Abstract
Limitations of printed, text-based, consent forms have long been documented and may be particularly problematic for persons at risk for impaired decision-making capacity, such as those with schizophrenia. We conducted a randomized controlled comparison of the effectiveness of a multimedia vs routine consent procedure (augmented with a 10-minute control video presentation) as a means of enhancing comprehension among 128 middle-aged and older persons with schizophrenia and 60 healthy comparison subjects. The primary outcome measure was manifest decisional capacity (understanding, appreciation, reasoning, and expression of choice) for participation in a (hypothetical) clinical drug trial, as measured with the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR) and the University of California San Diego (UCSD) Brief Assessment for Capacity to Consent (UBACC). The MacCAT-CR and UBACC were administered by research assistants kept blind to consent condition. Additional assessments included standardized measures of psychopathology and cognitive functioning. Relative to patients in the routine consent condition, schizophrenia patients receiving multimedia consent had significantly better scores on the UBACC and on the MacCAT-CR understanding and expression of choice subscales and were significantly more likely to be categorized as being capable to consent than those in the routine consent condition (as categorized with several previously established criteria). Among the healthy subjects, there were few significant effects of consent condition. These findings suggest that multimedia consent procedures may be a valuable consent aid that should be considered for use when enrolling participants at risk for impaired decisional capacity, particularly for complex and/or high-risk research protocols.
Keywords: bioethics, mental competency, informed consent, multimedia learning, cognition disorders, schizophrenia
Introduction
An important question confronting researchers and Institutional Review Boards (IRBs) is how to effectively disclose information relevant to consenting to research.1–8 Empirical data in the educational and cognitive psychology literature suggest that effective learning can be facilitated by multimodal presentation—eg, combining auditory-verbal and visual-pictorial presentations.9 Unfortunately, the commonly practiced consent process continues to rely on written and auditory representations only. The limitations of standard text-based consent forms have been long and well documented.10,11 This issue becomes particularly problematic for persons with suboptimal decision-making capacity. Studies suggest that individuals with serious mental illness (SMI) are at risk for impaired decisional capacity, although there is considerable heterogeneity in this regard.12–22
Several studies suggest that computer- or video-aided consent yields superior postconsent understanding relative to standard text-based consent conditions.23–25 However, a recent review of empirical studies of enhanced medical research consent26 concluded that multimedia and enhanced consent form interventions did not consistently improve research participants’ understanding and that person-to-person interactions, especially the extended discussion interventions, might be more effective in improving understanding. We believe that multimedia tools could be beneficial when integrated as an aide to such dialogue (wherein understanding can be checked and accommodated during the process), rather than employing such tools as a replacement for personal interaction. Also, multimedia consent might have greater benefits in patient populations with consent-relevant cognitive impairments.26 Given the neurocognitive deficits that characterize schizophrenia,27 as well as the normal age-related changes in cognition,28 middle-aged and older patients with schizophrenia seem a particularly appropriate population for evaluating the added value of multimedia consent procedures.29 Although we previously examined the efficacy of a Powerpoint-aided consent,23 and others have examined the utility of computer- or video-facilitated consent,26,30 there have been no large-scale randomized controlled trials (RCTs), in SMI patients, of an enhanced consent procedure fully grounded in the principles of multimedia learning.9
The present study was an RCT of multimedia consent vs routine consent procedure for research among middle-aged and older people with schizophrenia as well as healthy comparison subjects (HCSs). We hypothesized that patients and HCSs provided with multimedia consent would demonstrate better understanding, appreciation, reasoning, and expression of choice compared with participants presented with routine consent. The HCSs were included to evaluate the degree to which effects of consent condition generalize to a nonimpaired population. To reduce type I errors, we chose not to directly compare the 2 diagnostic groups because the differences between HCSs and schizophrenia patients in decisional capacity are already well established.16 Based on the literature regarding clinical characteristics associated with impaired decisional capacity,16,29,31 we also hypothesized that greater benefit of multimedia consent compared with routine paper consent format would be associated with higher severity of psychopathology or cognitive impairment.
Methods
Participants
We determined, based on previously published data,32 to test a difference between routine and multimedia consent procedures; a sample size of 120 patients with schizophrenia and 60 HCSs would be required to detect a medium effect size with a minimum of 80% power.33 We recruited 128 community-dwelling outpatients aged >40 years, with schizophrenia, from outpatient clinics in San Diego County's Adult and Older Adult Mental Health Services, University of California, San Diego (UCSD), and Veterans Affairs San Diego Healthcare System, local board-and-care facilities, as well as private physicians. Other inclusion criteria were fluency in English and an absence of a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV),34 diagnosis of current substance use disorder, dementia, or other known conditions likely to influence decisional capacity independent of the effects of schizophrenia. Sixty age-comparable HCSs were recruited through newspaper advertisements, flyers, and word of mouth. Absence of major neuropsychiatric disorders among HCSs was determined with the Mini-International Neuropsychiatric Interview,35 administered by the research coordinator (D.G.).
The diagnosis of schizophrenia was based on that made by the patient's treating clinician using DSM-IV criteria.34 We determined the patients’ clinical diagnoses as recorded in available medical or research records and/or by verbal report from the patients’ treating clinicians. Clinician-identified diagnosis is often the basis on which persons are first approached and consented for possible participation in research.
The research protocol was approved by UCSD's IRB, and all study subjects provided a written informed consent before participation. No potential participants were excluded on the basis of consent capacity. We should, however, point out that, as this was a minimal risk study, a lower level of manifest decisional capacity was permitted for enrollment in this study than would be needed for enrollment in a greater-than-minimal-risk protocol (as typifies most pharmacological RCTs).36–38
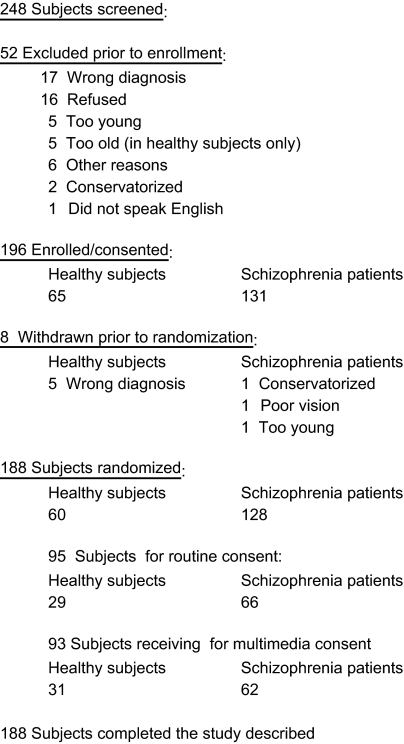
Subject flow is shown in figure 1.
