Abstract
Background
Influenza virus and respiratory syncytial virus (RSV) are among the most common viruses causing infections of the lower respiratory tract in young children. Although there are important differences in the immunopathogenesis of these 2 viral pathogens, little is known about how they affect antigen-presenting cells in children with acute infections.
Methods
To characterize the immune cells that are mobilized to the respiratory tract by influenza virus and RSV, we analyzed nasal wash and blood samples obtained from children hospitalized with acute respiratory infections.
Results
Influenza virus and RSV mobilize immune cells, including myeloid dendritic cells (mDCs) and plasma-cytoid dendritic cells (pDCs), to the nasal mucosa. Patients with influenza virus infection had greater numbers of mDCs, pDCs, and monocytes in nasal wash samples than did patients with RSV infection. The frequencies of respiratory tract and blood T cell subsets were not affected by infection with influenza virus or RSV. Monocyte chemoattractant protein–1 concentrations in nasal wash samples were significantly increased in patients with influenza virus infection but not in those with RSV infection. RANTES (regulated on activation, normally T cell expressed and secreted) concentrations were increased only in the blood of patients with influenza virus infection.
Conclusions
Infection with influenza virus or RSV mobilizes antigen-presenting cells to the respiratory tract. The differences in antigen-presenting cell numbers and cytokine concentrations suggest that there are distinctive, early immune responses to these 2 viruses.
Influenza virus is the major cause of respiratory morbidity worldwide. Despite effective vaccines, influenza epidemics result in ~30,000 deaths annually in the United States [1, 2]. Although most deaths occur among patients who are elderly, are immunocompromised, or have underlying cardiopulmonary disease, influenza virus infections are common in children, who are thought to perpetuate influenza outbreaks. In infants and children <5 years of age, influenza virus most frequently causes infection of the upper respiratory tract [3], but 10%–50% of children develop lower respiratory tract involvement, including bronchiolitis and pneumonia.
Respiratory syncytial virus (RSV) is the leading viral respiratory pathogen in young children, resulting in >126,000 hospitalizations annually in the United States [4 –7]. Like influenza, RSV infection in infants causes upper respiratory tract infection. Up to one-third of infants with RSV infection develop lower respiratory tract involvement, including bronchiolitis, which is the most common syndrome associated with RSV. Although there are some similarities in the clinical manifestations of these infections in young children, there are also differences in the clinical responses to these viruses. For example, RSV infection is more frequently associated with recurrent wheezing and airway hyperreactivity [8 –10]. In contrast, influenza virus infection has not been associated with long-term airway hyperreactivity. In addition, both viruses induce only incomplete immunity and are commonly associated with recurrent infections. It is also important to note that, although there currently is no licensed vaccine for RSV infection, vaccines for influenza are available and are quite effective, even in young children [11, 12].
Little is known about the role of antigen-presenting cells in RSV and influenza virus infections in young children. We previously demonstrated recruitment of both myeloid dendritic cells (mDCs) and plasmacytoid dendritic cells (pDCs) to the respiratory tract of children with acute viral respiratory infections [13]. Our initial studies focused on RSV and demonstrated that children infected with RSV had increased numbers of mDCs and pDCs in nasal wash samples, compared with healthy controls. We also studied patients infected with other viruses, including influenza virus, parainfluenza virus, and cytomegalovirus, and we found that all these other viruses were also associated with recruitment of increased numbers of dendritic cells (DCs) to the nasal mucosa. The small numbers of such patients included in our previous study, however, precluded comparisons among patients with different viruses.
The present study was designed to further characterize the participation of antigen-presenting cells in acute viral respiratory infections. Accordingly, we conducted a comparative analysis of the numbers of immune cell subsets and concentrations of cytokines/chemokines present in both the upper respiratory tract and the blood of children infected with 1 of 2 major respiratory viruses: influenza virus and RSV. Our results provide evidence that acute influenza virus infection in young children results in increased numbers of mDCs, pDCs, and monocytes in the upper respiratory tract, compared with acute RSV infection in children. Acute influenza virus infection also results in greater concentrations of monocyte chemoattractant protein (MCP)–1 in nasal wash samples and increased concentrations of RANTES (regulated on activation, normally T cell expressed and secreted) in blood samples than does RSV infection.
SUBJECTS AND METHODS
Patient population
Inclusion criteria for enrollment into the groups of patients with viral infection in the study were (1) age <36 months, (2) acute illness requiring hospitalization, and (3) diagnosis of infection with influenza virus or RSV by use of a direct fluorescent antibody (DFA) test (Diagnostic Hybrids) and/or viral culture (of HEp-2 or A549 cells). The DFA test and viral culture routinely performed by the clinical virology laboratory identify RSV; influenza viruses A and B; parainfluenza viruses 1, 2, and 3; adenovirus; and rhinovirus. Our control population consisted of healthy infants with no history of upper respiratory tract illness within the preceding 3 weeks. These children were typically enrolled in the study while in the operating room undergoing scheduled surgical procedures that did not involve the lung or respiratory tract. Nasopharyngeal viral cultures were performed at enrollment for all healthy controls, despite the lack of respiratory symptoms. Exclusion criteria for all groups included (1) age >36 months, (2) use of immunosuppressive medications, and (3) history of chronic lung disease. Written informed consent was obtained for all patients; and the study was approved by the institutional review board (IRB) of the University of Texas Southwestern Medical Center (IRB file 1096 –36300).
