Abstract
Objective: Providing incentives for research participation is widely practiced but minimally studied. In schizophrenia research, questions about capacity to consent and potential vulnerability may raise concerns when offering incentives for participation. Despite empirical attention focused on consent and decision-making capacity in schizophrenia, the issue of incentives has been essentially ignored. We examined willingness to participate in research, in relation to perceived risks and benefits, among people with schizophrenia and schizoaffective disorder. Method: Forty-six people with schizophrenia or schizoaffective disorder rated perceived risks and benefits of 5 hypothetical research vignettes. They also indicated whether they would be willing to participate at each of 5 incentive levels (including no compensation). Cognition was assessed with Mattis Dementia Rating Scale. Results: Ratings of risk and potential personal benefit were inversely correlated. For all scenarios, significant correlations were found between perceived risk and willingness to participate for greater compensation. Conversely, lower perceived likelihood of benefit was associated with a higher compensation threshold for participation in each scenario. Even at the highest proffered payment level for each scenario, however, a substantial proportion of respondents were not willing to participate. Risk assessment and willingness to participate (at all levels of compensation) were not associated with demographic variables or cognitive status. Conclusions: Determining whether incentives impede voluntarism remains an important task for empirical ethics research. Assessing potential research participants’ understanding and perceptions of risks, benefits, and alternatives to participation will help ensure that informed consent fulfills its mission—embodying the ethical principle of respect for persons.
Keywords: ethics, informed consent, research participation, incentives, risk perception, voluntarism
Introduction
Clinical research hinges, in part, upon recruitment of adequate numbers of volunteers who willingly forego standard treatments to help investigators in their efforts to learn more about illness and to improve the health and well-being of future patients. These volunteers deserve—and public trust in biomedical research relies upon—unswerving commitment to the ethical principles and protocol safeguards that must be upheld by investigators, institutions, and review boards.
Recruitment of research participants is a focus of increased scrutiny. Specific concerns relate to pressures from industry on investigators and recruiters, the potential for investigator conflict of interest, “professional” research subjects, and the potential for payments to serve as “undue inducements.”1–4 Yet, limited empirical data exist to help clarify these important issues.5
Financial incentives for clinical research participation, though widespread,6 remain controversial due to concerns that payment goes beyond compensation for time and inconvenience—serving rather as a direct incentive that could induce people to accept risks that they otherwise would not—and that incentives may disproportionately entice the socioeconomically disadvantaged, violating the principle of justice.7–11 Moreover, a key question is whether larger incentives affect or even impair participants’ judgment or reasoning about research risks10,12—eg, common psychological processes such as cognitive dissonance could influence perceived risk when more money is offered.
Empirical studies of the effects of compensation on research-related decision making by different populations are few.12 Two studies in nonpsychiatric populations suggest that although higher amounts of compensation serve as incentives, they do not cause people to overlook research risks.13,14 When persons with serious mental illnesses are recruited for clinical research, undue inducement may become a particularly salient issue.15 Relative to the general population, those with schizophrenia are at greater risk for impaired consent capacity,16 may be more vulnerable due to economic circumstances,17 and often have cognitive impairments that conceivably could affect risk assessments.18 For these reasons, incentives for participation by persons in this group raise ethical concerns.15,19–21 Given the large numbers of clinical trials enrolling people with serious mental illnesses and the substantial public health contributions deriving from these research endeavors, this topic carries immense importance.
Although over a decade of empirical work has illuminated the considerable heterogeneity of research-related decision-making abilities among people with schizophrenia,22–24 little is known about how incentives influence their research decisions.15,17,25,26 Moreover, despite emerging work examining perspectives on research risks among people with serious mental illnesses18,21,25,27—amidst a backdrop of decades of work on risk perception, cognitive biases, and influences on choice28—to our knowledge, no previous study has jointly assessed perspectives of people with schizophrenia on risks, benefits, and willingness to participate in reference to financial compensation.