Fig. 1.
Subject Flow.
Materials and Procedures
Demographic Information
Data on age, gender, self-identified ethnicity, education, age of onset of schizophrenia, duration of illness, and current medications were collected for each participant via interview or review of medical records.
We wished to minimize a potential for confusion among participants about the (initial) actual consent to participate in the proposed study and the (subsequent) presentation of the hypothetical consent protocol. We inserted a short (about 30 minutes) break between the actual consent to the study and the presentation of the hypothetical protocol. Also, at the beginning of the latter presentation, the research assistant (RA) clarified the difference between the 2 consent procedures. The subjects were reminded that this was a study of the consent process itself and that while another study would be described to them they would not actually be participating in that study.
Description of Hypothetical Protocol
The hypothetical study, in reference to which the capacity to consent was being evaluated, was described as a 14-week double-blind, placebo-controlled RCT to determine the effectiveness of a cognition-enhancing drug for cognitive deficits associated with schizophrenia or with normal aging. The procedures described as being included in that RCT consisted of randomized assignment, drug administration, safety monitoring, repeated neuropsychological assessments, functional neuroimaging (functional magnetic resonance imaging [MRI]), as well as medical and laboratory tests involving blood draws.
Description of Simulated Consent Form
As a part of the development of the hypothetical protocol, we also prepared a realistic consent form and provided the structure and content in this form exactly as if it were an actual study being conduced at our institution. We modeled it after the consent form for an actual pilot study that was being conducted at UCSD regarding the effects of a cholinomimetic agent for cognitive deficits in schizophrenia, and it was similar to a simulated consent form used in our prior research.19 Two IRB members reviewed the consent form during its development to help insure content validity. It included all the mandated elements for documentation of informed consent under federal law, (Title 21 Code of Federal Regulations Part 50; Title 45 Code of Federal Regulations Part 46) as well as those required by our local institutional IRB, including a description of the purpose and procedures of the study, the potential risks from the study procedures and medications (those typical of cholinomimetics), potential benefits (including possible lack of direct personal benefits), the limits of payment for treatment for research-related injuries, the voluntary nature of participation, procedures for withdrawal from the study, confidentiality (and procedures to foster it), and means of addressing any questions or concerns.
Description of DVD
The DVD was designed to present the same key information as in the printed consent form. However, in developing our DVD, we incorporated several key principles of multimedia learning (details of which are described in detail by Mayer9). A key component of multimedia learning theory, derived from the prevailing model of working memory,39 is that learning is facilitated when information is simultaneously provided through both verbal and visual-spatial/pictorial channels. The emphasis on value of multiple channels is known as the multiple representation principle, and the emphasis on simultaneous presentation is known as the contiguity principle.9 We incorporated the multiple representation and contiguity principles throughout the DVD by presenting consent-relevant information through audio of a narrator verbally explaining key points, with simultaneous visual presentation using graphics, still pictures, and animations, as well as summary (bullet-pointed) text, to depict the narrated information. For instance, while the audio track presented the narrator describing the process and purpose of randomized assignment, the screen included a short animated sequence wherein participants were shown balls being put into a hat and then being pulled out one by one, corresponding to treatment assignment. As the narrator/audio track described neuropsychological testing, the screen/visual track included a short video clip illustrating an example of neuropsychological testing. As the narrator described the MRI, the screen showed a video clip of someone undergoing an MRI examination, as well as a brain MRI picture. The latter also included audio presentation of the sound made by the MRI machine in operation. Our inclusion of noise from the MRI machine as part of the DVD explanation of that procedure is also an example of the coherence principle,9 which suggests learning is facilitated when extraneous words and sounds are excluded but relevant words and sounds are included. Some participants find the MRI sound quite aversive, so we believe it to be a particularly relevant part of disclosed information when consenting to a study involving MRI evaluations. It is much easier to present the sound of the MRI aurally than it is to describe it in words or text.
We also attended to the personalization principle,9 which suggests that learning is facilitated when information is provided in a conversational rather than formal tone. To achieve the latter, we prepared and revised the narrator script by repeatedly speaking it aloud (rather than just preparing it with written text) until we found phrasing that had the sound of appropriate/natural conversational tone. Yet another key principle of multimedia learning is that of signaling,9 which suggests that learning is facilitated when the learner is signaled (cued) regarding the content about to be presented. (Such signaling is thought to prime schematic knowledge that fosters efficient processing of subsequent information.) We incorporated the signaling principle by having the narrator describe the content she was about to explain, while using slides with bullet-pointed text on the screen to also indicate (signal) the topic of each upcoming section, as well as using follow-up bulleted summary slides to reiterate key points, which we and others have previously found to facilitate comprehension.23,24,40
Utilizing DVD technology also allowed us to break up the presentation into 13 different “chapters,” making it easy for a subject to review the information missed. Giving the participant such control is consistent with the interactivity principle, which suggests that learning is facilitated when learners are allowed to control the presentation. The inclusion of chapters also permitted us to incorporate further signaling because each of the 13 chapters was signaled by the screen turning to blue with the title of the chapter in bolded white font, accompanied by music specifically used at the beginning of each chapter.
Routine and Multimedia Consent Procedures
All the participants were informed that they would receive a simulated consent process for a hypothetical trial and were asked to imagine that they were actually being invited to participate in that trial. Subjects were then randomly assigned to either of 2 simulated consent procedures: (a) routine consent or (b) multimedia consent.
Routine Consent
Our previous pilot data suggested that the multimedia consent took approximately 10 minutes longer than the routine consent. To control for time spent with an RA and the novelty of a DVD, we added a 10-minute control DVD (describing general information about research) to the routine consent procedure. Subjects assigned to the routine consent procedure first met individually with a trained RA to view this DVD. Next, the RA provided the subjects with the printed simulated consent form and encouraged them to read along while the RA read it aloud. Participants were encouraged to stop the RA and ask for clarification any time during this review.
