Abstract
Poor social and vocational outcomes have long been observed in schizophrenia. Two of the most consistent predictors are negative symptoms and cognitive impairment. We investigate the hypothesis that cognitive content—defeatist beliefs regarding performance—provides a link between cognitive impairment, negative symptoms, and poor functioning in schizophrenia. A total of 77 individuals (55 patients diagnosed with schizophrenia or schizoaffective disorder and 22 healthy controls) participated in a cross-sectional study of psychopathology. Tests of memory, abstraction, attention, and processing speed, as well as current psychopathology, functioning, and endorsement of defeatist beliefs, were employed. Greater neurocognitive impairment was associated with elevated defeatist belief endorsement, higher negative symptom levels, and worse social and vocational functioning. Notably, statistical modeling indicated that defeatist belief endorsements were mediators in the relationship between cognitive impairment and both negative symptoms and functioning. These effects were independent of depression and positive symptom levels. The results add to the emerging biopsychosocial understanding of negative symptoms and introduce defeatist beliefs as a new psychotherapeutic target.
Keywords: neurocognitive impairment, negative symptoms, functioning
Introduction
For a substantial proportion of patients diagnosed with schizophrenia, social and vocational disability diminishes their quality of life and significantly limits the extent of their recovery.1,2 Negative symptoms and neurocognitive impairment, in contrast to psychotic symptoms, predict poor social and vocational functioning in schizophrenia.3–6 A moderate cross-sectional association has been observed between negative symptoms and cognitive impairment,7 each showing minimal association with positive symptoms. Antipsychotic medications have not demonstrated significant efficacy relative to either negative symptoms or cognitive impairment, a fact that is underscored in the National Institute of Mental Health's recent initiatives (Measurement and Treatment Research Initiative to Improve Cognition in Schizophrenia—MATRICS) designed to improve treatment and measurement in both domains.8,9
Models of negative symptoms, cognitive impairment, and functioning, whether emphasizing neurobiological pathology in the manner of Hughlings Jackson10 and Kraepelin11 or cognitive dysfunction in the tradition of Bleuler,12 have received mixed empirical support.13 Moreover, a direct connection between negative symptoms and cognitive impairment is not self-evident: neurocognitive problems such as mental inflexibility, memory, and attention difficulties are not isomorphic with the behavioral and affective symptoms such as anhedonia, asociality, and lack of motivation. Additionally, the process by which cognitive problems lead to negative symptoms remains unclear.
We propose that the lack of a clear fit between impairment and symptoms occurs because the connection is indirect. Put in terms of the conceptual scheme proposed by Harvey et al14 for understanding the “true” relationship between these constructs, we suggest that cognitive impairment and negative symptoms are linked through another construct. Recent research provides a clue to the identity of this common correlate: patients with higher negative symptom levels have been found to endorse defeatist beliefs regarding task performance (eg, “if I fail partly, it is as bad as being a complete failure”) to a greater extent than patients with lesser negative symptomatology—an association that holds when depression levels are controlled.15
Might defeatist beliefs also link cognitive impairment with negative symptoms and functioning in schizophrenia? Such a finding is predicted by the cognitive-behavioral model of psychopathology16,17 and suggests that specific performance-related cognitive content can serve as a bridge between compromised cognitive processing of attention, memory, and abstraction, on the one hand, and the emotional, motivational, and behavioral deficits of negative symptoms and poor functioning, on the other. Because neurocognitive performance, negative symptom levels, and functioning are all abnormal in schizophrenia, our hypothesis first requires, at minimum, that individuals with schizophrenia endorse defeatist beliefs to a greater extent than healthy individuals. While previous research suggests that defeatist beliefs correlate with negative symptoms,15 patients with schizophrenia have not, heretofore, been directly compared with healthy controls on the measure. A second requirement of our hypothesis is that the statistical relationship between defeatist beliefs, neurocognitive impairment, and negative symptoms is consistent with mediation. A similar relationship is predicted to hold between defeatist beliefs, neurocognitive impairment, and functioning. We, thus, conducted a cross-sectional investigation in which outpatients diagnosed with schizophrenia and healthy controls were administered measures of symptomatology, neurocognition, functioning, and defeatist beliefs.
Methods
Participants
The sample included 55 stable outpatients, with a diagnosis of schizophrenia or schizoaffective disorder, and 22 healthy controls. Patients with negative symptoms were referred by the Schizophrenia Research Center at the University of Pennsylvania. All recruitment contacts were made blind to current patient symptomatology and level of functioning. Nonpatients were recruited so as to match the patient sample on demographic variables, including gender and socioeconomic status (see table 1). The patient group was, on average, older than controls (F1,76 = 4.3, P < .05); age was controlled in the group statistical comparison.
Table 1.