Nasal wash sampling and cell isolation
Nasal wash samples were collected from patients by nasal suctioning performed according to a validated protocol [14]. In brief, after instillation of 3 mL of sterile saline into the patients’ nares, a suction catheter (connected to a mucus trap) was inserted 5 cm deep into the nasopharynx, and suction was applied during slow withdrawal of the catheter. Samples were collected into a mucus trap connected to the suction catheter, and a total of 3 mL of viral transport media was subsequently suctioned into the mucus trap to salvage any material remaining in the catheter. The nasal wash samples were then transported and maintained at 4°C, centrifuged at 300 g for 10 min, and were stored at −80°C for subsequent analysis. The nasal wash samples remaining after supernatant removal were then processed to single cell suspensions before staining was performed as described elsewhere [13].
Cell staining for flow cytometric analysis
For staining of cells in nasal wash samples, 200,000 cells per tube were incubated with antibodies. For staining of cells in blood, 100 μL of blood and 3–10 μL of each antibody were incubated as described elsewhere [13]. The following antibodies were used for staining of cells in both nasal wash and whole-blood samples: Lineage-FITC cocktail (containing CD3, CD14, CD16, CD19, CD20, and CD56), CD123-PE, HLA-DR-PerCP, CD11c-APC, CD4-FITC, CD8-PE, CD3-PerCP, and CD14-APC (BD Biosciences).
Quantification of cytokines and chemokines in nasal wash samples
Concentrations of cytokines (including interleukin [IL]–1α, IL-1β, IL-2–IL-8, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, granulocyte-macrophage colony-stimulating factor [GM-CSF], interferon [IFN]–γ, tumor necrosis factor [TNF]– α, eotaxin, MCP-1, RANTES, macrophage inflammatory protein [MIP]–1α, IFN-γ–inducible protein–10 [IP-10], and IFN–α) in nasal wash and plasma samples were measured using Luminex XMAP technology (Luminex), by use of commercially available kits (Upstate USA) according to the manufacturer’s instructions.
Statistical analysis
Data were displayed graphically by use of box-and-whisker plots. The solid dark line in the middle of each box denoted the median value; the lower and upper edges of the box, the 25th and 75th percentiles, respectively; and the whiskers, the range of values that fell within 1.5 box lengths from the 25th and 75th percentiles [15]. Because the data were not normally distributed, statistical testing of the overall group differences was performed using Kruskal-Wallis tests (a nonparametric alternative to 1-way analysis of variance), followed by post hoc pairwise comparisons conducted using Mann-Whitney U tests with Bonferroni corrections for family-wise multiple comparisons.
RESULTS
Patient characteristics
Twenty-two patients with influenza virus infection, 82 patients with RSV infection, and 37 healthy controls were enrolled in the present study between December 2000 and April 2005. Demographic characteristics of and clinical data for the patients are summarized in table 1. Clinical presentations of patients infected with influenza virus included bronchiolitis, dehydration, fever (≥38.5 C), and pneumonia. All patients infected with RSV had bronchiolitis. The median duration of symptoms (i.e., the number of days of illness) at the time of study enrollment was 3 days for patients infected with influenza virus versus 5 days for patients infected with RSV (P < .05). The median length of hospitalization (LOH) was 3 days (range, 0 –16 days) for patients infected with influenza virus (P = .14) versus 4.5 days (range, 0 –25 days) for patients infected with RSV. During hospitalization, patients were treated symptomatically with oxygen, intravenously administered fluids, and β-adrenergic agents for variable periods, but they did not receive antiviral agents or corticosteroids. Patients infected with influenza virus received supplemental oxygen for a median of 0 days (range, 0 –13 days), and patients infected with RSV received supplemental oxygen for a median of 1 day (range, 0 –21 days) (P = .09). Age distribution was similar for patients with influenza virus infection and those with RSV infection, whereas the healthy control population had a median age higher than that of the patients with viral infections (table 1) (P < .05).
Table 1.
Patient demographic characteristics and clinical data.
| Days, median (range) |
||||||
|---|---|---|---|---|---|---|
| Subject group | Patients, no. | Age, median (range), months | Male, % of patients | Of illness at enrollment | Receiving oxygena | Of hospitalization |
| Infected | ||||||
| With influenza virus | 22 | 4.3 (0.5–35) | 73 | 3 (2–9) | 0 (0–13) | 3.0 (0–16) |
| With RSV | 81 | 2.8 (0.5–33) | 51 | 5 (1–20) | 1 (0–21) | 4.5 (0–25) |
| Control | 37 | 8.6 (0–37) | 82 | NA | NA | NA |
NOTE. RSV, respiratory syncytial virus.
Supplemental oxygen.
Recruitment of mDCs to the upper respiratory tract and reduction in their numbers in the blood of patients with acute infection with influenza virus or RSV
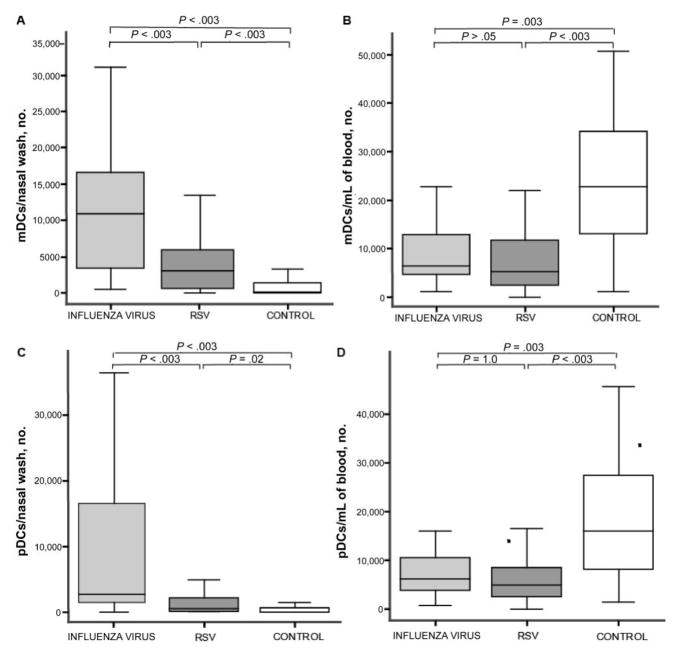
DCs were identified by flow cytometry as being lineage (CD3, CD14, CD16, CD19, CD20, and CD56) negative, HLA-DR positive, and either CD11c positive (mDCs) or CD123 positive (pDCs) [13]. The median number of mDCs in nasal wash samples (hereafter referred to as “nasal wash mDCs”) was 10,880 mDCs/sample for patients with influenza, 3040 mDCs/sample for patients with RSV infection, and 177 mDCs/sample for healthy controls (P < .05) (figure 1A). Patients in both the influenza virus and RSV infection groups had significantly increased numbers of nasal wash mDCs, compared with healthy controls (P < .003). Patients with influenza virus infection had significantly greater numbers of nasal wash mDCs than did the patients with RSV infection (P < .003).
