Abstract
Background
Myocardial systolic strain patterns in dilated cardiomyopathy are felt to be nonhomogeneous but have not been investigated with MRI-based multiparametric systolic strain analysis. Left ventricular (LV) three-dimensional (3D) multiparametric systolic strain analysis is sensitive to regional contractility and is generated from sequential magnetic resonance imaging (MRI) of tissue tagging gridline point displacements.
Methods
Sixty normal human volunteers underwent MRI-based 3D systolic strain analysis to supply normal average and standard deviation values for each of three strain parameters at each of 15,300 individual LV grid points. Patient-specific multiparametric systolic strain data from each dilated cardiomyopathy patient (n = 10) were then subjected to a point-by-point comparison (n = 15,300 LV points) to the normal strain database for 3 individual strain components (45,900 database comparisons per patient). The resulting composite multiparametric Z-score values (standard deviation from normal average) were color contour mapped over patient-specific 3D LV geometry to detect the normalized regional contractile patterns associated with dilated cardiomyopathy.
Results
Average multiparametric strain Z-score values varied significantly according to ventricular level (p = 0.001) and region (p = 0.003). Apical Z-scores were significantly less than those in both the base (p = 0.037) and mid-ventricle (p = 0.002), while anterolateral wall Z-scores were less than those in the anteroseptal (p = 0.023) and posteroseptal walls (p = 0.028).
Conclusions
MRI-based multiparametric systolic strain analysis suggests that myocardial systolic strain in patients with dilated cardiomyopathy has a heterogeneous regional distribution and on average falls almost two standard deviations from normal.
The uniform nature of the circumferential myocardial thinning and symmetrical short axis cross-sectional shape that characterizes the LV geometry of patients with non-ischemic, non-valvular dilated cardiomyopathy has long suggested a homogeneous myocardial process of injury. It is now recognized, however, that the pathological influence of secondary remodeling on both adjacent and distant non-injured myocardium can render uniform and homogeneous global ventricular geometrical changes from a heterogeneous injury process. Previous investigations have indeed suggested heterogeneous contractile changes (1–3) in the catchall category of myocardial dysfunction, ventricular dilation, and globular ventricular remodeling that is labeled non-ischemic, non-valvular dilated cardiomyopathy (4–7).
In addition to excellent temporal and spatial resolution, MRI also has the unique ability to track tissue tagging grids throughout systole. These attributes allow measurement of 3D LV systolic point displacement, and thus systolic strain, throughout contraction (8–12). These unique capabilities make this methodology ideally suited to regionally quantify contractile function in patients with dilated cardiomyopathy. Utilizing custom software, tag surface deformation can be utilized to generate accurate, LV systolic strain maps (10). A fitting of displacement data measured from the deformation of the tag surfaces provides a continuous description of displacement throughout the model domain. Utilizing the results of this fitting, strain can be computed at any point in the model.
To allow the application of a multiparametric systolic strain analysis in dilated cardiomyopathy patients, a normal human strain database was established by the application of MRI-based systolic strain analysis in 60 normal human volunteers. By characterizing all systolic strain parameters at each of 15,300 LV points in all volunteers, the normal averages and standard deviations for each strain parameter at each point were firmly established. Since systolic strain is nonhomogeneous throughout the human LV, this normal human strain database allows the comparison of each patient-specific systolic strain parameter from each LV grid point to the normal average and standard deviation at that position in the myocardium. This clinically applicable methodology provides the ideal manner in which to examine the heterogeneity of LV contractile function in the setting of non-ischemic, non-valvular dilated cardiomyopathy.
METHODS
Patient Characteristics
A group of 60 healthy volunteers underwent MRI-based strain analysis and contributed complete LV systolic strain information to a normal subject strain database. A separate group of ten patients with severe heart failure secondary to non-ischemic, non-valvular dilated cardiomyopathy also underwent MRI-based multiparametric strain analysis making the total study group 70 subjects. Select clinical data from the patients are presented in Table 1. All of the heart failure patients were on optimal medical therapy as determined by Washington University Medical School heart failure subspecialists. The Human Research Protection Office at Washington University, St. Louis, MO, approved the study and all subjects gave informed written consent.
Table 1.
