Abstract
Chronic exposure to manganese (Mn) leads to a neurological disorder, manganism, which shares multiple common features with idiopathic Parkinson disease (IPD). 17β-Estradiol (E2) and some selective estrogen receptor modulators, including tamoxifen (TX), afford neuroprotection in various experimental models of neurodegeneration. However, the neuroprotective effects and mechanisms of E2/TX in Mn-induced toxicity have yet to be documented. Herein, we studied the ability of E2/TX to protect rat cortical primary neuronal and astroglial cultures from Mn-induced toxicity. Cell viability, Western blot, and reactive oxygen species (ROS) generation were assessed. Results established that both E2 (10nM) and TX (1μM) attenuated Mn-induced toxicity. The protective effects of E2/TX were more pronounced in astrocytes versus neurons. The E2-mediated attenuation of Mn-induced ROS generation in astrocytes at 6-h treatment (where no cell death was detected) was mediated by a classical estrogen receptor (ER) pathway and the TX-mediated effect on Mn-induced ROS generation was not mediated via classical ER-dependent mechanisms and likely by its antioxidant properties. The phosphatidylinositol-3 kinase (PI3K)/Akt signaling pathway was involved in both E2- and TX-induced attenuation of Mn-induced ROS formation (6 h) in astrocytes. Treatments with Mn for a longer duration (24 h) led to significant cell death, and the protective effects of E2 and TX were (1) not mediated by a classical ER pathway and (2) associated with activation of both mitogen-activated protein kinase/extracellular signal-regulated kinase and PI3K/Akt signaling pathways. Taken together, the results suggest that both E2 and TX offer effective therapeutic means for neuroprotection against Mn-induced toxicity.
Keywords: neuroprotection, oxidative stress, kinase (MAPK/ERK), PI3K/Akt, tamoxifen, 17β-estradiol
Manganese (Mn) is an essential element, but chronic exposures to high levels of this metal can induce irreversible neurological damage, referred to as manganism. Clinically manganism and Parkinson disease (PD) share multiple common characteristics, including the presence of generalized bradykinesia and widespread rigidity (Barbeau, 1984). Although the pathological changes and symptoms of Mn exposure are well known, the primary mechanisms of neurotoxicity have yet to be established.
There is growing evidence that astrocytes mediate Mn-induced neurotoxicity. Mn primarily accumulates within astrocytes, reaching intracellular levels > 50–75μM (Tholey et al., 1988), where it serves as an essential component of glutamine synthetase, an astrocyte-specific enzyme involved in the metabolism of glutamate to glutamine (Gorovits et al., 1997). Excessive accumulation of Mn in astrocytes is associated with compromised energy metabolism, oxidative stress, and impairment of astrocytic-neuronal communication (Hazell, 2002; Milatovic et al., 2007; Taylor et al., 2006). Thus, neuronal vulnerability to Mn may be due to loss of functional integrity of astrocytes and their failure to control the extracellular milieu (Normandin and Hazell, 2002). Oxidative stress (Desole et al., 1997; Stokes et al., 2000) and glutamate transporter impairment in astrocytes have been proposed as mediators of Mn neurotoxicity (Brouillet et al., 1993; Desole et al., 1997). Notably, Mn effectively induces oxidative stress and free radical formation in both neurons and astrocytes (Chen and Liao, 2002; Gunter et al., 2005; Sun et al., 1993; Worley et al., 2002).
17β-Estradiol (E2), an ovarian steroid hormone, exerts neuroprotective effects in various experimental models of neurodegenerative diseases, such as Alzheimer disease (AD) (Barron et al., 2006), PD (Ramirez et al., 2003), and ischemic stroke (Dhandapani and Brann, 2002). However, since E2 induces undesirable peripheral side effects, characterized by hyperplasia of breast and uterine tissues, selective estrogen receptor modulators, such as tamoxifen (TX) and raloxifen, have received extensive attention as alternative neuroprotectants. TX possesses agonistic functions to the estrogen receptors (ERs) and has been documented to afford neuroprotection in experimental models of ischemia (Dhandapani et al., 2005; Kimelberg, 2005) and AD (O'Neill et al., 2004).
Although E2-induced neuroprotection is well established, the cellular and molecular mechanisms underlying its effectiveness have yet to be established. The genomic effects of E2 binding to its nuclear ERs and subsequent regulation of transcription and translation typically require several hours prior to manifestation (O'Lone et al., 2004). However, there is growing evidence that E2-mediated effects may also occur within minutes, which cannot be attributed to its genomic action. These rapid (nongenomic) E2-mediated effects likely target ERs at the plasma membrane via kinase signaling pathways (Raz et al., 2008). Notably, (1) E2 rapidly activates (within minutes) extracellular signal-regulated kinases (ERKs) (Bryant et al., 2005; Singh et al., 1999; Watters et al., 1997) and enhances phosphorylation of Akt in various neural cell types (Dominguez et al., 2007; Honda et al., 2000; Wilson et al., 2002); (2) MAPK kinase inhibitors effectively block E2-induced neuroprotection in cultured neurons (Numakawa et al., 2007; Wu et al., 2005) and in the CA1 region of the hippocampus (Jover-Mengual et al., 2007); and (3) E2 rapidly activates neuronal Akt (Honda et al., 2000), and inhibition of phosphatidylinositol-3 kinase (PI3K)/Akt attenuates the E2-induced neuroprotective effects, indicating a role for PI3K/Akt pathway in E2-mediated neuroprotection.
Oxidative stress has been implicated in various neurological disorders including AD (Moosmann and Behl, 1999) and idiopathic Parkinson's disease (Ebadi et al., 1996). Oxidative stress is also an established mechanism of Mn-induced neurotoxicity (Chen and Liao, 2002; Gunter et al., 2005; Worley et al., 2002). E2 itself exerts antioxidative effects via ER-independent mechanism, possibly due to its phenolic chemical structure that shares antioxidant properties with many other phenolic compounds (Moosmann and Behl, 1999; Wang et al., 2006). TX has also exerted antioxidant action in ischemia animal models (Kimelberg et al., 2000; Wakade et al., 2008). The mechanism of E2-mediated attenuation of reactive oxygen species (ROS) generation and the possible involvement of signaling pathways in E2-mediated neuroprotection from Mn toxicity remain to be established. Accordingly, in the present study, we tested the hypothesis that E2 and TX protect neurons and astrocytes from Mn toxicity. We further addressed whether the neuroprotective effects of E2/TX against Mn toxicity are mediated via their antioxidant properties and/or activation of nongenomic signaling pathways including the mitogen-activated protein kinase (MAPK)/ERK and PI3K/Akt pathways.
