Abstract
Alpha-naphthyl isothiocyanate (ANIT) is a hepatotoxicant that produces acute intrahepatic cholestasis in rodents. Farnesoid X receptor (FXR) and pregnane X receptor (PXR) are two major bile acid sensors in liver. The purpose of this study was to characterize the regulation of hepatic transporters by FXR and PXR during ANIT-induced liver injury. Wild-type, FXR-null, and PXR-null mice were administered ANIT (75 mg/kg, po) and evaluated 48 h later for hepatotoxicity and messenger RNA (mRNA) expression of basolateral uptake (sodium taurocholate–cotransporting polypeptide, organic anion transporting polypeptide [Oatp] 1a1, Oatp1a4, Oatp1b2) and efflux transporters (organic solute transporter [Ost] α, Ostβ, multidrug resistance–associated protein [Mrp] 3, Mrp4), as well as canalicular transporters (bile salt export pump [Bsep], Mrp2, multidrug resistance protein 2 [Mdr2], ATPase, class I, type 8B, member 1 [Atp8b1]). Livers from wild-type and PXR-null mice had comparable multifocal necrosis 48 h after ANIT. However, ANIT-treated FXR-null mice have fewer and smaller necrotic foci than wild-type mice but had scattered single-cell hepatocyte necrosis throughout the liver. Serum alanine transaminase, alkaline phosphatase (ALP), and direct bilirubin were increased in all genotypes, with higher ALP levels in FXR-null mice. Serum and liver unconjugated bile acids were higher in ANIT-treated FXR-null mice than the other two genotypes. ANIT induced mRNA expression of Mdr2, Bsep, and Atp8b1 in wild-type and PXR-null mice but failed to upregulate these genes in FXR-null mice. mRNA expression of uptake transporters declined in livers of all genotypes following ANIT treatment. ANIT increased Ostβ and Mrp3 mRNA in livers of wild-type and PXR-null mice but did not alter Ostβ mRNA in FXR-null mice. In conclusion, FXR deficiency enhances susceptibility of mice to ANIT-induced liver injury, likely a result of impaired induction of hepatobiliary efflux transporters and subsequent hepatic accumulation of unconjugated bile acids.
Keywords: ANIT, FXR, PXR, transporters, bile acids, liver
Cholestasis is the disruption of bile flow that can occur at the cellular level of the hepatocyte, at the level of the intrahepatic biliary ductules, or as a result of extrahepatic obstruction of the bile ducts. Interruption of bile flow leads to the accumulation of bile acids and other bile components in the liver and, ultimately, hepatobiliary toxicity. Cholestasis is often divided into two categories, intrahepatic and extrahepatic, based upon etiology. Extrahepatic cholestasis is typically observed in patients with gallstones or tumors of the common biliary tract and is often recapitulated in rodents by bile duct ligation (BDL). Intrahepatic cholestasis is caused by physiological and pathological factors, including inborn errors of bile acid synthesis or transport (Colombo et al., 2000), pregnancy (de Pagter et al., 1976; Leevy et al., 1997; Lucena et al., 2003), chemicals (Plaa and Priestly, 1976), primary biliary cirrhosis (Jansen, 2000), and hepatitis (Trinchet et al., 1994). Whereas liver transplantation is appropriate for some patients with cholestasis, therapeutic options for treating intrahepatic cholestasis are limited (Burdelski and Rogiers, 1999). A better understanding of the molecular mechanisms underlying intrahepatic cholestasis is required to identify novel drug targets and improve current therapies.
Alpha-naphthyl isothiocyanate (ANIT) is a hepatotoxicant used in rodents to model human intrahepatic cholestasis. A single dose of ANIT produces cholestasis that is dose dependent (Plaa and Priestly, 1976). ANIT is conjugated with glutathione (GSH) in hepatocytes (Carpenter-Deyo et al., 1991) and transported into bile by the canalicular efflux transporter, multidrug resistance–associated protein (Mrp) 2 (Abcc2) (Dietrich et al., 2001). The ANIT-GSH conjugate dissociates upon crossing the canalicular membrane, yielding free GSH and ANIT in the bile (Dietrich et al., 2001). ANIT then injures bile duct epithelium leading to cholestasis. This liver injury is evident by detecting elevated serum bile acids and bilirubin, increased activities of aspartate aminotransferase, as well as histopathological lesions (Dahm et al., 1991).
Bile acids are synthesized in liver via two pathways, namely the classic and the alternative pathway (Chiang, 2004). In humans, cholic acid (CA) and chenodeoxycholic acid (CDCA) are the major primary bile acids synthesized via the classic pathway, whereas CDCA is the primary product synthesized by the alternative pathway (Russell, 2003). In mouse liver, CDCA is further converted to α- and β-muricholic acid (MCA) in liver. Therefore, the main bile acids in mice are CA and MCA (Elliott and Hyde, 1971), which are then conjugated with taurine (major cosubstrate in mice) or glycine and excreted into bile. Levels of bile acids in liver and serum are widely used biomarkers for cholestasis. Recently, a simple and sensitive ultra-performance liquid chromatography-mass spectrometry/mass spectrometry (UPLC-MS/MS) method for the simultaneous quantification of individual bile acids was developed in our laboratory (Alnouti et al., 2008) and was used in the present study to determine the composition of bile acids after ANIT administration in mice.
The basolateral uptake transporters, sodium taurocholate–cotransporting polypeptide (Ntcp, Slc10a1), as well as organic anion transporting polypeptide (Oatp, Slco) 1a1, 1a4, and 1b2, transport bile acids, and other substrates from portal blood into hepatocytes (Trauner and Boyer, 2003). The organic solute transporter (Ost) α and Ostβ function as a dimer, and efflux bile acids from hepatocytes into blood (Ballatori et al., 2005). Mrp3 (Abcc3) and Mrp4 (Abcc4) are expressed on the basolateral membrane and efflux conjugated bile acids and xenobiotics into blood (Hirohashi et al., 2000; Mennone et al., 2006). Multidrug resistance protein 2 (Mdr2, Abcb4) flips phospholipids from the interior to the exterior bileaflet of the canalicular membrane. The phospholipids enter the bile to form micelles with bile acids and protect the biliary tree from injury (Smit et al., 1993). Bile salt export pump (Bsep, Abcb11) and Mrp2 (Abcc2) transport bile acids into bile. Mrp2 also transports GSH and conjugated bilirubin. ATPase, class I, type 8B, member 1 (Atp8b1) transports aminophospholipids from the outer to the inner portion of the bileaflet of the canaliculi, which plays an important role in maintaining bile flow (Alvarez et al., 2004). Expression of uptake and efflux transporters is altered during various forms of liver injury. In general, the expression of uptake transporters is reduced, and levels of efflux transporters are increased in damaged livers (Slitt et al., 2007; Trauner and Boyer, 2003). This adaptive response is thought to reduce the intracellular concentration of bile acids and other toxicants in an attempt to protect against further injury and promote cellular recovery.
Farnesoid X receptor (FXR) and pregnane X receptor (PXR) are hepatic nuclear receptors which regulate gene transcription. Bile acids are endogenous ligands of both FXR and PXR (Chiang, 2002). Once activated by bile acids, FXR downregulates the bile acid–synthesizing enzyme, cytochrome P450 (Cyp) 7a1; downregulates Ntcp (Zollner et al., 2005); and upregulates Bsep (Plass et al., 2002). PXR increases the expression of Cyp enzymes, conjugation enzymes, and transporters involved in the metabolism and elimination of potentially toxic chemicals from the body (Chiang, 2002). FXR and PXR are two negative feedback mechanisms to reduce hepatic bile acid concentrations.
Previous studies have examined the roles of FXR and PXR in extrahepatic cholestasis using the BDL model (Stedman et al., 2006). However, little is known about the roles of these two nuclear receptors in modulating intrahepatic cholestasis. One potential mechanism for PXR- and FXR-mediated protection against cholestasis may be the regulation of liver transporters because ANIT and BDL alter the expression of uptake and efflux transport proteins (Hirohashi et al., 2000; Slitt et al., 2007). In order to identify the roles of FXR and PXR in intrahepatic cholestasis, a loss-of-function approach was employed in this study. FXR- and PXR-null mice were used to determine whether susceptibility to ANIT-induced toxicity is altered in the absence of these nuclear receptors. In addition, the contribution of PXR and FXR to the adaptive regulation of transporters during intrahepatic cholestasis was characterized.