We describe here a study in people with schizophrenia and schizoaffective disorder, in which we examined the relationships among perceived research risks, perceived benefits, and willingness to participate in a series of research vignettes, presented in the context of varying levels of payment. Based on an economic model of decision making (which is also consistent with the classic tenets of social cognitive theory),12 we hypothesized that schizophrenia patients’ willingness to participate would decrease with increased risk and increase with level of perceived benefit (including monetary or clinical benefits) and as the subjective probability of costs (whether physical, psychological, or monetary) decrease. We also examined interactions among perceived risk, benefit, and payment on willingness to participate, but given the dearth of prior empirical research on the interaction among motivating factors for research participation, the latter analyses were exploratory in nature (ie, we did not have a priori hypotheses about the specific presence and nature of such interactions). In addition, we examined participants’ overall attitudes toward research, general interest in research participation, and level of trust in researchers, hypothesizing that increased willingness to participate at lower levels of compensation would be associated with positive attitudes toward research and trust in researchers, as well as general interest in research participation.
Methods
This project was approved by the Institutional Review Board of University of California, San Diego, as part of a larger study of informed consent for research, focused on middle-aged and older people with schizophrenia.29 All participants resided in board-and-care facilities in the San Diego area, had a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, diagnosis of schizophrenia or schizoaffective disorder, and were 40 years or older. (Schizophrenia and schizoaffective disorder were combined in the present sample because the bulk of empirical evidence indicates no substantive differences in terms of cognitive functioning, functional disability, and/or decision making; indeed, there exists more heterogeneity within and between schizophrenia clinical subtypes than between schizophrenia and schizoaffective disorder).30
Five research vignettes, modeled on previous work by Robert et al,25 described briefly (<90 words) hypothetical research scenarios, including 1 minimal risk protocol (as defined in applicable federal regulations) involving a blood draw and 4 greater than minimal risk studies. These were presented in the following order for each participant: (1) blood draw, (2) safety-related drug trial, (3) efficacy-related drug trial, (4) lumbar puncture, and (5) symptom provocation protocol (see table 2 for more details). Each scenario was read aloud, and participants were handed a large-font, printed card with the same description.
Table 2.
Risk and Benefit Ratings and Correlations With Willingness/Compensation Threshold (n = 46)
| Correlation Coefficients (Spearman ρ) |
|||||
| Hypothetical Research Scenario Descriptions | Risk Ratinga, Mean (SD) | Benefit Ratingb, Mean (SD) | Correlation Between Risk and Benefit Ratings | Correlation Between Risk and Willingness/Compensation Threshold | Correlation Between Benefit and Willingness/Compensation Threshold |
| 1—Goal: “learn more about schizophrenia.” Blood draw only. Possible discomfort, very small chance of infection (minimal risk study) | 3.91 (1.21) | 2.46 (1.50) | −.190, P = .207 | −.389, P = .008 | .502, P < .001 |
| 2—Goal: safety study of new experimental medication for schizophrenia. Six weeks on new experimental medication instead of usual psychiatric medications. Not previously tested in people. Believed to be safe. Believe it “may help” symptoms. “Potential risk of serious medical complications.” (greater than minimal risk study) | 2.74 (1.37) | 3.09 (1.49) | −.432, P = .003 | −.604, P < .001 | .753, P < .001 |
| 3—Goal: efficacy study of new experimental medication for schizophrenia. Six weeks on experimental medication instead of usual medications. Previously shown safe for most people, but 10% of people in previous studies have gained significant weight (risk for future health). (greater than minimal risk study) | 2.57 (1.39) | 2.85 (1.56) | −.602, P < .001 | −.599, P < .001 | .709, P < .001 |
| 4—Goal: “learn more about schizophrenia.” Spinal tap (procedure and risks described). (greater than minimal risk study) | 2.11 (1.46) | 3.73 (1.56) | −.401, P = .006 | −.392, P = .008 | .651, P < .001 |
| 5—Goal: “learn more about schizophrenia.” Take experimental drug that “causes temporary symptoms of schizophrenia for a few hours.” (greater than minimal risk study) | 2.20 (1.36) | 3.47 (1.69) | −.689, P < .001 | −.512, P < .001 | .683, P < .001 |
Perception of risk was rated on the following scale: 1 = “Very risky” to 5 = “Very safe.”
Perception of likelihood of personal benefit was rated as follows: 1 = “Very likely” to 5 = “Very unlikely.”
Using anchored 5-point Likert rating scales, also printed in large font and handed to participants, subjects rated how risky and personally beneficial they believed each protocol would be. Willingness to participate (yes, no, maybe) was assessed for each of 5 incrementally higher levels of compensation (asked aloud, one option at a time): no money, $50, $100, $200, and $500 (for scenarios 2–5) and $0, $5, $10, $50, and $100 (for scenario 1). (The blood draw compensation values were lower to maintain credibility of compensation values for this minimal risk scenario.) Open-ended questions were posed for each scenario to gather additional information about the reasoning behind participants’ decisions.
