Abstract
In the late 1960s, studies by George Duncan explained many of the basic principles that underlie lens ion homeostasis. The experiments pointed to a permeability barrier close to the surface of the lens and illustrated the requirement for continuous Na,K-ATPase-mediated active sodium extrusion. Without active sodium extrusion, lens sodium and calcium content increases resulting in lens swelling and deterioration of transparency. Later, Duncan's laboratory discovered functional muscarinic and purinergic receptors at the surface of the lens. Recent studies using intact lens suggest purinergic receptors might be involved in short-term regulation of Na,K-ATPase in the epithelium. Purinergic receptor agonists ATP and UTP selectively activate certain Src family tyrosine kinases and stimulate Na,K-ATPase activity. This might represent part of a control mechanism capable of adjusting, perhaps fine tuning, lens ion transport machinery.
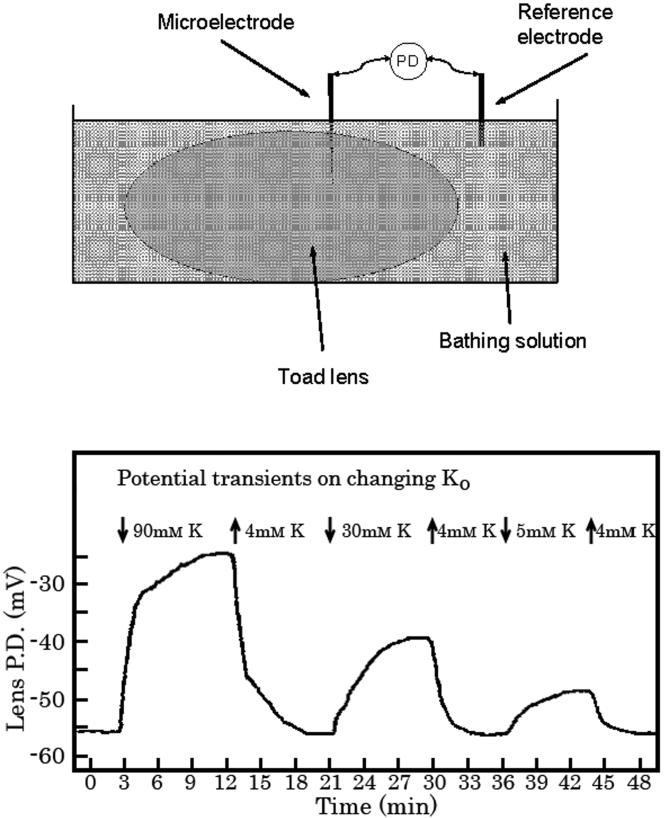
Close to forty years ago George Duncan published a number of seminal papers that were to become the foundation for our thinking on lens ion transport. These articles, which came at the beginning of Duncan's academic career, reported experimental findings and theoretical analysis which addressed issues that included the kinetics of potassium movement across lens membranes (Duncan, 1969a), mechanisms of volume regulation (Duncan and Croghan, 1969), the relative permeability of lens membranes to sodium and potassium (Duncan, 1969b) and water permeability of the lens (Duncan, 1970). The papers explained many of the basic principles that underlie lens ion homeostasis. At the time the work was being carried out there was discussion on the role of fiber cell membranes within the lens cell mass. It was understood that the bulk of the lens comprises tightly packed fiber cells layered one on top of the other but there were questions about which cells form the cellular barrier that enables the lens to maintain internal sodium and potassium concentrations distinct from the concentrations found in aqueous or vitreous humor. To examine the site of ion restricting membranes in the toad lens Duncan devised an ingenious experimental approach in which a voltage-sensing microelectrode was positioned in the cortex 1000 or more microns beneath the lens surface then used to measure the time course of depolarization of the lens potential that occurred when the concentration of potassium in the bathing medium was abruptly increased from 4 to 15, 30 or 90 mM. The depolarization was rapid (Fig 1.). Based on the observed half-time of the response, the estimated unstirred layer at the lens surface and the diffusion coefficient for potassium, Duncan calculated that the lens electrical potential is determined not by cells at the tip of the microelectrode but by cell membranes that lie within 10-20 microns of the surface (Duncan, 1969c). This conclusion, which he also supported with experimental evidence from electrical resistance measurements, was interpreted to signify the barrier to passage of ions into or out of the lens comprises the membranes of only outermost few layers of lens cells at the anterior and posterior surface. For this to be the case, the passage of ions and electrical current between adjacent fiber cells in the lens cortex and nucleus must be relatively unrestricted and in, subsequent years, research by Duncan and others confirmed a high degree of junctional coupling between fibers.
