Abstract
One mechanism to control immune responses following infection is to rapidly down regulate antigen presentation, which has been observed in acute viral and bacterial infections. Here we describe experiments designed to address whether antigen presentation is decreased after an initial response to Leishmania major. Naïve α-β-Leishmania-specific (ABLE) T cell receptor transgenic T cells were adoptively transferred into mice at various times after L. major infection to determine the duration of presentation of parasite-derived antigens. ABLE T cells responded vigorously at the initiation of infection, but the ability to prime these cells quickly diminished, independent of IL-10, regulatory T cells or antigen load. However, antigen-experienced clonal and polyclonal T cell populations could respond, indicating that the diminution in naïve ABLE cell responses was not due to lack of antigen presentation. Since naïve T cell priming could be restored by removal of the endogenous T cell population, or adoptive transfer of antigen pulsed dendritic cells, it appears that T cells that have previously encountered antigen during infection compete with naïve antigen-specific T cells. These results suggest that during L. major infection antigen-experienced T cells, rather than naïve T cells, may be primarily responsible for sustaining the immune response.
Keywords: T cells, Parasitic-Protozoan, Antigen Presentation/Processing
Introduction
Control of the intracellular protozoan Leishmania major requires a sustained T cell response, since in contrast to many acute viral and bacterial infections leishmaniasis is associated with a high parasite burden for many weeks. Moreover, after resolution of disease low numbers of parasites persist indefinitely, thus requiring the maintenance of T cell dependent immunity (1). We found that both central memory and effector CD4+ T cells mediate long-term resistance to leishmaniasis, and that L. major specific central memory T cells could be maintained in the absence of parasites, while the effector T cell population could not (2). The requirement for parasite persistence to maintain effector T cells would suggest that antigen presentation continually occurs during infection. Whether naïve T cells contribute to the maintenance of the protective response is unknown, although this would also require continued antigen presentation in persistently infected animals. To date, the issue of how long antigen presentation occurs in leishmaniasis has not been directly investigated.
Several recent studies have used adoptive transfer of naïve TCR transgenic T cells into previously infected mice as an indicator of ongoing antigen presentation. For example, antigen presentation following infection with Listeria monocytogenes was found to be extremely short-lived (3–5). Thus, within several days naïve Listeria specific TCR transgenic T cells were unable to proliferate when transferred into Listeria infected mice, in spite of the continued presence of bacteria. Similarly, the ability of naïve T cells to respond to malaria was lost within 3 days of infection (6). In contrast, another recent study found that following influenza infections, naïve TCR transgenic T cells were able to proliferate if transferred into infected animals even after the virus was eliminated, suggesting that antigen presentation was maintained throughout the course of infection and beyond (7). Furthermore, the continued recruitment of naïve T cells into the viral response during infection was associated with an increased number of memory T cells, suggesting that recruitment of naïve T cells may not only be important in sustaining an ongoing immune response, but important for memory T cell development. Taken together, these divergent results suggest that pathogen specific characteristics may influence whether antigen presentation is ongoing after infection.
Infections induce several immunoregulatory mechanisms that are likely to influence the ability of both previously activated and naïve T cells to respond. For example, L. major infection induces the production of IL-10 by macrophages and T cells–in particular T regulatory cells–which can decrease antigen presentation (8, 9). Another factor that may influence how well naïve T cells respond once a primary response has been induced is the degree of competition that occurs between naïve and primed T cells. Effector and/or memory T cells differ from naïve T cells with respect to faster kinetics in their response to antigen (10, 11), decreased requirement for co-stimulation (12, 13), their capacity to mediate effector functions (14), and their increased sensitivity to antigen compared to naïve cells (12, 15–17). All of these characteristics might make previously activated T cells better able to respond during the course of a L. major infection than naïve cells. Indeed, several studies of homeostatic induced proliferation indicate that the responsiveness of naïve T cells is significantly influenced by the presence of either additional naïve or activated T cells (18–20). On the other hand, since naïve T cells migrate through lymph nodes (LNs), while effector T cells home to sites of infection, it might be argued that there is little opportunity for competition to occur between naïve and effector T cells. Competition might occur in the LNs, however, between naïve and central memory or lymph node homing antigen-experienced T cells (2).
In this report, we used Leishmania-specific TCR transgenic mice, termed ABLE mice, to determine how responses of naïve T cells are influenced by an ongoing L. major infection (21). This TCR, which was originally derived from a protective T cell clone (22), recognizes the leishmanial antigen, termed LACK (23). In BALB/c mice, a robust LACK-specific response is initiated shortly after infection with L. major, leading to a 100-fold expansion of LACK-reactive T cells by 4 days (24). By adoptively transferring naïve ABLE T cells into mice at various times after L. major, we found that the ability of naïve Leishmania-specific T cells to proliferate rapidly diminishes following infection, independent of IL-10, regulatory T cells or antigen load. In contrast, previously activated T cells could respond, indicating that the loss of reactivity was not due to lack of antigen presentation. Moreover, after adoptive transfer naïve T cells did proliferate in previously infected animals if the endogenous responding T cell population was absent. Taken together, these data indicate that antigen presentation continues after infection, but that T cells that have previously been activated by infection compete with naïve antigen-specific T cells. Thus, our results suggest that during L. major infection antigen-experienced T cells, rather than naïve T cells, may be primarily responsible for sustaining the immune response.
Materials and Methods
Mice
BALB/cByJ, C57BL/6, and C57BL/6 Thy1.1 mice were obtained from the Jackson Laboratory (Bar Harbor, ME). ABLE T cell receptor transgenic mice bred onto a Cα−/− background (21), WT15 T cell receptor transgenic mice (kindly provided by Dr. Nigel Killeen, University of California, San Francisco, CA)(25), BALB/c Thy1.1 mice, and BALB/c IL-10−/− (kindly provided by Donna Rennick, DNAX Palo Alto, CA) were maintained in the animal colony. DO11.10 RAG2−/− mice were obtained from Taconic Laboratories as part of their emerging models program (26). Animals were maintained in a specific-pathogen-free environment. All experiments were conducted following the guidelines of the University of Pennsylvania institutional animal care and use committee.
Parasites and Antigens
L. major parasites (MHOM/IL/80/Friedlin) were grown in Grace's insect medium (Life Technologies) supplemented with 20% heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. Mice were infected in the hind footpad with 5×106 stationary phase promastigotes. Soluble leishmanial antigen (SLA) was prepared as previously described (22). Ovalbumin (OVA323–339) and LACK (LACK156–173) peptides were obtained from the Protein Chemistry Lab at the University of Pennsylvania (Philadelphia, PA). CpG DNA (1826) was obtained from Coley Pharmaceutical Group (Ottawa, Canada). Immunization of mice was achieved by administering 50 µg of SLA along with 50 µg CpG DNA in 50 µl of PBS in the hind footpad. Mice were boosted with a second dose of SLA and CpG in the same footpad.
L. major parasites expressing ovalbumin (Leishmania-OVA)(submitted; Sara Prickett, Peter M. Gray, Sara L. Colpitts, Phillip Scott, Paul M. Kaye, and Deborah F. Smith), were grown in Schneider’s medium (Sigma) supplemented with 20% heat-inactivated FBS, 2mM glutamine, 100U/ml penicillin, and 100ug/ml/streptomycin. Mice were infected in the hind footpad with 5×106 stationary phase promastigotes.
Regulatory T cell Depletion
Regulatory T cells (Tregs)(CD4+CD25+) were depleted in vivo with the administration of an anti-CD25 (PC61) monoclonal antibody as previously described (27, 28). Briefly, mice were injected i.p. with 1mg of PC61 seven days prior to infection. Control mice were treated with 1 mg of an isotype control (Rat IgG1) antibody. Depletion of Tregs was assessed by flow cytometry using a monoclonal antibody specific for a distinct CD25 epitope (7D4). Depletion was routinely >80% compared to mice treated with the isotype control antibody.
Adoptive Transfers
Lymphocytes from the spleens and lymph nodes of mice were stained with 6-carboxyfluoresceindiacetate succinimidyl ester (CFSE) as previously described (29) and various doses of cells were transferred via the retro-orbital plexus into recipient mice at d1, d7, d14 or d21 following L. major infection. Antigen-experienced ABLE T cells were generated as previously described with minor modifications (30). Briefly, ABLE lymphocytes were stimulated in vitro at 8 × 106 cell/ml in 24 well plates with 1µg/ml of LACK peptide in the presence of 5 ng/ml of rIL-12 and 10µg/ml of anti-IL-4 for four days. After stimulation cells were washed 3 times with PBS and then re-plated in fresh media at 1 × 106 cells/ml in 24 well plates for 5 days T cells were purified by negative selection using mouse T cell enrichment columns (R&D Systems, Minneapolis, MN), and 2 × 106 purified antigen-experienced ABLE cells were then CFSE labeled and adoptively transferred into mice.