Multimedia Consent
The RA provided participants assigned to the multimedia consent with the printed consent form, and rather than listening to the RA read the consent aloud, subjects watched a DVD that explained the protocol. [We considered having the RA read over the consent form with the subjects in addition to showing them the DVD but rejected this alternative because (a) we were concerned that the resulting consent process would become exceptionally lengthy and impractical in many settings and (b) our primary goal was to compare the effectiveness of routine vs enhanced consent, not routine vs routine + enhanced consent.] Subjects were encouraged to have the RA stop the DVD and repeat any segments that were unclear. Participants were also encouraged to discuss and clarify issues with the RAs (and not simply have the DVD rerun). Such discussion is important in that multimedia consent aids appear to be more effective as aids to than as substitutes for person-to-person interaction.26
Measures of Effectiveness of Consent Procedures
Immediately after completing the simulated consent process, participants met with the research coordinator, who was blind to the assigned consent condition. She administered the UCSD Brief Assessment of Capacity to Consent (UBACC),41 a recently developed and validated 10-item questionnaire that assesses consent capacity. Next, she administered a modified version of the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR).42 The MacCAT-CR is a 20-minute semistructured interview, consisting of 4 subscales for primary components of capacity to consent, including the ability to (a) understand relevant information (such as asking the participant to explain what he or she understands about the purpose, procedures, risks, and potential benefits of the study), (b) appreciate the applicability and significance of the information for one's own condition and situation (eg, recognizing the difference between treatment and clinical research, and the voluntary nature of research participation), (c) reason or manipulate information rationally (such as being able to describe potential consequences of participation in the study), and (d) communicate a choice (ie, being able to state clearly at the end of the MacCAT-CR interview whether he/she is willing to participate in that study). The content of the participants’ response to the “expression of a choice” item was also used to compare the rates of agreement to participate (vs declining participation) in the hypothetical protocol.
Because decisional capacity is a context-specific construct, the MacCAT-CR was designed in a manner requiring tailoring of items for each specific protocol.42 For the present study, the disclosures and scoring of items were based on the hypothetical study described above. This modification or tailoring of the MacCAT-CR was done jointly by the study authors, including Dr P.S.A., who was the principal author of the original instrument.42 Also, in applied settings, the MacCAT-CR manual recommends providing participants with a card summarizing key information prior to the query sections of the MacCAT-CR interview. We did not include these cards, although participants were told they could refer to the printed consent forms that had been provided to them. Also, standard MacCAT-CR procedures score participants after one reexplanation of initially misunderstood information. (This reexplanation involved rereading the information missed by the participant, such as the purpose of the study or risks and benefits of study participation.) However, consistent with our prior research with this instrument,20 we scored participants’ initial response (Trial 1), their response after reexplanation of any initially misunderstood information (Trial 2), and after another reexplanation when required (Trial 3). The decision to use 3 trials, rather than 2 or 4 is somewhat arbitrary, but the authors’ impression from prior experience with this instrument has been that some participants do benefit from the additional reexplanation (third trial).
MacCAT-CR administration involves providing participants a brief disclosure of key elements associated with the hypothetical study followed by questions that assess comprehension of the protocol. The first subscale of the MacCAT-CR assesses understanding the purpose of the project and key procedural elements. The disclosure for this subscale of our hypothetical study included the following information:
You were recently asked to participate in a research project to study whether an investigational medication called NDZ-674 is safe and effective for treating memory and attention problems.
Before anyone is enrolled in the study, there will be a screening period of up to 28 days, during which the researchers will determine whether patients qualify to participate. Screening procedures involve an interview to gather some general information about patients and their caregivers, a medical examination, blood and urine tests, ECG, and an MRI of the brain. They will also be interviewed to screen for depression, and have tests which examine thinking, attention and memory, some of which will be done on a computer.
The total duration of the study is 12 weeks, consisting of 10 visits (or more if needed) of up to 3 hours each.
At the end of the study, a board-certified psychiatrist (T.M.), blind to the consent condition and diagnosis, reviewed the MacCAT-CR Trial 1 and Trial 3 assessments of all subjects and made categorical determinations of the presence or absence of adequate decisional capacity to participate in the research protocol, as described elsewhere.41
Additional Assessments
Psychiatric symptom severity was measured with the Positive and Negative Syndrome Scale (PANSS)43 and the 17-item Hamilton Depression Rating Scale (HAM-D).44 Cognitive functioning was examined with the Repeatable Battery for Assessment of Neuropsychological Status (RBANS).45 The RBANS scores were converted to an Index Score Scale using the normative tables provided in the RBANS manual; this Index Score has a normative mean of 100 and SD of 15, with higher Index Scores reflecting better cognitive performance.
Statistical Analysis
Because the MacCAT-CR and UBACC scores were skewed, and showed significant heterogeneity of variance between the 2 consent groups, consent group differences were evaluated within diagnostic groups using the Fisher exact test or generalized Wilcoxon test The latter is a nonparametric test of difference in central tendency between 2 groups; unlike the standard Wilcoxon test, it does not require that the 2 groups have the same distribution or variance.
Because of the contextual nature of decisional capacity, there is no established algorithm46,47 for classifying participants as having adequate vs inadequate decisional capacity to consent based on the MacCAT-CR42 or UBACC.41 However, to explore the influence of consent condition on such categorical determinations, we employed 3 standards: (a) the definition of “adequate capacity” recently employed in the National Institute of Mental Health–sponsored Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia trial (MacCAT-CR understanding score ≥16 of 26 possible points),22 (b) a previously validated cut score for the UBACC (total score ≥14 of 20 possible points),41 and (c) categorical determinations by an independent board-certified psychiatrist (T.M.) who reviewed the MacCAT-CR protocols, as described above. Although we cannot be certain that any of these approaches would yield results identical to judicial determinations of whether or not an individual is legally competent to make such decisions, we used them here solely for comparisons of the proportions of subjects with adequate vs inadequate capacity, based on each of these criteria, employing Fisher exact test within each diagnostic group. The 95% confidence intervals for relative risks were constructed using bootstrap resampling.
To test the hypothesis regarding participant characteristics associated with differential benefit of the multimedia consent procedures, we computed Spearman rank correlation coefficients to examine the association of MacCAT-CR Trial 1 understanding and UBACC total scores with specific subject-related characteristics (eg, severity of psychopathology, cognitive impairment) within each consent group, and the 2 sets of correlation coefficients were then compared using Fisher Z-transform test.48 We postulated that significant differences between correlations in the routine vs multimedia consent groups would indicate differential effect with respect to those characteristics.
All analyses were 2 tailed, where applicable, with α = .05.
Results
There were no significant differences in demographic or clinical characteristics between subjects in the routine vs multimedia consent conditions within either diagnostic group (table 1).
Table 1.