A willingness/compensation threshold—the lowest level of payment, for each scenario, at which the subject indicated willingness to participate—was coded as follows for scenarios 2–5: 0 = willing to participate for no payment; 1 = not willing for no payment, willing for $50; 2 = not willing for $50, willing for $100; 3 = not willing for $100, willing for $200; 4 = not willing for $200, willing for $500; 5 = not willing, even at $500. For scenario 1 (blood draw), the amounts corresponding to each of these categories were no money, $5, $10, $50, and $100. Responses of “Unsure” were categorized as “Unwilling” for the purpose of this categorization.
Three questions inquired about level of interest in research participation, trust in researchers, and prior research experience. All participants were administered Mattis Dementia Rating Scale (DRS)31, a standardized measure of cognitive impairment. The DRS total score provides a global evaluation of cognitive impairment; lower scores indicate worse cognitive deficits.
Statistical Analysis
Within each scenario, bivariate correlations (Spearman rank correlation) were calculated to examine the relationships of the willingness/compensation threshold with demographic variables, cognitive impairment (DRS total), perceived risk, and perceived benefit for each scenario. Multivariate analyses of variance were (MANOVAs) were used to evaluate differences among protocols in risk ratings, benefit ratings, and willingness/compensation thresholds for scenarios 2–5 only. (The minimal risk, blood draw, and protocol were excluded from the MANOVA because the objective risk was substantially distinct from the other protocols and thereby would likely have disproportionately affected the overall results of that analysis.) Significance level (2 tailed) was defined as P < .05.
Results
Participant Characteristics
Participant demographics and research-related attitudes and experience are described in table 1. On average, patients manifested a mild degree of cognitive impairment, but consistent with prior research,32,33 there was substantial within-group heterogeneity (mean DRS total = 129, SD = 10.6). This population of patients was drawn from a larger study of middle-aged and older outpatients, whose clinical symptoms were overall in the mild range.29 Participants were receiving a median of $1000 per month in disability income, although most participants had approximately $100 per month in disposable income after board-and-care expenses.
Table 1.
Characteristics of Participants (n = 46)
| Demographics and Cognitive Characteristics | Mean (SD) | n (%) |
| Age, y | 53.4 (6.6) | |
| Education, y | 12.7 (2.3) | |
| Gender | ||
| Male | 34 (73.9) | |
| Female | 12 (26.1) | |
| Diagnosis | ||
| Schizophrenia | 37 (80.4) | |
| Schizoaffective disorder | 9 (19.6) | |
| Ethnicity | ||
| Caucasian | 37 (80.4) | |
| African American | 1 (2.2) | |
| Hispanic/Latino | 4 (8.7) | |
| Other | 4 (8.7) | |
| Monthly income, $ (n = 35) | 935 (209) | |
| Median: 1000 | ||
| DRS totala | 129.6 (10.6) | |
| Research attitudes and experiences | ||
| How interested are you personally in participating in research?b | 4.24 (1.16) | |
| How trusting are you of medical researchers?c | 4.24 (1.12) | |
| How much experience have you had in participating in research?d | 3.28 (1.46) | |
DRS = Mattis Dementia Rating Scale.
Rated from 1 =“Not interested at all” to 5 = “Very interested.”
Rated from 1 = “Do not trust them at all” to 5 = “Trust them completely.”
Rated from 1 = “None/no experience,” 2 = “1 previous study,” 3 = “2 to 3 previous studies,” 4 = “4 to 5 previous studies,” to 5 = “More than 5 previous studies.”
Research Attitudes and Experience
Participants were interested in research participation in general (mean 4.2 out of 5), trusted medical researchers (mean 4.2 out of 5), and had participated in a mean of approximately 3 previous research studies.