Selected demographic and clinical data from the ten dilated cardiomyopathy patients that were included in this study.
| Patient | Age | Gender | NYHA Class | EF | Duration of DCM (months) |
|---|---|---|---|---|---|
| 1 | 40 | F | II | 20–25% | 5 |
| 2 | 41 | F | III | <25% | 2 |
| 3 | 35 | F | III | <25% | 3 |
| 4 | 57 | M | II | <25% | 20 |
| 5 | 53 | M | III | 25% | 6 |
| 6 | 39 | M | III | 17% | 1 |
| 7 | 33 | M | III | 20% | 2 |
| 8 | 48 | F | III | 21% | 3 |
| 9 | 23 | M | III | <10% | 1 |
| 10 | 42 | F | III | 20% | 2 |
DCM = dilated cardiomyopathy; EF = ejection fraction; NYHA = New York Heart Association.
MR Imaging
Imaging was performed in a 1.5 Tesla MR scanner (Sonata, Siemens Medical Systems, Malvern, PA). Short axis image sets were acquired in parallel planes at 8mm intervals extending from the plane of the mitral valve to the apex of the heart (Figures 1A–B). Additionally, four sets of long axis images oriented radially and intersecting the centroid of the LV were obtained (Figures 1C–D). For each imaging plane, a single-slice tagged image was collected with a sequence consisting of a SPAMM (Spatial Modulation of Magnetization) radiofrequency tissue-tagging preparation (13, 14) followed by a 2-D balanced steady-state free precession cine image acquisition. Image acquisition was synchronized with real-time electrocardiogram at the time of the MRI scanning. Typical imaging parameters were: tag spacing 8mm; slice thickness 8mm; repetition time (TR) 30.3 ms, echo time (TE) 2.2 ms, field of view 306 × 350 mm and image matrix 168 × 256.
Figure 1. Radiofrequency Tagging Planes at End-Diastole and End-Systole.
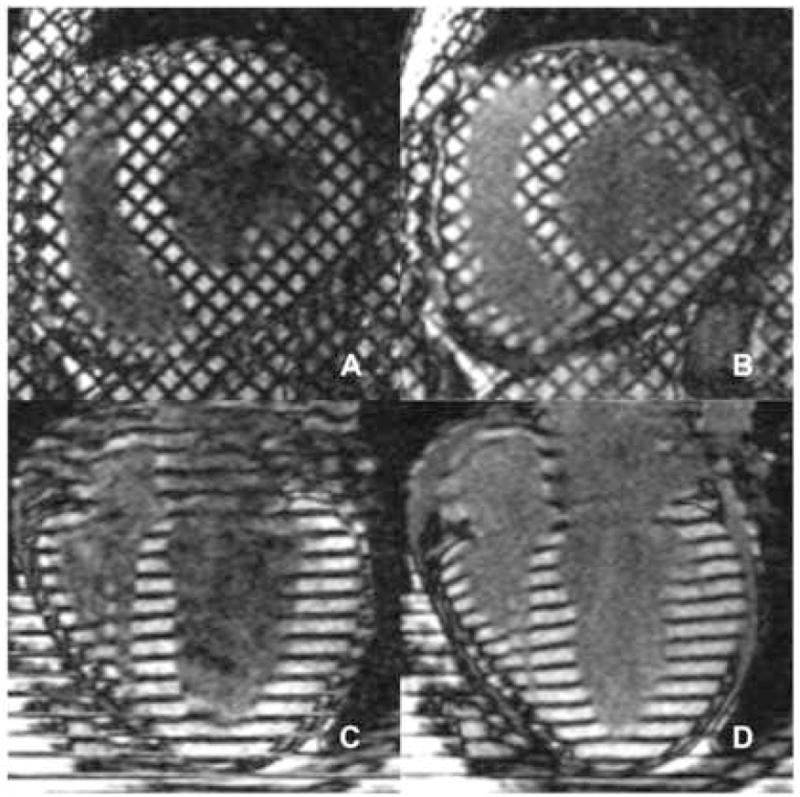
A uniform three-dimensional grid of radiofrequency tissue-tagging cubes encompasses the entirety of the left ventricular myocardium. The displacement of the three-dimensional tag plane grid is tracked at intervals from end-diastole to end-systole. The magnetic resonance tracking of each of these discrete tagging cubes allows the interpolation of displacement and strain data to the fraction of the 15,300 total left ventricular points that are enclosed within its six planar surfaces. Figures 1A and 1B show MRI scans demonstrating midventricular cross-sections (orthogonal to the long axis of the ventricle) obtained at end-diastole and end-systole, respectively. Figures 1C and 1D show MRI scans demonstrating left ventricular long axis tags obtained at end-diastole and end-systole, respectively.