Fig. 1.
(Duncan, 1969c) reported studies that were conducted with toad lenses implanted with a microelectrode that measured the potential difference (PD) between the interior of the lens and a reference electrode in the bath (upper diagram). The lenses were immersed in bathing solution containing 4 mM potassium and the time course of PD changes was recorded (lower panel) when potassium concentration in the bathing solution was increased to 90, 30 or 15 mM. High potassium solutions depolarized the lens. The short half-time of the PD changes (100 - 120 sec) signified ion restricting membranes are located very close to the surface of the lens. The lower panel is taken from (Duncan, 1969c). Permission requested.
In 1969, Duncan and his close colleague at the University of East Anglia, Peter Croghan, presented a theoretical argument for fixed negative charges in the lens that give it a tendency to accumulate water and swell unless an active transport mechanism continuously extrudes sodium ions and imports potassium ions (Duncan and Croghan, 1969). The active sodium-potassium transport mechanism is Na,K-ATPase. Many of the pioneering biochemical studies on Na,K-ATPase in mammalian tissues were carried out by Bonting, with whom Duncan did postdoctoral training. Bonting and his group showed Na,K-ATPase enzyme activity in the lens (Bonting et al., 1963) and indeed ouabain, an Na,K-ATPase inhibitor, depolarizes the lens (Duncan et al., 1980) and causes lens sodium to increase and potassium to decrease. Citing the possible disruption of cell-cell packing when the lens swells or changes secondary to the rise of calcium that invariably accompany lens sodium gain, Duncan argued that failure to maintain normal sodium-potassium balance was a threat to lens transparency. Indeed, in an ion composition analysis of human cataracts, Duncan and Bushell demonstrated a convincing association between abnormal lens ion levels and cortical opacification (Duncan and Bushell, 1975). While human lenses with nuclear cataract had normal ion composition, the severity of cortical cataract went hand in hand with the severity of the increase in lens sodium and decrease in potassium. In cortical cataract it became apparent that active sodium-potassium transport fails to keep pace with ion leaks. It is not yet clear whether this is the result of an overwhelming increase in membrane permeability or a defect in Na,K-ATPase function.
The monolayer of epithelium which covers the anterior lens surface has a high Na,K-ATPase activity compared to lens fibers. The epithelium appears specialized for active sodium-potassium transport and probably contributes to the maintenance of ion balance in the entire fiber mass. In the epithelium monolayer, recent studies indicate Na,K-ATPase activity is highest at the periphery and lowest at the center, the anterior pole of the lens (Candia and Zamudio, 2002; Tamiya et al., 2003). The asymmetric distribution of Na,K-ATPase activity might contribute to a flow of current around the lens. Such a current could speed the diffusion of materials in the fiber mass (Mathias et al., 2007). Fibers at the lens equator have significant Na,K-ATPase activity but at the anterior and posterior surface, and deeper within the lens, fiber Na,K-ATPase activity is low or undetectable (Alvarez et al., 1985). Curiously, however, western blot analysis experiments indicate Na,K-ATPase protein is plentiful even in fibers with low Na,K-ATPase activity (Delamere and Dean, 1993). This points to the possibility that the activity of Na,K-ATPase protein in the lens is regulated. Studies on porcine lenses revealed differences between tyrosine phosphorylation of membrane proteins, including the Na,K-ATPase catalytic subunit, in the epithelium and fibers. Importantly, dephosphorylation by protein tyrosine phosphatase 1B caused an increase in fiber Na,K-ATPase activity (Bozulic et al., 2004). This raises the possibility that tyrosine phosphorylation may be one of perhaps several mechanisms that cause fiber Na,K-ATPase activity to exist in a partially inhibited state. In lens epithelium, Na,K-ATPase activity is responsive to certain members of the Src family of tyrosine kinases. Some members of the Src kinase family have been found to inhibit Na,K-ATPase while others stimulated activity (Bozulic et al., 2005). Tamiya and Delamere, both former doctoral students of George Duncan, found several different members of the Src kinase family expressed in the lens epithelium, with differences between the center and periphery of the epithelial sheet (Tamiya and Delamere, 2005). They reasoned that selective activation of a particular Src-family kinase, perhaps in response to receptor activation, could be a mechanism for modulation of Na,K-ATPase activity.
