Abstract
The hematopoietic system is one of the first complex tissues to develop in the mammalian conceptus. Of particular interest within the field of developmental hematopoiesis is the origin of adult bone marrow hematopoietic stem cells. Tracing their origin is complicated because blood is a mobile tissue, and because hematopoietic cells emerge from multiple embryonic sites. The origin of the adult mammalian blood system remains a topic of lively discussion and intense research. Interest is also focused on developmental signals that induce the adult hematopoietic stem cell program, as these may prove useful for generating and expanding these clinically important cells ex vivo. This review presents a historical overview of, and the most recent data on the developmental origins of hematopoiesis.
Defining the embryonic origins of specific cell lineages is important for understanding how tissues of the adult organism develop. The signaling events that induce the molecular programs governing lineage-specific fate decisions in embryonic cells provide insight into the complexity of lineage relationships, cell diversity, and ultimately tissue function in the adult. The process of blood cell development in the mammalian conceptus is particularly complex, as it occurs in multiple sites that are separated both temporarily and spatially. Furthermore, unlike stationary tissues, cells of the hematopoietic system circulate, and thus their ancestry and the distinct characteristics associated with their site of origin are confounded by the natural mobility of the system. Recent studies have begun to reveal the lineage relationships between, and molecular programs controlling hematopoietic cell emergence in the conceptus and the legacy of the cells emerging from distinct anatomic sites. This review focuses on the embryonic origins of the hematopoietic system, and the environments and molecules affecting the development of adult mammalian hematopoietic stem cells.
Sites and cells: Where does it start?
The conceptus consists of embryonic tissues that will ultimately become part of the fetus, and extra-embryonic tissues that support fetal development. It has been long recognized that the first blood cells in the vertebrate conceptus appear in the extra-embryonic yolk sac concomitant with the developing vasculature. The yolk sac of early chick embryos was shown by histological studies to harbor the first visible hematopoietic cells, primitive erythrocytes 1. The close physical association of primitive erythrocytes and their synchronous appearance with endothelial cells led to the postulate of a common mesodermal precursor for these two lineages coined the hemangioblast 2. Studies utilizing the in vitro differentiation of totipotent mouse embryonic stem cells (ES) produced the first functional evidence for mammalian hemangioblasts 3, 4, and later analyses of early stage mouse conceptuses revealed presumptive hemangioblasts expressing both the mesodermal marker Brachyury and fetal liver kinase 1 (Flk1) in the posterior region of the primitive streak to the yolk sac 5. These hemangioblasts migrate to the yolk sac, at which point they become committed endothelial and hematopoietic progenitors (Fig 1a (left panel)), several of which contribute to the formation of each blood island 6, 7. Thus, during mammalian embryonic development, the earliest cohort of mesodermal cells emigrating from the primitive streak take on endothelial and hematopoietic fate prior to blood island formation, and give rise to primitive red blood cells and some of the yolk sac vasculature. The remainder of the yolk sac vasculature is derived from angioblasts that also emerge from the posterior primitive streak and do not contribute to blood 8.
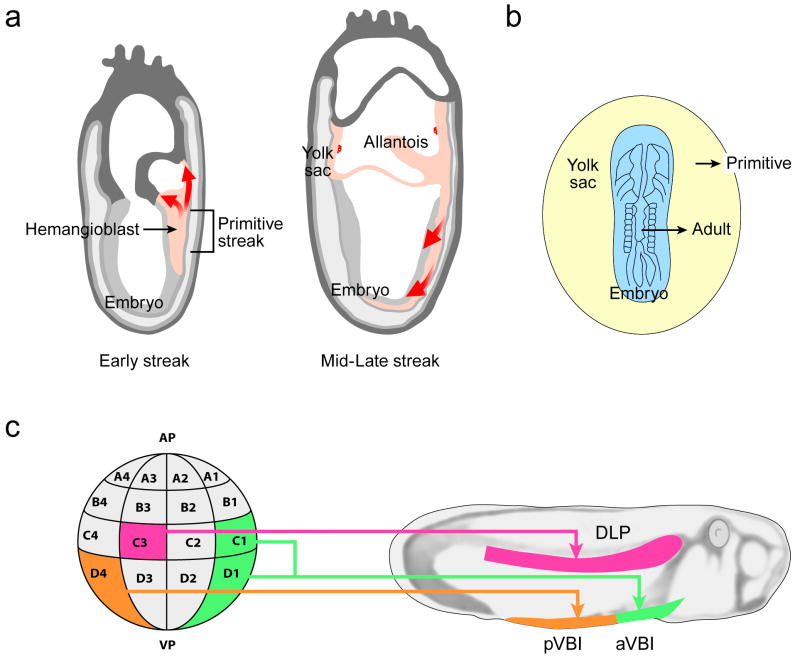
Figure 1.
Vertebrate hematopoietic development. (a) Mesodermal migration during the early streak (left panel) and mid/late streak stage (right panel) in the mouse conceptus. In the early streak stage, mesoderm emerging from the primitive streak forms the extraembryonic yolk sac and slightly later, the allantois. At the mid/late streak stage, mesoderm emerging from the anterior primitive streak forms the axial, paraxial and lateral mesoderm in the rostral region of the embryo. The mesoderm from the posterior primitive streak forms the paraxial and lateral mesoderm of the remaining trunk region of the embryo. The red arrows depict the emigration of mesoderm after egressing from the primitive streak. (b) Inter- and intra-species grafting in early pre-circulation avian embryos (for example a quail embryo body grafted onto a chick yolk sac) revealed the origins of the adult blood in the embryo body and not the yolk sac. (c) Amphibian embryos at the 32 cell stage (left) were genetically marked and the progeny traced to larval stages (right). The C3 blastomere gives rise to the DLP (dorsal lateral plate) mesoderm and more specifically to the dorsal aorta and the hematopoietic clusters within the lumen. The D4 blastomere gives rise to the pVBI (posterior ventral blood island) and the C1 + D1 blastomeres to the aVBI (anterior ventral blood island). Drawings adapted from 11, 15; and the Edinburgh mouse atlas website (http://genex.hgu.mrc.ac.uk/).
It was suggested in the 1970s that cells of the yolk sac were the source of the hematopoietic system in the adult mammal and that yolk sac cells emigrate to the fetal liver and thereafter to the bone marrow where they reside throughout adulthood 9, 10. However, tissue grafting approaches conclusively demonstrated that the yolk sac was not the source of adult blood in non-mammalian vertebrates 11, 12 (Fig 1b, c). Inter- and intra-species grafting of avian embryo body and yolk sac (and more recently the allantois), prior to the emergence of blood cells and the onset of circulation, showed that the adult hematopoietic system originates from cells within the body of the embryo and from the extra-embryonic allantois, and not from the yolk sac 11, 13, 14 (Fig 1b). Likewise, orthotopic grafting of the ventral blood island (VBI; yolk sac equivalent) and dorsal lateral plate (DLP; intrabody region) in amphibian embryos revealed that the DLP is the source of adult hematopoietic cells 12. Molecular marking experiments in xenopus embryos at the early cleavage stages demonstrated that the separation of presumptive VBI and DLP mesoderm occurs very early 15 (Fig 1c). The progeny of the C3 blastomere of regularly cleaving embryos contributes to intraembryonic (DLP) hematopoiesis while completely distinct blastomeres contribute to the anterior (C1 & D1) and posterior (D4) VBI. Separation at this early stage implies that the progeny of these cells experience spatially and temporally distinct signalling events during gastrulation and embryonic patterning. Indeed, the three blood compartments in xenopus (anterior VBI, posterior VBI, DLP) are specified from mesoderm that encounters different levels of the bone morphogenic protein (BMP) 16. In addition, the morphogen fibroblast growth factor (FGF) differentially affects the timing of Scl and Runx1 expression, both of which encode pivotal transcription factors that specify hematopoietic fate in the anterior versus posterior VBI 17. Interestingly, by grafting VBI or DLP cells to the reciprocal site in the amphibian embryo, the prospective hematopoietic cells can be reprogrammed to the alternate adult or primitive hematopoietic fate. However, this is possible only within an early developmental window of time 18, after which point primitive and adult states are thought to be epigenetically fixed.
