1038/sj.leu.2403201. [DOI] [PubMed] [Google Scholar]
- 156.Fu Z, Smith PC, Zhang L, Rubin MA, Dunn RL, Yao Z, Keller ET. Effects of raf kinase inhibitor protein on suppression of prostate cancer metastasis. JNCI. 2003;95:878–889. doi: 10.1093/jnci/95.12.878. [DOI] [PubMed] [Google Scholar]
- 157.Keller ET, Fu Z, Yeung K, Brennan M. Raf kinase inhibitor protein: a prostate cancer metastasis suppressor gene. Cancer Letters. 2004;207:131–137. doi: 10.1016/j.canlet.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 158.Shelton JG, Steelman LS, Abrams SL, Bertrand FE, Franklin RA, McMahon M, McCubrey JA. The epidermal growth factor receptor gene family as a target for therapeutic intervention in numerous cancers--what’s genetics got to do with it? Exp Opin Ther Targets. 2005;92:1009–1030. doi: 10.1517/14728222.9.5.1009. [DOI] [PubMed] [Google Scholar]
- 159.Wu G. The functuional interactions between p53 and MAPK signaling pathways. Cancer Biol & Ther. 2004;3:156–161. doi: 10.4161/cbt.3.2.614. [DOI] [PubMed] [Google Scholar]
- 160.Lee SW, Fang L, Igarashi M, Ouchi T, Lu KP, Aaronson SA. Sustained activation of Ras/Raf/mitogen-activated protein kinase cascade by the tumor suppressor p53. PNAS. 2000;97:8302–8305. doi: 10.1073/pnas.150024397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fang L, Li G, Liu G, Lee SW, Aaronson SA. P53 induction of heparin-binding EGF-like growth factor counteracts p53 growth suppression through activation of MAPK and PI3K/Akt signaling cascades. EMBO J. 2001;20:1931–1939. doi: 10.1093/emboj/20.8.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Blagosklonny MV, Prabhu NS, El-Deiry WS. Defects in p21WAF1/CIP1, Rb and c-myc signaling in phorbol ester-resistant cancer cells. Cancer Res. 1997;57:320–325. [PubMed] [Google Scholar]
- 163.Blagosklonny MV. The mitogen-activated protein kinase pathway mediates growth arrest or E1A-dependent apoptosis in SKBR3 human breast cancer cells. Int J Cancer. 1998;78:511–517. doi: 10.1002/(sici)1097-0215(19981109)78:4<511::aid-ijc19>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 164.Ravi RK, McMahon M, Yangang Z, Williams JR, Dillehay LE, Nelkin BC, Mabry M. Raf-1-induced cell cycle arrest in LNCap human prostate cancer cells. J Cell Biochem. 1999;72:458–469. doi: 10.1002/(sici)1097-4644(19990315)72:4<458::aid-jcb2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 165.Kojima K, Konopleva M, Samudio IJ, Shikami M, Cabreira-Hansen M, McQueen T, Ruvolo V, Tsao T, Zeng Z, Vassilev LT, Andreeff M. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106:3150–3159. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Heimbrook DC, Huber HE, Stirdivant SM, Claremon D, Liverton N, Patrick DR, Selnick H, Ahern J, Conroy R, Drakas R, Falconi N, Hancock P, Robinson R, Smith G, Oliff A. Identification of potent, selective kinase inhibitors of Raf. Proc Amer Assoc Cancer Res Annual Meeting. 1998;39:558. [Google Scholar]
- 167.Hall-Jackson CA, Eyers PA, Cohen P, Goedert M, Boyle FT, Hewitt N, Plant H, Hedge P. Paradoxical activation of Raf by a novel Raf inhibitor. Chemistry & Biology. 1999;6:559–568. doi: 10.1016/s1074-5521(99)80088-x. [DOI] [PubMed] [Google Scholar]
- 168.Lyons JF, Wilhelm S, Hibner B, Bollag G. Discovery of a novel Raf kinase inhibitor. Endocrine-Related Cancer. 2001;8:219–225. doi: 10.1677/erc.0.0080219. [DOI] [PubMed] [Google Scholar]
- 169.Lee JT, Steelman LS, McCubrey JA. PI3K Activation leads to MRP1 expression and subsequent chemoresistance in advanced prostate cancer cells. Cancer Res. 2004;64:8397–8404. doi: 10.1158/0008-5472.CAN-04-1612. [DOI] [PubMed] [Google Scholar]
- 170.Workman P. Altered states: selectively drugging the Hsp90 cancer chaperone. Trends in Molecular Medicine. 2004;10:47–51. doi: 10.1016/j.molmed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 171.Neckers L. Hsp90inhibitors as novel cancer chemotherapeutic agents. Trends in Molecular Medicine. 2002;8:S55–61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- 172.Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pages G, Valverde O, Marowsky A, Porrazzo A, Orban PC, Maldonado R, Ehrengruber MU, Cestari V, Lipp HP, Chapman PF, Pouyssegur J, Brambilla R. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34:807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 173.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functiuonal inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 174.Shaw RJ, CantleyRas LC. PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 175.Fornari FA, Randolph JK, Yalowich JC, Ritke MK, Gewirtz DA. Interference by doxorubicin with DNA unwinding in MCF-7 breast tumor cells. Mol Pharm. 1994;45:649–656. [PubMed] [Google Scholar]