Dendritic Cell Generation and Transfer
Dendritic cells (DC) were generated as previously described (31). Briefly, bone marrow was collected from the femurs of BALB/cByJ mice, and clusters within the bone marrow suspensions were dispersed by passing through a 20-gauge needle using a syringe. Cells were seeded into 6-well plates at ~2.5 × 105/ml in 2.5 ml of medium (RPMI 1640, Invitrogen) supplemented with 10% heat-inactivated FBS (HyClone), 2mM L-glutamine (Mediatech, Herndon, VA), 100 U/ml penicillin plus 100 µg/ml streptomycin (Mediatech, Herndon, VA), and 50 µM 2-ME (Invitrogen). In addition, 20 ng/ml of GM-CSF (Peprotech, Rocky Hill, NJ) was added. At d3, 6, and 8, a further 2.5 mls of medium containing 20 ng/ml of GM-CSF was added. At d10, 90–95% of cells were comprised of DCs (MHCII+, CD11c+). For maturation of DCs, cells were harvested at d10 and incubated with 100 ng/ml of LPS (Sigma) for 18–24 hours in the presence of 5 ng/ml of GM-CSF in round bottom polypropylene tubes. Maturation of cells was confirmed by surface staining of cells for CD40 (HM40-3), MHCII (M5/114,15.2), CD80 (16-10A1), CD86 (GL1), analyzed by flow cytometry. Cell surface marker expression was compared to immature DCs that were cultured at d10 in the absence of LPS.
Adoptive transfer of DCs was performed as previously described (32, 33). Briefly, 5 × 106 CFSE-labeled ABLE Thy1.1 cells were transferred into d7 L. major infected mice. One day later, mice were given 5 × 105 LPS matured DCs that had been pulsed with 5 µg/ml of LACK156–173 in the same footpad that had been infected. After three days, ABLE cells in non-draining and dLNs were analyzed by flow cytometry. Negative control mice were given DCs that were unpulsed.
Flow Cytometry, Intracellular Cytokine Staining, and Analysis
Cells isolated from draining and non-draining lymph nodes were analyzed by flow cytometry directly ex vivo as previously described (34). Briefly, cells were washed in staining buffer (PBS containing 0.1% BSA and 0.1% sodium azide) and incubated with Fc block (50 µg/ml 2.4G2 and 500 µg/ml rat Ig) prior to incubation with specific fluorochrome-conjugated monoclonal antibodies against CD4 (RM4-5), CD44 (IM7), Thy 1.2 (53-2.1), Thy1.1 (OX-7) or isotype control antibodies (all from BD Pharmingen, San Diego, CA). Cells stained with anti-mouse DO11.10 TCR (KJ1-26)(Caltag Laboratories, Burlingame, CA) were pre-incubated with normal mouse serum prior to incubation with specific monoclonal antibodies. For intracellular cytokine staining, cells were stimulated with 50 ng/ml PMA, 500 ng/ml ionomycin and 10ug/ml Brefeldin A (all from Sigma-Aldrich, St. Louis, MO) for 4 hours before surface staining. Fixed and surface-stained cells were permeabilized with 0.2% saponin (Sigma-Aldrich) in staining buffer before staining with specific fluorochrome-conjugated monoclonal antibodies against IFN-γ (XMG1.2)(BD Pharmingen). Samples were acquired on a FACSCalibur and analyzed using CellQuest Pro (Becton Dickinson).
T cells labeled with CFSE, and the subsequent dilution of this fluorescent dye was detected by flow cytometry and used to calculate the responder frequency (number of original T cells that divided due to stimulus) and the proliferative capacity (average number of daughter cells generated per responder) as described in detail in previous work (35).
Results
Activation of naïve T cells diminishes following L. major infection
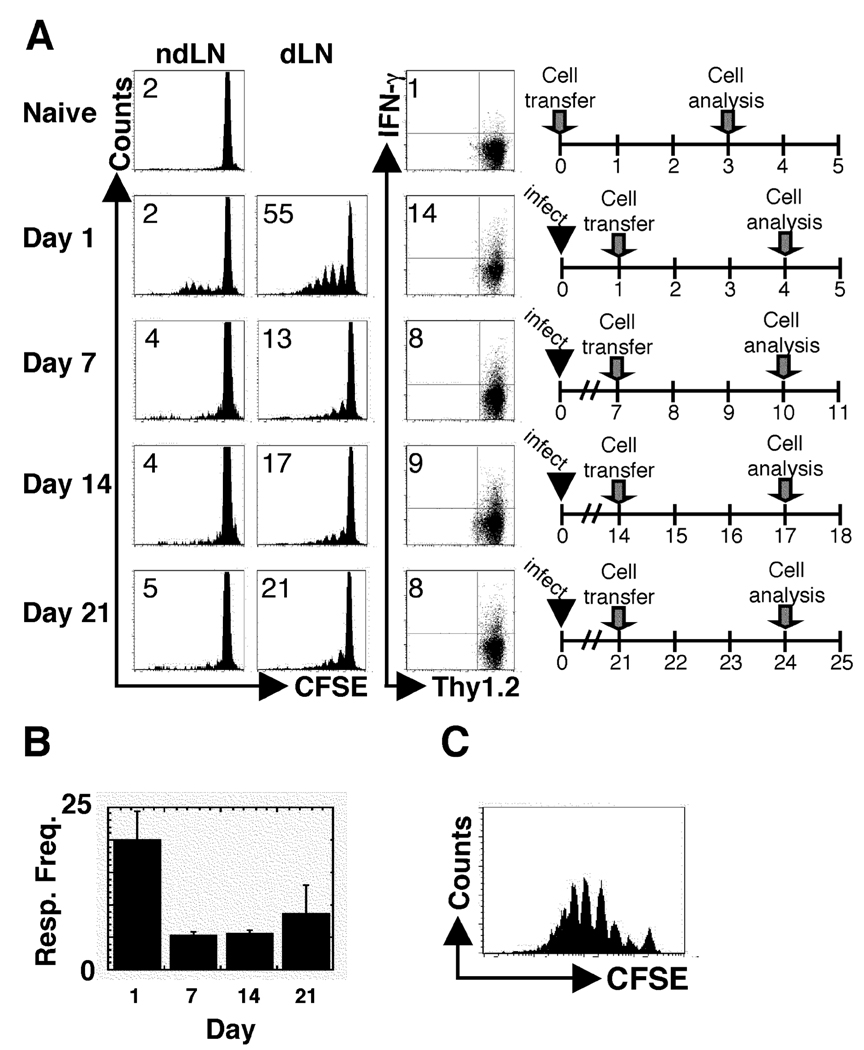
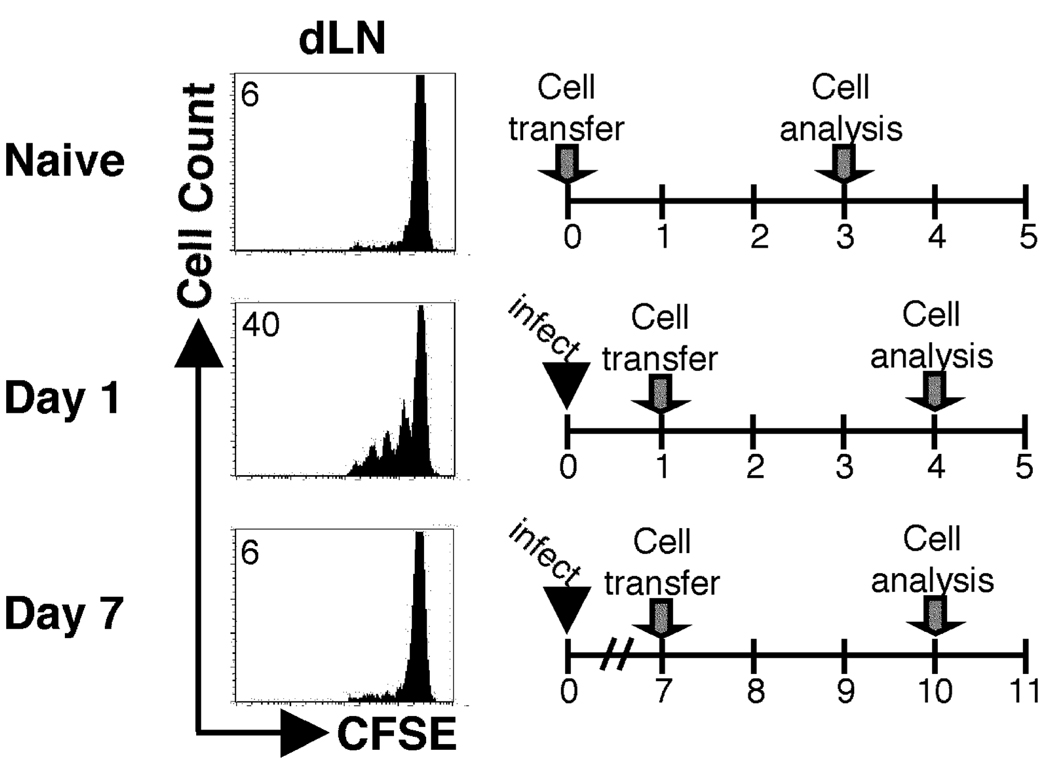
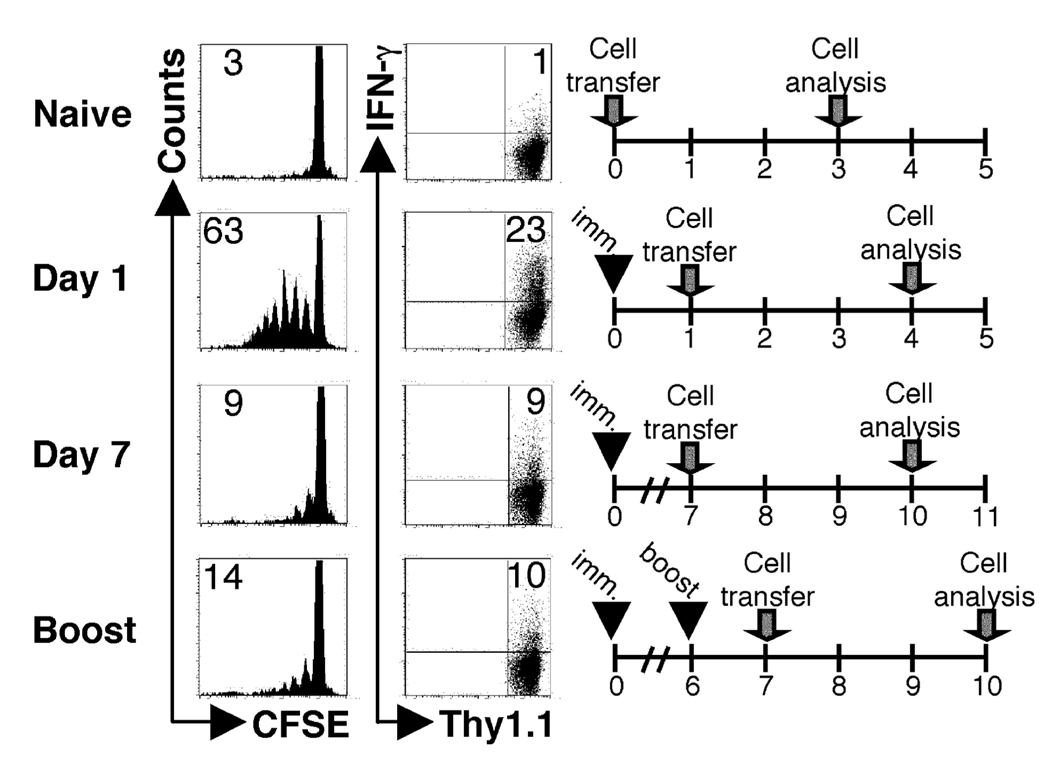
Our initial goal was to use Leishmania-specific TCR transgenic (ABLE) T cells as a monitor of ongoing antigen presentation following L. major infection, as has been done with several other pathogens (3, 6, 7). BALB/c Thy1.1 mice were infected, and at different times following infection, CFSE-labeled ABLE T cells were transferred into the animals. After three days, the donor cells in the lymph nodes were analyzed by flow cytometry for proliferation and IFN-γ production. No proliferation of ABLE T cells was observed in mice that were not infected. When ABLE T cells were adoptively transferred into mice infected with L. major for one day, more than 50% of the cells diluted CFSE in the draining LN, and 14% of the cells acquired the ability to produce IFN-γ (Fig. 1A). However this robust response was significantly diminished, although still detectable, when the naïve ABLE T cells were transferred into mice that were infected for one or more weeks with L. major. The significance, if any, of the detected response in mice that received cells a week after infection is