Evidence for the separation of presumptive mesoderm in the mouse epiblast was provided through single cell marking experiments. By labelling prospective mesodermal cells at early primitive streak stages (E6.5/E7), cells from the posterior primitive streak were found to contribute to extraembryonic hematopoietic tissue, i.e. yolk sac and allantois 19 (Fig 1a, left panel), and not to the body of the embryo. These results were confirmed using an orthotopic grafting method 8 which further showed that at mid-streak stages, the mesoderm derivatives within the rostral embryo arise from epiblast cells that ingress through the anterior primitive streak prior to the cells that give rise to axial, paraxial and lateral (blood forming) mesoderm of the anterior trunk (Fig 1a, right panel). Lastly, the mesoderm emigrating from more caudal regions of the streak forms the paraxial and lateral mesoderm of the remaining trunk regions 8 (Fig 1a, right panel). Interestingly, the entire epiblast of the early- and mid-streak stage embryos contains hematogenic potential, but that potential thereafter becomes restricted to the trunk and posterior region of the embryo 20. Thus, in remarkable similarity to the mesodermal fate map of the chick 8, presumptive extraembryonic blood-forming mesoderm in the mouse conceptus is the first to emerge from the primitive streak during gastrulation, followed by the intraembryonic mesoderm in a rostral to caudal sequence.
From primitive erythrocytes to adult HSCs
In vivo transplantation assays in mammals have provided great insight into the specific cells that can generate a complete functional adult hematopoietic system. Hematopoietic stem cells (HSCs) are at the foundation of the adult blood differentiation hierarchy, and provide continuous hematopoietic cell production throughout life. Transplantations of cells from different regions of the embryonic day 8 (E8) to E12 mouse conceptus demonstrated that HSCs conferring complete, long term, multilineage, high level hematopoietic repopulation of irradiated adult recipient mice appear only beginning at E10.5 in the aorta-gonad-mesonephros (AGM) region of the embryo body, and in the vitelline and umbilical arteries 21-23 (Fig. 2, right panel). The appearance of HSCs three days after primitive erythrocytes are generated in the yolk sac (Table 1, Fig. 3) makes it unlikely that they share a recent common ancestry. These adult-repopulating HSCs (which are as potent as adult bone marrow HSCs) are autonomously generated in the AGM, as shown by explant cultures 21 and are located in the ventral aspect of the dorsal aorta 24-26. Slightly thereafter, HSCs are found in other tissues; the placenta, yolk sac, and liver 21, 22, 27, 28. The liver does not generate hematopoietic cells de novo but is instead colonized beginning at late E9 by hematopoetic cells made in other tissues 29, 30 (Fig. 3). The identification of the yolk sac and placenta as de novo generators of HSCs is precluded by the circulation (which is established at the 4-6 somite pair stage, approximately E8.25-8.5 31), which easily distributes these cells throughout the conceptus. However, quantitative spatial and temporal analyses of HSCs suggest that the yolk sac 32 and placenta 27, 28 do contribute to the HSC pool in the liver either through expansion of pre-existing HSCs or by de novo generation of HSCs. It has been shown that there are more HSCs in the fetal liver than can be accounted for through HSC generation in the AGM alone 32. The additive production of HSCs by AGM, yolk sac and placenta 27 most likely is responsible for the high numbers of fetal liver HSCs, although the liver itself may expand these cells 33. Thus, within a span of three days, the mouse conceptus generates at least two very distinct and unrelated classes of functional hematopoietic cells – primitive erythrocytes at E7.5 and definitive adult repopulating HSCs at E10.5.
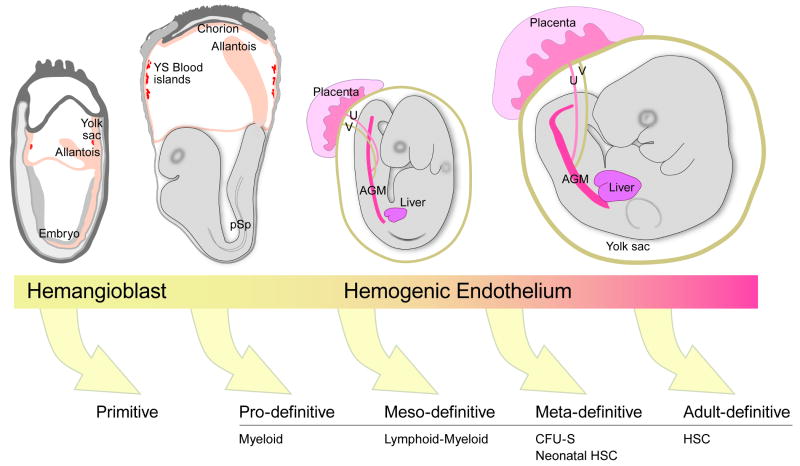
Figure 2.
Mouse conceptuses at E (embryonic day) 7.5, 8.25, 9.0 and 10.5 (respectively from left to right). At least five classes of hematopoietic cells (as defined by function) are progressively generated in the mouse conceptus as indicated by the arrows. The primitive class is derived from hemangioblasts and the pro, meso, meta and adult classes are thought to be derived from specialized vascular cells called hemogenic endothelium. Some of these cells are derived from distinct mesodermal lineages emigrating from the primitive streak (see Fig. 1). The E7.5 and E8.25 conceptuses show the outgrowing allantois that will fuse with the chorion to form the placenta. The circulation is established at E8.25-8.5. The E9 embryo has turned and is enveloped in the yolk sac. Colonization of the liver by hematopoietic progenitors begins at late E9. The E10.5 conceptus contains hematopoietic clusters in the dorsal aorta within the AGM regions, the vitelline (V) and umbilical (U) arteries and the first adult HSCs are found in these vessels. Drawings adapted from the Edinburgh mouse atlas website (http://genex.hgu.mrc.ac.uk/).