MATERIALS AND METHODS
Materials and reagents.
ANIT was purchased from Sigma-Aldrich (St Louis, MO). GW4064 was synthesized by Dr Jeffery Aube (University of Kansas, Lawrence). Serum alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activities were quantified by colorimetric assays from Pointe Scientific, Inc. (Canton, MI). Mrp2 and Bsep antibodies were provided by Bruno Stieger (University Hospital, Zurich, Switzerland). Anti-rabbit Alexa 488 secondary antibodies were purchased from Invitrogen (Carlsbad, CA).
Animals.
Male C57BL/6 mice (aged 8–10 weeks, n = 5) were purchased from Charles River Laboratories (Wilmington, MA) and used as wild-type controls. Mice were housed according to the American Animal Association Laboratory Animal Care guidelines. Breeding pairs of FXR-null mice (Sinal et al., 2000) and PXR-null mice (Staudinger et al., 2001) on the C57BL/6 background were kindly provided by Dr Frank Gonzalez (National Cancer Institute, Bethesda, MD) and were bred under standard conditions at the University of Kansas Medical Center. Male FXR-null and PXR-null offspring were used in the present study (aged between 8–10 weeks, n = 5). Studies were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
A single dose of ANIT (75 mg/kg, 10 ml/kg in corn oil, po) or vehicle was administered to mice. Following euthanasia with pentobarbital (100 mg/kg, ip), blood was withdrawn by cardiac puncture. In the time course study, livers from wild-type mice were harvested 24 and 48 h after ANIT administration, whereas livers from FXR-null and PXR-null mice were collected only at 48 h post-dose. A portion of liver was placed in 10% buffered formalin. The remaining liver was frozen in liquid nitrogen and stored at − 80°C.
In the protection study, C57BL/6 mice (aged 8–10 weeks, n = 3) were treated with the FXR agonist GW4064 or vehicle for 4 days (30 mg/kg, ip in corn oil). On day 2, a single dose of ANIT (75 mg/kg, po in corn oil) was administered 4 h after GW4064 treatment. Livers were collected for histopathology on day 4 (4 h after the final dose of GW4064 and 48 h after ANIT).
Serum ALT and ALP quantification.
Serum samples were analyzed by standard enzymatic-colorimetric assays using ALT and ALP kits according to the manufacturer's protocols (Pointe Scientific, Inc.). Absorbance was quantified spectrophotometrically at wavelengths of 340 and 555 nm, respectively.
Characterization of serum and liver bile acid composition.
To determine the serum bile acid composition in control and ANIT-treated mice (n = 5 per group, 48 h), protein was precipitated by ice-cold acetonitrile (ACN). Briefly, 1 ml of ice-cold ACN was added to 100 μl serum spiked with 20 μl internal standards (2H4-G-CDCA and 2H4-CDCA), vortexed, and centrifuged at 11,000 × g for 10 min. The supernatant was aspirated, evaporated under vacuum, and reconstituted in 100 μl of 50% methanol (H2O). For liver samples, approximately 100 mg of liver was homogenized in two volumes of 50% MeOH (individual samples, n = 3). In all, 200 μl of the liver homogenate was spiked with 20 μl internal standards (2H4-G-CDCA and 2H4-CDCA), 2 ml of ice-cold alkaline ACN (5% NH4OH in ACN) was added, vortexed, and shaked continuously for 1 h, and centrifuged at 11,000 × g for 10 min. The supernatant was aspirated and precipitant was extracted with another 1 ml of ice-cold alkaline ACN. Supernatants from the two extraction steps were pooled, evaporated, and reconstituted in 100 μl of 50% MeOH. Different compositions of bile acids in serum and liver were determined by UPLC-MS/MS as described previously (Alnouti et al., 2008).
RNA isolation.
Total RNA was isolated using RNA Bee reagent (Tel-Test, Inc., Friendswood, TX) per the manufacturer's protocol. RNA concentrations were quantified using a NanoDrop Spectrophotometer (NanoDrop Technologies, Wilmington, DE) at a wavelength of 260 nm.
Branched DNA signal amplification technology.
The messenger RNA (mRNA) expression of Ntcp, Oatp1a1, Oatp1a4, Oatp1b2, Mrp3, Mrp4, Mdr2, Bsep, Mrp2, small heterodimer partner [SHP], Cyp7a1, and Cyp3a11 was determined by branched DNA amplification (bDNA) technology (QuantiGene 1.0 bDNA signal amplification kit; Panomics, Fremont, CA [Hartley and Klaassen, 2000]). Multiple oligonucleotide probe sets for Atp8b1, Cyp7a1, SHP, Ostα, and Ostβ (including capture, label, and blocker probes) were designed using ProbeDesigner Software v1.0 as described previously (Cheng et al., 2005b) and are shown in Supplementary Table 1. Probe sets for Ntcp, Oatp1a1, Oatp1a4, Oatp1b2, Mrp2-4 (Aleksunes et al., 2005), Mdr2, Bsep (Cheng and Klaassen, 2006), and Cyp3a11 (Cheng et al., 2005b) have been described previously. Data are reported as relative light units per 10 μg total RNA.
Histological processing.
Paraffin-embedded liver sections (5 μm) were stained with hematoxylin and eosin using standard protocols and examined for histopathologic changes (n = 3–5) by a board-certified veterinary pathologist.
Immunofluorescence analysis.
Livers were embedded in optimal cutting temperature compound and rapidly frozen at −80°C; 6-μm sections were generated with a Leica CM3050 Cryostat (Meyer Instruments, Inc., Pittsburg, PA) and fixed with 4% paraformaldehyde in PBS. Briefly, slides were blocked with 5% goat serum in 0.1% Triton X-100 in PBS and incubated with primary antibody diluted 1:100 for both Mrp2 and Bsep. After washing, sections were incubated with a species-appropriate Alexa 488 IgG secondary antibody (1:200). Sections were mounted in Prolong Gold (Invitrogen). Fluorescent staining was visualized on an Olympus B×41 microscope (Olympus Optical, Tokyo, Japan). Images were captured using an Olympus DP70 camera (×40) and DP Controller software.
Statistical analysis.
Data were analyzed by ANOVA, followed by Duncan's multiple range post hoc test. Statistical significance was considered at p < 0.05.
RESULTS
Serum and Liver Biomarkers 24 and 48 h after ANIT Administration
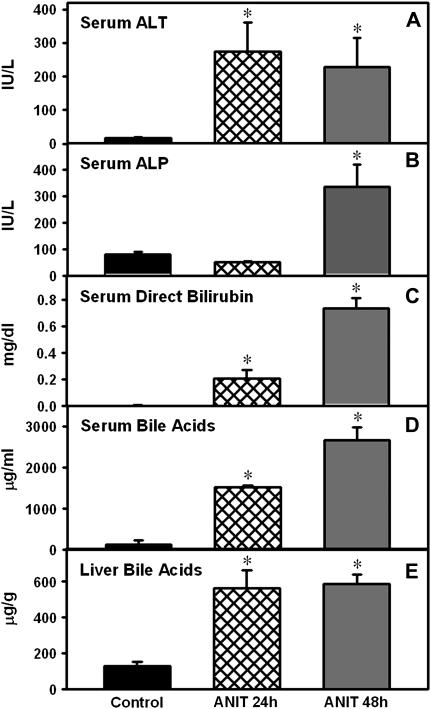
Twenty-four and forty-eight hours after vehicle administration, serum and liver biomarkers were within normal ranges in control mice and, thus, combined as a single group (Fig. 1). After ANIT administration, serum ALT was increased at both 24 (16-fold) and 48 h (13-fold) in wild-type mice (Fig. 1A), whereas serum ALP remained unchanged at 24 h but was elevated 3.2-fold at 48 h (Fig. 1B). ANIT increased serum direct bilirubin and bile acids at 24 h (42-fold and 13-fold), with a further increase at 48 h (144-fold and 23-fold, respectively) (Figs. 1C and D). ANIT increased bile acids in liver 4- to 5 fold at both 24 and 48 h after administration (Fig. 1E). In general, the maximum increase in these biomarkers was observed 48 h after ANIT.