currently unknown. Similar results were obtained with cells from another LACK-specific TCR transgenic mouse (data not shown) (25). It remained possible that the inability to optimally activate naïve LACK-specific T cells was peculiar to the LACK antigen. Therefore experiments were also performed using transgenic L. major parasites that expressed the model antigen, ovalbumin (Leishmania-OVA). In these experiments, instead of transferring ABLE T cells, ovalbumin-specific, DO11.10 T cells were adoptively transferred one or seven days following infection infection with Leishmania-OVA. Similar to wild type parasites, the Leishmania-OVA experiments showed a similar phenotype in that there was a reduction in proliferation in DO11.10 cells when transferred at d7 (6%) compared to d1 (40%) following infection (Fig 2). The diminished activation of naïve T cells observed after L. major infection was reflected in the percentage of donor cells in the dLN that responded by proliferating (responder frequency) (Fig. 1B), although an analysis of the proliferative capacity (the number of daughter cells produced by each dividing cell)(35) indicated that once cells initiated their proliferative cycle they responded similarly (data not shown). These results suggest that the activation signal received by cells 7 days following infection is similar to the signal received by cells one day after infection, but fewer of the cells after 7 days of infection are receiving this activation signal. The diminished ability of naïve T cells to proliferate during L. major infection was not due to any intrinsic deficit in the T cells adoptively transferred into L. major infected mice, since LACK peptide induced substantial in vitro proliferation by ABLE T that had been parked in L. major infected mice from day 7 to day 10 (Fig. 1C). Since transfer of lower doses of TCR transgenic T cells has been shown to increase proliferation (36, 37) we also decreased the number of ABLE T cells transferred into infected animals. At the lowest dose of donor T cells that could still be detected in the lymph nodes, proliferation of naïve TCR transgenic cells was also diminished at d7 of infection compared to d1 (data not shown).
Figure 1.
The ability to activate naïve L. major-specific T cells rapidly diminishes following infection. BALB/c recipient mice (Thy1.1+) were infected with 5×106 stationary phase L. major promastigotes and CFSE-labeled ABLE transgenic T cells (Thy1.2+) were transferred at the indicated times following infection. Three days following transfer, cells were harvested and analyzed by flow cytometry. (A) Histogram plots were gated on donor cells (Thy1.2+). The number in the corner represents the percentage of donor cells that were CFSEdim in the non-draining or dLNs. The numbers in the corners of the dot plots indicated the percentage of donor cells that acquired the ability to produce IFN-γ in the dLN of the recipient animals. (B) The percentage of donor cells that responded by proliferating in the dLN at each time point was calculated (see Materials and Methods). (C) Whole dLNs from mice that received ABLE cells seven days after infection and harvested 3 days later were isolated and restimulated in vitro with 1µg of LACK peptide for 3 days. Donor cells were gated on by Thy1.2 expression. The data are representative of three or more experiments.
Figure 2.
The ability to activate naïve T cells specific for an alternative antigen rapidly diminishes following infection. BALB/c recipient mice (Thy1.2+) were infected with transgenic Leishmania promastigotes expressing ovalbumin (Leishmania-OVA) and CFSE-labeled DO11.10 transgenic T cells were transferred at the indicated times following infection. Three days following transfer, cells were harvested and analyzed by flow cytometry. Histogram plots were gated on donor cells (KJ126+CD4+). The number in the corner represents the percentage of donor cells that were CFSEdim in the non-draining or dLNs.
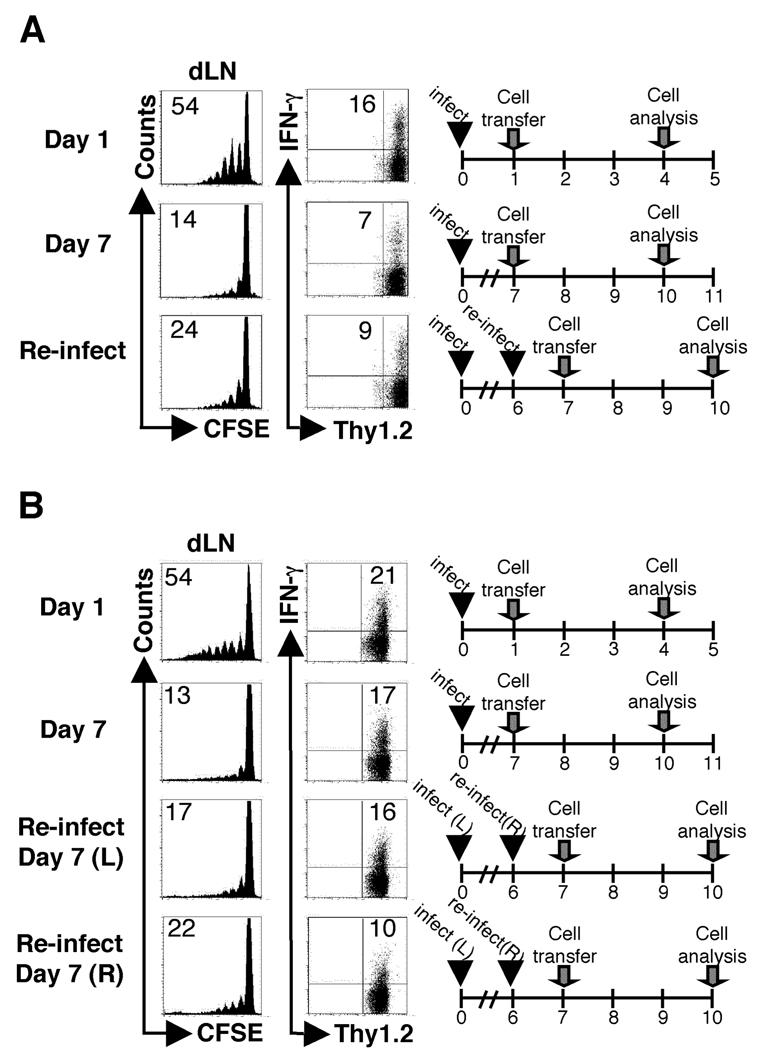
One explanation of these results could be that antigen presentation has ceased because of decreased levels of antigen. To test this, mice were given a second administration of live parasites six days after the initial infection, and CFSE-labeled ABLE cells were adoptively transferred one day later. As with a single infection, the ABLE T cell response was significantly diminished at day 7 when compared to cells that were transferred into mice on day 1 (Fig. 3A). Taken together, these data indicated that during infection with L. major the ability to optimally activate naïve T cells is a transient event.