Table 1. Spatial and temporal appearance of hematopoietic potential/cells in the mouse conceptus.
| Developmental time | Site | Functional activity | Conditions | Reference | |
|---|---|---|---|---|---|
| Primitive | E7.5 | Yolk saca | Primitive erythroid | - | 35 |
| E7.5/8 (0-4sp) | Yolk sacb | Primitive erythroid | Ncx1-/- | 43 | |
| Pro-definitive | E7.5/8 (EHF-2sp) | Allantoisa | Erythroid-myeloid progenitor | Explant pre-culture | 39 |
| E7.5/8 (0-7sp) | Allantoisa | Erythroid-myeloid progenitor | Explant pre-culture | 38 | |
| E8.25 | Yolk saca | Erythroid-myeloid progenitor | - | 35 | |
| E8.25 | Yolk sacb | Erythroid-myeloid progenitor | Ncx1-/- | 43 | |
| E9.5 | Yolk sacb | Erythroid-myeloid progenitor | Vegf-/- | 42 | |
| E9.0 | Placenta | Erythroid-myeloid progenitor | - | 40 | |
| Meso-definitive | E7.5/8 (0-8sp) | PSpa | Erythroid-myeloid-lymphoid progenitor | Explant pre-culture | 37 |
| E7.5/8 (0-5sp) | PSpa | Multipotent low level repopulating progenitor | Explant pre-culture | 45 | |
| Meta-definitive | E9.0 | YSc | Neonatal repopulating HSC | - | 47 |
| E9.0 | AGMc | Neonatal repopulating HSC | - | 47 | |
| E9.0 | YSc | CFU-S | - | 41 | |
| E9.0 | AGMc* | CFU-S | - | 41 | |
| Adult-definitive | E10.5 | AGMd* | Adult repopulating HSC | - | 21, 22, 32 |
| E10.5 | Umbilical and Vitelline Vesselsd | Adult repopulating HSC | - | 23 | |
| E10.5/11 | Placenta | Adult repopulating HSC | - | 27, 28 |
These data are from experiments in which hematopoietic activities in specific tissues were examined
before the onset of circulation between the extraembryonic and intraembryonic tissues at E8.25;
in conceptuses that lack circulation between these sites due to a genetic mutation;
in midgestation conceptuses, although the time earliest time or first site of appearance has not yet been determined;
in midgestation conceptuses identifying the first site in which the specific hematopoietic activity appears.
Autonomous expansion of CFU-S and HSCs in explant culture of these tissues suggests de novo generation.
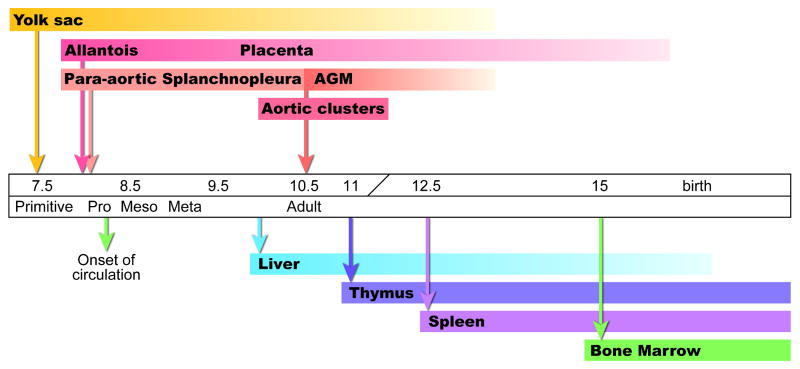
Figure 3.
Time line of hematopoietic events in the mouse conceptus. The arrows above the time line indicate the onset of specific hematopoietic cell generation and/or appearance and the arrows below indicate the earliest time of colonization of the secondary hematopoietic territories.
Other classes of hematopoietic cells are also generated/detected in the mouse conceptus between the time primitive erythrocytes and HSCs appear, including myeloid progenitors, lymphoid-myeloid (multipotent) progenitors, CFU-S and neonatal repopulating HSCs (Table 1). At E8.25, following the first wave of primitive erythropoiesis and before the circulation is established, myeloid progenitors are detected in the yolk sac and are thus generated in that site 34, 35. After the circulation is established myeloid progenitors are also found in the trunk region 36. However, prior to circulation, both the yolk sac and pSp (para-aortic splanchnopleura; prospective AGM region) contain cells with the potential to become myeloid progenitors as revealed in explant cultures 37. Similar cultures of pre-circulation allantoises 38, 39 also revealed cells with myeloid potential. By E9, the placenta contains an abundance of myeloid progenitors 40. More potent myeloid progenitors capable of forming colonies on the spleen following injection into irradiated mice (CFU-S progenitors) are present in both the yolk sac and the AGM beginning at E9 41. Quantitative analyses show more CFU-S in the AGM than in the YS, suggesting that the AGM generates these cells 21. More recently, studies of two mutant mice, VE-cadherin (Cdh5-/-) and Ncx1-/-, have provided strong in vivo evidence for the de novo production of definitive myeloid progenitors in the yolk sac. Conceptuses deficient for these genes do not establish a circulation between the yolk sac and embryo body; in Cdh5-/- conceptuses there is no vascular connection, while in Ncx1-/- conceptuses the vitelline vessels are intact but there is no heartbeat to promote the circulation. E9.5 Cdh5-/- conceptuses contained similar numbers of myeloid progenitors in the yolk sac as wild-type conceptuses, although macrophage and mixed colony-forming progenitors were decreased in number 42. In the Ncx1-/- conceptus, the numbers of myeloid progenitors of all types in the yolk sac were found to be equivalent to the cumulative number of progenitors in the Ncx1+/+ conceptus in all anatomic sites 43. No progenitors were found in the Ncx1-/- pSp suggesting that the yolk sac normally generates all of these progenitors and distributes them to the pSp and liver. Alternatively, it is possible that the Ncx1-deficient conceptuses, which lack hemodynamic stress, do not produce the proper signals to induce myeloid progenitor formation in the pSp 44. Notwithstanding, it is clear that several types of definitive myeloid progenitors are generated de novo in the yolk sac. It would be interesting to examine the allantois in such circulation defective embryos for the de novo generation of myeloid progenitors.
A progenitor with more complex lymphoid-myeloid potential is found prior to the onset of circulation in the E8 pSp-AGM of the embryo following explant culture 37 (Table 1). When transplanted into irradiated immunodeficient recipients, this lymphoid-myeloid progenitor contributes to low-level, long-term, multilineage hematopoietic repopulation 45. Yolk sac explants, on the other hand do not contain such cells until the circulation is established, suggesting that cells with lymphoid-myeloid potential are generated de novo in the pSp-AGM and could theoretically emigrate to the yolk sac. Similarly, in explant cultures with human yolk sac and pSp-AGM tissues isolated prior to the onset of circulation only the pSp-AGM tissue was found to contain cells with lymphoid-myeloid potential 46.
A potent neonatal engrafting HSC was identified in the E9 mouse yolk sac and AGM. Both tissues contain c-kit+CD34+ cells that, when injected directly in the liver of neonatal recipient mice, can yield high amounts of multilineage engraftment 47. The yolk sac contains quantitatively more of these cells than the AGM. In contrast to E10.5 AGM HSCs (also c-kit+CD34+), which fully engraft adult recipients, the E9 c-kit+CD34+ neonatal repopulating cells are incapable of engraftment when injected directly into adult mice 47. Hence, there are at least five broad classes of hematopoietic cells in the mammalian conceptus as defined by activity in in vitro clonogenic or transplantation assays; primitive, pro-definitive (myeloid progenitors), meso-definitive (lymphoid-myeloid progenitors), meta-definitive(neonatal repopulating HSCs) and adult-definitive (adult repopulating HSCs) (Fig. 2). These classes of cells appear to be generated independently of each other and are generated in distinct hematopoietic sites.
