SUMMARY
We recently described an in vivo model of posttraumatic epilepsy (PTE) in the rat where chronic spontaneous recurrent seizures appear following a single episode of fluid percussion injury (FPI; D'Ambrosio et al., 2003). PTE, studied during the first 2 months post-injury, was focal and seizures predominantly originated from the frontal-parietal neocortex at the injury site. However, rarer bilateral seizures originating from a different and undefined focus were also observed. To shed light on the posttraumatic epileptogenic mechanisms and on the generation of bilateral seizures, we studied rats up to 7 months post-injury. In-vivo paired epidural and depth-electrode recordings indicated the anterior hippocampus evolves into an epileptic focus which initiates bilateral seizures. The rate of frontal-parietal seizures remained constant over time post-injury, while the rate of hippocampal seizures greatly increased over time, suggesting different mechanisms mediate neocortical and hippocampal posttraumatic epileptogenesis. Because of different temporal evolution of these foci, the epileptic syndrome was characterized by predominant frontal-parietal seizures early after injury, but by predominant mesio-temporal seizures at later time points. Pathological analysis demonstrated progressive hippocampal and temporal cortex pathology that paralleled the increase in frequency and duration of bilateral seizures. These results demonstrate that FPI-induced frontal-parietal epilepsy (FPE) progresses to mesial-temporal lobe epilepsy (MTLE) with dual pathology. These observations establish numerous similarities between FPI-induced and human PTE and further validate it as a clinically relevant model of PTE.
Keywords: Trauma, drug screening, electrocorticography, partial seizures, gliosis
INTRODUCTION
Traumatic brain injury (TBI) is one of the most important causes of acquired epilepsy in western societies (Annegers et al., 1996, 1998) and often manifests itself as complex partial seizures, which are often resistant to treatment with antiepileptic drugs (AEDs; Juul-Jensen, 1986; Mattson et al., 1985). To compound this problem, no known treatment or agent decreases the progression to epilepsy (Temkin et al., 2001). The hippocampus is at the center of PTE research, and different potentially epileptogenic mechanisms have been proposed, including acute hilar neuron loss (Lowenstein et al., 1992), intrinsic membrane depolarization of hilar neurons (Ross and Soltesz, 2000), axonal sprouting (Salin et al., 1995; Golarai et al., 2001; Santhakumar et al., 2001), saturation of synaptic long term potentiation of Schaffer collaterals (D'Ambrosio et al., 1998), and impaired K+ buffering by posttraumatic glia (D'Ambrosio et al., 1999). However, the clinical significance of these mechanisms in the onset of posttraumatic partial seizures and their pharmacoresistace has yet to be defined (D'Ambrosio, 2003, 2004). Furthermore, while the hippocampus has a role in human PTE and TLE, as indicated by control of seizures enjoyed by a sub-group of patients following amygdalo-hippocampectomy (Mathern et al., 1994; Marks et al., 1995; Arruda et al., 1996), head injury patients often develop neocortical foci, suffer from progressive temporal cortex pathology and may not be seizure-free after hippocampectomy (Marks et al., 1995; Diaz-Arrastia et al., 2000; Arruda et al., 1996). Therefore, understanding the mechanisms by which neocortical and subcortical epileptic foci arise, interact and progress following TBI is crucial for the development of novel treatments. We have recently developed a model of PTE in the rat where chronic spontaneous recurrent seizures follow a single event of a clinically-relevant model of closed head injury, the FPI (D'Ambrosio et al., 2003). This model represents a significant departure from previous models of acquired epilepsy because the initiating insult, a transient compression of the dura mater without penetration, is mechanically very similar to human cases of closed head injury (McIntosh et al., 1989; Schmidt and Grady, 1993; Schneider et al., 2002). Our previous work demonstrated that, in the first 2 months after injury, FPI induces a predominant epileptic focus in the frontal-parietal neocortex at the injury site while another epileptic focus, possibly subcortical, was responsible for about 4% of the seizures, suggesting the FPI-based model of PTE had the neuropathophysiological depth needed to reproduce the complex pathophysiology of the human posttraumatic epileptic brain. In the present study we addressed the following questions: 1) how does the epileptic condition evolve following the first 2 months post-injury? 2) where is the second epileptic focus located? 3) does the activity of these two epileptic foci change over time? We show that multiple epileptic foci develop following FPI and their different temporal evolution results in the progression of FPE into MTLE with dual pathology.
MATERIALS AND METHODS
Animals
56 outbred male CD Sprague-Dawley rats (colony H-41, Charles Rivers, Hollister, CA), post-natal days 33−35, were used for this study (36 FPI, 20 Sham). All procedures were approved by the University of Washington Animal Care and Use Committee.
Rostral Parasaggital Fluid Percussion Injury
Rostral parasaggital fluid percussion injury (rpFPI) was performed as previously described (D'Ambrosio et al., 2003). Rats were anesthetized with halothane, intubated and mounted on a stereotaxic frame with incisor bar adjusted to set bregma 1.5mm below lambda. A burr hole of 3 mm in diameter was drilled 2 mm posterior to bregma and 3 mm lateral to midline, on the right convexity. A 10 ms pressure pulse of 3.25−3.5 atm was delivered through the FPI device (Scientific Instruments, University of Washington). After a 10-second pause in breathing upon injury, the animal was re-connected to the ventilator. Shams underwent the same procedure but the pressure pulse was generated with the stopcock of the FPI device closed. The righting time of FPI animals was 12.2±0.7 min (mean±SEM), while sham rats righted within seconds. The mortality rate by posttraumatic complications was 11%.
Electrophysiology
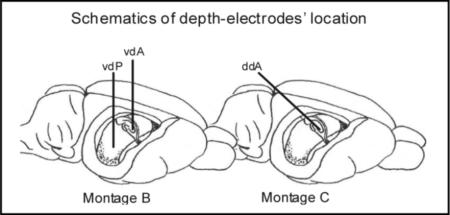 MontageA consisted of 5 epidural electrodes, stainless-steel screws of ϕ=1.4mm drilled down to the dura to prevent any breach-rhythm artifact (Kelly, 2004): a reference electrode placed midline anteriorly over the frontal sinus, over the and two electrodes per parietal bone at coordinates bregma 0 mm and −6.5 mm, 4 mm lateral from midline. Montage B utilized, in addition, two depth-electrodes (Teflon-insulated stainless steel wire, ϕ=280μm) vertically lowered at bregma − 3.5 mm (3.5 mm lateral; vdA) and at bregma −6 mm (4.5 mm lateral; vdP) to sample the activity of ipsilateral anterior and posterior hippocampal CA3 subregion. Montage C was similar, but incorporated only one depth-electrode lowered at a 45° angle from bregma −7.25mm (3.5 mm lateral) to sample the activity of the ipsilateral anterior hippocampal CA3 subregion at bregma −3.5 mm (ddA). The correct position of the depth-electrodes was verified by pathology in all animals used in the study. Montage A was implanted one week post-injury. In pilot experiments we observed a drop in hippocampal firing acutely after depth-electrode implantation, which subsided in the ensuing days; therefore montage B and C were implanted 6−18 days before recordings. The electrodes’ headset, video-electrocorticography (video-ECoG) acquisition, amplification and storage were as previously described (D'Ambrosio et al., 2003). Electrophysiological data were acquired and analyzed with SciWorks with Experimenter V2 software (Datawave Technologies Inc., Longmont, CO) and DT3010 acquisition boards (DataTranslation Inc.,Marlboro, MA). Eight hours of recordings were performed per rat (Sham and FPI) per week. For the study of duration and frequency of seizure, at least 24 hours of recordings were performed per rat per time point.
MontageA consisted of 5 epidural electrodes, stainless-steel screws of ϕ=1.4mm drilled down to the dura to prevent any breach-rhythm artifact (Kelly, 2004): a reference electrode placed midline anteriorly over the frontal sinus, over the and two electrodes per parietal bone at coordinates bregma 0 mm and −6.5 mm, 4 mm lateral from midline. Montage B utilized, in addition, two depth-electrodes (Teflon-insulated stainless steel wire, ϕ=280μm) vertically lowered at bregma − 3.5 mm (3.5 mm lateral; vdA) and at bregma −6 mm (4.5 mm lateral; vdP) to sample the activity of ipsilateral anterior and posterior hippocampal CA3 subregion. Montage C was similar, but incorporated only one depth-electrode lowered at a 45° angle from bregma −7.25mm (3.5 mm lateral) to sample the activity of the ipsilateral anterior hippocampal CA3 subregion at bregma −3.5 mm (ddA). The correct position of the depth-electrodes was verified by pathology in all animals used in the study. Montage A was implanted one week post-injury. In pilot experiments we observed a drop in hippocampal firing acutely after depth-electrode implantation, which subsided in the ensuing days; therefore montage B and C were implanted 6−18 days before recordings. The electrodes’ headset, video-electrocorticography (video-ECoG) acquisition, amplification and storage were as previously described (D'Ambrosio et al., 2003). Electrophysiological data were acquired and analyzed with SciWorks with Experimenter V2 software (Datawave Technologies Inc., Longmont, CO) and DT3010 acquisition boards (DataTranslation Inc.,Marlboro, MA). Eight hours of recordings were performed per rat (Sham and FPI) per week. For the study of duration and frequency of seizure, at least 24 hours of recordings were performed per rat per time point.
