Abstract
Dropout is often used as an outcome measure in clinical trials of antipsychotic medication. Previous research is inconclusive regarding (a) differences in dropout rates between first- and second-generation antipsychotic medications and (b) how trial design features reduce dropout. Meta-analysis of randomized controlled trials (RCTs) of antipsychotic medication was conducted to compare dropout rates for first- and second-generation antipsychotic drugs and to examine how a broad range of design features effect dropout. Ninety-three RCTs that met inclusion criteria were located (n = 26 686). Meta-analytic random effects models showed that dropout was higher for first- than second-generation drugs (odds ratio = 1.49, 95% confidence interval: 1.31–1.66). This advantage persisted after removing study arms with excessively high dosages, in flexible dose studies, studies of patients with symptom exacerbation, nonresponder patients, inpatients, and outpatients. Mixed effects models for meta-analysis were used to identify design features that effected dropout and develop formulae to derive expected dropout rates based on trial design features, and these assigned a pivotal role to duration. Collectively, dropout rates are lower for second- than first-generation antipsychotic drugs and appear to be partly explained by trial design features thus providing direction for future trial design.
Keywords: dropout, second-generation antipsychotic, first-generation antipsychotic
Introduction
Dropout occurs frequently in clinical trials of antipsychotic treatment. It is an important outcome because it may reflect drug tolerability, adverse effects, and lack of compliance. For instance, in the recent clinical antipsychotic trials of intervention effectiveness (CATIE) study discontinuation was a primary outcome measure. Seventy four percent of CATIE trial participants discontinued their assigned study medication before study completion at 18 months,1 and dropout rates were roughly equivalent for first-(ie, typical) and second-generation (ie, atypical) antipsychotics. Indeed, high dropout rates are not uncommon in RCTs of antipsychotic medication. Across studies of different durations, meta-analysis has estimated that dropout rates exceed a third of patients treated with antipsychotic medication in RCTs.2
Meta-analyses have reported lower dropout rates for second-generation antipsychotics than placebo.2–4 Such reviews, however, are inconclusive regarding differences in dropout rates between first- and second-generation medications. One meta-analysis of studies up to the year 2000 has reported that only clozapine shows significantly lower dropout rates than first-generation medications.2 Another meta-analysis covering studies conducted through 19985 has found differences favoring amisulpride, clozapine, risperidone, and olanzapine over first-generation medications. A meta-analysis of 28 published studies covering 4 of the major second-generation antipsychotics in Western populations through 2003 reported lower dropout rates for second-than first-generation treatment but only for flexible dose studies.6 Thus, research, based on meta-analyses shows lower dropout rates for second-generation antipsychotic drugs than placebo. Research, however, is inconclusive regarding differences in dropout rates between second- and first-generation antipsychotic treatment.
Research has examined how study design features of antipsychotic trials correspond with dropout rates. Wahlbeck et al2 have reported that dropout increases with trial length and year of publication. Yet, Kemmler et al3, who examined placebo-controlled trials up to 12 weeks long, did not find a significant association of dropout and duration, publication year, or use of multiple dosages. Yet, they3 did find that the presence of a placebo arm relates to a higher dropout rate in the active treatment arm. Furthermore, conclusions regarding second-generation antipsychotic medications differ between active- and placebo-controlled trials, highlighting the appropriateness of this comparson.7 Also, flexible rather than fixed dosage6 and higher dosages of first-generation medications5 have been reported to increase the difference in dropout rates between first- and second-generation medications. To provide a more comprehensive consideration of design features than has been covered previously, it is appropriate to consider patients levels of symptomatology and whether patients treated were in or outpatients because these may effect dropout rates.
The current meta-analysis compares dropout rates between first- and second-generation antipsychotic drugs and examines the effects of trial design features on dropout rates. Specifically, we examine the effects of trial duration, presence of placebo arm, number of trial arms, fixed vs flexible dosing, dosage, inpatient vs outpatient, symptom severity, and publication year on dropout rates. All published and unpublished studies irrespective of duration and sample size are included.
Methods
Literature Search
Trial reports were retrieved by an extensive literature search of the Cochrane Central Register of Controlled Trials and PubMed. The former includes published and nonpublished clinical trials and is based on extensive database searches, reference lists of published trials, and contacts with drug manufacturers and primary researchers.8 The search aimed to identify all double-blind randomized clinical trials of second-generation antipsychotic medications (risperidone, olanzapine, clozapine, quetiapine, amisulpride, ziprasidone, sertindole and aripiprazole) fulfilling the following criteria: being published or presented between the years 1990 and 2006, consisting of any adult patient population with a diagnosis of schizophrenia, schizoaffective or schizophreniform disorder.
Cumulatively our searches rendered 202 references using the following search string “(efficacy or effectiveness or relapse or remission or safety) and (schizophrenia or schizoaffective disorder or schizophreniform disorder) and (clozapine or olanzapine or risperidone or amisulpride or aripiprazole or quetiapine or sertindole or ziprasidone) and (adult and double mind)” in either the title, abstract, or keyword for the years 1990–2006. The removal of open-label trials rendered 162 references available. Sixty-two of these references were secondary publications of studies previously presented in a primary publication, and 7 additional references were excluded for missing information on dosing and or on dropout rates. This left 93 trials that met the inclusion criteria (see Appendix). Eight studies compared placebo, first-, and second-generation medications; 44 compared first- and second-generation without placebo; 19 compared second-generation and placebo; and 22 compared second-generation antipsychotic medications. Seven studies (7.5%) were published prior to 1996, 73 (78.5%) from 1997 to 2003, and 13 (14%) from 2004 to 2006.
Data Acquisition
The following information was extracted from each trial study report: the number of patients randomized to the different treatment arms, the total number of dropouts in the individual treatment arms, trial duration, patient type (stable responder, symptom exacerbation, nonresponder), hospitalization status (inpatients, outpatients, or both), study year, fixed vs flexible dosing, dose for fixed dose studies, and mean dose for flexible dose studies. In some studies, mean dose was not provided and so was estimated from the dosage range. To provide an overview, table 1 includes all studies with at least 100 patients per treatment (see Appendix for data from all studies), and Table 2 presents all placebo-controlled trials.
Table 1.
Summary of Studies Comparing Dropout in First- and Second-Generation Antipsychotic Medications With a Sample Size of At Least 100 Patients Per Treatment Arm
| Reference | Duration in wk | n | Treatment and Dosage | Dropout n | % Dropout | n | Dropout | % Dropout | Treatment and Dosage | Arms Overdose Nonsignificant and Dropout Notes Supplemented Where Applicable |
| Colonna et al9 | 52 | 370 | Am 605 | 167 | 45.1 | 119 | 62 | 52.1 | H 14.6 | Both arms overdosed |
| Copolov et al10 | 6 | 221 | Qu 455 | 69 | 31.2 | 227 | 80 | 35.2 | H 8 | |
| Csernansky et al11 | 52 | 179 | Ri 3 | 104 | 58.1 | 188 | 142 | 75.5 | H 7.5 | |
| Daniel et al12 | 52 | 141 | SE 24 | 27 | 19.1 | 141 | 43 | 30.5 | H 10 | |
| Emsley et al13 | 12 | 143 | Qu 600 | 32 | 22.4 | 145 | 28 | 19.3 | H 20 | Qu overdosed only |
| Hirsch et al14 | 28 | 148 | Zi 116.5 | 82 | 55.4 | 153 | 89 | 58.2 | H 8.6 | |
| Kane et al15,16 | 6 | 154 | Pe 39.10 | 44 | 28.6 | 146 | 31 | 21.2 | Ar 28.8 | Ar overdosed |
| Kane et al17 | 4 | 384 | Ar 15, 30 | 149 | 38.8 | 104 | 42 | 40.4 | H 10 | Ar is overdosed |
| Kasper et al18 | 52 | 861 | Ar 30 | 494 | 57.4 | 433 | 305 | 70.4 | H 10 | Ar is overdosed |
| Lieberman et al19,20 | 12 | 131 | Ol 9.1 | 42 | 32.1 | 132 | 61 | 68.1 | H 4.4 | |
| Lieberman et al1 | 78 | 1175 | Ol 11.25, Qu 300, Ri 2.25, Zi 60 | 869 | 74.0 | 257 | 192 | 74.7 | Pe 12 | |
| Peuskens21 | 8 | 1136 | Ri 1, 4, 8, and 16 | 284 | 25.0 | 226 | 63 | 27.9 | H 10 | Ri > 4 overdosed, (r = 8, n = 230, dropout = 24.35%, r = 12, n = 226, dropout = 27.43%, r = 16, n = 224, dropout = 28.3%) |
| Rosenheck et al22 | 52 | 205 | Cl 552 | 88 | 42.9 | 218 | 157 | 72.0 | H 28 | Both arms overdosed |
| Rosenheck et al23 | 52 | 159 | Ol 15.8 | 91 | 57.2 | 150 | 96 | 64.0 | H 14.3 | H overdosed |
| Schooler et al24 | 104 | 278 | Ri 3.3 | 117 | 42.1 | 277 | 101 | 36.5 | H 2.9 | |
| Tollefson et al25,26 | 6 | 1336 | Ol 13.2 | 448 | 33.5 | 660 | 351 | 53.2 | H 11.8 | H overdosed |
Note: H = Haloperidol, Qu = Quetiapine, Ri = Risperidone, SE = Sertindole, Zi = Ziprasidone, Ol = Olanzapine, Rinj = Risperidone Injectable, Cl = Clozapine, Pe = Perphenzine, Ar = Aripiprazole, Ch = Chlorpromazine. Trials with more than 2 treatments are ordered by drug and corresponding n, dropout number, and % dropout. Placebo arm details omitted.
