SUMMARY
BACKGROUND
The safety and efficacy of nevirapine (NVP) and efavirenz (EFV) based highly active antiretroviral treatment (ART) with concurrent anti-tuberculosis treatment in sub-Saharan Africa has not been well established.
METHODS
We performed a retrospective study comparing human immunodeficiency virus (HIV) infected adults exposed and not exposed to tuberculosis (TB) treatment with similar baseline HIV-1 RNA levels who were started on ART as part of Botswana's ART Programme. ART regimens, HIV-1 RNA, CD4+ cell count, and liver function tests were reviewed for 12 months following ART initiation.
RESULTS
Among 155 patients on ART only and 155 exposed to TB treatment, there was no difference in virologic or immunologic response throughout the first year of ART. Furthermore, there remained no differences in virologic or immunologic outcomes when NVP and EFV groups were stratified by TB treatment exposure status. While more hepatotoxic events occurred in the group exposed to TB treatment than in those not exposed (9% vs. 3%, P = 0.05), there was no difference between patients treated with NVP and those treated with EFV.
CONCLUSIONS
Patients co-infected with HIV and TB in Botswana can be treated effectively with either NVP-or EFV-based ART and TB treatment. As hepatotoxic events were more common in the group exposed to TB treatment, liver function tests should be monitored closely.
Keywords: tuberculosis, HIV, co-infection, Africa
APPROXIMATELY 60% of adults with active tuberculosis (TB) in Botswana are also infected by human immunodeficiency virus type 1 (HIV-1).1 The Botswana National Antiretroviral Treatment Programme (or Masa Programme, meaning `new dawn' in Setswana) provides highly active antiretroviral treatment (ART) to all adults with the presence of an acquired immune-deficiency syndrome (AIDS) defining illness (including suspected or confirmed extra-pulmonary TB) and/or a CD4+ cell count of <200 cells/mm3.2 For HIV and TB co-infected adults with advanced immune suppression (CD4+ cell count <100 cells/mm3) who are most at risk for potentially life-threatening opportunistic infections, ART is begun as early as 2 weeks following the initiation of TB treatment. Co-infected adults with a recent CD4+ cell count of 100-200 cells/mm3 are offered ART once they have completed the first 2-month intensive phase of TB treatment.2 Standard TB treatment in Botswana consists of 2 months of daily isoniazid (INH), rifampicin (RMP), pyrazinamide, and ethambutol, followed by 4 months of daily INH and RMP (2HRZE/4HR).
The concentrations of both widely used non-nucleoside reverse transcriptase inhibitors (NNRTIs), nevirapine (NVP) and efavirenz (EFV), are lowered with concurrent RMP use.3-5 Data from Asia and Africa suggest that immunologic and virologic responses are preserved despite concurrent use of EFV and RMP-based TB treatment.6-9 However, few reports have been published on the safety and efficacy of NVP with concurrent TB treatment.10-13 RMP, INH, NVP and EFV are associated with hepatotoxicity, and little is known about the relative rates of hepatotoxicity with either NVP or EFV in the setting of TB treatment.
In this study, we retrospectively analyzed the Masa Programme Database of ART-treated adults receiving longitudinal care at the Infectious Disease Care Clinic (IDCC), Princess Marina Hospital, in the capital city of Gaborone, Botswana, between 2001 and 2004. We identified patients concurrently treated for both HIV and TB while comparing their outcomes with those of adults treated for HIV but not TB. Our aim was to assess whether virologic outcomes, immunologic outcomes and rates of hepatotoxicity were affected by concurrent use of either NVP- or EFV-based ART with TB treatment.
METHODS
Study population
Three hundred and eighty patients were identifiable by database and chart review as having received TB treatment. For the study, TB treatment-exposed patients were defined as starting TB treatment before or at the same time as starting ART. Of these, we included all patients who were ART naïve; started either NVP-based ART (200 mg daily for 2 weeks, increased to twice daily thereafter) or EFV-based ART (600 mg per day) in conjunction with two nucleoside reverse transcriptase inhibitors (NRTIs); had a baseline plasma HIV-1 RNA (baseline defined as the most recent value before ART initiation); and had at least one HIV-1 RNA and CD4+ cell count value after ART initiation. All patients included were adults (age ≥ 16 years) and citizens of Botswana. Using the Masa database and manual chart review, patients not exposed to TB treatment were selected from a random subset of patients in the same period with a similar distribution of baseline HIV-1 RNA values (median and range) as the group exposed to TB treatment. This was done by creating bins for the TB treatment-exposed group based on the value of baseline viral loads. The control group was chosen by generating a uniform random variable, and ensuring an equal number of subjects within each bin. Patients not exposed to TB treatment had the same inclusion criteria as the exposed patients, but were confirmed by manual chart review to have received no overlapping TB treatment during the first year after ART initiation.