MATERIALS AND METHODS
Primary cortical cultures.
The protocol for animal use was approved by the institutional committee for animal research and was performed in accordance with the guidelines of the National Institutes of Health for the care and use of laboratory animals. Neonatal rat primary astrocyte cultures were prepared according to previously established protocols (Aschner et al., 1994) with a minor modification. Briefly, astrocytes were isolated from cerebral cortices of newborn (1 day old) Sprague-Dawley rats (Harlan, IN). Pups were decapitated under halothane-anesthesia, and the cerebral cortices were dissected out. The meninges were removed followed by digestion of the cortices with bacterial neutral protease (Dispase; Invitrogen, Carlsbad, CA). Cells were plated at a density of 2 × 105 cells/ml and cultured in medium containing minimum essential media (MEM) with Earle's salts supplemented with 10% fetal bovine serum, 100 U/ml of penicillin, and 100 μg/ml of streptomycin. The medium was changed twice a week. Our experience dictates that the purity of these astrocyte cultures is > 95% positive for the astrocyte marker, glial fibrillary acidic protein (GFAP). All experiments were performed 3 weeks postisolation when the astrocyte culture became confluent. Neurons were prepared in a similar fashion from cortices derived from embryonic day 17 pups, except that they were plated on poly-D-lysine–coated plates. Neurons were grown in Neurobasal medium (Invitrogen) supplemented with B27, 5 U/ml penicillin, 5 mg/ml streptomycin, 0.5mM glutamine, and 25μM glutamate (Gibco, Rockville, MD), at 37°C in 5% CO2. Cytosine arabinoside (20μM) was added 24 h postisolation to prevent glial proliferation. Neurons were used for experiments after 1 week in culture.
Cell viability assay.
Cell viability was measured with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Astrocytes were grown in 150-mm flasks for 3 weeks and subsequently detached with 0.25% trypsin. Cells (2 × 105 cells/ml) were seeded in 96-well poly-D-lysine–coated plates (100 μl/well) and allowed to attach overnight. Neurons (2 × 105 cells/ml) were grown for 1 week in 96-well poly-D-lysine–coated plates (100 μl/well). The medium was then replaced with 100 μl fresh medium containing the designated drug or vehicle control, followed by incubation for the indicated times. Three hours before the end of the incubation period, 10 μl of PBS containing MTT (5 g/l) was added to each well. Next, 100 μl of isopropanol containing 1.0N HCl was added to each well to dissolve the crystals formed by the MTT reagent. Absorbance was measured with a plate reader (Molecular Devices, Sunnyvale, CA) at 590 nm using a reference wavelength of 630 nm.
Detection of intracellular ROS formation.
Levels of intracellular free radical were analyzed by the fluorescent signal after the oxidation of a nonfluorescent dye 2,7-dichlorodihydrofluorescein diacetate (H2DCF-DA; Molecular Probe, Carlsbad, CA) to fluorescent DCF by ROS, as described previously (Fu et al., 1998). Cells (2 × 105 cells/ml) were incubated in 96-well plates for 24 h in low serum Opti-MEM (Invitrogen) media, then H2DCF-DA was added to the cells at a final concentration of 5μM for 30 min at 37°C. After three washes with PBS, cells were dissolved in 1% Triton X-100. Fluorescence was measured at wavelengths of 480/530 nm (excitation/emission) in a fluorometer (FlexStation; Molecular Devices).
Western blot.
At the end of the various treatments, astroglial cultures were washed twice with cold Hank's balanced salt solution, then 50 μl of a radioimmunoprecipitation assay buffer (150mM NaCl, 1.0% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% SDS, and 50mM Tris, pH 8.0) containing a protease inhibitor cocktail (10 μl in 1 ml of lysis buffer, Sigma-Aldrich) was added to each well. The cells were scraped-transferred to microcentrifuge tubes, followed by sonication. Next, the cell suspensions were centrifuged at 3000 × g for 10 min at 4°C, and protein solutions from the supernatant were obtained. The protein content of the cell lysates was measured with bicinchoninic acid reagents, and samples of 30-μg protein were resolved by SDS-polyacrylamide gel electrophoresis (10%) under reducing conditions. Proteins were electrophoretically transferred to a polyvinylidenefluoride membrane (Millipore, Bedford, MA). The membranes were blocked for 1 h at room temperature (RT) in Tris-buffered saline (TBS) with 0.1% Tween 20 (TBST) containing 5% nonfat milk. The blots were incubated with primary antibodies (pERK, 1: 2000; pAkt, 1:2000; total ERK, 1:3000; total Akt, 1: 3000; Santa Cruz Biotechnology, Santa Cruz, CA) in TBST containing 5% nonfat milk for 2 h at RT and subsequently washed three times in TBST. Then, the membranes were incubated with the secondary antibodies (1:2000, anti-rabbit or anti-mouse immunoglobulin G (IgG)-peroxidase conjugates; Santa Cruz Biotechnology) in TBST containing 5% nonfat milk for 1 h at RT. After three washes with TBST, the immunoreactive proteins were detected with an enhanced chemiluminescence Western blotting detection kit (Pierce, Rockford, IL).
Immunocytochemistry.