FIG. 1.
Levels of serum and liver biomarkers 24 and 48 h after ANIT administration. Serum ALT (A) and ALP (B) are expressed as mean IU/l ± SEM. Serum direct bilirubin (C) is expressed as mean mg/dl ± SEM. Serum total bile acids (D) are expressed as μg/ml ± SEM. Liver total bile acids (E) are expressed as μg/g ± SEM; “*” Represents significant differences (p < 0.05) compared to control mice (n = 5 animals).
Hepatic mRNA Expression of Uptake and Efflux Transporters in Wild-Type Mice after ANIT Administration
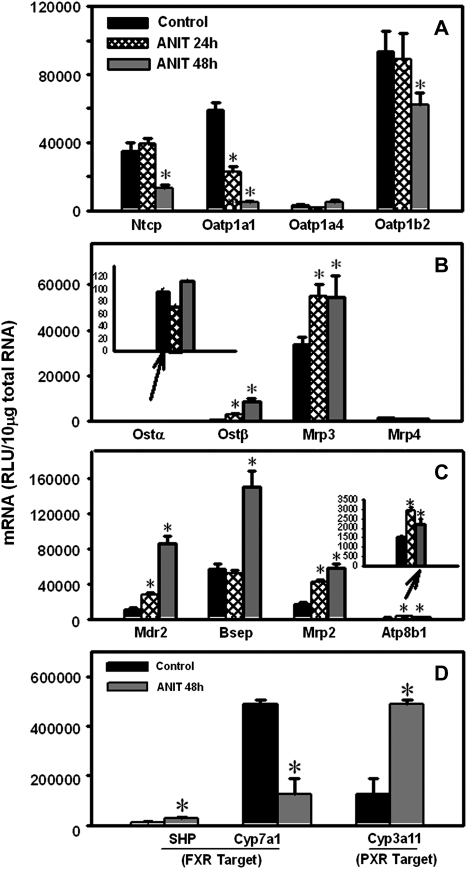
ANIT decreased Oatp1a1 mRNA (62%) at 24 h after ANIT administration. The expression of other uptake transporters was unchanged at this time point (Fig. 2A). By 48 h after ANIT treatment, the mRNA levels of Ntcp, Oatp1a1, and Oatp1b2 were reduced (63, 92, and 33%, respectively), whereas Oatp1a4 remained unchanged.
FIG. 2.
Time-dependent regulation of liver transporters in wild-type mice at 24 and 48 h after ANIT treatment (A, basolateral uptake; B, basolateral efflux; C, canalicular efflux). (D) Activation of FXR- and PXR-dependent pathways in wild-type livers 48 h after ANIT treatment. SHP and Cyp7a1, classic target genes for FXR; Cyp3a11, PXR target gene. Total RNA was isolated from control and ANIT-treated mouse livers and analyzed by the bDNA assay as described in the “Materials and Methods” section. Data are presented as mean relative light units ± SEM (n = 5 animals); “*” Represents significant differences (p < 0.05) compared with control.
The mRNA expression of the basolateral efflux transporter Ostβ increased 10-fold 24 h after ANIT, with a higher increase (35-fold) at 48 h (Fig. 2B). Mrp3 mRNA was upregulated 60% in livers from ANIT-treated wild-type mice at both 24 and 48 h. The mRNA expression of Ostα and Mrp4 was not altered by ANIT exposure. Canalicular efflux transporter expression was upregulated in ANIT-treated mice: Mdr2 (2.5-fold at 24 h; 7.8-fold at 48 h), Mrp2 (2.6-fold at 24 h; 3.6-fold at 48 h), and Atp8b1 (2-fold at 24 h; 1.5-fold at 48 h) (Fig. 2C). ANIT did not change Bsep mRNA at 24 h, but increased it 1.7-fold at 48 h. In general, maximal changes in serum biomarkers and the mRNA expression of transporters occurred 48 h after ANIT; therefore, 48 h post-dose was selected for subsequent experiments.
Hepatic mRNA Expression of Prototypical FXR and PXR Target Genes in Wild-Type Mice after ANIT Treatment
ANIT increased SHP mRNA 1.3-fold and Cyp7a1 mRNA decreased 74% in wild-type mice, which both indicate FXR activation (Fig. 2D). Cyp3a11 mRNA was increased 2.9-fold after ANIT treatment, demonstrating activation of PXR. Thus, both FXR- and PXR-dependent pathways were activated in livers of wild-type mice 48 h after ANIT administration.
Hepatotoxicity in Wild-Type, FXR-Null, and PXR-Null Mice after ANIT Administration
Because FXR and PXR target genes were activated by ANIT treatment in wild-type mice, FXR-null and PXR-null mice were administered ANIT to determine the role of these nuclear receptors in protecting against intrahepatic cholestasis.
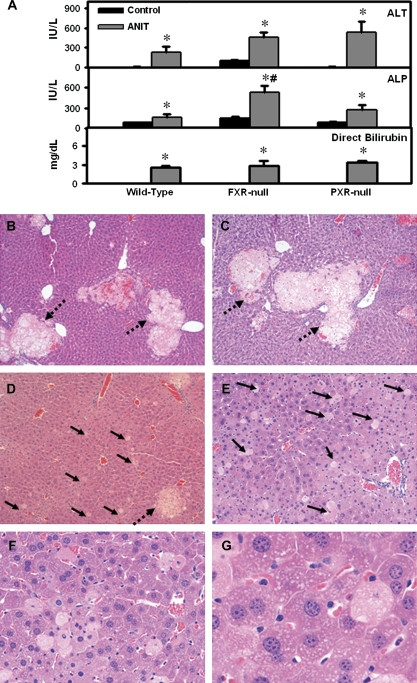
Serum ALT, ALP, and direct bilirubin levels were elevated in all ANIT-treated genotypes (Fig. 3A), with a higher increase in ALP observed in FXR-null mice (Fig. 3A, middle panel). Livers from vehicle-treated wild-type, FXR-null, and PXR-null mice had normal histology (data not shown). Representative histological sections are presented for ANIT-treated wild-type (Fig. 3B), PXR-null (Fig. 3C), and FXR-null mice (Figs. 3D–F).
FIG. 3.
(A) Serum alanine aminotransferase (ALT), ALP, and direct bilirubin 48 h after ANIT administration to wild-type, FXR-null, and PXR-null mice. Serum ALT and ALP are expressed as mean IU/l ± SEM. Serum direct bilirubin is expressed as mean mg/dl ± SEM. “*” Represents significant differences (p < 0.05) compared with vehicle-treated mice. “#” Represents significant differences (p < 0.05) compared with ANIT-treated wild-type mice (n = 5 animals). (B–F) Histopathological analysis of livers from wild-type, FXR-null, and PXR-null mice 48 h after ANIT administration. (B) Mild, multifocal periportal necrosis in wild-type liver (×10); (C) similar periportal necrosis in PXR-null liver (×10); (D) minimal multifocal periportal necrosis and minimal to mild single-cell necrosis in FXR-null liver (×10); (E) FXR-null remaining parenchyma (×10); (F) higher magnification of ANIT-treated FXR-null liver highlighting single-cell necrosis (×20); (G) pale vesicular cytoplasm of periportal hepatocytes (×40). Dashed arrow, focal necrosis; solid arrow, single-cell hepatocellular necrosis.