Figure 3.
Subsequent exposure to live parasites does not rescue the inability to activate naïve cells at d7. BALB/c recipient mice (Thy1.1+) were infected as described in Fig. 1. At d6 post primary infection one group of mice was re-infected in the same (A) or contralateral (B) footpad with L. major, control mice were given PBS in place of parasites at d6. CFSE-labeled ABLE transgenic T cells (Thy1.2+) were adoptively transferred following infection as indicated. Cells were harvested and analyzed as in Fig. 1 with the exception that the primary (L) and secondary (R) dLNs were analyzed from mice challenged in the contralateral footpad. Donor cells were gated on by Thy1.2 expression. The data are representative of three experiments.
Finally, to determine if the diminished response by naïve T cells during infection was limited to the draining lymph node of the primary infection, we assessed the ability of naïve T cells to respond in another site following a secondary infection. Mice were infected with a second dose of parasites in the contralateral footpad prior to the adoptive transfer of ABLE cells seven days following primary infection. In lymph nodes draining the primary and secondary sites of infection, a lower frequency of donor cells responded by proliferating compared to mice that received ABLE cells one day after primary infection (Fig. 3B). This was also associated with a decrease in IFN-γ+ donor cells following secondary exposure compared to the activation of ABLE cells observed one day following infection. Although there is only a modest decrease in the frequency of IFN-γ+ cells at d7 compared to d1 (Fig. 3B) (17% compared to 21%, respectively) a comparison of all the mice tested at each time point showed a significant difference (13.1 ± 1.7 at d7 compared to 21.6 ± 1.2 at d1, p<.0001). These data suggest that the diminished response of naïve T cells that occurs during L. major infection is a systemic event.
The diminished response of naïve T cells in L. major infected animals is not due to immunosuppression
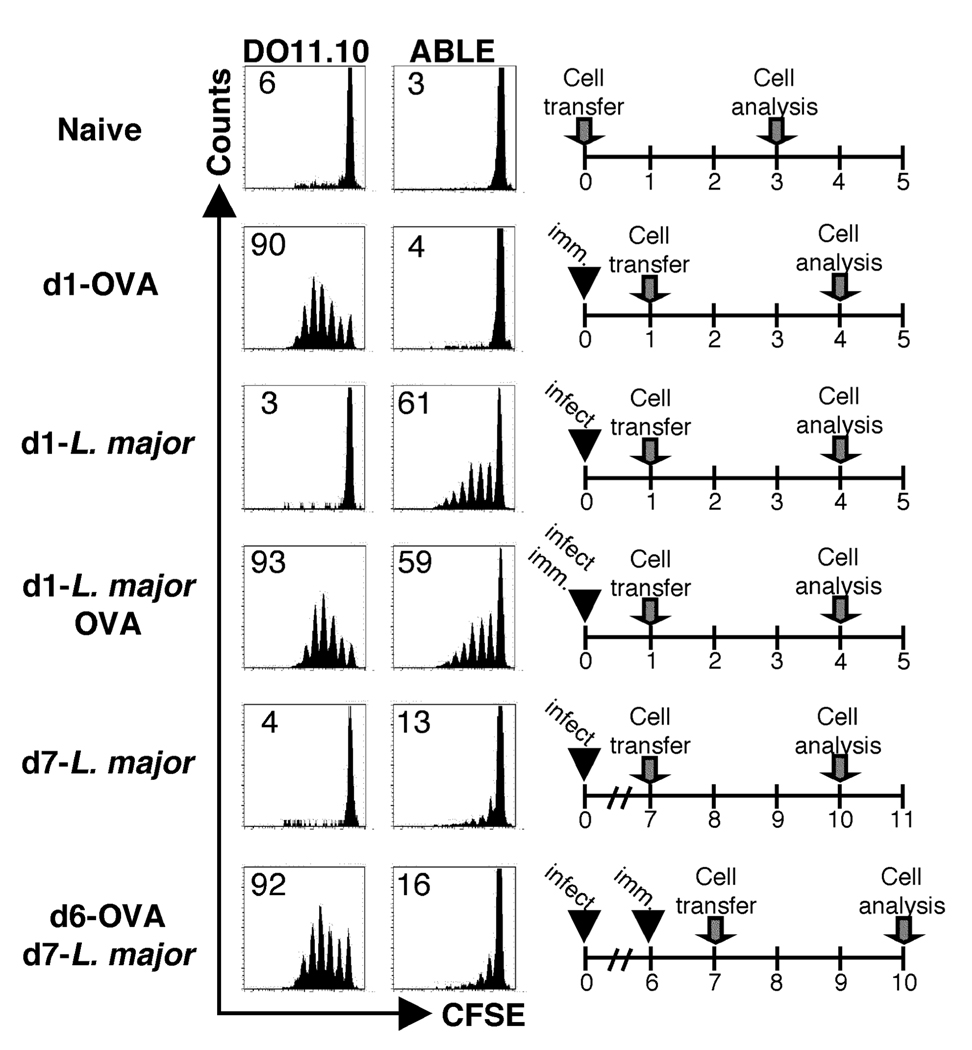
Both specific and non-specific mechanisms of immunosuppression operate during infection with L. major, and may contribute to the reduced response of naïve T cells once a L. major infection is established (8, 9, 38, 39). Therefore, we tested whether naïve T cells with specificity to an antigen unrelated to Leishmania exhibited decreased responses in mice with an established L. major infection. BALB/c mice infected with L. major for six days, or naïve animals, were immunized with OVA protein. The following day, CFSE labeled DO11.10 and ABLE Thy1.1 transgenic T cells were adoptively transferred into the immunized mice, and proliferation of the donor cells in the dLN was analyzed by flow cytometry. The proliferation of DO11.10 cells in L. major infected mice that were immunized with OVA six days after infection (d6-OVA/d7 L. major) was similar to control mice that were immunized with OVA alone. However, the ABLE cells were unable to respond in both cases, suggesting that a global decrease in antigen presentation was not responsible for the diminished response of naïve T cells in L. major infected mice (Fig. 4). Importantly, donor cells responded to their cognate antigen in control mice that were immunized with OVA protein, infected with L. major, or immunized and infected at the same time one day prior to receiving donor cells.
Figure 4.
Antigen presentation to an unrelated antigen is intact at seven days following L. major infection. BALB/c recipient mice (Thy1.2+) were infected with L. major. Six days following infection one group of mice was injected with 25ug of OVA protein in the same footpad (d6-OVA/d7-Leish). As controls, mice were given protein alone (d1-OVA), infected with L. major for one or seven days (d1-Leish and d7-Leish, respectively), or infected and immunized at the same time (d1-Leish/OVA). CFSE-labeled OVA-specific (DO11.10) transgenic T and ABLE Thy1.1+ cells were adoptively transferred into the recipient mice at the indicated time points. The dLNs were harvested and analyzed by flow cytometry three days following transfer. Histogram plots were gated on KJ1-26+CD4+ (DO11.10) or Thy1.1+ (ABLE) donor cells in the dLNs of recipient mice. The data are representative of three experiments.