Risk and Benefit Perceptions
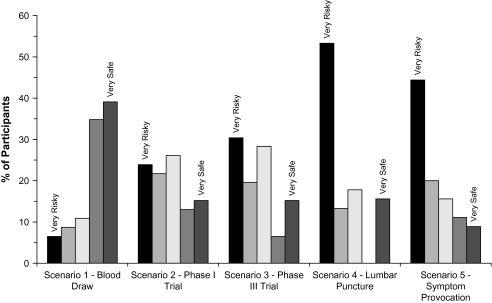
Figure 1 shows the proportion of participants endorsing each risk rating for each scenario; mean risk and benefit ratings are given in table 2. The difference in risk ratings across the 4 greater than minimal risk protocols (2–5) did not reach statistical significance (Wilks λ = 2.597, P = .065), although benefit ratings differed across these 4 scenarios (Wilks λ = 94.238, P < .001). Significant inverse correlations between mean perceived risk and benefit ratings, except for scenario 1 (the blood draw protocol), indicate that as perceived risk increased, perceived potential for personal benefit decreased (table 2).
Fig. 1.
Risk Ratings for Hypothetical Research Scenarios (n = 46).
Willingness to Participate
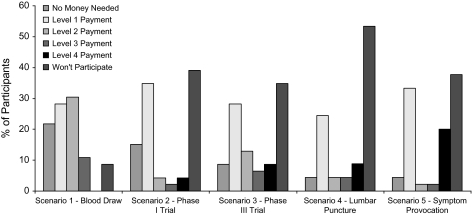
The proportion of participants at each willingness/compensation threshold level for each scenario is provided in figure 2. At the highest payment level for each vignette, the following proportions of participants were unwilling to participate (or were uncertain): scenario 1, 8.7%; scenario 2, 39.1%; scenario 3, 34.8%; scenario 4, 53.3%; and scenario 5, 37.8%. There were no significant differences in the willingness/compensation thresholds among scenarios 2–5 (Wilks λ = 2.258, P = .096) and no significant correlations between willingness/compensation thresholds (for any of the scenarios) and age, education, ethnic minority status, cognitive impairment, and income or interest, trust, or prior experience in research. Of the 46 participants, 8 (17.4%) were willing to participate in all 5 protocols for either no money or the lowest offered amount ($50) for the 4 greater than minimal risk protocols; one participant indicated willingness to participate in all 4 for no compensation. Nine (19.6%) participants refused all 4 of the greater than minimal risk protocols even for the highest amount.
Fig. 2.
Willingness/Compensation Thresholds for Hypothetical Research Scenarios (n = 46). Notes: Scenario 2–5 levels: 0 = willing to participate for no payment; 1 = not willing for no payment, willing for $50; 2 = not willing for $50, willing for $100; 3 = not willing for $100, willing for $200; 4 = not willing for $200, willing for $500; will not participate = not willing to participate even at $500. (corresponding scenario 1 payment levels: 0 = no money, 1 = $5, 2 = $10, 3 = $50, and 4 = $100).
Perceived Risk and Benefit in Relation to Willingness/Compensation Threshold
As shown in table 2, there was no significant correlation between perceived risk and perceived benefit in the minimal risk scenario (r = −.19, P = .21), but within the 4 greater than minimal risk scenarios, the level of perceived benefit was inversely related to the level of perceived risk (r's ranged from −.40 to −.69, all P's <.004). As also shown in table 2, within each of the 5 scenarios, participants’ willingness/compensation thresholds were negatively correlated with their level of perceived risk (r's ranged from −.39 to −.60, all P's <.009) and positively correlated with level of perceived benefit (r's ranged from −.50 to −.75, all P's <.001).
Discussion
This study provides preliminary evidence regarding the relationships among incentives and perceptions of risks and benefits, augmenting the existing literature concerning influences on research participation decision making by people with schizophrenia.26,34,35 Across a range of hypothetical research vignettes, both perceived risk and perceived potential personal benefit were related to the willingness/compensation threshold (the lowest level of compensation at which a subject indicated willingness to enroll in a particular protocol).
The finding that participants’ compensation thresholds correlated with perceived risk adds to a limited database on how risk and payment affect research decisions.13,14 Bentley and Thacker13 found that pharmacy students were more willing to participate if offered more money, regardless of risk level. Halpern et al14 found that hypertensive patients’ willingness to participate decreased as payment levels decreased and as (described) risks increased; these variables did not interact, suggesting that risks remained salient even at higher payment levels in these nonmentally ill individuals.
Our findings overlap those of Bentley and Thacker,13 as well as Halpern et al.14 Similar to the findings in those studies, and as hypothesized, schizophrenia patients’ compensation “thresholds” increased as perceived risks increased, suggesting that both risk and payment affected willingness to participate. Similar results were also reported by Roberts et al27 who found an inverse relationship between risk perceptions and willingness to participate among patients with schizophrenia. On the other hand, the finding that numerous participants would not participate even for substantial payments suggests that perceived risk was not disregarded even at high payment levels. The proportion declining was substantial (ranging from 35% to 53%) for all protocols except the blood draw, suggesting that at least some of these participants were sensitive to protocols they perceived as conferring greater or unknown risks.