Sample Characteristics
| Patient (N = 55) |
Control (N = 22) |
|||
| Mean | SD | Mean | SD | |
| Age (y)a | 36.9 | 9.9 | 31.8 | 7.8 |
| Gender (% male) | 65 | (N = 36) | 68 | (N = 15) |
| Parent education (y)b | 13.0 | 2.8 | 13.9 | 2.7 |
| Illness onset (age, y) | 22.0 | 5.8 | — | — |
| Length of Illness (y) | 14.4 | 9.8 | — | — |
| Diagnosis | N | % | ||
| Schizophrenia | ||||
| Paranoid | 22 | 40 | ||
| Undifferentiated | 26 | 47 | ||
| Disorganized/ catatonic | 2 | 4 | ||
| Schizoaffective | ||||
| Bipolar | 4 | 7 | ||
| Unipolar | 1 | 2 | ||
P <.05.
Parent education is average of father's and mother's education level.
Procedure
All subjects participated in a single research session lasting between 2 and 4 hours. After the procedure was fully explained, written informed consent was obtained from all subjects. All participants were compensated for participation. This procedure was approved by the Institutional Review Board at the University of Pennsylvania.
Trained interviewers (MA and/or PhD) administered symptom, attitude, and functioning measures. These measures are a mix of interviewer-scored and self-report instruments. Collateral information from family members and treating psychiatrists was factored into clinician ratings of symptoms and functioning. Neurocognitive impairment was assessed via computerized tasks. All ratings of symptoms and functioning were made blind to neurocognitive performance and degree of defeatist belief endorsment.
Materials
Clinical Measures.
The total score, sans the attention subscale and inappropriate affect, of the Scale for the Assessment of Negative Symptoms18 was used as an index of negative symptoms. The total scores of the psychotic factor and the disorganized factor of the Scale for the Assessment of Positive Symptoms19 were employed to measure positive symptoms. Quality of Life Scale, Abbreviated20 total score assessed current functioning. Finally, depression and anxiety were self-reported on the Beck Depression Inventory II21 and Beck Anxiety Inventory,22 respectively.
Belief Endorsement.
Beliefs were assessed with the Dysfunctional Attitude Scale (DAS).23 The defeatist performance belief subscale consists of 15 statements describing overgeneralized conclusions about one's ability to perform tasks (eg, “If you cannot do something well, there is little point in doing it at all”; see Appendix). The dysfunctional need for acceptance subscale consists of 10 statements that exaggerate the importance of being accepted by other people (eg, “I cannot be happy unless most people I know admire me”).
Neurocognitive Performance.
Cognitive impairment was assessed via 5 computerized tasks that have previously been applied to healthy participants24 and individuals diagnosed with schizophrenia.25 Tasks were selected to assess the following neurocognitive domains that meta-analysis26 indicates are related to poor outcome in schizophrenia: abstraction/mental flexibility,27,28 verbal memory,29 and attention/vigilance.30 Additionally, processing speed was also assessed. The tests, programmed in Flash Media, were presented in a window within a web browser (Mozilla Firefox) on either a laptop or desktop computer. Following previously established procedures,31 (a) accuracy and median response times were computed from raw scores of each test and converted to z scores using normative data; (b) abstraction, verbal memory, and attention/vigilance domain scores were computed by averaging the appropriate standardized accuracy values; (c) the standardized median response times for the 5 tests were averaged to compute the processing speed domain; and (d) the 3 accuracy domain scores were averaged along with the negative of the processing speed domain score to create a general index of neurocognitive performance.
Statistical Analysis
Statistical analyses were conducted to address 2 hypotheses: first, patients will differ from controls by evidencing greater defeatist belief endorsement; second, the patient data will be consistent with defeatist beliefs as a potential mediating variable between cognitive impairment and negative symptoms, on the one hand, and cognitive impairment and functioning, on the other. Because the present investigation was a partial replication of Rector's15 earlier work, a comparable sample size (N = 55) was employed.
Hypothesis 1: Abnormal Defeatist Belief Endorsement.
One-way analysis of covariance (ANCOVA) was employed: the covariate was age, group served as independent variable, and belief endorsement served as the dependent variable. ANCOVA models were tested for both defeatist belief and need for acceptance endorsements. Additionally, patient-control effect sizes (ie, Cohen's d)32were estimated to allow comparison across study measures. We followed Cohen's scheme in categorizing effect sizes: small = 0.3, medium = 0.5, and large = 0.8.
Hypothesis 2: Mediation.
Correlation
Pearson product-moment correlations were calculated for all study variables. Only statistically reliable correlations were retained for subsequent statistical analysis. Partial correlations were also calculated to clarify interrelationships between variables.
Path analysis
Three component models composed of independent (X), dependent (Y), and mediator (M) variables were estimated as (a) an unelaborated model consisting of the direct relationship between independent and dependent variables and (b) an elaborated model including the mediator.33 We followed Shrout and Bolger's34 5-step procedure for determining if a 3-variable model is consistent with mediation of a proximal effect. Two 3-variable models were assessed: (a) neurocognitive performance (X), defeatist beliefs (M), and negative symptoms (Y); (b) neurocognitive performance (X), defeatist beliefs (M), and functioning (Y). Because it is generally advised to have a 20:1 ratio of subjects to parameters in path analysis,35models composed of more than 3 freely varying parameters were not estimated. Additionally, nonparametric bootstrapping, which has been found to be more sensitive in mediation analyses when sample sizes are less than 100,34 was employed to estimate SEs of the direct and indirect effects for significance testing. For each model, SEs were SDs of a pseudopupulation based upon 5000 samples drawn with replacement from the patient data. A final index of mediation is the change in variance accounted for by the inclusion of the mediator in the model. This was determined by significance test of the change in the square of the multiple correlation coefficient (R2 change) associated with the mediator being included in the model.