At the end of the treatments, astrocytes and neurons were washed twice with TBS and fixed with 100% methanol for 10 min at RT. After additional rinsing in TBS containing 50mM Tris and 150mM NaCl, pH 7.4, the cells were incubated for 30 min at RT with TBS containing 5% normal goat serum and 0.1% Triton X-100 (TGT). Cultures were incubated overnight at 4°C in TGT containing mouse monoclonal anti-GFAP or tubulin β-III (1:200; Millipore, Temecula, CA). Next, the cells were rinsed in TBS containing 0.1% Triton X-100 and incubated for 2 h at RT in TGT containing anti-mouse IgG-fluorescein and anti-rabbit IgG-rhodamine conjugates (1:200; Chemicon). In separate experiments, primary or secondary antibodies were omitted to test for the specificity and possible cross-reaction. To visualize cell nuclei, cultures were rinsed and then incubated for 10 min at RT in 4′,6-diamidino-2-phenylindole dihydrochloride hydrate/antifade diluted 1:1000 in TBS. The coverslips were mounted with Immuomount, and pictures were taken with a wide-field fluorescence microscope (Olympus, Pittsburgh, PA).
Statistical analysis.
The mean and SEM were determined for each set of data, and one-way ANOVA followed by a Tukey post hoc test was used for statistical analysis. A probability of 0.05 or less was considered statistically significant.
RESULTS
Mn-Induced Cytotoxicity in Rat Cortical Neuronal and Astroglial Cultures
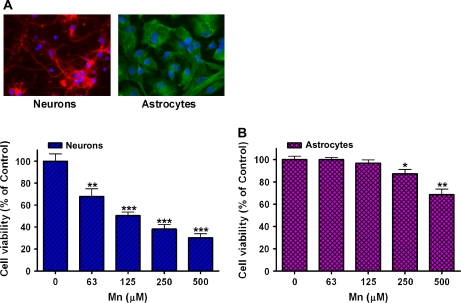
To better understand the susceptibility of various cell types to Mn-induced neurotoxicity, the experiments were carried out in primary neuronal and astroglial cultures derived from rat cortical regions (Fig. 1A). While the basal ganglia represent the main target for Mn neurotoxicity, reflecting upon its preferential accumulation in this region (Dorman et al., 2006), Mn is also well known to affect the cerebral cortex (Guilarte and Chen, 2007), thus providing a rationale for examining cells derived from this brain region. Recent studies have shown that chronic Mn exposure caused neurodegenerative changes and diffuse amyloid-β plaques in the frontal cortex (Guilarte et al., 2008). Initial studies established Mn-induced cell death in both neurons and astrocytes after 24-h treatment (2 × 105 cells/ml), and the results showed that Mn decreased cell viability in a concentration-dependent manner (Fig. 1B). Cell viability was assessed by the MTT assay. As shown in Figure 1B, at the lethal concentration that led to 50% cell death (LC50) in neurons (125μM), there was no apparent cell death in cultured astrocytes, suggesting that astrocytes are more resistant to Mn compared to neurons.
FIG. 1.
Mn induces cytotoxicity in neurons and astrocytes in a concentration-dependent manner. (A) Cells from the cortical regions of rat brain were prepared as described in the “Materials and Methods” section. Immumocytochemistry was performed to identify neurons (tubulin β-III) and astrocytes (GFAP). Nuclei were identified with DAPI. (B) Mn (24 h incubation) induced cytotoxicity in a concentration-dependent manner in both cell types, assessed with the MTT assay. (*p < 0.05, **p < 0.01, ***p < 0.001 vs. control; Tukey test following ANOVA.) Values are expressed as mean ± SEM (N = 8). Experiments were performed in three independently isolated sets of cultures.
E2 and TX Protect Both Neurons and Astrocytes against Mn Toxicity
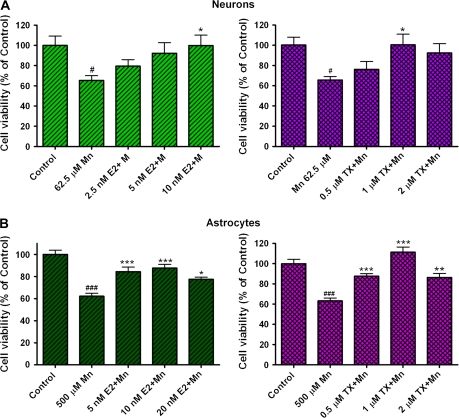
Since both E2 and TX are neuroprotective in several experimental neurodegenerative models, we tested their ability in attenuating the toxic effects of Mn. E2 and TX alone did not induce toxicity in either of the culture systems (data not shown). Neuronal and astroglial cultures were preincubated for 24 h with E2 (5–20nM) or TX (0.5–2μM) prior to Mn treatment (in the presence of E2 or TX). Cell viability was measured after 24-h treatment with Mn by the MTT assay. The results established that E2 and TX led to a statistically significant (p < 0.05) attenuation in Mn-induced cell death in both neurons and astrocytes (Fig. 2). Both E2 and TX showed greater efficacy in rescuing astrocytes from Mn-induced cell death compared to neurons.
FIG. 2.
E2 and TX protect cells against Mn toxicity in neuronal and astroglial cultures. Cell viability was assessed with the MTT assay as described in the “Methods” section. Cells were preincubated for 24 h with E2 or TX prior to Mn treatment for 24 h (in the presence of E2 or TX), followed by the MTT assay. (#p < 0.05, ###p < 0.001 vs. control; *p < 0.05, **p < 0.01, ***p < 0.001 vs. the Mn treatment; Tukey test following ANOVA.) Values are expressed as mean ± SEM (N = 8). Experiments were performed in three independently isolated sets of cultures.
Time-Dependent Protective Effects of E2 and TX against Mn Toxicity
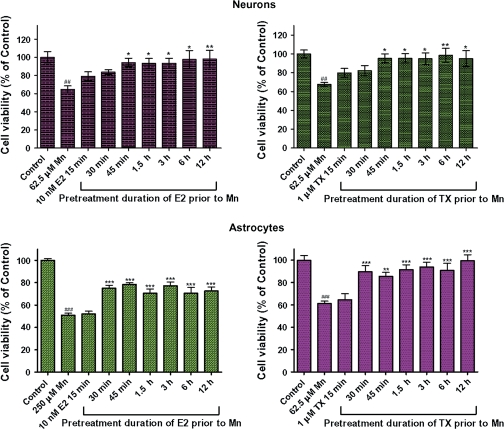
Results from a pretreatment time course study on the ability of E2/TX to protect astrocyte and neuron cultures from Mn-induced toxicity are shown in Figure 3. In neuronal cultures, 45-min preincubation with E2/TX was required to protect the cells from Mn-induced cell death when cells were treated with Mn for 24 h (in the presence of E2 or TX) (p < 0.05), whereas astroglial cultures required 30-min preincubation to achieve a similar protective effect (Fig. 3).