The association of perceived potential benefit with willingness/compensation thresholds suggests that participants weigh not only risk but also perceive direct benefit. It was notable, however, that although protocols were only briefly described and were generally vague about potential for personal benefit, the mean benefit ratings ranged from 2.5 to 3.7 (1 = highest perceived likelihood of personal benefit). Means in this range may represent uncertainty or a belief that personal benefit is a matter of chance. Because of limited data regarding underlying reasons for responses, we cannot speculate to what degree the subjects may have been responding to wording, reacting to the description of risks, or responding to some other unmeasured variables. Nevertheless, these findings should, at minimum, be viewed in the context of a substantial body of literature on informed consent emphasizing the need to attend carefully to the possibility that participants may overestimate the possible personal benefits of the research—a form of therapeutic misconception.36
The inverse correlation between risk and benefit perceptions was unexpected, and we interpret this finding cautiously. In a simple conceptualization of risk:benefit ratios, risks and benefits are treated as independent values, but our data suggest that perception of one may at least partially affect the perception of the other. Definitive conclusions about this possibility require studies in which the risk or benefit description is held constant while varying the other component. Nonetheless, given the general nature of the potential benefit descriptions, it is possible that the descriptions of risk were interpreted via a framing effect—in other words, benefits seemed lower in the context of higher risk studies. This would not be surprising given the literature on how framing effects can alter perceptions and decision making.37 It is unclear whether people with schizophrenia are more likely to be influenced by framing effects or other common sources of errors in reasoning, with several studies providing conflicting findings.38,39
As illustrated in figure 2, there was a bimodal pattern of willingness responses for all 4 greater than minimal risk protocols. This bimodal pattern of “willingness distributions” raises the possibility that patients might have a priori inclinations about participating in greater than minimal risk medical research. There was little evidence of people willing to participate for no compensation; in contrast, that nearly 20% of the sample would not participate in any of the 4 greater than minimal risk protocols is a finding that merits further exploration in diverse clinical populations. We found no correlation, however, between “trust” in researchers and willingness/compensation thresholds.
The finding that approximately two-thirds of the participants rated the lumbar puncture (66.7%) and symptom provocation (64.4%) studies as risky or very risky parallels research from Roberts et al,18 in which lumbar puncture and symptom provocation vignettes received the highest mean risk ratings by people with schizophrenia. It is possible that heuristic biases play a role in such perceptions: invasiveness (in the case of lumbar puncture) may be more salient, as may the immediacy of symptom provocation (as opposed to the possibility of future medical complications). It is also possible that previous experiences and/or framing of the information, although unintended, biased participants in their risk perceptions. It is also possible that familiarity with specific negative outcomes may affect perceptions of risks related to various types of procedures used in research.
This study's limitations include the use of hypothetical, briefly described research protocols. The conditions likely resembled an initial recruitment discussion more than an actual consent procedure. Empirical studies suggest, however, that most research participants, however, decide whether to enroll before the formal review of the printed consent form.40,41 Recruitment strategies frequently use only brief descriptions of studies—which often mention compensation or “reimbursement.” Therefore, brief scenario descriptions may have ecological validity.
While we cannot make inferences about whether patients’ willingness to participate for different incentive levels would substantially differ from that of healthy persons, due to the absence of a nonpsychiatrically ill comparison group, patients with psychotic disorders are nevertheless a particularly important group in which to examine these relationships. In addition to the primary psychotic symptoms characterizing these illnesses, impairments in cognition and everyday functioning are highly prevalent.32,42,43 Although the present data suggest that ability to distinguish among risk levels—at least in terms of gross risk categories—may be less influenced by cognition, future research should examine the role of more specific cognitive functions, such as those under the rubric of “executive functioning.”24 In the absence of empirical data, it is difficult to be certain precisely how deficits in executive functioning, as well as perceptions and weighing of risks and benefits, may interact and influence willingness to participate. Although we did not measure psychiatric symptoms (in order to reduce the interview burden on participants), this sample consisted of stable, chronically ill outpatients with mild-moderate symptom severity who had participated in the parent study of consent.29,44
Although findings from these individuals may not generalize to the wider population of patients with schizophrenia who consider enrolling in clinical trials, over two-thirds stated that they had previously participated in at least 2 other research studies. These participants, all board-and-care residents, were uniformly receiving modest fixed incomes; thus, further information on the interaction of economic pressures, perceived risks and benefits, and financial incentives should compare people with different income levels (as was done by Halpern et al14). Finally, the hypothetical scenarios were presented in a standard order to participants, and payment levels were presented in increasing order. Random presentation may have resulted in different findings.