Direct precursors to definitive hematopoietic cells
The “definitive” classes of hematopoietic progenitor-stem cells illustrated (Fig. 2) are thought to arise through a slightly different process than the primitive erythroid progenitors. Discrete subsets of vascular endothelial cells appear to exhibit hemogenic potential during development 1. In many species, histological and immuno staining studies have shown hematopoietic clusters tightly adherent to the ventral endothelium of the dorsal aorta, plus the umbilical and umbilical arteries (reviewed in 48). These hematopoietic clusters appear to be emerging from the endothelium during the time the first definitive HSCs are detected. Metabolic lineage tracing (AcLDL-DiI) or retroviral labelling of endothelial cells prior to hematopoietic cell appearance performed in chick embryos has confirmed the endothelial-hematopoietic lineage relationship of aortic hematopoietic clusters 49. One day after labelling of endothelial cells, AcLDL-DiI+CD45+ hematopoietic cells were found in the lumen adhering to the ventral aspect of the aorta. Slightly later, the underlying aortic mesenchyme also contained AcLDL-DiI+CD45+ cells, suggesting that labelled endothelial cells ingressed into the tissue. Clonal retroviral marking of the avian embryonic vasculature provided additional data confirming the fate commitment of an endothelial cell to the hematopoietic lineage 50. Similar attempts have been made in ex utero cultured mouse embryos to establish lineage relationships between endothelial cells and hematopoietic cells. Twelve hours following intracardiac injection of AcLDL-DiI into E10 mouse embryos, marked definitive erythroid cells were found in the circulation 51. Taken together, these data strongly indicate an endothelial origin for definitive hematopoietic cells.
In the mid-gestation mouse embryo, the phenotypic profile of adult HSCs and the spatial localization of these cells in the AGM have been useful in providing information about their direct precursors. Indeed, all HSCs in the AGM are CD45+ 25, Ly-6A (Sca-1) GFP+ 26, c-kit+CD34+ 52, Runx1+ 25, SCL+ 53 and Gata2+ 54, 55. These markers (with the exception of CD45) are also expressed by some or all endothelial cells in the ventral aspect of the dorsal aorta at E10/11. Most or all AGM HSCs express cell surface VE-cadherin 25, 56, which is typically thought of as an endothelial marker. Whether the “hemogenic” endothelium provides full endothelial function in the mid-gestation mouse aorta or if instead it is comprised of cells from the underlying mesenchyme that temporarily assume endothelial characteristics on their way to forming hematopoietic clusters is a matter of conjecture and/or semantics.
Some studies have suggested that HSCs are generated from mesenchyme located directly underneath endothelial cells in the ventral aspect of the dorsal aorta 25, or in discrete patches ventral-lateral to the dorsal aorta (sub-aortic patches) 57. Runx1 is expressed in mesenchymal cells underlying the ventral aspect of the dorsal aorta, and Runx1+ cells isolated from Runx1 haploinsufficient embryos based on a mesenchymal phenotype (CD45-, CD31- and VE-cadherin-) were found to possess HSC activity 25. However, CD45-, CD31- and VE-cadherin- cells similarly isolated from wild-type embryos did not contain HSCs 25. Transplantation data showed that cells from the subaortic patches (CD45-ckit+AA4.1+) have some repopulating activity in immunodeficient adult recipients (0.4-1.9% engraftment) 57, however they are not as potent as the Runx1+ or Ly-6A(Sca-1)GFP+ aortic endothelial/cluster HSCs that provide up to 100% engraftment of irradiated adult recipients 25, 26. The hematopoietic cells localized in the subaortic patches may be precursors to the fully potent HSCs found in the aortic endothelial hematopoietic clusters, or may represent differentiated progeny of hemogenic endothelium that has ingressed (as in the chick embryo) into this site, or may be an unrelated population of hematopoietic cells. Taken together, these mouse data strongly indicate that the direct precursors of HSCs are predominantly “hemogenic endothelial” cells. In addition, the vascular endothelium of the human embryo has blood-forming potential 58.
It appears that cells in the aortic hematopoietic clusters are not homogeneous. For example, only some cells in chick aortic clusters are CD41+ 59. Also, the Ly-6A GFP transgene marker associated with mouse aortic HSCs is expressed by only some cells in the aortic clusters and endothelium 28, 60. This could indicate that some emergent Ly-6A-GFP+ HSCs are undergoing differentiation (becoming GFP-) while residing in the cluster, or that adjacent Ly-6A GFP- hemogenic endothelium is contributing to Ly-6A GFP- hematopoietic cells in the clusters. In contrast to the strict ventral localization of hematopoietic clusters in the chick aorta, recent studies in the mouse have identified hematopoietic clusters on both the ventral and dorsal aspects of the dorsal aorta 24. Functional studies indicate that definitive hematopoietic progenitors reside on both aspects of the aorta, but only the ventral aspect contains fully potent HSCs 24. Interestingly, the chick aortic endothelium has dual origins: the dorsal aspect is derived from paraxial (somitic) mesoderm and the ventral aspect from splanchnic mesoderm 61. Recent data 62 demonstrate that chick somitic endothelial cells replace the splanchnopleural hemogenic endothelium in the floor of the aorta once the hematopoietic cluster phase has ended. In the mouse, a small contribution of somitic-derived endothelial cells is also found in the aorta but in the lateral aspect 63. To facilitate studies of the genetic programs that regulate hematopoietic specification, it will be important to establish the specific mesodermal origins of the hemogenic versus non-hemogenic endothelium in the mouse conceptus.
Extrinsic factors and master regulators
Prospective hematopoietic cells are specified through sets of intrinsic master regulators (transcription factors) and influenced by morphogens and factors emanating from the surrounding cellular environment; i.e. developing adjacent germ cell layers and tissues. The de novo generation of murine hematopoietic cells in the bilaminar yolk sac (endoderm and mesoderm), the chorio-allantoic placenta (mesoderm and trophectoderm) and the more complex AGM region (dorsal ectoderm, mesoderm and ventral endoderm) suggests distinct interactions and/or genetic programs are operative in each of these sites. Yet, the genetic program leading to hematopoietic specification should overlap to some degree between the distinct anatomical territories.
Interactions between endoderm and prospective hematopoietic mesoderm are necessary for hemogenic induction in the chick embryo. Blood island generation occurs only when the mesothelial and endoderm germ layers are cultured together – when cultured separately no primitive erythroblasts form 64-66. Similarly, somitic mesoderm, which normally only contributes to endothelium in the dorsal aspect of the dorsal aorta and not to the ventral endothelium or hematopoietic clusters, could be reprogrammed to assume the latter fates following transient exposure to endoderm prior to grafting 67. Several signalling molecules, including VEGF, bFGF, and TGFβ1 could substitute for this endodermal signal 67.