Seizure assessment
Seizure assessment and artifact management were performed with off-line analysis of video-ECoG or depth-electrode recordings as previously described (D'Ambrosio et al., 2003). Posttraumatic epileptic ECoG events were categorized as grade 1 if appearing to be limited to an originating focus (Fig. 1A). Grade 2 activity appeared to originate from a focus and then spread (Fig. 1B). Grade 3 events appeared bilateral in onset (Fig. 1C). In addition to posttraumatic epilepsy, we observed a total of 37 idiopathic seizures in the whole FPI-rat population during this study. They all occurred at 27−28 weeks post-FPI (≅3.6% of all cortical discharges at that time point) and were excluded from analysis. Animals showing a minimum of 2 seizures were considered epileptic. Headsets were occasionally lost; when possible they were re-implanted, otherwise animals were included in the study only until the last day of recording. Epileptic ECoG events occurring within 5 seconds of each other were defined as belonging to the same seizure event.
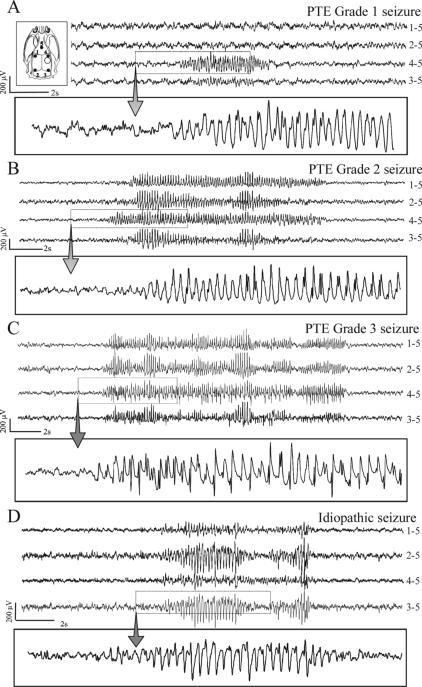
Figure 1. Different types of chronic recurrent seizures as revealed by ECoG following FPI.
Inset top, left) Schematic of the locations of the five cortical electrodes (filled circles) and of the injury site (hollow circle) in respect to the rat skull. All panels represent continuous ECoG monitoring at 7 months post-FPI in wake animals. A) A representative grade 1 posttraumatic seizure detected exclusively by the peri-lesion electrode. B) A representative grade 2 posttraumatic seizure first detected by the peri-lesion electrode and then by multiple channels. C) A representative grade 3 posttraumatic seizure detected simultaneously by multiple channels. D) A representative idiopathic seizure, detected bilaterally and characterized by larger occipital epileptic discharge. These idiopathic seizures were absent until 5.5 months of age and represented 3.6% of all cortical discharge of FPI animals at 8 months of age. ECoG calibration bars are on the left of each panel. Dotted boxes highlight the portion of ECoG shown at higher temporal resolution in the rectangle underneath each panel. The numbers next to each ECoG trace indicate the electrodes by which the trace is recorded, and its reference.
Behavioral seizure severity was assessed off-line by ranking the behavior concurrent to epileptiform ECoG events according to the following scale: 0= no behavioral change (sub-clinical), 1= freeze-like pause in behavior without impaired posture, 2= freeze-like pause in behavior without impaired posture and accompanied by facial automatisms (twitching of vibrissae, sniffing, eye blinking, or jaw automatisms), 3= head nodding; 4= body myoclonus; 5= loss of posture, atonia; 6= loss of posture followed by motor manifestations (facial automatisms, contralateral limb dystonia); 7= tonic-clonic convulsion; 8=partial status-like electroclinical syndrome. Cases when animals’ behavior could not be appreciated because of their unfavorable position with respect to the camera were excluded from analysis.
Histology
Histological analyses of brains were performed as previously described (D'Ambrosio et al., 2003). Animals were deeply anesthetized and perfused transcardially with 4% paraformaldehyde in PBS. Brains were removed, post-fixed, and cryoprotected in sucrose in phosphate buffer. GFAP immunoreactivity: Free floating sections (30μm) were treated to block non-specific staining and incubated with rabbit anti-GFAP antibody (1:4000 dilution; Dako). Secondary antibody treatment included overnight incubation in a 1:300 solution of biotinylated goat anti-rabbit immunoglobulin (IgG). Biotin/avidin/horseradish peroxidase complexes were formed by incubation in a 1:500 Elite ABC kit (Vector Labs, Burlingam, CA). Sections were then developed in 3,3-diaminobenzidine (DAB) with H2O2, mounted on glass slides, air dried, dehydrated through alcohols, cleared in xylene and cover slipped. At least six coronal sections were examined per animal. Cresyl-violet staining: sections (30μm) were mounted on glass slides, airdried, defatted in xylene, stained in cresyl violet solution, differentiated in 95% ethanol, dehydrated through graded alcohols, cleared in xylene, and coverslipped.
Morphological analysis
Hippocampal and temporal cortex asymmetry was assessed in 2X micrographs of cresyl-violet stained coronal sections obtained from bregma −4 mm through bregma −5 mm. Hippocampal area, perimeter, maximum and minimum axes, and temporal neocortex area and perimenter, were measured using Object Image 2. 10 (http://simon.bio.uva.nl). Duplicate measurements were averaged to reduce variability. The morphological measurements of the hippocampus ipsilateral to the injury were then divided by those of the contralateral hippocampus and multiplied by 100 to obtain a “symmetry percentage”. For the temporal neocortex, morphology was assessed by first computing the ratio of each cortical area and its perimeter, which is proportional to the thickness of the neocortex, and then by computing the ipsilateral vs contralateral ratio. In perfect coronal sections from symmetrical brains all morphological parameters would have a symmetry percentage of 100%. Symmetry percentage decreases as the ipsilateral hippocampus and temporal neocortex shrink due to tissue loss. The highest percentage deviation from 100% among the different morphological parameters examined in the hippocampus and neocortex was taken as their asymmetry. Asymmetry of sham-operated animals ranged 0−7%, and this variability was therefore not considered a pathological sign. A scale was constructed defining asymmetry as “negligible” for asymmetry <=7%, “mild” if >7% and <=15%, “moderate” if >15% and <=30% and “pronounced” if >30%.
Statistical analysis
All data are presented as mean±S.E.M. Statistical comparisons were performed with SPSS 12.0 (SPSS inc., Chicago, IL). All tests were performed two-tailed, except for the progression of pathology. A p<0.05 was considered significant.
RESULTS
Probability of developing PTE
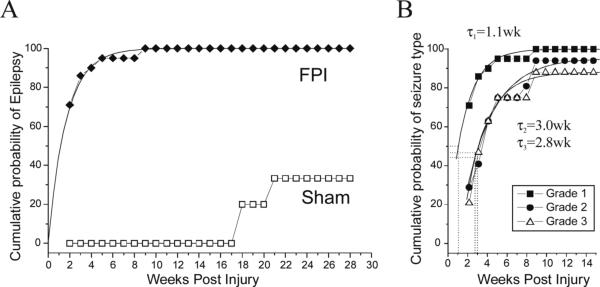
Video-ECoG monitoring based on montage A was performed in 24 rpFPI and 20 sham animals for a total of 1,882 and 784 hours, respectively. Montage A allowed for the detection of three different types of late posttraumatic seizures. Grade 1 and 2 seizures were invariably first detected by the perilesional electrode (Fig. 1A, B) while grade 3 seizures appeared, at the best of our spatial-temporal resolution, bilateral in their cortical onset (Figure 1C). The cumulative probability that rpFPI rats developed epilepsy reached 100% at 9 weeks post-injury (Figure 2A). Age-matched shams were recorded at the same time points after surgery for a comparable number of hours and showed no epileptiform ECoG events up to 5.5 months of age (4.5 months post-surgery), consistent with our previous report (D'Ambrosio et al., 2003). In this study we extended the temporal analysis and found that ∼33% of control animals manifested recurrent non-convulsive idiopathic generalized epilepsy at 7−8 months of age. These events, typically 2−10 second long and bilateral in onset, were characterized by a sharp-wave pattern. They were easily distinguished from posttraumatic grade 3 seizures because they were significantly larger in amplitude in the parietal-occipital cortex (Figure 1D), and were therefore disregarded. At 27−28 weeks post-injury these idiopathic seizures represented just 3.6% of all cortical discharges.
Figure 2. Probability of unprovoked seizures following severe lateral FPI.