Demographic, Cognitive, and Clinical Characteristics of the Subject Groups
| Routine (N = 29) | Multimedia (N = 31) | df | t test/Fisher Exact P Value | |
| Healthy Comparison Subjects | ||||
| Age (y) | 54.2 (9.3) | 54.7 (7.3) | 53.2 | 0.820 |
| Education (y) | 14.3 (2.3) | 14.1 (1.8) | 52.7 | 0.689 |
| Gender (% women) | 48 | 55 | 0.796 | |
| Ethnic background | 0.903 | |||
| % Caucasian | 83 | 74 | ||
| % African American | 3 | 6 | ||
| % Latino | 7 | 6 | ||
| % Other | 7 | 13 | ||
| Age of onset of schizophrenia (y) | N/A | N/A | ||
| RBANS total score | 94.8 (11.8) | 98.0 (14.3) | 57.2 | 0.326 |
| PANSS | ||||
| Overall total (N = 29,30) | 35.8 (3.9) | 36.7 (5.7) | 51.8 | 0.478 |
| Positive total | 8.4 (1.5) | 8.6 (2.0) | 54.5 | 0.717 |
| Negative total | 8.4 (1.5) | 8.6 (2.0) | 54.2 | 0.798 |
| General total | 18.9 (2.4) | 19.4 (3.3) | 54.0 | 0.514 |
| HAM-D Total | 3.9 (3.6) | 3.7 (3.7) | 57.9 | 0.871 |
| Schizophrenia patients | ||||
| Age (y) | 51.2 (6.5) | 52.40 (8.0) | 117.8 | 0.370 |
| Education (y) | 12.2 (1.9) | 12.4 (2.1) | 122.1 | 0.556 |
| Gender (% women) | 36 | 35 | 1.000 | |
| Ethnic background | 0.954 | |||
| % Caucasian | 61 | 65 | ||
| % African American | 18 | 16 | ||
| % Latino | 11 | 11 | ||
| % Other | 11 | 8 | ||
| Age of onset of schizophrenia (y) (N = 60, 58) | 27.0 (9.8) | 27.7 (13.2) | 104.9 | 0.748 |
| RBANS total score index (N = 63, 60) | 74.7 (14.7) | 77.4 (13.4) | 120.8 | 0.289 |
| PANSS (N = 65, 62) | ||||
| Overall total | 60.0 (16.6) | 59.0 (14.5) | 123.9 | 0.723 |
| Positive total | 15.7 (6.5) | 16.1 (6.0) | 124.9 | 0.694 |
| Negative total | 15.7 (4.8) | 14.6 (4.0) | 122.6 | 0.172 |
| General psychopathology total | 28.7 (8.6) | 284 (7.2) | 123.1 | 0.811 |
| HAM-D total (N = 65, 61) | 9.6 (6.2) | 10.1 (5.9) | 124.0 | 0.654 |
Note: Values above represent means (and SD), unless otherwise indicated. N/A, not applicable; RBANS, Repeatable Battery for Assessment of Neuropsychological Status; PANSS, Positive and Negative Syndrome Scale; HAM-D, Hamilton Depression Rating Scale
Effectiveness of Multimedia Vs Routine Consent Procedure
The average time for the 2 consent procedures was similar (26 minutes for the enhanced routine consent and 22 minutes for multimedia consent). Among the HCSs, the only significant difference on MacCAT-CR or UBACC was higher MacCAT-CR Trial 1 understanding score among those in the multimedia consent group (table 2). Among the schizophrenia patients, those in the multimedia consent group had significantly better scores on MacCAT-CR understanding Trial 1, Trial 2, and Trial 3; MacCAT-CR expression of a choice; and UBACC total. The mean score of the schizophrenia patients on MacCAT-CR understanding Trial 3 in the multimedia consent group (23.7) was similar to that of HCSs on MacCAT-CR understanding Trial 1 in the routine consent group (23.8). There was no significant difference in the rates of agreement to participate vs not participate in the hypothetical protocol between the 2 consent conditions within each subject group: 24.1% HCSs with routine consent and 25.8% HCSs with multimedia consent declined to participate in the study. Comparable values for persons with schizophrenia were 32.8% and 33.9%, respectively.
Table 2.
Effectiveness of Consent Condition
| Routine Consent (N = 29) | Multimedia Consent (N = 31) | Treatment Effecta | 95% Confidence Interval | df | Generalized Wilcoxon Test Statistic | Generalized Wilcoxon P Value | |
| Healthy comparison subjects | |||||||
| MacCAT-CR understandingb | |||||||
| Trial 1 | 23.8 (2.9) | 25.2 (1.5) | 0.6557 | 0.52, 0.79 | 51.4 | 2.38 | 0.0209 |
| Trial 2 | 25.4 (1.8) | 25.9 (0.4) | 0.5083 | 0.43, 0.59 | 53.4 | 0.21 | 0.8346 |
| Trial 3 | 25.8 (0.7) | 25.9 (0.2) | 0.5228 | 0.45, 0.60 | 48.9 | 0.62 | 0.5366 |
| MacCAT appreciation | 5.3 (1.1) | 5.5 (0.9) | 0.5423 | 0.43, 0.66 | 56.2 | 0.73 | 0.4715 |
| MacCAT reasoning | 5.9 (0.4) | 5.8 (0.6) | 0.4705 | 0.38, 0.56 | 57.0 | −0.66 | 0.5084 |
| MacCAT expression of choice | 2.0 (0.2) | 2.0 (0.2) | 0.5011 | 0.45, 0.55 | 57.4 | 0.05 | 0.9626 |
| UBACC total | 17.4 (2.9) | 18.5 (2.0) | 0.6179 | 0.48, 0.76 | 56.8 | 1.66 | 0.1026 |
| Schizophrenia patients | |||||||
| MacCAT-CR understandingb | |||||||
| Trial 1 | 16.3 (6.5) | 19.2 (5.9) | 0.6384 | 0.54, 0.74 | 125.0 | 2.82 | 0.0055 |
| Trial 2 | 20.3 (6.4) | 23.0 (4.8) | 0.6108 | 0.52, 0.71 | 122.1 | 2.29 | 0.0237 |
| Trial 3 | 21.1 (6.2) | 23.7 (4.4) | 0.6117 | 0.52, 0.70 | 119.7 | 2.42 | 0.0169 |
| MacCAT appreciation | 4.32 (1.8) | 4.82 (1.3) | 0.5676 | 0.47, 0.66 | 122.0 | 1.44 | 0.1526 |
| MacCAT reasoning | 4.92 (1.6) | 5.37 (0.9) | 0.5657 | 0.47, 0.66 | 125.7 | 1.42 | 0.1577 |
| MacCAT expression of choice | 1.89 (0.4) | 2.00 (0.0) | 0.5379 | 0.51, 0.57 | 65.0 | 2.31 | 0.0242 |
| UBACC total | 13.8 (4.2) | 16.1 (3.8) | 0.6795 | 0.59, 0.77 | 122.6 | 3.77 | 0.0003 |
Note: Values above represent means (and SD), unless otherwise indicated. MacCAT-CR, MacArthur Competence Assessment Tool for Clinical Research; UBACC, University of California, San Diego Brief Assessment of Capacity to Consent.