Studies in mouse conceptuses showed that contact with visceral endoderm is necessary for primitive hematopoiesis in yolk sac explants, and exposure to endoderm could respecify prospective neurectoderm to assume a hematopoietic fate 68, 69. This endoderm signal can be replaced in vitro by heparin-acrylic beads soaked in Indian Hedgehog (Ihh). Ihh is normally produced by the visceral endoderm, and this expression pattern, together with the explant data, suggests that hedgehog signaling is essential for primitive erythropoiesis 69. However, deletion of Ihh or its receptor Smoothened (Smo) in mice does not eliminate primitive erythropoiesis in the yolk sac, although it does profoundly affect yolk sac vascularization 70.
Hedgehog signaling is essential for hematopoiesis in the zebrafish equivalent of the AGM. Hedgehog is situated at the beginning of a signaling cascade that includes the downstream effectors VEGF, Notch, GATA-2 and Runx1 and culminates in the formation of blood cells in the dorsal aorta 71. VEGF, together with factors identified in the chick (bFGF, TGFβ and BMP4) are generally thought of as ventralizing factors, while dorsalizing factors that antagonize hematopoietic induction include EGF and TGFα 67. Most of the ventralizing factors also appear to play a role in hematopoiesis in the mouse. ES cell differentiation cultures and gene-targeting studies have revealed a role for the FGF, TGF and the VEGF/Flk-1 signaling axes in vasculogenesis and hematopoiesis 3, 72-74. VEGF is expressed by the yolk sac endoderm while Flk-1 is expressed by the mesoderm, and both are expressed intraembryonically as well 75, 76. BMP signaling is also important for initiating the blood program in the mouse conceptus. Bmp4-/- embryos generally die around the gastrulation stage, and those that do survive exhibit profound decreases in yolk sac mesoderm and erythropoiesis 74. Addition of BMP4 to ES cell differentiation cultures 77 and presumptive anterior head fold explants induces hematopoietic cell formation 20. BMP4 also increases the number of HSCs in AGM explants 60. Interestingly, BMP4 is localized in the mesenchyme underlying aortic clusters in the mouse 60 and human 78 embryo. Importantly, ventralizing factors control the expression of pivotal hematopoietic transcription factors such as SCL and GATA-1 that are important in hematopoiesis (reviewed in 79).
Notch1 signaling is selectively important for AGM but not yolk sac hematopoiesis. Mutations that affect Notch signaling in zebrafish eliminate Runx1 expression and hematopoietic cluster formation in the AGM 71, 80. Notch1-deficient mouse conceptuses die at E10 and contain almost normal numbers of yolk sac primitive erythroid and erythroid-myeloid progenitors, but have no AGM hematopoiesis or HSCs 81. Notch1, Notch4, and their ligands Delta-like 4, Jagged 1 and Jagged 2 are expressed in endothelial cells lining the dorsal aorta 82. Overexpression of Runx1 in Notch signaling mutants in both zebrafish and mice will restore AGM hematopoiesis, indicating that Runx1 is genetically downstream of Notch 80, 83.
Mice lacking the transcription factor GATA-2 suffer from severely impaired primitive erythropoiesis and a complete lack of other committed progenitors and HSCs and die at E10.5 84. GATA-2 is expressed in the aortic endothelium 55 and is thought to affect the expansion of the hemogenic population emerging from these cells 54. Interestingly, GATA-2 haploinsufficiency profoundly decreases the number of AGM HSCs, but yolk sac HSCs are only slightly affected. Runx1, another pivotal transcription factor for definitive hematopoiesis, is expressed ventrally in the mesenchyme, endothelium and hematopoietic clusters of the dorsal aorta 25, 85. Runx1-deficient conceptuses have essentially normal primitive erythropoiesis but have no myeloid or lymphoid-myeloid progenitors of any sort, and no AGM HSCs 86-88. The Ets-family transcription factor PU.1, which is required for definitive hematopoiesis, is a critical downstream target of Runx1 89-92. Haploinsufficiency of Runx1 leads to an increase in AGM HSCs when these are directly isolated from the embryo and transplanted into irradiated adult mice 25. However, when hematopoietic tissues of Runx1+/- conceptuses are first cultured as explants and then transplanted, they display interesting differential responses to Runx1 haploinsufficiency. HSCs were profoundly decreased in AGM explants but were increased in both yolk sac and placenta, suggesting that different regulatory networks, downstream targets, interacting molecules, or altered developmental timing are operative in these tissues 93. Since transcription factors work in complexes and act at several points in hematopoietic development, the interplay between specific transcription factors is an important aspect in hematopoietic specification. The finding that hematopoietic stem cell-specific enhancers of Scl and Runx1 can bind multiprotein complexes containing GATA and Ets 94 and GATA, Ets and SCL factors 95, respectively, suggests a higher order of regulatory complexity. In addition, prostaglandin E2 and IL-1, which are normally associated with the regulation of inflammation molecules, also affect hematopoiesis in the zebrafish and mouse AGM 96 (Orelio, personal communication). Thus, an understanding of how the master regulators are controlled, and are fine-tuned with respect to their levels in different hematopoietic subpopulations and sites, will provide insight into the genetic network that governs hematopoietic emergence in the conceptus. By analogy to the ES cell program 97, it is likely that just a small set of factors (Runx1, GATA, Ets and SCL) establishes hematopoietic cell identity in the conceptus.
Tracing cells to secondary hematopoietic territories
Once the various hematopoietic progenitors and HSCs emerge from their anatomically distinct sites, they are thought to enter the circulation and colonize the fetal liver (Fig. 3). In zebrafish, CD41+ hematopoietic cells enter the circulation by intravasation via the posterior cardinal veins 98. The mode of entry of mammalian progenitors and HSCs into the circulation is presumed to occur through release of cells from hematopoietic clusters into the artery or yolk sac vasculature, but this has not been directly demonstrated. In the mouse, the liver rudiment is colonized from late E9 onwards, and later the spleen and thymus are seeded either directly or from the fetal liver 99, 100. While it seems peculiar that the embryo first produces hematopoietic cells without the full potency of an adult HSC, these early classes of cells that are limited in potency and life span may provide maturation signals to the rudiments of the secondary hematopoietic territories. Indeed in the chick, several waves of early lymphocyte-like cells enter the thymus at receptive times and promote thymic growth 101. Similarly, mouse genetic models have demonstrated interactive dialogues between early lymphocytes entering the developing thymus and thymic epithelium 102. Thus, in addition to the well-known function of primitive erythrocytes in gaseous exchange, definitive myeloid and lymphoid-myeloid progenitors may function to stimulate the growth of secondary hemato-lymphoid territories.
Attempts have been made to lineage trace cells in the conceptus that give rise to the permanent hematopoietic system in the adult mouse. In lieu of the ex utero studies that can be performed on non-mammalian vertebrates, lineage tracing in the mouse has relied on Cre-lox recombination marking technology together with tissue specific and inducible transgenes 103. Several transcriptional control elements have been used to direct the expression of Cre recombinase to hematopoietic cells in the conceptus, including CD41, which is expressed in a subset of definitive hematopoietic progenitors 34, 104; SCL, which is expressed in endothelial cells and definitive HSCs 53; and Runx1 which is expressed in all definitive hematopoietic cells, hemogenic endothelium, and some mesenchymal cells 25. All three genes are also expressed in the mesodermal precursors of the primitive erythrocytes. Rosa26 reporter strains were used to detect the cells in which Cre was active, and the progeny of those cells.