Table 2.
Description of Placebo-Controlled Studies
| Publication | Publication Year | Duration in wk | n | % Dropout | Study Arms |
| Arvanitis and Miller27 | 1997 | 6 | 51 | 68.6 | 7 |
| Beasley and Sanger28 | 1996 | 6 | 50 | 80.0 | 3 |
| Beasley and Tollefson29 | 1996 | 6 | 68 | 67.6 | 5 |
| Beasley et al30 | 2003 | 52 | 102 | 53.9 | 2 |
| Borison et al31 | 1996 | 6 | 55 | 60.0 | 2 |
| Boyer et al32 | 1995 | 6 | 34 | 26.5 | 3 |
| Chouinard et al33 | 1993 | 8 | 22 | 72.7 | 6 |
| Cooper et al34 | 1999 | 8 | 53 | 47.2 | 3 |
| Cooper et al35 | 2000 | 26 | 58 | 84.5 | 2 |
| Corrigan et al36 | 2004 | 6 | 86 | 25.6 | 8 |
| Daniel et al37 | 1999 | 6 | 92 | 46.7 | 3 |
| Danion et al38 | 1999 | 12 | 83 | 39.8 | 3 |
| Kane et al17 | 2002 | 4 | 106 | 45.3 | 4 |
| Kane et al15,16 | 2003 | 12 | 98 | 67.3 | 6 |
| Keck et al39 | 1998 | 4 | 48 | 50.0 | 3 |
| Lecrubier et al40 | 2006 | 26 | 34 | 64.7 | 4 |
| Loo et al41 | 1997 | 26 | 72 | 68.1 | 2 |
| Marder et al42 | 1994 | 8 | 66 | 68.2 | 6 |
| Meltzer et al43 | 2004 | 6 | 98 | 79.6 | 6 |
| Pigott et al44,45 | 2003 | 26 | 155 | 71.0 | 2 |
| Potkin et al46 | 2003 | 4 | 103 | 49.5 | 4 |
| Small et al47 | 1997 | 6 | 96 | 59.4 | 3 |
| Tollefson et al48 | 1999 | .70 | 53 | 15.1 | 2 |
| Van-Kammen et al49 | 1996 | 5.70 | 38 | 39.5 | 4 |
| Arato et al50 | 2002 | 52 | 71 | 85.9 | 4 |
| Woods et al51 | 2003 | 8 | 29 | 27.6 | 2 |
Note: k = 26 studies.
Data Analysis
To compare the dropout rates within study arms between first- and second-generation antipsychotic medications, meta-regression (random effects meta-analysis) was conducted in R52 with the rmeta package.53 For comprehensiveness, this analysis was conduced first for all studies and then for those with at least 30, 50, and 100 patients per treatment. To test whether dropout differences might relate to the use of excessive dosages, analysis was rerun after removing study arms using excessive dosages and then for each second-generation drug. To see whether differences persisted, additional subanalyses were conducted of studies using flexible nonexcessive dosing, studies of nonresponder patients, studies of inpatients, and studies of outpatients.
Excessive dosing was operationalized as doses over the maximal effective dose based on the Davis et al54 meta-analysis of dose responses. That meta-analysis aimed to identify the near-maximal effective dose, namely, the threshold dose required to cumulate in all or almost all clinical responses for each drug. For example, the near-maximal efficacy dose for chlorpromazine is 450 mg/day and for risperidone is 4 mg/day.
In the second part of the analysis to predict dropout, mixed effects models for meta-analysis were conduced with the Mima function in the R statistical software environment.55 Covariates included were duration in weeks, number of study arms, presence of placebo, fixed vs flexible dosing, patient type (stable responder, symptom exacerbation, nonresponder), whether study was conducted on inpatients only, whether or not a study arm used excessively high dosages, and study year. Separate models were conducted for first-generation, second-generation, and placebo arms. This permitted us to derive equations for the prediction of dropout that operate much in the manner of typical regression models.
Results
Descriptive Statistics
The 94 studies constituted a total sample size of 26 686 subjects. They received first- (n = 5465) or second-generation antipsychotic medications (n = 19 400) or placebo (n = 1821). Participants allocated to first-generation medication ranged from 21 to 660 in each trial. The number treated with second-generation drugs ranged from 21 to 1336, and the number treated with placebo ranged from 22 to 155. The distribution of number of study arms was 2 arms (k = 66; 70.2%), 3 arms (k = 10; 10.6%), 4 arms (k = 6; 6.4%), and 5–8 arms (k = 12; 12.8%). The sample size for each dose arm ranged from 21 to 1336.
Comparing Dropout in First- and Second-Generation Antipsychotic Drugs
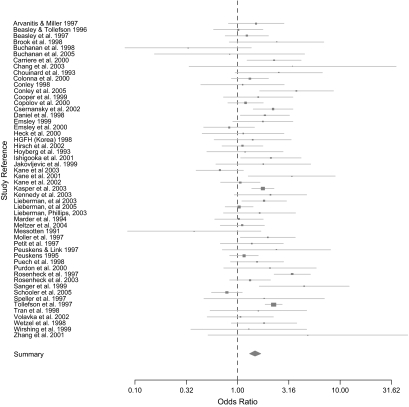
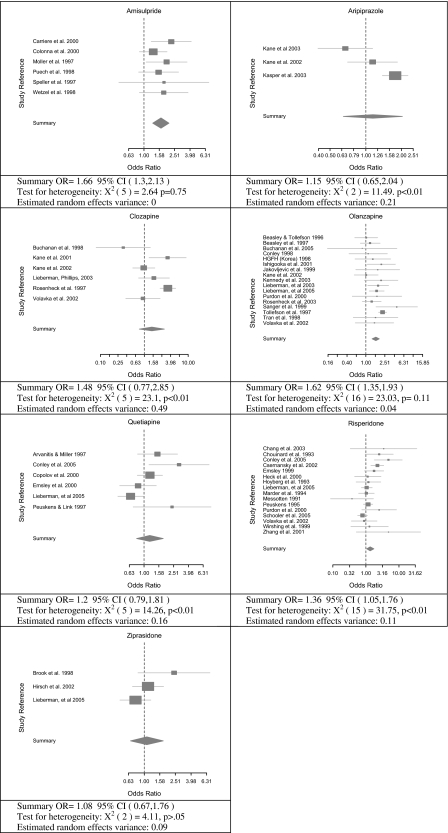
First- and second-generation treatments were compared utilizing random effects meta-analysis. To enable comparison of first- and second-generation medications, if the number of treatment conditions was not 2 (ie, one first- and one second-generation arm) then the arms were aggregated. The inclusion of all 52 studies comparing first- and second-generation medications, in figure 1, showed significantly lower dropout rates for second-generation drugs (OR = 1.49, 95% CI = 1.31, 1.66; test for heterogeneity: = 116.39, P = 0, estimated random effects variance = 0.1). As shown in table 3, this finding replicated in the 43 trials with over 30 participants in each treatment condition, the 33 trials with over 50 participants in each treatment condition, and the 16 trials with over 100 participants in each condition. It is noted that although the odds ratios dropped slightly in magnitude with arm sample size, the results suggested that second-generation treatment persistently had lower dropout rates than first-generation treatments. Dropout rates of specific second-generation drugs were compared with first-generation drugs in figure 2. These results showed a significant difference for amisulpride, olanzapine, risperidone, and an almost significant difference for clozapine and quetiapine.
Fig. 1.
Forest Plots Comparing All First- and Second-Generation Studies.
Fig. 2.
Forest Plots Comparing First- and Second-Generation Antipsychotic Drugs.
Dropout rates of first- and second-generation drugs were further compared in table 3 by examining subgroups of studies. First, differences were retested after removing study arms with excessive doses (see Methods section) from both first- and second-generation drug arms. After removing 52 study arms in 53 studies due to excessive dosing, 21 studies remained available for analysis that compared first- and second-generation medication. While based on only a small number of studies, these results showed a significant advantage for olanzapine and a nearly significant difference for risperidone but not for ziprasidone, clozapine, and quetiapine. No data were available to examine amisulpride and aripiprazole after removing excessive dosing. This analysis was repeated after removing fixed dose studies with excessive dosages. Collectively, 122 fixed and 84 overdosed study arms were removed leaving 17 studies available for analysis. Advantages for second-generation drugs were observed (see table 3). Next studies of patients with symptom exacerbation, nonresponder patients, inpatient, and outpatient were examined. These too all showed an advantage for second-generation drugs. Collectively, therefore, the current results consistently demonstrate at the aggregate level, for specific drugs even if not overdosed, and accounting for relevant moderators a unitary trend of higher dropout for first- than second-generation antipsychotic treatment.