P(multimedia > routine) + 0.5 × P(multimedia = routine). (Treatment effect was measured by the estimated probability that a subject given the multimedia consent would have a higher score than a subject given the routine consent plus half of the probability that they would score exactly the same on the test [area under the curve].56 Thus, a treatment effect greater than 1/2 indicates that subjects given the multimedia consent had improved scores relative to the routine consent subjects.)
Ranges of Scores for Different Scales—MacCAT-CR: understanding, 0 to 26; appreciation, 0 to 6; reasoning, 0 to 8; expression of choice, 0 to 2. UBACC: 0 to 20.
Effects of Consent Condition on Categorical Capacity Determinations
Percentages of HCSs classified as “capable,” using the criteria described above, did not differ between the consent conditions (table 3). Among schizophrenia patients, however, significantly more persons assigned to the multimedia consent condition were found to have adequate capacity to consent under all 3 methods of classification.
Table 3.
Proportions of Subjects Meeting MacCAT-CR and UBACC Capability Criteria With the 2 Consent Conditions
| Routine Consent, % Capable (N = 29) | Multimedia Consent, % Capable (N = 31) | Relative “Risk” of Capabilitya | 95% Confidence Interval | Fisher Exact Test/P Value | |
| Healthy Comparison Subjects | |||||
| CATIE criterion | |||||
| Trial 1 | 100 | 100 | 1.000 | N/A | N/A |
| Trial 2 | 100 | 100 | 1.000 | N/A | N/A |
| Trial 3 | 100 | 100 | 1.000 | N/A | N/A |
| UBACC criterion | 86 | 94 | 1.085 | 0.94, 1.32 | 0.417 |
| Capacity determination by psychiatrist based on MacCAT-CRb | |||||
| Trial 1 | 90 | 100 | 1.115 | 0.107 | |
| Trial 3 | 97 | 100 | 1.036 | 0.483 | |
| Schizophrenia patients | |||||
| CATIE criterion | |||||
| Trial 1 | 59 | 81 | 1.365 | 1.09, 1.75 | 0.012 |
| Trial 2 | 77 | 92 | 1.190 | 1.03, 1.40 | 0.028 |
| Trial 3 | 77 | 94 | 1.211 | 1.05, 1.42 | 0.012 |
| UBACC criterion | 47 | 76 | 1.614 | 1.22, 2.22 | 0.001 |
| Capacity determination by psychiatrist based on MacCAT-CRb | |||||
| Trial 1 | 47 | 66 | 1.408 | 1.04, 1.97 | 0.034 |
| Trial 3 | 70 | 89 | 1.273 | 1.06, 1.55 | 0.010 |
Note: MacCAT-CR, MacArthur Competence Assessment Tool for Clinical Research; UBACC, University of California, San Diego Brief Assessment of Capacity to Consent; CATIE, Clinical Antipsychotic Trials of Intervention Effetiveness; N/A, not applicable. Values in bold are the P values for those Fisher Exact tests that were statistically significant.
Relative “risk” = P (capable|multimedia)/P (capable|routine).
Clinical determination of capacity by an independent psychiatrist based on MacCAT-CR responses.
Among HCSs, there were few significant associations of MacCAT-CR or UBACC scores with demographic or clinical variables (not shown). Among schizophrenia patients, the only consistent significant associations of MacCAT-CR understanding Trial 1 and UBACC total scores in either consent condition were with level of cognitive functioning (RBANS total) and severity of negative symptoms (PANSS) (table 4). There were, however, no significant differences in the magnitude of these correlations between the 2 consent conditions.
Table 4.
Spearman Correlation Coefficients and P Values Within and Between Consent Conditions for Schizophrenia Patients
| Fisher Z-Transform Homogeneity Test |
||||||||
| Routine Consent (N = 66) |
Multimedia Consent (N = 62) |
MacCAT-CR Understanding |
UBACC Total |
|||||
| Schizophrenia Patients | MacCAT-CR Understanding | UBACC Total | MacCAT-CR Understanding | UBACC Total | Chi-Square df = 1 | P Value | Chi-Square df = 1 | P Value |
| Age | 0.030 | 0.112 | 0.107 | 0.035 | 0.183 | 0.669 | 0.183 | 0.669 |
| Gender | 0.015 | 0.119 | 0.094 | 0.059 | 0.191 | 0.662 | 0.112 | 0.738 |
| PANSS overall total (N = 65, 62) | −0.051 | −0.223 | −0.354a | −0.327a | 3.076 | 0.079 | 0.384 | 0.536 |
| PANSS positive total (N = 65,62) | −0.009 | −0.154 | −0.168 | −0.206 | 0.780 | 0.377 | 0.087 | 0.768 |
| PANSS negative total (N = 65,62) | −0.179 | −0.303b | −0.402a | −0.283b | 1.816 | 0.178 | 0.014 | 0.904 |
| PANSS general psychopathology total (N = 65, 62) | 0.043 | −0.114 | −0.331a | −0.244 | 4.527 | 0.033 | 0.547 | 0.460 |
| HAM-D total 1-17 (N = 65, 61) | 0.099 | −0.059 | 0.023 | −0.005 | 0.175 | 0.676 | 0.088 | 0.767 |
| RBANS total (N = 63, 60) | 0.545a | 0.575a | 0.566a | 0.360a | 0.028 | 0.868 | 2.317 | 0.128 |
Note: MacCAT-CR, MacArthur Competence Assessment Tool for Clinical Research; UBACC, University of California, San Diego Brief Assessment for Capacity to Consent; PANSS, Positive and Negative Syndrome Scale; HAM-D, Hamilton Depression Rating Scale; RBANS, Repeatable Battery for Assessment of Neuropsychological Status. Values in bold is the only significant P value for chi-square test.
Correlation is significant at the 0.01 level (2 tailed).
Correlation is significant at the 0.05 level (2 tailed).
Discussion
As hypothesized, we found that outpatients with schizophrenia provided with a multimedia-aided consent procedure demonstrated better comprehension of a research protocol and were more likely to be categorized as being capable of consent under 3 different standards examined, compared with those presented with an enhanced routine consent procedure. However, there were few differences between the 2 consent conditions among the HCSs. The inverse correlations between decisional capacity and severity of cognitive impairment and of negative symptoms are consistent with prior research.14,15,17–22,49 However, these and most other putatively relevant patient characteristics were not associated with a greater benefit from the multimedia consent.