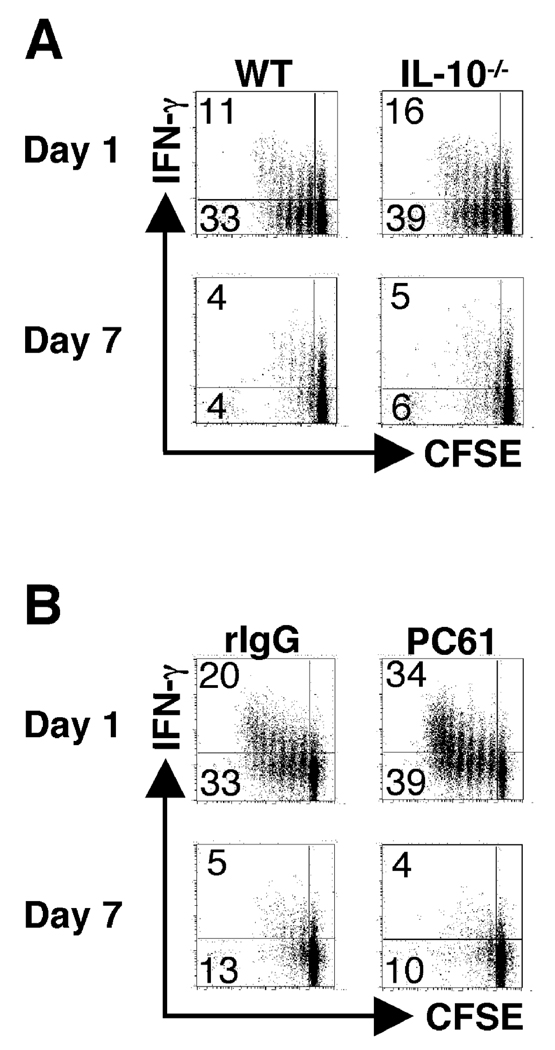
L. major infection is associated with a T regulatory response and increased production of IL-10, both of which may significantly impair T cell responses (8, 9). Therefore, we tested whether IL-10 or regulatory T cells might modulate the responses of naïve T cells during L. major infection. IL-10−/− animals were infected and received CFSE-labeled ABLE T cells at one and seven days following infection as described in figure 1. Three days following transfer the mice were sacrificed, dLNs were harvested, and analyzed for proliferation and IFN-γ production by flow cytometry. In the absence of IL-10 ABLE cells exhibited slightly enhanced proliferation, but naïve T cells transferred at seven days of infection still displayed decreased proliferation compared to cells transferred at the initiation of the infection (Fig. 5A). Depletion of IL-4 also had no effect on the ability of naïve T cells to respond at day 7 (data not shown). In a similar set of experiments, we depleted mice of Tregs prior to infection. Depletion of Tregs failed to enhance the ability of naïve ABLE T cells to respond when transferred into mice infected for 1 week with L. major (Fig. 5B), although the depletion of CD25+ cells also led to a more robust proliferative and IFN-γ response one day after infection compared to control mice. Taken together, these data demonstrate that the diminished response of naïve T cells in L. major infected mice is unrelated to IL-10, IL-4 or Treg cells.
Figure 5.
The suboptimal ability to activate naïve T cells at seven days following L. major infection is not dependent upon immunosuppression. (A) BALB/c IL-10−/− recipient mice (Thy1.2+) were infected with L. major and CFSE-labeled ABLE transgenic T cells (Thy1.1+) were transferred at one or seven days following infection. As controls, experiments were also performed with wild type recipient mice as described in Fig. 1. Dot plots are gated on donor cells in the dLNs of recipient mice (Thy1.1+). The numbers in the corners of the dot plots represent the percentage of events in either of the CFSEdim quadrants, and the numbers in parentheses represent the percentage of cells that produce IFN-γ that are CFSEdim. (B) BALB/c recipient mice (Thy1.2) were depleted of Tregs by administration of 1mg of anti-CD25 (PC61) antibody intraperitoneally 7 days prior to infection, and control mice were treated with 1mg of rat IgG. Following infection, CFSE-labeled ABLE transgenic T cells (Thy1.1+) cells were transferred, harvested, and analyzed as described in Fig. 1. Dot plots were gated on donor cells in the dLNs of recipient mice (Thy1.1+).
Antigen-experienced T cells are more efficiently activated during L. major infection compared to naïve cells
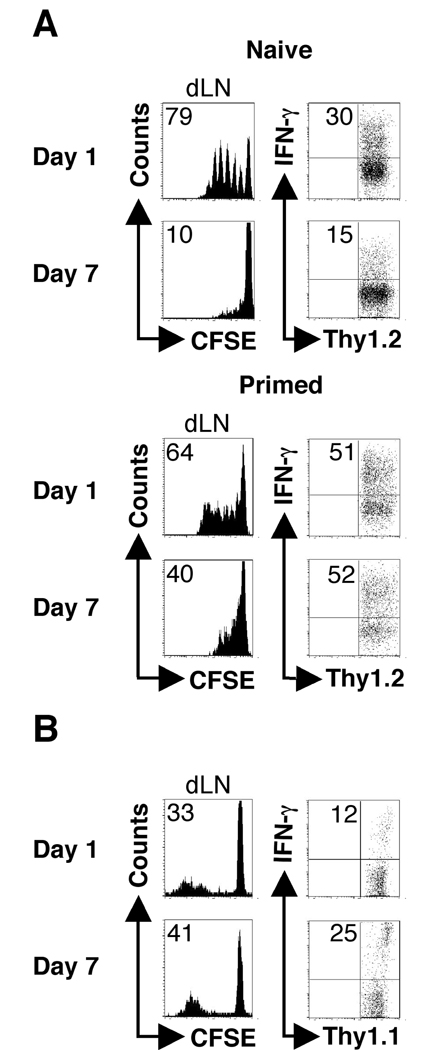
It is widely accepted that antigen-experienced T cells have a lower threshold for activation compared to naïve T cells (12, 15–17). Therefore, we considered the possibility that our results were not due to loss of antigen presentation, but competition between naïve and primed T cells. To test this, we first asked if antigen-experienced T cells responded better during an ongoing infection. Thus, ABLE transgenic T cells that had been stimulated in vitro with LACK peptide were adoptively transferred into infected mice. There were robust responses elicited in mice that received either naïve or previously activated cells one day following infection (Fig. 6A). In mice that received antigen experienced cells seven days after infection 40% of the cells had diluted CFSE, while only 10% of the naïve cell counterparts had done so. Thus, while the proliferative response by primed T cells was less at d7 compared to d1, the response was significantly enhanced compared with naïve T cells.
Figure 6.
Previously activated ABLE transgenic T cells and immune polyclonal cells are less susceptible to the suboptimal activation observed seven days following infection. (A) BALB/c recipient mice (Thy1.1+) were infected as described in Fig. 1. CFSE-labeled ABLE transgenic naïve or previously activated (primed)(Thy1.2+)(see Materials and Methods) were adoptively transferred into recipient mice at d1 or d7 following infection. The dLNs were harvested and analyzed by flow cytometry three days following transfer. Histogram plots were gated on donor cells (Thy1.2+). The number in the corner represents the percentage of donor cells that were CFSEdim in the dLNs. The numbers in the corners of the dot plots indicated the percentage of donor cells that acquired the ability to produce IFN-γ in the dLN of the recipient animals. (B) Naïve C57BL/6 mice (Thy1.2+) were infected as described in Fig. 1. Immune cells from C57BL/6 mice (Thy1.1+) that had healed infection (>12weeks following infection) were transferred at the indicated times following infection. Seven days following transfer cells were harvested and analyzed by flow cytometry. Donor cells were gated on by Thy1.1 expression. The data are representative of three experiments.
To determine if polyclonal, antigen-experienced T cells would proliferate in L. major infected mice, T cells from C57BL/6 Thy1.1 mice that had resolved infection (>12 weeks) were harvested, CFSE labeled and then adoptively transferred into C57BL/6 mice that had been infected for one or seven days. When harvested one week later, approximately 30% of the polyclonal immune cells transferred into mice one day following infection had diluted CFSE in the dLN and >10% of those cells had acquired the ability to produce IFN-γ (Fig. 6B). In mice that received immune cells after being infected for seven days more than 40% of the donor cells in the dLN had diluted CFSE. In addition, the percentage of IFN-γ producing cells was also enhanced in mice infected for seven days compared to one day (Fig. 6B). However, when similar experiments were attempted with the transfer of naïve polyclonal cells into mice infected for one or seven days, there was no detectable proliferation in either group at three, or even seven days following transfer (data not shown). It is likely that this is due to the low precursor frequency of antigen specific cells present in a naïve polyclonal population. Taken together, these data indicate that both TCR transgenic T cells and polyclonal antigen-experienced T cells are efficiently recruited into the response even when transferred following infection, suggesting that antigen-experienced cells have an advantage compared to naïve T cells in maintaining the immune response to L. major.