Figure 1.
Recruitment of myeloid dendritic cells (mDCs) and plasmacytoid dendritic cells (pDCs) to the upper respiratory tract and the subsequent decrease in the numbers of these cells in the blood of patients with acute influenza virus or respiratory syncytial virus (RSV) infection. The absolute numbers of mDCs and pDCs, as determined by flow cytometry in patients with influenza virus infection, RSV infection, or no viral infection (control), are displayed in columns 1–3 of each graph, respectively. Controls are healthy, uninfected subjects. The line in the center of each box denotes the median value; the lower and upper edges of the box, the 25th and 75th percentiles, respectively; and the whiskers, the range of values that fall within 1.5 box lengths from the 25th and 75th percentiles. P values denote post hoc pairwise comparisons made using Mann-Whitney U tests with Bonferroni corrections for family-wise multiple comparisons. Numbers of CD11c+ mDCs in nasal wash (A) and blood (B) samples obtained from each patient group. Absolute numbers of pDCs in nasal wash (C) and blood (D) samples obtained from each patient group.
Patients with influenza virus infection had decreased numbers of mDCs in blood samples (hereafter referred to as “blood mDCs”) (6450 mDCs/mL), compared with healthy controls (22,780 mDCs/mL; P < .003) (figure 1B). Likewise, patients with RSV infection had decreased numbers of blood mDCs, compared with healthy controls (median count, 5320 mDCs/mL, compared with controls; P < .003). There was no significant difference between the decreased number of blood mDCs in patients with influenza virus infection and patients with RSV infection, suggesting that acute respiratory infection with either virus results in the emigration of mDCs out of the blood.
Increase of pDCs in nasal wash samples (hereafter referred to as “nasal wash pDCs”) and decrease in pDCs in blood samples obtained from patients infected with influenza virus or RSV
The median number of nasal wash pDCs was 2720 pDCs/sample for patients with influenza virus infection, 628 pDCs/sample for patients with RSV infection, and 101 mDCs/sample for the healthy control group (P < .05) (figure 1C). Both the patients with influenza virus infection and the patients with RSV infection had significantly increased numbers of nasal wash pDCs, compared with healthy controls (P = .02 and P = .003, respectively). Patients infected with influenza virus had significantly greater numbers of nasal wash pDCs than did patients infected with RSV (P < .003). As we observed for blood mDCs, the numbers of pDCs in blood (hereafter referred to as “blood pDCs”) were decreased in patients with influenza virus infection or RSV infection, compared with healthy controls. The median number of pDCs per milliliter of blood was 6300 for patients with influenza virus infection, 4900 for patients with RSV infection, and 15,980 for healthy controls (P = .003, for patients with influenza virus infection vs. controls; P < .003, for patients with RSV infection vs. controls; and P = 1.0, for patients with RSV infection vs. those with influenza virus infection) (figure 1D).
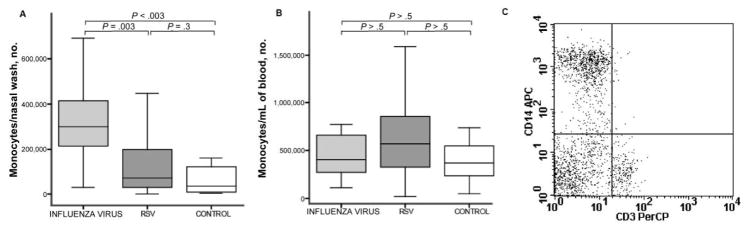
Increase in the number of monocytes in nasal wash samples obtained from patients infected with influenza virus
Monocytes were defined, by means of flow cytometry, by an expression of CD14 and a lack of CD3. The median number of monocytes in nasal wash samples (hereafter referred to as “nasal wash monocytes”) was significantly increased in patients with influenza virus infection (299,430 monocytes/nasal wash), compared with patients with RSV infection (73,640 monocytes/nasal wash; P = .003) and healthy controls (36,051 monocytes/nasal wash; P < .003) (figure 2A). The number of nasal wash monocytes was not increased in patients with RSV infection, compared with controls (P = .3). Unlike mDCs and pDCs, monocytes were not decreased in the blood of patients with influenza virus infection, compared with healthy controls (364,060 monocytes/mL of blood in patients with influenza virus infection and 399,350 monocytes/mL in controls; P > .5) (figure 2B). Although the median number of monocytes in blood was slightly higher in patients with RSV infection (567,000 monocytes/mL), it was not significantly different than the numbers of monocytes in blood in the other groups.
Figure 2.