FIG. 3.
Temporal relationship between E2 and TX pretreatment and their protective effects against Mn-induced cell death. Cultures were pretreated with E2 (10nM) or TX (1μM) for various times prior to 24 h exposure with Mn (in the presence of E2 or TX). Significant protection was noted at 30 and 45 min preincubations in astrocytes and neurons, respectively. (##p < 0.01, ###p < 0.001 vs. control; *p < 0.05, **p < 0.01, ***p < 0.001 vs. the Mn treatment; Tukey test following ANOVA.) Results are expressed as mean ± SEM (N = 8.) Experiments were performed in three independently isolated sets of cultures.
E2/TX Attenuate Mn-Induced ROS Generation in Astrocytes
Since E2 and TX possess antioxidant properties (Moosmann and Behl, 1999), we determined if E2/TX protection against Mn-induced toxicity might be mediated by antioxidant mechanism. Astrocytes were exposed to E2 (10nM) or TX (1μM) for either 5 min or 6 h prior to Mn treatment for 6 h (in the presence of E2 or TX). At the end of the treatments, intracellular ROS generation was assayed with H2DCF-DA (see “Materials and Methods” section). In the absence of E2 or TX, Mn treatments led to a concentration-dependent increase in astrocytic ROS generation (Fig. 4, left panel). Five minutes of preincubation with E2 or TX failed to attenuate the Mn-induced ROS generation when cells were treated with Mn for 6 h (in the presence of E2 or TX) (Fig. 4, left panel); however, 6-h preincubation with E2 (Fig. 4A, right panel) or TX (Fig. 4B, right panel) prior to Mn treatment for 6 h (in the presence of E2 or TX) decreased (p < 0.01 for E2 and p < 0.001 for TX) astrocytic Mn-induced ROS generation. Similar results were obtained in studies in neuronal cultures (data not shown).
FIG. 4.
Pretreatment with E2 and TX is required for their efficient attenuation of Mn-induced ROS generation in astrocytes. Cells were pretreated with E2 10nM (A) or TX 1μM (B) for 5 min or 6 h prior to Mn treatment for 6 h (in the presence of E2 or TX), followed by the measurement of ROS by DCF fluorescence. (*p < 0.05, **p < 0.01, ***p < 0.001 vs. control; Tukey test following ANOVA.) Results are expressed as mean ± SEM (N = 8). Experiments were performed in three independently isolated sets of cultures.
Activation of PI3K/Akt Signaling Pathway Might Play a Role in E2/TX–Mediated Attenuation of Mn-Induced ROS Generation
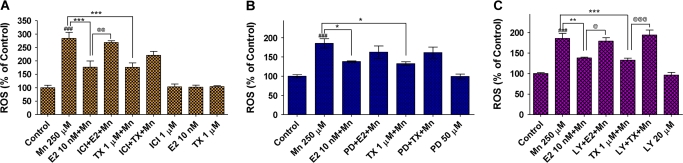
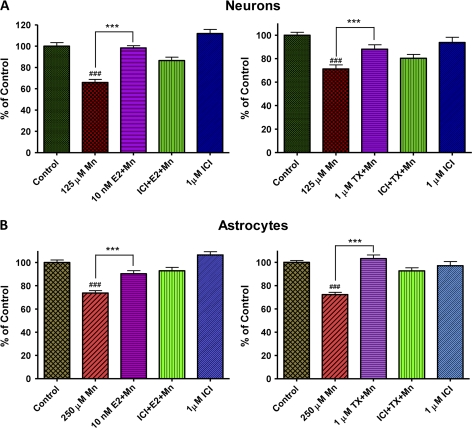
In additional studies, we examined whether E2/TX–mediated attenuation of Mn-induced ROS was ER dependent. The ER antagonist, ICI182,780, blocked the E2-mediated attenuation in Mn-induced ROS in astrocytes (Fig. 5A). However, ICI182,780 failed to block the TX-induced attenuation of Mn-induced ROS (Fig. 5A), suggesting that the effect of TX is ER independent.
FIG. 5.
Signaling pathways associated with E2- and TX-mediated attenuation on Mn-induced ROS generation in astrocytes. (A) The ER antagonist, ICI182,780 (1μM), was added to the media along with E2 or TX for 6 h, followed by Mn treatment for additional 6 h (in the presence of E2 or TX) in astrocytes. (B) MAPK/ERK inhibitor, PD98059 (50μM), was to the media along with E2 or TX for 6 h, followed by Mn treatment for additional 6 h (in the presence of E2 or TX) in astrocytes. (C) PI3K/Akt inhibitor, LY294002 (20μM), was to the media along with E2 or TX for 6 h, followed by Mn treatment for additional 6 h (in the presence of E2 or TX) in astrocytes. (###p < 0.001 vs. control; *p < 0.05, **p < 0.01, ***p < 0.001 vs. the Mn treatment; @p < 0.05, @@p < 0.01, @@@p < 0.001; Tukey test following ANOVA.) Results are expressed as mean ± SEM (N = 8). Experiments were performed in three independently isolated sets of cultures.
Next, we tested whether intracellular signaling pathways such as MAPK/ERK and PI3K/Akt are involved in the E2/TX–mediated actions. The MAPK/ERK inhibitor, PD98059, failed to block either E2- or TX-mediated attenuation of Mn-induced ROS generation (Fig. 5B), while, using the same experimental protocol, the PI3K/Akt inhibitor, LY294002, effectively blocked both the E2- and TX-mediated attenuation of Mn-induced ROS in astroglial cultures (Fig. 5C). These results suggest that E2 exerts its protective effects against Mn-induced ROS via classical ER and PI3K/Akt-dependent pathways, whereas TX mediates its effects via PI3K/Akt pathway that is not a classical ER-dependent mechanism. Similar results were obtained in studies in neuronal cultures (data not shown).