Strain Calculations
The method used to compute strain in this study has been described previously (10, 15), so only a summary is provided here. Manually identified LV boundaries from the tagged MR images were used to construct a finite element model of the LV. Using the attachment points of the right ventricular free wall to the septum as landmarks, a finite element mesh was constructed consisting of six hexahedral elements for the basal and middle regions of the LV and six pentahedral elements covering the apex (Figure 2). Three-dimensional systolic displacements were computed from the deformation of the tag surfaces using a previously described and validated method (10). Analysis of the displacement data was carried out in the finite element analysis software package StressCheck (ESRD, Inc., St. Louis, Missouri). Predicted displacements at any point within the domain of the model were obtained from a least squares fitting of the measured displacements. Strain values were computed from the results of this fitting.
Figure 2. Region-based Left Ventricular Finite Element Model.
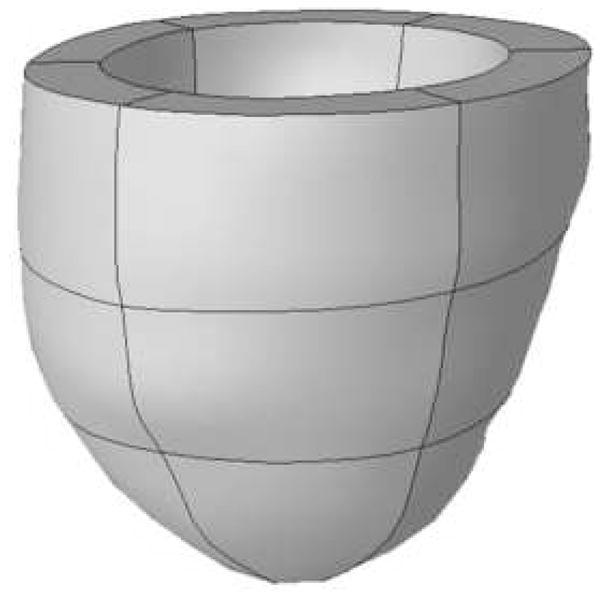
The attachment points of the right ventricular free wall to the LV septum serve as fiducial landmarks to consistently and uniformly divide the left ventricle into six standardized clinically relevant regions. Each region is then divided into basal, mid-ventricular, and apical segments. The standardized finite element mesh for the LV model therefore consists of six hexahedral elements each for the basal and mid-ventricular regions of the LV and six pentahedral elements covering the apex.
Multiparametric Systolic Strain Z-Scores
Myocardial systolic strain information draws clinical meaning and utility only as it relates the individual patient’s point-specific regional contractile function to “normal.” To define “normal,” a normal human strain database was developed. Utilizing MRI data from 60 normal volunteers, average and standard deviation values were computed for circumferential strain, longitudinal strain and the minimum principal strain angle (16) at each of 15,300 grid points distributed evenly over the entire LV model. Since systolic strain parameters are regionally and transmurally nonhomogeneous, the comparison of patient-specific strain values at a discrete point in the myocardium must therefore be in relation to the normal average value of that parameter at that position in the LV.
The normal human strain database allows the “normalization” of patient-specific strain values by their direct correlation to normal average and standard deviations for each strain component at each of 15,300 discrete myocardial points. Mathematically, this is expressed by calculation of a Z-score. The Z-score is a simple numerical representation of the number of standard deviations a raw strain value falls from the normal average and is computed as the difference between the measured strain value and the normal average divided by the normal standard deviation at a particular point. A Z-score of 1.87, for example, means that the raw patient value for that strain component at that discrete point in the LV falls 1.87 standard deviations from the normal average of that particular strain component at that point.
The normalization of individual strain components allows the combination of multiple strain parameters into a single multiparametric strain composite index. Three strain components (circumferential strain magnitude, longitudinal strain magnitude, and the minimum principal strain angle (16)) are utilized to perform the multiparametric strain analysis. Each individual cardiomyopathy patient’s multiparametric strain analysis therefore requires a total of 45,900 comparisons of their individual raw strain values to the established normal human strain component sub-databases (normal averages and standard deviations) that comprise our normal human strain database.
The multiparametric Z-score for any one of the 15,300 points throughout the LV therefore relates that patient’s contractility at that point in the myocardium to normal by relating its multiparametric strain, as expressed by a composite of three equally weighted strain components, to the “normal” multiparametric strain at that point in the LV. The patient-specific multiparametric composite Z-score values for each of 15,300 LV grid points are then subjected to 3D color contour mapping (Figure 3).
Figure 3. MRI-based Multiparametric Strain Z-Score Color Contour Modeling.