- 176.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 177.Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem. 2000;207:77–86. doi: 10.1023/a:1007094214460. [DOI] [PubMed] [Google Scholar]
- 178.Friesen C, Fulda S, Debatin KM. Induction of CD95 ligand and apoptosis by doxorubicin is modulated by the redox state in chemosensitive- and drug-resistant tumor cells. Cell Death Differ. 1999;6:471–480. doi: 10.1038/sj.cdd.4400512. [DOI] [PubMed] [Google Scholar]
- 179.Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res. 1997;57:3963–3971. [PubMed] [Google Scholar]
- 180.Kobayashi D, Tokino T, Watanabe N. Contribution of caspase-3 differs by p53 status in apoptosis induced by X-irradiation. Jpn J Cancer Res. 2001;92:475–481. doi: 10.1111/j.1349-7006.2001.tb01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Oleinick NL, Evans HH. The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res. 1998;150:S146–156. [PubMed] [Google Scholar]
- 182.Matroule JY, Carthy CM, Granville DJ, Jolois O, Hunt DW, Piette J. Mechanism of colon cancer cell apoptosis mediated by pyropheophorbide-a methylester photosensitization. Oncogene. 2001;20:4070–4084. doi: 10.1038/sj.onc.1204546. [DOI] [PubMed] [Google Scholar]
- 183.Tong Z, Singh G, Rainbow AJ. Sustained activation of the extracellular signal-regulated kinase pathway protects cells from photofrin-mediated photodynamic therapy. Cancer Res. 2002;62:5528–5535. [PubMed] [Google Scholar]
- 184.Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, Hagan MP, Grant S, Schmidt-Ullrich R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003b;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 185.Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003a;22:5885–5896. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- 186.Abdelmohsen K, von Montfort C, Stuhlmann D, Gerber PA, Decking UK, Sies H, Klotz LO. Doxorubicin induces EGF receptor-dependent downregulation of gap junctional intercellular communication in rat liver epithelial cells. Biol Chem. 2005;386:217–223. doi: 10.1515/BC.2005.027. [DOI] [PubMed] [Google Scholar]
- 187.Papaiahgari S, Kleeberger SR, Cho HY, Kalvakolanu DV, Reddy SP. NADPH oxidase and ERK signaling regulates hyperoxia-induced Nrf2-ARE transcriptional response in pulmonary epithelial cells. J Biol Chem. 2004;279:42302–42312. doi: 10.1074/jbc.M408275200. [DOI] [PubMed] [Google Scholar]
- 188.Matsuzawa A, Nishitoh H, Tobiume K, Takeda K, Ichijo H. Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice. Antioxid Redox Signal. 2002;4:415–425. doi: 10.1089/15230860260196218. [DOI] [PubMed] [Google Scholar]
- 189.Minamino T, Yujiri T, Papst PJ, Chan ED, Johnson GL, Terada N. MEKK1 suppresses oxidative stress-induced apoptosis of embryonic stem cell-derived cardiac myocytes. Proc Natl Acad Sci USA. 1999;96:15127–15132. doi: 10.1073/pnas.96.26.15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Modur V, Zimmerman G, Prescott S, McIntyre T. Endothelial cell inflammatory responses to tumor necrosis factor alpha. Ceramide-dependent and -independent mitogen-activated protein kinase cascades. J Biol Chem. 1996;271:13094–13102. doi: 10.1074/jbc.271.22.13094. [DOI] [PubMed] [Google Scholar]
- 192.Teramoto H, Coso OA, Miyata H, Igishi T, Miki T, Gutkind JS. Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. J Biol Chem. 1996;271:27225–27228. doi: 10.1074/jbc.271.44.27225. [DOI] [PubMed] [Google Scholar]
- 193.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 194.Touyz RM, Yao G, Viel E, Amiri F, Schiffrin EL. Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens. 2004;22:1141–1149. doi: 10.1097/00004872-200406000-00015. [DOI] [PubMed] [Google Scholar]
- 195.Kamakura S, Moriguchi T, Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J Biol Chem. 1999;274:26563–26571. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- 196.Cavanaugh JE, Ham J, Hetman M, Poser S, Yan C, Xia Z. Differential regulation of mitogen-activated protein kinases ERK1/2 and ERK5 by neurotrophins, neuronal activity, and cAMP in neurons. J Neurosci. 2001;21:434–443. doi: 10.1523/JNEUROSCI.21-02-00434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]