These findings, taken together, offer a mixed picture—providing general reassurance while also indicating the potential for financial payments to motivate a subset of participants with schizophrenia to participate in studies they consider risky. Respect for persons, part of the foundation of ethical conduct of research,45 involves supporting autonomous decision making by potential research participants, as well as protecting those with diminished autonomy. Numerous studies have consistently shown that, while the diagnosis of schizophrenia increases risk for impaired capacity, there is considerable within-group heterogeneity. Participant characteristics beyond diagnosis, particularly cognitive functioning, are more salient considerations when considering risk for capacity impairments.19,24,46 The present observations similarly argue against categorically viewing people with schizophrenia as easily induced to enroll in risky protocols. Also, investigators and IRBs, in designing and reviewing recruitment procedures, consent documents, and incentives, should pay heightened attention to how protocols may be perceived by those considering participation, rather than simply assessing risks and benefits through the federally mandated lens of “minimal risk” or “greater than minimal risk.” Because these perceptions are among the important factors affecting willingness to participate, they are highly relevant to discussions about autonomy and incentives.
Funding
National Institute of Mental Health (Career Development Award MH66062 to L.B.D.); National Institute of Mental Health (R01 MH64722 to B.W.P., NIA R01 AG028827 to B.W.P.).
References
- 1.DeRenzo EG. Coercion in the recruitment and retention of human research subjects, pharmaceutical industry payments to physician-investigators, and the moral courage of the IRB. IRB. 2000;35(4):730–737. [PubMed] [Google Scholar]
- 2.Department of Health and Human Services Office of Inspector General. Recruiting Human Subjects: Pressures in Industry-Sponsored Clinical Research. OEI-01-97-00195. 2000. http://oig.hhs.gov/oei/reports/oei-01-97-00195.pdf. [Google Scholar]
- 3.Pace C, Miller FG, Danis M. Enrolling the uninsured in clinical trials: an ethical perspective. Crit Care Med. 2003;31(suppl 3):S121–S125. doi: 10.1097/01.CCM.0000054907.33928.48. [DOI] [PubMed] [Google Scholar]
- 4.Rosenstein DL, Miller FG. Ethical considerations in psychopharmacological research involving decisionally impaired subjects. Psychopharmacology (Berl) 2003;171(1):92–97. doi: 10.1007/s00213-003-1503-1. [DOI] [PubMed] [Google Scholar]
- 5.Gross CP, Mallory R, Heiat A, Krumholz HM. Reporting the recruitment process in clinical trials: who are these patients and how did they get there? Ann Intern Med. 2002;137(1):10–16. doi: 10.7326/0003-4819-137-1-200207020-00007. [DOI] [PubMed] [Google Scholar]
- 6.Grady C, Dickert N, Jawetz T, Gensler G, Emanuel E. An analysis of U.S. practices of paying research participants. Contemp Clin Trials. 2005;26(3):365–375. doi: 10.1016/j.cct.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Macklin R. ‘Due’ and ‘undue’ inducements: on paying money to research subjects. IRB. 1981;3(5):1–6. [PubMed] [Google Scholar]
- 8.Emanuel EJ. Ending concerns about undue inducement. J Law Med Ethics. 2004;32(1):100–105. doi: 10.1111/j.1748-720x.2004.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 9.Nelson RM, Merz JF. Voluntariness of consent for research: an empirical and conceptual review. Med Care. 2002;40(suppl 9):V69–V80. doi: 10.1097/01.MLR.0000023958.28108.9C. [DOI] [PubMed] [Google Scholar]
- 10.Grant RW, Sugarman J. Ethics in human subjects research: do incentives matter? J Med Philos. 2004;29(6):717–738. doi: 10.1080/03605310490883046. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson M, Moore A. Inducement in research. Bioethics. 1997;11(5):373–389. doi: 10.1111/1467-8519.00078. [DOI] [PubMed] [Google Scholar]