This study was approved by both the Health Research and Development Committee of the Ministry of Health, Gaborone, Botswana and the Harvard School of Public Health Human Subjects Committee, Boston, Massachusetts, USA.
Study design
This is a retrospective study evaluating patients who were treated concomitantly with ART and TB treatment, and a comparison population of patients treated with ART alone. Patients were selected as outlined above, with a chart review performed for all patients in the study to manually confirm their exposure status and to identify missing data. We also accessed Botswana's National Tuberculosis Database to obtain missing dates of TB treatment. If the missing start or end date for TB treatment was not documented in either database, it was imputed to be 6 months prior to the documented TB treatment discontinuation date, or 6 months after the documented TB treatment initiation date. Primary endpoints were CD4+ cell count and plasma HIV-1 RNA values at 3 (range 1.5-4.5), 6 (range 4.5-7.5), 9 (range 7.5-10.5) and 12 (range 10.5-13.5) months following ART initiation. If more than one CD4+ cell count or HIV-1 RNA was obtained during each time interval, the value used for analysis was the one closest to the midpoint. The range of quantification for HIV-1 RNA in Botswana is 400-750 000 copies/ml. Virologic failure was defined as any HIV-1 RNA >400 copies/ml. Liver function testing, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin, was evaluated up to 1 year from ART initiation. Hepatotoxicity was defined as any AST or ALT value more than 5 times the upper limit of normal (ULN) or total bilirubin more than 2.5 times the ULN.
Statistical analysis
Statistical analyses were performed using the SAS version 9.1 statistical package (SAS Institute, Cary, NC, USA). Differences in virologic outcomes were assessed by comparing the per cent of subjects with unsuppressed viral loads (>400 copies/ml) in the group exposed to TB treatment with those in the ART-only group at each time point using Fisher's exact test. The Wilcoxon rank sum test was used to evaluate differences in immunologic outcomes within each group.
Although our primary analyses compared those with any overlap of ART and TB treatment with those on ART only, a secondary analysis was performed by dividing the TB treatment-exposed group into three sub-exposure groups: <2 months, 2-4 months and >4 months of simultaneous exposure to ART and TB treatment. Differences in the percentage of subjects with unsuppressed viral loads and differences in median CD4+ cell counts between each sub-exposure group and the ART-only group were assessed using Fisher's exact test and the Wilcoxon rank sum test, respectively.
Differences in rates of hepatotoxicity were calculated by comparing the percentage of patients with at least one hepatotoxic event during follow-up in the TB treatment-exposed and ART-only groups. Their statistical significance was determined using Fisher's exact test. All statistical tests were two-sided at the α = 0.05 level.
RESULTS
One hundred and fifty-five patients exposed to TB treatment were identified and 155 patients on ART only were selected as a comparison group. Table 1 lists the baseline characteristics in the TB treatment-exposed and ART-only groups. There was no difference in age or sex. Despite controlling for baseline HIV-1 RNA values, baseline weights and CD4+ cell counts were slightly lower in the TB treatment-exposed group than the ART-only group. More patients in the exposed group were started on an EFV-based ART regimen than the ART-only group, which may reflect physicians' concerns about combining NVP-based ART and TB treatment. There were no differences in the baseline median HIV-1 RNA, CD4+ cell counts or weights between NVP- and EFV-treated patients (506 456 vs. 521 843 copies/ml, P = 0.51, 81 vs. 76 cells/mm3, P = 0.99, and 52 kg vs. 54 kg, P = 0.14, respectively). However, more females were started on NVP-containing than EFV-containing ART (96% vs. 35%, P ≤ 0.0001). This is consistent with Botswana's National ART Programme, which states that women of child-bearing age should preferentially be started on a NVP-containing regimen. Probably due to the female predominance, the baseline age of patients started on NVP-containing ART was lower than for those started on EFV-containing ART (32 vs. 39 years, P ≤ 0.0001). For 23% of the patients in the TB treatment-exposed group, either the start date or the end date of TB treatment needed to be estimated. The baseline HIV-1 RNA and CD4+ cell counts were obtained after the initiation of TB treatment in respectively 87% and 78% of patients. Twenty-one per cent had <2 months of overlapping TB treatment after starting ART, 33% had 2-4 months and 46% had >4 months. TB treatment was initiated a median of 81 days prior to ART.