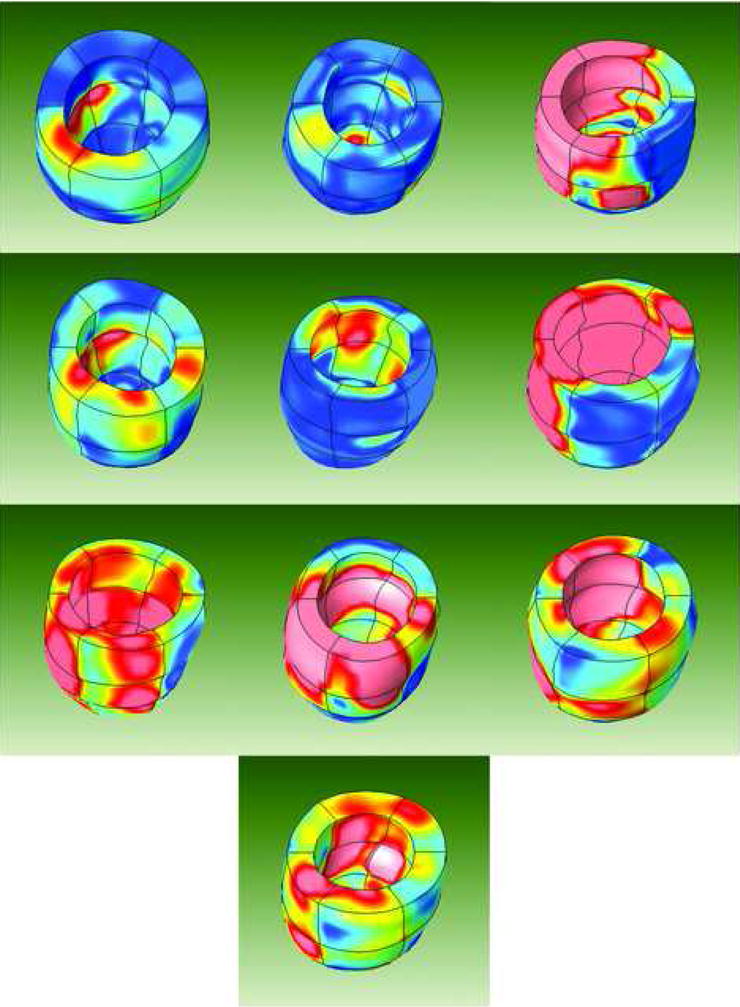
3D left ventricular strain maps obtained from all 10 dilated cardiomyopathy patients are demonstrated. The heterogeneity of the regional contractile dysfunction as it relates to the regional expected norm is well demonstrated. Areas represented by the color red have regional contractile function that deviates from the expected norm to the same degree as nonviable myocardium in patients with ischemic cardiomyopathy.
Statistical Analysis
Average multiparametric strain Z-scores from each of the eighteen model regions (see Figure 2) were compared using a two-way ANOVA. Post hoc comparisons were done using Tamhane’s T2 test. All calculations were carried out using the SPSS statistical package (SPSS, Inc., Chicago, Illinois). P-values of less than 0.05 were considered significant.
RESULTS
The average MRI-based multiparametric strain Z-score value for the dilated cardiomyopathy patients placed the composite group’s global LV contractile function 1.93 standard deviations from the expected norm.
Regional average multiparametric Z-scores were compared using a two-way ANOVA with ventricular level (base, mid-ventricle and apex) and region (anteroseptal, anterior, anterolateral, posterolateral, posterior and posteroseptal) included as fixed factors. Average Z-scores were found to vary significantly both by level (p = 0.001) and by region (p = 0.003), while the interaction between level and region was not found to be significant (p = 0.735). Average Z-scores grouped by ventricular level and region are presented in Figures 4 and 5, respectively. Post hoc comparisons showed that Z-scores in the apex (1.39 ± 1.09) were significantly less than those in the mid-ventricle (2.40 ± 1.94, p = 0.002) and the base (2.00 ± 1.50, p = 0.037). In the comparisons by region, Z-scores from the anterolateral wall (1.20 ± 0.66) were found to be significantly less than those in the anteroseptal (2.54 ± 2.03, p = 0.023) and posteroseptal walls (2.52 ± 2.05, p =0.028).
Figure 4. Average Multiparametric Z-scores Grouped by Ventricular Level.