Though livers from ANIT-treated mice in all three genotypes had multifocal periportal coagulative necrosis (intact cells with intense eosinophilic staining and distorted nuclei near bile ducts as a result of bile duct damage), wild-type and PXR-null mice had 7–8% multifocal necrosis compared to 2% in FXR-null mice (Figs. 3B–D). Most of the parenchyma surrounding the necrotic regions in wild-type and PXR-null mice was normal (Figs. 3B and C). More importantly, livers from ANIT-treated FXR-null mice had diffuse single-cell necrosis and apoptosis instead of normal hepatocellular parenchyma (Figs. 3E–G). Furthermore, ANIT-treated FXR-null mice had increased periportal hyperplasia (higher number of small hepatocytes and mitotic figures) (Fig. 3F) and the remaining hepatocytes were pale with vesicular cytoplasm (Fig. 3G). Diffuse biliary and hepatocellular damage of FXR-null mice livers after ANIT treatment is consistent with higher serum ALP activity in these mice. In addition, there was minimal multifocal biliary hyperplasia as well as neutrophil infiltration in and adjacent to necrotic areas in all three genotypes after ANIT administration (data not shown).
Similar to the histopathology, gross observation of livers revealed differences between the three genotypes after ANIT (Supplementary Fig. 1). Vehicle-treated livers from all three genotypes were normal with a dark red color (Supplementary Figs. 1A, 1C, and 1E). Likewise, the liver lobes from ANIT-treated wild-type and PXR-null mice appeared similar to controls (Supplementary Figs. 1B and 1D). In contrast, livers from ANIT-treated FXR-null mice had a nutmeg pattern (diffuse pallor punctuated by miliary red foci) (Supplementary Fig. 1F). The gross appearance of livers from FXR-null mice corresponds with periportal necrosis rimmed by pale vacuolated hepatocytes. In addition, ANIT-treated FXR-null mice also had significantly decreased body weight, impaired righting reflex, and reduced motor activity, whereas wild-type and PXR-null mice generally showed normal activity following ANIT treatment but did have decreased body weights (data not shown).
Serum and Liver Bile Acid Concentrations in Wild-Type, FXR-Null, and PXR-Null Mice after ANIT Administration
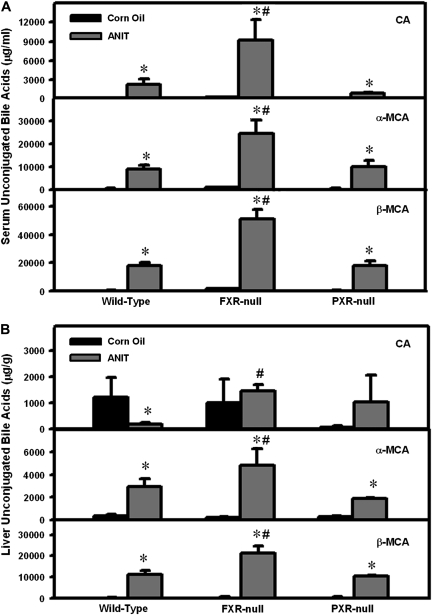
To aid in determining the extent of ANIT-induced cholestasis, total bile acids, including their taurine and glycine conjugates and unconjugated bile acids, were determined in serum and livers from all three genotypes by UPLC-MS/MS. There was a mild elevation in serum total bile acid concentrations in control FXR-null mice. Forty-eight hours after ANIT administration, serum total bile acids were markedly elevated to a similar extent in all three genotypes (Supplementary Fig. 2). ANIT increased the major serum unconjugated bile acids, including CA, as well as α- and β-MCA, in all genotypes, with a further increase in ANIT-treated FXR-null mice (Fig. 4A). CA concentrations in liver were lower in ANIT-treated wild-type mice but remained unchanged in FXR-null and PXR-null mice after ANIT. However, both α- and β-MCAs were increased by ANIT in all genotypes, with higher levels in FXR-null mice (Fig. 4B). Other unconjugated bile acids in serum and liver were close to background levels (data not shown). Profiles of serum and liver conjugated bile acids in all three genotypes are shown in Supplementary Figures 3 and 4. In summary, ANIT increased bile acids in both serum and livers of mice, and although the total bile acid levels were comparable among all ANIT-treated genotypes, there was a further increase in the profiles of unconjugated bile acids in both serum and livers of FXR-null mice.
FIG. 4.
Serum and liver unconjugated bile acids in control and ANIT-treated wild-type, FXR-null, and PXR-null mice. Bile acids were quantified by UPLC-MS/MS as described in the “Materials and Methods” section. (A) Serum unconjugated bile acids are expressed as μg/ml ± SEM. (B) Liver unconjugated bile acids are expressed as μg/g ± SEM. “*” Represents significant differences (p < 0.05) compared to control mice (n = 5 animals). “#” Represents significant differences (p < 0.05) compared with ANIT-treated wild-type mice.
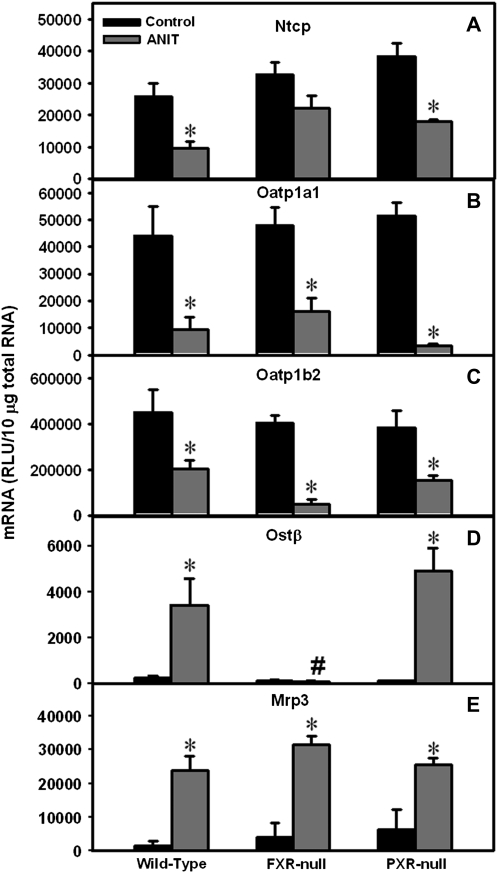
Hepatic mRNA Expression of Basolateral Transporters in Wild-Type, FXR-Null, and PXR-Null Mice after ANIT Administration
Based on the results in Figure 2, only the transporters that were altered by ANIT administration were considered in subsequent experiments. For basolateral uptake transporters, ANIT decreased Ntcp mRNA levels in wild-type, FXR-null, and PXR-null mice 63, 32, and 53%, respectively (Fig. 5A); however, the decrease in Ntcp mRNA in FXR-null mice was not statistically significant. Similarly, ANIT downregulated Oatp1a1 mRNA in wild-type, FXR-null, and PXR-null mice (79, 67, and 94% decrease, respectively) (Fig. 5B) and down-regulated Oatp1b2 mRNA in all three genotypes (55% decrease in wild-type; 88% in FXR-null; 61% in PXR-null mice) (Fig. 5C).
FIG. 5.
Expression of basolateral transporters Ntcp, Oatp1a1, and Oatp1b2 (uptake), as well as Ostβ and Mrp3 (efflux) in wild-type, FXR-null, and PXR-null mice 48 h after ANIT administration. Total RNA was isolated from control and ANIT-treated mouse livers and analyzed by the bDNA assay as described in the “Materials and Methods” section. Data are presented as mean relative light units ± SEM (n = 5 animals). “*” Represents significant differences (p < 0.05) compared with genotype-matched control mice. “#” Represents significant differences (p < 0.05) compared with ANIT-treated wild-type mice.
For basolateral efflux transporters, hepatic Ostβ mRNA was low in vehicle-treated mice of all three genotypes, but after ANIT administration, it was markedly increased in livers of wild-type and PXR-null mice (14- and 40-fold, Fig. 5D). However, ANIT did not change Ostβ mRNA in livers of FXR-null mice. ANIT increased Mrp3 mRNA in livers of wild-type (17-fold), FXR-null (8-fold), and PXR-null (4-fold) mice (Fig. 5E). Taken together, mRNA expression of the basolateral uptake transporters was decreased in all genotypes, whereas basolateral efflux transporters were induced in livers of ANIT-treated wild-type and PXR-null mice, with no change in Ostβ in FXR-null mice.