Increase in the number of monocytes in nasal wash samples obtained from patients with influenza. Absolute numbers of CD14+ cells (monocytes) identified by flow cytometry in the nasal wash (A) and blood (B) samples obtained from patients infected with influenza virus or respiratory syncytial virus or from control subjects. The line in the center of each box denotes the median value; the lower and upper edges of the box, the 25th and 75th percentiles respectively; and the whiskers, the range of values that fall within 1.5 box lengths from the 25th and 75th percentiles. P values denote post hoc pairwise comparisons made using Mann-Whitney U tests with Bonferroni corrections for family-wise multiple comparisons. C, Flow cytometry plot demonstrating how monocytes were identified in nasal wash samples by use of flow cytometry.
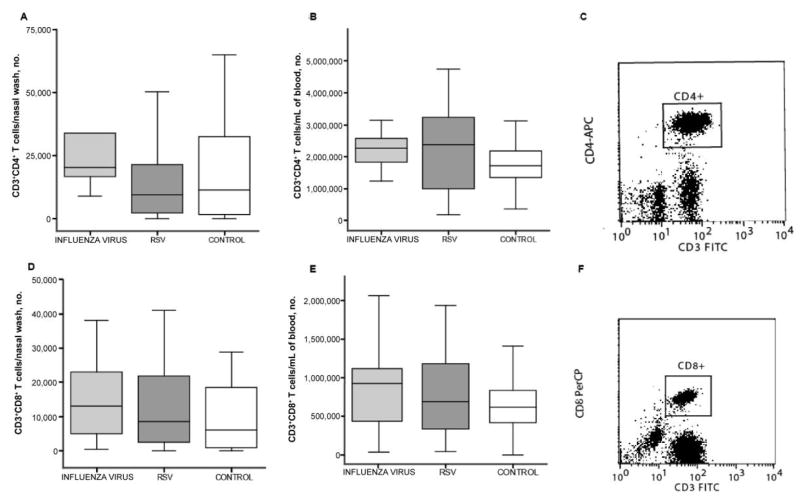
No significant alteration in the numbers of CD3+CD4+ and CD3+CD8+ T cells in nasal wash and blood samples from patients infected with influenza virus or RSV
Unlike frequencies of DC subsets and monocytes, frequencies of CD3+CD4+T cells were not significantly altered by either influenza virus infection or RSV infection. The median numbers of CD3+CD4+ T cells measured in nasal wash samples obtained from patients with influenza virus infection, patients with RSV infection, and healthy controls were 20,290, 9500, and 11,448 cells/sample, respectively (figure 3A). Similarly, the median numbers of CD3+CD4+ T cells in blood samples did not differ significantly among patients and controls (2.27 million cells/mL for patients with influenza virus infection; 2.37 million cells/mL for patients with RSV infection; and 1.72 million cells/mL for healthy controls) (figure 3B). Likewise, the numbers of CD3+CD8+ T cells in nasal wash samples were not significantly different among groups (13,070 cells/sample for patients with influenza; 8475 cells/sample for patients with RSV infection; and 6138 cells/sample for controls) (figure 3D). In addition, the numbers of blood CD3+CD8+ T cells in blood were also similar among all groups: 925,830 cells/mL in patients with influenza, 693,260 cells/mL in patients with RSV infection, and 618,340 cells/mL in healthy controls (figure 3E).
Figure 3.
No significant alteration in the numbers of T lymphocytes in the nasal wash and blood samples obtained from patients infected with influenza virus or respiratory syncytial virus (RSV). Absolute numbers of CD3+CD4+ T cells identified by flow cytometry in the nasal wash (A) and blood (B) samples obtained from patients with influenza virus or RSV infection. The line in the center of each box denotes the median value; the lower and upper edges, the 25th and 75th percentiles, respectively; and the whiskers, the range of values that fall within 1.5 box lengths from the 25th and 75th percentiles. C, Flow cytometry plot demonstrating how CD3+CD4+ T cells were identified in nasal wash samples. CD3+CD8+ T cell numbers in nasal wash (D) and blood (E) samples obtained from patients with influenza virus or RSV infection. F, Flow cytometry plot demonstrating how CD3+CD8+ T cells were identified in blood samples. No significant differences were observed between the study groups.
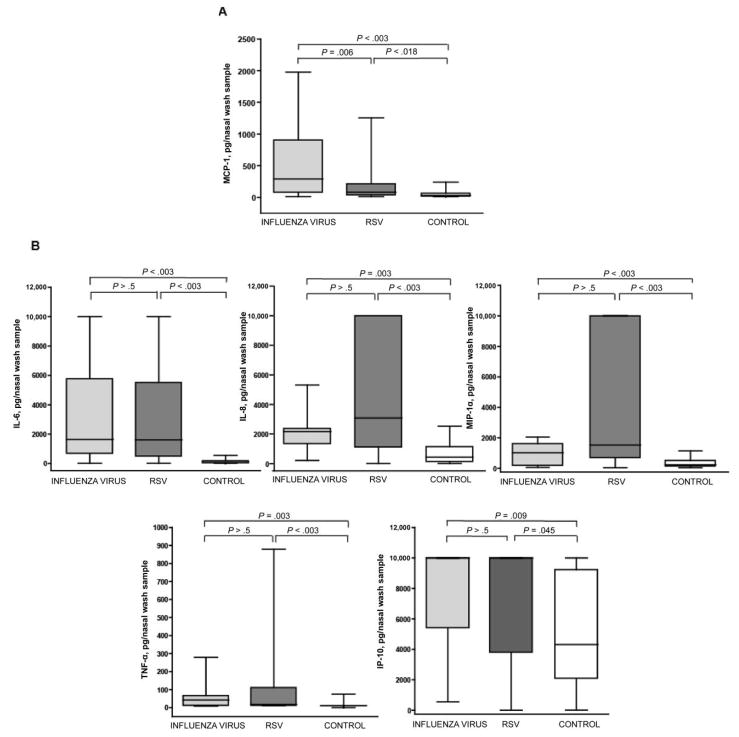
Increases in concentrations of MCP-1 in nasal wash samples obtained from children with acute influenza
Of the 23 different cytokines measured in nasal wash samples, only the concentrations of MCP-1 in patients with influenza virus infection (median, 380 pg/nasal wash sample) were significantly increased, compared with those of both patients with RSV infection (86 pg/nasal wash sample; P = .006) and healthy controls (47 pg/nasal wash sample; P < .003) (figure 4A). MCP-1 concentrations in nasal wash samples obtained from patients with RSV infection were also significantly higher than those noted in samples from controls (P = .018). As shown in figure 4B, patients with influenza virus or RSV infection had significantly increased nasal wash concentrations of several cytokines, including IL-6, IL-8, MIP-1α, IP-10, and TNF-α. There were no significant differences in the concentrations of these cytokines between patients with influenza virus infection and patients with RSV infection, suggesting that these cytokines are produced in a similar manner in response to infection with these 2 viruses.