Table 3.
Results of Meta-analysis for All Studies and Subgroups of Studies
| Grouping | OR (95% CI) | Test for Heterogeneity (χ2); Estimated Random Effects Variance (REV) |
| All studies (n = 52) | 1.49 (1.31, 1.69) | 116.39, df = 51, P = 0; REV = 0.1 |
| Studies with at least 30 patients per arm | 1.46 (1.27, 1.66) | 106.02, df = 42, P = 0, REV = 0.1 |
| Studies with at least 50 patients per arm | 1.43 (1.24, 1.64) | 90.14, df = 32, P = 0, REV = 0.1 |
| Studies with at least 100 patients per arm | 1.38 (1.11, 1.71) | 90.14, df = 15, P = 0, REV = 0.15 |
| Studies not using excessive doses for first- and second-generation drugs (n = 21) | 1.34 (1.14, 1.57) | 30.72, df = 20, P = .059; REV = 0.04 |
| Clozapine (n = 3) | 0.97 (0.49, 1.92) | 3.81, df = 2, P = .149; REV = 0.17 |
| Olanzapine (n = 8) | 1.74 (1.38, 2.2) | 1.57, df = 7, P = .98; REV = 0 |
| Quetiapine (n = 3) | 1.04 (0.58, 1.87) | 6.84, df = 2, P = .03; REV = 0.17 |
| Risperidone (n = 5) | 1.30 (0.88, 1.93) | 15.3, df = 4, P = .004; REV = 0.13 |
| Ziprasidone (n = 2) | 0.93 (0.65, 1.34) | 1.26, df = 1, P = .26; REV = 0.01 |
| Flexible dose studies not using excessive dosing (n = 17) | 1.37 (1.12, 1.68) | 27.77, df = 16, P = .03; REV = 0.07 |
| Studies of patients with symptom exacerbation (n = 40) | 1.40 (1.26, 1.57) | 48.64, df = 39, P = .14; REV = 0.02 |
| Studies of nonresponder patients (n = 10) | 1.53 (1.01, 2.32) | 47.77, df = 9, P = 0; REV = 0.31 |
| Inpatient studies (n = 23) | 1.54 (1.3, 1.84) | 38.4, df = 22, P = .02; REV = 0.06 |
| Outpatient studies (n = 29) | 1.45 (1.2, 1.74) | 77.69, df = 28, P = 0; REV = 0.13 |
Predictors of Dropout
Mixed effects regression models presented in table 4 were conducted separately for first generation, second generation, and placebo to examine the association of trial design features and dropout. Trial duration was consistently significant (<.01) in the 3 models. Specifically, the longer the trial the higher the dropout rate. Duration had a large effect size (Zs > 2.56), indicating its influence. In second-generation trials, flexible vs fixed dose also significantly reduced dropout, and for first-generation drugs, there was a nearly significant effect (P = .06) for excessive dosing which increased dropout. Numbers of study arms, presence of a placebo arm, and study year were not significantly associated with dropout.
Table 4.
Mixed Effects Regression for Meta-analysis (Mima in R) Predicting Dropout
| Second Generation (Arms = 171)a |
First Generation (Arms = 52)b |
Placebo (Arms = 26)c |
||||||||||
| Estimate | SE | z | P | Estimate | SE | z | P | Estimate | SE | z | P | |
| Intercept | −1063.80 | 774.72 | −1.37 | 0.17 | −1760.18 | 1726.94 | −1.02 | 0.31 | 2226.70 | 2296.50 | 0.97 | 0.33 |
| Publication year | 0.54 | 0.39 | 1.41 | 0.16 | 0.89 | 0.86 | 1.03 | 0.30 | −1.10 | 1.15 | −0.96 | 0.34 |
| Symptom leveld | 1.85 | 3.20 | 0.58 | 0.56 | −3.75 | 5.91 | −0.63 | 0.52 | 4.67 | 11.13 | 0.42 | 0.67 |
| Inpatient | −1.49 | 2.98 | −0.50 | 0.62 | −0.75 | 5.99 | −0.12 | 0.90 | 5.96 | 8.23 | 0.72 | 0.47 |
| With placebo | 4.23 | 3.57 | 1.19 | 0.24 | 11.75 | 10.42 | 1.13 | 0.26 | — | — | — | — |
| Number of arms | 0.52 | 0.92 | 0.56 | 0.57 | 3.13 | 2.69 | 1.16 | 0.24 | 1.86 | 2.47 | 0.75 | 0.45 |
| Duration in weeks | 0.38 | 0.08 | 4.99 | 0.0001 | 0.31 | 0.12 | 2.57 | 0.01 | 0.94 | 0.33 | 2.88 | 0.004 |
| Fixed dose | 8.23 | 3.39 | 2.43 | 0.01 | −4.18 | 7.03 | −0.59 | 0.55 | 10.02 | 10.57 | 0.95 | 0.34 |
| High dosage | −3.50 | 2.68 | −1.30 | 0.19 | 11.05 | 5.92 | 1.87 | 0.06 | — | — | — | — |
Estimate of (residual) heterogeneity: 257.92; test for (residual) heterogeneity: QE = 83076.29; df = 162; P < .0001; omnibus test of all moderators: QME = 50.32; df = 8; P < .0001.
Estimate of (residual) heterogeneity: 307.14; test for (residual) heterogeneity: QE = 23856.1; df = 43; P < .0001; omnibus Test of all Moderators: QME = 20.00; df = 8; P = .01.
Estimate of (residual) heterogeneity: 314.62; test for (residual) heterogeneity: QE = 9494.47; df = 19; P < .0001; omnibus Test of all Moderators: QME = 10.62; df = 6; P = .10.
Based on study inclusion criteria: 0 nonresponder patients, 1 patient with symptom exacerbation, 2 stable responder patients.
The regression models in table 4 may be applied to derive expected dropout rates. Caution is warranted because they have not been validated in trials not included in the analysis. To illustrate the use of the equations, the following are examples based on a placebo, first-, and second-generation study arms. Based on the placebo study arm of Pigott et al44 in 2003 the placebo equation is applied as follows: (intercept 2226.70) + (year 2003 × −1.10) + (symptom level 0 × 4.67) + (in patient study 1 × 5.96) + (arms 2 × 1.86) + (duration in weeks 26 × 0.94) + (fixed dosing 1 × 10.02). This produces an estimated dropout rate of 67.5% where the actual rate was 71.0%. The first-generation drug equation is illustrated using the haloperidol arm from Lieberman et al (2003)19 as follows: (intercept − 1760.18) + (year 2003 × 0.89) + (symptom level 1 × −3.75) + (in patient study 0 × −0.75) + (with placebo arm 1 × 11.75) + (number of arms 6 × 3.13) + (duration in weeks 12 × 0.31) + (fixed dosing 0 × 4.18) + (excessive dosing 0 × 11.05). This produces an estimated dropout rate of 53.0% where the rate was 46.2%. The second-generation drug equation is illustrated using the olanzapine arm from CATIE1 as follows: (intercept − 1063.80) + (year 2005 × 0.54) + (symptom level 1 × 1.85) + (in patient study 0 × −1.49) + (with placebo arm 0 × 4.23) + (arms 5 × 0.52) + (duration in weeks 78 × 0.38) + (fixed dosing 0 × 8.23) + (excessive dosing 0 × −3.50). This produces an estimated dropout rate of 52.99% where actual dropout rate was 63.64%.
The average difference between the estimated and actual percentage dropout across study arms was 6.44 (SD = 16.26). Differences were observed by drug and are presented in ascending order as follows: chlorpromazine, M = −6.64 (SD = 12.69, k = 5), placebo, M = −5.85 (SD = 15.48, k = 26), amisulpride, M = −0.95 (SD = 11.38, k = 19), ziprasidone, M = 1.87 (SD = 17.76, k = 15), haloperidol, M = 7.78 (SD = 16.45, k = 41), olanzapine, M = 8.52 (SD = 14.94, k = 43), risperidone, M = 8.69 (SD = 2.46, k = 40), clozapine, M = 8.87 (SD = 15.65, k = 9), aripiprazole, M = 10.80 (SD = 13.55, k = 10), quetiapine, M = 14.87 (SD = 3.65, k = 15). This highlighted that the formula was generally accurate but estimated dropout for some drugs with greater accuracy than others.
Discussion
The current meta-analysis indicates that the use of second-generation antipsychotic medication has lower dropout rates than first-generation treatments. Several design features of randomized clinical trials of antipsychotic medications are identified that are significantly associated with dropout rates. Among these, a longer duration was most consistently and strongly associated with dropout, although effects are observable also for dosing (fixed vs flexible) and excessive dosing. Significant effects are not found for publication year, number of study arms, the presence of a placebo study arm, inpatient study, or symptom level. The trial design features that we examined were used to develop an equation to estimate expected dropout rates. The equation shows a reasonable correspondence between predicted and actual dropout rates.