A) The cumulative probability of detecting unprovoked epileptiform ECoG events in post-FPI (filled circles) and sham-injured (hollow squares) rats is plotted versus time after injury. The incidence of unprovoked seizures in the sham-injured CD Sprague-Dawley rats used for this study is zero up to 4.5 months post-surgery (5.5 months old). B) The cumulative probability of developing grade 1 (filled squares), grade 2 (filled circles), and grade 3 (hollow triangles) seizures by chronic ECoG is plotted versus time after injury. The temporal increase in probability of developing each seizure type had half time τ1=1.1 weeks for grade 1 seizures, τ2=3.0 weeks for grade 2, and τ3=2.8 weeks for grade 3 seizures.
The cumulative probability that FPI rats developed each grade of posttraumatic seizures was also computed (Figure 2B). The probability of developing grade 1 seizures increased over time post-injury in a manner identical to the time course of the epileptic condition itself (Figure 2B), and could be well fit with a single exponential with half time τ1=1.1 weeks. The probability of developing grade 2 or 3 seizures was lower at all times and fit a single exponential with half time τ2=3.0 and τ3=2.8 weeks, respectively. This suggests different mechanisms mediate the genesis of grade 1 vs grade 2 and 3 seizures.
Remission from PTE
During these ECoG studies based on montage A, we identified an animal that presented 6 grade 1 seizures 13 days after injury, but no abnormal ECoG activity thereafter. We considered this animal a case of remission from PTE; it was examined for pathology but was not included in the studies of seizure frequency and duration.
Temporal evolution of electrical and behavioral seizures
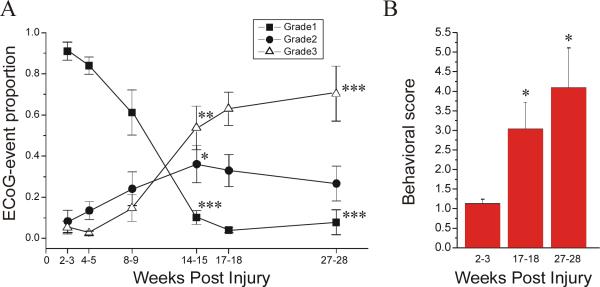
We previously determined that grade 2 seizures increase in proportion over the first 2 months post-FPI, while grade 3 seizures do not (D'Ambrosio et al., 2003). To determine if FPI-induced PTE continues evolving at later time points after injury, we examined the time-dependence of 1) the proportion of each seizure grade, and 2) the behavioral seizure severity. The first electrophysiological parameter was examined in 8 epileptic animals that were recorded weekly from 2 to 28 weeks post-injury (Figure 3A). At 2−3 weeks postinjury, grade 1 seizures accounted for 91±4.4% of all seizures. This proportion progressively decreased over time, reaching 7.8±6.1% at 27−28 weeks postinjury (p<<0.001). At 2−3 weeks postinjury grade 2 seizures were only 8.3±5.4% and increased over time peaking at 36±9.1%% at 14−15 weeks postinjury (p=0.026). Grade 3 seizures were also rare at 2−3 weeks postinjury, being only 5.3±3.1%, and increased over time to 54±11% and 70±13%, at 14−15 and 27−28 weeks postinjury, respectively (p<<0.001 vs 2−3wks; statistics with paired t-test).
Figure 3. Temporal evolution of the FPI-induced posttraumatic epileptic syndrome.
Electrical and behavioral correlates of PTE progression are assessed in 8 epileptic animals. A) The proportions of ECoG events of grade 1, 2, and 3 are plotted over time from 2 to 28 weeks post-injury. Focal frontal-parietal seizures (grade 1; filled square) represented the most common seizure type 2−3 weeks post-injury, but then progressively decreased in proportion over time. Focal neocortical spreading seizures (grade 2; filled circles) were rare at 2−3 weeks post-injury, but then increased in proportion over time and peaked at 14−15 weeks post-injury. Seizures that did not originate from the frontal-parietal cortex and appeared bilateral in onset (grade 3; hollow triangle) were rare up to 8−9 weeks post-injury, and then increased up to 27−28 weeks post-injury. Note the overall increase in seizures’ bilateral spread over time post-injury (grades 2&3 combined). B) The behavioral score during epileptiform ECoG events is plotted versus time post-injury. Note the progression of the severity of behavioral seizures over time post-injury. Data are presented as Mean±S.E.M. Statistics with paired t-test (* p<0.05; ** P≤0.01; *** P≤0.001, vs 2−3 weeks).
Ictal behavior was examined in the same 8 animals. The most common behavioral correlates of electrical seizures during the early weeks post-FPI were stereotyped freeze-like pauses, that were sometimes followed by facial automatisms, and during which the animal did not lose body posture. However, a different behavioral manifestation of electrical seizures became increasingly common over time post-injury. The animal would suddenly interrupt its normal exploratory or grooming behavior, crawl on the bottom of the cage and stop with its head propped on its forelimbs, staying motionless for 1 to 10 seconds, after which grade 3 electrical seizures and sometimes facial automatisms or dystonic posturing of the left hindlimb would appear. We interpret this electro-clinical syndrome as being a complex partial seizure not initiating in the frontal-parietal neocortex and starting during the phase of crawling. We also observed rarer cases of ictal atonia, during which an animal engaged in normal grooming behavior would suddenly fall head-down to the bottom of the cage, remain motionless for several seconds, and after which righting would be hindered by prolonged atonia of the forelimbs. These events were also consistent with partial seizures. At 7 months post-injury three animals exhibited prolonged (≥30 minutes) sequences of electrical seizures during abnormally quiet behavior and without ECoG sleep activity. These events were similar to human non-convulsive partial status epilepticus. The behavioral seizure severity associated with electrical seizures was 1.1±0.2 (range 0−4) at 2−3 weeks post-injury. It increased to 3.1±0.7 (range 1−6) at 17−18 weeks post-injury (p=0.04) and to 4.1±1.0 (range 1−8) at 27−28 weeks post-injury (p=0.02; with Mann-Whitney U test vs 2−3 weeks).
Localization of epileptic foci initiating different seizure types
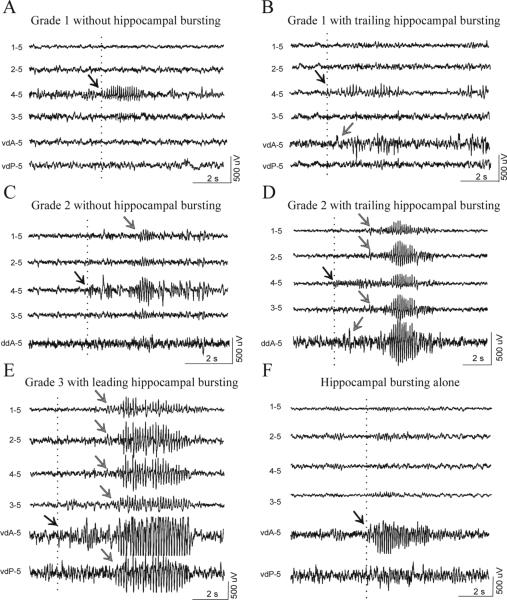
We previously proposed that grade 1 and grade 2 seizures originated from the frontal-parietal neocortex, while a second epileptic focus was responsible for grade 3 seizures (D'Ambrosio et al., 2003). To better determine the location of this second focus we acquired 336 hours of paired epidural and depth-electrode video-recordings using montages B and C in 6 animals at 2−4 weeks post-FPI (192hr) and in another 6 animals 6.5−7 months post-FPI (144hr). We classified all epileptic events by the location of the first detected epileptic activity. In the late group (Table 1), we observed discharge of the frontal-parietal neocortex in absence of abnormal hippocampal activity (Fig. 4A, C), and of the hippocampus in absence of abnormal neocortical activity (Fig 4 F), demonstrating that independent neocortical and hippocampal epileptic foci co-exist in the post-FPI epileptic brain chronically after injury. In addition, grade 1 and 2 seizures, always first detected in the frontal-parietal cortex, sometimes recruited the hippocampal focus (Fig 4B, D). Grade 3 seizures were always detected in the presence of either leading (Fig. 4E) or simultaneous hippocampal discharge (not shown), but were never observed to precede it, indicating that grade 3 seizures are never initiated by the frontal-parietal focus, but often by the hippocampus. Epileptiform activity detected in the hippocampus was mostly first detected in the anterior hippocampus (Fig. 4F), with or without trailing discharge of posterior hippocampus (Fig. 4E).
Table 1.