Table.
Baseline characteristics of patients on ART only and those exposed to TB treatment
| ART only | n* | TB treatment-exposed | n | P value | |
|---|---|---|---|---|---|
| Age, years, median (IQR) | 36 (31.2-40.9) | 155 | 35 (29.9-41.2) | 154 | 0.51 |
| Sex (% male) | 39 | 155 | 40 | 155 | 0.91 |
| Baseline weight, kg, median (range) | 54 (47.3-60.5) | 152 | 51 (45-58) | 148 | 0.03 |
| Baseline HIV-1 RNA,† copies/ml, median (range) | 579 583 (291000-750000) | 155 | 589 000 (283000-750000) | 155 | 0.96 |
| Days prior to antiretroviral treatment initiation baseline HIV-1 RNA obtained, median (range) | 16 (14-21) | 155 | 15 (14-30) | 155 | 0.89 |
| Baseline CD4+ cell count, cells/mm3, median (range) | 85 (50-152) | 155 | 72 (33.5-122.5) | 152 | 0.02 |
| Days prior to ART initiation baseline CD4+ cell count obtained, median (range) | 24 (14-57) | 155 | 33 (15-65) | 152 | 0.16 |
| Initial ART regimen (%EFV; n) | 52; 80 | 155 | 65; 100 | 155 | 0.03 |
Variation due to missing data.
ART-only patients selected to have a distribution of baseline HIV-1 RNA values similar to that of the TB treatment-exposed group.
ART = antiretroviral treatment; TB = tuberculosis; IQR = interquartile range; HIV = human immunodeficiency virus.
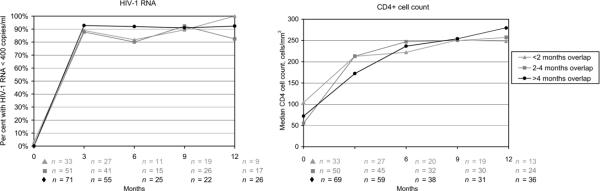
Rates of virologic failure were low in both groups during the first year of ART (Figure 1A). While median baseline HIV-1 RNA levels were high, ≥90% of both groups achieved virologic suppression by 3 months. At 12 months, 82% of the ART-only group and 91% in the TB treatment-exposed group had HIV-1 RNA levels <400 copies/ml (P = 0.28). There was no statistically significant difference in the percentage of virologic failures noted at any time point during the first year by TB treatment exposure status. While patients in the TB treatment-exposed group had a lower baseline median CD4+ cell count (72 vs. 85 cells/mm3, P = 0.02), this difference was no longer evident after 3 months of ART (Figure 1A). Thereafter, the increase in CD4+ cell counts remained similar during the first year of treatment, with the ART-only group achieving a median CD4+ cell count of 270 cells/mm3 and the TB treatment-exposed group achieving a median CD4+ cell count of 275 cells/3 by 12 months (P = 0.80). There remained no difference in immunologic and/or virologic outcomes when the NVP and EFV groups were stratified by TB treatment exposure (Figure 1B, C). Furthermore, the length of overlap of ART and TB treatment (<2, 2-4, or >4 months) did not predict virologic failure or immunologic outcome (Figure 2).
Figure 1.
HIV-1 RNA and CD4+ cell count by tuberculosis treatment exposure status and non-nucleoside reverse transcriptase inhibiter status. A. Total cohort: tuberculosis treatment, unexposed vs. exposed. B. Nevirapine treated: tuberculosis treatment, unexposed vs. exposed. C. Efavirenz treated: tuberculosis treatment, unexposed vs. exposed. n = number of patients with an HIV-1 RNA or CD4+ cell count at the designated time point. HIV = human immunodeficiency virus; ART = antiretroviral treatment.
Figure 2.
HIV-1 RNA and CD4+ cell count by antiretroviral treatment and tuberculosis treatment overlap among tuberculosis treatment-exposed patients. P values not significant among the three groups for follow-up (3, 6, 9 or 12 months) HIV-1 RNA or CD4+ cell counts when compared to the tuberculosis treatment-unexposed group. HIV = human immunodeficiency virus.