Results
Group Differences
Table 2 contains group comparison data across all study measures. Of note, and consistent with prediction, the patient group endorsed defeatist performance beliefs to a greater extent than the healthy controls (F1,74 = 15.48, P < .001). The effect size for this difference is large and comparable to patient-control differences for the other study measures. Patients, additionally, endorsed more need for acceptance attitudes than healthy controls (F1,74 = 9.5, P < .01). As compared with the healthy controls, the patient sample evidenced greater symptomatology, worse functioning, poorer cognitive performance, and elevated dysfunctional belief endorsement.
Table 2.
Group Differencesa
| Patients (N = 55) |
Controls (N = 22) |
||||
| Mean | SD | Mean | SD | Effect Size | |
| Negative | 23.7 | 12.1 | — | — | — |
| Psychotic | 14.3 | 13.4 | — | — | — |
| Disorganized | 5.4 | 7.6 | — | — | — |
| BDI | 13.0 | 11.5 | 4.9 | 4.6 | 0.74 |
| BAI | 12.0 | 11.3 | 1.4 | 2.1 | 0.97 |
| QOLA | 22.5 | 9.3 | 39.2 | 2.8 | −1.5 |
| NP | −0.84 | 0.98 | 0.11 | 0.4 | −0.99 |
| Defeatist beliefs | 43.2 | 14.1 | 30.1 | 9.9 | 0.92 |
| Need for acceptance | 35.3 | 10.4 | 27.4 | 9.6 | 0.73 |
Negative = total score, Scale for the Assessment of Negative Symtpoms18; psychotic = sum of hallucinations and delusions items, Scale for the Assessment of Positive Symptoms19; disorganized = sum of bizarre behavior and formal thought disorder items, Scale for the Assessment of Positive Symptoms19; BDI = Beck Depression Inventory21; QOLA = Quality of Life Inventory, Abridged20; NP = neurocognitive performance, average standardized score; defeatist beliefs = subscale, Dysfunctional Attitude Scale23; need for acceptance = subscale, Dysfunctional Attitude Scale.23
Correlations
Table 3 presents Pearson correlations between all the primary study measures in the patient sample.
Table 3.
Correlations: Patient Data (N = 55)a
| Neurocognitive Performance | Negative Symptoms | Functioning | Defeatist Beliefs | Psychotic | Disorganized | Depression | Anxiety | |
| Negative symptoms | −0.37b | — | — | — | — | — | — | — |
| Functioning | 0.47b | −0.81b | — | — | — | — | — | — |
| Defeatist beliefs | −0.32c | 0.49b | −0.45b | — | — | — | — | — |
| Psychotic | 0.01 | 0.22 | −0.30c | 0.20 | — | — | — | — |
| Disorganized | −0.32c | 0.24 | −0.32c | 0.34c | 0.36b | — | — | — |
| Depression | −0.03 | 0.20 | −0.24 | 0.41b | 0.27c | −0.10 | — | — |
| Anxiety | −0.17 | 0.25 | −0.25 | 0.34c | 0.31c | 0.00 | 0.66b | — |
| Need for acceptance | −0.15 | 0.12 | −0.22 | 0.64b | 0.18 | 0.35b | 0.26 | 0.14 |
Neurocognitive performance = average standardized score; negative symptoms = total score, Scale for the Assessment of Negative Symptoms18; functioning = Quality of Life Inventory, Abridged20; defeatist beliefs = subscale, Dysfunctional Attitude Scale23; psychotic = sum of hallucinations and delusions items, Scale for the Assessment of Positive Symptoms19; disorganized = sum of bizarre behavior and formal thought disorder items, Scale for the Assessment of Positive Symptoms19; depression = Beck Depression Inventory21; anxiety = Beck Anxiety Inventory.22
P < .01.
P < .05.
Defeatist Beliefs.
Consistent with the literature, neurocognitive performance correlates significantly both with functioning and negative symptoms and does not correlate with psychotic symptoms (P > .90). Defeatist beliefs also correlate with negative symptoms and functioning but not with psychotic symptoms (P > .10). The direction of this pattern of correlation suggests that patients who endorse defeatist beliefs tend to have elevated negative symptoms and poorer functioning and perform poorly on the neurocognitive tests. These variables will be subject to the mediation analysis.
Depression and Need for Acceptance.