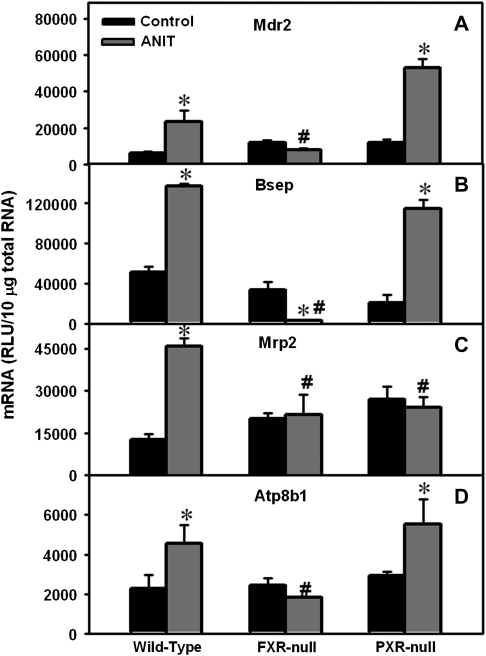
Hepatic mRNA Expression of Canalicular Transporters in Wild-Type, FXR-Null, and PXR-Null Mice after ANIT Administration
Mdr2 mRNA was increased in livers from ANIT-treated wild-type (4-fold) and PXR-null mice (4-fold), with no change in FXR-null mice (Fig. 6A). ANIT increased Bsep mRNA in livers from wild-type and PXR-null mice (2.7- and 5.5-fold, Fig. 6B). In contrast, ANIT actually decreased Bsep mRNA 92% in livers of FXR-null mice. ANIT increased Mrp2 mRNA expression in livers from wild-type mice (3.6-fold) but did not change it in FXR- and PXR-null mice (Fig. 6C). ANIT induced Atp8b1 mRNA 2-fold in livers of wild-type and PXR-null mice, with no induction in FXR-null mice (Fig. 6D). To summarize, ANIT induced the mRNA expression of most of the examined canalicular transporters in livers of wild-type and PXR-null mice but failed to upregulate the mRNA expression of these genes in FXR-null mice.
FIG. 6.
Expression of canalicular efflux transporters Mdr2, Bsep, Mrp2, and Atp8b1 in wild-type, FXR-null, and PXR-null mice 48 h after ANIT administration. Total RNA was isolated from control and ANIT-treated mouse livers and analyzed by the bDNA assay as described in the “Materials and Methods” section. Data are presented as mean relative light units ± SEM (each group, n = 5 animals). “*” Represents statistically significant differences (p < 0.05) compared with control. “#” Represents significant differences (p < 0.05) compared with ANIT-treated wild-type mice.
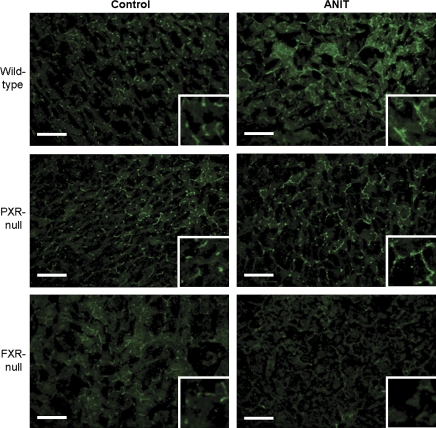
Immunofluorescent Localization of Mrp2 and Bsep Proteins in Livers from Wild-Type, FXR-Null, and PXR-Null Mice after ANIT Treatment
Mrp2 and Bsep immunofluorescence was performed on frozen liver sections from vehicle and ANIT-treated groups of all three genotypes to determine cellular and zonal transporter expression. In Figure 7, Mrp2 (green) was localized to the canalicular junctions between adjacent hepatocytes in all three control groups. Following ANIT treatment, enhanced canalicular Mrp2 staining throughout the liver lobule was observed only in wild-type mice. Bsep (green) was localized to the canalicular membrane of hepatocytes in control groups (Fig. 8) with enhanced canalicular Bsep staining in both ANIT-treated wild-type and PXR-null mice. Subapical and cytosolic Bsep staining was also observed in the vicinity of the canalicular membrane in livers of ANIT-treated wild-type mice. Conversely, a marked reduction of Bsep staining was observed in livers of ANIT-treated FXR-null mice, which correlates with decreased Bsep mRNA expression. Ostβ immunofluorescence was not detectable in any groups, likely due to low expression in liver (data not shown). There are no specific antibodies available commercially to detect mouse Mdr2 protein.
FIG. 7.
Immunofluorescence of Mrp2 protein in livers from wild-type, FXR-null, and PXR-null mice 48 h after ANIT. Immunofluorescence against canalicular Mrp2 (green) was conducted on liver cryosections as described in the “Materials and Methods” section. Portions of images were enlarged and provided as inserts. Representative images are shown. Bar, 50 μm.
FIG. 8.
Immunofluorescence of Bsep protein in livers from wild-type, FXR-null, and PXR-null mice 48 h after ANIT. Immunofluorescence against canalicular Bsep (green) was conducted on liver cryosections as described in “Materials and Methods” section. Portions of images were enlarged and provided as inserts. Representative images are shown. Bar, 50 μm.
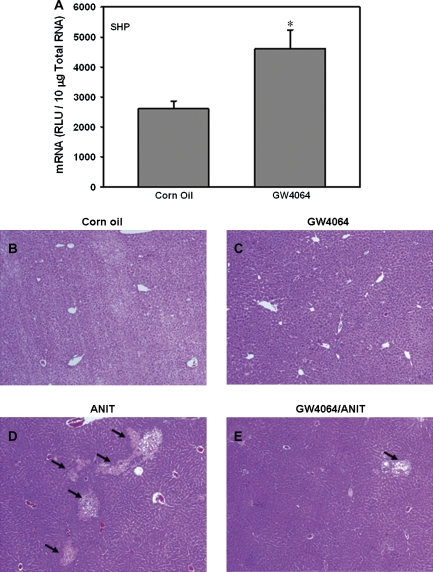
ANIT Hepatoxicity in Wild-Type Mice Pretreated with the FXR Agonist GW4064
GW4064 treatment induced SHP mRNA expression (twofold) in wild-type mice, demonstrating activation of FXR (Fig. 9A). To determine the protective effects in ANIT-induced hepatic injury, wild-type mice were treated with GW4064 for 4 days, and on the second day, a single dose of ANIT was administered. Mice were sacrificed 4 h after the final dose of GW4064. Livers from vehicle and GW4064-treated mice were histologically normal (Figs. 9B and C, respectively), whereas after ANIT administration, numerous necrotic foci were observed in mouse livers (Fig. 9D). GW4064 pretreatment markedly reduced the area of hepatocyte necrosis (Fig. 9E).
FIG. 9.
Pharmacological activation of FXR pathway by GW4064 induces the prototypical FXR target gene SHP mRNA expression (A) and protects liver from ANIT toxicity in wild-type mice (B–E). “*” Represents statistically significant differences (p < 0.05) compared with control; (B) (vehicle control) and (C) (GW4064 treated) have no lesions; (D) (ANIT treatment only) has mild necrosis, while (E) (ANIT and GW4064) had minimal necrosis. Magnification: ×10.
DISCUSSION
This study demonstrates that ANIT-induced liver injury results in the adaptive downregulation of hepatic uptake transporters and upregulation of efflux transporters in a time-dependent pattern. Changes in transporter expression were most prominent at 48 h and correlated with activation of FXR and PXR signaling pathways. Compared with ANIT-treated wild-type and PXR-null mice, FXR-null mice exhibited nutmeg liver on gross examination, higher serum ALP levels, as well as diffuse hepatocellular single-cell necrosis, however, fewer and smaller periportal necrotic foci. Enhanced ANIT toxicity in FXR-null mice is likely due to impaired induction of a number of efflux transporters and subsequent accumulation of unconjugated bile acids inside hepatocytes. Pharmacological activation of mouse FXR by the agonist GW4064 protected the liver from ANIT-induced injury, indicating that FXR is, indeed, beneficial and may be a good therapeutic target for intrahepatic cholestasis.