Clinical Implications
A previous meta-analysis2 covering studies up to the year 2000 reported an effect only for clozapine. Unlike that review, but like a review of 36 selected studies through 2003,6 our results show significant effects spanning second-generation drugs. Beyond those reviews, the results identify drug-specific effects. Our results also differ from another meta-analysis covering studies through 19985 that reports differences favoring amisulpride, clozapine, risperidone, and olanzapine. In that meta-analysis for all but amisulpride, these differences are found only in the fixed and not random effects analysis, and no evidence supporting differences for quetiapine are identified. Like Wahlbeck et al2 we found that dropout increases with trial length, however, we did not find a significant effect of publication year. While Kemmler et al3 reports an effect for placebo arms in studies up to 12 weeks long, we did not find a significant placebo arm effect, which may be related to our inclusion of studies regardless of length. We also did not find an effect of multiple dosage regimes. Corresponding to others,6 flexible rather than fixed dosage was found to effect dropout in second-generation and not first-generation arms. Like Geddes et al5, we found a nearly significant effect of high dosages on dropout for first-generation medications. Collectively, therefore, our findings support the use of second-generation treatment over first-generation treatment where dropout is the outcome.
Limitations
Several limitations are notable. It is not possible to estimate the possible bias introduced by studies not published, although our review was used by the Cochrane database that includes unpublished studies. Also, our meta-analysis does not eliminate studies due to a priori criteria that may have biased the results (eg, including only large clinical trials or only trials of a certain duration). Another limitation is that the current data contain limited clinical information. Such information is likely to influence the study outcomes, although the clinical information our study contained did not (ie, hospitalization status and symptom severity). It is noted that there is a payoff in meta-analysis between number of variables and the number of studies. The approach taken here was to opt for more studies in an unbiased manner, thus maximizing statistical power. Statistical power was, however, small when examining the specific effects of some of the second-generation medications. The number of studies included in the current meta-analysis, however, is much larger than previous reviews. The available data do not yet permit the analysis of long-acting injectable second-generation antipsychotics (eg, risperidone injectable) and thus highlight a direction for future research once enough studies become available. The formula to derive expected dropout rates may be useful to plan trials and compare study results. The formula should, however, be used with caution until it is validated by predicting dropout rates in future studies.
Conclusions
The current results demonstrate that dropout rates are moderately yet consistently reduced by second- rather than first-generation antipsychotic medication. This trend replicates across medications and irrespective of a series of moderators investigated (eg, hospitalization status, dosage). The current meta-analysis represents, to our knowledge, the largest study of how methodological factors effect dropout in clinical trials of antipsychotic medication. The results indicate that dropout rates are significantly influenced by the trial duration, fixed vs flexible dosing, and excessive dosing. These findings provide a significant increment in understanding dropout rates in clinical trials and may contribute to the design of future clinical trials. Collectively, these results show moderate and consistent benefits, indicated by dropout reduction, that favor second- over first-generation medications and provide formulae to assist future trial designs.
Supplementary Material
Supplementary material for this article is available online at http://schizophreniabulletin.oxfordjournals.org/.
Acknowledgments
Author Rabinowitz has received research grants, travel support, and or consulting fees from Janssen Pharmaceuticals, Eli Lilly, Pfizer, and Novartis. Other authors have no conflicts of interest. This work was done as part of a PhD conducted in the Department of Mathematics at Bar Ilan University.
Appendix
Appendix.
Randomized Controlled Trials of Second Generation Antipsychotic Medications Summary
| Publication | Total n | Total Dropout | Duration in wk | Arms | Placebo n | Placebo Dropout | First Generation n | First Generation Dropout | Second Generation n | Second Generation Dropout | Placebo | Symptoms | Fixed | Dosage | In Hospital |
| Addington et al56 | 296 | 98 | 8 | 2 | 296 | 98 | SE | In | |||||||
| Arato et al50 | 278 | 179 | 52 | 4 | 71 | 61 | 207 | 118 | P | SE | fix | ||||
| Arvanitis and Miller27 | 361 | 212 | 6 | 7 | 51 | 35 | 52 | 34 | 258 | 143 | P | SE | fix | NME | In |
| Azorin et al57 | 273 | 72 | 12 | 2 | 273 | 72 | SE | NME | |||||||
| Beasley Sanger et al28 | 152 | 107 | 6 | 3 | 50 | 40 | 102 | 67 | P | NR | fix | In | |||
| Beasley et al29 | 335 | 196 | 6 | 5 | 68 | 46 | 69 | 39 | 198 | 111 | P | SE | fix | NME | |
| Beasley et al58 | 431 | 184 | 6 | 5 | 81 | 38 | 350 | 146 | SE | fix | NME | In | |||
| Beasley et al30 | 326 | 85 | 52 | 2 | 102 | 55 | 224 | 30 | P | SR | |||||
| Bitter et al59 | 150 | 63 | 18 | 2 | 150 | 63 | SE | NME | In | ||||||
| Borison et al31 | 109 | 59 | 6 | 2 | 55 | 33 | 54 | 26 | P | SE | In | ||||
| Boyer et al32 | 104 | 19 | 6 | 3 | 34 | 9 | 70 | 10 | P | SE | fix | NME | In | ||
| Brook et al60 | 132 | 16 | 1 | 2 | 42 | 8 | 90 | 8 | SE | ||||||
| Buchanan et al62 | 75 | 11 | 10 | 2 | 37 | 3 | 38 | 8 | NR | ||||||
| Buchanan et al61 | 63 | 6 | 16 | 2 | 34 | 3 | 29 | 3 | SE | ||||||
| Carriere et al63 | 199 | 70 | 16 | 2 | 105 | 46 | 94 | 24 | SE | NME | |||||
| Casey et al64 | 207 | 55 | 8 | 3 | 207 | 55 | SR | fix | NME | In | |||||
| Chan et al65 | 60 | 8 | 8 | 2 | 60 | 8 | SE | In | |||||||
| Chang et al66 | 62 | 4 | 8 | 2 | 30 | 3 | 32 | 1 | SE | NME | |||||
| Chouinard et al33 | 135 | 65 | 8 | 6 | 22 | 16 | 21 | 13 | 92 | 36 | P | SE | fix | In | |
| Chue et al67 | 640 | 527 | 12 | 2 | 640 | 527 | SE | fix | |||||||
| Colonna et al9 | 489 | 229 | 52 | 2 | 119 | 62 | 370 | 167 | SE | NME | |||||
| Conley and Mahmoud69 | 377 | 96 | 8 | 2 | 377 | 96 | SE | NME | |||||||
| Conley 199870 | 84 | 25 | 8 | 2 | 42 | 13 | 42 | 12 | NR | fix | NME | In | |||
| Conley et al68 | 114 | 54 | 12 | 3 | 38 | 26 | 76 | 28 | NR | fix | In | ||||
| Cooper et al34 | 159 | 69 | 8 | 3 | 53 | 25 | 53 | 25 | 53 | 19 | P | SE | |||
| Cooper et al35 | 119 | 90 | 26 | 2 | 58 | 49 | 61 | 41 | P | SR | fix | ||||
| Copolov et al10 | 448 | 149 | 6 | 2 | 227 | 80 | 221 | 69 | SE | In | |||||
| Corrigan et al36 | 735 | 154 | 6 | 8 | 86 | 22 | 649 | 132 | P | SE | In | ||||
| Csernansky et al11 | 367 | 246 | 52 | 2 | 188 | 142 | 179 | 104 | SR | ||||||
| Daniel et al12 | 282 | 70 | 52 | 2 | 141 | 43 | 141 | 27 | SE | fix | |||||
| Daniel et al37 | 302 | 86 | 6 | 3 | 92 | 43 | 210 | 43 | P | SE | fix | ||||
| Danion et al38 | 242 | 62 | 12 | 3 | 83 | 33 | 159 | 29 | P | SE | fix | ||||
| Emsley71 | 183 | 46 | 6 | 2 | 84 | 26 | 99 | 20 | SE | NME | |||||
| Emsley et al13 | 288 | 60 | 12 | 2 | 145 | 28 | 143 | 32 | NR | fix | |||||
| Gureje et al72 | 65 | 36 | 30 | 2 | 65 | 36 | SE | NME | |||||||