E2- and TX-Mediated Protection against Mn-Induced Cell Death Is Not Mediated by ER
Since we have obtained the signaling mechanisms of E2- and TX-mediated attenuation against the short-time exposure to Mn (6 h)-induced ROS, it was of interest if longer exposure (24 h, at the time to induce cell death) to Mn and E2/TX would involve similar signaling pathways. First, we determined whether E2/TX protection from Mn-induced cell death at 24 h was ER dependent, determined by cell viability using the MTT assay. As shown in Figure 6, cotreatment of E2 or TX with the ER inhibitor ICI182,780 (1μM) failed to block E2- and TX-induced protection against Mn toxicity both in neuronal (Fig. 6A) or astroglial cultures (Fig. 6B). ICI182,780 (1μM) alone did not alter cell viability. Taken together, these results suggest that E2 and TX, at least in part, do not exert their protective effects via classical ER-dependent mechanisms.
FIG. 6.
Role of ER in the protective effects of E2/TX against Mn-induced cell death at 24 h in neurons (A) and astrocytes (B). The ER antagonist, ICI182,780 (1μM), was added to the media along with E2 or TX for 1 h, followed by Mn treatment for additional 24 h (in the presence of E2 or TX). E2 and TX exerted protective effects against Mn-induced cytotoxicity in both neurons and astrocytes, but ICI182,780 did not block the E2/TX–induced protective effects. (###p < 0.001 vs. control; ***p < 0.001 vs. the Mn treatment; Tukey test following ANOVA.) Results are expressed as mean ± SEM (N = 8). Experiments were performed in three independently isolated sets of cultures.
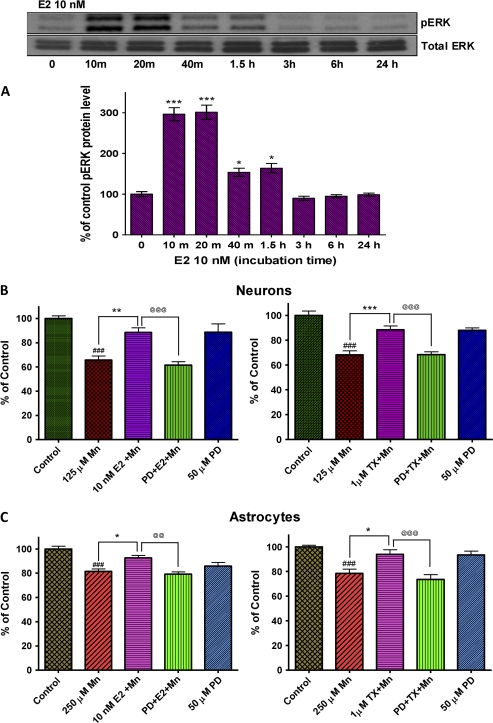
Activation of MAPK/ERK Pathway Mediates E2/TX–Mediated Protection against Mn-Induced Toxicity
Since earlier studies have established that E2 exerted neuroprotective effects against quinolinic acid–induced toxicity via activation of MAPK/ERK pathway (Kuroki et al., 2001), we determined whether this pathway is also involved in E2/TX–induced protective effects against Mn toxicity. Western blot analysis confirmed that treatment with E2 (10nM) activated the MAPK/ERK pathway in astrocytes (Fig. 7A). Neurons showed a similar effect (data not shown). The MAPK/ERK pathway inhibitor, PD98059 (50μM), blocked E2- and TX-mediated protective effects against Mn-induced cell death in both neurons (Fig. 7B) and astrocytes (Fig. 7C).
FIG. 7.
Role of the MAPK/ERK pathway in E2- and TX-mediated protection against Mn-induced cell death. E2 induced phosphorylation of ERK in astrocytes within 10 min of its addition to the media in astrocytes (A) and an inhibitor of this pathway, PD98059 (50μM), blocked E2/TX protective effect against Mn-induced cell death in neurons (B) as well as astrocytes (C). PD98059 (50μM) was added to the media along with E2 or TX for 1 h, followed by Mn treatment for additional 24 h (in the presence of E2 or TX) in astrocytes. (###p < 0.001 vs. control; *p < 0.05, **p < 0.01, ***p < 0.001 vs. the Mn treatment; @@p < 0.01, @@@p < 0.001; Tukey test following ANOVA.) Results are expressed as mean ± SEM (N = 8). Experiments were performed in three independent sets of cultures for verification of results.
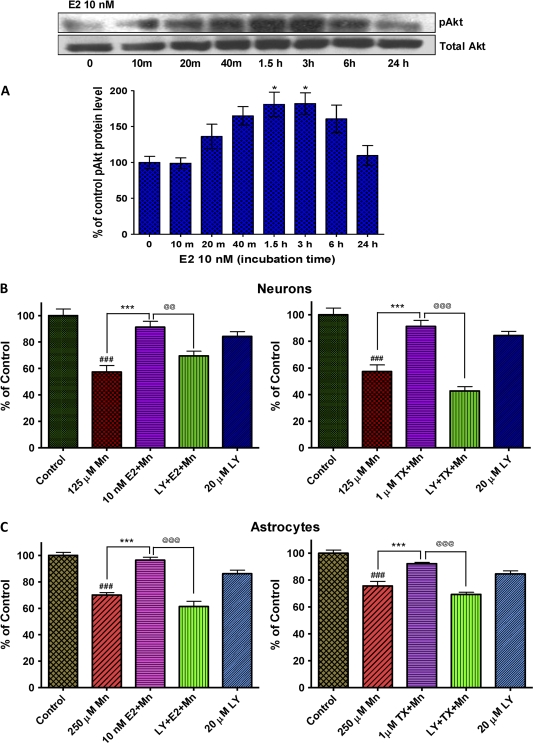
Activation of PI3K/Akt Pathway Mediates E2/TX–Mediated Protection against Mn Toxicity
Since previous studies have shown that activation of the PI3K/Akt pathway was involved in E2- and TX-mediated neuroprotective effects against camptothecin-induced neurotoxicity (Dhandapani et al., 2005), we have also tested whether this pathway plays a role in E2/TX protection against Mn-induced toxicity. Cells were pretreated with E2 or TX in the presence of LY294002 (20μM), an inhibitor of PI3K/Akt pathway for 1 h, since this time point (1 h) was sufficient to protect cells against Mn-induced toxicity (Fig. 3). Cultures were then exposed to Mn for 24 h (in the presence of E2 or TX), followed by assessment of cell viability. As shown in Figure 8, LY294002 blocked E2/TX–mediated protection against Mn-induced cell death in both neurons (Fig. 8B) and astrocytes (Fig. 8C). Western blot analysis confirmed that the E2 (10nM) effect in astrocytes was associated with the PI3K/Akt activation (Fig. 8A). Neuronal cells exerted a similar effect (data not shown).