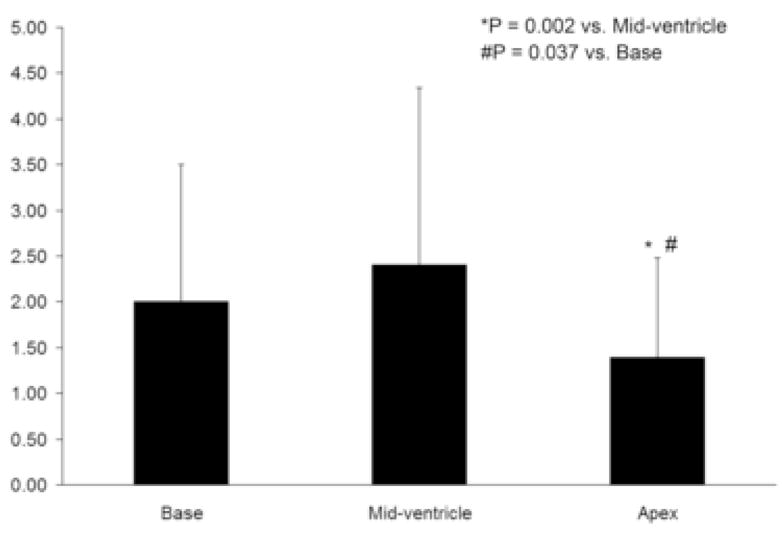
Average multiparametric Z-scores were smaller in the apex compared to both the mid-ventricle (p = 0.002) and the base (p = 0.037).
Figure 5. Average Multiparametric Z-scores Grouped by Ventricular Region.
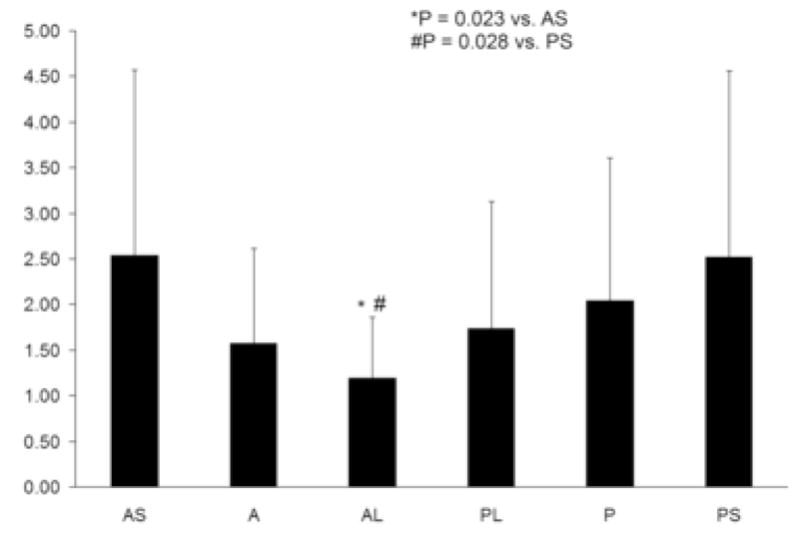
Average multiparametric Z-scores were smaller in the anterolateral wall compared to both the anteroseptal wall (p = 0.023) and the posteroseptal wall (p = 0.028). AS = anteroseptal; A = anterior; AL = anterolateral; PL = posterolateral; P = posterior; PS = posteroseptal.
Uniquely, the results from this study can be visually displayed in a format that relates the composite regional contractile information that has been obtained from these 10 cardiomyopathy patients to the normal average obtained from the 60 volunteers who contributed to the normal human strain database. To generate a single multiparametric strain Z-score 3D LV color contour map that represents the composite strain information from the entire dilated cardiomyopathy group, the 10 patient-specific multiparametric strain Z-score values for each of 15,300 points were averaged together. This generated a single set of 15,300 LV grid point multiparametric strain Z-score values that uniquely represents those found in the non-ischemic, non-valvular dilated cardiomyopathy patient population.
These LV average multiparametric Z-score values were then subjected to color contour mapping to generate the composite average multiparametric strain 3D color contour map shown in Figure 6. In this color contour map, different colors were assigned to designate a known level of “clinically relevant” deviation from normal. Areas with blue coloration represent areas with multiparametric strain Z-score values less than 1.5 standard deviations from the normal average. In a similar fashion, areas with yellow and red coloration represent average multiparametric strain average Z-score values between 1.5 and 2.25, and greater than 2.25, respectively.
Figure 6. Composite (n = 10) MRI-based Multiparametric Strain Z-Score Color Contour Left Ventricular 3D Mapping.