Figure 4.
Nasal wash concentrations of monocyte chemoattractant protein (MCP)–1 are increased in children with acute infection with influenza virus, whereas interleukin (IL)– 6, IL-8, macrophage inflammatory protein (MIP)–1α, interferon (IFN)– γ–inducible protein–10 (IP-10), and tumor necrosis factor (TNF)– α concentrations are increased in children with acute viral respiratory infection with influenza virus or respiratory syncytial virus (RSV). Cytokines/chemokines were significantly increased in nasal wash samples obtained from patients with viral infection (due to influenza virus or RSV), compared with controls. The line in the center of each box denotes the median value; the lower and upper edges of the box, the 25th and 75th percentiles, respectively; and the whiskers, the range of values that fall within 1.5 box lengths from the 25th and 75th percentiles. P values denote post hoc pairwise comparisons made using Mann-Whitney U tests with Bonferroni corrections for family-wise multiple comparisons. A, Increased nasal wash concentrations of MCP-1 in children with acute infection with influenza virus. B, Increased nasal wash concentrations of cytokines/chemokines in patients with acute viral respiratory infection. The increased concentrations were not significantly different between the groups infected with influenza virus and RSV.
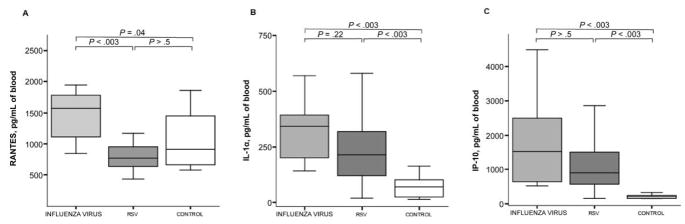
Increases in RANTES concentrations noted exclusively in the blood samples of children infected with influenza virus
As shown in figure 5A, serum concentrations of RANTES were significantly elevated in the blood of patients with influenza virus infection (median, 1572 pg/mL vs. 771 pg/mL in patients with RSV infection and 904 pg/mL in controls; P < .05). There were no differences in serum concentrations of RANTES between patients with RSV infection and controls. As shown in figure 5B, serum concentrations of IL-1α were significantly increased both in patients with influenza virus infection (median, 342 pg/mL) and in patients with RSV infection (213 pg/mL), compared with controls (71 pg/mL). Similarly, serum concentrations of IP-10 were significantly increased in patients with influenza virus infection (552 pg/mL) and in those with RSV infection (569 pg/mL), compared with controls (161 pg/mL) (figure 5C). However, there were no differences in serum concentrations of either IL-1α or IP-10 between patients infected with influenza virus and those infected with RSV.
Figure 5.
Serum concentrations of RANTES (regulated on activation, normally T cell expressed) increased exclusively in the blood of children with influenza; interleukin (IL)–1α and interferon (IFN)– γ–inducible protein–10 (IP-10) increased in patients with both influenza virus and respiratory syncytial virus (RSV) infections. Serum concentrations of RANTES, IL-1α, and IP-10 in samples obtained from patients with influenza or RSV infection and from healthy controls are displayed. The line in the center of each box denotes the median value; the lower and upper edges of the box, the 25th and 75th percentiles, respectively; and the whiskers, the range of values that fall within 1.5 box lengths from the 25th and 75th percentiles. P values denote post hoc pairwise comparisons made using Mann-Whitney U tests with Bonferroni corrections for family-wise multiple comparisons. A–C, Comparison of serum concentrations of RANTES, IL-1α, and IP-10 between study groups, respectively.
Comprehensive results of all nasal wash and serum cytokine/chemokine concentrations are presented in table 2, which is available only in the electronic version of the Journal.
Table 2.
Supplementary concentrations of cytokines and chemokines in nasal wash and blood samples, by subject group.
| The table is available in its entirety in the online edition of the Journal. |
Correlations between cell and cytokine/chemokine parameters and clinical disease
Data were reviewed for potential correlations between cells, chemokine/cytokine concentrations, and clinical disease severity. Correlation coefficients for all significant correlations (P < .05) are shown in table 3. We did find significant, although modest, positive correlations between LOH and nasal wash concentrations of IL-6, IL-8, MCP-1, TNF-α, and MIP-1α. Similarly, we found positive correlations between clinical disease severity (LOH, presence of fever, and number of days that supplemental oxygen was required) and serum IL-1α and IP-10 concentrations. The numbers of pDCs and mDCs measured in the upper respiratory tract correlated with the presence of fever (temperature, >38.5°C during hospitalization). The numbers of the same cell types measured in blood were inversely correlated with the presence of fever, suggesting that febrile patients had greater recruitment of DCs from blood to the site of viral replication. Lower numbers of pDCs and mDCs in blood were also associated with increases in LOH and the number of days that supplemental oxygen was required, although no corresponding significant positive correlations were found between the respiratory DCs and these clinical parameters. There were a number of significant positive correlations between concentrations of several cytokines/chemokines in the upper respiratory tract and clinical parameters, as shown in table 3.