George Duncan maintained a career-long interest in cytoplasmic calcium in lens cells. Along with studies on calcium in relation to lens transparency and lens cell growth, he and his colleagues demonstrated a role for calcium in signaling. In isolated epithelium as well as epithelial cells in the intact lens, the Norwich eye group found muscarinic and purinergic receptor agonists elicit a transient rise in cytoplasmic calcium (Collison et al., 2000; Collison and Duncan, 2001). Based on the selectivity of the calcium response to different purinergic agonists and antagonists, Duncan and his colleagues argued that the epithelium has functional P2U, now known as P2Y2, purinergic receptors (Riach et al., 1995). Later, in situ hybridization studies by a different laboratory confirmed the expression of P2Y2 receptors (Cowlen et al., 2003). Recently, Tamiya et al discovered that activation of purinergic receptors in the intact rabbit lens by ATP or UTP leads to stimulation of active sodium-potassium transport (Tamiya et al., 2007). The mechanism of the Na,K-ATPase activity response, described in detail by Tamiya et al (Tamiya et al., 2007), was examined as follows.
UTP and ATP both were found to increase the rate of ouabain-sensitive 86Rb uptake indicating activation of Na,K-ATPase-mediated cation transport by intact rabbit lenses. The purinergic antagonist suramin prevented the response. Importantly, pre-incubation with the Src family tyrosine kinase inhibitor PP2 also prevented the change of active sodium-potassium transport. Na,K-ATPase activity was found to be stimulated in the epithelium removed from lenses that had been exposed to purinergic agonists. Lenses exposed to ATP or UTP displayed activation of Src family kinases in the epithelium as judged by an increase in phosphorylation at the active loop (Y416) and concomitant decrease in phosphorylation at the inhibitory C-terminal. Although the Src kinase family has nine members that have slightly different molecular weights, a single PY416-Src immunoreactive band at ~60kDa was observed. Lack of multiple bands indicates not all Src family members are activated. The Src-family kinase response to purinergic receptor activation is selective. Immunoprecipitation studies were conducted to further investigate activation of the candidate kinases that have a molecular weight around 60 kDa. The results (Fig. 2) showed that band densities of the activated form of Src kinase, and to a lesser extent active Fyn kinase, were significantly increased while there was no change in the activated form of Yes kinase. Taken together the results suggest selective activation of Src kinase and/or Fyn kinase is part of a signaling mechanism initiated by purinergic agonists that increases Na,K-ATPase-mediated transport in the organ-cultured lens.
Fig. 2.
Selective activation of Src family members following ATP treatment. Rabbit lenses were incubated for 5 minutes in the presence or absence (control) of 100μM ATP then the epithelium was isolated from each lens and lysed. Yes, Src or Fyn protein was isolated from the lysate by immunoprecipitation, subjected to electrophoresis on an SDS-polyacrylamide gel, transferred to nitrocellulose and probed for active Src family kinases with an antibody against Src family kinases phosphorylated at the active loop tyrosine. Total Yes, Src, or Fyn protein was probed as loading control. Src and Fyn phosphorylation increased significantly, pointing to activation of these tyrosine kinases following ATP treatment of the lens. The data are the mean ± SE (vertical bar) of results from 12, 9 and 7 pools of lysates for Yes, Src and Fyn, respectively. * Indicates significant difference from control (p < 0.05).From (Tamiya et al., 2007); permission requested.
The link between tyrosine phosphorylation and stimulation of Na,K-ATPase activity is not exclusive to the lens or to purinergic receptors. In proximal tubule cells Feraille and colleagues have shown stimulation of ouabain-sensitive 86Rb uptake is tyrosine kinase dependent and requires the phosphorylation of the Y10 residue of the Na,K-ATPase α subunit (Feraille et al., 1999; Feraille et al., 1997). In lung alveolar epithelial cells and cortical neurons Src family kinases have also been implicated in stimulation of Na,K-ATPase activity (Lei et al., 2004; Wang and Yu, 2005). Tyrosine kinases also play a role in Na,K-ATPase inhibition responses. Inhibition of Na,K-ATPase by dopamine in the non-pigmented ciliary epithelium as well as by thrombin or endothelin in the lens is suppressed by the tyrosine kinase inhibitor genistein (Nakai et al., 1999; Okafor et al., 1999; Okafor and Delamere, 2001).
More studies are required before we fully understand the factors that control Na,K-ATPase activity in the lens. Src family tyrosine kinases could represent part of a control mechanism capable of adjusting, perhaps fine tuning, lens ion transport machinery. ATP and endothelin have been shown to be released by lens cells (Eldred et al., 2003; Okafor et al., 2002) and it is possible they could be part of an autocrine mechanism for regulation of the lens Na,K-ATPase. Duncan and his group determined ATP is released from lens epithelial cells when they are placed under stress (Eldred et al., 2003). ATP is likely to leak out of damaged or injured cells. If stimulation of Na,K-ATPase activity was to occur in response to receptor activation by released ATP, it would re-establish the sodium gradient which is vital for co-transport and counter-transport of osmolytes, calcium, glucose or amino acids required for volume regulation following osmotic stress or for injury responses.