There was no difference in baseline liver function tests (LFTs) between the TB treatment-exposed and ART-only groups. Ninety per cent of the TB treatment patients and 94% of the ART-only patients had at least one follow-up liver test monitored. Each TB treatment patient had LFTs checked on average 2.3 times compared to 1.7 times for ART-only patients. Overall, 18 patients were noted to have a hepatotoxic event. There was a trend toward more hepatotoxic events in the TB treatment group as compared to the ART-only group (9%. vs. 3%, P = 0.05). However, within each group, there were no differences in rates of hepatotoxicity between patients treated with either NVP or EFV (10% vs. 9% in the TB treatment groups and 4% vs. 3% in the ART-only groups). Physicians' responses to hepatotoxicity were individualized, and both ART and TB treatment were at times suspended or modified.
Overall, 6% of the ART-only group (5% EFV-treated vs. 7% NVP-treated) and 7% of the TB treatment group (8% EFV-treated vs. 6% NVP-treated) had their NNRTI switched during the first year of ART. Among patients with detectable HIV-1 RNA at 3, 6, 9 or 12 months, 2/28 (7%) in the NVP-treated group and 4/31 (13%) in the EFV-treated group were no longer on their initial NNRTI, due either to regimen change or to default from treatment. No patients had been switched or had defaulted from their original NNRTI at the time of hepatotoxicity.
exposed and ART-only groups. There was no difference in age or sex. Despite controlling for baseline HIV-1 RNA values, baseline weights and CD4+ cell counts were slightly lower in the TB treatment-exposed group than the ART-only group. More patients in the exposed group were started on an EFV-based ART regimen than the ART-only group, which may reflect physicians' concerns about combining NVP-based ART and TB treatment. There were no differences in the baseline median HIV-1 RNA, CD4+ cell counts or weights between NVP- and EFV-treated patients (506 456 vs. 521 843 copies/ml, P = 0.51, 81 vs. 76 cells/mm3, P = 0.99, and 52 kg vs. 54 kg, P = 0.14, respectively). However, more females were started on NVP-containing than EFV-containing ART (96% vs. 35%, P ≤ 0.0001). This is consistent with Botswana's National ART Programme, which states that women of child-bearing age should preferentially be started on a NVP-containing regimen. Probably due to the female predominance, the baseline age of patients started on NVP-containing ART was lower than for those started on EFV-containing ART (32 vs. 39 years, P ≤ 0.0001). For 23% of the patients in the TB treatment-exposed group, either the start date or the end date of TB treatment needed to be estimated. The baseline HIV-1 RNA and CD4+ cell counts were obtained after the initiation of TB treatment in respectively 87% and 78% of patients. Twenty-one per cent had <2 months of overlapping TB treatment after starting ART, 33% had 2-4 months and 46% had >4 months. TB treatment was initiated a median of 81 days prior to ART.
Rates of virologic failure were low in both groups during the first year of ART (Figure 1A). While median baseline HIV-1 RNA levels were high, ≥90% of both groups achieved virologic suppression by 3 months. At 12 months, 82% of the ART-only group and 91% in the TB treatment-exposed group had HIV-1 RNA levels <400 copies/ml (P = 0.28). There was no statistically significant difference in the percentage of virologic failures noted at any time point during the
DISCUSSION
In this selected cohort of 310 HIV-1 infected adults receiving NNRTI-based ART at a large, urban HIV clinic in Botswana, we documented excellent virologic and immunologic response rates through 1 year of follow-up among those who received or did not receive TB treatment. Concurrent TB treatment did not reduce the efficacy of either NVP-based or EFV-based ART when compared to a similar group of adults treated with ART alone. While there was a trend toward more hepatotoxic events in the TB treatment-exposed group, this was independent of the NNRTI they were receiving (NVP or EFV). While it has been suggested that the EFV dose should be increased to 800 mg daily and NVP dose increased to 400 mg twice daily to overcome the pharmacokinetic interaction with RMP, all patients in this study received standard drug dosing.3,14
Like other ART programs in the developing world, Botswana relies on NNRTI-based ART for first-line treatment. Few studies have been performed in Africa evaluating EFV-based ART in the setting of concurrent TB treatment. Two recent studies from South Africa have described co-infected patients receiving EFV (600 mg/day) and TB treatment: excellent immunologic and virologic outcomes were reported.8,9 However, in both of these studies the total number of treated patients was small and no comparisons were made with a control group. In accordance with our results, a recent study from South Africa noted that concomitant TB treatment increased the rate of hepatotoxicity in patients receiving EFV-based ART.15
Many public ART programs in Africa rely on fixed-dose NVP-containing ART combinations,16 and EFV-based ART may not be suitable or available in all settings. Our findings are consistent with data from Thailand and Spain, where it has been shown that co-infected patients can be safely and effectively treated with NVP-based ART while receiving concomitant RMP-based TB treatment.10-12
However, recently published data from Cape Town, South Africa, differ from our findings and suggest that receiving RMP-based TB treatment at the start of NVP-based ART was associated with up to a twofold increased risk of virologic failure in the first 18 months on ART compared to those receiving NVP-based ART without TB treatment.13 It is important to note that, despite this difference, high rates of virologic suppression were noted in the NVP-RMP group, with 85% at 6 months and 80% at 18 months remaining virologically suppressed. One recent study from South Africa evaluated pharmacokinetic data in patients receiving NVP-based ART during and after RMP-based anti-tuberculosis treatment.5 The authors were appropriately concerned by the finding of a lower minimal (Cmin) and maximal (Cmax) serum concentration and area under the curve (AUC)1-12 of NVP in participants receiving anti-tuberculosis treatment. In addition, while receiving anti-tuberculosis treatment, three of the 16 participants had an NVP Cmin below the recommended lower limit. As there were only 16 participants in the pharmacokinetic study, it was not powered to evaluate immunologic or virologic outcomes.