Independent firing and interaction of the frontal-parietal neocortical and hippocampal epileptic foci as observed by paired epidural and depth-electrode recordings 6.5−7 months post-FPI.
| Type of occurrence | Proportion | Evidence for focus in: | ||
|---|---|---|---|---|
 |
Grade 1 seizures in absence of hippocampal discharge: | 66.7% | Frontal-parietal cortex | |
| Grade 1 seizures leading hippocampal discharge: | 13.9% | Frontal-parietal cortex | ||
| Grade 1 seizures simultaneous to hippocampal discharge: | 19.4% | Undefined | ||
| Grade 1 seizures led by hippocampal discharge: | 0.0% | Not hippocampus | ||
 |
Grade 2 seizures in absence of hippocampal discharge: | 2.2% | Frontal-parietal cortex | |
| Grade 2 seizures leading hippocampal discharge: | 80.4% | Frontal-parietal cortex | ||
| Grade 2 seizures simultaneous to hippocampal discharge: | 17.4% | Undefined | ||
| Grade 2 seizures led by hippocampal discharge: | 0.0% | Not hippocampus | ||
 |
Grade 3 seizures in absence of hippocampal discharge: | 0.0% | Not frontal-parietal cortex | |
| Grade 3 seizures leading hippocampal discharge: | 0.0% | Not frontal-parietal cortex | ||
| Grade 3 seizures simultaneous to hippocampal discharge: | 56.3% | Undefined | ||
| Grade 3 seizures led by hippocampal discharge: | 43.7% | Hippocampus | ||
 |
Anterior hippocampus in absence of posterior hippocampus: | 71% | Anterior hippocampus | |
| Anterior hippocampus leading posterior hippocampus: | 7.9% | Anterior hippocampus | ||
| Anterior hippocampus simultaneous to posterior hippocampus: | 21.1% | Undefined | ||
| Anterior hippocampus led by posterior hippocampus: | 0.0% | Not posterior hippocampus | ||
| w/ s.e. | w/o s.e. | |||
 |
Frontal-parietal seizures in absence of hippocampal discharge: | 14.6% | 24.4% | Frontal-parietal cortex |
| Frontal-parietal seizures leading hippocampal discharge: | 16.1% | 26.9% | Frontal-parietal cortex | |
| Hippocampal seizures in absence of cortical discharge: | 1.8% | 3.0% | Hippocampus | |
| Hippocampal seizures recruiting only frontal-parietal cortex: | 0.0% | 0.0% | Hippocampus | |
| Hippocampal seizures recruiting neocortex: | 27.4% | 45.7% | Hippocampus | |
| Events simultaneous in cortex and hippocampus: | 40.1% | — | Undefined |
The relative proportion of cortical and hippocampal involvement is shown for each type of seizure (grades 1, 2, 3 and hippocampal). Interaction refers to the reciprocal recruitment of neocortex and hippocampus and it is computed by combining all seizure types and including (w/s.e.) or not (w/o s.e.) events with simultaneous hippocampal and cortical discharge.
Figure 4. Independent firing and crosstalk of the frontal-parietal neocortical and antero-hippocampal epileptic foci.
Paired epidural and depth-electrode recordings performed in 6 epileptic animals 6.5−7 months post-injury. A) A grade 1 seizure detected by the peri-lesion epidural electrode does not recruit the hippocampus, indicating the existence and independent firing of a focus in the frontal-parietal neocortex. B) Epileptic activity is first detected by the peri-lesion epidural electrode during a grade 1 seizure, and then in the anterior hippocampus, indicating the neocortical focus recruited the hippocampus. C) The hippocampus shows no epileptiform activity during the occurrence of a grade 2 seizure that originated around the injury site and then propagated to the frontal contralateral cortex. D) Epileptic activity is first detected by the perilesional epidural electrode in the frontal-parietal cortex, and then spreads ipsi- and contralaterally, during a grade 2 seizure, and to the anterior hippocampus, indicating the neocortical focus recruited the hippocampus. E) Epileptiform activity is first detected in the anterior hippocampus and then simultaneously in the ipsi- and contralateral neocortex, indicating that grade 3 seizures originate in the hippocampus. F) Epileptiform activity is detected only in the hippocampus, in absence of any neocortical discharge, indicating the existence and independent firing of an epileptic focus in the hippocampus. Scale bars apply to all four traces in each panel. Epileptic bursts shown in E and F are cut off at ±500μV by gain saturation. Dotted lines and black arrows indicate the beginning of epileptiform activity. Gray arrows indicate propagated epileptic activity. vdA = vertical depth electrode placed in the anterior hippocampus; vdP = vertical depth electrode placed in the posterior hippocampus; ddA = diagonal depth electrode placed in the anterior hippocampus. The text to the left of each ECoG trace indicate the electrode by which the trace is recorded and its reference.
Temporal changes in cortical discharge rate and duration
We examined the time course of the rate and duration of cortical discharge as detected by montage A. The total cortical discharge rate, as computed as the count, in epileptic animals, of all seizure grades was 2.1±0.51 event/hour (9 rats; range 0.05−4.4 events/hour) at 2−4 weeks and progressively increased to 6.0±1.8 (8 rats; range 0.22−16.4 events/hour) by 27−28 weeks post-injury (p=0.044; Fig. 5A). The rate of grade 1 seizures decreased from 1.79±0.49 events/hour at 2−4 weeks to 0.22±0.1 events/hour at 17−18 weeks post-injury (p=0.008), and persisted at 0.20±0.095 events/hour at 27−28 weeks post-injury (p=0.018; Fig. 5B). The rate of grade 2 seizures rose from 0.24±0.10 events/hour at 2−4 weeks to 1.84±0.78 events/hour at 17−18 weeks (p=0.015) and 2.4±1.29 events/hour at 27−28 weeks post-injury (p=0.12; Fig. 5B). Because grade 1 and 2 electrical seizures appear to originate from the same neocortical epileptic focus, we also evaluated their combined rate as an assessment of the overall activity of that neocortical focus. The combined frontal-parietal seizure rate was 2.04±0.49 events/hour at 2−4 weeks and did not significantly change for the duration of the study, being 2.05±0.86 events/hour at 17−18 weeks (p=0.68) and 2.6±1.53 events/hour at 27−28 weeks post-injury (p=0.24; Fig. 5 C). Conversely, the rate of grade 3 seizure progressively increased from 0.06±0.027 events/hour at 2−4 weeks to 3.7±0.76 at 27−28 weeks post-injury (p=0.017; Fig. 5C; statistics with Wilcoxon Signed Rank test vs 2−4 weeks).
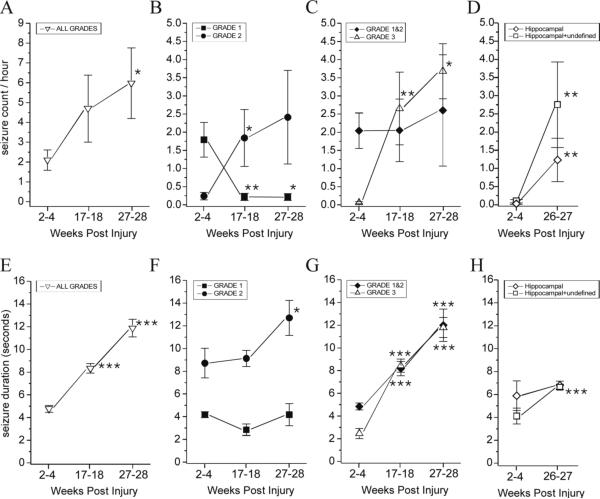
Figure 5. Temporal evolution of rate of occurrence and duration of cortical and hippocampal discharge.
Cortical and hippocampal discharge rate of occurrence (A-D) and duration (E-H) are shown from 2 to 28 weeks post-injury. Cortical discharge occurring during all seizure grades (1, 2, and 3) increased in frequency over time (A). Grade 1 seizures decreased, while grade 2 seizures increased in frequency over time (B). The rate of seizures originating from the frontal-parietal neocortex (grade 1 and 2 combined) did not change from 2−4 weeks to 27−28 weeks post-injury, while the rate of seizures not originating from that focus (grade 3) dramatically increased over time post-injury (C). The frequency of hippocampal seizures increased over time post-injury. Because seizures detected simultaneously in both hippocampus and cortex may also be originating from the hippocampus, the frequency of hippocampal+undefined seizures is shown (D). Cortical discharge duration (all seizure grades) increased over time post-injury (E). The duration of grade 1 seizures did not significantly increase over time, while grade 2 seizures did (F). The duration of seizures originating from the frontal-parietal focus (grades 1 and 2) increased, as did the duration of grade 3 seizures that do not originate from it (G). We did not detect a significant increase over time post-injury in the duration of hippocampal discharge during hippocampal seizures. However, the duration of hippocampal+undefined seizures significantly increased over time post-injury (H). Data are presented as mean±SEM. Statistical comparisons were performed with Wilcoxon Signed Rank test (A-C) and With Mann-Whitney U test (D-H) vs 2−4 wks time point (* p<0.05; ** P≤0.01; *** P≤0.001).