Endorsement of need for acceptance beliefs, according to prediction, is not reliably correlated with key study variables of neurocognitive performance (P > .25), negative symptoms (P > .40), and functioning (P > .10). The association between depression and neurocognitive performance is also not reliably different from zero (P > .80); negative symptoms, moreover, do not correlate significantly with levels of depressive symptoms (P > .15). The correlation between depression and functioning is a nonsignificant trend (.10 > P > .05). Both depression and need for acceptance correlate moderately with defeatist beliefs. However, the partial correlation between defeatist beliefs and negative symptoms, while controlling both for depression levels and need for acceptance endorsements, is statistically significant (r51 = .53, P < .001), as is the partial correlation between defeatist beliefs and neurocognitive impairment when depression and need for acceptance are statistically controlled (r50 = −.33, P < .05). Accordingly, need for acceptance beliefs and depression will not be included in the mediation analysis.
Disorganized Symptoms.
Neurocognitive performance correlates significantly with disorganized symptoms. However, the partial correlation is significant between neurocognitive performance and negative symptoms while statistically controlling disorganized symptoms (r51 = −.32, P < .05). Disorganized symptoms will not be included in the mediation analysis.
Functioning and Negative Symptoms.
The measures of functioning and negative symptoms are highly intercorrelated in this sample (r53 = .80, P < .001). Such a high level of colinearity precludes modeling the 2 variables together because very little variance will be left for other variables.36,37
Mediation: Neurocognitive Performance, Negative Symptoms, and Defeatist Beliefs.
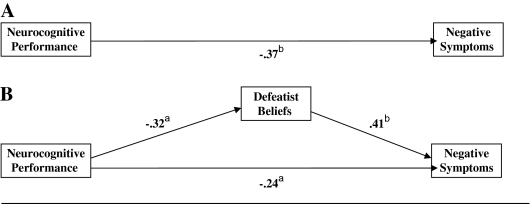
Figure 1 contains the results of the path analysis that forms the basis of the test of mediation: the independent variable is neurocognitive performance, the dependent variable is negative symptoms, and defeatist beliefs are the putative mediating variable.
Fig. 1.
Path Analysis (N = 54). Figure 1A depicts the simple model of neurocognitive performance and negative symptoms. Figure 1B depicts the full model that also includes defeatist beliefs. Neurocognitive performance = average standardized score; negative symptoms = total score, Scale for the Assessment of Negative Symptoms18; defeatist beliefs = subscale, Dysfunctional Attitude Scale.23 aP < .05. bP < .01.
Step 1
Figure 1A depicts the simple path model representing the relationship between neurocognitive performance and negative symptom levels. This path is statistically significant (z = −3.22, P < .01), satisfying step 1 of Shrout and Bolger's34 revised version of Baron and Kenny's 33 method.
Step 2
The path on the left side of figure 1B depicts the association between neurocognitive performance and defeatist beliefs. This path is also statistically significant (z = −2.49, P < .05) satisfying step 2: participants who perform more poorly on the tasks tend to endorse defeatist attitudes to a greater extent.
Step 3
On the right side of figure 1B appears the path representing the association between defeatist beliefs and negative symptoms. In accord with step 3 of mediation, this path is statistically reliable (z = 3.38, P < .001). Patients with more severe negative symptoms tend to endorse more defeatist beliefs. This relationship is independent of the association of neurocognitive performance upon negative symptoms.
Step 4
The path at the bottom of figure 1B represents the relationship between neurocognitive performance and negative symptoms, controlling for the effects of defeatist beliefs. This path is reduced in magnitude, relative to the comparable path in figure 1A, which suggests mediation according to Baron and Kenny's method.33 Table 4 contains the effect decomposition based on the bootstrap analysis. The indirect effect of neurocognitive performance on negative symptoms through defeatist beliefs is statistically reliable (90% confidence interval [CI] = −0.28 to −0.04, P < .05), confirming that the data are consistent with mediation.
Table 4.
Bootstrapping Mediation Effect Decompostion: Neurocognitive Performance, Defeatist Beliefs, and Negative Symptoms (Patient Data, N = 54)a
| Negative Symptoms |
|||
| Neurocognitive Performance | Unstandardized Estimate | Standard Error | Standardized Estimate |
| Direct effect | −3.00b | 1.48 | −0.24 |
| Indirect effect | −1.66b | 0.88 | −0.13 |
| Total effect | −4.66c | 1.45 | −0.37 |
Neurocognitive performance = average standardized score; negative symptoms = total score, Scale for the Assessment of Negative Symptoms18; direct effect = neurocognitive performance → negative symptoms; indirect effect = neurocognitive performance → defeatist beliefs → negative symptoms.
P < .05.
P < .01.
Step 5
The path coefficient at the bottom of figure 1B is statistically reliable (z = −1.97, P ≤ .05), suggesting that the data are consistent with partial mediation. The bootstrapping estimate (table 4) of the direct path between neurocognitive impairment and negative symptom is also statistically reliable (90% CI = −0.41 to −0.05, P < .05). The data are consistent with partial mediation. The ratio of the indirect effect to total effect is 0.35, indicating that defeatist beliefs account for about one-third of the effect of neurocognition on negative symptoms.