There are several limitations to our study. Its retrospective nature allows for potential sources of bias, although this was minimized through extensive chart review. Missing data was another potential problem, although only 10% in the ART-only group and 14% in the TB treatment-exposed group had <1 year of follow-up information available. Because HIV-1 RNA and CD4+ cell counts were not reliably checked in all patients at 3-month intervals, our total number for each follow-up time period is less than at baseline. The start or end date for 23% of the TB treatment-exposed patients needed to be estimated. However, we believe this estimate was accurate, as Botswana's TB treatment program employs directly observed treatment and most patients receive exactly 6 months of TB treatment. The default rate for TB treatment in Botswana in 2002-2004 was only 8-9%.17-19
Because our inclusion criteria required at least one follow-up plasma HIV-1 RNA level and CD4+ cell count, we cannot comment on safety issues for those patients experiencing early loss to follow-up who did not meet our study entry criteria. However, in a sub-analysis of the 17 TB treatment-exposed patients who were excluded from the cohort for not having a follow-up plasma HIV-1 RNA level and CD4+ cell count, but who did have at least one follow-up LFT following ART initiation, none were found to have developed hepatotoxicity. Information documenting ART adherence rates and previous use of single-dose NVP for prevention of mother-to-child transmission, which could have affected virologic outcomes in either group independently of concurrent TB treatment status, was not available in the Masa database or chart review.20 Finally, more women were treated with NVP and men with EFV, in accordance with Botswana's National ART Programme. We cannot thus rule out that this population difference confounded a true difference in hepatotoxic events between NVP- and EFV-treated patients.
Despite these limitations, we believe that this analysis compared similar groups of patients, and that our control of baseline plasma HIV-1 RNA levels in the selection of ART only patients and our detailed chart reviews eliminated most sources of bias in the study.
In conclusion, our data suggest that HIV and TB in Botswana can be effectively treated concomitantly with either NVP- or EFV-based ART in adults receiving RMP-based TB treatment. Because rates of hepatotoxicity were greater in the co-infected patients, LFTs should be monitored closely. The apparent safety and efficacy of NVP-based ART in the setting of TB treatment is reassuring for the many patients in Africa who require concurrent treatment for TB and HIV where only NVP-based ART is available or appropriate.