| Heck et al73 | 77 | 30 | 7 | 2 | 37 | 15 | 40 | 15 | SE | NME | |||||
| HGFH Korea74 | 104 | 29 | 6 | 2 | 51 | 16 | 53 | 13 | SE | ||||||
| Hirsch et al14 | 301 | 171 | 28 | 2 | 153 | 89 | 148 | 82 | SR | ||||||
| Hoyberg et al75 | 107 | 29 | 8 | 2 | 52 | 15 | 55 | 14 | SE | In | |||||
| Huttunen et al76 | 98 | 40 | 6 | 2 | 98 | 40 | SE | In | |||||||
| Ishigooka et al77 | 182 | 48 | 8 | 2 | 89 | 30 | 93 | 18 | SE | ||||||
| Jakovljevic et al78 | 60 | 22 | 6 | 2 | 30 | 13 | 30 | 9 | SE | In | |||||
| Jeste et al79 | 175 | 41 | 8 | 2 | 175 | 41 | SE | ||||||||
| Kane et al15,16 | 300 | 75 | 6 | 2 | 146 | 31 | 154 | 44 | NR | NME | |||||
| Kane et al80 | 71 | 35 | 26 | 2 | 34 | 22 | 37 | 13 | NR | NME | |||||
| Kane et al17 | 594 | 239 | 4 | 4 | 106 | 48 | 104 | 42 | 384 | 149 | P | SE | fix | NME | |
| Kane et al15,16 | 400 | 222 | 12 | 6 | 98 | 66 | 302 | 156 | P | SE | fix | ||||
| Kasper et al18 | 1294 | 799 | 52 | 2 | 433 | 305 | 861 | 494 | SE | fix | In | ||||
| Keck et al39 | 139 | 64 | 4 | 3 | 48 | 24 | 91 | 40 | P | SE | fix | ||||
| Kennedy et al81 | 117 | 47 | 6 | 2 | 34 | 18 | 83 | 29 | SE | ||||||
| Kudo et al82 | 180 | 59 | 8 | 2 | 180 | 59 | SE | ||||||||
| Lecrubier et al40 | 244 | 143 | 26 | 4 | 34 | 22 | 210 | 121 | P | SE | fix | ||||
| Lee et al83 | 54 | 9 | 6 | 2 | 54 | 9 | SE | ||||||||
| Lieberman et al19,20,84 | 263 | 103 | 12 | 6 | 132 | 61 | 131 | 42 | P | SE | |||||
| Lieberman et al1 | 1432 | 1061 | 78 | 5 | 257 | 192 | 1175 | 869 | SE | ||||||
| Lieberman et al20 | 160 | 30 | 52 | 2 | 80 | 18 | 80 | 12 | SE | In | |||||
| Loo et al41 | 141 | 80 | 26 | 2 | 72 | 49 | 69 | 31 | P | SE | fix | ||||
| Marder et al42 | 388 | 199 | 8 | 6 | 66 | 45 | 66 | 32 | 256 | 122 | P | SE | fix | In | |
| Martin et al85 | 377 | 81 | 26 | 2 | 377 | 81 | SE | NME | |||||||
| McQuade et al86 | 317 | 230 | 26 | 2 | 317 | 230 | SE | ||||||||
| Meltzer et al43 | 481 | 337 | 6 | 6 | 98 | 78 | 98 | 68 | 285 | 191 | P | SE | fix | In | |
| Messotten87 | 60 | 9 | 8 | 2 | 32 | 3 | 28 | 6 | SE | NME | In | ||||
| Moller et al88 | 191 | 64 | 6 | 2 | 96 | 39 | 95 | 25 | SE | fix | NME | In | |||
| Mortimer et al89 | 377 | 135 | 26 | 2 | 377 | 135 | SE | NME | In | ||||||
| Mullen et al90 | 728 | 235 | 16 | 2 | 728 | 235 | SE | ||||||||
| Naber et al91 | 114 | 71 | 26 | 2 | 114 | 71 | SE | ||||||||
| Petit et al (1997) | 126 | 55 | 8 | 2 | 63 | 30 | 63 | 25 | SE | In | |||||
| Peuskens and Link93 | 201 | 13 | 6 | 2 | 100 | 9 | 101 | 4 | SE | In | |||||
| Peuskens21 | 1362 | 347 | 8 | 6 | 226 | 63 | 1136 | 284 | SE | fix | In | ||||
| Peuskens et al92 | 228 | 69 | 8 | 2 | 228 | 69 | SE | fix | NME | ||||||
| Pigott et al44,45 | 310 | 194 | 26 | 2 | 155 | 110 | 155 | 84 | P | SR | fix | NME | In | ||
| Potkin et al46 | 404 | 162 | 4 | 4 | 103 | 51 | 301 | 111 | P | SE | fix | NME | In | ||
| Puech et al94 | 319 | 82 | 4 | 5 | 64 | 21 | 255 | 61 | SE | fix | NME | In | |||
| Purdon et al95 | 65 | 32 | 54 | 3 | 23 | 14 | 42 | 18 | SE | ||||||
| Ritchie et al96 | 66 | 14 | 4 | 2 | 66 | 14 | SR | ||||||||
| Rosenheck et al22 | 423 | 245 | 52 | 2 | 218 | 157 | 205 | 88 | NR | NME | In | ||||
| Rosenheck et al23 | 309 | 187 | 52 | 2 | 150 | 96 | 159 | 91 | NR | ||||||
| Sanger et al97 | 83 | 31 | 6 | 2 | 24 | 15 | 59 | 16 | SE | ||||||
| Schooler et al24 | 555 | 218 | 104 | 2 | 277 | 101 | 278 | 117 | SE | ||||||
| Sechter et al98 | 310 | 123 | 26 | 2 | 310 | 123 | SR | NME | |||||||
| Simpson et al99 | 269 | 115 | 6 | 2 | 269 | 115 | SE | In | |||||||
| Small et al47 | 286 | 159 | 6 | 3 | 96 | 57 | 190 | 102 | P | SE | fix | In | |||
| Speller et al100 | 60 | 11 | 52 | 2 | 31 | 7 | 29 | 4 | SE | NME | In | ||||
| Tollefson et al25,26 | 1996 | 799 | 6 | 2 | 660 | 351 | 1336 | 448 | NR | ||||||
| Tollefson et al48 | 106 | 11 | 1 | 2 | 53 | 8 | 53 | 3 | P | SE | fix | ||||
| Tran et al102 | 339 | 161 | 28 | 2 | 339 | 161 | SE | NME | |||||||
| Tran et al101 | 54 | 24 | 14 | 2 | 28 | 14 | 26 | 10 | SE | ||||||
| Van-Kammen et al49 | 153 | 53 | 6 | 4 | 38 | 15 | 115 | 38 | P | SE | fix | In | |||
| Volavka et al103 | 157 | 66 | 14 | 4 | 37 | 16 | 120 | 50 | SE | NME | In | ||||
| Wetzel et al104 | 132 | 44 | 6 | 2 | 62 | 25 | 70 | 19 | SE | NME | In | ||||
| Wirshing et al (1999)105 | 67 | 11 | 8 | 2 | 33 | 6 | 34 | 5 | NR | NME | |||||
| Woods et al51 | 60 | 19 | 8 | 2 | 29 | 8 | 31 | 11 | P | SE | |||||
| Zhang et al106 | 78 | 5 | 12 | 2 | 37 | 4 | 41 | 1 | SE | fix | NME | In |
References
- 1.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 2.Wahlbeck K, Tuunainen A, Ahokas A, Leucht S. Dropout rates in randomised antipsychotic drug trials. Psychopharmacology (Berl) 2001;155(3):230–233. doi: 10.1007/s002130100711. [DOI] [PubMed] [Google Scholar]
- 3.Kemmler G, Hummer M, Widschwendter C, Fleischhacker WW. Dropout rates in placebo-controlled and active-control clinical trials of antipsychotic drugs: a meta-analysis. Arch Gen Psychiatry. 2005;62(12):1305–1312. doi: 10.1001/archpsyc.62.12.1305. [DOI] [PubMed] [Google Scholar]
- 4.Labelle A, Boulay LJ, Lapierre YD. Retention rates in placebo- and nonplacebo-controlled clinical trials of schizophrenia. Can J Psychiatry. 1999;44(9):887–892. doi: 10.1177/070674379904400904. [DOI] [PubMed] [Google Scholar]
- 5.Geddes J, Freemantle N, Harrison P, Bebbington P. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ. 2000;321(7273):1371–1376. doi: 10.1136/bmj.321.7273.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin JL, Perez V, Sacristan M, Rodriguez-Artalejo F, Martinez C, Alvarez E. Meta-analysis of drop-out rates in randomised clinical trials, comparing typical and atypical antipsychotics in the treatment of schizophrenia. Eur Psychiatry. 2006;21(1):11–20. doi: 10.1016/j.eurpsy.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Woods SW, Gueorguieva RV, Baker CB, Makuch RW. Control group bias in randomized atypical antipsychotic medication trials for schizophrenia. Arch Gen Psychiatry. 2005;62(9):961–970. doi: 10.1001/archpsyc.62.9.961. [DOI] [PubMed] [Google Scholar]
- 8.Cochrane Library. Available at http://www3.interscience.wiley.com/cgi-bin/mrwhome/106568753/HOME. Accessed January 9, 2008. [Google Scholar]
- 9.Colonna L, Saleem P, Dondey-Nouvel L, Rein W. Long-term safety and efficacy of amisulpride in subchronic or chronic schizophrenia. Amisulpride Study Group. Int clin psychopharmacol. 2000;15(1):13–22. doi: 10.1097/00004850-200015010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Copolov DL, Link CG, Kowalcyk B. A multicentre, double-blind, randomized comparison of quetiapine (ICI 204,636, ‘Seroquel’) and haloperidol in schizophrenia. Psychol Med. 2000;30(1):95–105. doi: 10.1017/s0033291799001476. [DOI] [PubMed] [Google Scholar]
- 11.Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med. 2002;346(1):16–22. doi: 10.1056/NEJMoa002028. [DOI] [PubMed] [Google Scholar]
- 12.Daniel DG, Wozniak P, Mack RJ, McCarthy BG. Long-term efficacy and safety comparison of sertindole and haloperidol in the treatment of schizophrenia. The Sertindole Study Group. Psychopharmacol Bull. 1998;34(1):61–69. [PubMed] [Google Scholar]
- 13.Emsley RA, Raniwalla J, Bailey PJ, Jones AM. A comparison of the effects of quetiapine (‘seroquel’) and haloperidol in schizophrenic patients with a history of and a demonstrated, partial response to conventional antipsychotic treatment. PRIZE Study Group. Int clin psychopharmacol. 2000;15(3):121–131. doi: 10.1097/00004850-200015030-00001. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch SR, Kissling W, Bauml J, Power A, O'Connor R. A 28-week comparison of ziprasidone and haloperidol in outpatients with stable schizophrenia. J Clin Psychiatry. 2002;63(6):516–523. doi: 10.4088/jcp.v63n0609. [DOI] [PubMed] [Google Scholar]