CD41-Cre marked a high percentage of lymphoid and myeloid cells in the fetus, but only 5% of adult bone marrow cells 105. Therefore, although CD41 marks the majority of progenitors in the yolk sac 34, 104 most adult hematopoietic cells do not transit through a CD41-expressing precursor. The Scl- and Runx1-marking experiments utilized a tamoxifen-inducible Cre (ERT) to control the temporal window of active recombination 106, 107. Activation of Scl-CreERT pregnant females at E10 and E11 of gestation marked approximately 10% of the cells in the adult BM, indicating that the progeny of SCL expressing cells at midgestation contribute to adult hematopoiesis. In the experiments utilizing Runx1-CreERT, pregnant females were injected with tamoxifen between E7.5 to E10.5 107, and depending on the day of injection, varying numbers of marked cells were found in the adult BM. The authors' conclude that the adult hematopoietic cells were derived from yolk sac cells, since Runx1 is highly expressed in the yolk sac at the time of tamoxifen injection. However, others have found Runx1 expressing cells at the base of the allantois and in the pSp within 0.5 to 1 day following the onset of Runx1 expression in the yolk sac 39, 95. The inability to precisely stage embryos in utero, together with the uncertain kinetics of CreERT activity and recombination, makes it difficult to discern from what anatomical site the marked cells were derived. Additionally, the Runx1-CreERT allele creates a Runx1 haploinsufficiency, known to affect both the temporal and spatial appearance of HSCs in the conceptus 86. Hence, while the data confirm that Runx1 expressing cells from the early conceptus contribute to hematopoiesis in the adult, improvements in directing Cre recombination to specific sites of hematopoietic cell emergence will be necessary to unambiguously identify the anatomic origins of adult HSCs.
Summary
Hematopoietic development in the mammalian conceptus occurs in several mesodermal lineages, as defined by cells emigrating from the primitive streak to three distinct hemogenic tissues; the yolk sac, pSp/AGM and chorio-allantoic placenta. At least five distinct classes of hematopoietic activities have been described, with an increasingly progressive generation of more complex hematopoietic activities that culminate in the de novo generation of adult repopulating HSCs. The direct precursors to hematopoietic cells in the conceptus are hemangioblasts and the hemogenic endothelium of the major embryonic vasculature. The genetic program directing hematopoietic fate determination involves known developmental signalling pathways that converge on the directed expression of a small set of pivotal hematopoietic transcription factors. It is the balance (levels and timing) of expression of these factors in the different embryonic tissues that drive the fate determination and emergence of hematopoietic progenitors and HSCs. And while the specific lineages of cells contributing the mammalian adult hematopoietic system are in question, the legacy of Runx1 and SCL expressing embryonic cells in the adult is certain. The future challenge is to determine the precise balance of factors necessary to direct HSC fate ex vivo in highly expandable and accessible populations of cells, such as embryonic (stem) cells or other somatic cells. This knowledge and insight gained from study of HSC emergence in vertebrate embryos will ultimately be directed towards the de novo production of fully potent transplantable HSCs for cellular and molecular clinical therapies of human blood-related genetic diseases and leukemias.
Acknowledgments
The authors thank lab members and colleagues for lively discussions, and the Fondation des Treilles (http://www.les-treilles.com) for supporting and nurturing scientific dialogue through the colloquium “Stem cells of the blood vascular system”. Tom de Vries Lentsch produced the figures and the National Institutes of Health (RO1 HL091724 and R37 DK54077) and Dutch BSIK Innovative (03038 SCDD) and ZonMW VICI (916.36.601) programs provided support.
Contributor Information
Elaine Dzierzak, Erasmus Medical Center, Dept of Cell Biology, PO Box 2040, 3000 CA Rotterdam, The Netherlands.
Nancy A. Speck, Dartmouth Medical School, Dept of Biochemistry, Hanover, NH, USA
References
- 1.Sabin F. Studies on the origin of blood vessels and of red blood corpuscles as seen in the living blastoderm of chicks during the second day of incubation. Carnegie Inst Wash Pub # 272, Contrib Embryol. 1920;9:214. [Google Scholar]
- 2.Murray P. The development in vitro of the blood of the early chick embryo. Proc Roy Soc London. 1932;11:497–521. [Google Scholar]
- 3.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 4.Fehling HJ, et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 5.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 6.Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33:1041–1047. doi: 10.1016/j.exphem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11:519–533. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Kinder SJ, et al. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development. 1999;126:4691–4701. doi: 10.1242/dev.126.21.4691. [DOI] [PubMed] [Google Scholar]
- 9.Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 10.Weissman I, Papaioannou V, Gardner R. Differentiation of Normal and Neoplastic Hematopoietic Cells. Cold Spring Harbor Laboratory Press; New York: 1978. [Google Scholar]
- 11.Dieterlen-Lievre F. On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J Embryol Exp Morphol. 1975;33:607–619. [PubMed] [Google Scholar]
- 12.Turpen JB, Knudson CM, Hoefen PS. The early ontogeny of hematopoietic cells studied by grafting cytogenetically labeled tissue anlagen: localization of a prospective stem cell compartment. Dev Biol. 1981;85:99–112. doi: 10.1016/0012-1606(81)90239-6. [DOI] [PubMed] [Google Scholar]
- 13.Cormier F, Dieterlen-Lievre F. The wall of the chick embryo aorta harbours M-CFC, G-CFC, GM-CFC and BFU-E. Development. 1988;102:279–285. doi: 10.1242/dev.102.2.279. [DOI] [PubMed] [Google Scholar]
- 14.Caprioli A, et al. Hemangioblast commitment in the avian allantois: cellular and molecular aspects. Dev Biol. 2001;238:64–78. doi: 10.1006/dbio.2001.0362. [DOI] [PubMed] [Google Scholar]
- 15.Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell. 2000;102:787–796. doi: 10.1016/s0092-8674(00)00067-2. [DOI] [PubMed] [Google Scholar]
- 16.Walmsley M, Ciau-Uitz A, Patient R. Adult and embryonic blood and endothelium derive from distinct precursor populations which are differentially programmed by BMP in Xenopus. Development. 2002;129:5683–5695. doi: 10.1242/dev.00169. [DOI] [PubMed] [Google Scholar]
- 17.Walmsley M, Cleaver D, Patient R. FGF controls the timing of Scl, Lmo2 and Runx1 expression during embryonic blood development. Blood. 2007 doi: 10.1182/blood-2007-03-081323. [DOI] [PubMed] [Google Scholar]
- 18.Turpen JB, Kelley CM, Mead PE, Zon LI. Bipotential primitive-definitive hematopoietic progenitors in the vertebrate embryo. Immunity. 1997;7:325–334. doi: 10.1016/s1074-7613(00)80354-4. [DOI] [PubMed] [Google Scholar]
- 19.Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- 20.Kanatsu M, Nishikawa SI. In vitro analysis of epiblast tissue potency for hematopoietic cell differentiation. Development. 1996;122:823–830. doi: 10.1242/dev.122.3.823. [DOI] [PubMed] [Google Scholar]