To our knowledge, the present study is the first large-scale RCT of the effectiveness of a multimedia-based consent procedure relative to routine text-based consent process in SMI patients. We made the comparison condition similar to the experimental consent by adding a 10-minute control video. Our routine consent was, therefore, “enhanced” routine consent and might have yielded somewhat better results than a truly routine consent practiced in everyday research studies. However, we believe this was necessary to ensure that better results with a multimedia consent were not primarily due to the effect of novel technology or comparison with a suboptimal routine consent procedure. In both consent conditions, subjects were encouraged to stop and ask questions anytime during the consent process. The average time for the 2 consent procedures was similar.
A review of 12 empirical studies of enhanced medical research consent26 indicated skepticism about the value of such multimedia tools, but our results demonstrate that multimedia tools can be effective when they are appropriately integrated into the overall consent process. Thus, this was not one of human interaction vs technology but rather of human interaction vs such interaction aided by technology. It is noteworthy that the mean score of the schizophrenia patients on MacCAT-CR understanding Trial 3 in the multimedia consent group was similar to that of HCSs on MacCAT-CR understanding Trial 1 in the routine consent group. This suggests that, by using multimedia procedures and a repetition of missed information, the understanding of consent-related information in many schizophrenia patients can be brought to the level common among HCSs who are given routine consent. Thus, a number of people who have inadequate comprehension of a research protocol when the consent-relevant information is provided by the usual means may show adequate comprehension if they are provided the same information in a user-friendly manner.
The present study has several limitations. Because decisional capacity is a context-dependent construct,50 our results may not generalize to situations involving different populations or protocols. A second potential concern is the feasibility of producing multimedia consent materials routinely; however, most PC's are now shipped with DVD-read/write drives, digital video cameras are available for under $500, and affordable and user-friendly software for high-quality home DVD production and editing is widely accessible. Another potential limitation is that we evaluated consent procedure effectiveness in the context of a hypothetical protocol. The ecologic validity of the findings could be greater with a real study, eg, people may agree to participate in a hypothetical study but not do so in real-life situations or vice versa.51,52 We were mindful of this limitation at the outset but felt the advantages of a hypothetical scenario outweighed its disadvantages. A study examining decision-making capacity for research found no differences with real vs hypothetical protocols.51 Moreover, if we had limited this study to an existing double-blind, placebo-controlled trial, the characteristics of the subjects in the consent study would have been restricted by the exclusion/inclusion criteria for the particular protocol selected. Such narrow enrollment criteria, common to most large-scale trials, lead to highly biased sample selection51 and would have limited the generalizability of our results. Furthermore, use of hypothetical scenarios allows replication of findings with the same methods. Finally, to increase the ecological validity of our study, we selected a hypothetical protocol based on an actual one that had been previously approved by the IRB and was being conducted as a pilot study. The scenario of our subjects considering participation in RCTs for cognitive deficits associated with their illness (for schizophrenia patients) or with normal aging (for HCSs) was realistic.
Another potential limitation of the present study is that the sample size was selected to provide 80% power. Although this level is widely used in clinical research, there nonetheless remains a possibility of an elevated type II error. With a larger sample, additional statistically significant differences (such as differences in the effectiveness of the consent formats among HCSs) might emerge. In general, however, examination of the effect sizes in table 2 suggests that even if additional significant differences among HCSs consent conditions emerged with a larger sample size, the magnitude of such differences of meaningful clinical significance would seem to be greater among the patients with schizophrenia than among HCSs. This appears at least partially attributable to a ceiling effect because HCSs performed relatively well under routine consent conditions. This finding is consistent with the conclusions of Flory and Emanuel,26 who suggested that enhanced consent procedures may be most valuable among neuropsychiatric or other groups at risk for impaired comprehension under routine consent conditions.
We did not find a significant effect of multimedia consent procedure in HCSs. This appears at least partially attributable to a ceiling effect because HCSs performed well at baseline. Among patients, there was no significant difference between the 2 consent procedures on MacCAT-CR appreciation or reasoning subscale scores. This could be a result of the small number of questions and consequent narrow range of scores on these subscales or may reflect the dependence of the abilities involved in appreciation and reasoning on cognitive skills that are unlikely to be improved by better presentation of study-related information.
One question is whether the full multimedia presentation is needed to effectively enhance consent capacity. As we and others have demonstrated, comprehension can be improved to some degree by procedures as simple as corrective feedback,20,21,53,54 and Powerpoint-augmented presentations,23,24,40 or educational sessions.24,55 Because of the context-specific nature of decisional capacity, it is difficult to compare the effectiveness of different techniques used in different studies—ie, a technique that is effective in one population or for one type of research protocol or method may be less suitable in another context.26 Given the dearth of multimedia-based consent research, we designed the present study as a test of the efficacy of the full multimedia technique in one of the most vulnerable groups (older people with schizophrenia) who were presented with a high-risk, high-complexity hypothetical research protocol, rather than seeking to compare the efficacy of varied ways of enhancing consent. Follow-up studies including comparisons of different types of enhanced consent are clearly warranted.
Despite the above interpretative caution, we believe that there is value added by a multimedia disclosure at least under some circumstances. Moser et al24 studied patients with schizophrenia in reference to a hypothetical (standard risk) cognitive enhancing drug trial. All the subjects completed a simulated consent procedure using routine consent methods, and then a MacCAT-CR (which itself includes reexplanation of material, thus aiding comprehension), following which 50% of the subjects received a 30-minute educational intervention to highlight key information. With this intervention, the effect size for improvement in understanding was small (Cohen d = 0.287), in contrast to the moderate effect size of d = 0.646 seen in the present study (comparing the MacCAT-CR understanding scores among the patients who received the routine vs enhanced consent procedures). We believe that this issue should not only be viewed as to which method of enhancing consent is the best one but also to develop an array or menu of choices, any one of which may prove most viable or effective in the context of a specific protocol or study population.