- 12.Dunn LB, Gordon NE. Improving informed consent and enhancing recruitment for research by understanding economic behavior. JAMA. 2005;293:609–612. doi: 10.1001/jama.293.5.609. [DOI] [PubMed] [Google Scholar]
- 13.Bentley JP, Thacker PG. The influence of risk and monetary payment on the research participation decision making process. J Med Ethics. 2004;30(3):293–298. doi: 10.1136/jme.2002.001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halpern SD, Karlawish JH, Casarett D, Berlin JA, Asch DA. Empirical assessment of whether moderate payments are undue or unjust inducements for participation in clinical trials. Arch Intern Med. 2004;164(7):801–803. doi: 10.1001/archinte.164.7.801. [DOI] [PubMed] [Google Scholar]
- 15.Shore D. Ethical issues in schizophrenia research: a commentary on some current concerns. Schizophr Bull. 2006;32(1):26–29. doi: 10.1093/schbul/sbj031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appelbaum PS. Decisional capacity of patients with schizophrenia to consent to research: taking stock. Schizophr Bull. 2006;32(1):22–25. doi: 10.1093/schbul/sbi063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marson DC, Phillips J. Financial capacity in persons with schizophrenia and severe mental illness: clinical and ethical aspects. Schizophr Bull. 2006;32(1):81–91. doi: 10.1093/schbul/sbj027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts LW, Warner TD, Hammond KG, Dunn LB. Assessments by patients with schizophrenia and psychiatrists of relative risk of research procedures. Psychiatr Serv. 2006;57(11):1629–1635. doi: 10.1176/ps.2006.57.11.1629. [DOI] [PubMed] [Google Scholar]
- 19.Dunn LB, Candilis PJ, Roberts LW. Emerging empirical evidence on the ethics of schizophrenia research. Schizophr Bull. 2006;32(1):47–68. doi: 10.1093/schbul/sbj012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts LW. Informed consent and the capacity for voluntarism. Am J Psychiatry. 2002;159(5):705–712. doi: 10.1176/appi.ajp.159.5.705. [DOI] [PubMed] [Google Scholar]
- 21.Roberts LW, Dunn LB, Green Hammond KA, Warner TD. Do research procedures pose relatively greater risk for healthy persons than for persons with schizophrenia? Schizophr Bull. 2006;32(1):153–158. doi: 10.1093/schbul/sbi055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grisso T, Appelbaum PS. The MacArthur Treatment Competence Study. III: abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav. 1995;19(2):149–174. doi: 10.1007/BF01499323. [DOI] [PubMed] [Google Scholar]
- 23.Roberts LW, Roberts B. Psychiatric research ethics: an overview of evolving guidelines and current ethical dilemmas in the study of mental illness. Biol Psychiatry. 1999;46(8):1025–1038. doi: 10.1016/s0006-3223(99)00205-x. [DOI] [PubMed] [Google Scholar]
- 24.Palmer BW, Savla GN. The association of specific neuropsychological deficits with capacity to consent to research or treatment. J Int Neuropsychol Soc. 2007;13(6):1047–1059. doi: 10.1017/S1355617707071299. [DOI] [PubMed] [Google Scholar]
- 25.Roberts LW, Warner TD, Brody JL, Roberts B, Lauriello J, Lyketsos C. Patient and psychiatrist ratings of hypothetical schizophrenia research protocols: assessment of harm potential and factors influencing participation decisions. Am J Psychiatry. 2002;159(4):573–584. doi: 10.1176/appi.ajp.159.4.573. [DOI] [PubMed] [Google Scholar]
- 26.Roberts LW, Warner TD, Nguyen KP, Geppert CM, Rogers MK, Roberts BB. Schizophrenia patients’ and psychiatrists’ perspectives on ethical aspects of symptom re-emergence during psychopharmacological research participation. Psychopharmacology (Berl) 2003;171(1):58–67. doi: 10.1007/s00213-002-1160-9. [DOI] [PubMed] [Google Scholar]
- 27.Roberts LW, Hammond KG, Hoop J. An inverse relationship between perceived harm and participation willingness in schizophrenia research protocols. Am J Psychiatry. 2006;163(11):2002–2004. doi: 10.1176/ajp.2006.163.11.2002. [DOI] [PubMed] [Google Scholar]