The duration of all cortical discharges combined was 4.8±0.30 seconds (range 0.5 to 57s) at 2−4 weeks post-injury (n= 389), that increased to 8.3±0.43 seconds (range 0.5 to 89s) at 17−18 weeks (n= 470; p<<0.001), and to 11.9±0.78 seconds (1 to 88s) at 27−28 weeks post-injury (n= 280; p<<0.001; Fig. 5 E). Grade 1 seizures lasted 4.2±0.26 seconds at 2−4 weeks post-injury (n= 322), and persisted at 2.84±0.50 seconds at 17−18 at weeks (n=29; p=0.11), and at 4.17±0.97 seconds at 27−28 weeks post-injury (n= 9; p=0.42). Grade 2 seizures lasted 8.7±1.3 seconds at 2−4 weeks post-injury (n=55), persisted at 9.13±0.71 seconds at 17−18 at weeks (n=162; p=0.13), and increased to 12.7±1.5 seconds at 27−28 weeks post-injury (n=101; p=0.02; Fig. 5F). The duration of frontal-parietal seizures (grades 1 and 2 combined) was 4.84±0.31 seconds at 2−4 weeks (n=377), and increased to 8.17±0.63 seconds at 17−18 weeks (n=191; p<<0.001), and to 12.0±1.4 seconds at 27−28 weeks post-injury (n= 110; p<<0.001; Fig. 5G). Similarly, grade 3 seizures lasted 2.46±0.44 seconds at 2−4 weeks post-injury (n=12), and increased in duration to 8.43±0.58 seconds at 17−18 weeks (n=279; p<0.001), and to 11.79±0.89 seconds at 27−28 weeks post-injury (n=170; p<0.001; Fig. 5G; all statistics with Mann-Whitney U test vs 2−4 weeks).
Temporal changes in hippocampal discharge rate and duration
We examined the occurrence of hippocampal discharge as detected by montages B and C. The number of animals showing hippocampal seizures, as defined as those in which the hippocampus fired first or alone, was ∼33% (2 out of 6) at 2−4 weeks post-FPI, and increased to 100% (6 out of 6) at 26−27 weeks post-injury, demonstrating progressive MTLE. We then examined the temporal changes in rate and duration of hippocampal discharge as detected by 312hr of recording from epileptic animals with montages B and C. The rate of hippocampal seizures was 0.033±0.02 event/hour (5 rats) at 2−4 weeks and increased to 1.24±0.60 event/hour (6 rats) at 26−27 weeks (Fig. 5D; p=0.004). Seizures appearing simultaneously in hippocampus and cortex (Table 1) have undefined origin, but some or all of these may originate from the hippocampus. Their inclusion brought our estimate of hippocampal seizure rate to 0.1±0.05 event/hour at 2−4 weeks and to 2.75±1.18 at 26−27 weeks (Fig. 5D; p=0.004, both statistics with Mann-Whitney U test).
The duration of hippocampal discharge during hippocampal seizures was 5.9±1.3 seconds (n=8; range 2 to 10 s) at 2−4 weeks and 6.8±0.36 seconds (n=147; range 2 to 28 s) at 26−27 weeks (Fig. 5H; p=0.55). The inclusion of seizures appearing simultaneously in hippocampus and cortex brought our estimate of the duration of hippocampal discharge to 4.1±0.7 seconds (n=20) at 2−4 weeks and to 6.6±0.24 seconds (n=339) at 26−27 weeks (Fig. 5D; p<0.001, both statistics with Mann-Whitney U test).
Structural substrates of the progression of posttraumatic epilepsy
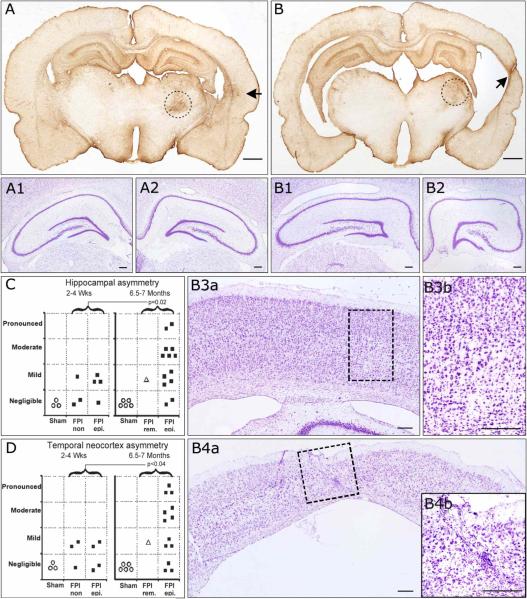
We examined time-dependent changes in brain pathology in 21 FPI and 8 sham animals. Coronal sections obtained from the early group (2−4 weeks post-FPI) and stained for cresyl violet showed remarkable neuronal loss and calcifications in the ipsilateral thalamus. The ipsilateral hippocampus and temporal neocortex presented either no or mild shrinkage without loss of laminar features (not shown). GFAP immuno-reactivity was markedly increased in the ipsilateral thalamus, hippocampus, and frontal-parietal cortex in all FPI animals, while a focus of glial reactivity was apparent in the temporal cortex of epileptic animals (D'Ambrosio et al., 2003). Coronal sections obtained from the late group (27−28 weeks post-FPI) and stained for cresyl violet showed remarkable neuronal depletion and calcifications in the thalamus of all FPI animals (Fig 6 A,B). The ipsilateral hippocampus (Fig. 6B2) and temporal neocortex (Fig 6B4) presented varying degrees of shrinkage among animals, ranging from negligible to pronounced with loss of laminar features (Fig. 6B4). Atrophic hippocampi were characterized by atrophy of CA1 and CA3 subfields (Fig. 6B1−2). Numerous small nuclei, presumably of glial cells, were observed in ipsilateral hippocampus and temporal cortex (Fig. 6B4, inset). We quantitatively assessed the temporal changes in asymmetry of hippocampus and temporal neocortex. In the early group, FPI animals, epileptics and non-epileptics, presented either no (∼43%) or mild (∼57%) hippocampal asymmetry (Figure 6C, left panel), and either no (∼43%) or mild (∼57%) asymmetry in the temporal cortex (Figure 6D, left panel). In the late group, FPI animals presented varying degrees of hippocampal asymmetry (Figure 6C, right panel) ranging from negligible (∼14%), mild (∼36%), moderate (∼36%) to pronounced (∼14%), as well as different degree of temporal cortex asymmetry (Figure 6D, right panel), ranging from negligible (∼21%), mild (∼29%), moderate (∼29%) to pronounced (∼21%). The differences in hippocampal and temporal cortex asymmetry between the early and late time points were statistically significant (p=0.02, and p<0.04 respectively; one-tailed Mann-Whitney U test). The degree of temporal cortex asymmetry correlated with the degree of hippocampal asymmetry (R=+0.76; p<0.005).
Figure 6. Progressive hippocampal and temporal cortex pathology in posttraumatic epileptic rat.
Coronal sections obtained from bregma −4 mm through bregma −5 mm and stained for cresyl violet and GFAP. A) GFAP immunoreactivity of an epileptic animal 7 months post-injury demonstrating no hippocampal and mild temporal cortex asymmetry. At higher magnification, cresyl violet staining shows symmetric contralateral (A1) and ipsilateral (A2) hippocampi and no loss of laminar features. B) GFAP immunoreactivity of another epileptic animal 7 months post-injury demonstrating pronounced hippocampal and temporal cortex asymmetry. At higher magnification, cresyl violet staining shows asymmetric contralateral (B1) and ipsilateral (B2) hippocampi, with evident atrophy of ipsilateral CA3 and CA1subregions. The contralateral temporal cortex showed no atrophy (B3a) and normal laminar structure (B3b). However, the ipsilateral temporal cortex was atrophic (B4a) and showed pronounced loss of neurons and laminar features, and increased small nuclei representing reactive glia (B4b), all typically associated with temporal cortex sclerosis. Hippocampal (C) and temporal cortex (D) asymmetry increased over time post-injury in the population of FPI animals. Statistics with one-tailed Mann-Whitney U test. FPI rem. = case of PTE remission following FPI. FPI epi. = persistently epileptic animals. FPI n.epi. = not epileptic animals. Scale bars: 1mm for A and B; 250 μm for A1−2 and B1-B4b. Black arrows in A and B indicate temporal foci of glial reactivity. Dotted circles in A and B delineate the thalamic injury present in all rpFPI animals. Dotted rectangles in B3a and B4a delineate the areas magnified in B3b and B4b, respectively.