The present results illustrate that multimedia consent procedures are feasible and effective in improving manifest capacity to consent to research among people with SMI. The limitations of common legalistic printed consent forms have long been documented10 and are widely recognized and acknowledged by researchers, participants, and IRB members alike. The time seems ripe for innovation in the consent process because the rapid expansion in widely available multimedia tools gives more general access to investigators to move beyond written consent forms. The IRBs should encourage investigators to employ new approaches to disclosure of consent-relevant information. In that regard, given the apparent added value of multimedia consent procedures, investigators conducting clinical research involving complex, high-risk protocols in potentially vulnerable subjects should strongly consider adding multimedia-aided consent procedures to the overall consent discussion and process to ensure adequate comprehension of that information by each participant. Multimedia consent is intended not to supplant, but rather to supplement, the researcher-participant in-person interaction involved in the consent process. As explained in the Methods section, no potential participants were excluded from the present study on the basis of impaired consent capacity because a lower level of decisional capacity is generally required to provide meaningful consent to a minimal risk and procedurally simple study than may be required for a complex and higher risk RCT.37 However, because some studies specifically target very low-functioning persons, the possibility that multimedia tools may have particular value in enhancing consent (or at least meaningful assent) capacity in lower functioning individuals warrants specific empirical attention.
Funding
National Institutes of Health (MH067902, T32 MH019934, P30 MH66248, R01 MH64722, R01 AG28827); The Department of Veterans Affairs.
References
- 1.Carpenter WT, Vasi H. NBAC process and recommendations: a critique from clinician investigators. Biolaw. 1999;2:S412–S416. [Google Scholar]
- 2.Fins JJ, Miller FG. Enrolling decisionally incapacitated subjects in neuropsychiatric research. CNS Spectr. 2000;5:32–42. doi: 10.1017/s1092852900007653. [DOI] [PubMed] [Google Scholar]
- 3.Roberts LW. Evidence-based ethics and informed consent in mental illness research. Arch Gen Psychiatry. 2000;57:540–542. doi: 10.1001/archpsyc.57.6.540. [DOI] [PubMed] [Google Scholar]
- 4.Shore D. Ethical issues in schizophrenia research: a commentary on some current concerns. Schizophr Bull. 2006;32:26–29. doi: 10.1093/schbul/sbj031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shore D, Hyman SE. An NIMH commentary on the NBAC report. Biol Psychiatry. 1999;46:1013–1016. doi: 10.1016/s0006-3223(99)00228-0. [DOI] [PubMed] [Google Scholar]
- 6.McCubbin M, Weisstub D. Toward a pure best interests model of proxy decision making for incompetent psychiatric patients. Int J Law Psychiatry. 1998;21:1–30. doi: 10.1016/s0160-2527(97)00056-3. [DOI] [PubMed] [Google Scholar]
- 7.Palmer BW. Informed consent for schizophrenia research: what is an investigator (or IRB) to do? Behav Sci Law. 2006;24:447–452. doi: 10.1002/bsl.695. [DOI] [PubMed] [Google Scholar]
- 8.Karlawish JH. Research involving cognitively impaired adults. N Engl J Med. 2003;348:1389–1392. doi: 10.1056/NEJMsb030172. [DOI] [PubMed] [Google Scholar]
- 9.Mayer RE. Multimedia Learning. New York, NY: Cambridge University Press; 2001. [Google Scholar]
- 10.Epstein LC, Lasagna L. Obtaining informed consent: form or substance. Arch Intern Med. 1969;123:682–688. [PubMed] [Google Scholar]
- 11.Roth LH, Lidz CW, Meisel A, et al. Competency to decide about treatment or research: an overview of some empirical data. Int J Law Psychiatry. 1982;5:29–50. [PubMed] [Google Scholar]
- 12.Appelbaum PS, Grisso T, Frank E, O'Donnell S, Kupfer DJ. Competence of depressed patients to consent to research. Am J Psychiatry. 1999;156:1380–1384. doi: 10.1176/ajp.156.9.1380. [DOI] [PubMed] [Google Scholar]
- 13.Candilis PJ, Geppert CM, Fletcher KE, Lidz CW, Appelbaum PS. Willingness of subjects with thought disorder to participate in research. Schizophr Bull. 2006;32:159–165. doi: 10.1093/schbul/sbj016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter WT, Gold JM, Lahti AC, et al. Decisional capacity for informed consent in schizophrenia research. Arch Gen Psychiatry. 2000;57:533–538. doi: 10.1001/archpsyc.57.6.533. [DOI] [PubMed] [Google Scholar]
- 15.Grisso T, Appelbaum PS. The MacArthur Treatment Competence Study. III. Abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav. 1995;19:149–174. doi: 10.1007/BF01499323. [DOI] [PubMed] [Google Scholar]
- 16.Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: an overview. Schizophr Bull. 2006;32:121–128. doi: 10.1093/schbul/sbj001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moser D, Schultz S, Arndt S, et al. Capacity to provide informed consent for participation in schizophrenia and HIV research. Am J Psychiatry. 2002;159:1201–1207. doi: 10.1176/appi.ajp.159.7.1201. [DOI] [PubMed] [Google Scholar]
- 18.Palmer BW, Dunn LB, Appelbaum PS, Jeste DV. Correlates of treatment-related decision-making capacity among middle-aged and older patients with schizophrenia. Arch Gen Psychiatry. 2004;61:230–236. doi: 10.1001/archpsyc.61.3.230. [DOI] [PubMed] [Google Scholar]
- 19.Palmer BW, Dunn LB, Appelbaum PS, et al. Assessment of capacity to consent to research among older persons with schizophrenia, Alzheimer disease or diabetes mellitus: comparison of a three-item questionnaire with a comprehensive standardized capacity instrument. Arch Gen Psychiatry. 2005;62:726–733. doi: 10.1001/archpsyc.62.7.726. [DOI] [PubMed] [Google Scholar]
- 20.Palmer BW, Jeste DV. Relationship of individual cognitive abilities to specific components of decisional capacity among middle-aged and older patients with schizophrenia. Schizophr Bull. 2006;32:98–106. doi: 10.1093/schbul/sbj002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer BW, Dunn LB, Depp C, Eyler LT, Jeste DV. Decisional capacity to consent to research among patients with bipolar disorder: comparison with schizophrenia patients and healthy subjects. J Clin Psychiatry. 2007;68:689–696. doi: 10.4088/jcp.v68n0505. [DOI] [PubMed] [Google Scholar]