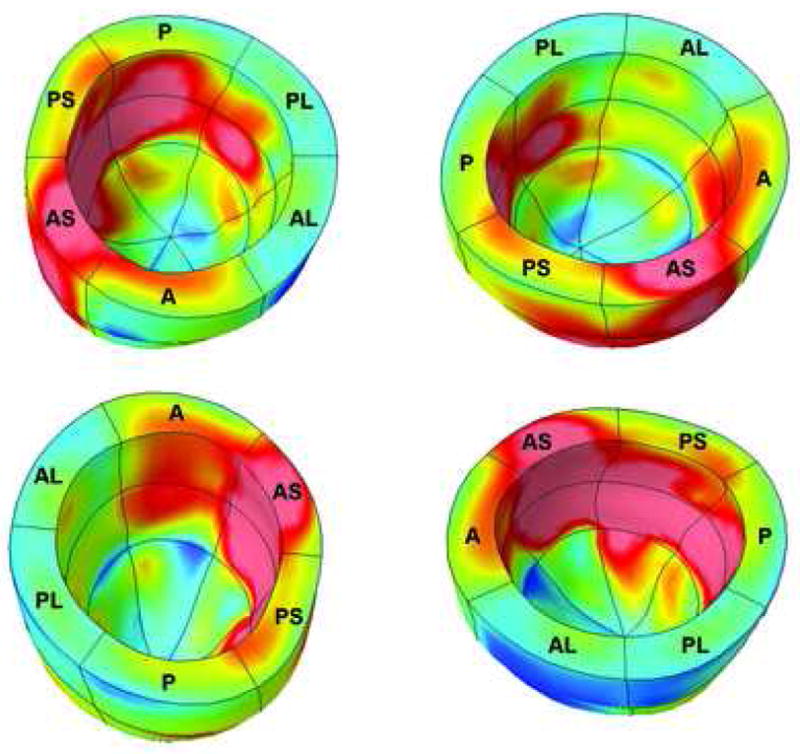
Multiparametric strain Z-scores from each of 15,300 points for each of ten dilated cardiomyopathy patients were averaged together to create a single representation of the composite regional and transmural myocardial contractile function that can be anticipated in patients in this heart failure subgroup. The regional heterogeneity of contractile dysfunction relative to the expected regional norm, including the predominant impairment of the ventricular septum, is demonstrated in this composite color contour map representing the entire group of non-ischemic, non-valvular dilated cardiomyopathy patients. AS = anteroseptal; A = anterior; AL = anterolateral; PL = posterolateral; P = posterior; PS = posteroseptal.
Multiparametric strain Z-score values that fell greater than 1.5 standard deviations from the normal average were therefore assigned a different (non-blue) coloration designating a clinically relevant deviation from normal. This assignment was based upon a recent study by our laboratory in patients with known nonviable myocardium secondary to myocardial infarction (17). Mean regional multiparametric strain Z-score values were correlated with currently utilized clinical standard viability testing methodologies. In that study, a receiver operator characteristic curve assessing the accuracy of the regional Z-scores in distinguishing viable from nonviable myocardium identified a Z-score viability threshold value of 1.525 (> 1.525 being nonviable; area under the curve 0.941 [P < 0.001] with a 95% confidence interval lower bound 0.897, upper bound 0.985; this resulted in an optimized sensitivity of 90% and a specificity of 90%). Figure 6 therefore offers a reasonable representation of the composite “normalized” regional contractile function—as it relates to the expected norm found in the general population—that can be anticipated in a representative group of patients with the clinical diagnosis of non-ischemic, non-valvular dilated cardiomyopathy. Non-blue colored areas in this dilated cardiomyopathy composite image therefore uniquely reveal regions whose contractile function was essentially equivalent to nonviable myocardium in an ischemic cardiomyopathy patient population.
DISCUSSION
The ability of sequential MR scanning to track tag surfaces throughout the course of ventricular systole enables the accurate characterization of regional and transmural LV contractile patterns. Finite element model predicted displacements were generated from a fitting of measured displacements obtained from tissue tagged MRI images. The resulting LV point displacement information enables the subsequent generation of detailed 3D systolic strain maps.
This mathematical expression of myocardial contractile function gains diagnostic and prognostic clinical significance only in its capability to relate patient-specific regional and transmural contractile function to the expected norm. To this end, our laboratory has established a normal human strain database by compiling comprehensive LV systolic strain data from a large number of normal human volunteers. This normal human strain database enables the normalization of individual patient-specific raw strain data thus allowing the subsequent combination of multiple strain parameters into powerful composite strain indices. These indices can then be utilized to express patient-specific regional and transmural strain contractile information in relation to the expected norm. No other cardiac imaging analysis does this. In addition, modalities such as tissue Doppler strain determinations are currently limited by their presentation of the strain data in two-dimensional format rather than the fully 3D format of multiparametric strain analysis. Most importantly, this allows MRI-based multiparametric strain analysis to overcome the primary problem of echo-based methodologies, which has to do with accounting for movement of the critical cardiac geometry in and out of the two-dimensional echo plane.