Endogenous responding cells inhibit the activation/expansion of naïve antigen-specific T cells
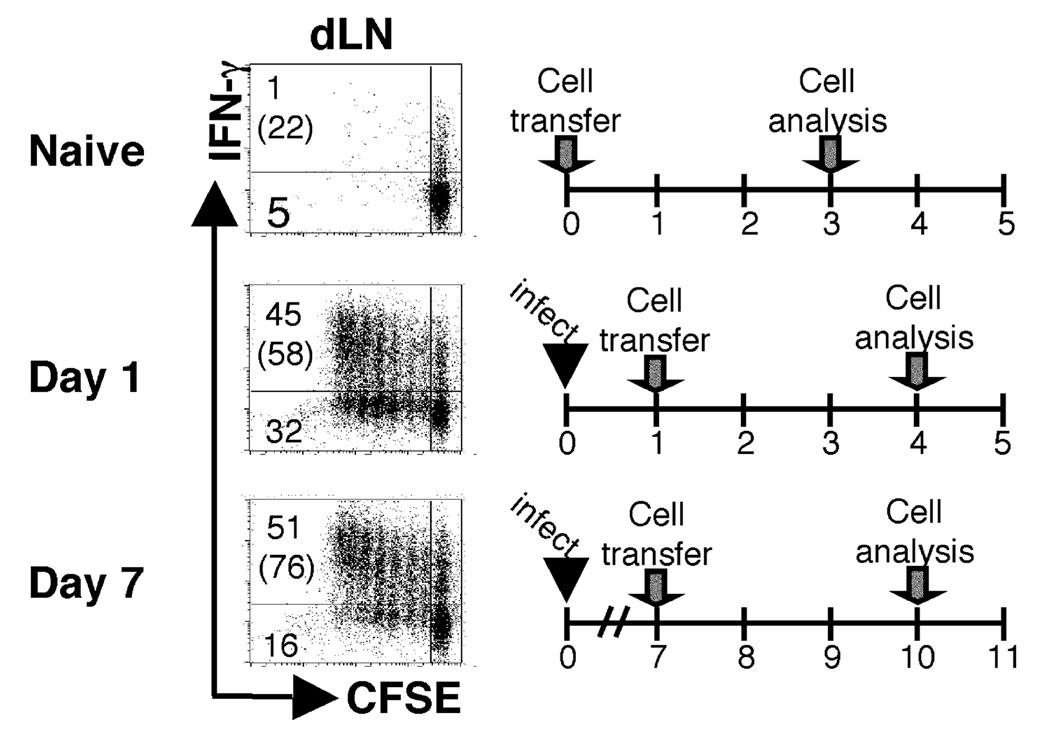
While our results with primed T cells indicates that antigen presentation does not cease after infection, it leaves open the question of why naïve T cells are less able to proliferate when transferred to infected mice. It is well known that following L. major infection in BALB/c mice a LACK-specific T cell response is initiated (24, 38). Having determined that antigen-experienced T cells are more readily activated than naïve T cells after infection we wanted to directly test if competition by the endogenously responding cells was responsible for the suboptimal activation of the naïve T cells. Therefore, naïve ABLE T cells were adoptively transferred into DO11.10 RAG2−/− mice infected with L. major. Since DO11.10 RAG2−/− mice only have T cells specific for an ovalbumin epitope, the Leishmania-specific endogenous response has been effectively removed. When naïve ABLE cells were transferred into DO11.10 infected mice that had been infected for one day, more than 70% of the ABLE cells detected in the dLNs of DO11.10 mice had diluted CFSE and almost 60% of the donor cells had acquired effector function (Fig. 7). Interestingly, in DO11.10 mice that had received donor cells at seven days following infection nearly 70% of the ABLE cells were CFSEdim in the dLN and 76% of the cells had gained the ability to produce IFN-γ. The expansion observed in infected animals was not due to homeostatic proliferation because none was detected upon adoptive transfer of ABLE cells into uninfected control mice. In addition, in vitro experiments were performed investigating the activation of naïve ABLE T cells in the presence of previously activated ABLE T cells, and found that in the presence of primed cells, naïve T cells exhibit a diminished response (data not shown). Together, these data provide evidence that naïve T cells are refractory to stimulation in the presence of antigen-experienced cells and indicate that during an ongoing immune response to a given antigen, previously activated cells may preferentially respond. They also suggest that antigen presentation is unimpaired during the first week of L. major infection.
Figure 7.
The previously activated antigen-specific cells prevent the optimal activation of naïve antigen-reactive cells. DO11.10 RAG2−/− recipient mice (Thy1.2+) were infected as described in Fig. 1. CFSE-labeled ABLE transgenic cells (Thy1.1+) were adoptively transferred into recipient mice at d1 or d7 following infection. The dLNs of recipient mice were harvested and analyzed by flow cytometry three days following transfer. Dot plots were gated on donor cells in the dLNs of recipient mice (Thy1.1+). The numbers in the corners represent the percentage of events in either of the CFSEdim quadrants, and the numbers in parentheses represent the percentage of cells that produce IFN-γ that are CFSEdim. The data are representative of three experiments.
Naïve and antigen-experienced T cells compete for access to antigen presenting cells
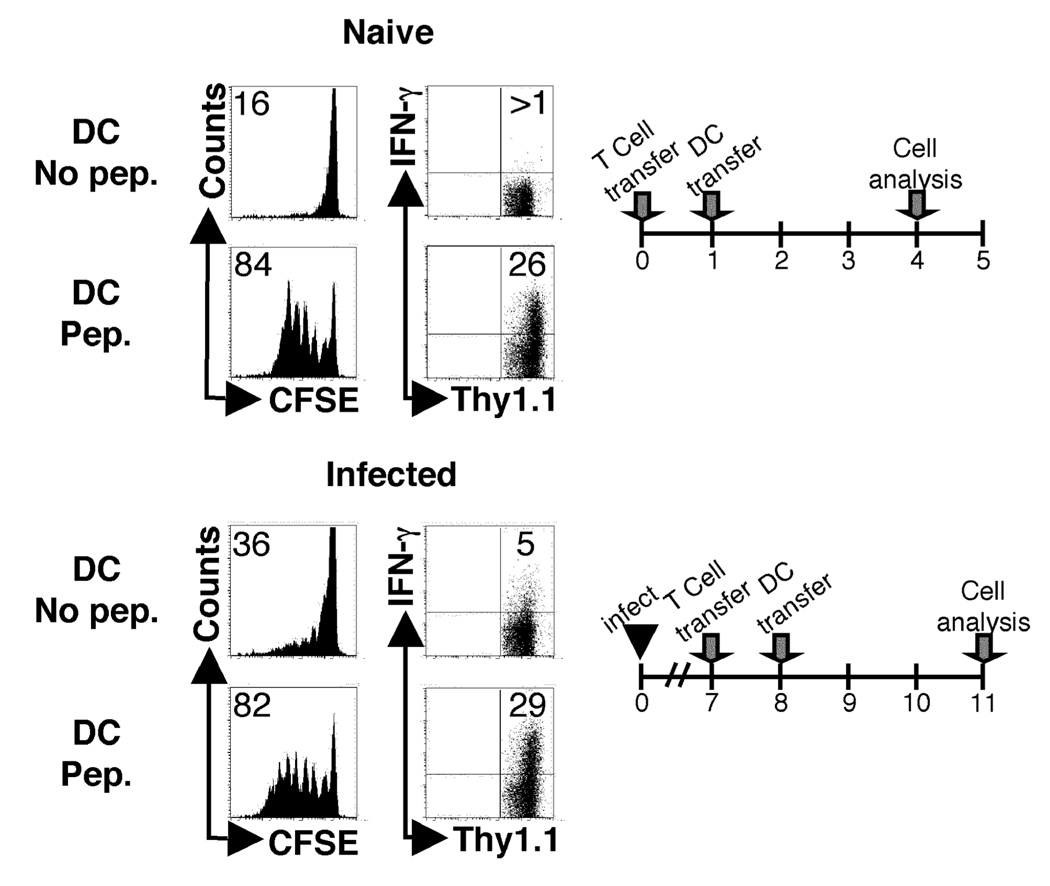
Since naïve ABLE T cells were able to respond both at the initiation and during L. major infection in DO11.10 mice that contained no other Leishmania specific cells, and since primed T cells have a lower threshold for activation than naïve T cells, it would appear that competition for antigen-presenting cells is a likely explanation for the diminished naïve T cell response observed during infection. Therefore, to determine if APCs were limiting after infection, we asked if dendritic cells could overcome the diminished naïve T cell response seen during infection. BALB/c mice were infected with L. major, and at day 7 CFSE labeled ABLE Thy1.1 T cells were adoptively transferred into the infected mice. One day later, infected mice that had received ABLE cells were injected with LACK-pulsed dendritic cells (DC). Three days after the adoptive transfer of DCs, the draining and non-draining lymph nodes were harvested and analyzed by flow cytometry. Infected animals that received unpulsed DCs had limited activation of naïve ABLE T cells in the dLN compared to positive control mice that were uninfected, but received antigen pulsed DCs (Fig. 8). In contrast, injection of LACK pulsed DCs restored the ability to effectively induce proliferation of naïve T cells. This was also reflected in the acquisition of effector function (Fig. 8). These data indicate that naïve and antigen experienced cells compete for access to antigen presenting cells during infection with L. major.
Figure 8.
Naïve and antigen-experienced T cells compete for access to antigen presenting cells. Naïve BALB/c mice (Thy1.2+) were either infected with 5e6 stationary phase promastigotes or left uninfected. Naïve CFSE-labeled ABLE transgenic T cells (Thy1.1+) were transferred into naïve or d7 infected mice. One day after transfer LPS matured BMDDC pulsed with 5ug of LACK peptide or left unpulsed were adoptively transferred directly into the recipient via the footpad. Three days following transfer of BMDDC, cells were harvested and analyzed by flow cytometry. Histogram plots were gated on donor cells (Thy1.1+). The number in the corner represents the percentage of donor cells that were CFSEdim in the dLNs. Non-draining lymph nodes were also harvested and analyzed for proliferation (insets). The numbers in the corners of the dot plots indicated the percentage of donor cells that acquired the ability to produce IFN-gamma in the dLN of the recipient animals. The data are representative of two experiments.