- 21.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 22.Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 23.de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. Embo J. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taoudi S, Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc Natl Acad Sci U S A. 2007;104:9399–9403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.North T, et al. Runx1 Expression Marks Long-Term Repopulating HSCs in the Midgestation Mouse Embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- 26.de Bruijn M, et al. HSCs localize to the endothelial layer in the midgestation mouse aorta. Immunity. 2002;16:673–683. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- 27.Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. Placenta is a niche for hematopoietic stem cells. Developmental Cell. 2005 doi: 10.1016/j.devcel.2004.12.016. In press. [DOI] [PubMed] [Google Scholar]
- 28.Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Developmental Cell. 2005 doi: 10.1016/j.devcel.2005.02.001. In press. [DOI] [PubMed] [Google Scholar]
- 29.Johnson GR, Moore MA. Role of stem cell migration in initiation of mouse foetal liver haemopoiesis. Nature. 1975;258:726–728. doi: 10.1038/258726a0. [DOI] [PubMed] [Google Scholar]
- 30.Houssaint E. Differentiation of the mouse hepatic primordium. II. Extrinsic origin of the haemopoietic cell line. Cell Differ. 1981;10:243–252. doi: 10.1016/0045-6039(81)90007-5. [DOI] [PubMed] [Google Scholar]
- 31.Downs KM. The murine allantois. Curr Top Dev Biol. 1998;39:1–33. doi: 10.1016/s0070-2153(08)60451-2. [DOI] [PubMed] [Google Scholar]
- 32.Kumaravelu P, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad- mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi M, Sekiguchi T, Hara T, Kinoshita T, Miyajima A. Cultivation of aorta-gonad-mesonephros-derived hematopoietic stem cells in the fetal liver microenvironment amplifies long-term repopulating activity and enhances engraftment to the bone marrow. Blood. 2002;99:1190–1196. doi: 10.1182/blood.v99.4.1190. [DOI] [PubMed] [Google Scholar]
- 34.Ferkowicz MJ, et al. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130:4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- 35.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 36.McGrath KE, Palis J. Hematopoiesis in the yolk sac: more than meets the eye. Exp Hematol. 2005;33:1021–1028. doi: 10.1016/j.exphem.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Cumano A, Dieterlen-Lievre F, Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 38.Corbel C, Salaun J, Belo-Diabangouaya P, Dieterlen-Lievre F. Hematopoietic potential of the pre-fusion allantois. Dev Biol. 2007;301:478–488. doi: 10.1016/j.ydbio.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 39.Zeigler BM, et al. The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Development. 2006;133:4183–4192. doi: 10.1242/dev.02596. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez-Silva M, Belo-Diabangouaya P, Salaun J, Dieterlen-Lievre F. Mouse placenta is a major hematopoietic organ. Development. 2003;130:5437–5444. doi: 10.1242/dev.00755. [DOI] [PubMed] [Google Scholar]
- 41.Medvinsky AL, Samoylina NL, Muller AM, Dzierzak EA. An early pre-liver intraembryonic source of CFU-S in the developing mouse. Nature. 1993;364:64–67. doi: 10.1038/364064a0. [DOI] [PubMed] [Google Scholar]
- 42.Rampon C, Huber P. Multilineage hematopoietic progenitor activity generated autonomously in the mouse yolk sac: analysis using angiogenesis-defective embryos. Int J Dev Biol. 2003;47:273–280. [PubMed] [Google Scholar]
- 43.Lux CT, et al. All primitive and definitive hematopoietic progenitor cells emerging prior to E10 in the mouse embryo are products of the yolk sac. Blood. 2007 doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yashiro K, Shiratori H, Hamada H. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature. 2007;450:285–288. doi: 10.1038/nature06254. [DOI] [PubMed] [Google Scholar]
- 45.Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15:477–485. doi: 10.1016/s1074-7613(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 46.Tavian M, Robin C, Coulombel L, Peault B. The human embryo, but not its yolk sac, generates lympho-myeloid stem cells: mapping multipotent hematopoietic cell fate in intraembryonic mesoderm. Immunity. 2001;15:487–495. doi: 10.1016/s1074-7613(01)00193-5. [DOI] [PubMed] [Google Scholar]
- 47.Yoder MC, et al. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7:335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- 48.Jaffredo T, Bollerot K, Sugiyama D, Gautier R, Drevon C. Tracing the hemangioblast during embryogenesis: developmental relationships between endothelial and hematopoietic cells. Int J Dev Biol. 2005;49:269–277. doi: 10.1387/ijdb.041948tj. [DOI] [PubMed] [Google Scholar]
- 49.Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125 doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- 50.Jaffredo T, Gautier R, Brajeul V, Dieterlen-Lievre F. Tracing the progeny of the aortic hemangioblast in the avian embryo. Dev Biol. 2000;224:204–214. doi: 10.1006/dbio.2000.9799. [DOI] [PubMed] [Google Scholar]
- 51.Sugiyama D, et al. Erythropoiesis from acetyl LDL incorporating endothelial cells at the preliver stage. Blood. 2003;101:4733–4738. doi: 10.1182/blood-2002-09-2799. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez MJ, Holmes A, Miles C, Dzierzak E. Characterization of the first definitive hematopoietic stem cells in the AGM and liver of the mouse embryo. Immunity. 1996;5:513–525. doi: 10.1016/s1074-7613(00)80267-8. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez MJ, Bockamp EO, Miller J, Gambardella L, Green AR. Selective rescue of early haematopoietic progenitors in Scl(-/-) mice by expressing Scl under the control of a stem cell enhancer. Development. 2001;128:4815–4827. doi: 10.1242/dev.128.23.4815. [DOI] [PubMed] [Google Scholar]
- 54.Ling KW, et al. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J Exp Med. 2004;200:871–882. doi: 10.1084/jem.20031556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Minegishi N, et al. The mouse GATA-2 gene is expressed in the para-aortic splanchnopleura and aorta-gonads and mesonephros region. Blood. 1999;93:4196–4207. [PubMed] [Google Scholar]
- 56.Taoudi S, et al. Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the foetal liver. Development. 2005;132:4179–4191. doi: 10.1242/dev.01974. [DOI] [PubMed] [Google Scholar]
- 57.Bertrand JY, et al. Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc Natl Acad Sci U S A. 2005;102:134–139. doi: 10.1073/pnas.0402270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oberlin E, Tavian M, Blazsek B, Peault B. Blood-forming potential of vascular endothelium in the human embryo. Development. 2002;129:4147–4157. doi: 10.1242/dev.129.17.4147. [DOI] [PubMed] [Google Scholar]
- 59.Ody C, Vaigot P, Quere P, Imhof BA, Corbel C. Glycoprotein IIb-IIIa is expressed on avian multilineage hematopoietic progenitor cells. Blood. 1999;93:2898–2906. [PubMed] [Google Scholar]
- 60.Durand C, Robin C, Bollerot K, Baron MH, Ottersbach K, Dzierzak E. Embryonic stromal clones reveal developmental regulators of definitive hematopoietic stem cells. PNAS. 2007 doi: 10.1073/pnas.0706923105. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pardanaud L, et al. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development. 1996;122:1363–1371. doi: 10.1242/dev.122.5.1363. [DOI] [PubMed] [Google Scholar]