George Duncan's early contributions on lens ion transport made it evident that preservation of lens transparency requires continuous active sodium export and potassium import. This paved the way for research on lens Na,K-ATPase. Nearly four decades later, our understanding of Na,K-ATPase in the lens has increased significantly. We know a lot more about which lens cells maintain a high Na,K-ATPase activity and which do not. We also know Na,K-ATPase activity is regulated, at least to a degree, and have started to unravel molecular mechanisms involved. Along the way we learned a functional role for one of the G protein-coupled receptors that interested George so much. Purinergic receptor activation changes Na,K-ATPase activity. There is still much more to learn.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez LJ, Candia OA, Grillone LR. Na+-K+ ATPase distribution in frog and bovine lenses. Curr Eye Res. 1985;4:143–152. doi: 10.3109/02713688508999980. [DOI] [PubMed] [Google Scholar]
- Bonting SL, Caravaggio LL, Hawkins NM. Studies on sodium-potassium-activated adenosinetriphosphatase. VI. Its role in cation transport in the lens of cat, calf and rabbit. Archives of Biochemistry & Biophysics. 1963;101:47–55. doi: 10.1016/0003-9861(63)90532-0. [DOI] [PubMed] [Google Scholar]
- Bozulic LD, Dean WL, Delamere NA. The influence of protein tyrosine phosphatase-1B on Na,K-ATPase activity in lens. J Cell Physiol. 2004;200:370–376. doi: 10.1002/jcp.20029. [DOI] [PubMed] [Google Scholar]
- Bozulic LD, Dean WL, Delamere NA. The influence of SRC-family tyrosine kinases on Na,K-ATPase activity in lens epithelium. Invest Ophthalmol Vis Sci. 2005;46:618–622. doi: 10.1167/iovs.04-0809. [DOI] [PubMed] [Google Scholar]
- Candia OA, Zamudio AC. Regional distribution of the Na(+) and K(+) currents around the crystalline lens of rabbit. Am J Physiol Cell Physiol. 2002;282:C252–262. doi: 10.1152/ajpcell.00360.2001. [DOI] [PubMed] [Google Scholar]
- Collison DJ, Coleman RA, James RS, Carey J, Duncan G. Characterization of muscarinic receptors in human lens cells by pharmacologic and molecular techniques. Invest Ophthalmol Vis Sci. 2000;41:2633–2641. [PubMed] [Google Scholar]
- Collison DJ, Duncan G. Regional differences in functional receptor distribution and calcium mobilization in the intact human lens. Invest Ophthalmol Vis Sci. 2001;42:2355–2363. [PubMed] [Google Scholar]
- Cowlen MS, Zhang VZ, Warnock L, Moyer CF, Peterson WM, Yerxa BR. Localization of ocular P2Y2 receptor gene expression by in situ hybridization. Exp Eye Res. 2003;77:77–84. doi: 10.1016/s0014-4835(03)00068-x. [DOI] [PubMed] [Google Scholar]
- Delamere NA, Dean WL. Distribution of lens sodium-potassium-adenosine triphosphatase. Invest Ophthalmol Vis Sci. 1993;34:2159–2163. [PubMed] [Google Scholar]
- Duncan G. Kinetics of potassium movement across amphibian lens membranes. Experimental Eye Research. 1969a;8:413–420. doi: 10.1016/s0014-4835(69)80007-2. [DOI] [PubMed] [Google Scholar]
- Duncan G. Experimental Eye Research. Vol. 8. Nijmegen, The Netherlands: 1969b. Relative permeabilities of the lens membranes to sodium and potassium: Paper presented at the Symposium on “The Biochemistry of the Eye”; pp. 315–325. June 1968. [DOI] [PubMed] [Google Scholar]
- Duncan G. The site of the ion restricting membranes in the toad lens. Experimental Eye Research. 1969c;8:406–412. doi: 10.1016/s0014-4835(69)80006-0. [DOI] [PubMed] [Google Scholar]
- Duncan G. Permeability of amphibian lens membranes to water. Experimental Eye Research. 1970;9:188–197. doi: 10.1016/s0014-4835(70)80075-6. [DOI] [PubMed] [Google Scholar]
- Duncan G, Bushell AR. Ion analyses of human cataractous lenses. Experimental Eye Research. 1975;20:223–230. doi: 10.1016/0014-4835(75)90136-0. [DOI] [PubMed] [Google Scholar]