Secondary immunizations do not recruit naïve T cells into the immune response
A logical extension of our findings is that the competition between naïve and primed T cells that we observed during infection might also influence the ability to recruit naïve T cells into an ongoing immune response induced by immunization. To assess this issue, we immunized mice with a soluble leishmanial antigen (SLA) fraction and the adjuvant CpG, boosted them by administration of a second injection and assessed the ability of transferred ABLE TCR transgenic T cells to proliferate. As expected, ABLE cells transferred into mice that had received SLA and CpG a day earlier exhibited a significant proliferative response (Fig. 9). However, when naïve T cells were transferred into mice that were given a second administration of antigen, there was an 80% reduction in the ability of the donor T cells to dilute CFSE.
Figure 9.
Secondary immunizations recruit few naïve T cells into the immune response. BALB/c recipient mice (Thy1.2+) were immunized in the hind footpad with 50µg of soluble Leishmania antigen (SLA) and 50µg of CpG DNA. In addition, a third group of mice was boosted with a second administration of SLA and CpG DNA in the same footpad at d6 following primary immunization (boost). CFSE-labeled ABLE transgenic T cells (Thy1.1+) were transferred at either d1 or d7 following the primary immunization. As a negative control, ABLE transgenic T cells were transferred into naïve BALB/c mice. The data are representative of two independent experiments.
Discussion
Analysis of the proliferation of TCR transgenic T cells following adoptive transfer into infected mice can be used as an indirect measure to assess how long antigen presentation continues after infection. A recent study using this approach found that antigen presentation persists for at least as long as the pathogen survives (7), while other studies indicate that antigen presentation is rapidly down-regulated after infection (3, 4, 6, 40). Using a similar approach, we found that adoptively transferred naïve TCR transgenic cells that recognize the leishmanial antigen LACK do not proliferate during an ongoing infection with L. major as well as at the initiation of the response. However, our data shows that this is not due to a loss of antigen presentation, but instead due to competition between naïve and antigen experienced T cells.
Infection of BALB/c mice with L. major is associated with increased IL-10 production (41, 42) and regulatory T cell activity (8), but neither of these were associated with the decreased naïve T cell response seen in L. major infected mice. However, elimination of endogenous T cells was able to restore the naïve T cell response to levels observed in mice infected for only one day, suggesting that the activated endogenous Leishmania-specific CD4+ T cells are competing with the naïve donor T cell population. In other systems, T cell competition has been shown to play a role during homeostatic proliferation (18, 20, 43), as well as priming of T cells of the same (44, 45) and different (46, 47) specificities during responses to antigen. In leishmaniasis, there is a substantial expansion of LACK-reactive T cells within four days (24, 48), which are likely to be responsible for inhibiting the activation of naïve LACK specific TCR transgenic cells. This inhibition was specific, since there was no decrease in the response of naïve T cells to ovalbumin during L. major infection. Therefore, it appears that competition for particular Class II molecules presenting LACK epitopes is responsible for the decreased naïve T cell response. Consistent with this hypothesis was our ability to enhance the naïve T cell response with LACK-pulsed DCs. Thus, our findings are similar to those showing that the activation of naïve T cells specific for a particular epitope can be inhibited in the presence of antigen-experienced cells of the same specificity through competition for access to antigen presenting cells (20, 46, 47, 49).
The inability to rescue the phenotype by the administration of more parasites or parasite-derived antigen indicates that the paucity of antigen is not responsible for the transient capacity to activate naïve T cells. Rather, since adoptive transfer of antigen pulsed DCs fully rescues the ability to prime naïve Leishmania-specific T cells, it would appear that LACK presenting APC are limiting, as the number of antigen-specific T cells expands during infection. Given the fact that there is a limited amount of surface area on DCs with which to interact with T cells, and it has been suggested that T cells with a lower threshold for activation preferentially occupy this space preventing the activation of T cells with a higher threshold for activation (47), it is likely that this plays a significant role in T cell activation. In addition, the ability of primed T cells to respond better that naïve T cells to APC other than DCs, such as macrophages and B cells, may also contribute to the capacity of primed T cells to respond. This hypothesis is consistent with our data especially since the ability of previously activated T cells to respond to lower doses of antigen (12, 15–17), as well as their less stringent requirements for costimulation (12, 13), may make them better able to respond when DCs are limiting compared to naïve T cells.
Why antigen presentation is maintained in some infections, but not others, is unknown. In the case of Listeria, it was found that antigen presentation to CD8 T cells is short lived due to the ability of cytotoxic T cells to eliminate antigen-presenting cells (3, 4). This is not the mechanism operating in leishmaniasis, since primed L. major T cells proliferated after transfer to L. major infected mice. Moreover, depletion of CD8+ T cells had no effect on the ability of naïve cells to proliferate (data not shown). The most likely explanation for the difference between the Listeria and L. major infections is that the magnitude of the CD8 T cell response is less following L. major infection, although we have not directly investigated this issue. Similar to Listeria, the CD8 response to a sporozoite antigen of malaria was also found to be short-lived (6). However, in this case whether antigen-presenting cells were killed due to the cytolytic activity of CD8 cells was not tested, and it is possible that competition between primed and naïve T cells may have contributed to this response. On the other hand, results with influenza virus suggests that competition does not always occur (7). Thus, when naïve influenza-specific CD4+ T cells were transferred into mice at different times after infection, they were able to proliferate for several weeks. It is unclear why the competition that we observed with L. major was not evident in the influenza system, although it might be explained by either a lower frequency of HA-specific T cells within the activated endogenous T cell population or differences in the affinity of the TCR-peptide interactions. Taken together these studies indicate that the ability to recruit naïve T cells into an ongoing immune response will vary, presumably depending upon specific characteristics of the infection.
One implication of our results is that continuous boosting with vaccines may not expand the pool of naïve antigen-specific cells beyond those that were primed initially. Rather, primed cells will have a significant advantage over those cells that have not encountered antigen. Our studies with both parasites and SLA suggest that once an endogenous pool of primed T cells is established, additional injections of antigen will recruit few naïve T cells into the immune response. This occurred in the lymph node given a primary infection, as well in other lymph nodes draining secondary sites of stimulation. These data suggest that antigen experienced T cells from the initial priming event in the recipient mouse home to a secondary lymph node upon reinfection, and compete with naïve T cells that were transferred into the mice (Fig 3). These cells may be the precursors of central memory T cells, which we have shown to be critical in maintaining immunity in the absence of persistent parasites (2). Whether this population is depleted with continued antigen administration has not yet been tested, but since few additional naïve T cells are recruited into the response it is possible that additional injections of antigen will increase the pool of short-lived effector T cells at the expense of central memory T cells. On the other hand, it is possible that those few naïve T cells that do respond in the face of competition by primed cells are better able to survive. In studies with Listeria monocytogenes, it was shown that competition decreased the attrition of CD8 T cells, and increased the percentage of cells that survived and became central memory T cells (5). Thus, those cells that received reduced stimulation were at a distinct advantage, consistent with the idea that signal strength dictates cell survival (50). While there was no evidence of competition in studies of CD4 T cells stimulated by influenza, naïve T cells that responded later in the infection—thus receiving less stimulation due to lower viral loads–were better memory T cells (7). Consequently, blocking the recruitment of those naïve T cells into the response diminished immunity. While our studies do not address this issue, they help highlight the importance of determining whether continued boosting leads to expansion of more memory T cells or depletion of a central memory T cell pool.
The finding that competition inhibited the ability of naïve T cells to respond following infection was unexpected, and indicates that the use of adoptively transferred naïve TCR transgenic T cells to assess antigen presentation may not always be appropriate. Based on our adoptive transfers we might have incorrectly concluded that antigen presentation ceased after the first week of L. major infection. At the same time, however, these studies allowed us to discover that significant competition exists between naïve T cells and previously primed T cells following L. major infection. Future studies will be required to determine if this is unique to LACK, or an attribute of other leishmanial antigens. In either event, the current study has important implications to consider for the optimal recruitment of T cells into a response following both a natural infection and a prime and boost regimen of immunization.
Acknowledgements
We thank Karen Joyce for the maintenance of the mouse colonies. We thank Nigel Killeen for providing the WT15 TCR transgenic mice. We thank members of the Scott lab for helpful discussions.
Footnotes
This work was supported by National Institutes of Health Grants AI 35914 (to P.S.) and AI 42370 (to S.L.R.). P.M.G. was supported by the National Research Training Award Grant AI 07518.