- 15.Kane J, Carson W, Kujawa M, Stringfellow J, Marcus R, Sanchez R. Annual Meeting of the American Psychiatric Association. San Francisco: 2003. Aripiprazole vs. perphenazine in treatment-resistant schizophrenia. [Google Scholar]
- 16.Kane JM, Eerdekens M, Lindenmayer JP, Keith SJ, Lesem M, Karcher K. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry. 2003;160(6):1125–1132. doi: 10.1176/appi.ajp.160.6.1125. [DOI] [PubMed] [Google Scholar]
- 17.Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763–771. doi: 10.4088/jcp.v63n0903. [DOI] [PubMed] [Google Scholar]
- 18.Kasper S, Lerman MN, McQuade RD, et al. Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J neuropsychopharmacol. 2003;6(4):325–337. doi: 10.1017/S1461145703003651. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman JA, Tollefson G, Tohen M, et al. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160(8):1396–1404. doi: 10.1176/appi.ajp.160.8.1396. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995–1003. doi: 10.1038/sj.npp.1300157. [DOI] [PubMed] [Google Scholar]
- 21.Peuskens J. Risperidone in the treatment of patients with chronic schizophrenia: a multi-national, multi-centre, double-blind, parallel-group study versus haloperidol. Risperidone Study Group. Br J Psychiatry. 1995;166(6):712–726. doi: 10.1192/bjp.166.6.712. discussion 727–733. [DOI] [PubMed] [Google Scholar]
- 22.Rosenheck R, Cramer J, Xu W, et al. A comparison of clozapine and haloperidol in hospitalized patients with refractory schizophrenia. Department of Veterans Affairs Cooperative Study Group on Clozapine in Refractory Schizophrenia. N Engl J Med. 1997;337(12):809–815. doi: 10.1056/NEJM199709183371202. [DOI] [PubMed] [Google Scholar]
- 23.Rosenheck R, Perlick D, Bingham S, et al. Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia: a randomized controlled trial. JAMA. 2003;290(20):2693–2702. doi: 10.1001/jama.290.20.2693. [DOI] [PubMed] [Google Scholar]
- 24.Schooler N, Rabinowitz J, Davidson M, et al. Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. The American journal of psychiatry. 2005;162(5):947–953. doi: 10.1176/appi.ajp.162.5.947. [DOI] [PubMed] [Google Scholar]
- 25.Tollefson GD, Beasley CM, Jr., Tran PV, et al. Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. Am J psychiatry. 1997;154(4):457–465. doi: 10.1176/ajp.154.4.457. [DOI] [PubMed] [Google Scholar]
- 26.Tollefson GD, Sanger TM. Negative symptoms: a path analytic approach to a double-blind, placebo- and haloperidol-controlled clinical trial with olanzapine. Am J psychiatry. 1997;154(4):466–474. doi: 10.1176/ajp.154.4.466. [DOI] [PubMed] [Google Scholar]
- 27.Arvanitis LA, Miller BG. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The Seroquel Trial 13 Study Group. Biol Psychiatry. 1997;42(4):233–246. doi: 10.1016/s0006-3223(97)00190-x. [DOI] [PubMed] [Google Scholar]
- 28.Beasley CM, Jr, Sanger T, Satterlee W, Tollefson G, Tran P, Hamilton S. Olanzapine versus placebo: results of a double-blind, fixed-dose olanzapine trial. Psychopharmacology (Berl) 1996;124(1–2):159–167. doi: 10.1007/BF02245617. [DOI] [PubMed] [Google Scholar]
- 29.Beasley CM, Jr, Tollefson G, Tran P, Satterlee W, Sanger T, Hamilton S. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14(2):111–123. doi: 10.1016/0893-133X(95)00069-P. [DOI] [PubMed] [Google Scholar]
- 30.Beasley CM, Jr, Sutton VK, Hamilton SH, et al. A double-blind, randomized, placebo-controlled trial of olanzapine in the prevention of psychotic relapse. J Clin Psychopharmacol. 2003;23(6):582–594. doi: 10.1097/01.jcp.0000095348.32154.ec. [DOI] [PubMed] [Google Scholar]
- 31.Borison RL, Arvanitis LA. Miller BG. ICI 204,636, an atypical antipsychotic: efficacy and safety in a multicenter, placebo-controlled trial in patients with schizophrenia. U.S. SEROQUEL Study Group. J Clin Psychopharmacol. 1996;16(2):158–169. doi: 10.1097/00004714-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Boyer P, Lecrubier Y, Puech AJ, Dewailly J, Aubin F. Treatment of negative symptoms in schizophrenia with amisulpride. Br J Psychiatry. 1995;166(1):68–72. doi: 10.1192/bjp.166.1.68. [DOI] [PubMed] [Google Scholar]
- 33.Chouinard G, Jones B, Remington G, et al. A Canadian multicenter placebo-controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. J Clin Psychopharmacol. 1993;13(1):25–40. [PubMed] [Google Scholar]
- 34.Cooper S, Tweed J, Raniwalla J, Welch C. A placebo-controlled comparison of zotepine versus chlorpromazine in patients with acute exacerbation of schizophrenia: cochrane Colloboration. 1999 [PubMed] [Google Scholar]
- 35.Cooper SJ, Tweed J, Raniwalla J, Butler A, Welch C. A placebo-controlled comparison of zotepine versus chlorpromazine in patients with acute exacerbation of schizophrenia. Acta psychiatr Scand. 2000;101(3):218–225. [PubMed] [Google Scholar]
- 36.Corrigan MH, Gallen CC, Bonura ML, Merchant KM. Effectiveness of the selective D4 antagonist sonepiprazole in schizophrenia: a placebo-controlled trial. Biol Psychiatry. 2004;55(5):445–451. doi: 10.1016/j.biopsych.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Daniel DG, Zimbroff DL, Potkin SG, Reeves KR, Harrigan EP, Lakshminarayanan M. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology. 1999;20(5):491–505. doi: 10.1016/S0893-133X(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 38.Danion JM, Rein W, Fleurot O. Improvement of schizophrenic patients with primary negative symptoms treated with amisulpride. Amisulpride Study Group. Am J Psychiatry. 1999;156(4):610–616. doi: 10.1176/ajp.156.4.610. [DOI] [PubMed] [Google Scholar]
- 39.Keck P, Jr, Buffenstein A, Ferguson J, et al. Ziprasidone 40 and 120 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 4-week placebo-controlled trial. Psychopharmacology (Berl) 1998;140(2):173–184. doi: 10.1007/s002130050755. [DOI] [PubMed] [Google Scholar]
- 40.Lecrubier Y, Quintin P, Bouhassira M, Perrin E, Lancrenon S. The treatment of negative symptoms and deficit states of chronic schizophrenia: olanzapine compared to amisulpride and placebo in a 6-month double-blind controlled clinical trial. Acta psychiatr Scand. 2006;114(5):319–327. doi: 10.1111/j.1600-0447.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- 41.Loo H, Poirier-Littre MF, Theron M, Rein W, Fleurot O. Amisulpride versus placebo in the medium-term treatment of the negative symptoms of schizophrenia. Br J Psychiatry. 1997;170:18–22. doi: 10.1192/bjp.170.1.18. [DOI] [PubMed] [Google Scholar]
- 42.Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151(6):825–835. doi: 10.1176/ajp.151.6.825. [DOI] [PubMed] [Google Scholar]
- 43.Meltzer HY, Arvanitis L, Bauer D, Rein W. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161(6):975–984. doi: 10.1176/appi.ajp.161.6.975. [DOI] [PubMed] [Google Scholar]
- 44.Pigott TA, Carson WH, Saha AR, Torbeyns AF, Stock EG, Ingenito GG. Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. J Clin Psychiatry. 2003;64(9):1048–1056. doi: 10.4088/jcp.v64n0910. [DOI] [PubMed] [Google Scholar]
- 45.Pigott TA, Carson WH, Saha AR, Torbeyns AF, Stock EG, Ingenito GG. Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. J Clinical Psychiatry. 2003;64(9):1048–1056. doi: 10.4088/jcp.v64n0910. [DOI] [PubMed] [Google Scholar]
- 46.Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60(7):681–690. doi: 10.1001/archpsyc.60.7.681. [DOI] [PubMed] [Google Scholar]