Three strain parameters (circumferential strain, longitudinal strain, and minimum principal strain angle (16)) have demonstrated accuracy in the characterization of regional and transmural myocardial function (15, 18, 19) and hold great potential as components of a composite multiparametric strain index. Their use as components of a single multiparametric strain index of myocardial contractile function therefore allowed the accurate characterization of regional contractile patterns in this group of patients with heart failure in the setting of non-ischemic, non-valvular dilated cardiomyopathy.
The application of this multiparametric strain analysis to a group of patients with dilated cardiomyopathy has not only demonstrated its clinical applicability in critically ill patients, but has also resulted in new insight into regional and global contractile patterns in this specific subgroup of heart failure patients. The unique perspective that is offered by the generation of a single composite image that summarizes the average regional and transmural myocardial contractile function from the entire group of patients ushers in a new diagnostic era in the visualization of the contractile patterns that can be anticipated in various patient subgroups.
The results of this study also confirm that which has been suggested by other studies of regional ventricular function in dilated cardiomyopathy patients. In 2001, Young and colleagues (3) used MRI with tissue tagging to study contractile function in 13 patients with dilated cardiomyopathy. They found abnormal values of both circumferential and longitudinal shortening in the septum compared to relatively normal values in the lateral wall. More recently, Potter et al. (2) measured minimum principal strain in nine mongrel dogs at baseline and after inducing dilated cardiomyopathy by rapid ventricular pacing. Strain values decreased in the septal and inferior walls compared to baseline, but were similar in the lateral and anterior walls. Despite short and long axis symmetry in pathological LV remodeling, regional and transmural myocardial contractile function is not affected in a homogeneous fashion in dilated cardiomyopathy patients. A consistent pattern of septal dominance in the abnormal regional deviation from the expected norm was confirmed in our patients by the composite representation shown in Figure 6. This pattern suggests that lateral wall function is impaired less than septal function when compared in a relative fashion to the expected norm.
Limitations
A fitting algorithm was utilized in this study to interpolate displacement information inside the walls of the uniform cubes formed by the tagging planes. The use of interpolation automatically engenders questions regarding the amount of interpolated displacement/strain data relative to the amount of measured data. When applied to hearts with normal wall thickness, currently utilized tissue tagging grid dimensions limit the number of tag lines that can fit transmurally between the epicardium and endocardium and thus results in sparse sampling of myocardial motion in the radial direction. Radial strain accuracy has indeed consistently lagged behind that of circumferential and longitudinal strain measurements in our clinical studies to date.
A multiparametric index uniquely allows the strain analysis to be focused upon the strengths of the MRI tissue tagging grid density. The three strain parameters that are based on the highest density of tag line data (the circumferential, longitudinal, and minimum principal strain directions) can be combined into a single multiparametric strain index with minimal reliance upon the less densely assessed radial direction. This ability to focus the strain analysis on these directions—which importantly also represent the directions of maximal fiber contraction—almost certainly contributed to the demonstrated accuracy of this methodology in the clinical detection of abnormal contractile patterns.
Conclusion
This study confirms a heterogeneous distribution of LV regional contractile dysfunction in patients with non-ischemic, non-valvular dilated cardiomyopathy. It also demonstrates the ready applicability of MRI-based multiparametric myocardial systolic strain analysis in this subset of critically ill heart failure patients. Of perhaps more importance, this study establishes the utility of the normalization of strain data using a normal volunteer human strain database in the quantification and color contour representation of regional and transmural contractile dysfunction relative to the expected norm. The composite multiparametric image representing the normalized strain data from all 10 patients supplies a unique visual representation of the strain heterogeneity that can be anticipated in this group of patients.