References
- 1.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 2.Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat Med. 2004;10:1104–1110. doi: 10.1038/nm1108. [DOI] [PubMed] [Google Scholar]
- 3.Wong P, Pamer EG. Feedback regulation of pathogen-specific T cell priming. Immunity. 2003;18:499–511. doi: 10.1016/s1074-7613(03)00081-5. [DOI] [PubMed] [Google Scholar]
- 4.van Faassen H, Dudani R, Krishnan L, Sad S. Prolonged antigen presentation, APC−, and CD8+ T cell turnover during mycobacterial infection: comparison with Listeria monocytogenes. J Immunol. 2004;172:3491–3500. doi: 10.4049/jimmunol.172.6.3491. [DOI] [PubMed] [Google Scholar]
- 5.van Faassen H, Saldanha M, Gilbertson D, Dudani R, Krishnan L, Sad S. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset. J Immunol. 2005;174:5341–5350. doi: 10.4049/jimmunol.174.9.5341. [DOI] [PubMed] [Google Scholar]
- 6.Hafalla JC, Sano G, Carvalho LH, Morrot A, Zavala F. Short-term antigen presentation and single clonal burst limit the magnitude of the CD8(+) T cell responses to malaria liver stages. Proc Natl Acad Sci U S A. 2002;99:11819–11824. doi: 10.1073/pnas.182189999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 9.Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001;166:1141–1147. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 10.Kedl RM, Mescher MF. Qualitative differences between naive and memory T cells make a major contribution to the more rapid and efficient memory CD8+ T cell response. J Immunol. 1998;161:674–683. [PubMed] [Google Scholar]
- 11.Zimmermann C, Prevost-Blondel A, Blaser C, Pircher H. Kinetics of the response of naive and memory CD8 T cells to antigen: similarities and differences. Eur J Immunol. 1999;29:284–290. doi: 10.1002/(SICI)1521-4141(199901)29:01<284::AID-IMMU284>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164:265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt CS, Mescher MF. Peptide antigen priming of naive, but not memory, CD8 T cells requires a third signal that can be provided by IL-12. J Immunol. 2002;168:5521–5529. doi: 10.4049/jimmunol.168.11.5521. [DOI] [PubMed] [Google Scholar]
- 14.Cho BK, Wang C, Sugawa S, Eisen HN, Chen J. Functional differences between memory and naive CD8 T cells. Proc Natl Acad Sci U S A. 1999;96:2976–2981. doi: 10.1073/pnas.96.6.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pihlgren M, Dubois PM, Tomkowiak M, Sjogren T, Marvel J. Resting memory CD8+ T cells are hyperreactive to antigenic challenge in vitro. J Exp Med. 1996;184:2141–2151. doi: 10.1084/jem.184.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–2346. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 17.Curtsinger JM, Lins DC, Mescher MF. CD8+ memory T cells (CD44high, Ly-6C+) are more sensitive than naive cells to (CD44low, Ly-6C−) to TCR/CD8 signaling in response to antigen. J Immunol. 1998;160:3236–3243. [PubMed] [Google Scholar]
- 18.Min B, Foucras G, Meier-Schellersheim M, Paul WE. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. Proc Natl Acad Sci U S A. 2004;101:3874–3879. doi: 10.1073/pnas.0400606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moses CT, Thorstenson KM, Jameson SC, Khoruts A. Competition for self ligands restrains homeostatic proliferation of naive CD4 T cells. Proc Natl Acad Sci U S A. 2003;100:1185–1190. doi: 10.1073/pnas.0334572100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge Q, Bai A, Jones B, Eisen HN, Chen J. Competition for self-peptide-MHC complexes and cytokines between naive and memory CD8+ T cells expressing the same or different T cell receptors. Proc Natl Acad Sci U S A. 2004;101:3041–3046. doi: 10.1073/pnas.0307339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiner SL, Fowell DJ, Moskowitz NH, Swier K, Brown DR, Brown CR, Turck CW, Scott PA, Killeen N, Locksley RM. Control of Leishmania major by a monoclonal alpha beta T cell repertoire. J Immunol. 1998;160:884–889. [PubMed] [Google Scholar]
- 22.Scott P, Pearce E, Natovitz P, Sher A. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987;139:221–227. [PubMed] [Google Scholar]
- 23.Mougneau E, Altare F, Wakil AE, Zheng S, Coppola T, Wang ZE, Waldmann R, Locksley RM, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563–566. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 24.Stetson DB, Mohrs M, Mallet-Designe V, Teyton L, Locksley RM. Rapid expansion and IL-4 expression by Leishmania-specific naive helper T cells in vivo. Immunity. 2002;17:191–200. doi: 10.1016/s1074-7613(02)00363-1. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Malherbe L, Zhang D, Zingler K, Glaichenhaus N, Killeen N. CD4 promotes breadth in the TCR repertoire. J Immunol. 2001;167:4311–4320. doi: 10.4049/jimmunol.167.8.4311. [DOI] [PubMed] [Google Scholar]
- 26.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 27.Oldenhove G, de Heusch M, Urbain-Vansanten G, Urbain J, Maliszewski C, Leo O, Moser M. CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J Exp Med. 2003;198:259–266. doi: 10.1084/jem.20030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taguchi O, Takahashi T. Administration of anti-interleukin-2 receptor alpha antibody in vivo induces localized autoimmune disease. Eur J Immunol. 1996;26:1608–1612. doi: 10.1002/eji.1830260730. [DOI] [PubMed] [Google Scholar]
- 29.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 30.Harbertson J, Biederman E, Bennett KE, Kondrack RM, Bradley LM. Withdrawal of stimulation may initiate the transition of effector to memory CD4 cells. J Immunol. 2002;168:1095–1102. doi: 10.4049/jimmunol.168.3.1095. [DOI] [PubMed] [Google Scholar]
- 31.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 32.Lugo-Villarino G, Maldonado-Lopez R, Possemato R, Penaranda C, Glimcher LH. T-bet is required for optimal production of IFN-gamma and antigen-specific T cell activation by dendritic cells. Proc Natl Acad Sci U S A. 2003;100:7749–7754. doi: 10.1073/pnas.1332767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaph C, Scott P. Th1 cell-mediated resistance to cutaneous infection with Leishmania major is independent of P- and E-selectins. J Immunol. 2003;171:4726–4732. doi: 10.4049/jimmunol.171.9.4726. [DOI] [PubMed] [Google Scholar]
- 35.Gudmundsdottir H, Wells AD, Turka LA. Dynamics and requirements of T cell clonal expansion in vivo at the single-cell level: effector function is linked to proliferative capacity. J Immunol. 1999;162:5212–5223. [PubMed] [Google Scholar]
- 36.Creusot RJ, Thomsen LL, Tite JP, Chain BM. Local cooperation dominates over competition between CD4+ T cells of different antigen/MHC specificity. J Immunol. 2003;171:240–246. doi: 10.4049/jimmunol.171.1.240. [DOI] [PubMed] [Google Scholar]
- 37.Badovinac VP, Messingham KA, Hamilton SE, Harty JT. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–4942. doi: 10.4049/jimmunol.170.10.4933. [DOI] [PubMed] [Google Scholar]
- 38.Launois P, Maillard I, Pingel S, Swihart KG, Xenarios I, Acha-Orbea H, Diggelmann H, Locksley RM, MacDonald HR, Louis JA. IL-4 rapidly produced by V beta 4 V alpha 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 1997;6:541–549. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 39.Himmelrich H, Launois P, Maillard I, Biedermann T, Tacchini-Cottier F, Locksley RM, Rocken M, Louis JA. In BALB/c mice, IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J Immunol. 2000;164:4819–4825. doi: 10.4049/jimmunol.164.9.4819. [DOI] [PubMed] [Google Scholar]
- 40.Norbury CC, Malide D, Gibbs JS, Bennink JR, Yewdell JW. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat Immunol. 2002;3:265–271. doi: 10.1038/ni762. [DOI] [PubMed] [Google Scholar]
- 41.Powrie F, Menon S, Coffman RL. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993;23:3043–3049. doi: 10.1002/eji.1830231147. [DOI] [PubMed] [Google Scholar]
- 42.Liu H, Hu B, Xu D, Liew FY. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-beta, and CTLA4. J Immunol. 2003;171:5012–5017. doi: 10.4049/jimmunol.171.10.5012. [DOI] [PubMed] [Google Scholar]
- 43.Troy AE, Shen H. Cutting edge: homeostatic proliferation of peripheral T lymphocytes is regulated by clonal competition. J Immunol. 2003;170:672–676. doi: 10.4049/jimmunol.170.2.672. [DOI] [PubMed] [Google Scholar]
- 44.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kedl RM, Schaefer BC, Kappler JW, Marrack P. T cells down-modulate peptide-MHC complexes on APCs in vivo. Nat Immunol. 2002;3:27–32. doi: 10.1038/ni742. [DOI] [PubMed] [Google Scholar]
- 46.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayball JD, Robinson BW, Lake RA. CD4+ T cells cross-compete for MHC class II-restricted peptide antigen complexes on the surface of antigen presenting cells. Immunol Cell Biol. 2004;82:103–111. doi: 10.1046/j.0818-9641.2004.01233.x. [DOI] [PubMed] [Google Scholar]
- 48.Malherbe L, Filippi C, Julia V, Foucras G, Moro M, Appel H, Wucherpfennig K, Guery JC, Glaichenhaus N. Selective activation and expansion of high-affinity CD4+ T cells in resistant mice upon infection with Leishmania major. Immunity. 2000;13:771–782. doi: 10.1016/s1074-7613(00)00075-3. [DOI] [PubMed] [Google Scholar]
- 49.Hafalla JC, Morrot A, Sano G, Milon G, Lafaille JJ, Zavala F. Early self-regulatory mechanisms control the magnitude of CD8+ T cell responses against liver stages of murine malaria. J Immunol. 2003;171:964–970. doi: 10.4049/jimmunol.171.2.964. [DOI] [PubMed] [Google Scholar]
- 50.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]