Two types of liver injury were observed in this study: focal necrosis and single-cell necrosis. Focal necrosis was observed in all three genotypes, although the number and size of necrotic foci were much smaller in ANIT-treated FXR-null mice. Periportal focal necrosis is a consequence of damage to biliary epithelium and subsequent obstruction/rupture of distended canaliculi or smaller cholangioles with inflammation, elevated extracellular pressure, and leakage of biliary contents. This type of pathology is typically striking and easy to quantify but is of little functional significance for the liver, even when foci are numerous (Jubb, 1993). This is because the remaining parenchyma is largely unaffected (∼90% in ANIT-treated wild-type and PXR-null livers) and hepatocytes in these regions have normal liver function as evidenced by healthy motor activity and righting reflex in these animals.
Single-cell necrosis (also referred to as necrobiosis) was also observed in the livers of ANIT-treated FXR-null mice. Though single-cell necrosis occurs at the cellular level and involves numerous hepatocytes, it is not easily quantified due to the rapid removal of individual cells by macrophages or adjacent hepatocytes. In the case of ANIT-treated FXR-null mice, single-cell necrosis was randomly scattered throughout the parenchyma and involved a mixture of necrotic and apoptotic hepatocytes. This pattern of cellular injury in FXR-null mice appears to cause greater deficits in hepatocyte function witnessed by global changes in animal behavior (poor motor activity and impaired righting reflex). In addition, FXR-null mice showed elevated mRNA expression of Gadd45α and β, which are central stress sensors involved in apoptosis (data not shown). Conversely, livers from ANIT-treated FXR-null mice expressed lower mRNA levels of constitutive androstane receptor (CAR, which is important for cellular homeostasis), peroxisome proliferator–activated receptor (PPAR) γ coactivator-1α (a critical metabolic regulator in glucose and lipid homeostasis), nuclear factor erythroid 2–related factor 2 transcription factor (a crucial transcription factor against oxidative stress), and liver receptor homolog-1 (important for bile acid homeostasis) compared to wild-type and PXR-null mice (data not shown). A combination of focal and single-cell hepatic necrosis in ANIT-treated FXR-null mice corresponded to a pale nutmeg-like liver appearance, higher serum ALP activity, and higher hepatic levels of unconjugated bile acids (likely a result of impaired induction of efflux transporters). Taken together, these data indicate that FXR deficiency in mice enhances susceptibility to ANIT-induced liver injury.
Clinical evidence indicates that impaired expression and/or function of liver efflux transporters causes intrahepatic cholestasis. For example, genetic defects in human ATP8B1, BSEP, or MDR3 (human ortholog of mouse Mdr2) lead to progressive familial intrahepatic cholestasis (type I, II, and III, respectively) (de Pagter et al., 1976; Smit et al., 1993). In addition, a genetic defect in human MRP2 results in Dubin-Johnson syndrome, which is characterized by chronic hyperbilirubinemia and jaundice (Hashimoto et al., 2002; Paulusma et al., 1997). The intrahepatic cholestasis observed with the antidiabetic drug troglitazone has been proposed to result from Bsep inhibition (Funk et al., 2001). An inability to induce Mrp2, Bsep, Mdr2, and Atp8b1 mRNA expression, as well as a further decrease in Bsep protein expression (as seen in ANIT-treated FXR-null mice) appears to reduce hepatocyte viability and function. Using NHR Scan software, we identified four putative binding sites for FXR (IR-1) within 10 kb upstream and 1 kb downstream of the promoter regions of both Mdr2 and Mrp2, which suggest that FXR may be important in mediating the transcription of these genes.
Bsep is considered the major bile acid efflux transporter on the canalicular membrane. In livers from ANIT-treated FXR-null mice, Bsep mRNA was not induced and was below basal levels. Reduced Bsep mRNA correlated with diminished canalicular staining of Bsep protein after ANIT treatment. Although the mechanism for this decrease in Bsep is unknown, the mRNAs of several other liver transcription factors are also lower in ANIT-treated FXR-null mice. These factors include the xenobiotic sensor, CAR, and the lipid sensor, PPARα (data not shown). Conversely, in ANIT-treated wild-type and PXR-null mice, both CAR and PPARα mRNAs were increased (data not shown). Within the 10-kb promoter plus 1-kb coding regions of the Bsep gene, 4 PPARα putative binding sites (direct repeat [DR]-1), and 14 CAR putative binding sites (one DR-2, two DR-3, four DR-4, and seven everted repeat-6 [ER-6]) were identified using NHR Scan software. This indicates that decreased expression of PPARα and/or CAR might cause the downregulation of Bsep mRNA in FXR-null mice. This being said, the effects of these transcription factors on Bsep gene regulation remain elusive.
The basal expression of liver transporters, including the basolateral uptake (Ntcp and Oatps) and efflux (Ostβ and Mrp3), and the canalicular transporters (Mdr2, Bsep, and Atp8b1) is similar among the three genotypes. It is only after ANIT administration that differential expression of transporters is observed. FXR-null mice have lower mRNA levels of canalicular transporters Bsep, Mdr2, Mrp2, and Atp8b1, as well as the basolateral transporter Ostβ after ANIT compared to WT mice. Therefore, it seems that the basal expression of liver transporters does not contribute to the susceptibility of the different genotypes to ANIT, but it is the loss of FXR, which impairs induction of these transporters and progresses liver injury in FXR-null mice. In addition, it has been demonstrated previously that Mrp2 is the primary transporter to eliminate GSH-conjugated ANIT via the biliary route (Dietrich et al., 2001). The basal expression of Mrp2 mRNA is similar among the three genotypes. The impaired induction of Mrp2 expression in FXR-null and PXR-null mouse livers 24–48 h after ANIT likely does not affect ANIT-GSH disposition. Although there are no reports of ANIT pharmacokinetics in mice, it has been demonstrated in rats that the biliary concentration of ANIT (100 mg/kg, po) falls from 78 to 29μM at 1 and 4 h post-dose, respectively (Jean and Roth, 1995). Therefore, it is likely that by 24–48 h (when Mrp2 mRNA is induced in wild-type mice), the majority of ANIT has been excreted.
Cholestasis is associated with increased levels of bile acids (substrates of Bsep and Ostβ) and conjugated bilirubin (Mrp2 substrate). The substrates of Bsep include tauro-chenodeoxycholic acid, tauro-ursodeoxycholic acid, tauro-cholic acid, glycocholic acid, and CA (Gerloff et al., 1998). Although total bile acid levels in serum and liver are comparable among the three genotypes after ANIT, FXR-null mice had higher accumulation of unconjugated bile acids (CA and MCAs) in liver and serum (Fig. 4) and a tendency of higher glycocholic acid (Supplementary Figs. 2 and 3) in liver and serum, indicating that less bile acids are excreted into bile due to lower Bsep expression in FXR-null mice. FXR-mediated regulation of efflux transporters is likely necessary to protect against progression of liver injury, by clearing toxic bile constituents from the liver. It has been shown that CA feeding results in marked liver necrosis in FXR-null mice (Zollner et al., 2003a) and another group also demonstrated that FXR is critical in enhancing the canalicular bile acid output and excretion of unconjugated bile acids, which protects against CA feeding–induced liver toxicity (Miyata et al., 2005). In the present study, excessive levels of CA and other unconjugated bile acids likely contribute to injury to individual hepatocytes (diffuse single-cell necrosis) throughout the livers of FXR-null mice after ANIT.