R2 change
The squared Pearson correlation between neurocognitive performance and negative symptoms is .14. The squared multiple correlation for the 3-variable model that also includes defeatist beliefs is .29. Thus, the change in R2 associated with adding defeatist beliefs to the model is .15, which is statistically different from zero (F-change1,51 = 11.0, P < .01), evidence that inclusion of defeatist beliefs in the model accounts for an additional 15% of the variance.
Mediation: Neurocognitive Performance, Functioning, and Defeatist Beliefs.
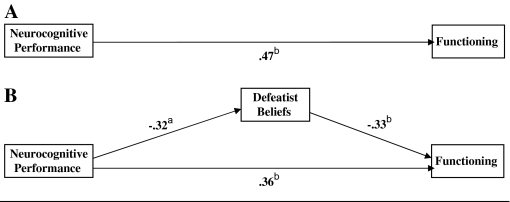
Figure 2 depicts the path analysis testing whether the relationship between neurocognitive performance and functioning is mediated by defeatist belief endorsements.
Fig. 2.
Path Analysis (N = 54). Figure 2A depicts the simple model of neurocognitive performance and functioning. Figure 2B depicts the full model that also includes defeatist beliefs. Neurocognitive performance = average standardized score; functioning = Quality of Life Scale, Abridged20; defeatist beliefs = subscale, Dysfunctional Attitude Scale.23 aP < .05. bP < .01.
Step 1
The path between neurocognitive performance and functioning (top of figure 2) is statistically significant (z = 4.69, P < .001); step 1 is satisfied.
Step 2
The statistically significant path between neurocognitive performance and defeatist beliefs (left side of figure 2; z = −2.49, P < .05) meets the stipulation of this step.
Step 3
The path between defeatist beliefs and functioning (right side of figure 2) is statistically reliable (z = −2.78, P < .01). Patients with poorer functioning tend to endorse more defeatist beliefs, a relationship that is independent of the effect of neurocognitive performance upon functioning.
Step 4
The path between neurocognitive performance and functioning, while the effect for defeatist beliefs is controlled (bottom of figure 2B), is reduced in magnitude, consistent with mediation. The effect decomposition resulting from the bootstrapping procedure appears in table 5. The indirect effect of neurocognitive performance on functioning through defeatist beliefs is statistically reliable (90% CI = −0.28 to −0.04, P < .05), confirming that the data are consistent with mediation.
Table 5.
Bootstrapping Mediation Effect Decompostion: Neurocognitive Performance, Defeatist Beliefs, and Functioning (Patient Data, N = 54)a
| Functioning |
|||
| Neurocognitive Performance | Unstandardized Estimate | Standard Error | Standardized Estimate |
| Direct effect | 3.44c | 1.05 | 0.36 |
| Indirect effect | 1.02b | 0.67 | 0.11 |
| Total effect | 4.46c | 0.95 | 0.47 |
Neurocognitive performance = average standardized score; functioning = Quality of Life Scale, Abridged20; direct effect = neurocognitive performance → functioning; indirect effect = neurocognitive performance → defeatist beliefs → functioning.
P < .05.
P < .01.
Step 5
The path linking neurocognitive performance and functioning, while controlling defeatist belief endorsement (bottom of figure 2B), is statistically reliable (z = 3.02; P < .01), suggesting that the data are consistent with partial mediation. The bootstrapping analysis of the direct path between neurocognitive impairment and negative symptom (table 5) is statistically reliable (90% CI = 0.18 to 0.52, P < .01). The data are consistent with partial mediation. The ratio of the indirect effect to total effect is 0.23; defeatist beliefs account for nearly a quarter of the effect of neurocognition on functioning.
R2 change
The relationship between neurocognitive performance and functioning has an R2 of .22. Adding defeatist beliefs to the model increase the value of R2 to .32. The change of .09 in R2 is statistically different from zero (F-change1,51 = 7.44, P < .01), evidence that inclusion of defeatist beliefs in the model accounts for an additional tenth of the variance.
Discussion
Our findings are consistent with the hypothesis that defeatist beliefs are a mediating variable between cognitive impairment, negative symptomatology, and poor functioning in schizophrenia. Patients showed more defeatist belief endorsement than healthy controls; furthermore, the inclusion of defeatist beliefs in the statistical modeling resulted in an attenuation of the association between cognitive impairment and both negative symptom levels and functioning in the patient sample. Bootstrapping procedures, moreover, confirm the statistical significance of the mediation effect. Defeatist beliefs appear to explain a significant proportion of the variance in both models. The present study, consequently, replicates and expands upon Rector's15original finding of an association between defeatist beliefs and negative symptoms.
Alternative Explanations
Several important competing explanations of the present result can be addressed by the pattern of the data.
Depression
Patient participants manifested mild to moderate levels of depression. However, depression levels are not reliably correlated with neurocognitive performance, negative symptoms, or functioning, and the association between defeatist beliefs and all 3 variables is still significant when depression levels are statistically controlled.
Positive Symptoms
Many of the patient participants had moderate to marked hallucinations and delusions. However, 2 observations are as follows: (a) defeatist beliefs are not associated with psychotic symptoms and (b) defeatist beliefs, and not psychotic symptoms, are associated with neurocognitive impairment and negative symptoms.