Table 3.
Correlation of data on upper respiratory tract cells and cytokines/chemokines with clinical data.
| Sample, parameter | LOH | DOI | Fevera | Day(s) receiving oxygenb |
|---|---|---|---|---|
| Nasal | ||||
| pDCs | … | −.241 | .279 | … |
| mDCs | … | … | .312 | … |
| IL-4 | … | … | … | .243 |
| IL-6 | .499 | … | .271 | .240 |
| IL-7 | … | … | … | … |
| IL-8 | .385 | … | .256 | … |
| IL-10 | … | … | … | .260 |
| IL-12 | … | … | … | … |
| IL-13 | … | … | … | … |
| GM-CSF | … | … | … | .259 |
| IFN-γ | … | … | … | .294 |
| MCP-1 | .377 | −.290 | … | .307 |
| TNF-α | .4 | … | … | … |
| IP-10 | … | … | .351 | … |
| MIP-1α | .531 | … | .293 | .345 |
| IFN-γ | … | … | … | … |
| Blood | ||||
| pDCs | −.309 | … | −.361 | −.325 |
| mDCs | −.382 | … | −.452 | −.417 |
| IL-1α | .588 | … | .471 | .445 |
| IP-10 | .556 | … | .458 | .390 |
NOTE. Data are correlation coefficients for which P < .05. DOI, day(s) of illness; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; IP-10, IFN-γ–inducible protein–10; LOH, length of hospitalization. mDCs, myeloid dendritic cells; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; pDCs, plasmacytoid dendritic cells; TNF, tumor necrosis factor.
Temperature, >38.5°C. Corrected by point-biserial correlation (dichotomous and continuous variables).
Supplemental oxygen.
DISCUSSION
Despite the enormous clinical influence of influenza virus and RSV, especially when they affect young children, little is known about the immunopathogenesis of these important viruses. Our investigative group has been interested in studying the role of DCs in the immune responses of young children infected with influenza virus and RSV, as well as whether differences in the numbers of DCs in the different compartments can, at least in part, provide insight into how immune responses to these 2 common viral infections are regulated.
The present study confirms our previous observations that both mDCs and pDCs are present in increased numbers in the upper respiratory tract of children with acute RSV infection [13]. It also provides the first evidence that acute infection with influenza virus also results in increased numbers of mDCs and pDCs in the upper respiratory tract, and that it is more effective than infection with RSV in this regard. Indeed, the numbers of both mDCs and pDCs in nasal wash samples of children with influenza virus infection were significantly higher than those in samples from children with RSV infection.
Studies of other immune cells offer additional insights into the similarities and differences in the immune responses associated with these 2 viral infections. The number of monocytes was also increased in nasal wash samples obtained from children infected with influenza virus, compared with those obtained both from children infected with RSV and from controls. Children with RSV infection, however, did not have increased numbers of monocytes in their upper respiratory tract samples. In contrast with the observations for DCs, we did not observe decreased numbers of monocytes in the blood of children infected with influenza virus or RSV, suggesting that a different mechanism(s) may regulate monocyte and DC migration into the respiratory tract after viral infection. It also is possible that variation in maturation from monocytes to mDCs within the respiratory tract itself could also contribute to the differences observed between influenza virus and RSV. An alternative explanation could be that RSV has a direct effect on monocytes, resulting in accelerated death of this cell type.
The lack of significant changes in the numbers of T cells suggests that the increased numbers of DCs detected in the upper respiratory tract of children with these viral infections do not simply denote a nonspecific recruitment of all immune cells but probably reflect more-selective mechanisms related to the primary role of DCs in orchestrating immune responses.
To understand which factors may influence the migration of DCs and other immune cells to the respiratory tract of these patients, we measured 23 cytokines and chemokines in both nasal wash and blood samples. As expected, there were significant similarities between the groups with the 2 infections in terms of increased nasal wash concentrations of IL-6, IL-8, MIP-1α, IP-10, and TNF-α, compared with controls. The concentrations of MCP-1, however, were significantly elevated in patients with influenza virus infection, compared with both patients with RSV infection and controls.
Previous studies by Casola et al. [16] and Garofalo et al. [17] demonstrated increased concentrations of MCP-1 in nasal secretions of children with RSV bronchiolitis, compared with controls. Additional studies showed that children with influenza virus infection have greater nasopharyngeal concentrations of MCP-1 than do those with RSV infection [18, 19]. The present study confirms those findings, including the higher values measured in children infected with influenza virus, further underscoring the consistency of this difference. In addition, we observed a positive correlation between nasopharyngeal MCP-1 concentrations and the numbers of mDCs, pDCs, and monocytes in the upper respiratory tract, although mediators other than MCP-1 were also correlated with the numbers of nasal wash immune cells (table 4, which is available only in the electronic version of the Journal).
Table 4.