Although extrahepatic and intrahepatic cholestasis are both characterized by decreased bile flow and increased accumulation of bile constituents in liver and serum, it has not been determined whether FXR and PXR exert similar roles in the two types of cholestasis. Numerous studies using the BDL model have yielded conflicting results regarding the influence of FXR and PXR in extrahepatic cholestasis. Whereas pretreatment with the selective FXR agonist GW4064 protected the liver from BDL-induced injury in rats (Liu et al., 2003), another study showed that FXR deficiency protected against BDL-induced liver injury in mice (Stedman et al., 2006). PXR deficiency in mice enhanced BDL-induced hepatotoxicity (Stedman et al., 2005), suggesting that PXR protects the liver from extrahepatic cholestasis. Less is known of the role of FXR and PXR in intrahepatic cholestasis. Decreased expression of FXR or PXR, or both, has been observed clinically in several types of intrahepatic cholestasis (Chen et al., 2004; Nagasaka et al., 2007; Van Mil et al., 2007), indicating that these nuclear receptors may play a role in the pathogenesis of intrahepatic cholestasis. The present study better defines the consequences of FXR and PXR deficiency in ANIT-induced intrahepatic cholestasis and demonstrates that FXR deficiency (not PXR deficiency) produces deleterious effects to the liver. Taken together, intrahepatic and extrahepatic cholestases have different injury patterns in mice lacking bile acid sensors.
The adaptive regulation of liver transporters is similar between extrahepatic and intrahepatic cholestasis. BDL decreased the mRNA expression of liver uptake transporters including Ntcp and several Oatps and increased the mRNA expression of efflux transporters, including Bsep and Mrps in mice (Slitt et al., 2007). ANIT also decreased the mRNA levels of uptake transporters Ntcp and Oatp1a1 and induced efflux transporters Mdr2, Mrp2, and Mrp3 in rats (Liu et al., 2003). The present study demonstrates that ANIT decreases the mRNA of Ntcp, Oatp1a1, Oatp1b2, and increases Ostβ, Mrp3, Mdr2, Bsep, and Mrp2 levels in wild-type mice. It should be noted that Mrp4 is differentially regulated in obstructive and intrahepatic cholestasis. Mrp4 mRNA was upregulated in wild-type mice after BDL (Stedman et al., 2006), whereas it was unchanged by ANIT administration in the current study. Clinically, patients with intrahepatic cholestasis due to primary biliary cirrhosis have decreased levels of uptake transporters (NTCP, OATP1B1), preserved expression of the efflux transporters BSEP and MRP2, and increased expression of MDR3 and MRP3 (Zollner et al., 2003b). Therefore, downregulation of uptake transporters and induction of some efflux transporters appear to be a general pattern in both intrahepatic and obstructive cholestasis.
When bile acid sensors are absent, the regulatory patterns of transporters in extrahepatic and intrahepatic cholestasis can be different. For example, in BDL-induced cholestasis, Bsep mRNA was not altered in wild-type mice but decreased in both FXR- and PXR-null mice (Stedman et al., 2006). However, in ANIT-induced intrahepatic cholestasis, Bsep mRNA was induced in both wild-type and PXR-null mice but was decreased in FXR-null mice.
A previous study demonstrated that pretreating rats with the potent FXR agonist GW4064 protects against ANIT- and BDL-induced liver injury (Liu et al., 2003). The present study is the first in mice to demonstrate that GW4064 is efficacious in protecting the liver from ANIT-induced injury. The reproducibility of the GW4064 protection against ANIT in mice is important for proof of concept supporting FXR as a possible therapeutic target for patients with intrahepatic cholestasis. With the dose utilized in the present study, the mRNA expression of the FXR target gene SHP was induced in wild-type mice treated with GW4064 (data not shown), indicating that FXR-mediated pathway is, indeed, activated by this compound. Interestingly, expression of FXR is decreased in patients with primary biliary cirrhosis-related intrahepatic cholestasis (Zollner et al., 2005) and in patients with progressive familial intrahepatic cholestasis type I (Chen et al., 2004; Nagasaka et al., 2007). In addition, novel FXR variants have been identified in patients with intrahepatic cholestasis of pregnancy (Van Mil et al., 2007). Taken together, FXR might be a therapeutic target for several types of intrahepatic cholestasis, and GW4064 may be useful to prevent cholestatic injury.
In conclusion, FXR deficiency enhances susceptibility to mice of ANIT-induced liver injury and is likely a result of hepatocyte accumulation of unconjugated bile acids due to impaired induction of hepatobiliary efflux transporters.
SUPPLEMENTARY DATA
Supplementary Figures 1–4 and Table 1 are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (ES-009649, ES-009716, ES-007079, and ES-013714 to C.D.K.).
Acknowledgments
The authors would like to thank K. Brian Lee for assistance with imaging and Dr Frank Gonzalez (NCI, Bethesda, MD) for providing the breeding pairs of the FXR-null and PXR-null mice. Portions of this work were presented at the national meeting of the Society of Toxicology for which Yue Julia Cui received the Carl C. Smith First Place Award, 16–20 March 2008.
References
- Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol. Sci. 2005;83(1):44–52. doi: 10.1093/toxsci/kfi013. [DOI] [PubMed] [Google Scholar]
- Alnouti Y, Csanaky IL, Klaassen CD. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008;873:209–217. doi: 10.1016/j.jchromb.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez L, Jara P, Sanchez-Sabate E, Hierro L, Larrauri J, Diaz MC, Camarena C, De la Vega A, Frauca E, Lopez-Collazo E, et al. Reduced hepatic expression of farnesoid X receptor in hereditary cholestasis associated to mutation in ATP8B1. Hum. Mol. Genet. 2004;13(20):2451–2460. doi: 10.1093/hmg/ddh261. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTalpha-OSTbeta: A major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42(6):1270–1279. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]
- Burdelski M, Rogiers X. Liver transplantation in metabolic disorders. Acta Gastroenterol. Belg. 1999;62(3):300–305. [PubMed] [Google Scholar]
- Carpenter-Deyo L, Marchand DH, Jean PA, Roth RA, Reed DJ. Involvement of glutathione in 1-naphthylisothiocyanate (ANIT) metabolism and toxicity to isolated hepatocytes. Biochem. Pharmacol. 1991;42(11):2171–2180. doi: 10.1016/0006-2952(91)90353-7. [DOI] [PubMed] [Google Scholar]
- Chen F, Ananthanarayanan M, Emre S, Neimark E, Bull LN, Knisely AS, Strautnieks SS, Thompson RJ, Magid MS, Gordon R, et al. Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid X receptor activity. Gastroenterology. 2004;126(3):756–764. doi: 10.1053/j.gastro.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Cheng X, Klaassen CD. Regulation of mRNA expression of xenobiotic transporters by the pregnane x receptor in mouse liver, kidney, and intestine. Drug Metab. Dispos. 2006;34(11):1863–1867. doi: 10.1124/dmd.106.010520. [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Chen C, Klaassen CD. Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps) Drug Metab. Dispos. 2005a;33(7):1062–1073. doi: 10.1124/dmd.105.003640. [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Dieter MZ, Klaassen CD. Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab. Dispos. 2005b;33(9):1276–1282. doi: 10.1124/dmd.105.003988. [DOI] [PubMed] [Google Scholar]
- Chiang JY. Bile acid regulation of gene expression: Roles of nuclear hormone receptors. Endocr. Rev. 2002;23(4):443–463. doi: 10.1210/er.2000-0035. [DOI] [PubMed] [Google Scholar]
- Chiang JY. Regulation of bile acid synthesis: Pathways, nuclear receptors, and mechanisms. J. Hepatol. 2004;40(3):539–551. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Colombo C, Okolicsanyi L, Strazzabosco M. Advances in familial and congenital cholestatic diseases. Clinical and diagnostic implications. Dig. Liver Dis. 2000;32(2):152–159. doi: 10.1016/s1590-8658(00)80403-x. [DOI] [PubMed] [Google Scholar]