DISCUSSION
The main finding of the present study is that a single episode of rpFPI is sufficient to induce independent cortical and hippocampal epileptic foci which activity evolves differently over time post-injury resulting in changes in the epileptic syndrome and a progressive development of MTLE.
rpFPI-induced PTE is a progressive disorder
rpFPI-induced PTE manifests itself at the cortical level with three different types of electrical seizures in the first weeks post-injury (D'Ambrosio et al., 2003). We now have followed their evolution up to 7 months post-injury and found their relative proportion, in each epileptic animal, dramatically changes over time. Grade 1 seizures, detected only in the neocortex at the injury site (Fig. 1 A), are the most common type in the first two months after injury (Figs. 2B) but rapidly drop in proportion to represent only ∼5% of all seizures by 7 months post-injury (Figure 3A). While it is possible that the thalamic injury found in all rpFPI animals may generate an epileptic thalamo-cortical loop, the findings of intrinsically hyperexcitable frontal-parietal cortex in slices in vitro and of non-epileptic (D'Ambrosio et al., 2003) and remission cases observed in spite of comparable thalamic injury argue that the epileptic focus responsible for grade 1 seizures lies within the frontal-parietal cortex itself. Grade 2 seizures spread from the injury site and represent secondarily generalized focal events. They progressively increased in proportion over the first 2 months post-injury (D'Ambrosio et al., 2003) indicating that focal seizures spread more easily over time. We now show that grade 2 seizures peak in proportion at about 14−15 weeks post-injury (Figure 3A). The cellular substrates of their increase are likely different from those responsible for the onset of grade 1 seizures and may include progression of pathology and/or kindling of cortico-cortical and cortico-subcortical pathways (Goddard, 1967). Consistent with this hypothesis, epileptogenesis responsible for grade 2 seizures had a half time longer than the one of grade 1 seizures (Fig. 2B). Grade 3 seizures, which are bilateral at their cortical onset (Figure 1 C), are rare in the first 2 months post-injury, representing only ∼5% of the overall seizure activity, but dramatically increase in proportion over the following months reaching ∼65% of all cortical discharge by 7 months post-injury (Figure 3A). Their simultaneous bilateral appearance demonstrates the frontal-parietal neocortex is not the focus generating them. Therefore, they likely propagate to the neocortex via subcortical pathways. Indeed, paired epidural- and depth-electrode recordings indicate they can originate from the hippocampus.
In addition to their relative proportion, seizures also changed in rate of occurrence and duration over the months post-injury. While grade 1 seizures decreased, grade 2 seizures increased in frequency over time (Fig. 5B). We interpret their opposite and complementary evolution as due to their common origin in the frontal-parietal focus, as indicated by our previous work (D'Ambrosio et al., 2003) and by paired epidural-depth-electrode recordings (Fig. 4; Table 1). We surmise the worsening of grade 1 seizures results in grade 2 seizures. We therefore can represent the overall activity of the frontal-parietal focus as the sum of the rates of grade 1 & 2 seizures. This activity was constant over the months post-FPI (Fig. 5C) suggesting that the frontal-parietal focus develops within 2 weeks post-injury and that the epileptogenic mechanisms responsible for its onset do not significantly affect its firing rate after this critical period. However, pro-epileptic mechanisms continue to work after this temporal window and result in their increase in duration of both frontal-parietal (grade 1&2 combined) and grade 3 seizures (Fig. 5G). Contrary to frontal-parietal seizures, cortical discharge during grade 3 seizures also increased in rate of occurrence (Fig. 5C) over the months post-injury. We have strong indication that 40−60% of grade 3 seizures originate in the anterior ipsilateral hippocampus and are therefore hippocampal seizures (Fig. 4E; Table 1). Indeed, we found that the firing rate of the anterior hippocampus (Fig. 5D) dramatically increases over time post-injury. The remainder of grade 3 seizures may be originating from either a different area of the hippocampus, possibly the contralateral one, or from a third epileptic focus (see below). The dramatic time-dependent increase in hippocampal firing rate may be due to kindling induced by the relentless activity of the frontal-parietal focus. Alternatively, it may be an epiphenomenon accompanying the underlying progression in brain pathology. Further experiments are needed to elucidate the issue and the different mechanisms responsible for seizure onset, maintenance and spread in the frontal-parietal neocortex and hippocampus.
The progression of FPI-induced epilepsy was also evident at the behavioral level and further demonstrated the evolution from frontal-parietal to TLE. At 2−3 weeks post-injury the behavioral correlate of electrical seizures was typically freeze-like pause in behavior, with or without facial automatisms, consistent with an epileptic focus located in the forebrain (Browning, 1987). However, at 5−7 months post-injury animals typically displayed stereotyped electro-clinical events consisting in a sudden interruption in behavior, followed by crawling, and then by trains of grade 3 electrical seizures during which motor manifestations, typically facial automatisms and contralateral hindlimb dystonia often occurred. Both of these behavioral seizures are similar to complex partial seizures in humans; the former sometimes observed when the focus is in the frontal cortex (Williamson and Spencer, 1986), the latter typically observed in TLE (Kuba et al., 2003). We also observed rarer events of ictal atonia during which animals engaged in grooming behavior would fall and remain unresponsive and motionless for several seconds. These events are similar to atonia occurring during partial seizures as observed in patients with either frontal or frontal-parietal epilepsy (Satow et al., 2002; Tinuper et al., 1998). We did not observe tonic-clonic convulsions, as expected due to the employment of rostral parasaggital FPI which does not result in significant damage to the brain stem, a structure involved in their onset (Browning 1987; Gale and Browning, 1988). In addition, most of our rats were recorded only 8 hours/week, and sufficiently rare tonic-clonic convulsions may have been missed. However, more caudal FPI is known to result in larger damage to the motor cortex, hippocampus, and brain stem and should result in more frequent tonic-clonic seizures. Further experiments are needed to assess the relationship between FPI site and epileptic syndrome.
Crosstalk between neocortical and hippocampal epileptic foci
In addition to the independent firing of the frontal-parietal and hippocampal epileptic foci, numerous cases of their interaction were observed (Table 1). The frontal-parietal cortex was capable of rather selectively recruiting the anterior hippocampus (Fig. 4B,D), but selective recruitment of the frontal-parietal cortex by the hippocampus was never observed. Hippocampal activity first detected in the hippocampus always propagated bilaterally to large extents of the cortex (Fig. 4E). Specific communication pathways, possibly mediated by thalamic nuclei (Dolleman-van der Weel et al., 1997; Bertram and Zhang, 1999), may underlie this preferential recruitment of the hippocampus by a frontal-parietal focus and may contribute to kindling-mediated secondary hippocampal epileptogenesis and/or hippocampal sclerosis in susceptible epileptic patients (Engel and Shewmon, 1991). Cortex and hippocampus also frequently appeared to fire simultaneously (Table 1). This may due to 1) depth-electrodes not always ideally located to detect the very first discharge, 2) epileptic activity also originating from a different area of the hippocampus, likely the contralateral one, or 3) a third epileptic focus. To estimate the maximum contribution of the first factor, one should consider that grade 1 and 2 seizures were detected simultaneously to hippocampal discharge in ∼20% of the cases, which therefore is an upper limit, while the percentage of simultaneous detection of cortical and hippocampal discharge during grade 3 seizures was ∼60%. This suggests that ∼40% of all the grade 3 seizures that appear simultaneous to hippocampal discharge may be propagated to both areas from a third location. Possible anatomical areas for this third location include other regions of the hippocampus itself, likely the contralateral one, but also piriform and/or perirhinal cortices (Piredda and Gale, 1985; Gale and Browning, 1988), other structures within the mesial temporal lobe such as the amygdale or the enthorinal cortex, and the temporal neocortex. Hippocampus and temporal neocortex are the most likely candidates because they undergo dramatic pathological changes over time post-injury (Fig. 6) that parallel the increase in proportion of grade 3 seizures (Fig. 3A). The temporal cortex was not found to originate obvious epileptic activity few months after injury (D'Ambrosio et al., 2003), but the progressive pathology described here suggests it may originate seizures at later times. Further experiments are needed to confirm the existence of a third epileptic focus.
Progressive hippocampal and temporal cortex pathology
Our data demonstrate progressive hippocampal and temporal cortex pathology following rpFPI that manifests itself as predominant ipsilateral atrophy and loss of laminar features (Fig. 6). While 2−4 weeks post-injury 57% of the animals have significant hippocampal and temporal cortex asymmetry, by 7 months post-FPI hippocampal and temporal cortex asymmetry becomes significant in 86% and 79% of the cases, respectively. Our choice to perform rpFPI is instrumental to this observation because a more posterior FPI causes greater acute damage to the hippocampus and temporal cortex (Floyd et al., 2002; Cortez et al., 1989) which hinders the observation of a later progressive sclerosis, typical of MTLE, by confounding it with acute and sub-acute posttraumatic neuronal loss. While the temporal cortex pathology progressed to a clear sclerosis, as defined by significant loss of neurons and laminar features with increased gliosis, in 79% of the animals, we did not observe loss of laminar features in the hippocampus. However, Grady and coworkers (2003), employing an identical rpFPI and stereological cell count, demonstrated ∼50% neuronal loss in the ipsilateral hilus and ∼23% in the ipsilateralCA3 sub-region two weeks post-injury. Therefore, neuronal loss occurred and the progressive hippocampal atrophy we observed is likely an early stage of progressive sclerosis. Indeed, human hippocampal sclerosis is a progressive disorder (Fuerst et al., 2003), and hippocampi studied are typically resected from MTLE patients ∼17−18 years from diagnosis (Benbadis et al., 2003; Salanova et al., 2002), and likely after several more years of sub-clinical seizures, therefore representing extremely chronic cases. In agreement with this view, atrophy in rpFPi-animals was mostly evident in CA1 and CA3 sub-fields, as it is expected in the earlier stages of human hippocampal sclerosis. Further experiments are needed to determine the cellular bases of this progressive hippocampal atrophy. Interestingly, the observed heterogeneity in hippocampal and temporal cortex pathology defines different sub-populations of rpFPI epileptic animals, just like different sub-populations of human epileptic patients suffering from TLE exist with different degrees of hippocampal or temporal cortex atrophy, cognitive or mnestic disturbances, and pharmacoresistant complex partial seizures (French et al., 1993; Mathern et al., 1995; Fuerst et al., 2003). We surmise that differences in the epileptic condition of the animal, and therefore in seizure-induced hippocampal kindling and neuronal loss (Sloviter, 1983; Cavazos and Sutula, 1990; Holmes 2002), and differences in genetic background (Schauwecker, 2002; McKhann et al., 2003) may all account for the different progression of hippocampal and temporal cortex pathology.