- 28.Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211(4481):453–458. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- 29.Dunn LB, Palmer BW, Appelbaum PS, Saks ER, Aarons GA, Jeste DV. Prevalence and correlates of adequate performance on a measure of abilities related to decisional capacity: differences among three standards for the MacCAT-CR in patients with schizophrenia. Schizophr Res. 2007;89(1–3):110–118. doi: 10.1016/j.schres.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans JD, Heaton RK, Paulsen JS, McAdams LA, Heaton SC, Jeste DV. Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry. 1999;60(12):874–882. [PubMed] [Google Scholar]
- 31.Mattis S. Dementia Rating Scale. Odessa, FL: Psychological Assessment Resources, Inc.; 1973. [Google Scholar]
- 32.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 33.Palmer BW, Heaton RK, Paulsen JS, et al. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11(3):437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- 34.Roberts LW, Warner TD, Brody JL. Perspectives of patients with schizophrenia and psychiatrists regarding ethically important aspects of research participation. Am J Psychiatry. 2000;157(1):67–74. doi: 10.1176/ajp.157.1.67. [DOI] [PubMed] [Google Scholar]
- 35.Candilis PJ, Geppert CM, Fletcher KE, Lidz CW, Appelbaum PS. Willingness of subjects with thought disorder to participate in research. Schizophr Bull. 2006;32(1):159–165. doi: 10.1093/schbul/sbj016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lidz CW, Appelbaum PS. The therapeutic misconception: problems and solutions. Med Care. 2002;40(suppl 9):V55–V63. doi: 10.1097/01.MLR.0000023956.25813.18. [DOI] [PubMed] [Google Scholar]
- 37.Redelmeier DA, Rozin P, Kahneman D. Understanding patients’ decisions. Cognitive and emotional perspectives. JAMA. 1993;270(1):72–76. [PubMed] [Google Scholar]
- 38.Kemp R, Chua S, McKenna P, David A. Reasoning and delusions. Br J Psychiatry. 1997;170:398–405. doi: 10.1192/bjp.170.5.398. [DOI] [PubMed] [Google Scholar]
- 39.Huq SF, Garety PA, Hemsley DR. Probabilistic judgements in deluded and non-deluded subjects. Q J Exp Psychol A. 1988;40(4):801–812. doi: 10.1080/14640748808402300. [DOI] [PubMed] [Google Scholar]
- 40.Verheggen FW, Nieman F, Jonkers R. Determinants of patient participation in clinical studies requiring informed consent: why patients enter a clinical trial. Patient Educ Couns. 1998;35(2):111–125. doi: 10.1016/s0738-3991(98)00060-3. [DOI] [PubMed] [Google Scholar]
- 41.Roberts LW, Warner TD, Anderson CT, Smithpeter MV, Rogers MK. Schizophrenia research participants’ responses to protocol safeguards: recruitment, consent, and debriefing. Schizophr Res. 2004;67(2–3):283–291. doi: 10.1016/S0920-9964(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 42.Twamley EW, Doshi RR, Nayak GV, et al. Generalized cognitive impairments, ability to perform everyday tasks, and level of independence in community living situations of older patients with psychosis. Am J Psychiatry. 2002;159(12):2013–2020. doi: 10.1176/appi.ajp.159.12.2013. [DOI] [PubMed] [Google Scholar]
- 43.Savla GN, Moore DJ, Palmer BW. Cognitive functioning in schizophrenia. In: Mueser KT, Jeste DV, editors. Clinical Handbook of Schizophrenia. New York, NY: Guilford Press; [Google Scholar]
- 44.Dunn LB, Palmer BW, Keehan M, Jeste DV, Appelbaum PS. Assessment of therapeutic misconception in older schizophrenia patients with a brief instrument. Am J Psychiatry. 2006;163(3):500–506. doi: 10.1176/appi.ajp.163.3.500. [DOI] [PubMed] [Google Scholar]
- 45.National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. http://www.hhs.gov/ohrp/humansubjects/guidance/belmont.htm. Accessed October 5, 2006. [PubMed] [Google Scholar]
- 46.Palmer BW, Dunn LB, Appelbaum PS, et al. Assessment of capacity to consent to research among older persons with schizophrenia, Alzheimer disease, or diabetes mellitus: comparison of a 3-item questionnaire with a comprehensive standardized capacity instrument. Arch Gen Psychiatry. 2005;62(7):726–733. doi: 10.1001/archpsyc.62.7.726. [DOI] [PubMed] [Google Scholar]