- Dahm LJ, Schultze AE, Roth RA. An antibody to neutrophils attenuates alpha-naphthyl isothiocyanate-induced liver injury. J. Pharmacol. Exp. Ther. 1991;256(1):412–420. [PubMed] [Google Scholar]
- de Pagter AG, van Berge Henegouwen GP, ten Bokkel Huinink JA, Brandt KH. Familial benign recurrent intrahepatic cholestasis. Interrelation with intrahepatic cholestasis of pregnancy and from oral contraceptives? Gastroenterology. 1976;71(2):202–207. [PubMed] [Google Scholar]
- Dietrich CG, Ottenhoff R, de Waart DR, Oude Elferink RP. Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology. 2001;167(1):73–81. doi: 10.1016/s0300-483x(01)00459-0. [DOI] [PubMed] [Google Scholar]
- Elliott WH, Hyde PM. Metabolic pathways of bile acid synthesis. Am. J. Med. 1971;51(5):568–579. doi: 10.1016/0002-9343(71)90281-6. [DOI] [PubMed] [Google Scholar]
- Funk C, Ponelle C, Scheuermann G, Pantze M. Cholestatic potential of troglitazone as a possible factor contributing to troglitazone-induced hepatotoxicity: In vivo and in vitro interaction at the canalicular bile salt export pump (Bsep) in the rat. Mol. Pharmacol. 2001;59(3):627–635. [PubMed] [Google Scholar]
- Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J. Biol. Chem. 1998;273:10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- Hartley DP, Klaassen CD. Detection of chemical-induced differential expression of rat hepatic cytochrome P450 mRNA transcripts using branched DNA signal amplification technology. Drug Metab. Dispos. 2000;28(5):608–616. [PubMed] [Google Scholar]
- Hashimoto K, Uchiumi T, Konno T, Ebihara T, Nakamura T, Wada M, Sakisaka S, Maniwa F, Amachi T, Ueda K, et al. Trafficking and functional defects by mutations of the ATP-binding domains in MRP2 in patients with Dubin-Johnson syndrome. Hepatology. 2002;36(5):1236–1245. doi: 10.1053/jhep.2002.36368. [DOI] [PubMed] [Google Scholar]
- Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3) J. Biol. Chem. 2000;275(4):2905–2910. doi: 10.1074/jbc.275.4.2905. [DOI] [PubMed] [Google Scholar]
- Jansen PL. The pathophysiology of cholestasis with special reference to primary biliary cirrhosis. Bailliere's Best Pract. Res. Clin. 2000;14(4):571–583. doi: 10.1053/bega.2000.0104. [DOI] [PubMed] [Google Scholar]
- Jean PA, Roth RA. Naphthylisothiocyanate disposition in bile and its relationship to liver glutathione and toxicity. Biochemical Pharmacology. 1995;50:1469–1474. doi: 10.1016/0006-2952(95)02051-9. [DOI] [PubMed] [Google Scholar]
- Jubb KVF, Kennedy PC, Palmer N. 1993. Pathology of domestic animals. Academic Press, San Diego. [Google Scholar]
- Leevy CB, Koneru B, Klein KM. Recurrent familial prolonged intrahepatic cholestasis of pregnancy associated with chronic liver disease. Gastroenterology. 1997;113(3):966–972. doi: 10.1016/s0016-5085(97)70193-8. [DOI] [PubMed] [Google Scholar]
- Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, MacKenzie KI, Mansfield TA, Kliewer SA, Goodwin B, et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J. Clin. Invest. 2003;112(11):1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucena JF, Herrero JI, Quiroga J, Sangro B, Garcia-Foncillas J, Zabalegui N, Sola J, Herraiz M, Medina JF, Prieto J. A multidrug resistance 3 gene mutation causing cholelithiasis, cholestasis of pregnancy, and adulthood biliary cirrhosis. Gastroenterology. 2003;124(4):1037–1042. doi: 10.1053/gast.2003.50144. [DOI] [PubMed] [Google Scholar]
- Mennone A, Soroka CJ, Cai SY, Harry K, Adachi M, Hagey L, Schuetz JD, Boyer JL. Mrp4−/− mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology. 2006;43(5):1013–1021. doi: 10.1002/hep.21158. [DOI] [PubMed] [Google Scholar]
- Miyata M, Tozawa A, Otsuka H, Nakamura T, Nagata K, Gonzalez FJ, Yamazoe Y. Role of farnesoid X receptor in the enhancement of canalicular bile acid output and excretion of unconjugated bile acids: A mechanism for protection against cholic acid-induced liver toxicity. J. Pharmacol. Exp. Ther. 2005;312(2):759–766. doi: 10.1124/jpet.104.076158. [DOI] [PubMed] [Google Scholar]
- Nagasaka H, Chiba H, Hui SP, Takikawa H, Miida T, Takayanagi M, Yorifuji T, Hasegawa M, Ota A, Hirano K, et al. Depletion of high-density lipoprotein and appearance of triglyceride-rich low-density lipoprotein in a Japanese patient with FIC1 deficiency manifesting benign recurrent intrahepatic cholestasis. J. Pediatr. Gastroenterol. Nutr. 2007;45(1):96–105. doi: 10.1097/MPG.0b013e3180331df9. [DOI] [PubMed] [Google Scholar]
- Paulusma CC, Kool M, Bosma PJ, Scheffer GL, ter Borg F, Scheper RJ, Tytgat GN, Borst P, Baas F, Oude Elferink RP. A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin-Johnson syndrome. Hepatology. 1997;25(6):1539–1542. doi: 10.1002/hep.510250635. [DOI] [PubMed] [Google Scholar]
- Plaa GL, Priestly BG. Intrahepatic cholestasis induced by drugs and chemicals. Pharmacol. Rev. 1976;28(3):207–273. [PubMed] [Google Scholar]
- Plass JR, Mol O, Heegsma J, Geuken M, Faber KN, Jansen PL, Muller M. Farnesoid X receptor and bile salts are involved in transcriptional regulation of the gene encoding the human bile salt export pump. Hepatology. 2002;35(3):589–596. doi: 10.1053/jhep.2002.31724. [DOI] [PubMed] [Google Scholar]
- Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102(6):731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Slitt AL, Allen K, Morrone J, Aleksunes LM, Chen C, Maher JM, Manautou JE, Cherrington NJ, Klaassen CD. Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim. Biophys. Acta. 2007;1768(3):637–647. doi: 10.1016/j.bbamem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75(3):451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl Acad. Sci. U.S.A. 2001;98(6):3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman C, Liddle C, Coulter S, Sonoda J, Alvarez JG, Evans RM, Downes M. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc. Natl Acad. Sci. U.S.A. 2006;103(30):11323–11328. doi: 10.1073/pnas.0604772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, Evans RM, Downes M. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc. Natl Acad. Sci. U.S.A. 2005;102(6):2063–2068. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauner M, Boyer JL. Bile salt transporters: Molecular characterization, function, and regulation. Physiol. Rev. 2003;83(2):633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- Trinchet JC, Gerhardt MF, Balkau B, Munz C, Poupon RE. Serum bile acids and cholestasis in alcoholic hepatitis. Relationship with usual liver tests and histological features. J. Hepatol. 1994;21(2):235–240. doi: 10.1016/s0168-8278(05)80401-5. [DOI] [PubMed] [Google Scholar]
- Van Mil SW, Milona A, Dixon PH, Mullenbach R, Geenes VL, Chambers J, Shevchuk V, Moore GE, Lammert F, Glantz AG, et al. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology. 2007;133(2):507–516. doi: 10.1053/j.gastro.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Zollner G, Fickert P, Fuchsbichler A, Silbert D, Wagner M, Arbeiter S, Gonzalez FJ, Marschall HU, Zatloukal K, Denk H, et al. Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J. Hepatol. 2003a;39(4):480–488. doi: 10.1016/s0168-8278(03)00228-9. [DOI] [PubMed] [Google Scholar]
- Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall HU, Zatloukal K, Denk H, Trauner M. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J. Hepatol. 2003b;38(6):717–727. doi: 10.1016/s0168-8278(03)00096-5. [DOI] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Fickert P, Geier A, Fuchsbichler A, Silbert D, Gumhold J, Zatloukal K, Kaser A, Tilg H, et al. Role of nuclear receptors and hepatocyte-enriched transcription factors for Ntcp repression in biliary obstruction in mouse liver. Am. J. Physiol. 2005;289(5):G798–G805. doi: 10.1152/ajpgi.00319.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.