A case of remission from PTE
During this study we identified a case of remission from PTE. At the pathological level the brain showed mild hippocampal asymmetry and pronounced injury to the ipsilateral thalamus (not shown) which are therefore entirely attributable to the acute injury and not to the epileptic condition. As expected, this animal did not present a focus of glial reactivity in the temporal cortex, as all other epileptic animals in this study did, and as previously reported (D'Ambrosio et al., 2003). Cases of remission also occur in human posttraumatic epilepsy (Frey, 2003) and the finding of similar cases in the FPI-rat population increases the numerous similarities found between this rodent model and the human condition (Table 2).
Table 2.
Similarities between rpFPI-induced PTE in the rat and human PTE.
| 1.The traumatic brain injury model is mechanically very similar to human cases of closed head injury. |
| 2.Chronic spontaneous recurrent seizures appear after a single event of traumatic brain injury. |
| 3.Seizure free “latent period” between the initiating injury and the onset of the epileptic condition. |
| 4.Seizures are focal, with or without secondary bilateral spread. |
| 5.The ictal behavior is consistent with human complex partial seizures. |
| 6.Neocortical and hippocampal epileptic foci (dual pathology). |
| 7.Preferential recruitment of hippocampus by frontal-parietal foci, but not vice versa. |
| 8.Progressive temporal lobe sclerosis in a subgroup of epileptic individuals, and not in others. |
| 9.Time-dependent changes in epileptic syndrome. |
| 10.Cases of remission. |
Cases of age-dependent idiopathic epilepsy in the colony
Sprague Dawley rats purchased from Charles Rivers’ colony H41, and used for this and our previous study (D'Ambrosio et al., 2003), did not exhibit idiopathic seizures within their first 5.5 months of life, but we found cases at later age. About 33% of the animals presented bilateral posterior epileptic ECoG events, associated with no obvious brain pathology at 7−8 months of age, that were likely a manifestation of age-dependent idiopathic epilepsy. These idiopathic seizures presented with a pattern of sharp waves that was similar to that seen in many other laboratory rat strains (Van Luijtelaar and Coenen, 1986; Inoue et al., 1990; Willoughby and Mackenzie, 1992; Vadasz et al., 1995), but it was different from true spike-and-wave discharge (a term often and erroneously considered interchangeable with idiopathic), as seen in the Strasbourg rat (Snead et al., 1999; Marescaux et al., 1992; Vergnes et al., 2003), which consist in high amplitude spikes followed by slow waves. The idiopathic sharp-wave seizures of the rats employed for this study only appeared at 5.5 months of age, when most of the progression of PTE had already occurred. These remained much rarer than the posttraumatic seizures, being only 3.6% of all cortical discharge in FPI animals at 7 months post-injury, and could easily be identified and excluded on the basis of their posterior dominant generalized distribution (Fig. 1D), as it was observed in the Wistar Furth rat (Willoughby and Mackenzie, 1992). Because of genetic drift, outbred rat colonies may randomly produce generations with an epileptic phenotype which may appear at variable age. This unpredictable variability stresses the need for appropriate ECoG monitoring of control siblings in acquired epilepsy research.
CONCLUSIONS
rpFPI-induced PTE in the rat reproduces many of the features of human PTE and shows faster epileptogenesis in the neocortex at the injury site than in the mesial temporal lobe, resulting in temporal evolution from FPE to dual pathology with MTLE. The temporal changes in type and frequency of neocortical and hippocampal partial seizures observed offer the unprecedented opportunity to study different aspects of clinically-relevant post-traumatic epileptogenesis and to examine the drug sensitivity of partial seizures that represent the real obstacle in the treatment of pharmacoresistant epilepsy.
ACKNOWLEDGEMENTS
This work was supported by grants to Raimondo D'Ambrosio from NIH (NS040823), University of Washington (UWRRF2985), and The Epilepsy Project foundation. We thank Drs. Massimo Avoli, Cliff Eastman, Richard Ellenbogen, Jeffrey Ojemann, Emilio Perucca and Nicholas Poolos for helpful discussion or review of the manuscript, and Janet Schukar for excellent photographic support.
BIBLIOGRAPHY
- Annegers JF, Rocca WA, Hauser WA. Causes of epilepsy: contributions of the Rochester epidemiology project. Mayo Clin Proc. 1996;71:570–575. doi: 10.4065/71.6.570. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Hauser WA, Coan SP, et al. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338:20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- Arruda F, Cendes F, Andermann F, Dubeau F, Villemure JG, Jones-Gotman M, Poulin N, Arnold DL, Olivier A. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40(3):446–50. doi: 10.1002/ana.410400314. [DOI] [PubMed] [Google Scholar]
- Benbadis SR, Heriaud L, Tatum WO, Vale FL. Epilepsy surgery, delays and referral patterns-are all your epilepsy patients controlled? Seizure. 2003;12(3):167–70. doi: 10.1016/s1059-1311(02)00320-5. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Zhang DX. Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neuroscience. 1999;92(1):15–26. doi: 10.1016/s0306-4522(98)00712-x. [DOI] [PubMed] [Google Scholar]
- Browning RA. Effects of lesions on seizures in experimental animals. In: Fromm GH, Faingold CL, Browning RA, Burnham WM, editors. Epilepsy and the reticular formation: the role of the reticular core in convulsive seizures. Alan R Liss; New York: 1987. p. 137. [Google Scholar]
- Cavazos JE, Sutula TP. Progressive neuronal loss induced by kindling: a possible mechanism for mossy fiber synaptic reorganization and hippocampal sclerosis. Brain Res. 1990;527(1):1–6. doi: 10.1016/0006-8993(90)91054-k. [DOI] [PubMed] [Google Scholar]
- Cortez SC, McIntosh TK, Noble LJ. Experimental fluid percussion brain injury: vascular disruption and neuronal and glial alterations. Brain Res. 1989;482(2):271–82. doi: 10.1016/0006-8993(89)91190-6. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R. Basic mechanisms of posttraumatic epilepsy. In: Winn HR, editor. Youman's Neurological Surgery. 5th Edition. Elsevier; 2003. pp. 2449–2460. [Google Scholar]
- D'Ambrosio R. The role of glial membrane ion channels in seizures and epileptogenesis. Pharmacology & Therapeutics. 2004 doi: 10.1016/j.pharmthera.2004.05.004. In Press. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Selective loss of hippocampal long-term potentiation, but not depression, following fluid percussion injury. Brain Res. 1998;786(1−2):64–79. doi: 10.1016/s0006-8993(97)01412-1. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Impaired K(+) homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J Neurosci. 1999;19(18):8152–62. doi: 10.1523/JNEUROSCI.19-18-08152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio Fairbanks, Fender Doyle, Born Miller. Posttraumatic epilepsy following fluid percussion injury in the rat. Brain. 2003 2003 Nov 7; doi: 10.1093/brain/awh038. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Arrastia R, Agostini MA, Frol AB, Mickey B, Fleckenstein J, Bigio E, Van Ness PC. Neurophysiologic and neuroradiologic features of intractable epilepsy after traumatic brain injury in adults. Arch Neurol. 2000;57(11):1611–6. doi: 10.1001/archneur.57.11.1611. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Lopes da Silva FH, Witter MP. Nucleus reuniens thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. J Neurosci. 1997;17(14):5640–50. doi: 10.1523/JNEUROSCI.17-14-05640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JJ, Shewmon DA. Impact of the kindling phenomenon on clinical epileptology. In: Morrell F, editor. Kindling and synaptic plasticity. Birkhauser; Boston: 1991. p. 196. [Google Scholar]