Artifact/Halo
The defeatist belief and need for acceptance measures were administered as a part of the same questionnaire (ie, the DAS). Yet, despite both sharing the same response scale and the items appearing interleaved with one another, the defeatist beliefs measure was associated with neurocognitive impairment, negative symptoms, and functioning, while need for acceptance was not associated with any of these variables. Additionally, the measures that have been shown to be related do not all occur in the same measurement modality: symptoms are interviewer scored, defeatist beliefs are self-reported, and cognitive impairment is determined by an objective computerized scoring algorithm.
Implications
In theoretical terms, our findings help to articulate key psychological features of an emerging biopsychosocial model of schizophrenia. While previous research has suggested a link between low self-esteem, cognitive impairment, and poor functioning,38,39 our finding identifies a proximal mechanism, defeatist beliefs regarding performance, that correlates with negative symptoms in addition to neurocognitive impairment and poor functioning. We propose that the intellectual rigidity of the emerging cognitive impairment imposes discouraging life experiences upon the patients leading them to develop negative expectancies and defeatist beliefs. Preliminary data support part of this model because defeatist performance beliefs are associated with negative symptom levels in individuals at high risk for developing psychosis (D. Perivoliotis, A. P. Morrison, P.M.G., P. French, A.T.B., unpublished manuscript, 2008). Ultimately, defeatist beliefs become a critical intermediary process between neurobiological factors and dysfunctional behavior seen in individuals with chronic schizophrenia. In this regard, our finding joins a number of recent reports of the impact of cognitive factors in negative symptoms and functioning.40,41
This emerging literature suggests that defeatist, negativistic beliefs and negative expectancies may contribute to the avoidance of constructive and pleasurable activity seen in individuals with schizophrenia.42 This formulation is consistent with general models of human motivation, in which belief and expectation play important roles,43 and efficacy beliefs, in particular, have been shown to be important mediators between task resources and task engagement.44 Because negative symptoms, cognitive impairment, and functional outcome are all aspects of schizophrenia that do not respond well to traditional pharmacotherapy, and defeatist performance attitudes are connected to all 3, the present results suggest the possibility of improving functional outcome in schizophrenia. Specifically, we propose that eliciting and changing the defeatist performance beliefs could lead to increased engagement in constructive activity in individuals with prominent cognitive impairment and negative symptoms. Defeatist beliefs are, furthermore, measurable targets that can be added to traditional rehabilitation efforts,45 cognitive remediation programs,46 social skills training,47 as well as cognitive behavioral therapy for schizophrenia.48
Limitations
The principal limitations of our study are related to the size and nature (ie, stable outpatients) of our sample, as well as to the cross-sectional methodology. Our data are consistent with other causal hypotheses: thus, negative symptoms could cause defeatist beliefs, defeatist beliefs could cause neurocognitive impairment, etc. A longitudinal study or clinical trial is required to more effectively test between these hypotheses. Two additional limitations of the study merit mention.
Measurement
The present research relied, to a great extent, upon self-report and clinician-rated measures. Behavioral demonstration of the impact of defeatist beliefs, perhaps in the manner analogous to that suggested by Horan et al49 for anhedonia, would buttress the present findings, as would more objective functioning indicators and use of a performance-based measure of functional capacity, such as the University of California Performance Skills Assessment.50
Primary Vs Secondary Negative Symptoms
A priori, negative symptoms can be primary expressions of illness or secondary to another factor.51 Because we cannot attribute the negative symptoms we have measured to psychosis, anxiety, or depression, we propose that neurocognitive deficits appear to be the principal causal factor in the negative symptoms we have observed.
Conclusion
Our findings suggest that further investigation of patient psychological reaction to their cognitive impairments may further clarify the nature of negative symptoms and poor psychosocial adjustment and facilitate treatment that improves functional outcome.
Funding
National Alliance for Research on Schizophrenia and Depression (Distinguished Investigator Award 2006 to A.T.B.); Foundation for Cognitive Therapy.
Acknowledgments
Most of all we would like to thank the patients who took the time to participate in this research and thereby make it possible. Additionally, we express gratitude to Raquel and Ruben Gur, Christian Kohler, Steve Siegel, Jennifer Greene, LaRiena Ralph, Jan Richards, Julie Thysen, Paul Hughett, and the staff of Penn's Neuropsychiatry Section. We are thankful to Greg Brown, Shannon Wiltsey-Stirman, Dara Freedman-Wheeler, Dimitri Perivoliotis, Danielle Farabaugh, Megan Spokas, Maureen Endres, Mary Tabit, and the staff of the Psychopathology Research Unit also at the University of Pennsylvania. We would also like to acknowledge input from Neil Rector, Neal Stolar, Steve Silverstein, Robert DeRubeis, Lois Gelfand, Robert Steer, as well as 5 anonymous reviewers.
Appendix
Defeatist Performance Beliefs
It is difficult to be happy unless one is good looking, intelligent, rich, and creative.
People will probably think less of me if I make a mistake.
If I do not do well all the time, people will not respect me.