- 47.Small JG, Hirsch SR, Arvanitis LA, Miller BG, Link CG. Quetiapine in patients with schizophrenia. A high- and low-dose double-blind comparison with placebo. Seroquel Study Group. Arch Gen Psychiatry. 1997;54(6):549–557. doi: 10.1001/archpsyc.1997.01830180067009. [DOI] [PubMed] [Google Scholar]
- 48.Tollefson GD, Dellva MA, Mattler CA, Kane JM, Wirshing DA, Kinon BJ. Controlled, double-blind investigation of the clozapine discontinuation symptoms with conversion to either olanzapine or placebo. The Collaborative Crossover Study Group. J Clin psychopharmacol. 1999;19(5):435–443. doi: 10.1097/00004714-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 49.van Kammen DP, McEvoy JP, Targum SD, Kardatzke D, Sebree TB. A randomized, controlled, dose-ranging trial of sertindole in patients with schizophrenia. Psychopharmacology (Berl) 1996;124(1–2):168–175. doi: 10.1007/BF02245618. [DOI] [PubMed] [Google Scholar]
- 50.Arato M, O'Connor R, Meltzer HY. A 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: the Ziprasidone Extended Use in Schizophrenia (ZEUS) study. Int Clin Psychopharmacol. 2002;17(5):207–215. doi: 10.1097/00004850-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Woods SW, Breier A, Zipursky RB, et al. Randomized trial of olanzapine versus placebo in the symptomatic acute treatment of the schizophrenic prodrome. Biological Psychiatry. 2003;54(4):453–464. doi: 10.1016/s0006-3223(03)00321-4. [DOI] [PubMed] [Google Scholar]
- 52.Team RDC. Vienna, Austria: R Foundation for Statistical Computing; 2006. R: a Language and Environment for Statistical Computing. [Google Scholar]
- 53.Schwarzer G. http://cran.r-project.org/doc/packages/meta.pdf. [Google Scholar]
- 54.Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol. 2004;24(2):192–208. doi: 10.1097/01.jcp.0000117422.05703.ae. [DOI] [PubMed] [Google Scholar]
- 55.Viechtbauer W. MiMa: an S-Plus/R function to fit meta-analytic mixed-, random-, and fixed-effects models [computer program]. Version 2006. Available from http://www.wvbauer.com [Google Scholar]
- 56.Addington DE, Pantelis C, Dineen M, Benattia I, Romano SJ. Efficacy and tolerability of ziprasidone versus risperidone in patients with acute exacerbation of schizophrenia or schizoaffective disorder: an 8-week, double-blind, multicenter trial. J Clin Psychiatry. 2004;65(12):1624–1633. doi: 10.4088/jcp.v65n1207. [DOI] [PubMed] [Google Scholar]
- 57.Azorin JM, Spiegel R, Remington G, et al. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry. 2001;158(8):1305–1313. doi: 10.1176/appi.ajp.158.8.1305. [DOI] [PubMed] [Google Scholar]
- 58.Beasley CM, Jr., Hamilton SH, Crawford AM, et al. Olanzapine versus haloperidol: acute phase results of the international double-blind olanzapine trial. Eur Neuropsychopharmacol. 1997;7(2):125–137. doi: 10.1016/s0924-977x(96)00392-6. [DOI] [PubMed] [Google Scholar]
- 59.Bitter I, Dossenbach MR, Brook S, et al. Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):173–180. doi: 10.1016/j.pnpbp.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 60.Brook S, Krams M, Gunn KP Ziprasidone_IM_Study_Group. The efficacy and tolerability of intramuscular (IM) ziprasidone versus IM haloperidol in patients with acute, non-organic psychosis. 151st American Psychiatric Association Meeting. 1998 [Google Scholar]
- 61.Buchanan RW, Ball MP, Weiner E, et al. Olanzapine treatment of residual positive and negative symptoms. Am J Psychiatry. 2005;162(1):124–129. doi: 10.1176/appi.ajp.162.1.124. [DOI] [PubMed] [Google Scholar]
- 62.Buchanan RW, Breier A, Kirkpatrick B, Ball P, Carpenter WT., Jr Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry. 1998;155(6):751–760. doi: 10.1176/ajp.155.6.751. [DOI] [PubMed] [Google Scholar]
- 63.Carriere P, Bonhomme D, Lemperiere T. Amisulpride has a superior benefit/risk profile to haloperidol in schizophrenia: results of a multicentre, double-blind study (the Amisulpride Study Group) Eur Psychiatry. 2000;15(5):321–329. doi: 10.1016/s0924-9338(00)00401-6. [DOI] [PubMed] [Google Scholar]
- 64.Casey DE, Carson WH, Saha AR, et al. Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology (Berl) 2003;166(4):391–399. doi: 10.1007/s00213-002-1344-3. [DOI] [PubMed] [Google Scholar]
- 65.Chan H, Chen C, Chen J, Sun H, Chiu H-J, Chang C. A comparison of olanzapine and risperidone for the schizophrenic patients intolerance of neuroleptic-induced extrapyramidal syndromes (eps) J Eur Coll Neuropsychopharmacol. 2003;13(4):S316. [Google Scholar]
- 66.Chang Fw, Wang CH, Zhao Z. Clinical effects of olanzapine vs chlorpromazine in treating positive symptoms of schizophrenia. Chin J New Drugs and Clin Remedies. 2003;22(6):357–359. [Google Scholar]
- 67.Chue P, Eerdekens M, Augustyns I, et al. Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol. 2005;15(1):111–117. doi: 10.1016/j.euroneuro.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Conley RR, Kelly DL, Nelson MW, et al. Risperidone, quetiapine, and fluphenazine in the treatment of patients with therapy-refractory schizophrenia. Clin Neuropharmacol. 2005;28(4):163–168. doi: 10.1097/01.wnf.0000172993.89879.0f. [DOI] [PubMed] [Google Scholar]
- 69.Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry. 2001;158(5):765–774. doi: 10.1176/appi.ajp.158.5.765. [DOI] [PubMed] [Google Scholar]
- 70.Conley RR, Tamminga CA, Bartko JJ, et al. Olanzapine compared with chlorpromazine in treatment-resistant schizophrenia. Am J Psychiatry. 1998;155(7):914–920. doi: 10.1176/ajp.155.7.914. [DOI] [PubMed] [Google Scholar]
- 71.Emsley RA. Risperidone in the treatment of first-episode psychotic patients: a double-blind multicenter study. Risperidone Working Group. Schizophr Bull. 1999;25(4):721–729. doi: 10.1093/oxfordjournals.schbul.a033413. [DOI] [PubMed] [Google Scholar]
- 72.Gureje O, Miles W, Keks N, et al. Olanzapine vs risperidone in the management of schizophrenia: a randomized double-blind trial in Australia and New Zealand. Schizophr Res. 2003;61(2–3):303–314. doi: 10.1016/s0920-9964(02)00226-8. [DOI] [PubMed] [Google Scholar]
- 73.Heck AH, Haffmans PM, de Groot IW, Hoencamp E. Risperidone versus haloperidol in psychotic patients with disturbing neuroleptic-induced extrapyramidal symptoms: a double-blind, multi-center trial. Schizophr Res. 2000;46(2–3):97–105. doi: 10.1016/s0920-9964(00)00009-8. [DOI] [PubMed] [Google Scholar]
- 74.HGFH_Korea. HGFH Korea. Data on file: Cochrane Schizophrenia Group. 1989. [Google Scholar]
- 75.Hoyberg OJ, Fensbo C, Remvig J, Lingjaerde O, Sloth-Nielsen M, Salvesen I. Risperidone versus perphenazine in the treatment of chronic schizophrenic patients with acute exacerbations. Acta psychiatr Scand. 1993;88(6):395–402. doi: 10.1111/j.1600-0447.1993.tb03480.x. [DOI] [PubMed] [Google Scholar]
- 76.Huttunen MO, Piepponen T, Rantanen H, Larmo I, Nyholm R, Raitasuo V. Risperidone versus zuclopenthixol in the treatment of acute schizophrenic episodes: a double-blind parallel-group trial. Acta psychiatr Scand. 1995;91(4):271–277. doi: 10.1111/j.1600-0447.1995.tb09781.x. [DOI] [PubMed] [Google Scholar]
- 77.Ishigooka J, Inada T, Miura S. Olanzapine versus haloperidol in the treatment of patients with chronic schizophrenia: results of the Japan multicenter, double-blind olanzapine trial. Psychiatry Clin Neurosci. 2001;55(4):403–414. doi: 10.1046/j.1440-1819.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- 78.Jakovljevic M, Dossenbach M. Olanzapine versus fluphenazine in the acute (six-week) treatment of schizophrenia. Psychiatr Danub. 1999;11(1–2):3–10. [Google Scholar]
- 79.Jeste DV, Barak Y, Madhusoodanan S, Grossman F, Gharabawi G. International multisite double-blind trial of the atypical antipsychotics risperidone and olanzapine in 175 elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2003;11(6):638–647. doi: 10.1176/appi.ajgp.11.6.638. [DOI] [PubMed] [Google Scholar]