- Floyd CL, Golden KM, Black RT, Hamm RJ, Lyeth BG. Craniectomy position affects morris water maze performance and hippocampal cell loss after parasagittal fluid percussion. J Neurotrauma. 2002;19(3):303–16. doi: 10.1089/089771502753594873. [DOI] [PubMed] [Google Scholar]
- French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34(6):774–80. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- Frey LC. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia. 2003;44(Suppl 10):11–7. doi: 10.1046/j.1528-1157.44.s10.4.x. [DOI] [PubMed] [Google Scholar]
- Fuerst D, Shah J, Shah A, Watson C. Hippocampal sclerosis is a progressive disorder: a longitudinal volumetric MRI study. Ann Neurol. 2003;53(3):413–6. doi: 10.1002/ana.10509. [DOI] [PubMed] [Google Scholar]
- Gale K, Browning RA. Anatomical and neurochemical substrates of clonic and tonic seizures. In: Dichter MA, editor. Mechanisms of Epileptogenesis. Plenum Press; New York: 1988. p. 111. [Google Scholar]
- Goddard GV. Development of epileptic seizures through brain stimulation at low intensity. Nature. 1967;214:1020. doi: 10.1038/2141020a0. [DOI] [PubMed] [Google Scholar]
- Golarai G, Greenwood AC, Feeney DM, Connor JA. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J Neurosci. 2001;21(21):8523–37. doi: 10.1523/JNEUROSCI.21-21-08523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady MS, Charleston JS, Maris D, Witgen BM, Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: analysis by stereological estimation. Journal of Neurotrauma. 2003;20(10):929–941. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- Holmes GL. Seizure-induced neuronal injury: animal data. Neurology. 2002;59(9 Suppl 5):S3–6. doi: 10.1212/wnl.59.9_suppl_5.s3. [DOI] [PubMed] [Google Scholar]
- Inoue M, Peeters BW, van Luijtelaar EL, Vossen JM, Coenen AM. Spontaneous occurrence of spike-wave discharges in five inbred strains of rats. Physiol Behav. 1990;48(1):199–201. doi: 10.1016/0031-9384(90)90285-c. [DOI] [PubMed] [Google Scholar]
- Juul-Jensen P. Epidemiology of intractable epilepsy. In: Schmidt D, Morselli P, editors. Intractable Epilepsy. Raven Press; New York: 1986. pp. 5–11. [Google Scholar]
- Kelly KM. Modeling traumatic brain injury and posttraumatic epilepsy. Epilepsy Currents. 2004;4(4):160–161. doi: 10.1111/j.1535-7597.2004.44015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba R, Rektor I, Brazdil M. Ictal limb dystonia in temporal lobe epilepsy: an invasive video-EEG finding. Eur J Neurol. 2003;10(6):641–9. doi: 10.1046/j.1468-1331.2003.00684.x. [DOI] [PubMed] [Google Scholar]
- Marescaux C, Vergnes M, Depaulis A. Genetic absence epilepsy in rats from Strasbourg--a review. J Neural Transm Suppl. 1992;35:37–69. doi: 10.1007/978-3-7091-9206-1_4. [DOI] [PubMed] [Google Scholar]
- Marks DA, Kim J, Spencer DD, et al. Seizure localization and pathology following head injury in patients with uncontrolled epilepsy. Neurology. 1995;45:2051–7. doi: 10.1212/wnl.45.11.2051. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Vickrey BG, Melendez M, Pretorius JK. Traumatic compared to non-traumatic clinical-pathologic associations in temporal lobe epilepsy. Epilepsy Res. 1994;19:129–139. doi: 10.1016/0920-1211(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Vickrey BG, Melendez M, Pretorius JK. The clinical-pathogenic mechanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilepsy. Brain. 1995;118(Pt 1):105–18. doi: 10.1093/brain/118.1.105. [DOI] [PubMed] [Google Scholar]
- Mattson RH, Cramer JA, Collins JF, Smith DB, Delgado-Escueta AV, Browne TR, Williamson PD, Treiman DM, McNamara JO, McCutchen CB. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic-clonic seizures. N Engl J Med. 1985;313(3):145–51. doi: 10.1056/NEJM198507183130303. [DOI] [PubMed] [Google Scholar]
- McKhann GM, 2nd, Wenzel HJ, Robbins CA, Sosunov AA, Schwartzkroin PA. Mouse strain differences in kainic acid sensitivity, seizure behavior, mortality, and hippocampal pathology. Neuroscience. 2003;122(2):551–61. doi: 10.1016/s0306-4522(03)00562-1. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28(1):233–44. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Piredda S, Gale K. Evidence that the deep prepiriform cortex contains a crucial epileptogenic site. Nature. 1985;317:623–625. doi: 10.1038/317623a0. [DOI] [PubMed] [Google Scholar]
- Salanova V, Markand O, Worth R. Temporal lobe epilepsy surgery: outcome, complications, and late mortality rate in 215 patients. Epilepsia. 2002;43(2):170–4. doi: 10.1046/j.1528-1157.2002.33800.x. [DOI] [PubMed] [Google Scholar]
- Salin P, Tseng GF, Hoffman S, Parada I, Prince DA. Axonal sprouting in layer V pyramidal neurons of chronically injured cerebral cortex. J Neurosci. 1995;15(12):8234–45. doi: 10.1523/JNEUROSCI.15-12-08234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Ratzliff AD, Jeng J, Toth Z, Soltesz I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann Neurol. 2001;50(6):708–17. doi: 10.1002/ana.1230. [DOI] [PubMed] [Google Scholar]
- Satow T, Ikeda A, Yamamoto J, Takayama M, Matsuhashi M, Ohara S, Matsumoto R, Begum T, Fukuyama H, Hashimoto N, Shibasaki H. Partial epilepsy manifesting atonic seizure: report of two cases. Epilepsia. 2002;43(11):1425–31. doi: 10.1046/j.1528-1157.2002.34501.x. [DOI] [PubMed] [Google Scholar]
- Schauwecker PE. Complications associated with genetic background effects in models of experimental epilepsy. Prog Brain Res. 2002;135:139–148. doi: 10.1016/s0079-6123(02)35014-3. [DOI] [PubMed] [Google Scholar]
- Schmidt RH, Grady MS. Regional patterns of blood-brain barrier breakdown following central and lateral fluid percussion injury in rodents. J Neurotraum. 1993;10(4):415–30. doi: 10.1089/neu.1993.10.415. [DOI] [PubMed] [Google Scholar]
- Schneider G, Fries P, Wagner-Jochem D, Thome D, Laurer H, Kramann B, Mautes A, Hagen T. Pathophysiological changes after traumatic brain injury: comparison of two experimental animal models by means of MRI. MAGMA. 2002;14(3):233–41. doi: 10.1007/BF02668217. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. “Epileptic” brain damage in rats induced by sustained electrical stimulation of the perforant path. I. Acute electrophysiological and light microscopic studies. Brain Res Bull. 1983;10(5):675–97. doi: 10.1016/0361-9230(83)90037-0. [DOI] [PubMed] [Google Scholar]
- Snead OC, Depaulis A, Vergnes M, Marescaux C. Jasper's Basic Mechanisms of Epilepsies. 3rd edition. 1999. Absence Epilepsy: advances in experimental animal models. pp. 253–278. [PubMed] [Google Scholar]
- Temkin NR, Jarell AD, Anderson GD. Antiepileptogenic agents. How close are we? Drugs. 2001;61(8):1045–1055. doi: 10.2165/00003495-200161080-00002. [DOI] [PubMed] [Google Scholar]
- Tinuper P, Cerullo A, Marini C, Avoni P, Rosati A, Riva R, Baruzzi A, Lugaresi E. Epileptic drop attacks in partial epilepsy: clinical features, evolution, and prognosis. J Neurol Neurosurg Psychiatry. 1998;64(2):231–7. doi: 10.1136/jnnp.64.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadasz C, Carpi D, Jando G, Kandel A, Urioste R, Horvath Z, Pierre E, Vadi D, Fleischer A, Buzsaki G. Genetic threshold hypothesis of neocortical spike-and-wave discharges in the rat: an animal model of petit mal epilepsy. Am J Med Genet. 1995;60(1):55–63. doi: 10.1002/ajmg.1320600111. [DOI] [PubMed] [Google Scholar]
- Van Luijtelaar EL, Coenen AM. Two types of electrocortical paroxysms in an inbred strain of rats. Neurosci Lett. 1986;70(3):393–7. doi: 10.1016/0304-3940(86)90586-0. [DOI] [PubMed] [Google Scholar]
- Willoughby JO, Mackenzie L. Nonconvulsive electrocorticographic paroxysms (absence epilepsy) in rat strains. Lab Anim Sci. 1992;42(6):551–4. [PubMed] [Google Scholar]
- Vergnes M, Boehrer A, Nehlig A. Developmental characteristics of picrotoxin-induced convulsions in rats with genetic absence epilepsy. Exp Neurol. 2003;184(1):549–51. doi: 10.1016/s0014-4886(03)00098-0. [DOI] [PubMed] [Google Scholar]
- Williamson PD, Spencer SS. Clinical and EEG features of complex partial seizures of extratemporal origin. Epilepsia. 1986;27(Suppl 2):S46–63. doi: 10.1111/j.1528-1157.1986.tb05740.x. [DOI] [PubMed] [Google Scholar]