References
- 1.Nelson LJ, Talbot EA, Mwasekaga MJ, et al. Anti-tuberculosis drug resistance and anonymous HIV surveillance in Botswana, 2002. Lancet. 2005;366:488–490. doi: 10.1016/S0140-6736(05)67062-6. [DOI] [PubMed] [Google Scholar]
- 2.Anabwani G, Jimbo W, editors. Botswana guidelines on antiretroviral treatment. Ministry of Health, Botswana; Gaborone, Botswana: 2005. [Google Scholar]
- 3.Lopez-Cortes LF, Ruiz-Valderas R, Viciana P, et al. Pharmacokinetic interaction between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clinical Pharmacokinet. 2002;41:681–690. doi: 10.2165/00003088-200241090-00004. [DOI] [PubMed] [Google Scholar]
- 4.Ribera E, Pou L, Lopez RM, et al. Pharmacokinetic interaction between nevirapine and rifampicin in HIV-infected patients with tuberculosis. J AIDS. 2001;28:450–453. doi: 10.1097/00042560-200112150-00007. [DOI] [PubMed] [Google Scholar]
- 5.Cohen K, Van Cutsem G, Boulle A, et al. Effect of rifampicin-based antitubercular treatment on nevirapine plasma concentrations in South African adults with HIV-associated tuberculosis. J Antimicrob Chemother. 2008;61:389–393. doi: 10.1093/jac/dkm484. [DOI] [PubMed] [Google Scholar]
- 6.Patel A, Patel K, Patel J, Shah N, Patel B, Rani S. Safety and antiretroviral effectiveness of concomitant use of rifampicin and efavirenz for antiretroviral-naïve patients in India who are coinfected with tuberculosis and HIV-1. J AIDS. 2004;37:1166–1169. doi: 10.1097/01.qai.0000135956.96166.f0. [DOI] [PubMed] [Google Scholar]
- 7.Manosuthi W, Sungkanuparph S, Thakkinstian A, et al. Efavirenz levels and 24-week efficacy in HIV-infected patients with tuberculosis receiving highly active antiretroviral therapy and rifampicin. AIDS. 2005;19:1481–1486. doi: 10.1097/01.aids.0000183630.27665.30. [DOI] [PubMed] [Google Scholar]
- 8.Friedland G, Khoos S, Jack C, Lalloo U. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J Antimicrob Chemother. 2006;58:1299–1302. doi: 10.1093/jac/dkl399. [DOI] [PubMed] [Google Scholar]
- 9.Cassol E, Page T, Mosam A, et al. Therapeutic response of HIV-1 subtype C in African patients coinfected with either Mycobacterium tuberculosis or human herpes virus-8. J Infect Dis. 2005;191:324–332. doi: 10.1086/427337. [DOI] [PubMed] [Google Scholar]
- 10.Manosuthi W, Sungkanuparph S, Thakkinstian A, et al. Plasma nevirapine levels and 24-week efficacy in HIV-infected patients receiving nevirapine-based highly active antiretroviral therapy with or without rifampicin. Clin Infect Dis. 2006;43:253–255. doi: 10.1086/505210. [DOI] [PubMed] [Google Scholar]
- 11.Oliva J, Moreno S, Sanz J, et al. Co-administration of rifampicin and nevirapine in HIV-infected patients with tuberculosis. AIDS. 2003;17:637–638. doi: 10.1097/00002030-200303070-00024. [DOI] [PubMed] [Google Scholar]
- 12.Manosuthi W, Ruxrungtham K, Likanonsakul S, et al. Nevirapine levels after discontinuation of rifampicin therapy and 60-week efficacy of nevirapine-based antiretroviral therapy in HIV-infected patients with tuberculosis. Clin Infect Dis. 2007;44:141–144. doi: 10.1086/510078. [DOI] [PubMed] [Google Scholar]
- 13.Boulle A, Van Cutsem G, Cohen K, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300:530–539. doi: 10.1001/jama.300.5.530. [DOI] [PubMed] [Google Scholar]
- 14.Ramachandran G, Hemanthkumar AK, Rajasekaran S, et al. Increasing nevirapine dose can overcome reduced bioavailability due to rifampicin coadministration. J AIDS. 2006;42:36–41. doi: 10.1097/01.qai.0000214808.75594.73. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann CJ, Charalambous S, Thio CL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS. 2007;21:1301–1308. doi: 10.1097/QAD.0b013e32814e6b08. [DOI] [PubMed] [Google Scholar]
- 16.South Africa National Department of Health . National anti-retroviral treatment guidelines. South Africa National Department of Health; Pretoria, South Africa: 2004. [Google Scholar]
- 17.Ministry of Health, Botswana . Botswana's National Tuberculosis Control Programme Annual Report 2002. Epidemiological and Disease Control Unit, Community Health Services Division, Ministry of Health; Gaborone, Republic of Botswana: 2002. [Google Scholar]
- 18.Ministry of Health, Botswana . Botswana's National Tuberculosis Control Programme Annual Report 2003. Epidemiological and Disease Control Unit, Community Health Services Division, Ministry of Health; Gaborone, Republic of Botswana: 2003. [Google Scholar]
- 19.Ministry of Health, Botswana . Botswana's National Tuberculosis Control Programme Annual Report 2004. Epidemiological and Disease Control Unit, Community Health Services Division, Ministry of Health; Gaborone, Republic of Botswana: 2004. [Google Scholar]
- 20.Lockman S, Shapiro RL, Smeaton LM, et al. Response to anti-retroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]