Supplementary correlations between cell numbers and cytokine/chemokine concentrations in nasal wash samples.
| The table is available in its entirety in the online edition of the Journal. |
MCP-1 (CCL2) is thought to play an important role in the pathogenesis of lung inflammatory disorders [20], including asthma. MCP-1 stimulates histamine and leukotriene release, enhances Th2 polarization, and mediates trafficking of effector and regulatory leukocytes, especially monocytes, to the lungs. Many immune cells express CCR2, the receptor for MCP-1, including >90% of macrophages, monocytes, and immature murine DCs. MCP-1 is secreted by epithelial cells and many immune cells, including monocytes and DCs [21]. It is possible that the greater concentrations of MCP-1 induced by influenza virus result in more effective recruitment of monocytes and DCs to the respiratory tract, because both monocytes and DCs respond to MCP-1 [22, 23]. Because DCs can also synthesize MCP-1 [24, 25], the increased MCP-1 concentrations may also be a reflection of the increased numbers of DCs present in the upper respiratory tract of patients infected with influenza virus.
Several in vitro studies demonstrated that RSV can infect monocyte-derived DCs and, more importantly, that, as a result of the infection (or interaction), DCs have decreased capacity to stimulate CD4+ T cells [26 –28]. Influenza virus, however, does not impair the capacity of DCs to induce T cell priming and proliferation in vitro [29 –31]. Other viruses, such as cytomegalovirus (CMV), have been shown to affect DC phenotype and function both in vitro and in patients with CMV infectious mononucleosis. [32–35]. In patients with CMV infection, DCs showed decreased expression of major histocompatibility complex class II and diminished capacity to induce T cell alloproliferation. Exposure of DCs to CMV in vitro resulted in decreased migration capacity as well [35].
To further define the role of DCs and cytokines in disease pathogenesis, we also analyzed the correlations between parameters of clinical disease severity and the number of DC subsets, as well as the concentrations of cytokines. Our results demonstrate a significant correlation between the concentrations of several inflammatory cytokines and chemokines, such as MIP-1α, IL-6, IL-8, MCP-1, and TNF-α, and the duration of hospitalization (table 3). This was not unexpected, because several studies have correlated these cytokines with disease severity. In addition, we documented mild but significant inverse correlations between the number of mDCs and pDCs in blood and the presence of fever, as well as the LOH and the number of days that supplementary oxygen was required. This is a novel and intriguing observation that will require confirmation by other investigators. Nevertheless, it strongly suggests that mDCs and pDCs play a major role in the immunopathogenesis that determines the clinical manifestations of these common respiratory infections.
In summary, we have documented quantitative differences in the numbers of DCs and monocytes in the respiratory tract of children with acute infection with influenza virus or RSV. Future studies of children are required to determine whether the observed quantitative differences in the numbers of DCs and monocytes correlate with differences in the expression of maturation markers and receptors, such as CCR2, which may regulate migration of these cells. In addition, future studies are required to determine whether the differences in mDC function observed after in vitro exposure to RSV and influenza also occur in cells obtained from children with naturally occurring infections. Further understanding the in vivo function of mDCs will provide important insight into the pathogenesis of these infections and should facilitate designing of novel, more effective vaccines, which are especially needed to reduce the influence of RSV infections in the pediatric population.
Acknowledgments
Children’s Medical Center of Dallas Foundation (grant to M.A.G.); Baylor Health Care System Foundation (grant); National Institutes of Health (grants K08 AI059379 – 02 [to M.A.G.], AI 54990 [to O.R.], and U19 AIO57234 [to J.B.]). J.B. is the recipient of the W.W. Caruth Chair for Immunology Research, at Baylor University.
We thank Elizabeth Kraus for her excellent technical assistance and Juanita Lozano-Hernandez for her assistance in enrolling patients in the study.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: Annual meeting of the Society for Pediatric Research, San Francisco, California, May 2006 (abstract 1904).
References
- 1.Glezen WP. Influenza vaccination for healthy children. Curr Opin Infect Dis. 2002;15:283–7. doi: 10.1097/00001432-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Glezen WP. Influenza control. N Engl J Med. 2006;355:79 – 81. doi: 10.1056/NEJMe068114. [DOI] [PubMed] [Google Scholar]
- 3.Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31– 40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 4.Chanock RM, Parrott RH. Acute respiratory disease in infancy and childhood: present understanding and prospects for prevention. Pediatrics. 1965;36:21–39. [PubMed] [Google Scholar]
- 5.Macasaet FF, Kidd PA, Bolano CR, Wenner HA. The etiology of acute respiratory infections. 3. The role of viruses and bacteria. J Pediatr. 1968;72:829 –39. doi: 10.1016/s0022-3476(68)80436-6. [DOI] [PubMed] [Google Scholar]