Acknowledgments
Supported by NIH Grants R01 HL069967 and R01 HL064869
The authors would like to acknowledge the assistance of Dr. Edward Geltman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bach DS, Beanlands RS, Schwaiger M, Armstrong WF. Heterogeneity of ventricular function and myocardial oxidative metabolism in nonischemic dilated cardiomyopathy. Journal of the American College of Cardiology. 1995;25:1258–62. doi: 10.1016/0735-1097(95)00019-Z. [DOI] [PubMed] [Google Scholar]
- 2.Potter DD, Araoz PA, Ng LL, et al. Cardiotropin-1 and myocardial strain change heterogeneously in cardiomyopathy. The Journal of surgical research. 2007;141:277–83. doi: 10.1016/j.jss.2006.12.539. [DOI] [PubMed] [Google Scholar]
- 3.Young AA, Dokos S, Powell KA, et al. Regional heterogeneity of function in nonischemic dilated cardiomyopathy. Cardiovascular research. 2001;49:308–18. doi: 10.1016/s0008-6363(00)00248-0. [DOI] [PubMed] [Google Scholar]
- 4.Collins MJ, Ozeki T, Zhuo J, et al. Use of diffusion tensor imaging to predict myocardial viability after warm global ischemia: possible avenue for use of non-beating donor hearts. J Heart Lung Transplant. 2007;26:376–83. doi: 10.1016/j.healun.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Rasmusson KD, Stehlik J, Brown RN, et al. Long-term outcomes of cardiac transplantation for peri-partum cardiomyopathy: a multiinstitutional analysis. J Heart Lung Transplant. 2007;26:1097–104. doi: 10.1016/j.healun.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Tjang YS, Stenlund H, Tenderich G, Hornik L, Bairaktaris A, Korfer R. Risk factor analysis in pediatric heart transplantation. J Heart Lung Transplant. 2008;27:408–15. doi: 10.1016/j.healun.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Yoda M, El-Banayosy A, Arusoglu L, Tendrich G, Minami K, Korfer R. Permanent use of a ventricle assist device for dilated cardiomyopathy in Friedreich’s ataxia. J Heart Lung Transplant. 2006;25:251–2. doi: 10.1016/j.healun.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Denney TS, Prince JL. Reconstruction of 3D left ventricular motion from planar tagged cardiac MR images: an estimation theoretic approach. IEEE Trans Med Imaging. 1995;14:413–21. doi: 10.1109/42.476104. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Abendschein D, Davila-Roman VG, Amini AA. Spatio-temporal tracking of myocardial deformations with a 4-D B-spline model from tagged MRI. IEEE Trans Med Imaging. 1999;18:957–72. doi: 10.1109/42.811299. [DOI] [PubMed] [Google Scholar]
- 10.Moulton MJ, Creswell LL, Downing SW, et al. Spline surface interpolation for calculating 3-D ventricular strains from MRI tissue tagging. Am J Physiol. 1996;270:H281–97. doi: 10.1152/ajpheart.1996.270.1.H281. [DOI] [PubMed] [Google Scholar]
- 11.Young AA, Axel L. Three-dimensional motion and deformation of the heart wall: estimation with spatial modulation of magnetization--a model-based approach. Radiology. 1992;185:241–7. doi: 10.1148/radiology.185.1.1523316. [DOI] [PubMed] [Google Scholar]
- 12.Young AA, Kraitchman DL, Dougherty L, Axel L. Tracking and finite element analysis of stripe deformation in magnetic resonance imaging. IEEE Trans Med Imaging. 1995;14:413–21. doi: 10.1109/42.414605. [DOI] [PubMed] [Google Scholar]
- 13.Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171:841–5. doi: 10.1148/radiology.171.3.2717762. [DOI] [PubMed] [Google Scholar]
- 14.Axel L, Dougherty L. Heart wall motion: improved method of spatial modulation of magnetization for MR imaging. Radiology. 1989;172:349–50. doi: 10.1148/radiology.172.2.2748813. [DOI] [PubMed] [Google Scholar]
- 15.Moustakidis P, Cupps BP, Pomerantz BJ, et al. Noninvasive, quantitative assessment of left ventricular function in ischemic cardiomyopathy. The Journal of surgical research. 2004;116:187–96. doi: 10.1016/j.jss.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Cupps BP, Pomerantz BJ, Krock MD, et al. Principal strain orientation in the normal human left ventricle. The Annals of thoracic surgery. 2005;79:1338–43. doi: 10.1016/j.athoracsur.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Cupps BP, Bree DR, Wollmuth JR, et al. Myocardial Viability Mapping by Magnetic Resonance-Based Multiparametric Systolic Strain Analysis. The Annals of thoracic surgery. 2008 doi: 10.1016/j.athoracsur.2008.06.072. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bree D, Wollmuth JR, Cupps BP, et al. Low-dose dobutamine tissue-tagged magnetic resonance imaging with 3-dimensional strain analysis allows assessment of myocardial viability in patients with ischemic cardiomyopathy. Circulation. 2006;114:I33–6. doi: 10.1161/CIRCULATIONAHA.105.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pomerantz BJ, Wollmuth JR, Krock MD, et al. Myocardial systolic strain is decreased after aortic valve replacement in patients with aortic insufficiency. The Annals of thoracic surgery. 2005;80:2186–92. doi: 10.1016/j.athoracsur.2005.05.095. [DOI] [PubMed] [Google Scholar]