- Duncan G, Croghan PC. Mechanisms for the regulation of cell volume with particular reference to the lens. Experimental Eye Research. 1969;8:421–428. doi: 10.1016/s0014-4835(69)80008-4. [DOI] [PubMed] [Google Scholar]
- Duncan G, Delamere NA, Paterson CA, Neville MC. Contribution of an electrogenic pump to the electrical characteristics of frog lens membranes. Experimental Eye Research. 1980;30:105–107. doi: 10.1016/0014-4835(80)90128-1. [DOI] [PubMed] [Google Scholar]
- Eldred JA, Sanderson J, Wormstone M, Reddan JR, Duncan G. Stress-induced ATP release from and growth modulation of human lens and retinal pigment epithelial cells. Biochem Soc Trans. 2003;31:1213–1215. doi: 10.1042/bst0311213. [DOI] [PubMed] [Google Scholar]
- Feraille E, Carranza ML, Gonin S, Beguin P, Pedemonte C, Rousselot M, Caverzasio J, Geering K, Martin PY, Favre H. Insulin-induced stimulation of Na+,K(+)-ATPase activity in kidney proximal tubule cells depends on phosphorylation of the alpha-subunit at Tyr-10. Mol Biol Cell. 1999;10:2847–2859. doi: 10.1091/mbc.10.9.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraille E, Carranza ML, Rousselot M, Favre H. Modulation of Na+,K(+)-ATPase activity by a tyrosine phosphorylation process in rat proximal convoluted tubule. J Physiol. 1997;498(Pt 1):99–108. doi: 10.1113/jphysiol.1997.sp021844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J, Mariash CN, Ingbar DH. 3,3',5-Triiodo-L-thyronine up-regulation of Na,K-ATPase activity and cell surface expression in alveolar epithelial cells is Src kinase- and phosphoinositide 3-kinase-dependent. J Biol Chem. 2004;279:47589–47600. doi: 10.1074/jbc.M405497200. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Kistler J, Donaldson P. The lens circulation. J Membr Biol. 2007;216:1–16. doi: 10.1007/s00232-007-9019-y. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Dean WL, Hou Y, Delamere NA. Genistein inhibits the regulation of active sodium-potassium transport by dopaminergic agonists in nonpigmented ciliary epithelium. Invest Ophthalmol Vis Sci. 1999;40:1460–1466. [PubMed] [Google Scholar]
- Okafor MC, Dean WL, Delamere NA. Thrombin inhibits active sodium-potassium transport in porcine lens. Invest Ophthalmol Vis Sci. 1999;40:2033–2038. [PubMed] [Google Scholar]
- Okafor MC, Delamere NA. The inhibitory influence of endothelin on active sodium-potassium transport in porcine lens. Invest Ophthalmol Vis Sci. 2001;42:1018–1023. [PubMed] [Google Scholar]
- Okafor MC, Mukhopadhyay P, Delamere NA. Studies on endothelin release and Na,K transport in porcine lens. Invest Ophthalmol Vis Sci. 2002;43:790–796. [PubMed] [Google Scholar]
- Riach RA, Duncan G, Williams MR, Webb SF. Histamine and ATP mobilize calcium by activation of H1 and P2u receptors in human lens epithelial cells. J Physiol. 1995;486(Pt 2):273–282. doi: 10.1113/jphysiol.1995.sp020810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiya S, Dean WL, Paterson CA, Delamere NA. Regional distribution of Na,K-ATPase activity in porcine lens epithelium. Invest Ophthalmol Vis Sci. 2003;44:4395–4399. doi: 10.1167/iovs.03-0287. [DOI] [PubMed] [Google Scholar]
- Tamiya S, Delamere NA. Studies of tyrosine phosphorylation and Src family tyrosine kinases in the lens epithelium. Invest Ophthalmol Vis Sci. 2005;46:2076–2081. doi: 10.1167/iovs.04-1199. [DOI] [PubMed] [Google Scholar]
- Tamiya S, Okafor MC, Delamere NA. Purinergic agonists stimulate lens Na-K-ATPase-mediated transport via a Src tyrosine kinase-dependent pathway. Am J Physiol Cell Physiol. 2007;293:C790–796. doi: 10.1152/ajpcell.00579.2006. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Yu SP. Novel regulation of Na, K-ATPase by Src tyrosine kinases in cortical neurons. J Neurochem. 2005;93:1515–1523. doi: 10.1111/j.1471-4159.2005.03147.x. [DOI] [PubMed] [Google Scholar]