- 6.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543– 6. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 7.Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J. 2002;21:629 –32. doi: 10.1097/00006454-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16:386 –92. doi: 10.1111/j.1399-3038.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 9.Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137– 41. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 10.Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–5. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 11.Halloran ME, Piedra PA, Longini IM, Jr, et al. Efficacy of trivalent, cold-adapted, influenza virus vaccine against influenza A (Fujian), a drift variant, during 2003–2004. Vaccine. 2007;25:4038 – 45. doi: 10.1016/j.vaccine.2007.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356:685–96. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 13.Gill MA, Palucka AK, Barton T, et al. Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J Infect Dis. 2005;191:1105–15. doi: 10.1086/428589. [DOI] [PubMed] [Google Scholar]
- 14.Malley R, DeVincenzo J, Ramilo O, et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis. 1998;178:1555– 61. doi: 10.1086/314523. [DOI] [PubMed] [Google Scholar]
- 15.SPSS Base 10.0 applications guide. Chicago: SPSS; 1999. [Google Scholar]
- 16.Casola A, Burger N, Liu T, Jamaluddin M, Brasier AR, Garofalo RP. Oxidant tone regulates RANTES gene expression in airway epithelial cells infected with respiratory syncytial virus: role in viral-induced interferon regulatory factor activation. J Biol Chem. 2001;276:19715–22. doi: 10.1074/jbc.M101526200. [DOI] [PubMed] [Google Scholar]
- 17.Garofalo RP, Hintz KH, Hill V, Ogra PL, Welliver RC., Sr Production of interferon gamma in respiratory syncytial virus infection of humans is not associated with interleukins 12 and 18. J Med Virol. 2004;73:289 –94. doi: 10.1002/jmv.20089. [DOI] [PubMed] [Google Scholar]
- 18.Garofalo RP, Hintz KH, Hill V, Patti J, Ogra PL, Welliver RC., Sr A comparison of epidemiologic and immunologic features of bronchiolitis caused by influenza virus and respiratory syncytial virus. J Med Virol. 2005;75:282–9. doi: 10.1002/jmv.20268. [DOI] [PubMed] [Google Scholar]
- 19.Welliver TP, Garofalo RP, Hosakote Y, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126 –36. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali-Ahmad D, Bonville CA, Rosenberg HF, Domachowske JB. Replication of respiratory syncytial virus is inhibited in target cells generating nitric oxide in situ. Front Biosci. 2003;8:a48 –53. doi: 10.2741/986. [DOI] [PubMed] [Google Scholar]
- 21.Julkunen I, Melen K, Nyqvist M, Pirhonen J, Sareneva T, Matikainen S. Inflammatory responses in influenza A virus infection. Vaccine. 2000;19:S32–7. doi: 10.1016/s0264-410x(00)00275-9. [DOI] [PubMed] [Google Scholar]
- 22.Sakai N, Wada T, Furuichi K, et al. MCP-1/CCR2-dependent loop for fibrogenesis in human peripheral CD14-positive monocytes. J Leukoc Biol. 2006;79:555– 63. doi: 10.1189/jlb.0305127. [DOI] [PubMed] [Google Scholar]
- 23.Henkel JS, Beers DR, Siklós L, Appel SH. The chemokine MCP-1 and the dendritic and myeloid cells it attracts are increased in the mSOD1 mouse model of ALS. Mol Cell Neurosci. 2006;31:427–37. doi: 10.1016/j.mcn.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Guo Z, Zhang M, Tang H, Cao X. Fas signal links innate and adaptive immunity by promoting dendritic-cell secretion of CC and CXC chemokines. Blood. 2005;106:2033– 41. doi: 10.1182/blood-2004-12-4831. [DOI] [PubMed] [Google Scholar]
- 25.Berndt BE, Zhang M, Chen GH, Huffnagle GB, Kao JY. The role of dendritic cells in the development of acute dextran sulfate sodium colitis. J Immunol. 2007;179:6255– 62. doi: 10.4049/jimmunol.179.9.6255. [DOI] [PubMed] [Google Scholar]
- 26.de Graaff PM, de Jong EC, van Capel TM, et al. Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate CD4 T cells. J Immunol. 2005;175:5904 –11. doi: 10.4049/jimmunol.175.9.5904. [DOI] [PubMed] [Google Scholar]
- 27.Jones A, Morton I, Hobson L, Evans GS, Everard ML. Differentiation and immune function of human dendritic cells following infection by respiratory syncytial virus. Clin Exp Immunol. 2006;143:513–22. doi: 10.1111/j.1365-2249.2005.03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartz H, Türkel O, Hoffjan S, Rothoeft T, Gonschorek A, Schauer U. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-gamma in naïve T cells. Immunology. 2003;109:49 –57. doi: 10.1046/j.1365-2567.2003.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhardwaj N, Bender A, Gonzalez N, Bui LK, Garrett MC, Steinman RM. Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J Clin Invest. 1994;94:797– 807. doi: 10.1172/JCI117399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bender A, Albert M, Reddy A, et al. The distinctive features of influenza virus infection of dendritic cells. Immunobiology. 1998;198:552– 67. doi: 10.1016/S0171-2985(98)80078-8. [DOI] [PubMed] [Google Scholar]
- 31.Larsson M, Messmer D, Somersan S, et al. Requirement of mature dendritic cells for efficient activation of influenza A—specific memory CD8+ T cells. J Immunol. 2000;165:1182–90. doi: 10.4049/jimmunol.165.3.1182. [DOI] [PubMed] [Google Scholar]
- 32.Frascaroli G, Varani S, Mastroianni A, et al. Dendritic cell function in cytomegalovirus-infected patients with mononucleosis. J Leukoc Biol. 2006;79:932– 40. doi: 10.1189/jlb.0905499. [DOI] [PubMed] [Google Scholar]
- 33.Frascaroli G, Varani S, Moepps B, Sinzger C, Landini MP, Mertens T. Human cytomegalovirus subverts the functions of monocytes, impairing chemokine-mediated migration and leukocyte recruitment. J Virol. 2006;80:7578 – 89. doi: 10.1128/JVI.02421-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varani S, Cederarv M, Feld S, et al. Human cytomegalovirus differentially controls B cell and T cell responses through effects on plasmacytoid dendritic cells. J Immunol. 2007;179:7767–76. doi: 10.4049/jimmunol.179.11.7767. [DOI] [PubMed] [Google Scholar]
- 35.Varani S, Frascaroli G, Homman-Loudiyi M, Feld S, Landini MP, Söderberg-Nauclér C. Human cytomegalovirus inhibits the migration of immature dendritic cells by down-regulating cell-surface CCR1 and CCR5. J Leukoc Biol. 2005;77:219 –28. doi: 10.1189/jlb.0504301. [DOI] [PubMed] [Google Scholar]