FIG. 8.
Role of the PI3k/Akt pathway in E2- and TX-mediated protection against Mn-induced cell death. E2 induced phosphorylation of Akt in astrocytes within 20 min of its addition to the media in astrocytes (A), and an inhibitor of this pathway, LY294002 (20μM), blocked the E2- and TX-mediated protective effect against Mn-induced cell death in neurons (B) as well as astrocytes (C). LY294002 (20μM) was added to the media 1 h prior to E2/TX preincubation, followed by Mn treatment for 24 h (in the presence of E2 or TX). (###p < 0.001 vs. control; *p < 0.05, ***p < 0.001 vs. the Mn treatment; @@p < 0.01, @@@p < 0.001; Tukey test following ANOVA.) Results are expressed as mean ± SEM (N = 8). Experiments were performed in three independent sets of cultures for verification of results.
DISCUSSION
Chronic exposure to high levels of Mn can induce manganism, a disorder sharing multiple pathological and clinical features with idiopathic PD. E2 and TX are neuroprotective in a variety of in vitro and in vivo models of neurodegenerative diseases, but no previous study has addressed the efficacy of E2 and/or TX as neuroprotective agents in attenuating Mn-induced neurotoxicity.
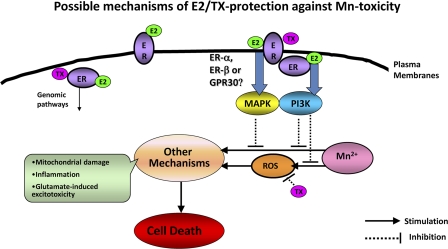
The present study shows, for the first time, that E2 and TX attenuate Mn-induced ROS generation (at 6-h treatment) in cultured neurons and astrocytes via activation of the PI3K/Akt signaling pathways. Moreover, at longer treatment (24 h), both E2 and TX exert protective effects in neurons and astrocytes against Mn-induced cell death. The mechanisms involved in this protective effect appear not to be mediated by classical ER since the classical ER inhibitor, ICI 182,780, does not block the protective effects of E2 and TX. Thus, the protective effects are mediated by ER-independent or nonclassical membrane-associated ER and subsequent activation of signaling proteins, such as MAPK/ERK and PI3K/Akt pathways (Fig. 9) (Singh et al., 1999).
FIG. 9.
Proposed mechanisms of E2- and TX-mediated protective effects against Mn-induced toxicity (details are in the “Discussion” section).
In recent years, the critical role of astrocytes in neuronal survival has received increased attention. Dysfunction of astrocytes and their failure to maintain proper control over the extracellular milieu has been invoked as an early event in triggering Mn-induced neurotoxicity in vivo (Liao et al., 2007). Astrocytes also participate in several processes crucial for optimal brain function, including scavenging of free radicals (Chen and Liao, 2002). Compared to neurons, astrocytes also have higher glutathione (GSH) levels (Rice and Russo-Menna, 1998); thus, they likely protect and shield neurons in early stages of exposure from Mn-induced ROS generation (Desagher et al., 1996). However, excessive accumulation of Mn in astrocytes may lead to their failure to perform a host of supportive roles and the ensuing demise of juxtaposed neurons (Chen and Liao, 2002).
The efficacy of E2 and TX as neuroprotectants has been shown in a variety of animal models of neurodegenerative diseases and the present study establishes the efficacy of E2 and TX also in reversing neurotoxic effects of Mn. Since Mn induces ROS generation and both E2 and TX have antioxidant action, we have tested whether E2 and/or TX attenuates Mn-induced ROS. Mn induced ROS effectively at 6-h treatment. Notably, results of the present study establish that the mechanisms of E2 and TX that mediate the attenuation of Mn-induced ROS formation are different. E2 attenuated Mn-induced ROS generation by an ER-dependent mechanism, whereas TX inhibited Mn-induced ROS via an ER-independent pathway, indicating that TX may activate a nonclassical ER pathway and/or exert antioxidant effects. This is consistent with earlier reports on TX-induced neuroprotection via ER-independent antioxidant mechanisms in rat focal cerebral ischemic injury model (Zhang et al., 2007). TX has also been shown to be neuroprotective against camptothecin-induced apoptotic cell death via ER-dependent mechanism in rat primary cultures (Wakade et al., 2008; Zhang et al., 2007). We have also tested what signaling pathways are involved in E2 and/or TX attenuation of Mn-induced ROS. Both E2 and TX exerted their attenuating effects of Mn-induced ROS via PI3K/Akt pathway. E2 activates this signaling pathway via ER activation, whereas TX does via ER-independent or nonclassical membrane-associated ER pathway (Fig. 9).
In addition to testing E2 and TX actions on Mn-induced ROS at a short exposure time (6 h), we have also examined whether longer exposure (24 h) to Mn shares similar mechanisms in E2 and/or TX protection against Mn-induced toxicity. At 24-h treatment, Mn induces cell death, and thus, we used cell viability as a parameter. We have specifically sought to identify molecular targets in intracellular signaling cascades which mediate E2- and TX-induced protection against Mn-induced cell death. Nongenomic action, possibly by the plasma membrane–associated ER and scaffold proteins that link ER to kinase signaling pathways, including PI3K/Akt and MARK/ERK, has been shown to play a role in mediating E2 and TX neuroprotection (Kuroki et al., 2001; Raz et al., 2008; Singer et al., 1999; Watters et al., 1997). Results from the present study show that E2 phosphorylated MAPK/ERK within 10 min of addition to the cells (Fig. 7), and an inhibitor of this pathway, PD98059, blocked the E2 and TX protective effects against Mn-induced cell death (24 h), indicating that the MAPK/ERK pathway plays a role in both E2 and TX protection against Mn-induced cell death.