- 80.Kane JM, Marder SR, Schooler NR, et al. Clozapine and haloperidol in moderately refractory schizophrenia: a 6-month randomized and double-blind comparison. Arch Gen Psychiatry. 2001;58(10):965–972. doi: 10.1001/archpsyc.58.10.965. [DOI] [PubMed] [Google Scholar]
- 81.Kennedy JS, Jeste D, Kaiser CJ, et al. Olanzapine vs haloperidol in geriatric schizophrenia: analysis of data from a double-blind controlled trial. Int J Geriatr Psychiatry. 2003;18(11):1013–1020. doi: 10.1002/gps.1007. [DOI] [PubMed] [Google Scholar]
- 82.Kudo YNJ, Ikawa G, Nakajima T, et al. Annual Meeting of the World Psychiatric Association. Hamburg, Germany: 1999. Clinical trial of quetiapine in schizophrenia - efficacy and tolerability of quetiapine: a comparative double-blind study with masopramine in schizophrenic patients. [Google Scholar]
- 83.Lee CT, Conde BJ, Mazlan M, et al. Switching to olanzapine from previous antipsychotics: a regional collaborative multicenter trial assessing 2 switching techniques in Asia Pacific. J Clinical Psychiatry. 2002;63(7):569–576. doi: 10.4088/jcp.v63n0706. [DOI] [PubMed] [Google Scholar]
- 84.Lieberman JA, Tollefson G, Tohen M, et al. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160(8):1396–1404. doi: 10.1176/appi.ajp.160.8.1396. [DOI] [PubMed] [Google Scholar]
- 85.Martin S, Ljo H, Peuskens J, et al. A double-blind, randomised comparative trial of amisulpride versus olanzapine in the treatment of schizophrenia: short-term results at two months. Curr Med Res Opin. 2002;18(6):355–362. doi: 10.1185/030079902125001128. [DOI] [PubMed] [Google Scholar]
- 86.McQuade R, Jody D, Kujawa M, Carson W, Iwamoto T, Archibald D. 156th Annual Meeting of the American Psychiatric Association. San Francisco: 2003. Long-term weight effects of aripiprazole vs. olanzapine. [Google Scholar]
- 87.Messotten F. Risperidone versus haloperidol in the teatment of chronic psychotic patients: a multicentre double-blind study. Unpublished data on file from Janssen: Cochrane Colloboration. 1991 [Google Scholar]
- 88.Moller HJ, Boyer P, Fleurot O, Rein W. Improvement of acute exacerbations of schizophrenia with amisulpride: a comparison with haloperidol. PROD-ASLP Study Group. Psychopharmacology (Berl) 1997;132(4):396–401. doi: 10.1007/s002130050361. [DOI] [PubMed] [Google Scholar]
- 89.Mortimer A, Martin S, Loo H, Peuskens J. A double-blind, randomized comparative trial of amisulpride versus olanzapine for 6 months in the treatment of schizophrenia. Int Clin Psychopharmacology. 2004;19(2):63–69. doi: 10.1097/00004850-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 90.Mullen J, Jibson MD, Sweitzer D. A comparison of the relative safety, efficacy, and tolerability of quetiapine and risperidone in outpatients with schizophrenia and other psychotic disorders: the quetiapine experience with safety and tolerability (QUEST) study. Clin Ther. 2001;23(11):1839–1854. doi: 10.1016/s0149-2918(00)89080-3. [DOI] [PubMed] [Google Scholar]
- 91.Naber D, Riedel M, Klimke A, et al. Randomized double blind comparison of olanzapine vs. clozapine on subjective well-being and clinical outcome in patients with schizophrenia. Acta Psychiatr Scand. 2005;111(2):106–115. doi: 10.1111/j.1600-0447.2004.00486.x. [DOI] [PubMed] [Google Scholar]
- 92.Peuskens J, Bech P, Moller HJ, Bale R, Fleurot O, Rein W. Amisulpride vs. risperidone in the treatment of acute exacerbations of schizophrenia. Amisulpride study group. Psychiatry Res. 1999;88(2):107–117. doi: 10.1016/s0165-1781(99)00075-x. [DOI] [PubMed] [Google Scholar]
- 93.Peuskens J, Link CG. A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta Psychiatr Scand. 1997;96(4):265–273. doi: 10.1111/j.1600-0447.1997.tb10162.x. [DOI] [PubMed] [Google Scholar]
- 94.Puech A, Fleurot O, Rein W. Amisulpride, and atypical antipsychotic, in the treatment of acute episodes of schizophrenia: a dose-ranging study vs. haloperidol. The Amisulpride Study Group. Acta Psychiatr Scand. 1998;98(1):65–72. doi: 10.1111/j.1600-0447.1998.tb10044.x. [DOI] [PubMed] [Google Scholar]
- 95.Purdon SE, Jones BD, Stip E, et al. Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. The Canadian Collaborative Group for research in schizophrenia. Arch Genl Psychiatry. 2000;57(3):249–258. doi: 10.1001/archpsyc.57.3.249. [DOI] [PubMed] [Google Scholar]
- 96.Ritchie CW, Chiu E, Harrigan S, et al. The impact upon extra-pyramidal side effects, clinical symptoms and quality of life of a switch from conventional to atypical antipsychotics (risperidone or olanzapine) in elderly patients with schizophrenia. Int J Geriatr Psychiatry. 2003;18(5):432–440. doi: 10.1002/gps.862. [DOI] [PubMed] [Google Scholar]
- 97.Sanger TM, Lieberman JA, Tohen M, Grundy S, Beasley C, Jr., Tollefson GD. Olanzapine versus haloperidol treatment in first-episode psychosis. Am J Psychiatry. 1999;156(1):79–87. doi: 10.1176/ajp.156.1.79. [DOI] [PubMed] [Google Scholar]
- 98.Sechter D, Peuskens J, Fleurot O, Rein W, Lecrubier Y. Amisulpride vs. risperidone in chronic schizophrenia: results of a 6-month double-blind study. Neuropsychopharmacology. 2002;27(6):1071–1081. doi: 10.1016/S0893-133X(02)00375-5. [DOI] [PubMed] [Google Scholar]
- 99.Simpson GM, Glick ID, Weiden PJ, Romano SJ, Siu CO. Randomized, controlled, double-blind multicenter comparison of the efficacy and tolerability of ziprasidone and olanzapine in acutely ill inpatients with schizophrenia or schizoaffective disorder. Am J Psychiatry. 2004;161(10):1837–1847. doi: 10.1176/ajp.161.10.1837. [DOI] [PubMed] [Google Scholar]
- 100.Speller JC, Barnes TR, Curson DA, Pantelis C, Alberts JL. One-year, low-dose neuroleptic study of in-patients with chronic schizophrenia characterised by persistent negative symptoms. Amisulpride v. haloperidol. Br J Psychiatry. 1997;171:564–568. doi: 10.1192/bjp.171.6.564. [DOI] [PubMed] [Google Scholar]
- 101.Tran PV, Dellva MA, Tollefson GD, Wentley AL, Beasley CM., Jr Oral olanzapine versus oral haloperidol in the maintenance treatment of schizophrenia and related psychoses. Br J Psychiatry. 1998;172:499–505. doi: 10.1192/bjp.172.6.499. [DOI] [PubMed] [Google Scholar]
- 102.Tran PV, Hamilton SH, Kuntz AJ, et al. Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol. 1997;17(5):407–418. doi: 10.1097/00004714-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 103.Volavka J, Czobor P, Sheitman B, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry. 2002;159(2):255–262. doi: 10.1176/appi.ajp.159.2.255. [DOI] [PubMed] [Google Scholar]
- 104.Wetzel H, Grunder G, Hillert A, et al. Amisulpride versus flupentixol in schizophrenia with predominantly positive symptomatology – a double-blind controlled study comparing a selective D2-like antagonist to a mixed D1-/D2-like antagonist. The Amisulpride Study Group. Psychopharmacology (Berl) 1998;137(3):223–232. doi: 10.1007/s002130050614. [DOI] [PubMed] [Google Scholar]
- 105.Wirshing DA, Marshall BD, Jr., Green MF, Mintz J, Marder SR, Wirshing WC. Risperidone in treatment-refractory schizophrenia. Am J Psychiatry. 1999;156(9):1374–1379. doi: 10.1176/ajp.156.9.1374. [DOI] [PubMed] [Google Scholar]
- 106.Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY, Shen YC. Risperidone versus haloperidol in the treatment of acute exacerbations of chronic inpatients with schizophrenia: a randomized double-blind study. Int Clin Psychopharmacol. 2001;16(6):325–330. doi: 10.1097/00004850-200111000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.