- 62.Pouget C, Gautier R, Teillet MA, Jaffredo T. Somite-derived cells replace ventral aortic hemangioblasts and provide aortic smooth muscle cells of the trunk. Development. 2006;133:1013–1022. doi: 10.1242/dev.02269. [DOI] [PubMed] [Google Scholar]
- 63.Esner M, et al. Smooth muscle of the dorsal aorta shares a common clonal origin with skeletal muscle of the myotome. Development. 2006;133:737–749. doi: 10.1242/dev.02226. [DOI] [PubMed] [Google Scholar]
- 64.Miura Y, Wilt FH. Tissue interaction and the formation of the first erythroblasts of the chick embryo. Dev Biol. 1969;19:201–211. doi: 10.1016/0012-1606(69)90055-4. [DOI] [PubMed] [Google Scholar]
- 65.Pardanaud L, Dieterlen-Lievre F. Emergence of endothelial and hemopoietic cells in the avian embryo. Anat Embryol (Berl) 1993;187:107–114. doi: 10.1007/BF00171741. [DOI] [PubMed] [Google Scholar]
- 66.Wilt FH. Erythropoiesis in the Chick Embryo: The Role of Endoderm. Science. 1965;147:1588–1590. doi: 10.1126/science.147.3665.1588. [DOI] [PubMed] [Google Scholar]
- 67.Pardanaud L, Dieterlen-Lievre F. Manipulation of the angiopoietic/hemangiopoietic commitment in the avian embryo. Development. 1999;126:617–627. doi: 10.1242/dev.126.4.617. [DOI] [PubMed] [Google Scholar]
- 68.Belaoussoff M, Farrington SM, Baron MH. Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo. Development. 1998;125:5009–5018. doi: 10.1242/dev.125.24.5009. [DOI] [PubMed] [Google Scholar]
- 69.Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128:1717–1730. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- 70.Byrd N, et al. Hedgehog is required for murine yolk sac angiogenesis. Development. 2002;129:361–372. doi: 10.1242/dev.129.2.361. [DOI] [PubMed] [Google Scholar]
- 71.Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell. 2005;8:389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 72.Faloon P, et al. Basic fibroblast growth factor positively regulates hematopoietic development. Development. 2000;127:1931–1941. doi: 10.1242/dev.127.9.1931. [DOI] [PubMed] [Google Scholar]
- 73.Shalaby F, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 74.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 75.Breier G, Clauss M, Risau W. Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests a paracrine regulation of murine vascular development. Dev Dyn. 1995;204:228–239. doi: 10.1002/aja.1002040303. [DOI] [PubMed] [Google Scholar]
- 76.Dumont DJ, et al. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn. 1995;203:80–92. doi: 10.1002/aja.1002030109. [DOI] [PubMed] [Google Scholar]
- 77.Johansson B, W M. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Molecular and Cellular Biology. 1995;15:141–151. doi: 10.1128/mcb.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marshall CJ, Kinnon C, Thrasher AJ. Polarized expression of bone morphogenetic protein-4 in the human aorta-gonad-mesonephros region. Blood. 2000;96:1591–1593. [PubMed] [Google Scholar]
- 79.Sadlon TJ, Lewis ID, D'Andrea RJ. BMP4: its role in development of the hematopoietic system and potential as a hematopoietic growth factor. Stem Cells. 2004;22:457–474. doi: 10.1634/stemcells.22-4-457. [DOI] [PubMed] [Google Scholar]
- 80.Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumano K, et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18:699–711. doi: 10.1016/s1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- 82.Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A. RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development. 2005;132:1117–1126. doi: 10.1242/dev.01660. [DOI] [PubMed] [Google Scholar]
- 83.Nakagawa M, et al. AML1/Runx1 rescues Notch1-null mutation-induced deficiency of para-aortic splanchnopleural hematopoiesis. Blood. 2006;108:3329–3334. doi: 10.1182/blood-2006-04-019570. [DOI] [PubMed] [Google Scholar]
- 84.Tsai FY, et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 85.North T, et al. Cbfa is required for the formation of intraaortic hematopoietic clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- 86.Cai ZL, et al. Haploinsufficiency of AML1/CBFA2 affects the temportal and spatial generation of hematopoietic stem cells in the mouse embryo. 2000;13:423–431. doi: 10.1016/s1074-7613(00)00042-x. [DOI] [PubMed] [Google Scholar]
- 87.Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 88.Wang Q, et al. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci U S A. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Okada H, et al. AML1(-/-) embryos do not express certain hematopoiesis-related gene transcripts including those of the PU.1 gene. Oncogene. 1998;17:2287–2293. doi: 10.1038/sj.onc.1202151. [DOI] [PubMed] [Google Scholar]
- 90.McKercher SR, et al. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. Embo J. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- 91.Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- 92.Huang G, et al. PU.1 is a major downstream target of AML1 (RUNX1) in adult mouse hematopoiesis. Nat Genet. 2007 doi: 10.1038/ng.2007.7. [DOI] [PubMed] [Google Scholar]
- 93.Robin C, et al. An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells. Dev Cell. 2006;11:171–180. doi: 10.1016/j.devcel.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Gottgens B, et al. Establishing the transcriptional programme for blood: the SCL stem cell enhancer is regulated by a multiprotein complex containing Ets and GATA factors. Embo J. 2002;21:3039–3050. doi: 10.1093/emboj/cdf286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nottingham WT, et al. Runx1-mediated hematopoietic stem cell emergence is controlled by a Gata/Ets/SCL-regulated enhancer. Blood. 2007 doi: 10.1182/blood-2007-07-100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 98.Kissa K, et al. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood. 2007 doi: 10.1182/blood-2007-07-099499. [DOI] [PubMed] [Google Scholar]
- 99.Bertrand JY, et al. Fetal spleen stroma drives macrophage commitment. Development. 2006;133:3619–3628. doi: 10.1242/dev.02510. [DOI] [PubMed] [Google Scholar]
- 100.Yokota T, et al. Tracing the first waves of lymphopoiesis in mice. Development. 2006;133:2041–2051. doi: 10.1242/dev.02349. [DOI] [PubMed] [Google Scholar]
- 101.Jotereau FV, Le Douarin NM. Demonstration of a cyclic renewal of the lymphocyte precursor cells in the quail thymus during embryonic and perinatal life. J Immunol. 1982;129:1869–1877. [PubMed] [Google Scholar]
- 102.van Ewijk W, Hollander G, Terhorst C, Wang B. Stepwise development of thymic microenvironments in vivo is regulated by thymocyte subsets. Development. 2000;127:1583–1591. doi: 10.1242/dev.127.8.1583. [DOI] [PubMed] [Google Scholar]
- 103.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- 104.Mikkola HK, Fujiwara Y, Schlaeger TM, Traver D, Orkin SH. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003;101:508–516. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- 105.Emambokus NR, Frampton J. The glycoprotein IIb molecule is expressed on early murine hematopoietic progenitors and regulates their numbers in sites of hematopoiesis. Immunity. 2003;19:33–45. doi: 10.1016/s1074-7613(03)00173-0. [DOI] [PubMed] [Google Scholar]
- 106.Gothert JR, et al. In vivo fate tracing studies using the SCL stem cell enhancer: embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood. 2004 doi: 10.1182/blood-2004-08-3037. [DOI] [PubMed] [Google Scholar]
- 107.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]