Activation of PI3K/Akt signaling pathway is also involved in both E2- and TX-mediated protection against Mn-induced cell death (24 h). E2 activated PI3K/Akt within 20 min of its addition to the cells (Fig. 8), and the selective PI3K inhibitor, LY294002, abolished the E2 and TX protective effect against Mn-induced cell death. Since the ER antagonist ICI182780 did not abolish either E2- or TX-induced protection against Mn toxicity, activation of PI3K/Akt pathway must be downstream of nonclassical membrane-associated ER, GPR30 or ER-X (Singh et al., 1999), or ER-independent pathway (Fig. 9). The neuroprotective effects of TX against apoptosis via nonclassical membrane-associated ER activation and the PI3K/Akt signaling pathway have been reported in rat cortical astrocytes (Dhandapani et al., 2005). E2 also rapidly enhances the phosphorylation of Akt in cortical neurons (Mannella and Brinton, 2006), supporting the role of PI3K/Akt pathway in E2 and TX-induced protective effects against Mn toxicity.
In conclusion, the present study shows that E2 and TX protect both neurons and astrocytes from Mn-induced cytotoxicity by attenuating oxidative stress and activating MAPK/ERK and PI3K/Akt pathways. The results suggest that both E2 and TX offer effective therapeutic means for neuroprotection against Mn-induced toxicity and potentially other neurodegenerative diseases. Future studies could be profitably directed at testing the efficacy of E2 and TX in in vivo models of Mn-induced neurodegeneration.
FUNDING
National Institute of Environmental Health Sciences (10563); Department of Defense W81XWH-05-1-0239 (to M.A.).
References
- Aschner M, Mullaney KJ, Wagoner D, Lash LH, Kimelberg HK. Intracellular glutathione (GSH) levels modulate mercuric chloride (MC)- and methylmercuric chloride (MeHgCl)-induced amino acid release from neonatal rat primary astrocytes cultures. Brain Res. 1994;664:133–140. doi: 10.1016/0006-8993(94)91963-1. [DOI] [PubMed] [Google Scholar]
- Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias) Neurotoxicology. 1984;5:13–35. [PubMed] [Google Scholar]
- Barron AM, Fuller SJ, Verdile G, Martins RN. Reproductive hormones modulate oxidative stress in Alzheimer's disease. Antioxid. Redox Signal. 2006;8:2047–2059. doi: 10.1089/ars.2006.8.2047. [DOI] [PubMed] [Google Scholar]
- Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp. Neurol. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Bryant W, Snowhite AE, Rice LW, Shupnik MA. The estrogen receptor (ER) alpha variant Delta5 exhibits dominant positive activity on ER-regulated promoters in endometrial carcinoma cells. Endocrinology. 2005;146:751–759. doi: 10.1210/en.2004-0825. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Liao SL. Oxidative stress involves in astrocytic alterations induced by manganese. Exp. Neurol. 2002;175:216–225. doi: 10.1006/exnr.2002.7894. [DOI] [PubMed] [Google Scholar]
- Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. J. Neurosci. 1996;16:2553–2562. doi: 10.1523/JNEUROSCI.16-08-02553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desole MS, Sciola L, Delogu MR, Sircana S, Migheli R, Miele E. Role of oxidative stress in the manganese and 1-methyl-4-(2′-ethylphenyl)-1,2,3,6-tetrahydropyridine-induced apoptosis in PC12 cells. Neurochem. Int. 1997;31:169–176. doi: 10.1016/s0197-0186(96)00146-5. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW. Protective effects of estrogen and selective estrogen receptor modulators in the brain. Biol. Reprod. 2002;67:1379–1385. doi: 10.1095/biolreprod.102.003848. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Wade FM, Mahesh VB, Brann DW. Astrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}-estradiol: Involvement of nonclassical genomic signaling pathways. Endocrinology. 2005;146:2749–2759. doi: 10.1210/en.2005-0014. [DOI] [PubMed] [Google Scholar]
- Dominguez R, Liu R, Baudry M. 17-Beta-estradiol-mediated activation of extracellular-signal regulated kinase, phosphatidylinositol 3-kinase/protein kinase B-Akt and N-methyl-D-aspartate receptor phosphorylation in cortical synaptoneurosomes. J. Neurochem. 2007;101:232–240. doi: 10.1111/j.1471-4159.2006.04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Marshall MW, Parkinson CU, James RA, Wong BA. Tissue manganese concentrations in young male rhesus monkeys following subchronic manganese sulfate inhalation. Toxicol. Sci. 2006;92:201–210. doi: 10.1093/toxsci/kfj206. [DOI] [PubMed] [Google Scholar]
- Ebadi M, Srinivasan SK, Baxi MD. Oxidative stress and antioxidant therapy in Parkinson's disease. Prog. Neurobiol. 1996;48:1–19. doi: 10.1016/0301-0082(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Fu W, Luo H, Parthasarathy S, Mattson MP. Catecholamines potentiate amyloid beta-peptide neurotoxicity: Involvement of oxidative stress, mitochondrial dysfunction, and perturbed calcium homeostasis. Neurobiol. Dis. 1998;5:229–243. doi: 10.1006/nbdi.1998.0192. [DOI] [PubMed] [Google Scholar]
- Gorovits R, Avidan N, Avisar N, Shaked I, Vardimon L. Glutamine synthetase protects against neuronal degeneration in injured retinal tissue. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7024–7029. doi: 10.1073/pnas.94.13.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J. Neurochem. 2008;105:1948–1959. doi: 10.1111/j.1471-4159.2008.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK. Manganese inhibits NMDA receptor channel function: Implications to psychiatric and cognitive effects. Neurotoxicology. 2007;28:1147–1152. doi: 10.1016/j.neuro.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter KK, Aschner M, Miller LM, Eliseev R, Salter J, Anderson K, Hammond S, Gunter TE. Determining the oxidation states of manganese in PC12 and nerve growth factor-induced PC12 cells. Free Radic. Biol. Med. 2005;39:164–181. doi: 10.1016/j.freeradbiomed.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Hazell AS. Astrocytes and manganese neurotoxicity. Neurochem. Int. 2002;41:271–277. doi: 10.1016/s0197-0186(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Honda K, Sawada H, Kihara T, Urushitani M, Nakamizo T, Akaike A, Shimohama S. Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J. Neurosci. Res. 2000;60:321–327. doi: 10.1002/(SICI)1097-4547(20000501)60:3<321::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Jover-Mengual T, Zukin RS, Etgen AM. MAPK signaling is critical to estradiol protection of CA1 neurons in global ischemia. Endocrinology. 2007;148:1131–1143. doi: 10.1210/en.2006-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK. Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia. 2005;50:389–397. doi: 10.1002/glia.20174. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Feustel PJ, Jin Y, Paquette J, Boulos A, Keller RW, Jr, Tranmer BI. Acute treatment with tamoxifen reduces ischemic damage following middle cerebral artery occlusion. Neuroreport. 2000;11:2675–2679. doi: 10.1097/00001756-200008210-00014. [DOI] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Neuroprotection by estrogen via extracellular signal-regulated kinase against quinolinic acid-induced cell death in the rat hippocampus. Eur. J. Neurosci. 2001;13:472–476. doi: 10.1046/j.0953-816x.2000.01409.x. [DOI] [PubMed] [Google Scholar]
- Liao SL, Ou YC, Chen SY, Chiang AN, Chen CJ. Induction of cyclooxygenase-2 expression by manganese in cultured astrocytes. Neurochem. Int. 2007;50:905–915. doi: 10.1016/j.neuint.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: A unified mechanism of estrogen action. J. Neurosci. 2006;26:9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, Aschner JL, Aschner M. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol. Sci. 2007;98:198–205. doi: 10.1093/toxsci/kfm095. [DOI] [PubMed] [Google Scholar]
- Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normandin L, Hazell AS. Manganese neurotoxicity: An update of pathophysiologic mechanisms. Metab. Brain Dis. 2002;17:375–387. doi: 10.1023/a:1021970120965. [DOI] [PubMed] [Google Scholar]
- Numakawa Y, Matsumoto T, Yokomaku D, Taguchi T, Niki E, Hatanaka H, Kunugi H, Numakawa T. 17Beta-estradiol protects cortical neurons against oxidative stress-induced cell death through reduction in the activity of mitogen-activated protein kinase and in the accumulation of intracellular calcium. Endocrinology. 2007;148:627–637. doi: 10.1210/en.2006-1210. [DOI] [PubMed] [Google Scholar]
- O'Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol. Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- O'Neill K, Chen S, Brinton RD. Impact of the selective estrogen receptor modulator, raloxifene, on neuronal survival and outgrowth following toxic insults associated with aging and Alzheimer's disease. Exp. Neurol. 2004;185:63–80. doi: 10.1016/j.expneurol.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Ramirez AD, Liu X, Menniti FS. Repeated estradiol treatment prevents MPTP-induced dopamine depletion in male mice. Neuroendocrinology. 2003;77:223–231. doi: 10.1159/000070277. [DOI] [PubMed] [Google Scholar]
- Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. Rapid estrogen signaling in the brain. Neurosignals. 2008;16:140–153. doi: 10.1159/000111559. [DOI] [PubMed] [Google Scholar]
- Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J. Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr, Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: Convergence of estrogen and neurotrophin signaling pathways. J. Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes AH, Lewis DY, Lash LH, Jerome WG, III, Grant KW, Aschner M, Vrana KE. Dopamine toxicity in neuroblastoma cells: Role of glutathione depletion by L-BSO and apoptosis. Brain Res. 2000;858:1–8. doi: 10.1016/s0006-8993(99)02329-x. [DOI] [PubMed] [Google Scholar]
- Sun AY, Yang WL, Kim HD. Free radical and lipid peroxidation in manganese-induced neuronal cell injury. Ann. N. Y. Acad. Sci. 1993;679:358–363. doi: 10.1111/j.1749-6632.1993.tb18322.x. [DOI] [PubMed] [Google Scholar]
- Taylor MD, Erikson KM, Dobson AW, Fitsanakis VA, Dorman DC, Aschner M. Effects of inhaled manganese on biomarkers of oxidative stress in the rat brain. Neurotoxicology. 2006;27:788–797. doi: 10.1016/j.neuro.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Tholey G, Ledig M, Mandel P, Sargentini L, Frivold AH, Leroy M, Grippo AA, Wedler FC. Concentrations of physiologically important metal ions in glial cells cultured from chick cerebral cortex. Neurochem. Res. 1988;13:45–50. doi: 10.1007/BF00971853. [DOI] [PubMed] [Google Scholar]
- Wakade C, Khan MM, De Sevilla LM, Zhang QG, Mahesh VB, Brann DW. Tamoxifen neuroprotection in cerebral ischemia involves attenuation of kinase activation and superoxide production and potentiation of mitochondrial superoxide dismutase. Endocrinology. 2008;149:367–379. doi: 10.1210/en.2007-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dykens JA, Perez E, Liu R, Yang S, Covey DF, Simpkins JW. Neuroprotective effects of 17beta-estradiol and nonfeminizing estrogens against H2O2 toxicity in human neuroblastoma SK-N-SH cells. Mol. Pharmacol. 2006;70:395–404. doi: 10.1124/mol.106.022384. [DOI] [PubMed] [Google Scholar]
- Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: Effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Liu Y, Wise PM. Estradiol enhances Akt activation in cortical explant cultures following neuronal injury. Brain Res. Mol. Brain Res. 2002;102:48–54. doi: 10.1016/s0169-328x(02)00181-x. [DOI] [PubMed] [Google Scholar]
- Worley CG, Bombick D, Allen JW, Suber RL, Aschner M. Effects of manganese on oxidative stress in CATH.a cells. Neurotoxicology. 2002;23:159–164. doi: 10.1016/s0161-813x(02)00028-1. [DOI] [PubMed] [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD. 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: A potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Milatovic D, Aschner M, Feustel PJ, Kimelberg HK. Neuroprotection by tamoxifen in focal cerebral ischemia is not mediated by an agonist action at estrogen receptors but is associated with antioxidant activity. Exp. Neurol. 2007;204:819–827. doi: 10.1016/j.expneurol.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]