- 22.Stroup S, Appelbaum P, Swartz M, et al. Decision-making capacity for research participation among individuals in CATIE schizophrenia trial. Schizophr Res. 2005;80:1–8. doi: 10.1016/j.schres.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Dunn LB, Lindamer LA, Palmer BW, Golshan S, Schneiderman LJ, Jeste DV. Improving understanding of research consent in middle-aged and elderly patients with psychotic disorders. Am J Geriatr Psychiatry. 2002;10:142–150. [PubMed] [Google Scholar]
- 24.Moser DR. Using a brief intervention to improve decisional capacity in schizophrenia research. Schizophr Bull. 2006;32:116–120. doi: 10.1093/schbul/sbi066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wirshing DA, Sergi MJ, Mintz J. A videotape intervention to enhance the informed consent process for medical and psychiatric treatment research. Am J Psychiatry. 2005;165:186–188. doi: 10.1176/appi.ajp.162.1.186. [DOI] [PubMed] [Google Scholar]
- 26.Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. JAMA. 2004;292:1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 27.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 28.Christensen H, Kumar R. Cognitive changes and the ageing brain. In: Sachdev PS, editor. The Ageing Brain: The Neurobiology and Neuropsychiatry of Ageing. Lisse, The Netherlands: Swets & Zeitlinger; 2003. pp. 75–95. [Google Scholar]
- 29.Palmer BW, Savla GN. The association of specific neuropsychological deficits with capacity to consent to research or treatment. J Int Neuropsychol Soc. 2007;13:1047–1059. doi: 10.1017/S1355617707071299. [DOI] [PubMed] [Google Scholar]
- 30.Dunn LB, Jeste DV. Enhancing informed consent for research and treatment. Neuropsychopharmacology. 2001;24:595–607. doi: 10.1016/S0893-133X(00)00218-9. [DOI] [PubMed] [Google Scholar]
- 31.Moye J, Marson DC. Assessment of decision-making capacity in older adults: an emerging area of practice and research. J Gerontol B Psychol Sci Soc Sci. 2007;62:P3–P11. doi: 10.1093/geronb/62.1.p3. [DOI] [PubMed] [Google Scholar]
- 32.Dunn LB, Lindamer LA, Palmer BW, Schneiderman LJ, Jeste DV. Enhancing comprehension of consent for research in older patients with psychosis: a randomized study of a novel educational strategy. Am J Psychiatry. 2001;158:1911–1913. doi: 10.1176/appi.ajp.158.11.1911. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- 34.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 35.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 36.UCSD Human Research Protections Program. Decision making capacity guidelines. 2003; 2004 Available at: http://irb.ucsd.edu/decisional.shtml. Accessed December 17, 2007. [Google Scholar]
- 37.Saks ER, Dunn LB, Palmer BW. Meta-consent in research on decisional capacity: a “Catch-22”? Schizophr Bull. 2006;32:42–46. doi: 10.1093/schbul/sbj017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SY, Caine ED, Swan JG, Appelbaum PS. Do clinicians follow a risk-sensitive model of capacity-determination? An experimental video survey. Psychosomatics. 2006;47:325–329. doi: 10.1176/appi.psy.47.4.325. [DOI] [PubMed] [Google Scholar]
- 39.Baddeley A. Working Memory, Thought, and Action. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 40.Mittal D, Palmer BW, Dunn LB, et al. Comparison of two enhanced consent procedures for patients with mild Alzheimer disease or mild cognitive impairment. Am J Geriatr Psychiatry. 2007;15:167. doi: 10.1097/JGP.0b013e31802dd379. [DOI] [PubMed] [Google Scholar]
- 41.Jeste DV, Palmer BW, Appelbaum PS, et al. A new brief instrument for assessing decisional capacity for clinical research. Arch Gen Psychiatry. 2007;64:966–974. doi: 10.1001/archpsyc.64.8.966. [DOI] [PubMed] [Google Scholar]
- 42.Appelbaum PS, Grisso T. MacCAT-CR: MacArthur Competence Assessment Tool for Clinical Research. Sarasota, FL: Professional Resource Press; 2001. [Google Scholar]
- 43.Kay S, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 44.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 45.Randolph C. RBANS—Manual—Repeatable Battery for the Assessment of Neuropsychological Status. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 46.Dunn LB, Nowrangi MA, Palmer BW, Jeste DV, Saks ER. Assessing decisional capacity for clinical research or treatment: a review of instruments. Am J Psychiatry. 2006;163:1323–1334. doi: 10.1176/ajp.2006.163.8.1323. [DOI] [PubMed] [Google Scholar]
- 47.Dunn LB, Palmer BW, Appelbaum PS, Saks ER, Aarons GA, Jeste DV. Prevalence and correlates of adequate performance on a measure of abilities related to decisional capacity: differences among three standards for the MacCAT-CR in patients with schizophrenia. Schizophr Res. 2007;89:110–118. doi: 10.1016/j.schres.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kraemer HC. Tests of homogeneity of independent correlation coefficients. Psychometrika. 1979;44:329–335. [Google Scholar]
- 49.Saks ER, Dunn LB, Marshall BJ, Nayak GV, Golshan S, Jeste DV. The California Scale of Appreciation: a new instrument to measure the appreciation component of capacity to consent to research. Am J Geriatr Psychiatry. 2002;10:166–174. [PubMed] [Google Scholar]
- 50.Appelbaum PS, Roth LH. Competency to consent to research: a psychiatric overview. Arch Gen Psychiatry. 1982;39:951–958. doi: 10.1001/archpsyc.1982.04290080061009. [DOI] [PubMed] [Google Scholar]
- 51.Schain WS. Barriers to clinical trials: knowledge and attitudes of potential participants. Cancer. 1994;74(suppl 9):2666–2671. doi: 10.1002/1097-0142(19941101)74:9+<2666::aid-cncr2820741814>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 52.Verheggen FWSM, van Wijmen FCB. Informed consent in clinical trials. Health Policy. 1996;36:131–153. doi: 10.1016/0168-8510(95)00805-5. [DOI] [PubMed] [Google Scholar]
- 53.Palmer BW, Cassidy EL, Dunn LB, Spira AP, Sheikh JI. Simple yet effective: questioning research participants during initial presentation of consent forms facilitates the consent process. IRB: Ethics Hum Res. In press. [Google Scholar]
- 54.Taub HA, Kline GE, Baker MT. The elderly and informed consent: effects of vocabulary level and corrected feedback. Exp Aging Res. 1981;7:137–146. doi: 10.1080/03610738108259796. [DOI] [PubMed] [Google Scholar]
- 55.Wirshing DA, Wirshing WC, Marder SR, Liberman RP, Mintz J. Informed consent: assessment of comprehension. Am J Psychiatry. 1998;155:1508–1511. doi: 10.1176/ajp.155.11.1508. [DOI] [PubMed] [Google Scholar]
- 56.Acion L, Peterson JJ, Temple S, Arndt S. Probabilistic index: an intuitive non-parametric approach to measuring the size of treatment effects. Stat Med. 2006;25:591–602. doi: 10.1002/sim.2256. [DOI] [PubMed] [Google Scholar]