Taking even a small risk is foolish because the loss is likely to be a disaster.
If a person asks for help, it is a sign of weakness.
If I do not do as well as other people, it means I am an inferior human being.
If I fail at my work, then I am a failure as a person.
If you cannot do something well, there is little point in doing it at all.
Making mistakes is fine because I can learn from them.
If I fail partly, it is as bad as being a complete failure.
People should have a reasonable likelihood of success before undertaking anything.
If I don't set the highest standards for myself, I am likely to end up a second-rate person.
If I am to be a worthwhile person, I must be truly outstanding in one major respect.
People who have good ideas are more worthy than those who do not.
If I ask a question, it makes me look inferior.
References
- 1.Warner R. Recovery From Schizophrenia: Psychiatry and Political Economy. 3rd ed. Hove, UK: Brunner-Routledge; 2004. [Google Scholar]
- 2.Neumann CS, Walker EF. Developmental origins of interpersonal deficits in schizophrenia. In: Mueser KT, Terrier N, editors. Handbook of Social Functioning in Schizophrenia. Boston, Mass: Ally and Bacon; 1998. pp. 121–133. [Google Scholar]
- 3.Hafner H. Prodrome, onset and early course of schizophrenia. In: Murray RM, Jones PB, Susser E, Van Os J, Cannon M, editors. The Epidemiology of Schizophrenia. Cambridge, UK: Cambridge University Press; 2003. pp. 124–147. [Google Scholar]
- 4.Bromet EJ, Naz B, Fochtmann LJ, Carlson GA, Tanenberg-Karant M. Long-term diagnostic stability and outcome in recent first-episode cohort studies of schizophrenia. Schizophr Bull. 2005;31(3):639–649. doi: 10.1093/schbul/sbi030. [DOI] [PubMed] [Google Scholar]
- 5.Robinson DG, Woerner MG, McMeniman M, Mendelowitz A, Bilder RM. Symptomatic and functional recover from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 2004;161(3):473–479. doi: 10.1176/appi.ajp.161.3.473. [DOI] [PubMed] [Google Scholar]
- 6.Milev P, Ho B, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- 7.O'Leary DS, Flaum M, Kesler ML, Flashman LA, Arndt S, Andreasen NC. Cognitive correlates of the negative, disorganized, and psychotic symptom dimensions of schizophrenia. J Neuropsychiatry Clin Neurosci. 2000;12(1):4–15. doi: 10.1176/jnp.12.1.4. [DOI] [PubMed] [Google Scholar]
- 8.Kirkpatrick B, Fenton W, Carpenter WTJ, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchanan RW, Davis M, Goff D, et al. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005;31(1):5–19. doi: 10.1093/schbul/sbi020. [DOI] [PubMed] [Google Scholar]
- 10.Hughlings Jackson J. Selected Writings. London, UK: Hodder & Stoughton; 1931. [Google Scholar]
- 11.Kraepelin E. Dementia Praecox and Paraphrenia. Huntington, NY: Robert E. Krieger Publishing; 1971. [Google Scholar]
- 12.Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York, NY: International Universities Press, Inc.; 1950. [Google Scholar]
- 13.Williamson P. Mind, Brain, and Schizophrenia. New York, NY: Oxford Press; 2006. [Google Scholar]
- 14.Harvey PD, Green MF, Bowie CR, Loebel A. The dimensions of clinical and cognitive change in schizophrenia: evidence for independence of improvements. Psychopharmacology (Berl) 2006;187(3):356–363. doi: 10.1007/s00213-006-0432-1. [DOI] [PubMed] [Google Scholar]
- 15.Rector NA. Dysfunctional attitudes and symptom expression in schizophrenia: differential associations with paranoid delusions and negative symptoms. J Cogn Psychother: Int Q. 2004;18(2):163–173. [Google Scholar]
- 16.Beck AT. Thinking and depression: idiosyncratic content and cognitive distortions. Arch Gen Psychiatry. 1963;9:324–333. doi: 10.1001/archpsyc.1963.01720160014002. [DOI] [PubMed] [Google Scholar]
- 17.Grant P, Young PR, DeRubeis RJ. Cognitive and behavioral therapies. In: Gabbard GO, Beck JS, Holmes J, editors. Oxford Textbook of Psychotherapy. New York, NY: Oxford University Press; 2005. pp. 15–25. [Google Scholar]
- 18.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, Iowa: University of Iowa; 1984. [Google Scholar]
- 19.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, Iowa: University of Iowa; 1983. [Google Scholar]
- 20.Bilker WB, Brensinger C, Kurtz MM, et al. Development of an abbreviated schizophrenia quality of life scale using a new method. Neuropsychopharmacology. 2003;28(4):773–777. doi: 10.1038/sj.npp.1300093. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA, Brown GK. The Beck Depression Inventory—Second Edition. San Antonio, Tex: The Psychological Corporation; 1996. [Google Scholar]
- 22.Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, Tex: The Psychological Corporation; 1990. [Google Scholar]
- 23.Weissman A. The Dysfunctional Attitudes Scale: A Validation Study. Philadelphia, Pa: University of Pennsylvania; 1978. [Google Scholar]
- 24.Gur RC, Ragland JD, Moberg PJ, et al. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25(5):766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 25.Gur RC, Ragland JD, Moberg PJ, et al. Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology. 2001;25(5):777–788. doi: 10.1016/S0893-133X(01)00279-2. [DOI] [PubMed] [Google Scholar]
- 26.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 27.Kurtz MM, Ragland JD, Moberg PJ, Gur RC. The Penn Conditional Exclusion Test: a new measure of executive-function with alternate forms for repeat administration. Arch Clin Neuropsychol. 2004;19(2):191–201. doi: 10.1016/S0887-6177(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 28.Glahn DC, Cannon TD, Gur RE, Ragland JD, Gur RC. Working memory constrains abstraction in schizophrenia. Biol Psychiatry. 2000;47(1):34–42. doi: 10.1016/s0006-3223(99)00187-0. [DOI] [PubMed] [Google Scholar]
- 29.Gur RC, Jaggi JL, Ragland JD, et al. Effects of memory processing on regional brain activation: cerebral blood flow in normal subjects. Int J Neurosci. 1993;72(1–2):31–44. doi: 10.3109/00207459308991621. [DOI] [PubMed] [Google Scholar]
- 30.Kurtz MM, Ragland JD, Bilker WB, Gur RC, Gur RE. Comparison of continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophr Res. 2001;48(2–3):307–316. doi: 10.1016/s0920-9964(00)00060-8. [DOI] [PubMed] [Google Scholar]
- 31.Gur RE, Nimgaonkar VL, Almsay L, et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164(5):813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 33.Baron RM, Kenny DA. The mediator-moderator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 34.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7(4):422–445. [PubMed] [Google Scholar]
- 35.Kline RB. Principles and Practice of Structural Equation Modeling. New York, NY: Guilford Press; 2005. [Google Scholar]
- 36.Frazier PA, Tix AP, Barron KE. Testing moderator and mediator effects in counseling psychology research. J Couns Psychol. 2004;51:115–134. [Google Scholar]
- 37.Kenny DA. Mediation. http://davidakenny.net/cm/mediate.htm. Accessed December 8, 2007. [Google Scholar]
- 38.Brekke JS, Kohrt B, Green MF. Neuropsychological functioning as a moderator of the relationship between psychosocial functioning and the subjective experience of the self and life in schizophrenia. Schizophr Bull. 2001;27(4):697–708. doi: 10.1093/oxfordjournals.schbul.a006908. [DOI] [PubMed] [Google Scholar]
- 39.Bradshaw W, Brekke JS. Subjective experience in schizophrenia: factors influencing self-esteem, satisfaction with life, and subjective distress. Am J Orthopsychiatry. 1999;69(2):254–260. doi: 10.1037/h0080427. [DOI] [PubMed] [Google Scholar]
- 40.Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93(1–3):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Granholm E, Verney SP, Perivoliotis D, Miura T. Effortful cognitive resource allocation and negative symptom severity in chronic schizophrenia. Schizophr Bull. 2007;33(3):831–842. doi: 10.1093/schbul/sbl040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rector NA, Beck AT, Stolar NM. The negative symptoms of schizophrenia: a cognitive perspective. Can J Psychiatry. 2005;50(5):247–257. doi: 10.1177/070674370505000503. [DOI] [PubMed] [Google Scholar]
- 43.Eccles JS, Wigfield A. Motivational beliefs, values and goals. Annu Rev Psychol. 2002;53:109–132. doi: 10.1146/annurev.psych.53.100901.135153. [DOI] [PubMed] [Google Scholar]
- 44.Llorens S, Schaufeli W, Bakker A, Salanova M. Does a positive gain spiral of resources, efficacy beliefs and engagement exist? Comput Human Behav. 2007;23(1):825–841. [Google Scholar]
- 45.Davis LW, Lysaker PH, Lancaster RS, Bryson GJ, Bell MA. The Indianapolis Vocational Intervention Program: a cognitive behavioral approach to addressing rehabilitation issues in schizophrenia. J Rehabil Res Dev. 2005;42(1):35–46. doi: 10.1682/jrrd.2003.05.0083. [DOI] [PubMed] [Google Scholar]
- 46.Wykes T, Reeder C. Cognitive Remediation Therapy for Schizophrenia. New York, NY: Routledge; 2005. [Google Scholar]
- 47.Kopelowicz A, Liberman RP, Zarate R. Recent advances in social skills training for schizophrenia. Schizophr Bull. 2006;32(suppl 1):12–23. doi: 10.1093/schbul/sbl023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrison AP, Renton JC, Dunn H, Williams S, Bentall RP. Cognitive Therapy for Psychosis: A Formulation-Based Approach. Hove, UK: Brunner-Routledge; 2004. [Google Scholar]
- 49.Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull. 2006;32(2):259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD performance-based skills assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. 2001;27(2):235–245. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- 51.Carpenter WTJ, Heinrichs DW, Wagman AMI. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145(5):578–583. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]